Abstract
Neurodegeneration in multiple sclerosis (MS) correlates with disease progression and reparative processes may be triggered. Growth-associated protein 43 (GAP-43) exhibits induced expression during axonal growth and reduced expression during MS progression. We aimed to evaluate if GAP-43 can serve as a biomarker of regeneration in relapsing-remitting MS (RRMS) and whether disease-modifying therapies (DMTs) influence GAP-43 concentration in cerebrospinal fluid (CSF). GAP-43 was measured using an enzyme-linked immunosorbent assay in 105 MS patients (73 RRMS, 12 primary progressive MS, 20 secondary progressive MS) and 23 healthy controls (HCs). In 35 of the patients, lumbar puncture, clinical assessment, and magnetic resonance imaging was performed before initiation of therapeutic intervention, and at follow-up. CSF GAP-43 concentration was significantly lower in progressive MS compared with HCs (p = 0.004) and RRMS (p = < 0.001) and correlated negatively with disability (p = 0.026). However, DMTs did not alter CSF GAP-43. Interestingly, in RRMS CSF GAP-43 levels were higher in patients with signs of active inflammatory disease than in patients in remission (p = 0.042). According to CSF GAP-43 concentrations, regeneration seems reduced in progressive MS, increased during disease activity in RRMS but is unaffected by treatment of highly active DMTs.
Subject terms: Diagnostic markers, Multiple sclerosis
Introduction
Multiple sclerosis (MS) is an autoimmune disease of the central nervous system (CNS) that exhibits neurodegenerative features. The immune attack causes multiple demyelinating lesions with axonal loss but, with time, degeneration takes over with astrogliosis and atrophy1,2. However, regenerative mechanisms promote the repair of tissue damage, including remyelination in order to restore conduction. These pathogenic processes may be reflected by alterations in biomarker concentrations in cerebrospinal fluid (CSF), such as (i) neurofilament light (NFL), a biomarker of axonal damage3–5, (ii) glial fibrillary acidic protein (GFAP), a biomarker of astrogliosis6, and (iii) neurogranin, a marker of synaptic integrity7.
Growth-associated protein 43 (GAP-43), also known as B-50 or neuromodulin, is a membrane-associated protein8 and a major component of the motile growth cones of elongating axons and immature synaptic terminals9. GAP-43 is widely used as a marker of neuronal growth and regeneration, as it is highly expressed during synaptogenesis and axonal outgrowth10,11. Upon axotomy and in experimental models of ischemia, traumatic brain injury, and MS, GAP-43 protein expression is temporarily induced adjacent to the lesions12–18. Altered GAP-43 expression has also been reported in MS brains post-mortem19,20, including decreased expression in the vicinity of white matter lesions and increased or unaltered expression adjacent to remyelinated lesions20. CSF GAP-43 concentration correlates negatively with the Expanded Disability Status Scale (EDSS)20, and lower levels of CSF GAP-43 have been found in secondary progressive MS (SPMS) compared to early stages of MS, controls, and other neurological diseases21. Using a novel enzyme-linked immunosorbent assay (ELISA), we recently measured CSF GAP-43 in clinically isolated syndrome, early MS patients, and controls; though no differences were found between these major groups, patients who progressed had lower CSF GAP-43 concentrations22. In the present study, we investigated whether disease-modifying therapies (DMTs) alter CSF GAP-43 concentrations in MS, which would suggest an impact on regeneration.
Results
CSF GAP-43 concentrations in MS patients and healthy controls
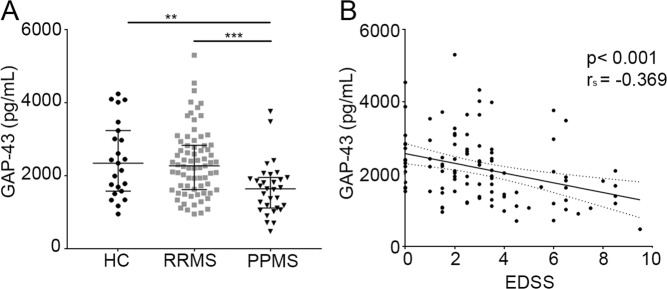
Significantly lower CSF GAP-43 concentrations were found in progressive MS [1640 (1120–1950) pg/mL, p = 0.004], but not in relapsing-remitting MS (RRMS) [2270 (1620–2830) pg/mL, p = 0.8], compared with healthy controls (HCs) [2340 (1580–3230) pg/mL] (Fig. 1A). The difference was still significant when dividing the progressive MS patients into primary progressive MS (PPMS) and SPMS compared with HCs (each p = < 0.05), and p = 0.003, and p = 0.002, respectively compared with RRMS. The diagnostic accuracy of CSF GAP-43 in diagnosing progressive MS, calculated with a ROC curve, gave an area under the curve (AUC) of 0.73 (p = 0.012).
Figure 1.
CSF GAP-43 differs across disease groups and correlates with EDSS: (A) CSF GAP-43 concentrations across disease groups in the MS population and HCs. **p = 0.0054, ***p < 0.0004. (B) Correlation between CSF GAP-43 concentration and EDSS in MS patients, p < 0.001. CSF, cerebrospinal fluid; GAP-43, growth-associated protein 43; MS, multiple sclerosis; HCs, healthy controls; RRMS, relapsing-remitting multiple sclerosis; PPMS, primary progressive multiple sclerosis; EDSS, Expanded Disability Status Scale.
The influence of clinical and demographic factors and blood-brain barrier function on CSF GAP-43 concentrations
While no correlation was found between CSF GAP-43 concentrations and age in HCs, it correlated negatively with age (rs = −0.339, p < 0.001), disease duration (rs = −0.303, p = 0.002), and EDSS (rs = −0.369, p < 0.001, Fig. 1B) in the MS population. Multiple regression analysis showed that only EDSS independently correlated with CSF GAP-43 concentration (p = 0.026). When dividing the MS population into RRMS, PPMS and SPMS patients, EDSS correlation was significant only in PPMS (PPMS: rs = −0.651, p = 0.03, RRMS: rs = −0.123, p = 0.301, SPMS: rs = −0.242, p = 0.304). After adjustment for age in the PPMS group the correlation was still significant (p = 0.009). No significant differences were found between CSF GAP-43 concentrations in females and males, and baseline GAP-43 concentration did not correlate with the albumin ratio.
CSF GAP-43 and disease activity
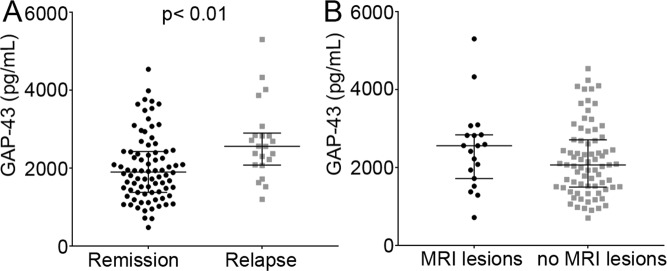
CSF GAP-43 concentration was higher in RRMS patients with a relapse within 3 months prior to CSF sampling [2560 (2080–2900) pg/mL], compared with CSF obtained in remission [1900 (1380–2420) pg/mL, p = 0.002, Fig. 2A]. The RRMS patients with a relapse within 3 months were significantly younger than those without relapse (p = 0.011), and the latter had a longer disease duration (p < 0.001, RRMS, relapse mean = 2.3 years, no relapse mean = 10 years), after adjusted analysis for age and disease duration, disease duration was still significant (p = 0.02). Although, CSF GAP-43 concentrations at baseline were higher in patients with gadolinum-enhancing magnetic resonance imaging (MRI) lesions [2560 (1720–2840) pg/mL] compared with those without lesions [2030 (1470–2470) pg/mL], this difference was not statistically significant (p = 0.088, Fig. 2B).
Figure 2.
CSF GAP-43 and clinical characteristics: (A) CSF GAP-43 concentrations in RRMS patients with a relapse within 3 months prior to CSF sampling compared to CSF obtained in remission, p < 0.01. (B) CSF GAP-43 concentrations at baseline in MS patients with gadolinum-enhancing MRI lesions compared to those without lesions, p = 0.088. CSF, cerebrospinal fluid; GAP-43, growth-associated protein 43; RRMS, relapsing-remitting multiple sclerosis; MS, multiple sclerosis; MRI, magnetic resonance imaging.
Effect of treatment on CSF GAP-43 concentration
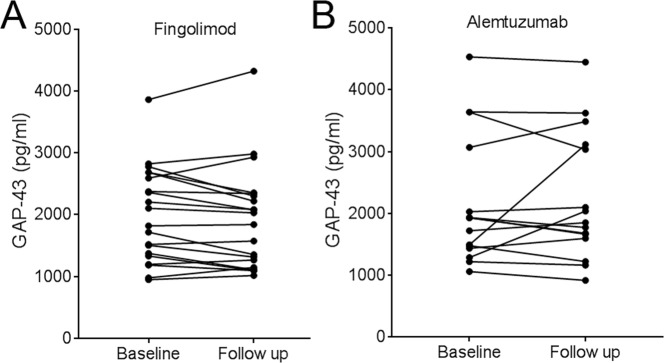
At baseline, CSF GAP-43 concentrations were not different in patients on DMTs (n = 44) and treatment-naïve patients (n = 61, p = 0.851), or in patients on first line compared with second line treatment (p = 0.935). In patients switching from treatment-naïve (n = 1), first-line (n = 15) or second-line (n = 19) to fingolimod (n = 20) or alemtuzumab (n = 15), CSF GAP-43 concentrations did not change and the follow-up CSF GAP-43 level [fingolimod: 1940 (1180–2330) pg/mL, alemtuzumab: 1850 (1600–3120) pg/mL] was highly dependent on baseline concentrations [fingolimod: 1960 (1340–2660) pg/mL, alemtuzumab: 1930 (1440–3070) pg/mL, fingolimod: rs = 0.958, p < 0.001, alemtuzumab: rs = 0.793, p < 0.001] (Fig. 3A,B), with a mean coefficient of variance (CV) between these time points of 7.8% and 11.6%, respectively. At baseline, no significant difference in CSF GAP-43 concentrations were found between RRMS patients who achieved no evidence of disease activity (NEDA-3) (n = 19) switching from treatment naïve (n = 1), first-line (n = 15) or second-line (n = 19) to fingolimod (n = 20) or alemtuzumab (n = 15), and those who did not [NEDA-3: 1669 (1298–2244) pg/mL, no NEDA-3: 2018 (1482–2788) pg/mL, p = 0.26]. At follow-up, we neither found a significant difference in CSF GAP-43 concentrations between patients with NEDA-3 [n = 23, 1619 (1322–2244) pg/mL] and those with no NEDA-3 [2201 (1569–2754) pg/mL, p = 0.160]. In the group of RRMS patients that switched to fingolimod or alemtuzumab there was a slight trend that those with higher baseline CSF GAP-43 concentration more often achieved clinical disability improvement (CDI; EDSS improvement of ≥1 point for those with an EDSS of ≥ 2 points at baseline, excluding 5 patients as they did not have EDSS ≥ 2 at baseline), but not significant [CDI at follow-up (n = 5): 1930 (1471–2999) pg/mL, no CDI at follow-up (n = 25): 1717 (1354–2398) pg/mL, p = 0.58]. Examining treatment effects on CSF GAP-43 concentrations in strata of patients who either started on first-line treatment and changed to second-line treatment or changed from one type of second-line treatment to another second-line treatment, did not reveal any significant changes in CSF GAP-43 concentration (p = 0.143 and p = 0.54, respectively).
Figure 3.
CSF GAP-43 at baseline and after DMTs: CSF GAP-43 concentrations at baseline and follow-up after fingolimod (A) and alemtuzumab (B) treatment. CSF, cerebrospinal fluid; GAP-43, growth-associated protein 43; DMTs, disease-modifying therapies.
Discussion
The data in this study are based on a heterogeneous group of MS patients in different stages of disease. We confirmed that CSF GAP-43 was significantly lower in progressive MS compared with HCs and RRMS patients21,22, with the lowest levels in PPMS. CSF GAP-43 concentrations correlated negatively with age, disease duration, and EDSS, but only independently with EDSS. However, we found that the CSF GAP-43 concentration was significantly higher in RRMS patients with signs of active inflammatory disease compared with RRMS patients in remission, whereas fingolimod and alemtuzumab treatment did not alter CSF GAP-43 concentrations.
We confirmed no difference between CSF GAP-43 concentrations of RRMS and HCs22. In contrast, the GAP-43 was reduced in progressive MS, suggesting lost or reduced regenerative potential in late MS. This ability seems to become more marked with increased disability and may be the result of atrophy development and progressive neuro-axonal loss. This finding is in contrast to our findings in Alzheimer’s disease, in which CSF GAP-43 was increased23. Thus, the nature of neurodegeneration seems to be more important than the degree of neurodegeneration, as the level of CSF GAP-43 was not increased in other neurodegenerative diseases23 and not related to the extent of atrophy24. Although the pathogenesis of MS progression is unknown, and clearly different from that of Alzheimer’s disease, our results suggest that regenerative processes such as synaptogenesis and axonal outgrowth, is reduced in progressive MS. This interpretation is supported by the decreased expression of GAP-43 in the vicinity of white matter lesions20.
Our results suggest that the CSF GAP-43 concentration increases in association with new inflammatory activity in MS patients. Increased CSF GAP-43 concentration was found in RRMS and patients with clinically isolated syndrome with >10 T2 lesions compared with those with fewer T2 lesions22. However, increase of CSF GAP-43 during relapses or in the presence of contrast enhancing lesions has not been reported before. This elevation seemed independent of blood-brain barrier function, since no correlation was found between CSF GAP-43 and the albumin ratio. Previous studies of axotomy and experimental models of ischemia, traumatic brain injury, and MS show that GAP-43 protein expression is induced temporarily and adjacent to neuro-axonal damage and the formation of new lesions12–18. Thus, immune-mediated damage of the CNS may explain the transient release of GAP-43 that we found in the CSF of MS patients with ongoing disease activity. Another explanation could be that the CSF GAP-43 concentration increases during MS relapse in an attempt to regenerate injured axons.
We could not show any significant impact of DMTs on CSF GAP-43 concentration. Similar CSF GAP-43 concentrations were observed at baseline in patients without prior treatment and those on first- or second-line treatment. MS treatments primarily reduce CNS inflammation in MS, and not the regenerative capacity. The lack of change in CSF GAP-43 across different therapies suggests that reduced inflammation does not influence regeneration involving GAP-43.
HCs were of younger age than the MS population. However, we found no association between CSF GAP-43 concentrations and age in HCs. While multiple regression analysis revealed an independent relationship between disability and the CSF GAP-43 concentration, this were not the case for disease duration and age. Thus, our study confirmed previous findings22,23,25, and thus, differences in age between HCs and patients should not have influenced the results. Similar differences existed between the gender distributions of the study groups. However, neither did gender seem to influence the CSF GAP-43 concentrations. Moreover, we could only report an association between ongoing inflammatory activity and increased CSF GAP-43 levels, but we lacked MRI data on lesion load or cerebral and spinal cord atrophy. Relationships between CSF GAP-43 concentrations and such MRI measures should be further explored to better characterize the possible role of GAP-43 in the pathogenesis of MS.
In conclusion, studies of GAP-43 in MS concordantly show that, this protein is decreased in CSF in progressive MS, and we found an association with disability and also with disease activity. However, effective DMTs had no effect on the CSF GAP-43 concentration. Previous studies have not shown correlation between GAP-43 and NFL in CSF22, indicating that axonal damage does not influence the release of GAP-43 in CSF. Although the clinical potential of GAP-43 as a biomarker in MS seems limited at this stage, it contributes to further understand the pathogenesis behind progression, and that of degeneration and regeneration in MS.
Methods
Patients and healthy control subjects
We included 23 HCs and 105 MS patients, including 73 with RRMS, 20 with SPMS, and 12 PPMS, fulfilling the revised McDonald criteria from 201026. Ninety of these patients had previously participated in studies of CSF biomarkers in MS6,27, including one investigating the influence of fingolimod treatment28. The remaining patients (n = 15) were recently recruited from consecutive patients at the MS Centre, Sahlgrenska University Hospital, Gothenburg, Sweden, to explore the influence of alemtuzumab therapy on CSF biomarker concentrations. At baseline, 24 patients received first-line treatment (19 interferon beta, 4 glatiramer acetate, 1 dimethyl fumarate), 20 received second-line treatment (6 fingolimod, 1 rituximab, 13 natalizumab) and 61 were treatment naïve. Descriptive clinical and demographic characteristics are presented in Table 1.
Table 1.
Descriptive clinical and demographic characteristics of patients and HCs.
| N | HCs | RRMS | PPMS | SPMS |
|---|---|---|---|---|
| 23 | 73 | 12 | 20 | |
| Mean age, years (SD) | 29.3 (10.1) | 39.2 (10.3)a | 52.4 (7.0)b | 53.3 (8.7)b |
| Gender, female/male | 14/9 | 48/25c | 6/6 | 7/13 |
| Disease duration, years | NA | 6 (2–12.5) | 5.5 (1.25–8) | 18 (12.3–22.8)d,e |
| EDSS | NA | 2 (1–3) | 4.5 (3–6)f | 6.5 (5.1–8.4)g |
| Relapse 3 months prior to LP, yes/no | NA | 22/51 | 0/12 | 0/20 |
| DMT | ||||
| No previous treatment | NA | 34 | 11 | 16 |
| First-line treatment | NA | 21 | 0 | 3 |
| Second-line treatment | NA | 18 | 1 | 1 |
| QAlb | 3.9 (3.3–5.8) | 4.9 (3.9–6.32) | 5.2 (4.4–6.0) | 6.9 (5.1–9.7) |
| Fingolimod | Alemtuzumab | |||
| N | 20 | 15 | ||
| Mean age, years (SD) | 38.5 (10.3) | 40.3 (7.7) | ||
| Gender, female/male | 9/11 | 9/6 | ||
| Disease duration, years | 7 (3–11.8) | 4 (3–13) | ||
| EDSS | 3 (1.1–3.5) | 2.5 (1.5–3.5) | ||
| Relapse 3 months prior to LP, yes/no | 6/14 | 0/15 | ||
| DMT baseline | ||||
| No previous treatment | 1 | 0 | ||
| First-line treatment | 13 | 2 | ||
| Second-line treatment | 6 | 13 | ||
| QAlb | 5.1 (3.2–6.6) | 5.2 (3.2–6.4) | ||
Data are presented as n or median (interquartile range) unless otherwise noted. HCs, healthy controls; RRMS, relapsing-remitting multiple sclerosis; PPMS, primary progressive multiple sclerosis; SPMS, secondary progressive multiple sclerosis; EDSS, Expanded Disability Status Scale; LP, lumbar puncture; DMT, disease-modifying therapies; QAlb, albumin ratio. First-line treatment = interferon beta, glatiramer acetate, dimethyl fumarate. Second-line treatment = natalizumab, fingolimod, rituximab.
ap < 0.05 RRMS versus HCs.
bp = 0.001 PPMS or SPMS versus HCs.
cp < 0.01 female versus male in RRMS.
dp < 0.001 SPMS versus RRMS.
ep < 0.01 SPMS versus PPMS.
fp < 0.05 PPMS versus RRMS.
gp < 0.001 SPMS versus RRMS.
Clinical evaluation, sampling of CSF, and magnetic resonance imaging
All patients were assessed clinically at baseline by MS-specialized neurologists. The EDSS29 was used to score neurological deficits and impairment. A relapse was defined as an episode of neurological disturbance lasting for at least 24 h that could not be better explained by another cause30. Lumbar puncture was performed at baseline (n = 127), and in the fingolimod (n = 20) and alemtuzumab (n = 15) treatment groups, CSF was obtained again after a median of 7 (range 3–13) and 24 (range 24–26) months, respectively. One patient had only a follow-up lumbar puncture sample. The CSF samples were handled according to the consensus protocol of the BioMS-EU network for CSF biomarker research in MS31. MRI of the brain was performed on 66 patients at baseline and in close association with the clinical neurological examinations and lumbar puncture (median 1 month, range 0–7 months). A standard MRI protocol for MS including intravenous gadolinium contrast was performed on a 1.5 or 3 Tesla MRI scanner and included T1, T2, and fluid attenuation inversion recovery (FLAIR) sequences, according to the Swedish guidelines32.
CSF GAP-43 analysis
The GAP-43 protein concentration in CSF was determined by an in-house ELISA as described previously22, with minor modifications. Briefly, plates were coated with NM4 monoclonal antibody (1.35 μg/mL, Fujirebio, Tokyo, Japan) in carbonate buffer (pH 9.6), and incubated over night at 4 °C. After three washes with phosphate-buffered saline with tween (PBST), wells were blocked with a solution of PBST-milk (2% non-fat dry milk, Biorad, Hercules, CA, US) for 1 hour on a shaker at room temperature and placed at −20 °C for at least 12 hours to enable higher throughput during sample runs. After thawing and three more washes, the detection antibody (polyclonal ABB-135, Nordic Biosite, Täby, Sweden), 50 μL of twofold prediluted samples, and calibrators (recombinant GAP-43) in PBST-milk were co-incubated overnight at 4 °C. Plates were washed three times and secondary antibody (anti-rabbit IgG HRP, Promega, Wisconsin, US) diluted in 1% bovine serum albumin (BSA)/PBST at 1:20000 added and incubated on the bench for 2 hours at room temperature. Wells were washed and 100 μL of 3,3′,5,5′-tetramethyl-benzidine (TMB One, KemEnTech Diagnostics, Taastrup, Denmark) added. Plates were incubated in the dark for 30 min before adding 100 μL of 0.2 M H2SO4 and measuring the absorbance at 450 nm immediately, with a 650 nm reference, on a SunriseTM microplate absorbance reader (Tecan group, Männedorf, Switzerland). The analysis was carried out using the same batch of reagents and the analyst was blind to disease condition. Quality control samples were run for estimation of intra- and inter-plate variations. The intra-assay CV for a sample with a mean concentration of 3597 pg/mL was 5.4% with an inter-assay CV of 8.9%, and for sample with a mean concentration of 571 pg/mL the intra- and inter-assay CVs were 10.7% and 11.1% respectively. The lower limit of quantification (LLoQ), determined as the lowest concentration at which GAP-43 could be detected reliably, was 475 pg/mL after adjusting for a twofold sample dilution. Further assay evaluation of precision, dilution linearity, spike recovery in matrix, and sample stability has been described previously25.
Albumin ratio
Serum and CSF albumin concentrations were measured by immunonephelometry on a Beckman Immage Immunochemistry system (Beckman Instruments, Beckman Coulter, Brea, CA, USA). QAlb was calculated as the ratio of CSF albumin (mg/L) to serum albumin (g/L)33.
Statistical analysis
Data were not normally distributed; therefore, non-parametric tests were used. Differences across patient groups, clinical measures, and treatments were evaluated as continuous variables using Kruskal-Wallis or Mann-Whitney U tests. The Wilcoxon signed ranks test for paired samples was used to evaluate changes in CSF GAP-43 over time in patients switching from other treatments to fingolimod or alemtuzumab. Possible correlations between biomarker concentrations and clinical measures were evaluated using Spearman correlation. The chi-squared test was used for categorical variables. Multiple regression analysis was performed to test the influence of age, disease duration, and EDSS on CSF GAP-43 concentration. SPSS version 23.00 (IBM, NY, US) and GraphPad Prism 5.0 (GraphPad Inc., California, USA) were used for statistical analyses. All tests were two-sided with a significance threshold of p < 0.05.
Ethical standards
All patients and HCs voluntarily participated in the study, and informed consent was obtained from all subjects. Measures were taken to minimize pain and discomfort for all participants in the study, and all methods were performed in accordance with the relevant ethical guidelines and regulations. The study conforms with The Code of Ethics of the World Medical Association (Declaration of Helsinki)34. For patient material from previous studies, reference is made to the respective publication6,27,28 for their ethical approval. For the remaining patients and for HCs, participating in the assessment of alemtuzumab, the study was approved by the Regional Ethics Review Board in Gothenburg, Sweden (Reference number 460–13).
Acknowledgements
The authors would like to thank and acknowledge all of the participants who have helped support and contribute to this study. This study was funded by grants from the Swedish Federal Government (LUA/ALF agreement, ALFGBG-722081), the Swedish Association of Persons with Neurological Disabilities, the Research Foundation of the Multiple Sclerosis Society of Gothenburg, the Edit Jacobson Foundation, the AFA Insurance Foundation, the Swedish Society for Medical Research, the Swedish Brain Foundation, NEURO Sweden, the Torsten Söderberg Foundation, as well as Novartis Foundation and Biogen (unconditional grants).
Author contributions
Å.S. and S.S. contributed equally. Å.S., S.S., M.A., C.M., L.N., K.B., H.Z. and J.L. participated in the primary data acquisition. Å.S., S.S., M.A., C.M., L.N., K.B., H.Z. and J.L. participated in the study concept design. Å.S., S.S. and M.A. did the statistical analysis. Å.S. did the figures. V.K. and M.V. performed method development. S.S. and J.L. drafted the report and all authors participated in the critical revision of the final version and approved its publication.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
S.S. has received compensation for lectures and/or advisory board membership from Merck and SanofiGenzyme. M.A. has received compensation for lectures and/or advisory board membership from Biogen, Genzyme, and Novartis. C.M. has received honoraria for lectures and advisory board membership from Biogen, Merck, Novartis, and SanofiAventis. V.K. and M.V. are employees of Fujirebio Europe N.V. K.B. holds the Torsten Söderberg Professorship, has served as a consultant or on advisory boards for Alzheon, BioArctic, Biogen, Eli Lilly, Fujirebio Europe, IBL International, Merck, Novartis, Pfizer, and Roche Diagnostics and is a cofounder of Brain Biomarker Solutions in Gothenburg AB, a GU Ventures-based platform company at the University of Gothenburg. HZ is a Wallenberg Academy Fellow, a cofounder of Brain Biomarker Solutions in Gothenburg A.B., a GU Ventures-based platform company at the University of Gothenburg, and has served on advisory boards for Roche Diagnostics, Wave, Samumed and CogRx. J.L. has received travel support and/or lecture honoraria from Biogen, Novartis, Teva, and Genzyme/SanofiAventis; has served on scientific advisory boards for Almirall, Teva, Biogen, Novartis, and Genzyme/SanofiAventis; serves on the editorial board of the Acta Neurologica Scandinavica; and has received unconditional research grants from Biogen, Novartis, and Teva. ÅS and LN declare no conflicts of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Åsa Sandelius and Sofia Sandgren.
References
- 1.Frischer JM, et al. The relation between inflammation and neurodegeneration in multiple sclerosis brains. Brain: a journal of neurology. 2009;132:1175–1189. doi: 10.1093/brain/awp070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Popescu BF, Pirko I, Lucchinetti CF. Pathology of multiple sclerosis: where do we stand? Continuum (Minneapolis, Minn.) 2013;19:901–921. doi: 10.1212/01.con.0000433291.23091.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Malmestrom C, Haghighi S, Rosengren L, Andersen O, Lycke J. Neurofilament light protein and glial fibrillary acidic protein as biological markers in MS. Neurology. 2003;61:1720–1725. doi: 10.1212/01.WNL.0000098880.19793.B6. [DOI] [PubMed] [Google Scholar]
- 4.Lycke JN, Karlsson JE, Andersen O, Rosengren LE. Neurofilament protein in cerebrospinal fluid: a potential marker of activity in multiple sclerosis. Journal of neurology, neurosurgery, and psychiatry. 1998;64:402–404. doi: 10.1136/jnnp.64.3.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teunissen CE, Khalil M. Neurofilaments as biomarkers in multiple sclerosis. Multiple sclerosis (Houndmills, Basingstoke, England) 2012;18:552–556. doi: 10.1177/1352458512443092. [DOI] [PubMed] [Google Scholar]
- 6.Axelsson M, et al. Glial fibrillary acidic protein: a potential biomarker for progression in multiple sclerosis. Journal of neurology. 2011;258:882–888. doi: 10.1007/s00415-010-5863-2. [DOI] [PubMed] [Google Scholar]
- 7.Novakova L, et al. Cerebrospinal fluid biomarkers as a measure of disease activity and treatment efficacy in relapsing-remitting multiple sclerosis. Journal of neurochemistry. 2017;141:296–304. doi: 10.1111/jnc.13881. [DOI] [PubMed] [Google Scholar]
- 8.Zwiers H, Schotman P, Gispen WH. Purification and some characteristics of an ACTH-sensitive protein kinase and its substrate protein in rat brain membranes. Journal of neurochemistry. 1980;34:1689–1699. doi: 10.1111/j.1471-4159.1980.tb11262.x. [DOI] [PubMed] [Google Scholar]
- 9.Skene JH, et al. A protein induced during nerve growth (GAP-43) is a major component of growth-cone membranes. Science (New York, N.Y.) 1986;233:783–786. doi: 10.1126/science.3738509. [DOI] [PubMed] [Google Scholar]
- 10.Goslin K, Schreyer DJ, Skene JH, Banker G. Development of neuronal polarity: GAP-43 distinguishes axonal from dendritic growth cones. Nature. 1988;336:672–674. doi: 10.1038/336672a0. [DOI] [PubMed] [Google Scholar]
- 11.Benowitz LI, Routtenberg A. GAP-43: an intrinsic determinant of neuronal development and plasticity. Trends in neurosciences. 1997;20:84–91. doi: 10.1016/S0166-2236(96)10072-2. [DOI] [PubMed] [Google Scholar]
- 12.Allegra Mascaro AL, et al. In vivo single branch axotomy induces GAP-43-dependent sprouting and synaptic remodeling in cerebellar cortex. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:10824–10829. doi: 10.1073/pnas.1219256110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chong MS, et al. GAP-43 expression in primary sensory neurons following central axotomy. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1994;14:4375–4384. doi: 10.1523/JNEUROSCI.14-07-04375.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goto S, Yamada K, Inoue N, Nagahiro S, Ushio Y. Increased expression of growth-associated protein GAP-43/B-50 following cerebral hemitransection or striatal ischemic injury in the substantia nigra of adult rats. Brain research. 1994;647:333–339. doi: 10.1016/0006-8993(94)91332-3. [DOI] [PubMed] [Google Scholar]
- 15.Kerschensteiner M, et al. Remodeling of axonal connections contributes to recovery in an animal model of multiple sclerosis. The Journal of experimental medicine. 2004;200:1027–1038. doi: 10.1084/jem.20040452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hulsebosch CE, DeWitt DS, Jenkins LW, Prough DS. Traumatic brain injury in rats results in increased expression of Gap-43 that correlates with behavioral recovery. Neuroscience letters. 1998;255:83–86. doi: 10.1016/S0304-3940(98)00712-5. [DOI] [PubMed] [Google Scholar]
- 17.Li, Y., Jiang, N., Powers, C. & Chopp, M. Neuronal damage and plasticity identified by microtubule-associated protein 2, growth-associated protein 43, and cyclin D1 immunoreactivity after focal cerebral ischemia in rats. Stroke29, 1972–1980; discussion 1980–1971 (1998). [DOI] [PubMed]
- 18.Stroemer RP, Kent TA, Hulsebosch CE. Acute increase in expression of growth associated protein GAP-43 following cortical ischemia in rat. Neuroscience letters. 1993;162:51–54. doi: 10.1016/0304-3940(93)90557-2. [DOI] [PubMed] [Google Scholar]
- 19.Schirmer L, Merkler D, Konig FB, Bruck W, Stadelmann C. Neuroaxonal regeneration is more pronounced in early multiple sclerosis than in traumatic brain injury lesions. Brain pathology (Zurich, Switzerland) 2013;23:2–12. doi: 10.1111/j.1750-3639.2012.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teunissen CE, et al. Growth-associated protein 43 in lesions and cerebrospinal fluid in multiple sclerosis. Neuropathology and applied neurobiology. 2006;32:318–331. doi: 10.1111/j.1365-2990.2006.00730.x. [DOI] [PubMed] [Google Scholar]
- 21.Haggmark A, et al. Antibody-based profiling of cerebrospinal fluid within multiple sclerosis. Proteomics. 2013;13:2256–2267. doi: 10.1002/pmic.201200580. [DOI] [PubMed] [Google Scholar]
- 22.Rot U, Sandelius Å, Emeršič A, Zetterberg H, Blennow K. Cerebrospinal fluid GAP-43 in early multiple sclerosis. Multiple Sclerosis Journal - Experimental, Translational and Clinical. 2018;4(3):205521731879293. doi: 10.1177/2055217318792931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sandelius Åsa, Portelius Erik, Källén Åsa, Zetterberg Henrik, Rot Uros, Olsson Bob, Toledo Jon B., Shaw Leslie M., Lee Virginia M.Y., Irwin David J., Grossman Murray, Weintraub Daniel, Chen-Plotkin Alice, Wolk David A., McCluskey Leo, Elman Lauren, Kostanjevecki Vesna, Vandijck Manu, McBride Jennifer, Trojanowski John Q., Blennow Kaj. Elevated CSF GAP-43 is Alzheimer's disease specific and associated with tau and amyloid pathology. Alzheimer's & Dementia. 2019;15(1):55–64. doi: 10.1016/j.jalz.2018.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sjogren M, et al. CSF levels of tau, beta-amyloid(1-42) and GAP-43 in frontotemporal dementia, other types of dementia and normal aging. Journal of neural transmission (Vienna, Austria: 1996) 2000;107:563–579. doi: 10.1007/s007020070079. [DOI] [PubMed] [Google Scholar]
- 25.Sandelius A, et al. Transient increase in CSF GAP-43 concentration after ischemic stroke. BMC neurology. 2018;18:202. doi: 10.1186/s12883-018-1210-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Polman CH, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Annals of neurology. 2011;69:292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Novakova L, et al. Searching for neurodegeneration in multiple sclerosis at clinical onset: Diagnostic value of biomarkers. PloS one. 2018;13:e0194828. doi: 10.1371/journal.pone.0194828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Novakova L, et al. Cerebrospinal fluid biomarkers of inflammation and degeneration as measures of fingolimod efficacy in multiple sclerosis. Multiple sclerosis (Houndmills, Basingstoke, England) 2017;23:62–71. doi: 10.1177/1352458516639384. [DOI] [PubMed] [Google Scholar]
- 29.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983;33:1444–1452. doi: 10.1212/WNL.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 30.McDonald WI, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Annals of neurology. 2001;50:121–127. doi: 10.1002/ana.1032. [DOI] [PubMed] [Google Scholar]
- 31.Teunissen CE, et al. A consensus protocol for the standardization of cerebrospinal fluid collection and biobanking. Neurology. 2009;73:1914–1922. doi: 10.1212/WNL.0b013e3181c47cc2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vagberg M, et al. Guidelines for the use of magnetic resonance imaging in diagnosing and monitoring the treatment of multiple sclerosis: recommendations of the Swedish Multiple Sclerosis Association and the Swedish Neuroradiological Society. Acta neurologica Scandinavica. 2017;135:17–24. doi: 10.1111/ane.12667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tibbling G, Link H, Ohman S. Principles of albumin and IgG analyses in neurological disorders. I. Establishment of reference values. Scand J Clin Lab Invest. 1977;37:385–390. doi: 10.1080/00365517709091496. [DOI] [PubMed] [Google Scholar]
- 34.World Medical Association Declaration of Helsinki ethical principles for medical research involving human subjects. Jama. 2013;310:2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.