Abstract
The microphthalmia (MiT) subfamily of transcription factors includes TFE3, TFEB, TFEC, and MITF. In the 2016 World Health Organization classification, MiT family translocation renal cell carcinoma (tRCC) including Xp11 tRCC and t(6;11) RCC, was newly defined as an RCC subtype. Xp11 and t(6;11) RCC are characterized by the rearrangement of the MiT transcription factors TFE3 and TFEB, respectively. Recent studies identified the fusion partner-dependent clinicopathological and immunohistochemical features in TFE3-rearranged RCC. Furthermore, RCC with TFEB amplification, melanotic MiT family translocation neoplasms, was identified may as a unique subtype of MiT family associated renal neoplasms, along with MITF associated RCC. In this review, we will collect available literature of these newly-described RCCs, analyze their clinicopathological and immunohistochemical features, and summarize their molecular and genetic evidences. We expect this review would be beneficial for the understanding of these rare subtypes of RCCs, and eventually promote clinical management strategies.
Keywords: Microphthalmia, TFE3, TFEB, MITF, Kidney, Renal cell carcinoma, Translocation
The microphthalmia (MiT) family of transcription factors comprises four distinctly encoded genes: MITF, TFEB, TFE3, and TFEC. All family members share sequence homology in their DNA-contacting basic domains and the transactivation domains, recognize similar DNA sequences, indicating potential overlap in their target gene repertoire. Additionally, these factors can heterodimerize with each other [1]. They are physiologic regulators of cell growth, differentiation, and survival in several tissue types. Several distinct tumors are associated with the dysregulation of this gene family, including renal cell carcinoma (RCC), melanoma, alveolar soft part sarcoma (ASPS), clear cell sarcoma, angiomyolipoma, and perivascular epithelioid cell neoplasms (PEComas). All these tumors have been considered to be members of the family of tumors, owing to their histological, immunohistochemical and molecular genetic similarities.
The new category of MiT family translocation RCC has been incorporated into the World Health Organization (WHO) classification in 2016 [2]. The MiT family RCC chiefly consists of Xp11 translocation RCC harboring TFE3 gene fusions and t(6;11) RCC harboring a MALAT1–TFEB gene fusion. RCCs associated with the other two MiT family members, MITF and TFEC, have rarely been reported. Recent findings indicate that MITF has an important oncogenic function in tumorigenesis of multiple tissues/melanocytes and kidney cells [3]. TFEC was first identified in cells of the mononuclear phagocyte lineage, coexpressed with other members of the MiT family. Within this lineage, TFEC's expression is restricted to macrophages, and its function has not been widely investigated. This review summarizes our current knowledge of these recently described RCCs.
1. Xp11 translocation RCCs (tRCCs)
Xp11 tRCCs are a distinctive subtype of RCC characterized by several different translocations that involve chromosome Xp11.2, resulting in gene fusions of the TFE3 transcription factor gene. In these tumors, the TFE3 gene is fused by translocation to one of several other genes, including ASPL (17q25), PRCC (1q21.2), NONO (P54NRB) (Xq12), CLTC (17q23), SFPQ (PSF) (1p34), LUC7L3 (17q21.33), KHSRP (19p13.3), PARP14 (3q21.1), DVL2 (17p13.1), RBM10 (Xp11.3), GRIPAP1 (Xp11.23), MED15 (22q11.21) and unknown partner genes on chromosomes 3, 10 and 19 (Table 1) [4], [5]. The three most common Xp11 tRCCs are those bearing the t(X;1)(p11.2;q21) which fuses the PRCC and TFE3 genes, the t(X;17)(p11.2;q25) which fuses the ASPSCR1 and TFE3 genes, and the t(X;1)(p11.2;p34) which fuses the SFPQ (PSF) and TFE3 genes. Of interest, both ASPL–TFE3 RCC and ASPS harbor the same ASPL–TFE3 fusion gene. However, the translocation is balanced in ASPL–TFE3 RCC and is unbalanced in ASPS, which may contribute to the differences seen at the clinical and histopathologic levels between Xp11 translocation RCC and ASPS.
Table 1.
TFE3, TFEB and MITF gene fusions.
| Neoplasm | Fusion | Translocation | Age range (year) |
|---|---|---|---|
| ASPS | ASPSCR1–TFE3 | der(17)(X;17)(p11.2q25) | 1–71 |
| RCC | ASPSCR1–TFE3 | t(X;17)(p11.2;q25) | 1–75 |
| RCC | PRCC–TFE3 | t(X;1)(p11.2;q21) | 2–69 |
| RCC | SFPQ–TFE3 | t(X;1)(p11.2;q34) | 3–68 |
| RCC | NonO–TFE3 | inv(X)(p11.2;q12) | 39 |
| RCC | CLTC–TFE3 | t(X;17)(p11.2;q23) | 14 |
| RCC | LUC7L3–TFE3 | t(X;17)(p11.2;q21) | 20 F |
| RCC | KHSRP–TFE3 | t(X;19)(p11.2;p13) | |
| RCC | PARP14–TFE3 | t(X;3)(p11.2;q23) | 45 F |
| RCC | DVL2–TFE3 | t(X;17)(p11.2;p13.1) | 73 M |
| RCC | RBM10–TFE3 | Inv(X)(p11.2;p11.23) | 32–61 |
| RCC | GRIPAP1–TFE3 | inv(X)(p11.23,p11.23) | 40 F |
| RCC | MED15–TFE3 | t(X;22)(p11.2;q11.2) | 34 F |
| RCC | Unknown | t(X;3)(p11.2;q23) | 32 |
| RCC | Unknown | t(X;10)(p11.2;q23) | 77 |
| Melanotic Xp11 translocation cancer | SFPQ(PSF)–TFE3 | t(X;1)(p11.2;q34) | 11–55 |
| ARID1B–TFE3 | T(X:6)(p11.2;q25.3) | ||
| Melanotic t(6;11) renal cell carcinoma | MALAT1(Alpha)–TFEB | t(6;11)(p21;q12) | |
| Xp11 PEComa | SFPQ–TFE3 and others | t(X;1)(p11.2;q34) and others | 9–55 |
| Subset of epithelioid hemangioendothelioma | YAP1–TFE3 | t(X;11)(p11.2;q13) | 14–50 |
| RCC | MALAT1(Alpha)–TFEB | t(6;11)(p21;q12) | 3–68 |
| RCC | CLTC–TFEB | t(6;17)(p21;q23) | |
| RCC | KHDRBS2–TFEB | inv(6)(p21q11) | |
| RCC | COL21A1–TFEB | inv(6)(p21p12) | |
| RCC | TFEB–CADM2 | t(3;6)(p12;p21) | |
| RCC | ACTG1–MITF | t(17;3)(q25.3;p13) | |
| RCC | PRCC–MITF | t(1;3)(q21;p13) | 45 |
ASPS, alveolar soft part sarcoma; PEComa, perivascular epithelioid cell neoplasm; RCC, renal cell carcinoma; F, female; M, male.
The function of chimeric TFE3 fusion proteins can also vary. Tumors with different specific gene fusions may have different clinical manifestations and morphological features.
1.1. Clinical features
Xp11 tRCC comprises 20%–40% of childhood RCC and 1%–4% of adult RCC with an average age of onset of 50 years [6]. The frequency of Xp11 tRCC in adults may be underestimated, perhaps due to morphological overlap with more common adult RCC subtypes, such a conventional clear cell RCC and papillary RCC [7]. Xp11.2 RCC is one of the fewer subtypes of RCC that occurs in females more frequently than males (2.5:1), and the age at presentation is typically earlier than with other RCC subtypes (i.e., in the third to fifth decades) [8]. Clinically, the typical presenting features of hematuria, flank pain, or abdominal pain are the most common presenting features for translocation carcinomas. As is common for other forms of renal cell neoplasia, approximately one-third of tumors present as an asymptomatic, painless renal mass, often identified accidentally during abdominal imaging.
Prior exposure to cytotoxic chemotherapy is currently the only known risk factor for development of Xp11 tRCC. tRCC has been documented in patients with a history of prior chemotherapy for various cancers or inflammatory conditions (Wilms tumor, Ewing sarcoma, systemic lupus erythematosus, acute leukemia, and bone marrow transplant), possibly accounting for 15%–20% of all pediatric tRCC cases [9]. The post chemotherapy interval ranged from 4 to 13 years, though more recent studies have documented occurrence of Xp11 tRCC within 2 years of chemotherapy. In adults, although Xp11 tRCC has been reported during pregnancy or in association with end stage renal diseases and hemodialysis, no studies have been carried out to identify particular risk factors.
1.2. Pathologic features
1.2.1. Gross findings
Gross examination of Xp11.2 tRCCs includes lesions that can range from small (1–2 cm) to quite large (up to 20 cm); smaller lesions tend to be well circumscribed, whereas larger lesions may be irregular in shape and extend beyond the confines of the kidney. They present as solitary cortical masses characterized by tan-yellow cut surfaces with foci of hemorrhage and necrosis, and occasionally focal cystic degeneration is present. Other cases are more grayer and appear grossly papillary.
1.2.2. Microscopic features
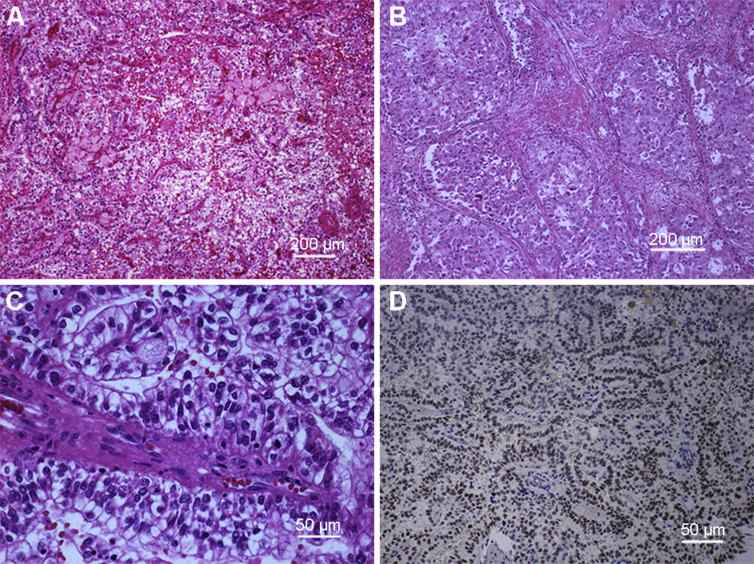
Histologically, Xp11.2 tRCCs are characterized by heterogeneous architectural and cytologic features. The most distinctive histologic pattern is that of a neoplasm featuring papillary architecture and epithelioid clear cells. Architecturally, these tumors can contain true papillae, or pseudopapillae, broad sheets, nested, trabeculae, and solid growth patterns. Tumor cells are clear to eosinophilic with varying amounts of cytoplasm (moderate to voluminous). The nuclei may show some variability in size and are generally large with a prominent eosinophilic nucleolus (typically International Society of Urologic Pathology [ISUP] 3 or 4) (Fig. 1). Eosinophilic hyaline droplets, psammoma bodies, and large calcifications may also be present. Focal necrosis is a common feature, and rarely there may be a lymphocytic infiltrate or aggregates of foamy macrophages [10], [11].
Figure 1.
Xp11 translocation renal cell carcinoma (RCC). (A–C: Hematoxylin and eosin stains) (A) Xp11 tRCC showing compact nested and papillary architecture at low power; (B) Xp11 tRCC showing solid nested architecture with eosinophilic cytoplasm; (C) Xp11 tRCC at higher power showing cells with voluminous clear cytoplasm and vesicular nuclei with prominent nucleoli; (D) Immunohistochemistry for TFE3 showing nuclear labeling of neoplastic cells; note the absence of labeling in native endothelial cells.
However, there has been increasing evidence that Xp11 tRCCs often present with unusual morphological features, which can mimic other types of RCC (e.g., clear cell RCC, multilocular cystic RCC, collecting duct carcinoma), and can also be mimicked by some other atypical tumors (e.g., high-grade urothelial carcinoma and well-developed fascicles of spindled neoplastic cells with bland nuclei and focal myxoid stroma). The wide spectrum of morphology seen in Xp11 tRCCs emphasizes the need to consider these carcinomas in the differential diagnosis of unusual, difficult to classify RCCs occurring in both children and adults.
Different gene fusions in Xp11 tRCC may be associated with differing morphologic features. Tumors with the ASPL–TFE3 fusion are predominantly nested with pseudopapillary architecture, and foam cells are not a feature, although psammoma bodies may be present [12]. Carcinomas with the PRCC–TFE3 fusion usually show a papillary or alveolar architecture [13]. Foam cells and more rarely psammoma bodies may be seen, and there is often focal tumor necrosis. However, these are only trends, and there can be morphologic overlap among tumors with different gene fusions. Typical morphologic features associated with the other identified gene fusions have not been described due to the rarity of cases.
1.2.3. Immunohistochemistry
Unlike other types of RCC, Xp11 tRCCs are either negative or only focally positive for epithelial markers such as cytokeratins, cytokeratin 7 (CK7), and epithelial membrane antigen (EMA) and have variable vimentin expression. CK7 is typically negative. These tumors are also generally positive for the RCC immunostaining (CD10, RCC marker, racemase [AMACR; P504S], PAX2 and PAX8) [14]. Occasionally, Xp11 tRCCs may express melanocytic markers such as Melan-A and HMB-45, particularly in cases associated with less common gene fusions. Cathepsin K to be a transcriptional target of the MITF family, an immunohistochemistry antibody to cathepsin K has been utilized in the diagnosis of MITF family tRCC. Cathepsin K is overexpressed in a subset of Xp11 tRCCs (approximately 60%) and in almost all t(6;11) RCCs, but not in other types of RCCs. Interestingly, PRCC–TFE3 RCC label more frequently for cathepsin K than do the ASPSCR1–TFE3 RCC in the limited number of cases tested, suggesting that there are functional differences between the resulting fusion proteins [15].
The most sensitive and specific marker for the Xp11 tRCC is strong nuclear TFE3 immunoreactivity, using an antibody to the C-terminal portion of TFE3 [16]. However, false-positive and false-negative results are quite frequent due to differences in fixation times, technical methods and scoring systems. Furthermore, ALK-rearranged RCCs often show positive nuclear TFE3 immunostaining, so the immunohistochemical positivity for TFE3 should be cautiously interpreted [17]. TFE3 break-apart fluorescence in situ hybridization (FISH) assay on formalin-fixed paraffin embedded tissue sections is currently the gold standard for identifying TFE3 rearrangements and often results in large-space split signals from a translocation [18].
1.3. Prognosis and treatment
From a clinical outcome perspective, outcome data on Xp11 tRCC remain at a premature stage because of its relatively rare incidence. Outcomes have been highly variable, with some patients surviving decades with indolent disease and others dying rapidly of progressive disease. Overall, Xp11 tRCC frequently shows lymph node metastasis and has a worse prognosis than papillary RCC and similar prognosis with clear cell RCC [19]. Several studies have demonstrated that Xp11 tRCCs in childhood patients have a relatively indolent course, despite their often advanced stage at presentation [20]. Xp11 tRCCs have the potential to metastasize as late as 20–30 years after diagnosis [21]. Therefore, good long-term follow-up data are necessary before any favorable short-term outcome can be confirmed. Among Xp11 tRCCs, Xp11 tRCC with an ASPSCR1–TFE3 fusion, which is also detected in alveolar soft part sarcoma, was reported to have a worse prognosis than Xp11 tRCCs with other fusion partners, but it is still unclear whether the fusion partner plays a prognostic role [19].
The optimal therapy for the Xp11 tRCCs remains to be determined, as clinical trials have been mainly conducted in patients with clear cell histology. For localized Xp11 tRCC, including patients with positive regional lymph nodes, surgery is the treatment of choice. For patients with hematogenous metastases, the current options are immunotherapy using cytokines, such as interleukin-2 and interferon-alfa, and multikinase inhibitors. However, Xp11 tRCC has poor prognosis regardless of treatment. Therefore, new, effective treatments are desperately needed for patients with this tumor type. Therapies targeting vascular endothelial growth factor receptor and mammalian target of rapamycin may benefit patients with Xp11 tRCC [22], [23]; the MET signaling pathway is another possible target, since it is activated by ASPL–TFE3 fusion [24]. A recent study finds that positive PD-L1 expression is independently associated with tumor progression and predicts an adverse prognosis for Xp11 tRCC patients. These suggest that immunotherapy targeting the PD-1/PD-L1 pathway may represent a potential novel treatment for Xp11 tRCC patients [25].
2. Melanotic MiT family translocation neoplasms
In recent years, an increasing number of MiT family translocation neoplasms with melanotic features have been reported, such as melanotic Xp11 translocation renal cancer, TFE3 rearrangement-associated PEComas, Xp11 neoplasm with melanocytic differentiation, and melanotic t(6;11) RCC. These tumors share similar morphologic characteristics, such as purely nested or sheet-like architectures separated by delicate vascular networks, purely epithelioid cells containing a clear or granular eosinophilic cytoplasm, uniform round or oval nuclei containing small visible nucleoli, and in most cases, melanin pigmentation. By immunohistochemistry, the tumor cells were immunoreactive for the melanocytic markers HMB45 and Melan A, but not for cytokeratins, muscle markers SMA, S100, and the renal tubule markers CD10, PAX2, and PAX8 [26].
In 2009 Argani et al. [27] first described a distinctive subset of MiT family renal tumors designated melanotic Xp11 translocation renal cancer. These authors later noted that melanotic Xp11 translocation renal cancer shares many common features with subsequently described extra-renal PEComas with TFE3 fusions [28]. However, all these lesions have overlapping features driven by the underlying TFE3 gene fusion and can all be placed in the category of MiT family associated cancers. To date only 20 cases have been described. Clinically these tumors tend to occur in younger patients (11–46 years, and mean age of 27 years) and had a propensity towards aggressive tumor behavior, where most cases are metastatic at initial presentation. It can present with flank pain, abdominal pain, constitutional symptoms, or metastasis. African and Asian ethnical groups are predominant among affected individuals. No associations with oncogenetic syndromes or specific environmental exposures have been found [29].
They are characterized by epithelioid nested morphology, cytoplasmic melanin pigment with positive immunoreactivity for melanocytic markers (HMB45 and Melan-A), negative staining for melanoma (S100 and MITF), epithelial, muscle and renal tubular markers, and positive staining for the MiT family transcription factor TFE3 (however negative for MITF which is usually positive in melanoma and PEComa). Most cases have demonstrated an SFPQ (PSF)–TFE3 gene fusion. Antic et al. [30] described a rare melanotic translocation RCC with a novel ARID1B–TFE3 gene fusion. Immunostains were positive for TFE3 and HMB45 and negative for PAX8, MITF, and CAIX; keratins Cam 5.2 and AE1/AE3 were focally positive. The patient is alive and without evidence of disease 7 years after his diagnosis. Cardili et al. [31] reported herein a rare case of TFE3-related melanotic renal cell tumor showing an unusual immune expression of cytokeratins (AE1/AE3) and RCC markers (RCC, CD10). Cathepsin K and vimentin were diffusely positive whereas melanocytic markers (HMB-45 and Melan-A) displayed weak and patchy expression. PAX-8, muscle markers and S-100 were negative. TFE3 fusion was confirmed by break-apart FISH. Different fusion partners might explain these intriguing phenotype variations.
Saleeb et al. [29] described the first reported case of melanotic t(6;11) RCC. Morphologically, this case was very similar to the melanotic Xp11 tRCC, having clear to eosinophilic epithelioid cells, nested alveolar architecture and intracytoplasmic melanin. This case exhibiting both epithelial and melonocytic differentiation was proven by positivity for epithelial markers and melanocytic markers (HMB45 and Melan-A).
This malignancy has various histologic and immunophenotypic features that overlap with melanoma, RCC, and PEComa. In summary, the expression of cytokeratins and RCC markers corroborates previous evidence for overlap in the MITF associated cancer family, remarkably for pigmented tumors. It may not be possible to set a clear cutoff between the epithelial and mesenchymal subgroups, at least in some cases.
3. t(6;11) RCCs
The t(6;11) RCC is an extremely rare variant and accounts for 0.02% of all renal carcinomas [32]. The t(6;11) RCC was first described by Argani et al. [33] in 2001. While their lineage was initially unclear, the t(6;11) RCC has now been accepted by the 2016 WHO Renal Tumor Classification as a subtype of the MiT family of translocation RCC [2]. The t(6;11) translocation fuses the gene for TFEB, a transcription factor related to MITF, with Alpha (MALAT1), an untranslated gene of unknown function, resulting in overexpression of native TFEB.
3.1. Clinical features
The t(6;11) RCCs are less common than the Xp11 tRCCs; only approximately 70 cases have been documented in the literature, the majority of which occurring in children and adolescents, however, more recent literature has demonstrated that these neoplasms can present in adults as well [34], [35], [36]. The mean age of presentation is 34 years, with a wide reported range of 3–77 years. The male-to-female ratio approximately is 1.1:1. The tumor size ranges from 1.0 cm to 27.0 cm with a mean of 7.5 cm. The patients may present with hematuria, abdominal pain or an abdominal mass. The tumor may be also incidentally. Similar to the Xp11 tRCC, a subset of cases have occurred in patients who have received cytotoxic chemotherapy for other disorders.
3.2. Pathologic features
3.2.1. Gross findings
The t(6;11) RCCs do not have a distinctive gross appearance. The tumors are generally well circumscribed and satellite nodules around the main tumor are often observed. Some cases have had a mahogany color and soft texture similar to that of oncocytoma, where others have been grayer and still others have been focal cystic change, hemorrhage or necrosis [37].
3.2.2. Microscopic features
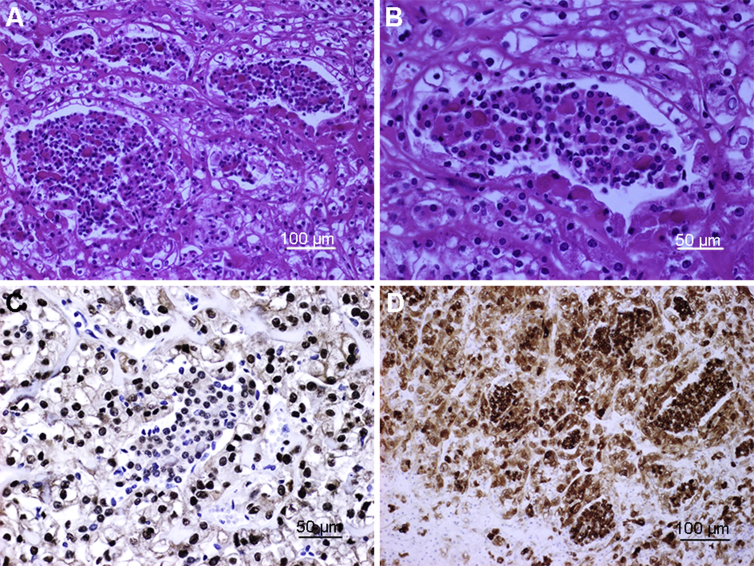
Histologically, t(6;11) RCCs typically show a biphasic morphology, composed of large and small epithelioid cells, which often form nests of rosettes clustered around the basement membrane. The larger cells have clear to eosinophilic cytoplasm, and the smaller cells have narrow cytoplasm with condensed chromatin. (Fig. 2). These neoplasms typically do not show prominent cytologic atypia or mitotic activity. The biphasic cell population (large and small cells) is an important diagnostic clue. More recently, however, other studies have found that this unique morphology is not always straight forward, and that some molecularly confirmed cases may have unusual morphological features, such as epithelioid angiomyolipoma-like, papillary, tubulocystic, oncocytoma-like, chromophobe cell RCC, clear cell RCC, multilocular cystic, higher grade unclassified, and extensive hyalinization or sclerotic, ossified features. In some tumors, pigment and psammoma bodies can be observed. These unusual features have expanded the morphologic spectrum of TFEB RCCs [38], [39].
Figure 2.
The t(6;11) renal cell carcinoma (RCC). (A,B) The t(6;11) RCCs typically show a biphasic morphology, with larger epithelioid cells at the periphery of the nests and smaller cells clustered around hyaline basement membrane material in the center; (C) Immunohistochemistry for TFEB showing strong nuclear labeling of neoplastic cells; note the absence of labeling in native endothelial cells; (D) Diffuse cytoplasmic staining with Melan A in both types of cells.
3.2.3. Immunohistochemistry
Immunohistochemically, previous literature reports that most t(6;11) RCCs express melanocytic markers, such as HMB-45 and Melan-A, but are either negative or only focally positive for epithelial markers, such as cytokeratins and EMA. Cathepsin K is overexpressed in almost all t(6;11) RCCs. Most cases express PAX8, supporting renal tubular differentiation. TFE3 is consistently negative in this tumor. Diffuse nuclear immunoreactivity for TFEB is a critical diagnostic marker for t(6;11) RCCs. Immunoreactivity for TFEB is highly sensitive and specific for this tumor, as exemplified by Argani et al. [40] who could not demonstrate any staining for TFEB in any of the 1089 other tumors, including Xp11 tRCCs. However, the results of immunostainings can be inconsistent among laboratories, mainly because of technical factors such as fixation time and differences in the methods of antigen retrieval, the scoring system, and antibody specificity and sensitivity. Ultimately, the identification of these neoplasms as t(6;11) RCC should be confirmed definitively by TFEB FISH studies [40], [41].
3.3. Molecular genetic feature
Genetically, t(6;11) RCC is characterized by the fusion of the 5′ portion of the MALAT1 (also known as Alpha) gene mapped at 11q12 with the TFEB gene located at 6p21 [42]. MALAT1 is a well-known long non-coding RNA that fuses to TFEB upstream of the translation initiation codon ATG in exon 3 or exon 4 (downstream of the wild-type ATG in exon 3) [43].Therefore, the fusion transcript of MALAT1–TFEB encodes full length TFEB.
More recently, Malouf et al. [44] identified a novel fusion partner of TFEB (TFEB–KHDRBS2) in the TCGA database of clear cell RCC. Durinck et al. [45] found an unreported gene fusion involving CLTC and TFEB (CLTC–TFEB) in a non-clear cell RCC sample that was designated as unclassified. The comprehensive molecular characterization by the TCGA research network identified two new fusion partners of TFEB (COL21A1–TFEB and TFEB–CADM2) in two cases of papillary RCC [46].
3.4. Prognosis and treatment
Most instances of t(6;11) RCC have an indolent clinical course with a few published cases demonstrating aggressive behavior. Most neoplasms have presented at low stage (pT1 or pT2) and have had benign follow-up. With respect to aggressive t(6;11) RCC, review of the literature provides some useful information [34], [35], [36]. RCC with TFEB rearrangement displayed an aggressive behavior in roughly 17% of cases (12 of 71), occurring as larger masses (12 vs. 7 cm) in older patients (46 vs. 31 years). It should be noted that hematogenous metastasis are more common than nodal metastasis (3 of 12). There are no well-established prognostic markers or protocols predicting biological behavior that are applicable for t(6;11) RCC.
The clinical behavior of t(6;11) RCC remains unestablished due to their rare incidence. Extirpative surgery remains the lone therapeutic strategy as no proven neoadjuvant or adjuvant therapies exist. Like the Xp11 tRCC, these neoplasms have demonstrated the capacity to recur late (up to 8 years after diagnosis), so long-term follow-up is important for these patients. However, given the more aggressive behavior in adult populations as well as the potential for recurrence and metastasis, a more aggressive postoperative follow-up may be warranted.
4. RCCs with TFEB amplification
More recently, RCC with TFEB amplification have been identified and appear to be associated with a more aggressive clinical course than TFEB tRCC. The first case of TFEB-amplified RCC was published in 2014 by Peckova et al. [47]. To date, 39 cases of high-level amplification of TFEB in RCCs have been reported [48]. Argani et al. [49] reported the first series of eight cases of TFEB-amplified RCC (six without TFEB rearrangement and two with TFEB rearrangement). Gupta et al. [50] noticed the proximity of TFEB and VEGFA at 6p21.1. The two genes were co-amplified in 11 TFEB amplified RCC.
Morphologically, TFEB-amplified RCC frequently shows nests of high ISUP grade epithelioid cells with eosinophilic cytoplasm associated with pseudopapillary formation and necrosis, or true papillary formations. These patterns raise the differential diagnosis of high grade clear cell and papillary RCC. Although all TFEB-amplified RCCs showed aberrant melanocytic marker expression, TFEB-amplified RCCs were different from t(6;11) RCC in some ways. For example, TFEB-amplified RCC occurred in older patients (23–83 years; mean age, 63 years) compared with unamplified t(6;11) RCC (3–77 years; mean age, 34 years). TFEB-amplified RCC was associated with a more aggressive clinical course, 62% of patients presented with pT2 disease or higher, and 46% presented with pT3 disease or higher. TFEB and melanocytic marker expression was more variable within the TFEB-amplified RCC. Given that TFEB immunoreactivity is less in TFEB-amplified RCC than unamplified t(6;11) RCC, it seems unlikely that an increased cellular level of TFEB in the TFEB-amplified RCC relative to that in the usual t(6;11) RCC is the key reason for the aggressive behavior. Instead, it seems likely that additional concurrent genetic alterations which are as yet unknown (such as genomic instability) drive the aggressive clinical behavior [51].
TFEB-amplified RCC may constitute a novel entity with a poor prognosis that warrants novel therapeutic approaches. Because of their relatively recent identification, such RCC with TFEB amplification have not been given a formal name or included in the WHO classification. Its pathogenesis should be further characterized to develop appropriate targeted therapy.
5. MITF associated RCC
MITF, one member of the MITF family of transcription factors, encodes a member of the Myc supergene family of transcription factors, which is thought to function as a melanoma oncogene. A germline missense variant of MITF (c.952G-A; p.E318K) has been identified at higher frequency in patients with family history of cutaneous malignant melanoma (CMM) or primary multiple melanomas relative to healthy controls [52]. MITF p.E318K occurs at a conserved SUMOylation position and this variant decreases the number of SUMO-modified MITF forms. As SUMOylation of MITF represses its transcriptional activity, p.E318K increases the MITF transcriptional activity and upregulates HIF-1α, which is known to drive renal tumorigenesis [3]. Interestingly, a significantly increased frequency of RCC compared to normal controls was detected in melanoma patients with the p.E318K germline mutation in MITF. The prevalence of p.E318K in melanoma and RCC patients ranges from 2.2% to 4%, whilst in a control population prevalence of this variant is 0.6% in French and Italian populations and 0.8% in UK and Australian populations [52], [53]. These findings suggest MITF as a possible connection point between melanoma and RCC.
Recent studies have shown that the same MITF mutation associated with increased risk of melanoma (E318K) also leads to increased risk of RCC. These studies indicate that the missense variant p.E318K is more often observed in patients affected by multiple primary cancers, such as melanoma + RCC or by multiple primary melanomas and associated odds ratios (ORs) ranged from 4.22, 95% confidence interval (CI) (1.52, 10.91) (Australian study), 7.79, 95% CI (3.12, 20.04) (French study) and 6.4, 95% CI (1.43, 28.58) (Italia study) for multiple melanomas to 14.46, 95% CI (3.74, 48.04) (French study) and 7.9, 95% CI (1.62, 39.4) (Italia study) for melanoma + RCC [52], [53], [54]. Collectively, MITF might be the missing link between melanoma and kidney cancer and as the first common inherited factor between these two cancers.
More recently, two studies reported that the MITF p.E318K mutation does not seem to play a major role in sporadic RCC development in Caucasians and Polish, but is possibly restricted to a rare subpopulation of inherited RCC [55], [56]. Although further research is necessary, the evidence strongly supports a role for the MITF p.E318K variant as a medium-penetrance, germline mutation that predisposes to melanoma and RCC and potentially other cancers as well.
Considering the overlapping functions of MiT transcription factor family members, MITF genetic abnormalities could create a neoplasm similar to the RCC resulting from TFE3 or TFEB gene fusions in theory. However, MITF has rarely been reported. To our knowledge, only two MITF gene fusion RCC was reported.
Durinck et al. [45] identified an ACTG1–MITF gene fusion in a papillary RCC sample (this case also exhibited oncocytic and papillary features), and found that the tumor expressing the fusion had a higher level of MITF expression than the matched normal sample. Xia et al. [57] reported another case of novel PRCC–MITF gene fusion RCC by RNA-sequencing and specifically described its clinicopathologic and molecular features. This tumor demonstrated the overlapping histology of Xp11 tRCC and t(6;11) RCC, including an alveolar or nested architecture, discrete cell borders, psammoma bodies, “pseudorosettes” formation and basement membrane material. The tumor was immunohistochemically negative for TFE3 and TFEB but strongly positive for cathepsin K. These two cases prove the recurring existence of MITF tRCC and expand the genotype spectrum of MiT family tRCCs. The clinicopathologic manifestations of MITF tRCC need to be further explored and elucidated. Altogether, MITF is the third member of the MiT family that might play a critical role in kidney cancer.
6. Conclusion
In summary, MiT family associated RCC is a rare subtype of RCC with genetic abnormalities (gene fusion, amplification, and mutation etc.) involving members of the MiT family of transcription factors, including TFE3, TFEB and MITF. This review highlights that MiT family associated RCCs are clinicopathologically and molecularly diverse. Several different tumor types can affect the kidney, ranging from indolent tumors treated primarily with surgery to more aggressive entities which require multimodal therapy. More common tumors such as Xp11 tRCC and t(6;11) RCC have included in the WHO classification. More rare entities will by definition have only case series and case reports (such as TFEB amplified RCC, MITF associated RCC, melanotic MiT family translocation neoplasms). There has been increasing awareness that renal cell tumors, which may seem histopathologically similar across many tumors, actually represents a group of molecularly distinct tumors. The increased understanding of morphological, immunohistochemical, molecular, and epidemiological features of RCCs enables us to categorize renal neoplasms into subtypes/entities with distinct characteristics. At the current time, there is standard treatment protocol for patients with MiT family associated RCC. Future studies will utilize emerging genomic technologies to identify prognostic factors and therapeutic targets in these neoplasms.
Conflicts of interest
The authors declare that they have no conflict of interest.
Author contributions
Conception and design: Chin-Lee Wu.
Review of the literature: Chin-Lee Wu, Yifen Zhang, Ling Xie.
Drafting the manuscript: Ling Xie.
Critical revision of the manuscript: Chin-Lee Wu, Yifen Zhang.
Footnotes
Peer review under responsibility of Second Military Medical University.
References
- 1.Haq R., Fisher D.E. Biology and clinical relevance of the micropthalmia family of transcription factors in human cancer. J Clin Oncol. 2011;29:3474–3482. doi: 10.1200/JCO.2010.32.6223. [DOI] [PubMed] [Google Scholar]
- 2.Moch H., Humphrey P.A., Ulbright T.M., Reuter V.E. 4th ed. IARC Press; Lyon: 2016. WHO classification of tumours of the urinary system and male genital organs; pp. 33–34. [Google Scholar]
- 3.Bertolotto C., Lesueur F., Giuliano S., Strub T., de Lichy M., Bille K. A SUMOylation-defective MITF germline mutation predisposes to melanoma and renal carcinoma. Nature. 2011;480:94–98. doi: 10.1038/nature10539. [DOI] [PubMed] [Google Scholar]
- 4.Argani P., Zhang L., Reuter V.E. RBM10–TFE3 renal cell carcinoma: a potential diagnostic pitfall due to cryptic intrachromosomal Xp11.2 inversion resulting in false-negative TFE3 FISH. Am J Surg Pathol. 2017;47:655–662. doi: 10.1097/PAS.0000000000000835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inamura K. Translocation renal cell carcinoma: an update on clinico-pathological and molecular features. Cancers. 2017;9:e111. doi: 10.3390/cancers9090111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kmetec A., Jeruc J. Xp 11.2 translocation renal carcinoma in young adults; recently classified distinct subtype. Radiol Oncol. 2014;48:197–202. doi: 10.2478/raon-2013-0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Argani P., Zhong M., Reuter V.E., Fallon J.T., Epstein J.I., Netto G.J. TFE3-fusion variant analysis defines specific clinicopathologic associations among Xp11 translocation cancers. Am J Surg Pathol. 2016;40:723–737. doi: 10.1097/PAS.0000000000000631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Srigley J.R., Delahunt B. Uncommon and recently described renal carcinomas. Mod Pathol. 2009;22(Suppl. 2):S2–S23. doi: 10.1038/modpathol.2009.70. [DOI] [PubMed] [Google Scholar]
- 9.Argani P., Laé M., Ballard E.T., Amin M., Manivel C., Hutchinson B. Translocation carcinomas of the kidney after chemotherapy in childhood. J Clin Oncol. 2006;24:1529–1534. doi: 10.1200/JCO.2005.04.4693. [DOI] [PubMed] [Google Scholar]
- 10.Rao Q., Xia Q.Y., Cheng L., Zhou X.J. Molecular genetics and immunohistochemistry characterization of uncommon and recently described renal cell carcinomas. Chin J Cancer Res. 2016;28:29–49. doi: 10.3978/j.issn.1000-9604.2016.01.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirsch M.S., Signoretti S., Dal Cin P. Adult renal cell carcinoma, a review of established entities from morphology to molecular genetics. Surg Pathol Clin. 2015;8:587–621. doi: 10.1016/j.path.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 12.Argani P., Antonescu C.R., Illei P.B., Lui M.Y., Timmons C.F., Newbury R. Primary renal neoplasms with the ASPL–TFE3 gene fusion of alveolar soft part sarcoma: a distinctive tumor entity previously included among renal cell carcinomas of children and adolescents. Am J Pathol. 2001;159:179–192. doi: 10.1016/S0002-9440(10)61684-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Argani P., Antonescu C.R., Couturier J., Fournet J.C., Sciot R., Debiec-Rychter M. PRCC–TFE3 renal carcinomas: morphologic, immunohistochemical, ultrastructural, and molecular analysis of an entity associated with the t(X;1)(p11.2;q21) Am J Surg Pathol. 2002;26:1553–1566. doi: 10.1097/00000478-200212000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Argani P., Hicks J., De Marzo A.M., Albadine R., Illei P.B., Ladanyi M. Xp11 Translocation renal cell carcinoma (RCC): extended immunohistochemical profile emphasizing novel RCC markers. Am J Surg Pathol. 2010;34:1295–1303. doi: 10.1097/PAS.0b013e3181e8ce5b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Magers M.J., Udager A.M., Mehra R. MiT family translocation-associated renal cell carcinoma. A contemporary update with emphasis on morphologic, immune-phenotypic, and molecular mimics. Arch Pathol Lab Med. 2015;139:1224–1233. doi: 10.5858/arpa.2015-0196-RA. [DOI] [PubMed] [Google Scholar]
- 16.Argani P., Lal P., Hutchinson B., Lui M.Y., Reuter V.E., Ladanyi M. Aberrant nuclear immunoreactivity for TFE3 in neoplasms with TFE3 gene fusions: a sensitive and specific immunohistochemical assay. Am J Surg Pathol. 2003;27:750–761. doi: 10.1097/00000478-200306000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Debelenko L.V., Raimondi S.C., Daw N., Shivakumar B.R., Huang D., Nelson M. Renal cell carcinoma with novel VCL–ALK fusion: new representative of ALK-associated tumor spectrum. Mod Pathol. 2011;24:430–442. doi: 10.1038/modpathol.2010.213. [DOI] [PubMed] [Google Scholar]
- 18.Green W.M.1, Yonescu R., Morsberger L., Morris K., Netto G.J., Epstein J.I. Utilization of a TFE3 break-apart FISH assay in a renal tumor consultation service. Am J Surg Pathol. 2013;37:1150–1163. doi: 10.1097/PAS.0b013e31828a69ae. [DOI] [PubMed] [Google Scholar]
- 19.Sukov W.R., Hodge J.C., Lohse C.M., Leibovich B.C., Thompson R.H., Pearce K.E. TFE3 rearrangements in adult renal cell carcinoma: clinical and pathologic features with outcome in a large series of consecutively treated patients. Am J Surg Pathol. 2012;36:663–670. doi: 10.1097/PAS.0b013e31824dd972. [DOI] [PubMed] [Google Scholar]
- 20.Ellis C.L., Eble J.N., Subhawong A.P., Martignoni G., Zhong M., Ladanyi M. Clinical heterogeneity of Xp11 translocation renal cell carcinoma: impact of fusion subtype, age, and stage. Mod Pathol. 2014;27:875–886. doi: 10.1038/modpathol.2013.208. [DOI] [PubMed] [Google Scholar]
- 21.Armah H.B., Parwani A.V. Xp11.2 translocation renal cell carcinoma. Arch Pathol Lab Med. 2010;134:124–129. doi: 10.5858/2008-0391-RSR.1. [DOI] [PubMed] [Google Scholar]
- 22.Malouf G.G., Camparo P., Oudard S., Schleiermacher G., Theodore C., Rustine A. Targeted agents in metastatic Xp11 translocation/TFE3 gene fusion renal cell carcinoma (RCC): a report from the Juvenile RCC Network. Ann Oncol. 2010;21:1834–1838. doi: 10.1093/annonc/mdq029. [DOI] [PubMed] [Google Scholar]
- 23.Choueiri T.K., Lim Z.D., Hirsch M.S., Tamboli P., Jonasch E., McDermott D.F. Vascular endothelial growth factor targeted therapy for the treatment of adult metastatic Xp11.2 translocation renal cell carcinoma. Cancer. 2010;116:5219–5225. doi: 10.1002/cncr.25512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsuda M., Davis I.J., Argani P., Shukla N., McGill G.G., Nagai M. TFE3 fusions activate MET signaling by transcriptional upregulation, defining another class of tumors as candidates for therapeutic MET inhibition. Cancer Res. 2007;67:919–929. doi: 10.1158/0008-5472.CAN-06-2855. [DOI] [PubMed] [Google Scholar]
- 25.Chang K., Qu Y., Dai B., Zhao J.Y., Gan H., Shi G. PD-L1 expression in Xp11.2 translocation renal cell carcinoma: indicator of tumor aggressiveness. Sci Rep. 2017;7:2074. doi: 10.1038/s41598-017-02005-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rao Q., Shen Q., Xia Q.Y., Wang Z.Y., Liu B., Shi S.S. PSF/SFPQ is a very common gene fusion partner in TFE3 rearrangement-associated perivascular epithelioid cell tumors (PEComas) and melanotic Xp11 translocation renal cancers: clinicopathologic, immunohistochemical, and molecular characteristics suggesting classification as a distinct entity. Am J Surg Pathol. 2015;39:1181–1196. doi: 10.1097/PAS.0000000000000502. [DOI] [PubMed] [Google Scholar]
- 27.Argani P., Aulmann S., Karanjawala Z., Fraser R.B., Ladanyi M., Rodriguez M.M. Melanotic Xp11 translocation renal cancers: a distinctive neoplasm with overlapping features of PEComa, carcinoma, and melanoma. Am J Surg Pathol. 2009;33:609–619. doi: 10.1097/PAS.0b013e31818fbdff. [DOI] [PubMed] [Google Scholar]
- 28.Argani P., Aulmann S., Illei P.B., Netto G.J., Ro J., Cho H.Y. A distinctive subset of PEComas harbors TFE3 gene fusions. Am J Surg Pathol. 2010;34:1395–1406. doi: 10.1097/PAS.0b013e3181f17ac0. [DOI] [PubMed] [Google Scholar]
- 29.Saleeb R.M., Srigley J.R., Sweet J., Doucet C., Royal V., Chen Y.B. Melanotic MiT family translocation neoplasms: expanding the clinical and molecular spectrum of this unique entity of tumors. Pathol Res Pract. 2017;213:1412–1418. doi: 10.1016/j.prp.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 30.Antic T., Taxy J.B., Alikhan M., Segal J. Melanotic translocation renal cell carcinoma with a novel ARID1B–TFE3 gene fusion. Am J Surg Pathol. 2017;41:1576–1580. doi: 10.1097/PAS.0000000000000927. [DOI] [PubMed] [Google Scholar]
- 31.Cardili L., Wrublevsky Pereira G., Viana C.R. A rare case of TFE-related pigmented renal tumor with overlapping features between melanotic Xp11 translocation renal cancer and Xp11 renal cell carcinoma with melanotic features. Pathol Int. 2017;67:208–213. doi: 10.1111/pin.12517. [DOI] [PubMed] [Google Scholar]
- 32.Geller J.I., Argani P., Adeniran A., Hampton E., De Marzo A., Hicks J. Translocation renal cell carcinoma: lack of negative impact due to lymph node spread. Cancer. 2008;112:1607–1616. doi: 10.1002/cncr.23331. [DOI] [PubMed] [Google Scholar]
- 33.Argani P., Hawkins A., Griffin C.A., Goldstein J.D., Haas M., Beckwith J.B. A distinctive pediatric renal neoplasm characterized by epithelioid morphology, basement membrane production, focal HMB45 immunoreactivity, and t (6;11) (p21.1;q12) chromosome translocation. Am J Pathol. 2001;158:2089–2096. doi: 10.1016/S0002-9440(10)64680-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caliò A., Brunelli M., Segala D., Pedron S., Tardanico R., Remo A. t (6;11) renal cell carcinoma: a study of seven cases including two with aggressive behavior, and utility of CD68 (PG-M1) in the differential diagnosis with pure epithelioid PEComa/epithelioid angiomyolipoma. Mod Pathol. 2018;31:474–487. doi: 10.1038/modpathol.2017.144. [DOI] [PubMed] [Google Scholar]
- 35.Kuroda N., Yorita K., Sasaki N., Ishihara A., Matsuura K., Daa T. Clinico pathological study of 5 cases of renal cell carcinoma with t(6;11)(p21;q12) Pol J Pathol. 2017;68:66–72. doi: 10.5114/pjp.2017.67617. [DOI] [PubMed] [Google Scholar]
- 36.Cutruzzula P., Cahn D., Kivlin D., Tong C., Edwards D., Amster M. Review of translocation T(6;11) renal cell carcinoma tumors in the adult patient. Curr Urol. 2017;10:69–71. doi: 10.1159/000447154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuroda N., Tanaka A., Sasaki N., Ishihara A., Matsuura K., Moriyama M. Review of renal carcinoma with t(6;11) (p21;q12) with focus on clinical and pathobiological aspects. Histol Histopathol. 2013;28:685–690. doi: 10.14670/HH-28.685. [DOI] [PubMed] [Google Scholar]
- 38.Argani P., Yonescu R., Morsberger L., Morris K., Netto G.J., Smith N. Molecular confirmation of t(6;11)(p21;q12) renal cell carcinoma in archival paraffin-embedded material using a break-apart TFEB FISH assay expands its clinicopathologic spectrum. Am J Surg Pathol. 2012;36:1516–1526. doi: 10.1097/PAS.0b013e3182613d8f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rao Q., Zhang X.M., Tu P., Xia Q.Y., Shen Q., Zhou X.J. Renal cell carcinomas with t(6;11)(p21;q12) presenting with tubulocystic renal cell carcinoma-like features. Int J Clin Exp Pathol. 2013;6:1452–1457. [PMC free article] [PubMed] [Google Scholar]
- 40.Argani P., Laé M., Hutchinson B., Reuter V.E., Collins M.H., Perentesis J. Renal carcinomas with the t(6;11)(p21;q12): clinicopathologic features and demonstration of the specific alpha-TFEB gene fusion by immunohistochemistry, RT-PCR, and DNA PCR. Am J Surg Pathol. 2005;29:230–240. doi: 10.1097/01.pas.0000146007.54092.37. [DOI] [PubMed] [Google Scholar]
- 41.Smith N.E., Illei P.B., Allaf M., Gonzalez N., Morris K., Hicks J. t(6;11) renal cell carcinoma (RCC): expanded immunohistochemical profile emphasizing novel RCC markers and report of 10 new genetically confirmed cases. Am J Surg Pathol. 2014;38:604–614. doi: 10.1097/PAS.0000000000000203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davis I.J., Hsi B.L., Arroyo J.D., Vargas S.O., Yeh Y.A., Motyckova G. Cloning of an alpha-TFEB fusion in renal tumors harboring the t(6;11)(p21;q13) chromosome translocation. Proc Natl Acad Sci U S A. 2003;100:6051–6056. doi: 10.1073/pnas.0931430100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Inamura K., Fujiwara M., Togashi Y., Nomura K., Mukai H., Fujii Y. Diverse fusion patterns and heterogeneous clinicopathologic features of renal cell carcinoma with t(6;11) translocation. Am J Surg Pathol. 2012;36:35–42. doi: 10.1097/PAS.0b013e3182293ec3. [DOI] [PubMed] [Google Scholar]
- 44.Malouf G.G., Su X., Yao H., Gao J., Xiong L., He Q. Next-generation sequencing of translocation renal cell carcinoma reveals novel RNA splicing partners and frequent mutations of chromatin-remodeling genes. Clin Cancer Res. 2014;20:4129–4140. doi: 10.1158/1078-0432.CCR-13-3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Durinck S., Stawiski E.W., Pavía-Jiménez A., Modrusan Z., Kapur P., Jaiswal B.S. Spectrum of diverse genomic alterations define non-clear cell renal carcinoma subtypes. Nat Genet. 2015;47:13–21. doi: 10.1038/ng.3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cancer Genome Atlas Research Network, Linehan W.M., Spellman P.T., Ricketts C.J., Creighton C.J., Fei S.S. Comprehensive molecular characterization of papillary renal-cell carcinoma. N Engl J Med. 2016;374:135–145. doi: 10.1056/NEJMoa1505917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peckova K., Vanecek T., Martinek P., Spagnolo D., Kuroda N., Brunelli M. Aggressive and nonaggressive translocation t(6;11) renal cell carcinoma: comparative study of 6 cases and review of the literature. Ann Diagn Pathol. 2014;18:351–357. doi: 10.1016/j.anndiagpath.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 48.Mendel L., Ambrosetti D., Bodokh Y., Ngo-Mai M., Durand M., Simbsler-Michel C. Comprehensive study of three novel cases of TFEB-amplified renal cell carcinoma and review of the literature: evidence for a specific entity with poor outcome. Genes Chromosomes Cancer. 2018;57:99–113. doi: 10.1002/gcc.22513. [DOI] [PubMed] [Google Scholar]
- 49.Argani P., Reuter V.E., Zhang L., Sung Y.S., Ning Y., Epstein J.I. TFEB-amplified renal cell carcinomas: an aggressive molecular subset demonstrating variable melanocytic marker expression and morphologic heterogeneity. Am J Surg Pathol. 2016;40:1484–1495. doi: 10.1097/PAS.0000000000000720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gupta S., Johnson S.H., Vasmatzis G., Porath B., Rustin J.G., Rao P. TFEB–VEGFA (6p21.1) co-amplified renal cell carcinoma: a distinct entity with potential implications for clinical management. Mod Pathol. 2017;30:998–1012. doi: 10.1038/modpathol.2017.24. [DOI] [PubMed] [Google Scholar]
- 51.Williamson S.R., Grignon D.J., Cheng L., Favazza L., Gondim D.D., Carskadon S. Renal cell carcinoma with chromosome 6p amplification including the TFEB gene: a novel mechanism of tumor pathogenesis? Am J Surg Pathol. 2017;41:287–298. doi: 10.1097/PAS.0000000000000776. [DOI] [PubMed] [Google Scholar]
- 52.Yokoyama S., Woods S.L., Boyle G.M., Aoude L.G., MacGregor S., Zismann V. A novel recurrent mutation in MITF predisposes to familial and sporadic melanoma. Nature. 2011;480:99–103. doi: 10.1038/nature10630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ghiorzo P., Pastorino L., Queirolo P., Bruno W., Tibiletti M.G., Nasti S. Prevalence of the E318K MITF germline mutation in Italian melanoma patients: associations with histological subtypes and family cancer history. Pigment Cell Melanoma Res. 2013;26:259–262. doi: 10.1111/pcmr.12047. [DOI] [PubMed] [Google Scholar]
- 54.Paillerets B.B., Lesueur F., Bertolotto C. A germline oncogenic MITF mutation and tumor susceptibility. Eur J Cell Biol. 2014;93:71–75. doi: 10.1016/j.ejcb.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 55.Gromowski T., Masojć B., Scott R.J., Cybulski C., Górski B., Kluźniak W. Prevalence of the E318K and V320I MITF germline mutations in Polish cancer patients and multiorgan cancer risk—a population-based study. Cancer Genet. 2014;207:128–132. doi: 10.1016/j.cancergen.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 56.Stoehr C.G., Walter B., Denzinger S., Ghiorzo P., Sturm R.A., Hinze R. The microphthalmia-associated transcription factor p.E318K mutation does not play a major role in sporadic renal cell tumors from Caucasian patients. Pathobiology. 2016;83:165–169. doi: 10.1159/000443311. [DOI] [PubMed] [Google Scholar]
- 57.Xia Q.Y., Wang X.T., Ye S.B., Wang X., Li R., Shi S.S. Novel gene fusion of PRCC–MITF defines a new member of MiT family translocation renal cell carcinoma: clinicopathologic analysis and detection of the gene fusion by RNA-sequencing and FISH. Histopathology. 2018;72:786–794. doi: 10.1111/his.13439. [DOI] [PubMed] [Google Scholar]