Highlights
-
•
The authors discuss the concept of atrial myopathy; its relationship to aging, electrophysiological remodeling, and autonomic remodeling; the interplay between atrial myopathy, AF, and stroke; and suggest how to identify patients with atrial myopathy and how to incorporate atrial myopathy into decisions about anticoagulation.
-
•
Atrial myopathy seen in animal models of AF and in patients with AF is the result of a combination of factors that lead to electrical and structural remodeling in the atrium. Although AF may lead to the initiation and/or progression of this myopathy, the presence of AF is by no means essential to the development or the maintenance of the atrial myopathic state.
-
•
Methods to identify atrial myopathy include atrial electrograms, tissue biopsy, cardiac imaging, and certain serum biomarkers. A promising modality is 4-dimensional flow cardiac magnetic resonance. The concept of atrial myopathy may help guide oral anticoagulant therapy in selected groups of patients with AF, particularly those with low to intermediate risk of strokes and those who have undergone successful AF ablation. This review highlights the need for prospective randomized trials to test these hypotheses.
Key Words: atrial fibrillation, atrial myopathy, electrophysiology, thrombosis
Abbreviations and Acronyms: 4D, 4 dimensional; AF, atrial fibrillation; APD, action potential duration; Ca2+, calcium; CMR, cardiac magnetic resonance; CRP, C-reactive protein; Cx, connexin; GDF, growth differentiation factor; IL, interleukin; K+, potassium; LA, left atrial; LAA, left atrial appendage; NADPH, nicotinamide adenine dinucleotide phosphate; NOX2, catalytic, membrane-bound subunit of NADPH oxidase; NT-proBNP, N-terminal pro B-type natriuretic peptide; OAC, oral anticoagulant; ROS, reactive oxygen species; TGF, transforming growth factor; TNF, tumor necrosis factor
Summary
This paper discusses the evolving concept of atrial myopathy by presenting how it develops and how it affects the properties of the atria. It also reviews the complex relationships among atrial myopathy, atrial fibrillation (AF), and stroke. Finally, it discusses how to apply the concept of atrial myopathy in the clinical setting—to identify patients with atrial myopathy and to be more selective in anticoagulation in a subset of patients with AF. An apparent lack of a temporal relationship between episodes of paroxysmal AF and stroke in patients with cardiac implantable electronic devices has led investigators to search for additional factors that are responsible for AF-related strokes. Multiple animal models and human studies have revealed a close interplay of atrial myopathy, AF, and stroke via various mechanisms (e.g., aging, inflammation, oxidative stress, and stretch), which, in turn, lead to fibrosis, electrical and autonomic remodeling, and a pro-thrombotic state. The complex interplay among these mechanisms creates a vicious cycle of ever-worsening atrial myopathy and a higher risk of more sustained AF and strokes. By highlighting the importance of atrial myopathy and the risk of strokes independent of AF, this paper reviews the methods to identify patients with atrial myopathy and proposes a way to incorporate the concept of atrial myopathy to guide anticoagulation in patients with AF.
Central Illustration
Atrial fibrillation (AF) is the most common arrhythmia in developed countries. Approximately 2.3 million people in the United States have been currently diagnosed with AF, and U.S. Census projections estimate that this count will more than double by 2050 (1). AF is associated with a 5-fold risk of stroke, and AF-related strokes are 2.5-fold likely to be fatal (2). AF-related strokes are usually attributed to clots forming in the left atrial appendage (LAA) due to local stasis, followed by dislodgement and embolization to the brain (particularly upon restoration of sinus rhythm). However, this classic concept has been challenged by observations from clinical trials of the past 2 decades. If local stasis during AF is the primary cause of stroke, maintenance of sinus rhythm should prevent embolism. This was not what early randomized clinical trials such as RACE (Rate Control versus Electrical Cardioversion for Persistent Atrial Fibrillation Study) and AFFIRM (Atrial Fibrillation Follow-up Investigation of Rhythm Management) demonstrated 16 years ago 3, 4. Recently, several clinical studies in which patients’ atrial rhythms were continuously monitored showed a temporal dissociation of the episodes of device-recorded subclinical AF and stroke 5, 6, 7, 8. For instance, in the ASSERT (Asymptomatic Atrial Fibrillation and Stroke Evaluation in Pacemaker Patients and the Atrial Fibrillation Reduction Atrial Pacing) trial that enrolled 2,580 patients with cardiac implantable electronic devices, only 8% of patients with stroke had AF events detected within 30 days before stroke, and 16% of patients with stroke had their first AF event after their strokes (5). The lack of a temporal relationship between the onset of AF and stroke suggests that additional factors may be important contributors to the occurrence of stroke and that the presence of AF is not necessary. The current paradigm of selecting individuals at elevated risk of stroke and who therefore warrant oral anticoagulation (OAC) therapy, as endorsed by major international societies 9, 10, is by the CHA2DS2-VASc score (congestive heart failure, hypertension, age older than 75 years, diabetes mellitus, previous stroke, or transient ischemic attack or thromboembolism, vascular disease, age 65 to 74 years, sex category), but not the properties of AF per se (frequency, duration, ventricular rates, and so on). All these risk factors are known to cause atrial myopathy. There is mounting evidence that supports that atrial myopathy not only leads to stasis, but also to endothelial and/or endocardial dysfunction and the hypercoagulable state, which are 3 key factors in thrombogenesis described by Virchow (11). AF may not be the root cause of stroke, but rather, a marker that the atria are diseased. This review seeks to: 1) introduce the concept of atrial myopathy—whether in the absence or presence of AF—and highlight the recent translational and clinical studies that have investigated the relationship among atrial myopathy, AF, and stroke; 2) discuss how to identify patients with atrial myopathy, even in the absence of AF; and 3) discuss whether severity of atrial myopathy can help guide the decision to anticoagulate patients with AF.
Atrial Myopathy: The Concept
“… that the arrhythmia (AF) causes a tachycardia-induced atrial cardiomyopathy (myopathy) that results in electrophysiological and anatomic remodeling of the atria.” (12)
Most of our mechanistic understanding of the atrial myopathic state comes from research conducted either in animal models of AF or from examination of tissue removed from patients with a history of AF. In 1997, Zipes (12) first used the term “atrial myopathy” to describe that AF can lead to myopathy through atrial remodeling. The past 2 decades have seen the concept of atrial myopathy evolving. Several recent studies have demonstrated that the relationship between AF and atrial myopathy is more complex. For example, atrial myopathy may exist without AF and can facilitate the development of AF (13). Anatomical or structural changes, particularly fibrosis, play a major role in the pathogenesis of AF, by increasing the conduction heterogeneity in the atria, thereby providing the substrate for re-entry 14, 15, 16, 17. Atrial interstitial fibrosis has been observed in patients with AF (18). In dogs with heart failure induced by rapid ventricular pacing, extensive atrial fibrosis underlies the mechanism by which AF is much easier to be induced and become sustained, despite unaltered atrial electrophysiological parameters (19). Fibroblast proliferation and extracellular matrix deposition in response to insults such as aging (20), atrial stretch (16), inflammation 21, 22, and oxidative stress 21, 23 may predispose to anisotropy and re-entry (24). The electrical derangement adds to a vicious cycle in which “AF begets AF” (25)—rapid atrial myocyte depolarization leads to intracellular calcium accumulation, triggering adaptive and inflammatory responses that potentiate myocyte apoptosis and accelerate atrial fibrosis (26).
Histologically, atrial fibrosis is characterized by cardiomyocytes exhibiting loss of sarcomeres as well as accumulation of glycogen storage granules, which are adaptive alterations in cellular metabolism not unlike those observed in response to ischemia (27). A principal mediator of AF-induced atrial cardiomyocyte apoptosis and necrosis is calpain (28), a protein concentrated in or near the nucleus, and intercalated discs, which are capable of both proteolysis and degradation of L-type calcium channels (29). Other potential mediators of structural remodeling include platelet-derived growth factor, atrial natriuretic peptide, and galectin-3 30, 31. In addition, the renin-angiotensin system appears to play a role, because angiotensin-converting enzyme inhibitors (or angiotensin-receptor blockers) blunt atrial fibrosis (32) and decrease the incidence of AF in heart failure in both animal 33, 34 and clinical studies 35, 36. Transforming growth factor-β (TGF-β) also has an important role. Mouse models that overexpress TGF-β1 have profound atrial fibrosis and AF (with normal ventricles) (15). In a canine model of heart failure with extensive atrial fibrosis, the drug pirfenidone significantly reduced atrial fibrotic remodeling and vulnerability, and was associated with a significant reduction in the expression of TGF-β1 (37). In a canine heart failure model, Kunamalla et al. (38) recently showed that targeted atrial expression of a plasmid expressing a dominant negative TGF-β1 receptor prevented atrial fibrosis, with a resulting homogenization of conduction and a decrease in inducible AF. To sum up, pathological atrial remodeling leads to the development of AF: which worsens the atrial myopathic processes that then help to sustain more AF? The following sections help to further highlight the concept that although AF may lead to the initiation and/or progression of this myopathy, the presence of AF is by no means essential to the development or the maintenance of this myopathic state.
Atrial Myopathy and the Aging Heart
Aging leads to advancing decline in the structure and function of the heart, and is a leading risk factor for cardiovascular diseases (39), including AF. Although 50% of patients with AF are older than 80 years of age (40), the molecular mechanisms that relate aging to atrial deterioration remain partly elucidated, and it is unclear how aging promotes atrial remodeling. Aging is commonly associated with cardiovascular comorbidities, oxidative stress, calcium dysregulation, atrial myopathy with apoptosis, and fibrosis, all of which contribute to the initiation and/or maintenance of AF, but the mechanisms have been poorly explored (41). Chronic inflammation is associated with several age-related diseases such as atherosclerosis, Alzheimer’s disease, sarcopenia, and arthritis (42). The genesis of chronic inflammation with aging is unclear, but inflammation could be an underlying mechanism that connects aging to atrial myopathy and AF (43). Clinical studies have connected various circulating inflammatory mediators, including C-reactive protein (CRP), interleukin (IL)-1β, IL-6, tumor necrosis factor (TNF)-α, and immune complement activation, with persistent AF (44). Epicardial fat is a major source of adipokines, inflammatory cytokines, and free fatty acids, which contributes to fibrotic remodeling within the atrial myocardium (45). Macrophages and neutrophils are key cellular mediators of inflammation that may contribute to AF by infiltrating the atria or epicardial fat (46), releasing reactive oxygen species (ROS), and producing inflammatory cytokines, chemokines, metalloproteinases, or myeloperoxidases (43). Inflammation is also critical for insulin resistance in experimental models of diet-induced obesity (47). Increasing adiposity leads to the recruitment of macrophages into fat depots. Macrophages, together with adipocytes, generate inflammatory mediators, including TNF-α, which may mediate insulin resistance. Thus, inflammation occurs in aging, obesity and AF, yet the inflammatory pathway linking obesity to AF has not been identified.
Comprehensive understanding of the molecular mechanisms of intrinsic cardiac aging, including atrial aging, will be required to improve understanding of the relationship among aging, inflammation, atrial myopathy, and AF. Such new understanding should guide the development and future translation of novel therapies to clinical application. Mechanistic insights may also identify other more specific therapeutic targets and provide guidance toward interventions for AF-related comorbidities.
Atrial Myopathy and Electrophysiological Remodeling
Besides structural changes, atrial myopathy is associated with alterations in calcium cycling (excitation−contraction coupling), ion channels, and gap junctions that, in turn, lead to electrophysiological remodeling in the atrium.
Oxidative stress associated with atrial myopathy leads to intracellular calcium overload, promoting triggered activity and apoptosis. However, during AF, the exceedingly high frequency of excitation of the atria is expected to lead to ryanodine receptor type 2 refractoriness (48), as well as down-regulation of calcium (Ca2+) handling proteins (49), which act to prevent triggered activity. Nevertheless, [Ca2+]i overload, together with atrial dilatation, mitochondrial ROS, and activation of inflammatory and pro-fibrotic pathways, progressively alters gene expression, with consequent myocyte hypertrophy, interstitial fibrosis, and ion channel remodeling, all of which occur slowly but reach critical levels when AF becomes persistent (49).
The spatial distribution of ion channel functional expression is heterogeneous throughout the atria 50, 51, 52. In addition, the amount of remodeling ion channels undergo with time in AF is also heterogeneous (53). Such heterogeneities are responsible for the different electrophysiological properties of different regions of the atria in terms of conduction velocity, action potential duration (APD), and the refractory period, all of which condition the ability of each region to harbor re-entrant circuits at different frequencies in response to the remodeling process. Cellular electrophysiological studies in atrial myocytes from patients in persistent or permanent AF have revealed marked reductions in the densities of the L-type voltage-gated Ca2+ current, the transient outward potassium (K+) current, and the ultra-rapid delayed rectifier K+ current (54). Sustained high-frequency excitation in a sheep model of long-term atrial tachypacing led to APD abbreviation secondary to ion channel gene expression changes (the main cardiac sodium channel, L-type calcium channel, and voltage gated potassium channel decrease; and stria specific inward rectifying potassium channel increase) (49). A study using specimens of human atrium demonstrated that chronic AF reduced the transient outward current and the ultrarapid delayed rectifier potassium channel (IKur) (55). In addition, the slow component of the delayed rectifier K+ current is increased, which results in significant APD abbreviation. Importantly, such changes are quantitatively different between right and left atria, which may explain the propensity of 1 atrium to sustain more stable re-entry at a higher frequency than the other.
Gap junctions are essential in atrial conduction, and gap junction remodeling, such as changes in distribution, intercellular orientation, and expression of gap junction proteins, is associated with electrophysiological and structural changes that result in sustained AF 56, 57. Two major gap junction proteins, connexin (Cx) 40 and Cx43, mediate cardiomyocyte-to-cardiomyocyte electrical coupling. Abnormal expression and heterogeneous distributions of Cxs have a strong association with AF in patients (58) and rapid pacing animal models 59, 60. In a dilated left atrium (LA) with chronic pressure overload and left ventricular hypertrophy, reduced expression and lateral distribution of Cx43, as well as interstitial fibrosis, lead to conduction abnormality, which increases the susceptibility to AF (61). Moreover, the laterally redistributed Cx43 does not form gap junction channels (62). Together with the significant reduction in sodium current associated with atrial remodeling (49), reduced Cx function should significantly reduce atrial conduction velocity. In patients with hypertension and left ventricular hypertrophy but no history of AF, global conduction slowing, focal conduction delay, and increased vulnerability to AF have been noted (63). In a subgroup of 485 patients from the ASSERT cohort (5), serial noninvasive programmed atrial stimulation showed those who went on to develop atrial tachyarrhythmias had P-wave prolongation and increased vulnerability to AF induction at baseline but no difference in atrial refractoriness (64).
Atrial Myopathy and Autonomic Remodeling
In addition to alterations in ion channels, gap junctions, and excitation−contraction coupling, the autonomic nervous system is another contributor to electrical remodeling in the fibrillating atrium. Animal studies have shown that atrial myopathy or LA distention caused by obesity and obstructive sleep apnea (65) can lead to remodeling of the autonomic nervous system, which is instrumental in pathogenesis of cardiac arrhythmias (66), including that of AF (67). Patterson et al. 68, 69 described “calcium transient triggering,” in that sympathetic activation causes an increasing calcium transient (70), whereas vagal activation can reduce the atrial effective refractory period (71). The discrepancy between APD and the intracellular calcium transient, which are normally tightly coupled, leads to an increased forward sodium/Ca exchanger current, which contributes to generation of early afterdepolarizations and triggered activity (68). This is particularly evident in the muscle sleeves of the pulmonary veins, of which focal activities are critically important in the initiation of AF in humans (72). Histologically, the pulmonary vein muscle sleeves are particularly richly innervated with both sympathetic and parasympathetic nerves 73, 74. With direct nerve recordings, the intrinsic cardiac nerve activities recorded from the fat pad next to the left superior pulmonary vein and LA junction were found to be in close temporal relationships, with nerve activities recorded from extracardiac nerve structures (e.g., the left stellate ganglion and the thoracic vagus nerve). Intrinsic cardiac nerve activities invariably preceded spontaneously occurring atrial tachyarrhythmias in a canine model of pacing-induced AF (75). In patients recovering from open heart surgery, these fat pad intrinsic cardiac nerve activities were associated with an increased burden of premature atrial complexes and might predict the development of post-operative AF (76).
Atrial Myopathy: Its Interplay between AF and Stroke
One major link between atrial myopathy, AF, and stroke is inflammation (77). Inflammatory markers, such as CRP, TNF-α, and IL-2, -6, and -8, increase in patients with AF (78). Several prospective epidemiological studies confirmed that inflammation confers an increased risk of AF. For example, in a large cohort that involved 25,883 participants of the Women's Health Study, inflammatory biomarkers, including CRP, soluble intercellular adhesion molecule-1, and fibrinogen, were independently associated with increased incidence of AF in initially healthy, middle-aged women during a median follow-up of 14.4 years, after controlling for traditional risk factors (79). In another large cohort study of 47,000 subjects, elevated plasma CRP levels were robustly associated with increased incidence of AF (80). In the absence of AF, it is possible that the pro-inflammatory state can contribute to the development of atrial myopathy, which, in turn, leads to endothelial dysfunction or other structural changes, and thereby more pro-inflammatory (81). Once AF occurs, the inflammatory state may help perpetuate AF, as evident by the observation that CRP levels are higher in patients who remained in AF, compared with those of patients who converted to sinus rhythm (82). Inflammation might also be involved in the creation of a pro-thrombotic state during AF (83). In a cohort of 880 patients with AF, CRP levels were correlated with risk of stroke and all-cause mortality (84).
Inflammatory cells such as monocytes, macrophages, and lymphocytes produce cytokines and chemokines and can trigger thrombosis in AF. One such cytokine, IL-6, induces the expression of tissue factor, fibrinogen, factor VIII, and von Willebrand factor, mediating a pro-thrombotic state. It may also cause endothelial activation and endothelial cell damage, which leads to platelet aggregation and sensitivity to thrombin (78). Activated platelets in patients with AF could, in turn, promote and sustain the pro-thrombotic state and increase inflammatory biomarkers. Altered endothelial function also contributes to inflammation and thrombosis in AF 85, 86. Upon endothelial activation, substances such as von Willebrand factor and soluble P-selectin are rapidly released onto the endothelial surface, promoting the attachment of rolling white blood cells to the endothelium and subsequently contributing to the development of a pro-inflammatory and pro-thrombotic environment. A recent interesting discovery was that not only AF could promote thrombosis, the hypercoagulable state itself promoted atrial fibrosis and thereby facilitated AF, at least in mouse models (87). OAC therapy might not only prevent strokes but inhibit the development of a substrate for AF. Another mechanism invoked in the creation of atrial myopathy that is closely related to inflammation is oxidative stress, in which ROS are believed to modify the function of key ion channels and Ca2+ cycling proteins 88, 89, 90, as well as activating pro-fibrotic signaling 91, 92. The major source of atrial ROS in patients with AF is NOX2, a membrane-bound protein containing nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (93). Reilly et al. (94) showed that NADPH oxidase is elevated early in AF (e.g., with post-operative AF), with mitochondrial oxidases and uncoupled NO syntheses being noted in long-standing AF. Yoo et al. (95) recently showed that ROS are preferentially elevated in the posterior LA in a heart failure model of AF, with a resulting increase in the substrate for both triggered activity (by Ca2+/calmodulin-dependent protein kinase II phosphorylation of ryanodine receptor type 2) and re-entry (via Ca2+/calmodulin-dependent protein kinase II phosphorylation of the main cardiac sodium channel). In response to oxidative stress, the expression of growth differentiation factor 15 (GDF-15) was shown to increase significantly (96). GDF-15 emerged as a novel biomarker to provide prognostic information regarding cardiovascular events, beyond traditional risk factors and other biomarkers, in patients with myocardial infarction (97) or heart failure (98). Moreover, GDF-15 was also shown to be an independent risk factor for stroke in patients with AF (99). After adjusting for potential clinical risk factors, GDF-15 was associated with LAA thrombus in patients with nonvalvular AF (100) (Central Illustration).
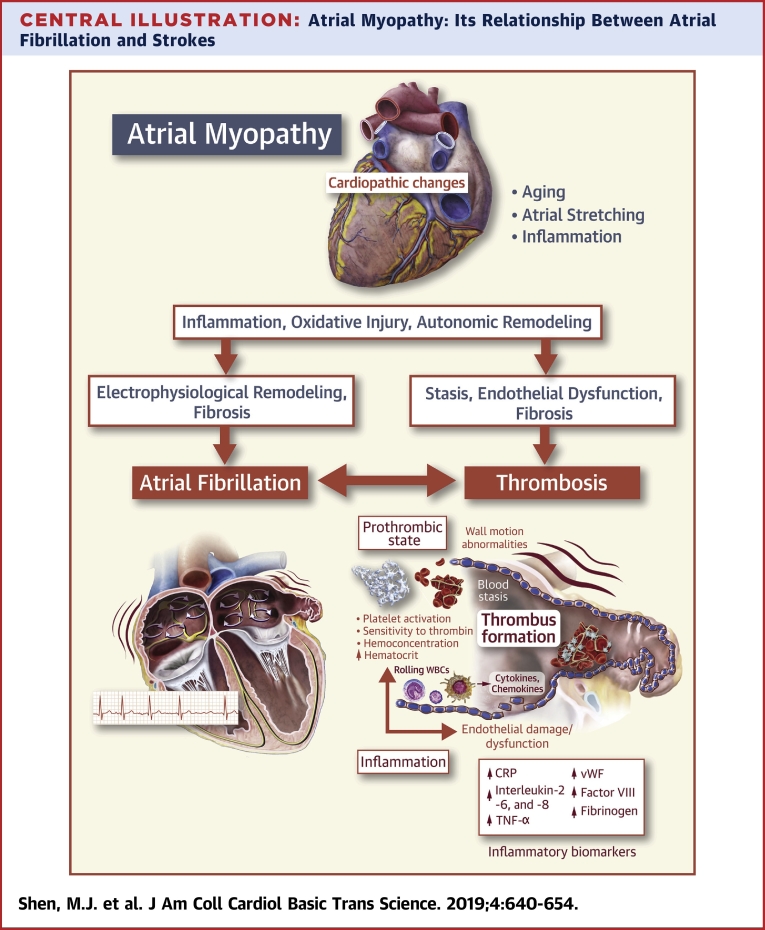
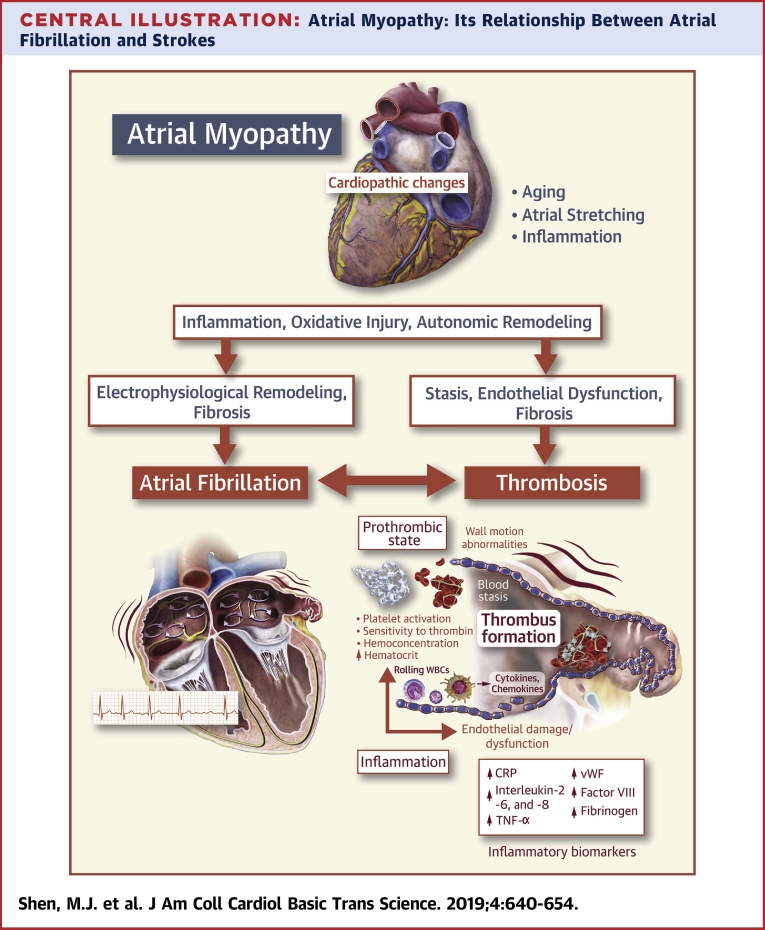
Central Illustration.
Atrial Myopathy: Its Relationship Between Atrial Fibrillation and Strokes
Atrial myopathy is typically caused by insults such as aging, inflammation, oxidative stress, and stretching of the atria. These myopathic changes alter the properties of myocardial electrophysiology and cardiac autonomic nervous system. They can also lead to architectural structural changes characterized by fibrosis. Furthermore, atrial myopathy results in endothelial dysfunction and stasis, thereby a prothrombotic state. Electrophysiological remodeling and fibrosis facilitate the development of atrial fibrillation, which leads to more inflammation, fibrosis and autonomic remodeling, all of which contribute to a worsening prothrombotic environment, mediated by circulating inflammatory cytokines, chemokines and other molecules such as C-reactive protein (CRP), interleukin (IL)- 2, -6 and -8, tumor necrosis factor (TNF)-α, etc. Atrial fibrillation and thrombosis can develop separately and interact closely to further aggravate the underlying atrial myopathic processes. CRP = C-reactive protein; vWF = von Willebrand's Factor; WBC = white blood cell.
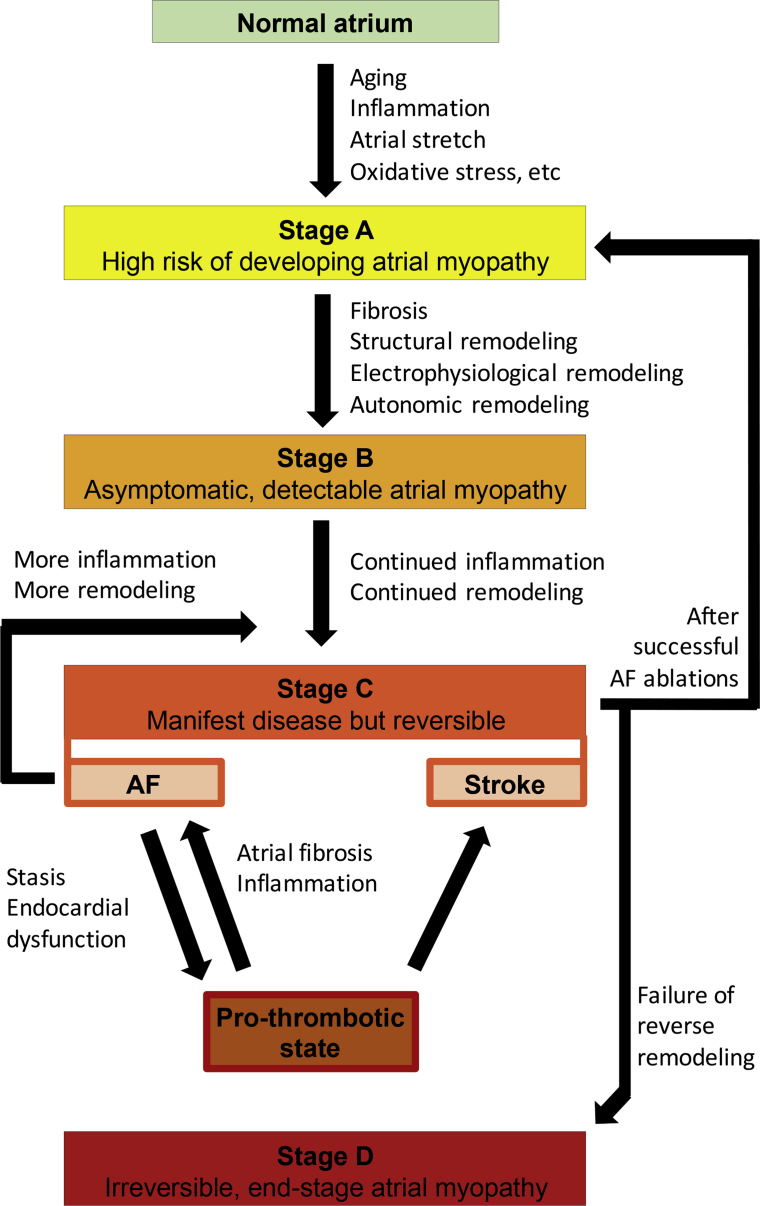
Figure 1 shows the proposed stages of atrial myopathy (staging in line with 2013 American College of Cardiology Foundation/American Heart Association guidelines for heart failure [101]). With aging, inflammation, atrial stretch from volume and/or pressure overload, and oxidative stress, atria may be at risk of developing atrial myopathy (stage A). At this stage, clinical atrial myopathy is not detectable (see the paragraph “How to identify patients with atrial myopathy”). Once fibrosis and various ways of atrial remodeling, including structural, electrophysiological, and autonomic remodeling have taken place, atrial myopathy is established and becomes detectable (stage B). At this stage, the individual remains asymptomatic. Nevertheless, with ongoing inflammation and remodeling, the myopathic atria become manifest in the form of AF and stroke that typically draw clinical attention. AF leads to a pro-thrombotic state that feeds back to cause more AF by facilitating atrial fibrosis and inflammation. At stage C, the manifest atrial myopathy can be reversed by aggressive interventions such as lifestyle modification and a successful AF ablation. If failure to reverse occurs, the disease progresses to stage D, or end-stage atrial myopathy. This illustration does not account for AF being the initiating stimulus underlying the development of the atrial myopathy. However, once paroxysmal or chronic AF has led to the development of some electrical and/or structural remodeling, several of the paradigms suggested in Figure 1 would again come into play to help perpetuate these myopathic changes, potentially even in the absence of the initiating stimulus (i.e., AF).
Figure 1.
Stages of Atrial Myopathy
AF = atrial fibrillation.
Atrial Myopathy: a Logical Explanation to the Lack of Temporal Relationships Between AF and Stroke
Key observations, including the apparent inability of AF rhythm control strategies to lower stroke risk 3, 4, and the lack of a strong temporal association between paroxysmal AF and stroke in patients with prolonged rhythm monitoring via cardiac implantable electronic devices 8, 102, 103 have heightened the search for additional factors that could account for AF-related strokes beyond the rhythm disturbance itself. Although strokes may occur in the absence of AF itself, AF burden is positively correlated with risk of stroke (104). Furthermore, it has been recently reported that patients with persistent or permanent AF have much more thromboembolic events compared with those with paroxysmal AF (105). If stroke can occur independently of AF, how could more AF be associated with more strokes? One possibility is that these strokes observed in clinical trials were not cardioembolic strokes but rather strokes related to aortic, carotid. or intracerebral atherosclerosis (106). A more likely explanation is that the presence of atrial myopathy, and its associated atrial remodeling (structural, electrical, and autonomic), endocardial dysfunction and pro-thrombotic state, may lead to cardioembolic strokes without the necessity of fibrillating atria. Higher AF burden (or persistent and/or permanent AF) may merely reflect underlying more severe atrial myopathy. In other words, the most logical explanation to all these clinical observations is that atrial myopathy facilitates the development of both AF and stroke (in parallel, not in series). Atrial myopathy may manifest periodically as AF but is always present and continually thrombogenic (107). Recent reviews and editorials have suggested the importance of atrial myopathy and have called for additional studies of its role in AF and AF-associated complications 106, 108, 109, 110. The following section will discuss how to identify patients with atrial myopathy and how to apply the concept of atrial myopathy in guiding OAC therapy in patients with AF.
Atrial Myopathy: Translating the Concept Into Clinical Practice
How to identify patients with atrial myopathy
Macroscopically, atrial myopathy may manifest as AF or non-AF atrial arrhythmias, atrial dilatation, impaired atrial systole, or abnormal cardiac imaging findings (111). Therefore, several methods have been reported to identify patients with atrial myopathy and who are at risk of developing AF and AF-related complications, particularly strokes.
Non-AF atrial arrhythmias, such as frequent atrial premature beats or paroxysmal atrial tachycardia, might indicate an abnormal atrial substrate and a predisposition to AF, and have been associated with increased risk of stroke in long-term follow-up, independent of diagnosed AF 112, 113. Atrial electrograms acquired either noninvasively or invasively may shed some light on underlying atrial myopathy or potentially elevated thromboembolic risk, even in the absence of AF. In a case−cohort analysis of the Northern Manhattan Study, which was a prospective cohort study of stroke risk factors, P-wave terminal force in lead V1 in sinus rhythm (a marker of LA abnormality) was associated with an increased risk of cardioembolic stroke independently of the presence of AF (114). Invasively, with high-density patch electrodes in the LA in canines with healthy hearts and pacing-induced heart failure with atrial fibrosis, the atrial electrograms during induced AF were markedly different: the overall AF electrograms in dogs with heart failure (and extensive atrial fibrosis) were slower and paradoxically more organized (115). However, in the autonomic nerve-rich posterior LA, the regional electrograms in dogs with heart failure exhibited more spatial heterogeneity and were less organized. Clinical and animal studies suggested that regions of complex fractionated atrial electrograms during AF might represent sites of high autonomic innervation 75, 116.
Imaging can be a useful tool in detecting patients with atrial myopathy. Echocardiography (2 dimensional, pulsed-wave Doppler, speckle-tracking echo, strain and strain rate imaging) and cardiac computed tomography are both useful in providing volumetric and functional assessment of the LA 117, 118. Larger LA size, assessed by echocardiography, is associated with a higher recurrence rate of AF after AF ablation (119), and with an increased risk of recurrent stroke in patients with nonvalvular AF and ischemic stroke (120).
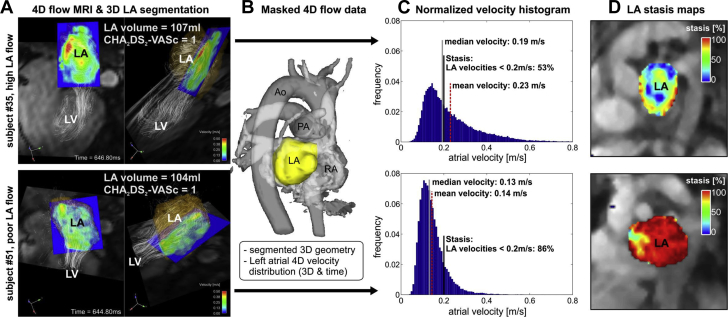
In recent years, cardiac magnetic resonance (CMR) has become the gold standard of assessment of chamber structure and function, mainly because of its superiority in tissue characterization, particularly in fibrosis. Delayed-enhanced CMR has been applied to assess the extent of LA fibrosis and was found to be correlated with surgical biopsy results and with AF recurrence after catheter ablation 121, 122. Furthermore, delayed-enhanced CMR−detected LA fibrosis was found to be an independent risk factor of stroke, after adjusting for other clinical risk factors, in patients with AF (123). Complimentary to this, a novel noninvasive approach is atrial 4-dimensional (4D) flow CMR, which provides a comprehensive characterization of atrial flow dynamics that can overcome limitations of transesophageal echocardiography. It measures 3-D blood flow velocities with full coverage of the LA and LAA, deriving stasis maps (124) that provide intuitive visualizations and quantification of stasis in the LA and LAA, the typical site of thrombus formation 124, 125, 126, 127. Moreover, 4-D flow CMR can detect physiological changes in LA hemodynamics in AF and reveal an increased predisposition to atrial thrombogenesis that is not discernible by the CHA2DS2-VASc score 124, 125, 128, 129. Figure 2 shows data from 2 patients with AF and with identically low CHA2DS2-VASc scores of 1 but substantially different LA blood flow velocity histograms (Figure 2C) and blood stasis maps (Figure 2D, color coded for an intuitive visualization of stasis, where the red color corresponds to a heightened risk for thrombogenesis). Therefore, 4-D flow CMR-derived atrial stasis may serve as an important new metric that measures predisposition to atrial thrombogenesis that is not captured with current clinical risk predictors and may provide guidance in anticoagulation therapy in selected groups of patients with AF.
Figure 2.
Atrial 4D Flow CMR in Patients With AF
(A) Atrial 4-dimensional (4D) flow cardiac magnetic resonance (CMR) for 2 patients with AF with comparable left atrial (LA) volume and identical CHA2DS2-VASc scores = 1 indicating low thromboembolic risk. Velocity histograms (C) quantify the LA velocity distribution inside the (B) LA. (D) LA stasis maps depict the relative amount of low LA flow velocities (<0.2 m/s). Note the substantially increased flow stasis (red) in subject #51 compared with subject #35 despite identical CHA2DS2-VASc scores. Ao = aorta; LV = left ventricle; PA = pulmonary artery; RA = right atrium; other abbreviation as in Figure 1.
Despite the promising data, several challenges limit routine application for patients with AF. Beyond the costs, a high level of expertise is required for image acquisition and the time required for image processing. Accurate 3-D imaging of the LA involves manual segmentation from other images and careful intensity threshold calibration to differentiate normal from fibrotic tissue (130). Anatomic variability and imaging artifacts may confound image processing. Among the relevant patient factors are body size and habitus that allow optimum image quality and adequate heart rate control to facilitate gating.
How to incorporate the concept of atrial myopathy in guiding anticoagulation therapy
Current guidelines endorsed the use of the CHA2DS2-VASc score to guide anticoagulation therapy in patients with AF. Because stroke, as discussed earlier, might occur independently of AF, individuals with an elevated risk of stroke and no history of AF might be identified and be protected by OAC therapy. The CHA2DS2-VASc score was found to be valuable in predicting stroke risk in the absence of AF. In patients with heart failure and sinus rhythm, the CHA2DS2-VASc score was found to provide prognostic information on future stroke risk (131). Similarly, the CHA2DS2-VASc score was associated with spontaneous echo contrast in the LA in patients with rheumatic mitral stenosis and no history of AF, who are at risk of LA thrombus formation and thromboembolism, despite being in sinus rhythm (132). On the other end of the spectrum, with AF, the lack of key atrial myopathic features might be helpful in identifying individuals who do not require OAC therapy, thereby sparing them the unnecessary risk of bleeding. The current guidelines recommend no need for anticoagulation if the CHA2DS2-VASc score is 0 or 1, if female. A logical integration of the concept of atrial myopathy to the present paradigm would be to examine the features of atrial myopathy in 2 groups of patients: male with a CHA2DS2-VASc score of 1 and female with a CHA2DS2-VASc score of 2. A review article by Calenda et al. (107) proposed that in these 2 groups of individuals with AF, the lack of atrial myopathic features might spare them of unnecessary OAC therapy. Conversely, individuals with a low CHA2DS2-VASc score but who have evidence of atrial myopathy might benefit from OAC therapy. A prospective, randomized clinical trial, ARCADIA (Atrial Cardiopathy and Antithrombotic Drugs In Prevention After Cryptogenic Stroke) sought to answer if OAC therapy compared with daily baby aspirin would prevent recurrent ischemic stroke in patients with cryptogenic stroke who possess at least 1 marker of atrial myopathy: an abnormal P wave, N-terminal pro B-type natriuretic peptide (NT-proBNP) level, and dilated LA on echocardiography (133).
Finally, observational studies showed that after a successful AF ablation, the stroke risk might be substantially reduced and cessation of anticoagulation might be safe in many patients 134, 135. It is possible that reverse remodeling can take place after a successful AF ablation. Therefore, the assessment of atrial myopathy after an AF ablation might aid in the identification of individuals who no longer require anticoagulation therapy. Conversely, failure of reverse remodeling after an AF ablation might support a need for indefinite OAC therapy despite the absence of AF recurrence. After AF ablation, elevated circulating fibrocytes were found to serve as a marker of LA fibrosis and predict the recurrence of AF (136). It is possible that persistently elevated serum fibrocytes may indicate the failure of reverse remodeling of an underlying atrial myopathic process, which may be continuously thrombogenic. Cessation of OAC therapy in this group of patients might not be safe. This hypothesis needs to be tested in prospective, randomized trials.
Serum biomarkers can further improve risk stratification for strokes beyond traditional risk factors. Although generally not specific to atrial myocardial disease, a growing body of evidence suggests that serum biomarkers might be useful to quantify stroke risk in patients with AF, in addition to clinical risk factors. Various biomarkers correlated with myocyte injury, oxidative stress, inflammation, and fibrosis (BNP, NT-proBNP, troponins, CRP, IL-6, among others) have been reported to be elevated in AF or predictive of development of AF (137) and linked to outcomes in AF, including strokes (138). Using troponins and NT-proBNP, the novel biomarker-based ABC (age, biomarkers, clinical history of stroke or transient ischemic attack) stroke risk score system has been shown to outperform the classic CHA2DS2-VASc score system in both the derivation cohort (c-statistic: 0.68 vs. 0.62) and in the validation cohort (c-statistic: 0.66 vs. 0.58) (139).
Conclusions
Atrial myopathy characterized atrial fibrotic remodeling, together with electrical and autonomic remodeling, facilitates the development of both AF and stroke. Various animal models of atrial myopathy, such as a canine model of pacing-induced heart failure and extensive atrial fibrosis (19), a mouse model that overexpresses TGF-β1 (15), and a rat model of obesity and artificially induced obstructive apnea (65), have helped demystify the complex interplay between atrial myopathy and AF. However, most animal studies do not have long enough follow-up to further determine a causal relationship between atrial myopathy and strokes. The chronicity of human clinical trial data allows bridging of this gap, showing that individuals with markers of atrial myopathy have an elevated risk of developing both AF and strokes 88, 99, 112, 113, 114, 123, 140, 141. Methods to identify atrial myopathy include atrial electrograms, tissue biopsy, cardiac imaging, and certain serum biomarkers. A promising modality is 4-D flow CMR. The concept of atrial myopathy may help guide OAC therapy in selected groups of patients with AF, particularly those with low-intermediate risk of strokes (male with a CHA2DS2-VASc score of 1 and female with a CHA2DS2-VASc score of 2) and those who have undergone a successful AF ablation. Prospective randomized trials are needed to test these hypotheses.
Footnotes
Dr. Arora is an owner of Rhythm Therapeutics, Inc. Dr. Jalife has received a research grant from Medtronic. Dr. Shen has reported that he has no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
References
- 1.Benjamin E.J., Chen P.S., Bild D.E. Prevention of atrial fibrillation: report from a National Heart, Lung, and Blood Institute workshop. Circulation. 2009;119:606–618. doi: 10.1161/CIRCULATIONAHA.108.825380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McGrath E.R., Kapral M.K., Fang J. Association of atrial fibrillation with mortality and disability after ischemic stroke. Neurology. 2013;81:825–832. doi: 10.1212/WNL.0b013e3182a2cc15. [DOI] [PubMed] [Google Scholar]
- 3.Van Gelder I.C., Hagens V.E., Bosker H.A. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med. 2002;347:1834–1840. doi: 10.1056/NEJMoa021375. [DOI] [PubMed] [Google Scholar]
- 4.Wyse D.G., Waldo A.L., DiMarco J.P. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825–1833. doi: 10.1056/NEJMoa021328. [DOI] [PubMed] [Google Scholar]
- 5.Brambatti M., Connolly S.J., Gold M.R. Temporal relationship between subclinical atrial fibrillation and embolic events. Circulation. 2014;129:2094–2099. doi: 10.1161/CIRCULATIONAHA.113.007825. [DOI] [PubMed] [Google Scholar]
- 6.Glotzer T.V., Daoud E.G., Wyse D.G. The relationship between daily atrial tachyarrhythmia burden from implantable device diagnostics and stroke risk: the TRENDS study. Circ Arrhythm Electrophysiol. 2009;2:474–480. doi: 10.1161/CIRCEP.109.849638. [DOI] [PubMed] [Google Scholar]
- 7.Ip J., Waldo A.L., Lip G.Y. Multicenter randomized study of anticoagulation guided by remote rhythm monitoring in patients with implantable cardioverter-defibrillator and CRT-D devices: rationale, design, and clinical characteristics of the initially enrolled cohort The IMPACT study. Am Heart J. 2009;158:364–370 e1. doi: 10.1016/j.ahj.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 8.Martin D.T., Bersohn M.M., Waldo A.L. Randomized trial of atrial arrhythmia monitoring to guide anticoagulation in patients with implanted defibrillator and cardiac resynchronization devices. Eur Heart J. 2015;36:1660–1668. doi: 10.1093/eurheartj/ehv115. [DOI] [PubMed] [Google Scholar]
- 9.January C.T., Wann L.S., Alpert J.S. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130:2071–2104. doi: 10.1161/CIR.0000000000000040. [DOI] [PubMed] [Google Scholar]
- 10.Kirchhof P., Benussi S., Kotecha D. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–2962. doi: 10.1093/eurheartj/ehw210. [DOI] [PubMed] [Google Scholar]
- 11.Watson T., Shantsila E., Lip G.Y. Mechanisms of thrombogenesis in atrial fibrillation: Virchow's triad revisited. Lancet. 2009;373:155–166. doi: 10.1016/S0140-6736(09)60040-4. [DOI] [PubMed] [Google Scholar]
- 12.Zipes D.P. Atrial fibrillation. A tachycardia-induced atrial cardiomyopathy. Circulation. 1997;95:562–564. doi: 10.1161/01.cir.95.3.562. [DOI] [PubMed] [Google Scholar]
- 13.Kottkamp H. Fibrotic atrial cardiomyopathy: a specific disease/syndrome supplying substrates for atrial fibrillation, atrial tachycardia, sinus node disease, AV node disease, and thromboembolic complications. J Cardiovasc Electrophysiol. 2012;23:797–799. doi: 10.1111/j.1540-8167.2012.02341.x. [DOI] [PubMed] [Google Scholar]
- 14.Boldt A., Wetzel U., Lauschke J. Fibrosis in left atrial tissue of patients with atrial fibrillation with and without underlying mitral valve disease. Heart. 2004;90:400–405. doi: 10.1136/hrt.2003.015347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Everett THt, Olgin J.E. Atrial fibrosis and the mechanisms of atrial fibrillation. Heart Rhythm. 2007;4:S24–S27. doi: 10.1016/j.hrthm.2006.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burstein B., Nattel S. Atrial structural remodeling as an antiarrhythmic target. J Cardiovasc Pharmacol. 2008;52:4–10. doi: 10.1097/FJC.0b013e3181668057. [DOI] [PubMed] [Google Scholar]
- 17.Hirsh B.J., Copeland-Halperin R.S., Halperin J.L. Fibrotic atrial cardiomyopathy, atrial fibrillation, and thromboembolism: mechanistic links and clinical inferences. J Am Coll Cardiol. 2015;65:2239–2251. doi: 10.1016/j.jacc.2015.03.557. [DOI] [PubMed] [Google Scholar]
- 18.Kostin S., Klein G., Szalay Z., Hein S., Bauer E.P., Schaper J. Structural correlate of atrial fibrillation in human patients. Cardiovasc Res. 2002;54:361–379. doi: 10.1016/s0008-6363(02)00273-0. [DOI] [PubMed] [Google Scholar]
- 19.Li D., Fareh S., Leung T.K., Nattel S. Promotion of atrial fibrillation by heart failure in dogs: atrial remodeling of a different sort. Circulation. 1999;100:87–95. doi: 10.1161/01.cir.100.1.87. [DOI] [PubMed] [Google Scholar]
- 20.Luo T., Chang C.X., Zhou X., Gu S.K., Jiang T.M., Li Y.M. Characterization of atrial histopathological and electrophysiological changes in a mouse model of aging. Int J Mol Med. 2013;31:138–146. doi: 10.3892/ijmm.2012.1174. [DOI] [PubMed] [Google Scholar]
- 21.Van Wagoner D.R. Oxidative stress and inflammation in atrial fibrillation: role in pathogenesis and potential as a therapeutic target. J Cardiovasc Pharmacol. 2008;52:306–313. doi: 10.1097/FJC.0b013e31817f9398. [DOI] [PubMed] [Google Scholar]
- 22.Korantzopoulos P., Letsas K.P., Tse G., Fragakis N., Goudis C.A., Liu T. Inflammation and atrial fibrillation: a comprehensive review. J Arrhythm. 2018;34:394–401. doi: 10.1002/joa3.12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Youn J.Y., Zhang J., Zhang Y. Oxidative stress in atrial fibrillation: an emerging role of NADPH oxidase. J Mol Cell Cardiol. 2013;62:72–79. doi: 10.1016/j.yjmcc.2013.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eckstein J., Verheule S., de Groot N.M., Allessie M., Schotten U. Mechanisms of perpetuation of atrial fibrillation in chronically dilated atria. Prog Biophys Mol Biol. 2008;97:435–451. doi: 10.1016/j.pbiomolbio.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 25.Nattel S., Harada M. Atrial remodeling and atrial fibrillation: recent advances and translational perspectives. J Am Coll Cardiol. 2014;63:2335–2345. doi: 10.1016/j.jacc.2014.02.555. [DOI] [PubMed] [Google Scholar]
- 26.Schotten U., Neuberger H.R., Allessie M.A. The role of atrial dilatation in the domestication of atrial fibrillation. Prog Biophys Mol Biol. 2003;82:151–162. doi: 10.1016/s0079-6107(03)00012-9. [DOI] [PubMed] [Google Scholar]
- 27.Corradi D. Atrial fibrillation from the pathologist's perspective. Cardiovasc Pathol. 2014;23:71–84. doi: 10.1016/j.carpath.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 28.Bukowska A., Lendeckel U., Bode-Boger S.M., Goette A. Physiologic and pathophysiologic role of calpain: implications for the occurrence of atrial fibrillation. Cardiovasc Ther. 2012;30:e115–e127. doi: 10.1111/j.1755-5922.2010.00245.x. [DOI] [PubMed] [Google Scholar]
- 29.Suzuki K., Imajoh S., Emori Y., Kawasaki H., Minami Y., Ohno S. Calcium-activated neutral protease and its endogenous inhibitor. Activation at the cell membrane and biological function. FEBS Lett. 1987;220:271–277. doi: 10.1016/0014-5793(87)80828-1. [DOI] [PubMed] [Google Scholar]
- 30.Jalife J. Mechanisms of persistent atrial fibrillation. Curr Opin Cardiol. 2014;29:20–27. doi: 10.1097/HCO.0000000000000027. [DOI] [PubMed] [Google Scholar]
- 31.Takemoto Y., Ramirez R.J., Yokokawa M. Galectin-3 regulates atrial fibrillation remodeling and predicts catheter ablation outcomes. J Am Coll Cardiol Basic Trans Science. 2016;1:143–154. doi: 10.1016/j.jacbts.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takemoto Y., Ramirez R.J., Kaur K. Eplerenone reduces atrial fibrillation burden without preventing atrial electrical remodeling. J Am Coll Cardiol. 2017;70:2893–2905. doi: 10.1016/j.jacc.2017.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shi Y., Li D., Tardif J.C., Nattel S. Enalapril effects on atrial remodeling and atrial fibrillation in experimental congestive heart failure. Cardiovasc Res. 2002;54:456–461. doi: 10.1016/s0008-6363(02)00243-2. [DOI] [PubMed] [Google Scholar]
- 34.Li D., Shinagawa K., Pang L. Effects of angiotensin-converting enzyme inhibition on the development of the atrial fibrillation substrate in dogs with ventricular tachypacing-induced congestive heart failure. Circulation. 2001;104:2608–2614. doi: 10.1161/hc4601.099402. [DOI] [PubMed] [Google Scholar]
- 35.Boldt A., Scholl A., Garbade J. ACE-inhibitor treatment attenuates atrial structural remodeling in patients with lone chronic atrial fibrillation. Basic Res Cardiol. 2006;101:261–267. doi: 10.1007/s00395-005-0571-2. [DOI] [PubMed] [Google Scholar]
- 36.Healey J.S., Baranchuk A., Crystal E. Prevention of atrial fibrillation with angiotensin-converting enzyme inhibitors and angiotensin receptor blockers: a meta-analysis. J Am Coll Cardiol. 2005;45:1832–1839. doi: 10.1016/j.jacc.2004.11.070. [DOI] [PubMed] [Google Scholar]
- 37.Lee K.W., Everett T.H.T., Rahmutula D. Pirfenidone prevents the development of a vulnerable substrate for atrial fibrillation in a canine model of heart failure. Circulation. 2006;114:1703–1712. doi: 10.1161/CIRCULATIONAHA.106.624320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kunamalla A., Ng J., Parini V. Constitutive expression of a dominant-negative TGF-beta type II receptor in the posterior left atrium leads to beneficial remodeling of atrial fibrillation substrate. Circ Res. 2016;119:69–82. doi: 10.1161/CIRCRESAHA.115.307878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chiao Y.A., Rabinovitch P.S. The aging heart. Cold Spring Harb Perspect Med. 2015;5:a025148. doi: 10.1101/cshperspect.a025148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zoni-Berisso M., Lercari F., Carazza T., Domenicucci S. Epidemiology of atrial fibrillation: European perspective. Clin Epidemiol. 2014;6:213–220. doi: 10.2147/CLEP.S47385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin Y.K., Chen Y.A., Lee T.I., Chen Y.C., Chen S.A., Chen Y.J. Aging modulates the substrate and triggers remodeling in atrial fibrillation. Circ J. 2018;82:1237–1244. doi: 10.1253/circj.CJ-17-0242. [DOI] [PubMed] [Google Scholar]
- 42.Chung H.Y., Cesari M., Anton S. Molecular inflammation: underpinnings of aging and age-related diseases. Ageing Res Rev. 2009;8:18–30. doi: 10.1016/j.arr.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anumonwo J.M., Jalife J., Goldstein D.R. Triple threat: adiposity, aging, atrial fibrillation. Aging (Albany NY) 2017;9:2235–2236. doi: 10.18632/aging.101318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kourliouros A., Savelieva I., Kiotsekoglou A., Jahangiri M., Camm J. Current concepts in the pathogenesis of atrial fibrillation. Am Heart J. 2009;157:243–252. doi: 10.1016/j.ahj.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 45.Pandit S.V., Anumonwo J., Jalife J. Atrial fibrillation susceptibility in obesity: an excess adiposity and fibrosis complicity? Circ Res. 2016;118:1468–1471. doi: 10.1161/CIRCRESAHA.116.308686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haemers P., Hamdi H., Guedj K. Atrial fibrillation is associated with the fibrotic remodelling of adipose tissue in the subepicardium of human and sheep atria. Eur Heart J. 2017;38:53–61. doi: 10.1093/eurheartj/ehv625. [DOI] [PubMed] [Google Scholar]
- 47.Wong C.X., Brooks A.G., Leong D.P., Roberts-Thomson K.C., Sanders P. The increasing burden of atrial fibrillation compared with heart failure and myocardial infarction: a 15-year study of all hospitalizations in Australia. Arch Intern Med. 2012;172:739–741. doi: 10.1001/archinternmed.2012.878. [DOI] [PubMed] [Google Scholar]
- 48.Wang L., Myles R.C., De Jesus N.M., Ohlendorf A.K., Bers D.M., Ripplinger C.M. Optical mapping of sarcoplasmic reticulum Ca2+ in the intact heart: ryanodine receptor refractoriness during alternans and fibrillation. Circ Res. 2014;114:1410–1421. doi: 10.1161/CIRCRESAHA.114.302505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martins R.P., Kaur K., Hwang E. Dominant frequency increase rate predicts transition from paroxysmal to long-term persistent atrial fibrillation. Circulation. 2014;129:1472–1482. doi: 10.1161/CIRCULATIONAHA.113.004742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vaquero M., Calvo D., Jalife J. Cardiac fibrillation: from ion channels to rotors in the human heart. Heart Rhythm. 2008;5:872–879. doi: 10.1016/j.hrthm.2008.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Voigt N., Trausch A., Knaut M. Left-to-right atrial inward rectifier potassium current gradients in patients with paroxysmal versus chronic atrial fibrillation. Circ Arrhythm Electrophysiol. 2010;3:472–480. doi: 10.1161/CIRCEP.110.954636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Swartz M.F., Fink G.W., Lutz C.J. Left versus right atrial difference in dominant frequency, K(+) channel transcripts, and fibrosis in patients developing atrial fibrillation after cardiac surgery. Heart Rhythm. 2009;6:1415–1422. doi: 10.1016/j.hrthm.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nattel S., Maguy A., Le Bouter S., Yeh Y.H. Arrhythmogenic ion-channel remodeling in the heart: heart failure, myocardial infarction, and atrial fibrillation. Physiol Rev. 2007;87:425–456. doi: 10.1152/physrev.00014.2006. [DOI] [PubMed] [Google Scholar]
- 54.Van Wagoner D.R. Basic mechanisms of atrial fibrillation. Cleve Clin J Med. 2003;70 Suppl 3:S2–S5. doi: 10.3949/ccjm.70.suppl_3.s2. [DOI] [PubMed] [Google Scholar]
- 55.Caballero R., de la Fuente M.G., Gomez R. In humans, chronic atrial fibrillation decreases the transient outward current and ultrarapid component of the delayed rectifier current differentially on each atria and increases the slow component of the delayed rectifier current in both. J Am Coll Cardiol. 2010;55:2346–2354. doi: 10.1016/j.jacc.2010.02.028. [DOI] [PubMed] [Google Scholar]
- 56.Chaldoupi S.M., Loh P., Hauer R.N., de Bakker J.M., van Rijen H.V. The role of connexin40 in atrial fibrillation. Cardiovasc Res. 2009;84:15–23. doi: 10.1093/cvr/cvp203. [DOI] [PubMed] [Google Scholar]
- 57.Severs N.J., Coppen S.R., Dupont E., Yeh H.I., Ko Y.S., Matsushita T. Gap junction alterations in human cardiac disease. Cardiovasc Res. 2004;62:368–377. doi: 10.1016/j.cardiores.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 58.Polontchouk L., Haefliger J.A., Ebelt B. Effects of chronic atrial fibrillation on gap junction distribution in human and rat atria. J Am Coll Cardiol. 2001;38:883–891. doi: 10.1016/s0735-1097(01)01443-7. [DOI] [PubMed] [Google Scholar]
- 59.Elvan A., Huang X.D., Pressler M.L., Zipes D.P. Radiofrequency catheter ablation of the atria eliminates pacing-induced sustained atrial fibrillation and reduces connexin 43 in dogs. Circulation. 1997;96:1675–1685. doi: 10.1161/01.cir.96.5.1675. [DOI] [PubMed] [Google Scholar]
- 60.van der Velden H.M., Ausma J., Rook M.B. Gap junctional remodeling in relation to stabilization of atrial fibrillation in the goat. Cardiovasc Res. 2000;46:476–486. doi: 10.1016/s0008-6363(00)00026-2. [DOI] [PubMed] [Google Scholar]
- 61.Shin S.Y., Jo W.M., Min T.J. Gap junction remodelling by chronic pressure overload is related to the increased susceptibility to atrial fibrillation in rat heart. Europace. 2015;17:655–663. doi: 10.1093/europace/euu294. [DOI] [PubMed] [Google Scholar]
- 62.Rucker-Martin C., Milliez P., Tan S. Chronic hemodynamic overload of the atria is an important factor for gap junction remodeling in human and rat hearts. Cardiovasc Res. 2006;72:69–79. doi: 10.1016/j.cardiores.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 63.Medi C., Kalman J.M., Spence S.J. Atrial electrical and structural changes associated with longstanding hypertension in humans: implications for the substrate for atrial fibrillation. J Cardiovasc Electrophysiol. 2011;22:1317–1324. doi: 10.1111/j.1540-8167.2011.02125.x. [DOI] [PubMed] [Google Scholar]
- 64.Healey J.S., Israel C.W., Connolly S.J. Relevance of electrical remodeling in human atrial fibrillation: results of the Asymptomatic Atrial Fibrillation and Stroke Evaluation in Pacemaker Patients and the Atrial Fibrillation Reduction Atrial Pacing Trial mechanisms of atrial fibrillation study. Circ Arrhythm Electrophysiol. 2012;5:626–631. doi: 10.1161/CIRCEP.112.970442. [DOI] [PubMed] [Google Scholar]
- 65.Iwasaki Y.K., Shi Y., Benito B. Determinants of atrial fibrillation in an animal model of obesity and acute obstructive sleep apnea. Heart Rhythm. 2012;9:1409–16 e1. doi: 10.1016/j.hrthm.2012.03.024. [DOI] [PubMed] [Google Scholar]
- 66.Shen M.J., Zipes D.P. Role of the autonomic nervous system in modulating cardiac arrhythmias. Circ Res. 2014;114:1004–1021. doi: 10.1161/CIRCRESAHA.113.302549. [DOI] [PubMed] [Google Scholar]
- 67.Shen M.J., Choi E.K., Tan A.Y. Neural mechanisms of atrial arrhythmias. Nat Rev Cardiol. 2011;9:30–39. doi: 10.1038/nrcardio.2011.139. [DOI] [PubMed] [Google Scholar]
- 68.Patterson E., Lazzara R., Szabo B. Sodium-calcium exchange initiated by the Ca2+ transient: an arrhythmia trigger within pulmonary veins. J Am Coll Cardiol. 2006;47:1196–1206. doi: 10.1016/j.jacc.2005.12.023. [DOI] [PubMed] [Google Scholar]
- 69.Patterson E., Jackman W.M., Beckman K.J. Spontaneous pulmonary vein firing in man: relationship to tachycardia-pause early afterdepolarizations and triggered arrhythmia in canine pulmonary veins in vitro. J Cardiovasc Electrophysiol. 2007;18:1067–1075. doi: 10.1111/j.1540-8167.2007.00909.x. [DOI] [PubMed] [Google Scholar]
- 70.Bers D.M. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 71.Wijffels M.C., Kirchhof C.J., Dorland R., Allessie M.A. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation. 1995;92:1954–1968. doi: 10.1161/01.cir.92.7.1954. [DOI] [PubMed] [Google Scholar]
- 72.Haissaguerre M., Jais P., Shah D.C. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–666. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 73.Tan A.Y., Li H., Wachsmann-Hogiu S., Chen L.S., Chen P.S., Fishbein M.C. Autonomic innervation and segmental muscular disconnections at the human pulmonary vein-atrial junction: implications for catheter ablation of atrial-pulmonary vein junction. J Am Coll Cardiol. 2006;48:132–143. doi: 10.1016/j.jacc.2006.02.054. [DOI] [PubMed] [Google Scholar]
- 74.Chou C.C., Nihei M., Zhou S. Intracellular calcium dynamics and anisotropic re-entry in isolated canine pulmonary veins and left atrium. Circulation. 2005;111:2889–2897. doi: 10.1161/CIRCULATIONAHA.104.498758. [DOI] [PubMed] [Google Scholar]
- 75.Choi E.K., Shen M.J., Han S. Intrinsic cardiac nerve activity and paroxysmal atrial tachyarrhythmia in ambulatory dogs. Circulation. 2010;121:2615–2623. doi: 10.1161/CIRCULATIONAHA.109.919829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shen M.J., Coffey A.C., Straka S. Simultaneous recordings of intrinsic cardiac nerve activity and skin sympathetic nerve activity from human patients during the postoperative period. Heart Rhythm. 2017;14:1587–1593. doi: 10.1016/j.hrthm.2017.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Aviles R.J., Martin D.O., Apperson-Hansen C. Inflammation as a risk factor for atrial fibrillation. Circulation. 2003;108:3006–3010. doi: 10.1161/01.CIR.0000103131.70301.4F. [DOI] [PubMed] [Google Scholar]
- 78.Guo Y., Lip G.Y., Apostolakis S. Inflammation in atrial fibrillation. J Am Coll Cardiol. 2012;60:2263–2270. doi: 10.1016/j.jacc.2012.04.063. [DOI] [PubMed] [Google Scholar]
- 79.Conen D., Ridker P.M., Everett B.M. A multimarker approach to assess the influence of inflammation on the incidence of atrial fibrillation in women. Eur Heart J. 2010;31:1730–1736. doi: 10.1093/eurheartj/ehq146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Marott S.C., Nordestgaard B.G., Zacho J. Does elevated C-reactive protein increase atrial fibrillation risk? A Mendelian randomization of 47,000 individuals from the general population. J Am Coll Cardiol. 2010;56:789–795. doi: 10.1016/j.jacc.2010.02.066. [DOI] [PubMed] [Google Scholar]
- 81.Tousoulis D., Zisimos K., Antoniades C. Oxidative stress and inflammatory process in patients with atrial fibrillation: the role of left atrium distension. Int J Cardiol. 2009;136:258–262. doi: 10.1016/j.ijcard.2008.04.087. [DOI] [PubMed] [Google Scholar]
- 82.Acevedo M., Corbalan R., Braun S., Pereira J., Gonzalez I., Navarrete C. Biochemical predictors of cardiac rhythm at 1 year follow-up in patients with non-valvular atrial fibrillation. J Thromb Thrombolysis. 2012;33:383–388. doi: 10.1007/s11239-012-0690-1. [DOI] [PubMed] [Google Scholar]
- 83.Conway D.S., Buggins P., Hughes E., Lip G.Y. Relationship of interleukin-6 and C-reactive protein to the prothrombotic state in chronic atrial fibrillation. J Am Coll Cardiol. 2004;43:2075–2082. doi: 10.1016/j.jacc.2003.11.062. [DOI] [PubMed] [Google Scholar]
- 84.Lip G.Y., Patel J.V., Hughes E., Hart R.G. High-sensitivity C-reactive protein and soluble CD40 ligand as indices of inflammation and platelet activation in 880 patients with nonvalvular atrial fibrillation: relationship to stroke risk factors, stroke risk stratification schema, and prognosis. Stroke. 2007;38:1229–1237. doi: 10.1161/01.STR.0000260090.90508.3e. [DOI] [PubMed] [Google Scholar]
- 85.Yacoub D., Hachem A., Theoret J.F., Gillis M.A., Mourad W., Merhi Y. Enhanced levels of soluble CD40 ligand exacerbate platelet aggregation and thrombus formation through a CD40-dependent tumor necrosis factor receptor-associated factor-2/Rac1/p38 mitogen-activated protein kinase signaling pathway. Arterioscler Thromb Vasc Biol. 2010;30:2424–2433. doi: 10.1161/ATVBAHA.110.216143. [DOI] [PubMed] [Google Scholar]
- 86.Raviele A., Ronco F. Endothelial dysfunction and atrial fibrillation: what is the relationship? J Cardiovasc Electrophysiol. 2011;22:383–384. doi: 10.1111/j.1540-8167.2010.01954.x. [DOI] [PubMed] [Google Scholar]
- 87.Spronk H.M., De Jong A.M., Verheule S. Hypercoagulability causes atrial fibrosis and promotes atrial fibrillation. Eur Heart J. 2017;38:38–50. doi: 10.1093/eurheartj/ehw119. [DOI] [PubMed] [Google Scholar]
- 88.Hool L.C. Reactive oxygen species in cardiac signalling: from mitochondria to plasma membrane ion channels. Clin Exp Pharmacol Physiol. 2006;33:146–151. doi: 10.1111/j.1440-1681.2006.04341.x. [DOI] [PubMed] [Google Scholar]
- 89.Zima A.V., Blatter L.A. Redox regulation of cardiac calcium channels and transporters. Cardiovasc Res. 2006;71:310–321. doi: 10.1016/j.cardiores.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 90.Nediani C., Raimondi L., Borchi E., Cerbai E. Nitric oxide/reactive oxygen species generation and nitroso/redox imbalance in heart failure: from molecular mechanisms to therapeutic implications. Antioxidants Redox Signaling. 2011;14:289–331. doi: 10.1089/ars.2010.3198. [DOI] [PubMed] [Google Scholar]
- 91.Lijnen P.J., van Pelt J.F., Fagard R.H. Stimulation of reactive oxygen species and collagen synthesis by angiotensin II in cardiac fibroblasts. Cardiovasc Ther. 2012;30:e1–e8. doi: 10.1111/j.1755-5922.2010.00205.x. [DOI] [PubMed] [Google Scholar]
- 92.Nabeebaccus A., Zhang M., Shah A.M. NADPH oxidases and cardiac remodelling. Heart Fail Rev. 2011;16:5–12. doi: 10.1007/s10741-010-9186-2. [DOI] [PubMed] [Google Scholar]
- 93.Kim Y.M., Guzik T.J., Zhang Y.H. A myocardial Nox2 containing NAD(P)H oxidase contributes to oxidative stress in human atrial fibrillation. Circ Res. 2005;97:629–636. doi: 10.1161/01.RES.0000183735.09871.61. [DOI] [PubMed] [Google Scholar]
- 94.Reilly S.N., Jayaram R., Nahar K. Atrial sources of reactive oxygen species vary with the duration and substrate of atrial fibrillation: implications for the antiarrhythmic effect of statins. Circulation. 2011;124:1107–1117. doi: 10.1161/CIRCULATIONAHA.111.029223. [DOI] [PubMed] [Google Scholar]
- 95.Yoo S., Aistrup G., Shiferaw Y. Oxidative stress creates a unique, CaMKII-mediated substrate for atrial fibrillation in heart failure. JCI Insight. 2018;3 doi: 10.1172/jci.insight.120728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schlittenhardt D., Schober A., Strelau J. Involvement of growth differentiation factor-15/macrophage inhibitory cytokine-1 (GDF-15/MIC-1) in oxLDL-induced apoptosis of human macrophages in vitro and in arteriosclerotic lesions. Cell Tissue Res. 2004;318:325–333. doi: 10.1007/s00441-004-0986-3. [DOI] [PubMed] [Google Scholar]
- 97.Wollert K.C., Kempf T., Peter T. Prognostic value of growth-differentiation factor-15 in patients with non-ST-elevation acute coronary syndrome. Circulation. 2007;115:962–971. doi: 10.1161/CIRCULATIONAHA.106.650846. [DOI] [PubMed] [Google Scholar]
- 98.Kempf T., von Haehling S., Peter T. Prognostic utility of growth differentiation factor-15 in patients with chronic heart failure. J Am Coll Cardiol. 2007;50:1054–1060. doi: 10.1016/j.jacc.2007.04.091. [DOI] [PubMed] [Google Scholar]
- 99.Wallentin L., Hijazi Z., Andersson U. Growth differentiation factor 15, a marker of oxidative stress and inflammation, for risk assessment in patients with atrial fibrillation: insights from the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial. Circulation. 2014;130:1847–1858. doi: 10.1161/CIRCULATIONAHA.114.011204. [DOI] [PubMed] [Google Scholar]
- 100.Hu X.F., Zhan R., Xu S. Growth differentiation factor 15 is associated with left atrial/left atrial appendage thrombus in patients with nonvalvular atrial fibrillation. Clin Cardiol. 2018;41:34–38. doi: 10.1002/clc.22844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yancy C.W., Jessup M., Bozkurt B. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 102.Al-Khatib S.M., Allen LaPointe N.M., Chatterjee R. Rate- and rhythm-control therapies in patients with atrial fibrillation: a systematic review. Ann Intern Med. 2014;160:760–773. doi: 10.7326/M13-1467. [DOI] [PubMed] [Google Scholar]
- 103.Healey J.S., Connolly S.J., Gold M.R. Subclinical atrial fibrillation and the risk of stroke. N Engl J Med. 2012;366:120–129. doi: 10.1056/NEJMoa1105575. [DOI] [PubMed] [Google Scholar]
- 104.Botto G.L., Padeletti L., Santini M. Presence and duration of atrial fibrillation detected by continuous monitoring: crucial implications for the risk of thromboembolic events. J Cardiovasc Electrophysiol. 2009;20:241–248. doi: 10.1111/j.1540-8167.2008.01320.x. [DOI] [PubMed] [Google Scholar]
- 105.Link M.S., Giugliano R.P., Ruff C.T. Stroke and mortality risk in patients with various patterns of atrial fibrillation: results from the ENGAGE AF-TIMI 48 Trial (Effective Anticoagulation With Factor Xa Next Generation in Atrial Fibrillation-Thrombolysis in Myocardial Infarction 48) Circ Arrhythm Electrophysiol. 2017;10 doi: 10.1161/CIRCEP.116.004267. pii:e004267. [DOI] [PubMed] [Google Scholar]
- 106.Piccini J.P., Daubert J.P. Atrial fibrillation and stroke: it's not necessarily all about the rhythm. Heart Rhythm. 2011;8:1424–1425. doi: 10.1016/j.hrthm.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 107.Calenda B.W., Fuster V., Halperin J.L., Granger C.B. Stroke risk assessment in atrial fibrillation: risk factors and markers of atrial myopathy. Nat Rev Cardiol. 2016;13:549–559. doi: 10.1038/nrcardio.2016.106. [DOI] [PubMed] [Google Scholar]
- 108.Goldberger J.J., Arora R., Green D. Evaluating the atrial myopathy underlying atrial fibrillation: identifying the arrhythmogenic and thrombogenic substrate. Circulation. 2015;132:278–291. doi: 10.1161/CIRCULATIONAHA.115.016795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kamel H., Okin P.M., Elkind M.S., Iadecola C. Atrial fibrillation and mechanisms of stroke: time for a new model. Stroke. 2016;47:895–900. doi: 10.1161/STROKEAHA.115.012004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Guichard J.B., Nattel S. Atrial cardiomyopathy: a useful notion in cardiac disease management or a passing fad? J Am Coll Cardiol. 2017;70:756–765. doi: 10.1016/j.jacc.2017.06.033. [DOI] [PubMed] [Google Scholar]
- 111.Kuppahally S.S., Akoum N., Burgon N.S. Left atrial strain and strain rate in patients with paroxysmal and persistent atrial fibrillation: relationship to left atrial structural remodeling detected by delayed-enhancement MRI. Circ Cardiovasc Imaging. 2010;3:231–239. doi: 10.1161/CIRCIMAGING.109.865683. [DOI] [PubMed] [Google Scholar]
- 112.Binici Z., Intzilakis T., Nielsen O.W., Kober L., Sajadieh A. Excessive supraventricular ectopic activity and increased risk of atrial fibrillation and stroke. Circulation. 2010;121:1904–1911. doi: 10.1161/CIRCULATIONAHA.109.874982. [DOI] [PubMed] [Google Scholar]
- 113.Larsen B.S., Kumarathurai P., Falkenberg J., Nielsen O.W., Sajadieh A. Excessive atrial ectopy and short atrial runs increase the risk of stroke beyond incident atrial fibrillation. J Am Coll Cardiol. 2015;66:232–241. doi: 10.1016/j.jacc.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 114.Kamel H., Hunter M., Moon Y.P. Electrocardiographic left atrial abnormality and risk of stroke: Northern Manhattan Study. Stroke. 2015;46:3208–3212. doi: 10.1161/STROKEAHA.115.009989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Koduri H., Ng J., Cokic I. Contribution of fibrosis and the autonomic nervous system to atrial fibrillation electrograms in heart failure. Circ Arrhythm Electrophysiol. 2012;5:640–649. doi: 10.1161/CIRCEP.111.970095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Katritsis D., Giazitzoglou E., Sougiannis D., Voridis E., Po S.S. Complex fractionated atrial electrograms at anatomic sites of ganglionated plexi in atrial fibrillation. Europace. 2009;11:308–315. doi: 10.1093/europace/eup036. [DOI] [PubMed] [Google Scholar]
- 117.Todaro M.C., Choudhuri I., Belohlavek M. New echocardiographic techniques for evaluation of left atrial mechanics. Eur Heart J Cardiovasc Imaging. 2012;13:973–984. doi: 10.1093/ehjci/jes174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rohner A., Brinkert M., Kawel N. Functional assessment of the left atrium by real-time three-dimensional echocardiography using a novel dedicated analysis tool: initial validation studies in comparison with computed tomography. Eur J Echocardiogr. 2011;12:497–505. doi: 10.1093/ejechocard/jer066. [DOI] [PubMed] [Google Scholar]
- 119.Njoku A., Kannabhiran M., Arora R. Left atrial volume predicts atrial fibrillation recurrence after radiofrequency ablation: a meta-analysis. Europace. 2018;20:33–42. doi: 10.1093/europace/eux013. [DOI] [PubMed] [Google Scholar]
- 120.Ogata T., Matsuo R., Kiyuna F. Left atrial size and long-term risk of recurrent stroke after acute ischemic stroke in patients with nonvalvular atrial fibrillation. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.117.006402. pii:e006402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.McGann C., Akoum N., Patel A. Atrial fibrillation ablation outcome is predicted by left atrial remodeling on CMR. Circ Arrhythm Electrophysiol. 2014;7:23–30. doi: 10.1161/CIRCEP.113.000689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Marrouche N.F., Wilber D., Hindricks G. Association of atrial tissue fibrosis identified by delayed enhancement MRI and atrial fibrillation catheter ablation: the DECAAF study. JAMA. 2014;311:498–506. doi: 10.1001/jama.2014.3. [DOI] [PubMed] [Google Scholar]
- 123.Daccarett M., Badger T.J., Akoum N. Association of left atrial fibrosis detected by delayed-enhancement magnetic resonance imaging and the risk of stroke in patients with atrial fibrillation. J Am Coll Cardiol. 2011;57:831–838. doi: 10.1016/j.jacc.2010.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Markl M., Lee D.C., Ng J., Carr M., Carr J., Goldberger J.J. Left atrial 4-dimensional flow magnetic resonance imaging: stasis and velocity mapping in patients with atrial fibrillation. Invest Radiol. 2016;51:147–154. doi: 10.1097/RLI.0000000000000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fluckiger J.U., Goldberger J.J., Lee D.C. Left atrial flow velocity distribution and flow coherence using four-dimensional FLOW MRI: a pilot study investigating the impact of age and pre- and postintervention atrial fibrillation on atrial hemodynamics. J Magn Reson Imaging. 2013;38:580–587. doi: 10.1002/jmri.23994. [DOI] [PubMed] [Google Scholar]
- 126.Foll D., Taeger S., Bode C., Jung B., Markl M. Age, gender, blood pressure, and ventricular geometry influence normal 3D blood flow characteristics in the left heart. Eur Heart J Cardiovasc Imaging. 2013;14:366–373. doi: 10.1093/ehjci/jes196. [DOI] [PubMed] [Google Scholar]
- 127.Lee D.C., Markl M., Ng J. Three-dimensional left atrial blood flow characteristics in patients with atrial fibrillation assessed by 4D flow MRI. Eur Heart J Cardiovasc Imaging. 2016;17:1259–1268. doi: 10.1093/ehjci/jev304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Markl M., Lee D.C., Furiasse N. Left Atrial and left atrial appendage 4d blood flow dynamics in atrial fibrillation. Circ Cardiovasc Imaging. 2016;9 doi: 10.1161/CIRCIMAGING.116.004984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lee D.C., Markl M., Ng J. Three-dimensional left atrial blood flow characteristics in patients with atrial fibrillation assessed by 4D flow MRI. Eur Heart J Cardiovasc Imaging. 2016;17:1259–1268. doi: 10.1093/ehjci/jev304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Appelbaum E., Manning W.J. Left atrial fibrosis by late gadolinium enhancement cardiovascular magnetic resonance predicts recurrence of atrial fibrillation after pulmonary vein isolation: do you see what I see? Circ Arrhythm Electrophysiol. 2014;7:2–4. doi: 10.1161/CIRCEP.114.001354. [DOI] [PubMed] [Google Scholar]
- 131.Wolsk E., Lamberts M., Hansen M.L. Thromboembolic risk stratification of patients hospitalized with heart failure in sinus rhythm: a nationwide cohort study. Eur J Heart Fail. 2015;17:828–836. doi: 10.1002/ejhf.309. [DOI] [PubMed] [Google Scholar]
- 132.Belen E., Ozal E., Pusuroglu H. Association of the CHA2DS2-VASc score with left atrial spontaneous echo contrast: a cross-sectional study of patients with rheumatic mitral stenosis in sinus rhythm. Heart Vessels. 2016;31:1537–1543. doi: 10.1007/s00380-015-0759-9. [DOI] [PubMed] [Google Scholar]
- 133.Kamel H., Longstreth W.T., Jr., Tirschwell D.L. The AtRial Cardiopathy and Antithrombotic Drugs In prevention After cryptogenic stroke randomized trial: rationale and methods. Int J Stroke. 2019;14:207–214. doi: 10.1177/1747493018799981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ha A.C., Hindricks G., Birnie D.H., Verma A. Long-term oral anticoagulation for patients after successful catheter ablation of atrial fibrillation: is it necessary? Curr Opin Cardiol. 2015;30:1–7. doi: 10.1097/HCO.0000000000000121. [DOI] [PubMed] [Google Scholar]
- 135.Deng L., Xiao Y., Hong H. Withdrawal of oral anticoagulants 3 months after successful radiofrequency catheter ablation in patients with atrial fibrillation: a meta-analysis. Pacing Clin Electrophysiol. 2018;41:1391–1400. doi: 10.1111/pace.13494. [DOI] [PubMed] [Google Scholar]
- 136.Liu Y., Niu X.H., Yin X. Elevated circulating fibrocytes is a marker of left atrial fibrosis and recurrence of persistent atrial fibrillation. J Am Heart Assoc. 2018;7 doi: 10.1161/JAHA.117.008083. pii:e008083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Smith J.G., Newton-Cheh C., Almgren P. Assessment of conventional cardiovascular risk factors and multiple biomarkers for the prediction of incident heart failure and atrial fibrillation. J Am Coll Cardiol. 2010;56:1712–1719. doi: 10.1016/j.jacc.2010.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hijazi Z., Oldgren J., Siegbahn A., Granger C.B., Wallentin L. Biomarkers in atrial fibrillation: a clinical review. Eur Heart J. 2013;34:1475–1480. doi: 10.1093/eurheartj/eht024. [DOI] [PubMed] [Google Scholar]
- 139.Hijazi Z., Lindback J., Alexander J.H. The ABC (age, biomarkers, clinical history) stroke risk score: a biomarker-based risk score for predicting stroke in atrial fibrillation. Eur Heart J. 2016;37:1582–1590. doi: 10.1093/eurheartj/ehw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hijazi Z., Siegbahn A., Andersson U. High-sensitivity troponin I for risk assessment in patients with atrial fibrillation: insights from the Apixaban for Reduction in Stroke and other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial. Circulation. 2014;129:625–634. doi: 10.1161/CIRCULATIONAHA.113.006286. [DOI] [PubMed] [Google Scholar]
- 141.Hijazi Z., Wallentin L., Siegbahn A. N-terminal pro-B-type natriuretic peptide for risk assessment in patients with atrial fibrillation: insights from the ARISTOTLE Trial (Apixaban for the Prevention of Stroke in Subjects With Atrial Fibrillation) J Am Coll Cardiol. 2013;61:2274–2284. doi: 10.1016/j.jacc.2012.11.082. [DOI] [PubMed] [Google Scholar]