Abstract
Background
Patients newly-diagnosed with advanced cancer often rely on family caregivers to provide daily support to manage healthcare needs and maintain quality of life. Early telehealth palliative care has been shown to effectively provide an extra layer of support to family caregivers, however there has been little work with underserved populations, especially African-Americans and rural-dwellers. This is concerning given the lack of palliative care access for these underserved groups.
Study design
Single-site, small-scale pilot randomized controlled trial (RCT) of Project ENABLE (Educate, Nurture, Advise, Before Life Ends) Cornerstone, a lay navigator-led, early palliative care coaching intervention for family caregivers of African-American and rural-dwelling patients with newly-diagnosed advanced cancer. Family caregivers are paired with a trained lay navigator overseen by specialist palliative care clinicians and receive a series of brief in-person and telehealth sessions focusing on stress management and coping, caregiving skills and organization, getting help, self-care, and preparing for the future/advance care planning. This pilot trial is assessing acceptability of the intervention, feasibility of recruitment and data collection procedures, and preliminary efficacy compared to usual care on caregiver and patient quality of life and mood over 24 weeks.
Conclusion
Once acceptability and feasibility are determined and issues addressed, the ENABLE Cornerstone intervention for underserved family caregivers of persons with advanced cancer will be primed for a fully powered efficacy RCT. Given its use of lay navigators and telehealth delivery, the intervention is potentially highly scalable and capable of overcoming many of the geographic, human resource, and cultural obstacles to accessing early palliative care support.
Keywords: Family caregivers, Advanced cancer, Telehealth, Palliative care, Rural
1. Introduction
By 2026, the number of U.S. individuals with cancer is expected to swell to over 20 million, and in 2019 [1], the number in their last year of life was over 600,000 [2]. Advanced cancer and its treatment can be debilitating, necessitating the assistance of unpaid close family members and friends. These cancer family caregivers represent a ‘hidden’ healthcare workforce providing an average of 8 h of daily assistance to care recipients with advanced metastatic cancers [3], including managing and monitoring symptoms, providing transportation, coordinating care, communicating with health care providers, and providing emotional and spiritual support [[4], [5], [6], [7]]. Providing these tasks while also coping with seeing a close relative or friend struggle with serious illness can result in psychological distress for family caregivers that often exceeds that reported by their care recipients with cancer [8,9]. Furthermore, distressed and underprepared family caregivers may experience poor overall physical and mental health that compromises their ability to provide high quality care to patients [[10], [11], [12]]. Recognizing this public health crisis, the National Institute for Nursing Research [13], the National Cancer Institute [14], and the National Academy of Medicine [15] among others have stressed the imperative that supportive and palliative care interventions be developed to assist family caregivers and patients.
While interventions to support cancer family caregivers have grown in number in the past decade, there have been noted limitations of not including underserved populations, particularly both African-Americans and rural-dwellers as two groups who evidence similar marked disparities in serious illness and access to palliative care support [14,16,17]. A large proportion of these groups live in the Southern U.S., which is concerning because rural areas and counties with high proportions of African-Americans in the U.S. South have poor access to palliative care [18,19]. For example, Alabama received a D grade in a 2019 State-by-State Report Card on Access to Palliative Care, which noted that only 39% of Alabama hospitals have a palliative care program [19]. This poor palliative care access is especially pernicious to the high proportion of rural-dwellers and African-Americans living in Alabama [20,21]. Because African-Americans have lower rates of advance care planning and hospice use and often receive more aggressive EOL care [[22], [23], [24], [25]], improving access to palliative care support is especially important in Southern U.S. states. As numerous statements of research priorities in family caregiving have highlighted [14,15,[26], [27], [28]], increased focus on developing and testing interventions for underserved rural and minority populations is critically needed.
To meet this need, we developed a lay navigator-led, early palliative care intervention designed specifically for underserved family caregivers called ENABLE (Educate, Nurture, Advise, Before Life Ends) Cornerstone that consists of a series of semi-structured weekly coaching sessions and long-term monthly follow-up. We tested the original ENABLE early palliative care caregiver intervention in a New England population of advanced cancer family caregivers [29,30]. While the randomized controlled trial (RCT) results showed that early intervention group family caregivers had lower depressive symptoms and stress burden, the trial sample was nearly all White. To adapt it to a more underserved and racially diverse population, we completed a qualitative formative evaluation study in the Southern U.S. to elicit feedback on the outline of an intervention from family caregivers and their rural-dwelling patients with advanced cancer and lay healthcare navigators [31]. Lay navigators were included to enhance scalability of the adapted intervention and its cultural appropriateness. The intervention outline was reflective of our prior ENABLE caregiver intervention and included elements of other evidence-based cancer caregiver interventions. Findings from this formative evaluation study were used to develop the protocol reported here for our ongoing small scale, pilot RCT of the newly-tailored ENABLE Cornerstone intervention which began recruitment in October 2018 and is anticipated to complete accrual in December 2019–January 2020.
2. Study objectives
The primary aim of the Project ENABLE Cornerstone pilot RCT, is to evaluate the acceptability of the intervention via post-intervention qualitative interviews and the feasibility of enrolling and retaining 60 caregivers and 60 patients over 24 weeks. We hypothesize that randomized participants will complete at least 80% of all study-related assessments and intervention sessions. Our secondary aim is to evaluate the potential efficacy of the intervention compared to usual care. We hypothesize that intervention group family caregivers and patients will demonstrate better quality of life and mood (depression/anxiety symptoms) by at least 0.3 standard deviation units over 24 weeks compared to usual care.
3. Conceptual basis of ENABLE Cornerstone
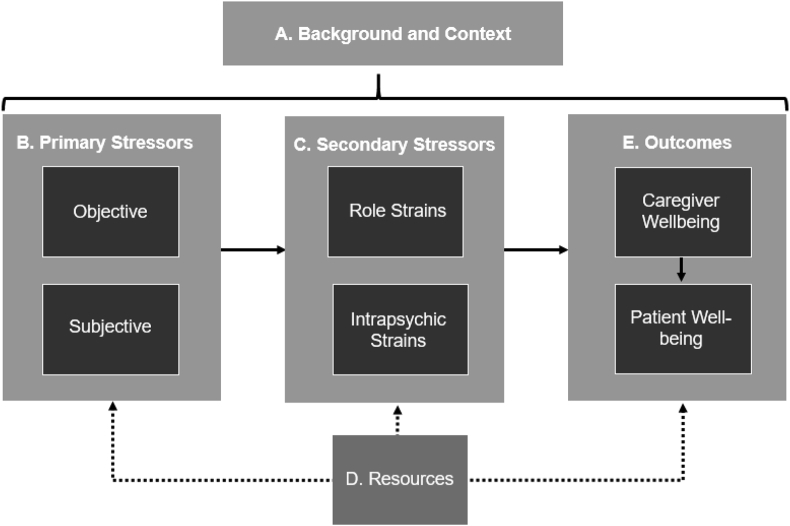
The ENABLE Cornerstone intervention is conceptually based on the Pearlin Stress Process Model of Family Caregiving (Fig. 1) [32]. There are 5 primary domains of the model: the caregiving context (A), primary stressors (B), secondary stressors (C), resources (D), and outcomes (E). The caregiving context includes sociodemographics of the caregiving individual (e.g., age, gender, race, educational background) and a history of their caregiving experience, including their relationship history with the care recipient. Primary stressors include objective stressors, such as the time spent caregiving and the care recipient's function and health state, and subjective stressors, which is the caregiver's perception of how distressing the objective stressors are for him or her. Secondary stressors include role strains and intrapsychic strains and are stressors that are a result of the “spillover” from primary stressors into other areas of the caregiver's life. Role strains are difficulties experienced by caregivers in other life roles, such as their job or within their social networks. Intrapsychic strains are feelings of distress from having one's self concept altered, such as low self-esteem or feelings of incompetence. Resources are strategies used by caregivers to lessen the impact of primary and secondary stressors, such as various coping tactics and leveraging social support. Outcomes reflect how well resources are being used by caregivers to counter stress and is indicated by caregiver's and patient's overall well-being and quality of life.
Fig. 1.
Pearlin stress process model of family caregiving.
The ENABE Cornerstone intervention is designed to enhance a caregiver's resources to effect better outcomes. Consisting primarily of a series of 6 weekly coaching sessions, each intervention session is intended to enhance caregiver resources in a way that targets different domains of stress in the model. Table 1 shows how the different Cornerstone sessions might impact each of the stress domains.
Table 1.
Pearlin Stress Process Domains and Cornerstone Components Targeting those Domains.
| Cornerstone intervention goal to enhance RESOURCES | Cornerstone component to meet goal | Targeted Pearlin Stress Process Model Domain |
|---|---|---|
| Establish therapeutic alliance by understanding and validating caregiving situation | Cornerstone Session 1: “Telling Your Story” | Caregiving Context (A) |
| Promote stress management skills | Cornerstone Session 2: “Coping with Stress” | Primary Stressors (B), Secondary Stressors (C) |
| Motivate effective social support through asking for and getting help | Cornerstone Session 3: “Getting Help” | Primary Stressors (B) |
| Enhance caregiving skills & organization | Cornerstone Session 4: “Improving your Support Skills” | Primary Stressors (B), Secondary Stressors (C) |
| Improve and reinforce self-care behaviors | Cornerstone Session 5: “Taking Care of Yourself” | Outcomes (E) |
| Help develop plans for the future to help mitigate future stressors and potential crises | Cornerstone Session 6: “Decision Making and Planning for the Future” | Primary Stressors (B) |
4. Methods
4.1. Overview of study design
This study is an NIH/National Institute of Nursing Research-funded single-site randomized controlled pilot trial comparing the ENABLE Cornerstone intervention to usual care among family caregivers of underserved patients with newly-diagnosed advanced cancer. The research protocol was approved by the University of Alabama at Birmingham Institutional Review Board (IRB-30000979) and the trial is registered as NCT03464188 on clinicaltrials.gov.
4.2. Setting and participants
The study is recruiting participants from the outpatient clinics of the University of Alabama at Birmingham Comprehensive Cancer Center. The UAB O'Neal Comprehensive Cancer Center is one of only two NCI-designated comprehensive cancer centers within a large six state area of the U.S. South that includes Alabama, Mississippi, Louisiana, Arkansas, Georgia, and South Carolina. The Center includes 400 clinicians, scientists, and clinician-scientists and treats 9500 new patients each year.
Target enrollment for the trial is 60 family caregivers and 60 of their care recipients newly-diagnosed with advanced cancer who live in rural area or who are African-American. Patients are invited (but not required) to participate for data collection purposes only. For the study, family caregiver is defined as “a relative, friend, or partner with whom one has a close relationship and who assists with medical care on a regular basis and who may or may not live in the same residence and who is not paid for their help.” The eligibility criteria for patients and caregivers is listed in Table 2.
Table 2.
Caregiver and patient eligibility criteria.
| Caregiver | Patient |
|---|---|
Inclusion criteria
|
Inclusion criteria
|
Exclusion criteria
|
Exclusion criteria
|
The rationale for targeting patients within 60–90 days of new advanced cancer diagnosis was to be consistent with American Society of Clinical Oncology guidelines for integration of palliative care early at diagnosis of advanced cancer even when patients continue to receive curative intent treatment [33]. We also exclude individuals with untreated, active severe mental illness because, while we believe Cornerstone can be helpful for caregivers of patients with subtle cognitive deficits, it is currently not designed for the special challenges that arise for caregivers caring for individuals with severe mental illness. Finally, we chose to target caregivers of six different common solid-tumor cancer types so as to refine an intervention that ultimately has the broadest applicability possible.
4.3. Recruitment and retention
Initial screening for potentially eligible patients is performed by reviewing the electronic medical record to identify patients meeting the eligibility criteria who have a planned office visit at the UAB Comprehensive Cancer Center in the subsequent 1–2 weeks. After gaining permission from oncologists via an opt out email, patients and their family members (who are typically present with the patient) are approached by study recruiters prior to their office visit to inform them about the study. Those interested and eligible sign informed consent. To promote retention, participating family caregivers and patients receive a check incentive escalating in amount after each of the 3 measurement occasions: $20 for completion of baseline measures, $30 for 8-week measures, and $40 for 24-week measures. Intervention group caregivers also receive an additional $30 for completing post-intervention acceptability interviews.
4.4. Randomization and blinding
Randomization occurs at the level of the caregiver participant. Patients are assigned to the group that caregivers are randomized to. Consistent with the randomization schemes used in our prior work that have yield balanced groups by demographic and illness variables [34], the randomization scheme is 1:1 in block lengths of six and executed via a computer-generated algorithm in REDCap, a clinical trials management software program [35]. The project manager is alerted to the assignment in REDCap and then informs the participants of their group assignment and directs the lay navigator interventionist to initiate intervention activities. All other members of the research team, including the principal investigator (JND-O) and all co-investigators remain blind to group assignment and participants are instructed not to discuss their assignment with the UAB data collector collecting outcome assessments. The allocation sequence will be concealed until the last participant completes 24-week data collection and the data have been checked for completeness and accuracy.
4.5. Intervention and usual care conditions
4.5.1. The ENABLE Cornerstone intervention
The elements of the intervention are as follows:
-
•
Early initiation with long term follow-up: The intervention is initiated within 60–90 days of being identified as having a new advanced cancer diagnosis and extends for 24 weeks or up to 24 weeks after the patient's death.
-
•
Lay navigator-led: The intervention is led by a lay navigator that has had additional training as a palliative care coach with weekly supervision by a board-certified palliative care nurse practitioner and health coach trained and palliative care board certified advanced practice nurse. Additional as-needed clinical support is provided by a palliative care clinical psychologist, a palliative care social worker, and two oncologists. The principal role of the lay navigator is to: 1) provide basic psychoeducation on relevant caregiving topics (see Table 3); 2) offer health coaching and problem solving support for caregiver identified problems and self-care goals; 3) perform caregiver distress screening; 4) bridge the communication gap between the healthcare team and the family and patient as needed; 5) provide families with the appropriate UAB, local, state, and national resources; 6) offer basic psychological and emotional support; and 7) serve as a continuity figure.
-
•
Six core in-person/telephone coaching sessions (Table 3): There are six weekly core sessions covering specific caregiver relevant topics identified from our prior formative evaluation study [31]. Sessions are designed to last 20 min at minimum but be flexible up to an hour if desired by the participant.
-
•
Caregiver distress thermometer screening: Each core session and monthly follow-up encounter begins with a caregiver distress thermometer screening (see Fig. 2). The thermometer was adapted from the NCCN Patient Distress Thermometer [36] by our study team and by the Caregiver and Bereavement Support Service in the UAB Center for Palliative and Supportive Care, consisting of UAB clinicians and administrators and patient and family stakeholders. Based on distress thermometer screening, navigators can provide additional informational materials and/or suggest referrals for additional support, including (but not limited to) psychological or spiritual counseling, social work consultation, financial guidance, home health, specialty palliative care or hospice services, and bereavement counseling. These screenings also help the lay navigator customize session discussions to the caregiver's specific challenges.
-
•
Project ENABLE Cornerstone Toolkit: Intervention group caregivers receive a study team-developed Project ENABLE Cornerstone Toolkit. This 3 ring, self-enclosed binder contains educational information pertaining to the 6 core sessions and additionally serves as an all-in-one organizational binder that includes business card holders, caregiver tracking sheets for patient medications, tests, and procedures, and a calendar. The Toolkit is hand delivered at or mailed just prior to the first core session.
Table 3.
Intervention session content.
| Title | Length | Topical Content | ||
| Introductory Call | 5–15 min |
|
||
| Psychoeducational Sessions | 1 | Caregiving Story | 20–60 min |
|
| 2 | Coping with Stress | 20–60 min |
|
|
| 3 | Getting Help | 20–60 min |
|
|
| 4 | Improving Your Support Skills | 20–60 min |
|
|
| 5 | Taking Care of Yourself | 20–60 min |
|
|
| 6 | Decision Making and Planning for the Future | 20–60 min |
|
|
| Monthly Follow-Up/ | 5–30 min |
|
||
| Bereavement Call (for patients who die) | 5–30 min |
|
||
Fig. 2.
Caregiver distress thermometer.
4.5.2. Usual care
Usual care at the UAB Comprehensive Cancer Center consists of resources focused primarily on the patient; no specific family caregiver direct services exist to support the needs of the study population. The usual care comparison was chosen to explore whether caregivers experiencing burden and distress needed more active support than what is currently offered by traditional services. All participants are informed about resources at the UAB Comprehensive Cancer Center upon enrollment and given a one-page summary of UAB and other local resources. To further examine and describe usual care, we are collecting caregiver healthcare utilization data from all participants (e.g., receipt of therapy or counseling, education and training, practical support, provider visits, support through UAB cancer center resources).
4.6. Interventionist training and treatment fidelity monitoring
Lay navigators were selected to lead the ENABLE Cornerstone intervention. The rationale for choosing lay navigators was to involve individuals of similar racial and cultural backgrounds to the populations being served in order to enhance trust and therapeutic alliance. Using job description language from a 5-state, lay navigator demonstration project funded by the Centers for Medicare and Medicaid coordinated at UAB [37,38], minimum requirements for the position included a bachelor's degree and 1 year previous experience in community outreach with cancer patients and families. Requisite skills included (but were not limited to) good communication and interpersonal skills, recognition of the rights and responsibilities of patient confidentiality, ability to convey empathy and compassion to those experiencing pain, physical and emotional distress, and grief, and ability to function within the boundaries of the job description while referring/coordinating care appropriately with other healthcare professionals. For interventionist training, we developed a structured orientation checklist and 102-page treatment manual that was used to train the lay navigator interventionist (CD). The PI and a palliative care nurse practitioner study co-investigator (RAT) oversaw training of the lay navigator to deliver the intervention. The training program was approximately 70 h in length and was modeled on procedures developed by our study team over the course of several clinical trials of behavioral interventions [34,39]. The training consisted of independent readings, interactive online modules, videos including demonstration of coaching techniques (e.g., active listening, single and double-sided reflections), review of all study protocols and procedures, provision of the treatment manual, and role play of six simulated training cases. These training sessions were audio-recorded and reviewed by the PI and palliative care nurse practitioner to debrief with the lay navigator. The lay navigator was certified as an interventionist and Project ENABLE Cornerstone coach after demonstrating competency in the training sessions and reporting feeling prepared.
Four strategies (consistent with NIH and TIDieR guidelines) [40,41] are being used to ensure intervention fidelity. First, the lay navigator interventionist training is standardized and overseen by the same trainers. Second, the lay navigator coach follows study-team developed intervention scripts for each of the core sessions and monthly follow-up. Third, for each intervention contact, structured charting templates are used to ensure that the interventionist addresses the essential topics of each session. Fourth, all intervention sessions are digitally-recorded and reviewed for treatment fidelity using a fidelity checklist. Protocol adherence is monitored for scores <80% after which a remediation plan is instituted to provide supplemental training.
4.7. Data collection and outcomes
The primary outcomes of this pilot trial are acceptability and feasibility. Acceptability is being assessed through one-on-one, semi-structured interviews conducted after intervention group caregivers complete 24-week data collection. Participants are asked about: their general impressions of the intervention overall; what they liked most and least; what they thought of the individual core sessions (including rating each session's relevance, helpfulness, and satisfaction); examples of how the intervention changed what they were thinking, feeling, and/or doing on a day-to-day basis and how they think the intervention affected their overall quality of life over the 24 weeks; what they thought of the Cornerstone Toolkit; what they thought of their lay navigator coach; and what changes they recommend for the program going forward. Responses will be used to further refine the content, format, and cultural appropriateness of the intervention.
Qualitative analysis procedures similar to our past formative evaluation study of patients and family caregivers [42] will be employed to conduct analysis. All interviews are digitally-recorded and will be transcribed verbatim by a professional transcription service and uploaded into Atlas.ti qualitative analysis software. Informed by the within and across case comparison approach of Miles, Huberman, and Saldana [43], the data coding approach will use concepts introduced by participants and using codes reflecting potential difficulties with the intervention; opinions about the length and number of sessions; the delivery method, etc. Codes emerging across cases will be operationally defined and entered into a formal codebook. Member checking will be ongoing from interview to interview as previous participant insights are asked of future participants (e.g. “A number of participants have said X. What is your perspective on this issue?“). To corroborate findings and establish trustworthiness, an audit trail will be kept and co-investigators will be convened to provide critical feedback on the emerging codes. We will then look within and across coded texts in order to extract converging themes and reach consensus on principal themes.
Feasibility is being assessed by intervention completion rate (# participants completing 6 sessions) and questionnaire assessment completion rate (# participants completing each study assessment [Baseline, 8, 24 weeks]). Consistent with other pilot intervention studies [44,45], ≥80% completion rates for intervention sessions and study assessments are being considered as evidence of feasibility. Screening, eligibility, and enrollment rates, reasons for non-consent of eligible individuals and reasons for participant drop out are also being tracked [46].
A secondary outcome of the study is preliminary efficacy through standardized assessments of caregiver and patient quality of life and mood (Table 4). A UAB data collector, blind to group assignment, administers standardized telephone interviews, scheduled at participants’ convenience. Data collection calls last 20–45 min and data is entered directly into the REDCap software system that our team has used for data collection in many studies [47]. The REDCap system is programmed with quality controls that facilitate rigorous data collection, such as not allowing interviewers to skip questions.
Table 4.
Preliminary efficacy outcome measures.a.
| Construct | Measure | Description |
|---|---|
| Family caregiver quality of life | Caregiver Quality of Life – Cancer [48] | 35 items; measure of a cancer caregiver's overall quality of life, including physical, social, emotional, and financial aspects of wellbeing and function. |
| Family caregiver mood (anxiety/depression symptoms) | Hospital Anxiety and Depression Scale [49,50] | 14 items total (α = .92), 7 items measure anxiety (e.g., feeling tense, restless, worry), 7 items measure depressive symptoms (e.g., cheerfulness, feeling slowed down). |
| Patient quality of life | Functional Assessment of Chronic Illness Therapy – Palliative Care (FACIT-Pal) [51] | 47 items; includes total QoL score and 5 subscales including: physical, social/family, emotional, functional well-being, end-of-life concerns. |
| Patient Mood | Hospital Anxiety and Depression Scale [49,50] |Same as above |
All measures administered at baseline, 8 and 24 weeks.
We will conduct longitudinal, intention-to-treat analyses of the study outcomes for family caregivers (QOL and distress [anxiety/depression]) and patients (QOL and distress [anxiety/depression]) using linear mixed random and fixed effect models to examine the relative impact of the intervention compared to usual care at over 24 weeks. These analyses will control for conceptually relevant factors identified as differing between the randomized groups or being predictive of missing data or adherence. Between-group differences in change from baseline of 0.3 standard deviation units favoring the intervention group will be considered as evidence of potential efficacy, which is consistent with the magnitude of improvements in other palliative care intervention trials [17,52]. Because the nature of the study is exploratory rather than confirmatory, the objective of the analysis is in-sample effect size estimation, rather than formal hypothesis testing [53,54], and statistical power is not a primary concern, as no inferential statements will be made, consistent with appropriate analytical approaches for pilot data.
5. Discussion
The ENABLE Cornerstone small scale, pilot randomized controlled trial is assessing the acceptability, feasibility, and preliminary efficacy of a lay navigator-led early palliative care coaching intervention to support underserved family caregivers of both African-Americans and rural-dwelling persons with newly-diagnosed advanced cancer. While the intervention is targeted solely at caregivers, the objectives of the intervention are to improve the health and skills of caregivers so that they can more proficiently provide high quality care in the home to patients, thus making optimization of patient outcomes an additional focus of this intervention. The design of ENABLE Cornerstone is guided by components of existing caregiver interventions [16,17], experience from the original ENABLE caregiver intervention trial [29], and formative evaluation work to adapt an early palliative care telehealth intervention for family caregivers to a Southern U.S. rural and minority population [31]. A common theme from this prior work is that every caregiver faces a unique set of challenges that are not easily addressed by a one-size-fits all intervention. Hence, ENABLE Cornerstone is a multicomponent package of intervention components that targets varying and multiple sources of potential distress, based on the Pearlin Stress Process Model [32].
There are several key innovations of this intervention. First, it is being designed and tested for both underserved African-American and rural-dwelling populations who have had historically poor access to palliative care. To date, existing interventions for cancer family caregivers have not specifically targeted these populations and have only shown minimal efficacy [17,52,55]. We believe our formative evaluation work and the lessons learned and qualitative feedback from this pilot trial will help our team further refine the intervention to be culturally appropriate and acceptable to these underserved populations.
Second, this intervention is the first for advanced cancer family caregivers to be led by lay navigators who are part of an interdisciplinary palliative care team. Typically, lay healthcare navigators provide one-on-one guidance to patients and families where services offered can include, assisting with insurance and financial issues, explaining treatments and healthcare options, providing emotional support, providing transportation and accompanying patients to office visits, coordinating social support networks, and communicating with the healthcare team [56,57]. They typically do not have formal healthcare backgrounds; however they are respected, trusted, and are cultural in-group members of the community, making them ideal for working with underserved populations [56,57]. Moreover, our lay navigators received additional training in principles of palliative care, family caregiving, health coaching, and problem solving support in a curriculum that took less than two weeks to complete. Hence, if our intervention proves efficacious in a fully powered trial, we believe it would be highly scalable, especially since navigation programs have proliferated in cancer centers since their inclusion in the Affordable Care Act [58].
Third, ENABLE Cornerstone is designed to follow caregivers over the entire length of a serious illness trajectory, from initial diagnosis of advanced cancer through bereavement. Reviews of caregiver interventions to date have noted that a key limitation of tested programs have been their relatively short duration and confinement to a single setting or context [15,55]. Finally, ENABLE Cornerstone will expand the paradigm of palliative care from its initial reactionary illness stress perspective to a proactive health-wellness paradigm. Not only will the intervention address acute caregiver distress, it also emphasizes self-care practices, coping skills, and tools to navigate and cope with future stressors.
In summary, we are ascertaining the acceptability, feasibility, and potential efficacy of a potentially beneficial intervention for family caregivers of underserved persons with newly diagnosed advanced cancer. The intervention has been and will continue to be culturally tailored to both African-Americans and rural-dwelling persons in the U.S. South, a region of the country that has had poor access to palliative care. Moreover, it leverages an untapped lay navigator workforce that may greatly augment the reach of specialty palliative care that will have severe personnel shortages over the coming decades [59]. The critical next step will be testing the ENABLE Cornerstone intervention in a fully powered, phase III efficacy trial where both caregiver and patient outcomes are assessed.
Declaration of Competing interest
The authors have no conflict of interest to report.
Acknowledgements
This study is funded by the National Institute of Nursing Research (NINR) (R00NR015903; PI: Dionne-Odom). Ms. Hendricks and Mr. Lofton are supported by funding through the Robert Wood Johnson Foundation Future of Nursing Scholars Program. Dr. Rocque is funded by an American Cancer Society Mentored Research Scholar Grant (MRSG-17-051-01-PCS). Dr. Williams receives support from the National Cancer Institute of the National Institutes of Health (K08CA234225). Dr. Taylor receives support from the Center for Disease Control and Prevention (1U58DP005412-01) and the American Cancer Society. Dr. Bakitas is funded by the NIH/NINR (R01NR013665). Dr. Dionne-Odom also receives support from the National Palliative Care Research Center. The authors would like to thank Julie Schach, Tawny Martin, and the UAB Recruitment and Retention Shared Facility and for partnering UAB oncologists who assisted with identification and recruitment of patient and family caregiver participants. The authors would also like to thank all family caregivers and their patients who participated in this study.
References
- 1.Miller K.D., Siegel R.L., Lin C.C. Cancer treatment and survivorship statistics, 2016. CA A Cancer J. Clin. 2016;66(4):271–289. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2019. CA A Cancer J. Clin. 2019;69(1):7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 3.Yabroff K.R., Kim Y. Time costs associated with informal caregiving for cancer survivors. Cancer. 2009;115(18 Suppl):4362–4373. doi: 10.1002/cncr.24588. [DOI] [PubMed] [Google Scholar]
- 4.National Alliance for Caregiving . National Alliance for Caregiving; Bethesda, MD: 2016. Cancer Caregiving in the U.S.: an Intense, Episodic, and Challenging Care Experience. [Google Scholar]
- 5.Stenberg U., Ruland C.M., Miaskowski C. Review of the literature on the effects of caring for a patient with cancer. Psycho Oncol. 2010;19(10):1013–1025. doi: 10.1002/pon.1670. [DOI] [PubMed] [Google Scholar]
- 6.AARP with the United Hospital Fund . AARP Public Policy Institute; Washington DC: 2012. Home Alone: Family Caregivers Providing Complex Chronic Care. [Google Scholar]
- 7.van Ryn M., Sanders S., Kahn K. Objective burden, resources, and other stressors among informal cancer caregivers: a hidden quality issue? Psycho Oncol. 2011;20(1):44–52. doi: 10.1002/pon.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hodges L.J., Humphris G.M., Macfarlane G. A meta-analytic investigation of the relationship between the psychological distress of cancer patients and their carers. Soc. Sci. Med. 2005;60(1):1–12. doi: 10.1016/j.socscimed.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 9.Palos G.R., Mendoza T.R., Liao K.P. Caregiver symptom burden: the risk of caring for an underserved patient with advanced cancer. Cancer. 2011;117(5):1070–1079. doi: 10.1002/cncr.25695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Institute of Medicine . The National Academies Press; Washington, DC: 2013. Delivering High-Quality Cancer Care: Charting a New Course for a System in Crisis. [PubMed] [Google Scholar]
- 11.Park S.M., Kim Y.J., Kim S. Impact of caregivers' unmet needs for supportive care on quality of terminal cancer care delivered and caregiver's workforce performance. Support. Care Cancer : Off. J. Multinatl. Assoc. Support. Care Cancer. 2010;18(6):699–706. doi: 10.1007/s00520-009-0668-5. [DOI] [PubMed] [Google Scholar]
- 12.Evercare and National Alliance for Caregiving . 2006. Caregivers in Decline: A Close-Up Look at the Health Risks of Caring for a Loved One. Bethesda, MD. [Google Scholar]
- 13.Littleton-Kearney M.T., Grady P.A. The science of caregiving bringing voices together: summary of National Institute of Nursing Research's 2017 summit. Nurs. Outlook. 2018;66(2):157–159. doi: 10.1016/j.outlook.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 14.Kent E.E., Rowland J.H., Northouse L. Caring for caregivers and patients: research and clinical priorities for informal cancer caregiving. Cancer. 2016;122(13):1987–1995. doi: 10.1002/cncr.29939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Academy of Medicine . The National Academies Press; Washington, DC: 2016. Families Caring for an Aging America. [PubMed] [Google Scholar]
- 16.Ferrell B., Wittenberg E. A review of family caregiving intervention trials in oncology. CA A Cancer J. Clin. 2017 doi: 10.3322/caac.21396. [DOI] [PubMed] [Google Scholar]
- 17.Northouse L.L., Katapodi M.C., Song L., Zhang L., Mood D.W. Interventions with family caregivers of cancer patients: meta-analysis of randomized trials. CA A Cancer J. Clin. 2010;60(5):317–339. doi: 10.3322/caac.20081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carlson M.D., Bradley E.H., Du Q., Morrison R.S. Geographic access to hospice in the United States. J. Palliat. Med. 2010;13(11):1331–1338. doi: 10.1089/jpm.2010.0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Center to Advance Palliative Care and the National Palliative Care Research Center . 2019. America's Care of Serious Illness: A State-By-State Report Card on Access to Palliative Care in Our Nation's Hospitals. New York, NY. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Center to Advance Palliative Care . 2015. America's Care of Serious Illness: 2015 State-By-State Report Card on Access to Palliative Care in Our Nation's Hospitals. New York, NY. [Google Scholar]
- 21.Alabama Rural Health Association . Alabama Department of Public Health; Montgomery, AL: 2007. Selected Indicators of Health Status in Alabama: Alabama's Rural and Urban Counties. [Google Scholar]
- 22.Barnato A.E., Anthony D.L., Skinner J., Gallagher P.M., Fisher E.S. Racial and ethnic differences in preferences for end-of-life treatment. J. Gen. Intern. Med. 2009;24(6):695–701. doi: 10.1007/s11606-009-0952-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mack J.W., Paulk M.E., Viswanath K., Prigerson H.G. Racial disparities in the outcomes of communication on medical care received near death. Arch. Intern. Med. 2010;170(17):1533–1540. doi: 10.1001/archinternmed.2010.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loggers E.T., Maciejewski P.K., Paulk E. Racial differences in predictors of intensive end-of-life care in patients with advanced cancer. J. Clin. Oncol. 2009;27(33):5559–5564. doi: 10.1200/JCO.2009.22.4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson K.S., Elbert-Avila K.I., Tulsky J.A. The influence of spiritual beliefs and practices on the treatment preferences of African Americans: a review of the literature. J. Am. Geriatr. Soc. 2005;53(4):711–719. doi: 10.1111/j.1532-5415.2005.53224.x. [DOI] [PubMed] [Google Scholar]
- 26.Dionne-Odom J., Hooker S., Bekelman D. Family caregiving for persons with heart failure at the intersection of heart failure and palliative care: a state-of-the-science review. Heart Fail. Rev. 2017 doi: 10.1007/s10741-017-9597-4. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schulz R. Research priorities in geriatric palliative care: informal caregiving. J. Palliat. Med. 2013;16(9):1008–1012. doi: 10.1089/jpm.2013.9483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson K.S. Racial and ethnic disparities in palliative care. J. Palliat. Med. 2013;16(11):1329–1334. doi: 10.1089/jpm.2013.9468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dionne-Odom J.N., Azuero A., Lyons K.D. Benefits of early versus delayed palliative care to informal family caregivers of patients with advanced cancer: outcomes from the ENABLE III randomized controlled trial. J. Clin. Oncol. 2015;33(13):1446–1452. doi: 10.1200/JCO.2014.58.7824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dionne-Odom J.N., Azuero A., Lyons K.D. Family caregiver depressive symptom and grief outcomes from the ENABLE III randomized controlled trial. J. Pain Symptom Manag. 2016;52(3):378–385. doi: 10.1016/j.jpainsymman.2016.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dionne-Odom J.N., Taylor R., Rocque G. Adapting an early palliative care intervention to family caregivers of persons with advanced cancer in the rural deep South: a qualitative formative evaluation. J. Pain Symptom Manag. 2018;55(6):1519–1530. doi: 10.1016/j.jpainsymman.2018.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pearlin L.I., Mullan J.T., Semple S.J., Skaff M.M. Caregiving and the stress process: an overview of concepts and their measures. Gerontol. 1990;30(5):583–594. doi: 10.1093/geront/30.5.583. [DOI] [PubMed] [Google Scholar]
- 33.Ferrell B.R., Temel J.S., Temin S. Integration of palliative care into standard oncology care: American society of clinical oncology clinical practice guideline update. J. Clin. Oncol. 2017;35(1):96–112. doi: 10.1200/JCO.2016.70.1474. [DOI] [PubMed] [Google Scholar]
- 34.Dionne-Odom J.N., Azuero A., Lyons K.D. Benefits of early versus delayed palliative care to informal family caregivers of patients with advanced cancer: outcomes from the ENABLE III randomized controlled trial. J. Clin. Oncol. 2015 doi: 10.1200/JCO.2014.58.7824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.National Comprehensive Cancer Network . 2013. Distress Management: Clinical Practice Guidelines in Oncology. [DOI] [PubMed] [Google Scholar]
- 37.Rocque G.B., Partridge E.E., Pisu M. The patient care connect program: transforming health care through lay navigation. J. Oncol. Pract. 2016 doi: 10.1200/JOP.2015.008896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rocque G.B., Pisu M., Jackson B.E. Resource use and Medicare costs during lay navigation for geriatric patients with cancer. JAMA Oncol. 2017;3(6):817–825. doi: 10.1001/jamaoncol.2016.6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wells R., Stockdill M.L., Dionne-Odom J.N. Educate, nurture, Advise, before life Ends comprehensive heartcare for patients and caregivers (ENABLE CHF-PC): study protocol for a randomized controlled trial. Trials. 2018;19(1):422. doi: 10.1186/s13063-018-2770-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bellg A.J., Borrelli B., Resnick B. Enhancing treatment fidelity in health behavior change studies: best practices and recommendations from the NIH Behavior Change Consortium. Health Psychol. 2004;23(5):443–451. doi: 10.1037/0278-6133.23.5.443. [DOI] [PubMed] [Google Scholar]
- 41.Hoffmann T.C., Glasziou P.P., Boutron I. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. 2014;348 doi: 10.1136/bmj.g1687. g1687. [DOI] [PubMed] [Google Scholar]
- 42.Dionne-Odom J.N., Kono A., Frost J. Translating and testing the ENABLE: CHF-PC concurrent palliative care model for older adults with heart failure and their family caregivers. J. Palliat. Med. 2014;17(9):995–1004. doi: 10.1089/jpm.2013.0680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miles M.B., Huberman A.M., Saldaña J. Thousand Oaks. third ed. Califorinia: SAGE Publications, Inc; 2014. Qualitative data analysis : a methods sourcebook. [Google Scholar]
- 44.Johns S.A., Brown L.F., Beck-Coon K., Monahan P.O., Tong Y., Kroenke K. Randomized controlled pilot study of mindfulness-based stress reduction for persistently fatigued cancer survivors. Psycho Oncol. 2015;24(8):885–893. doi: 10.1002/pon.3648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Northouse L.L., Rosset T., Phillips L., Mood D., Schafenacker A., Kershaw T. Research with families facing cancer: the challenges of accrual and retention. Res. Nurs. Health. 2006;29(3):199–211. doi: 10.1002/nur.20128. [DOI] [PubMed] [Google Scholar]
- 46.Conn V.S., Algase D.L., Rawl S.M., Zerwic J.J., Wyman J.F. Publishing pilot intervention work. West. J. Nurs. Res. 2010;32(8):994–1010. doi: 10.1177/0193945910367229. [DOI] [PubMed] [Google Scholar]
- 47.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weitzner M., Jacobsen P.B., Wagner H., Friedland J., Cox C. The caregiver quality of life index-cancer (CQOL-C) scale: development and validation of an instrument to measure quality of life of the family of caregiver of patients with cancer. Qual. Life Res. 1999;8:55–63. doi: 10.1023/a:1026407010614. [DOI] [PubMed] [Google Scholar]
- 49.Bjelland I., Dahl A.A., Haug T.T., Neckelmann D. The validity of the Hospital Anxiety and Depression Scale: an updated literature review. J. Psychosom. Res. 2002;52(2):69–77. doi: 10.1016/s0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 50.Snaith R.P. The hospital anxiety and depression scale. Health Qual. Life Outcomes. 2003;1:29. doi: 10.1186/1477-7525-1-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lyons K.D., Bakitas M., Hegel M.T., Hanscom B., Hull J., Ahles T.A. Reliability and validity of the functional assessment of chronic illness therapy-palliative care (FACIT-Pal) scale. J. Pain Symptom Manag. 2009;37(1):23–32. doi: 10.1016/j.jpainsymman.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Candy B., Jones L., Drake R., Leurent B., King M. Interventions for supporting informal caregivers of patients in the terminal phase of a disease. Cochrane Database Syst. Rev. 2011;6 doi: 10.1002/14651858.CD007617.pub2. CD007617. [DOI] [PubMed] [Google Scholar]
- 53.Lancaster G.A., Dodd S., Williamson P.R. Design and analysis of pilot studies: recommendations for good practice. J. Eval. Clin. Pract. 2004;10(2):307–312. doi: 10.1111/j..2002.384.doc.x. [DOI] [PubMed] [Google Scholar]
- 54.Conn V.S., Algase D.L., Rawl S.M., Zerwic J.J., Wyman J.F. Publishing pilot intervention work. West. J. Nurs. Res. 2010;32(8):994–1010. doi: 10.1177/0193945910367229. [DOI] [PubMed] [Google Scholar]
- 55.Ferrell B., Wittenberg E. A review of family caregiving intervention trials in oncology. CA A Cancer J. Clin. 2017;67(4):318–325. doi: 10.3322/caac.21396. [DOI] [PubMed] [Google Scholar]
- 56.Palos G.R., Hare M. Patients, family caregivers, and patient navigators: a partnership approach. Cancer. 2011;117(15 Suppl):3592–3602. doi: 10.1002/cncr.26263. [DOI] [PubMed] [Google Scholar]
- 57.Natale-Pereira A., Enard K.R., Nevarez L., Jones L.A. The role of patient navigators in eliminating health disparities. Cancer. 2011;117(15 Suppl):3543–3552. doi: 10.1002/cncr.26264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.The Patient Protection and Affordable Care Act. Vol. 119. 2010. [Google Scholar]
- 59.Kamal A.H., Wolf S.P., Troy J. Policy changes key to promoting sustainability and growth of the specialty palliative care workforce. Health Aff. 2019;38(6):910–918. doi: 10.1377/hlthaff.2019.00018. [DOI] [PubMed] [Google Scholar]