Abstract
Background
Specific immunotherapy is the only type of disease-modifying treatment, which induces rapid desensitization and long-term sustained unresponsiveness in patients with seasonal allergic rhinoconjunctivitis. The safety and tolerability of a new cumulative dose regimen of 35600 SU Grass MATA MPL for subcutaneous immunotherapy were assessed in pre-seasonal, single-blind, placebo controlled Phase I clinical study. Underlying immunological mechanisms were explored using transcriptome analysis of peripheral blood mononuclear cells.
Methods
Study subjects with a history of moderate to severe seasonal allergic rhinitis and/or conjunctivitis (SAR) due to grass (Pooideae) pollen exposure were randomized on a 1:1 ratio to receive either six 1.0 mL injections of cumulative dose regimen 35600 SU of Grass MATA MPL or placebo. The study consisted of three periods: screening, randomization and treatment and End of Study period. Blood samples were taken for clinical safety laboratory assessments and for the assessment of gene expression analysis during screening visit and End of Study visit. The safety statistics was calculated using Fisher's exact test. Delta Delta Ct method analysis of RT2 Profiler PCR Array gene expression results was used to calculate changes in gene expression level. Genes with the absolute value of log2 fold change greater than ±1.1 and p-value less than 0.05 were identified as differentially expressed and underwent IPA data analysis.
Results
The results of the study indicated that the higher cumulative dose regimen of the immunotherapy was well-tolerated. Changes in gene expression profile were associated with early immune responses implicating innate and adaptive immune mechanisms. Pathways and mechanistic network analysis via IPA mapped differentially expressed genes onto canonical pathways related to T cell differentiation, cytokine signalling and Th1/Th2 activation pathways. The transcriptome findings of the study could be further verified in large-scale field studies in order to explore their potential as predictive markers of successful immunotherapy.
Conclusions
The higher dose cumulative regime 35600 SU of Grass MATA MPL vaccine was well tolerated and safe. Molecular markers IL-27, IL-10, IL-4, TNF, IFNγ, TGFβ and TLR4 were the main predicted molecular drivers of the observed gene expression changes following early stages of SIT with Grass MATA MPL immunotherapy.
Keywords: Grass pollen, Allergen immunotherapy, Allergoid, Safety, Transcriptome
Abbreviations: ARC, adverse reaction complex; ADRs, adverse drug reactions; AE, adverse events; AIT, allergen mmunotherapy; DC, dendritic cell; EAACI, European Academy of Allergy and Clinical Immunology; FEV, forced expiratory volume; FVC, forced vital capacity; IPA, Ingenuity Pathway Analysis; MATA, modified allergen tyrosine adsorbate; MCT, microcrystalline tyrosine; MPL, monophosphoryl lipid A; mRNA, messenger ribosomal nucleic acid; SAEs, serious adverse events; SAR, seasonal allergic rhinoconjunctivitis; SD, standard deviation; SIT, specific immunotherapy; SU, standardized units; TEAEs, treatment-emergent adverse events; TLR, toll-like receptor; TSS, total symptom score; URA, Upstream Regulator Analysis
Introduction
Allergic rhinoconjunctivitis is a Type I allergic disease caused by common allergens such as pollen, mold spores, animal hair and dust mite residue. Seasonal allergic rhinoconjunctivitis (SAR) is most commonly triggered by allergy to pollen from trees, grasses or weeds, while perennial allergic rhinoconjunctivitis is associated with allergy to dust mite residue, mold spores or animal dander.1
Specific immunotherapy (SIT) with Grass MATA MPL combines broad spectrum modified grass allergens adsorbed on the depot adjuvant system microcrystalline tyrosine (MCT) in combination with Monophosphoryl Lipid A (MPL), offering a short course form of treatment for IgE-mediated SAR.2,3 The efficacy and safety of Grass MATA MPL, which is based on a cumulative dose of 5100 SU, has previously been reported in a Phase III clinical trials2,4 and is recommended grade 1A according to EAACI guidelines.5 There is increasing evidence that indicates the efficacy of allergen-specific immunotherapy correlates with the total cumulative dose of allergen or allergoid administered during a course of subcutaneous injections or sublingual administration.6, 7, 8, 9 As such, a new seven-fold increase in cumulative dose (35600 SU) of Grass MATA MPL was a subject of further evaluation as part of Phase II clinical development.10
Grass MATA MPL formulation is based on the chemical modification of 13 grass pollens (Grass family Poaceae) with glutaraldehyde, resulting in a reduction in allergenicity due to disruption of conformational IgE-binding epitopes but maintenance of immunogenicity, which has been demonstrated in a recent proof-of-principle study of the formulation.11 Grass allergoids are adsorbed onto the depot adjuvant – MCT, which allows slow release of antigen at the injection site for extended immune exposure and stimulus of immune deviation towards the Th1 cellular pathway and the induction of allergen-specific IgG4.12 The biodegradable nature of MCT limits formation of granulomas at the injection site and has been shown to exhibit a 48 hour half-life, which makes it a suitable candidate depot for a short course therapy.13,14
MPL is a second-generation adjuvant/immunomodulator, which is derived from Salmonella minnesota 595 using acid and base hydrolysis of lipid A. The predominant species created by this process is 3-O-deacyl-4-monophosphoryl lipid A with a main characteristic of substantially reduced toxicity in comparison with native lipopolysaccharide (LPS).13,14 As a result MPL has unique biological characteristics of combining attenuated pro-inflammatory activity with retained immunomodulatory properties and therefore is used as immunoadjuvant to enhance efficacy of the grass allergoid vaccine via TLR-4 signalling pathway.15,16
Several studies have shown therapeutic potential and a favorable safety profile of Grass MATA MPL2,3,15,17,18 with the most recent Phase II dose finding study based on higher cumulative dose of 35600 SU.10 It is stipulated that clinical improvement as a result of SIT is accompanied by suppression of Th2 immunity, an increase in allergen-specific IgG4 and inhibition of IgE, a predominant Th1 response with increased IFNγ production and induction of iTregs with secretion of anti-inflammatory cytokines – TGFß and IL-10.19, 20, 21 However, the exact or dominant underlying immune mechanisms implicating immunotherapy with Grass MATA MPL is a topic of active research. Understanding immunological mechanisms, which underlie induction and persistence of tolerance, will help to identify potential biomarkers that could predict therapeutic efficacy of allergen specific immunotherapy. Candidate biomarkers will be an essential tool in the diagnosis and classification of patients based on disease severity and prognosis as well as improving the safety profile by reducing likelihood of side-effects and adverse reactions. Identified biomarkers could improve the efficacy of SIT by targeting patients, who are most likely to respond to treatment. It may also help to reduce the population size required for clinical trials and guide development of treatment modalities to induce long-lasting protection after completion of a vaccination regime.22
In this current study, the tolerability and safety profile of a cumulative dose regimen of 35600 SU Grass MATA MPL for subcutaneous immunotherapy was evaluated in adults with seasonal allergic rhinoconjunctivitis associated with grass pollen. Assessment of gene expression profiles from clinical subjects one-week post-treatment allowed to infer early-phase immunological responses induced by Grass MATA MPL (35600 SU) immunotherapy.
Methods
The study was a randomized, single-blind, placebo-controlled Phase I Grass Study (ClinicalTrials.gov registration no. NCT 03931993) that was conducted at 4 study centers in the USA outside the grass pollen season. The study was conducted in accordance with the ethical principles that have their origin in the Declaration of Helsinki and the International Conference on Harmonization (ICH) guideline E6: Good Clinical Practice (GCP). Written informed consent was obtained from all subjects and the protocol was approved by the relevant ethics and regulatory authorities.
Subject population
Male and female subjects (aged 18–50 years) with a history of moderate to severe seasonal allergic rhinitis and/or conjunctivitis (SAR) due to grass (Pooideae) pollen exposure that required repeated use of antihistamines, nasal steroids, and/or leukotriene modifiers for relief of symptoms during the last 2 consecutive seasons prior to the study were eligible. All subjects had a positive case history for grass (Pooideae) pollen induced SAR, positive skin prick test for grass pollen allergen (wheal diameter ≥ 5 mm) and positive class of ≥2 grass-specific IgE level (>0.70 kU/L) to grass pollen mix defined by ImmunoCAP test (Phadia).
Main exclusion criteria were moderate to severe allergy symptoms during screening and treatment periods caused by perennial allergens or seasonal allergens as verified by medical history and positive SPT, presence of moderate to severe asthma, history of immunological disorders, presence of non-atopic rhinitis and/or rhino-sinusitis, presence of any skin conditions that might interfere with the interpretation of the SPT results, or any other conditions that could have affected the subject's safety or compromise the interpretation of results.
Study materials
Grass MATA MPL allergoid of 13 grass pollens (Pooideae) (900, 2700, 8000 standardized units [SU] and 50 μg of MPL/1.0 mL) adsorbed onto L-tyrosine (2% w/v) and 0.5% phenol.
Placebo vehicle: 2% w/v L-tyrosine, 0.5% phenol.
Active and placebo formulations were manufactured at Allergy Therapeutics (UK) Ltd, Worthing, UK.
Study design
Subjects were randomly assigned on a 1:1 ratio to receive either 6 1.0 mL injections of cumulative dose regimen 35600 SU of subcutaneous immunotherapy – Grass MATA MPL or placebo.
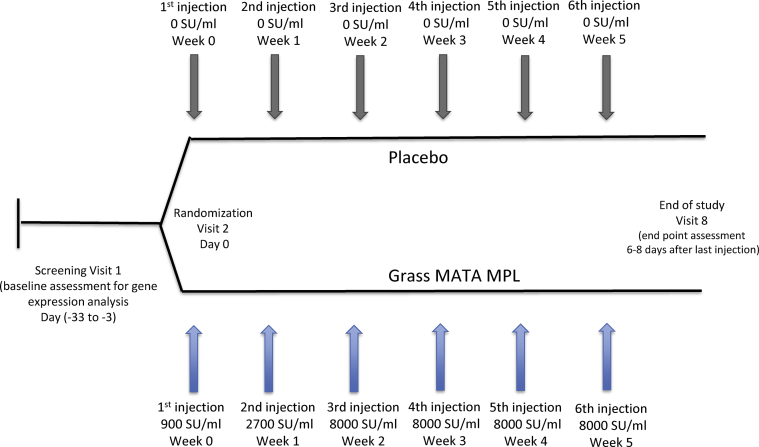
The study consisted of 3 periods: screening, randomization and treatment and post-treatment safety follow-up – End of Study (Fig. 1).
Fig. 1.
Study design. Study consisted of three periods: screening, randomization and treatment, end of study (EoS) with subjects randomization on 1:1 ratio to receive Grass MATA MPL or placebo
Period 1 included the screening visit (Visit 1) to assess the patients’ eligibility for the study and blood samples were taken for clinical safety laboratory assessments and for the baseline assessment for gene expression analysis. Eligible patients proceeded to Period 2 for enrolment and were randomly allocated to the Grass MATA MPL 35600 SU treatment group or placebo group to receive the first of the 6 weekly injections. Injections 2 to 6 were administered on a weekly basis (6–8 days between injections) at Visits 3 to 7. Period 3 (Visit 8 – End of Study) was conducted 6–8 days after Visit 7 to review any adverse events and to perform end-of-treatment assessments, which included blood sample collection for safety laboratory tests and for gene expression analysis.
Study objectives
The primary objective of this study was to assess the tolerability and safety of a new cumulative dose for Grass MATA MPL 35600 SU for subcutaneous immunotherapy compared with placebo. An exploratory objective of the study was focused on transcriptome analysis of peripheral blood mononuclear cells (PBMCs) in order to evaluate the effect of subcutaneous immunotherapy with Grass MATA MPL on innate and adaptive immune mechanisms during an early-phase clinical response.
Safety assessments
Primary safety evaluation included number and frequency of adverse events (AE) that started within the time period from the first injection of study medication up to and including Visit 8 or early termination visit, if applicable. In addition, monitoring of changes in vital sign parameters and changes in routine clinical laboratory parameters (serum chemistry, hematology, urinalysis) were assessed. For asthmatic subjects changes in Peak Expiratory Flow Rate before and after injections were monitored.
Transcriptome analysis
Transcriptome analysis was performed using RT2 Profiler PCR Arrays technology (Qiagen, USA) to allow simultaneous multi-gene expression profiling in clinical samples. Blood samples were collected at screening Visit 1 (baseline) and at Visit 8. Total RNA purification was measured on an automated QIAcube system (Qiagen) using PAXgene Blood RNA kit IVD (Qiagen, Frederick, USA). RNA quality and concentration was determined by using a nanodrop spectrophotometer to measure the concentration and OD260/280 ratio of the samples. An OD260/280 ratio of between 1.8 to 2.0 and RNA concentration >40 μg/mL were used for high quality isolated RNA. RNA integrity was assessed using an RNA ScreenTape on Agilent TapeStation 2200 (Agilent Technologies).
The first strand cDNA synthesis was performed using QIAGEN RT2 First Strand Kit (Qiagen, USA). RT2 Profiler PCR Array gene expression analysis was performed by RT2 Profiler PCR Array Service (Qiagen, Frederick, USA) using RT2 SYBR® Green qPCR Mastermix and Custom RT2 Profiler PCR Array (Qiagen, CLAH24893).
Statistical methods
The safety statistics comprised the safety population, which included all patients, who received any dose of study drug, grouped according to the treatment they received.
Summary statistics were provided for the following metric variables: number of subjects with data available, arithmetic mean, standard deviation, median, minimum, maximum and 95% confidence intervals. Categorical data were summarized with frequency and percentage (%). The percentage of subjects with events was compared between treatment groups applying Fisher's exact test in a descriptive way. In addition, 95% confidence intervals for the difference in percentage for types of AEs were presented as well as the odds ratio and the corresponding 95% confidence intervals.
Delta Delta Ct method analysis of RT2 Profiler PCR Array gene expression results was used to obtain an overview of gene expression changes before the start of immunotherapy and one week after completion of the treatment. Genes with the absolute value of log2 fold change greater than ±1.1 and p-value less than 0.05 were identified as differentially expressed and underwent IPA data analysis (v. 01–12) (Ingenuity Systems Inc., CA): Venn diagram comparison, Canonical Pathway Analysis and Upstream Regulator Analysis.
Results
Demographics and baseline characteristics
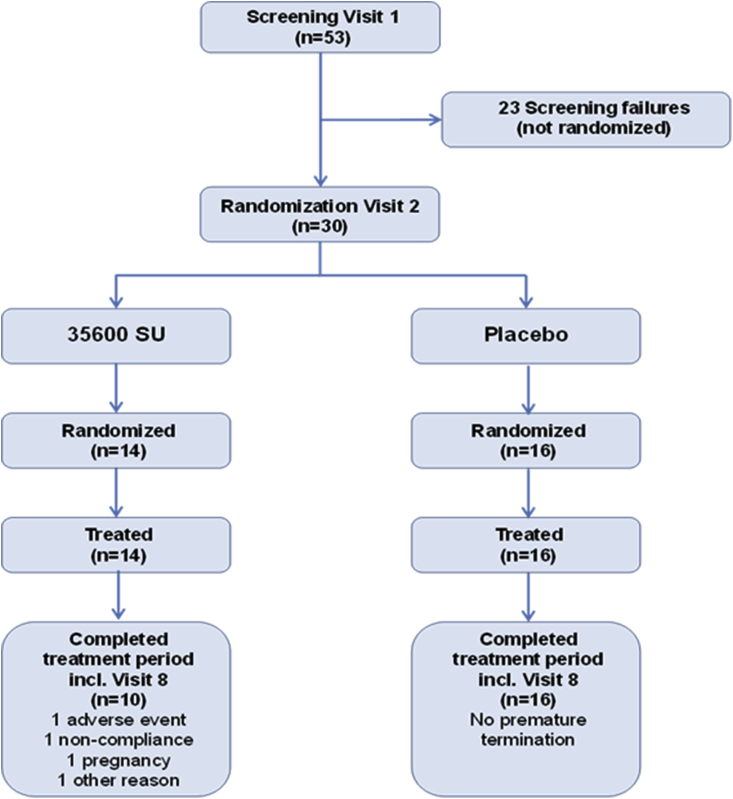
A total of 53 patients were enrolled and screened, of which 30 patients were randomized to receive study medication: 14 received 35600 SU Grass MATA MPL and 16 received placebo (Fig. 2).
Fig. 2.
Flow diagram for subjects disposition. All subjects from placebo group completed treatment. Four subjects from 35600 SU Grass MATA MPL group terminated prematurely
A total of 26 subjects (86.7%) received the full course of therapy (six injections): 16 (100%) from the placebo group and 10 (71.4%) from 35600 SU Grass MATA MPL group. Four subjects in the 35600 SU group received less than 6 injections, with 3 of the subjects missing injection 6 due to premature study termination (adverse event, a missed dose and weather conditions respectively) and 1 subject missing last 2 injections due to family emergency and pregnancy.
The 2 treatment groups were comparable with respect to most demographic variables with an average age (mean ± SD) 32.1 ± 9.56 years old, body mass index (mean ± SD) 29.22 ± 6.82 kg/m2 and duration of the disease 14.90 ± 10.41. An average age of the subjects from the placebo group was younger than those in the 35600 SU treatment group (median age 26.0 vs. 35.5 years), which have influenced the duration of the disease (Table 1). The differences in the baseline values for the age and disease duration parameters had no influence on the outcome of the safety statistics. Both treatment groups satisfied the requirements for the planned sample size in the safety set.
Table 1.
Demographics and Baseline characteristics (n = 30)
| Total (N = 30) |
35600 SU (N = 14) |
Placebo (N = 16) |
|
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| Gender [n (%)] | |||
| Female | 16 (53.3%) | 7 (50.0%) | 9 (56.3%) |
| Male | 14 (46.7%) | 7 (50.0%) | 7 (43.8%) |
| Age [y] | |||
| Mean (SD) | 32.1 (9.56) | 37.1 (9.37) | 27.8 (7.52) |
| Median | 28.5 | 35.5 | 26.0 |
| Range | 18–49 | 23–49 | 18–46 |
| Race [n(%)] | |||
| Black or African American | 3 (10.0%) | 1 (7.1%) | 2 (12.5%) |
| White | 27 (90.0%) | 13 (92.9%) | 14 (87.5%) |
| Ethnicity [n(%)] | |||
| Hispanic or Latino | 8 (26.7%) | 2 (14.3%) | 6 (37.5%) |
| Not Hispanic or Latino | 22 (73.3%) | 12 (85.7%) | 10 (62.5%) |
| Body mass index at screening [kg/m2] | |||
| Mean (SD) | 29.22 (6.82) | 30.44 (6.93) | 28.15 (6.75) |
| Median | 28.62 | 29.01 | 26.69 |
| Range | 15.8–47.4 | 22.7–47.4 | 15.8–40.3 |
| Duration of grass pollen allergy [years] | |||
| Mean (SD) | 14.90 (10.41) | 19.74 (12.85) | 10.67 (5.05) |
| Median | 11.60 | 20.25 | 8.60 |
| Range | 2.6–41.8 | 2.6–41.8 | 3.8–23.9 |
Safety profile
Overall, summary statistics for treatment emergent adverse events (TEAE) and adverse drug reactions (ADR) is reported in Table 2. In total, 85.7% (12/14) of subjects in 35600 SU group and 25% (4/16) subjects in placebo group had at least 1 TEAE. The majority of TEAEs were local after injection of study drug and most patients experienced only mild TEAEs: 10 out of 12 in the 35600 SU group and 3 out of 4 in the placebo group. One patient had a severe injection site swelling related to treatment, which resulted in subject withdrawal from the 35600 SU group. Two patients with non-mild TEAE experienced moderate injection site pain and swelling related to study drug (35600 SU group). None of the TEAEs were classified as serious.
Table 2.
Summary statistics of adverse events and adverse drug reactions.
| 35600 SU (N = 14) |
Placebo (N = 16) |
|||
|---|---|---|---|---|
| n (%) | Ev. | n(%) | Ev. | |
| Any TEAE | 12 (85.7%) | 55 | 4 (25.0%) | 10 |
| Any ADR | 11 (78.6%) | 53 | 2 (12.5%) | 5 |
| Any severe TEAE | 1 (7.1%) | 1 | 0 (0.0%) | 0 |
| Any severe ADR | 1 (7.1%) | 1 | 0 (0.0%) | 0 |
| Any serious AE | 0 (0.0%) | 0 | 0 (0.0%) | 0 |
| Any non-serious treatment emergent AE | 12 (85.7%) | 55 | 4 (25.0%) | 10 |
| Any AE leading to study drug discontinuation | 1 (7.1%) | 1 | 0 (0.0%) | 0 |
| Any ADR leading to study drug discontinuation | 1 (7.1%) | 1 | 0 (0.0%) | 0 |
| Any TEAE leading to premature discontinuation from study | 1 (7.1%) | 1 | 0 (0.0%) | 0 |
| Any local AE | 9 (64.3%) | 49 | 1 (6.3%) | 3 |
| Any local AE within 24 h of injection | 9 (64.3%) | 43 | 1 (6.3%) | 3 |
| Any local AE > 24 h of injection | 1 (7.1%) | 6 | 0 (0.0%) | 0 |
| Any systemic AE | 1 (7.1%) | 1 | 1 (6.3%) | 2 |
| Any systemic AE within 24 h of injection | 1 (7.1%) | 1 | 1 (6.3%) | 2 |
Ev. = number of adverse events in corresponding class and treatment group; TEAE = treatment emergent adverse event; n = number of subjects with AE = adverse event; ADR = adverse drug reaction
Treatment-emergent ADRs were reported in 11 subjects (78.6%, 53 ADRs) in the 35600 SU group and in 2 subjects (12.5%, 5 ADRs) of the placebo group. None of the ADRs were classified as serious. No deaths, serious TEAEs and TEADRs, neuro-inflammatory or new onset autoimmune disease were reported during the treatment period of the study. Systemic AEs occurred in 1 subject in the 35,600 SU group (mild allergic rhinitis) and in 1 subject in the placebo group (mild nasal congestion and mild throat irritation).
Immunotherapy-induced molecular mechanisms
Gene expression analysis of a panel of disease and pathway-focused molecular markers was conducted using Peripheral Blood Mononuclear cells (PBMCs) to explore the underlying immunological mechanisms related to the treatment with 35600 SU Grass MATA MPL. Transcriptome profiling of 179 genes 1 week post-treatment in comparison to baseline expression levels at the screening visit identified 99 genes where expression level exhibited statistical significance (p < 0.05, fold-change greater than ±1.1). A tertiary analysis using Ingenuity Pathway Analysis (IPA) mapped differentially expressed genes from the study data set onto canonical pathways and enabled prediction of upstream molecular regulators.
Selection strategy of differentially expressed genes associated with grass MATA MPL immunotherapy
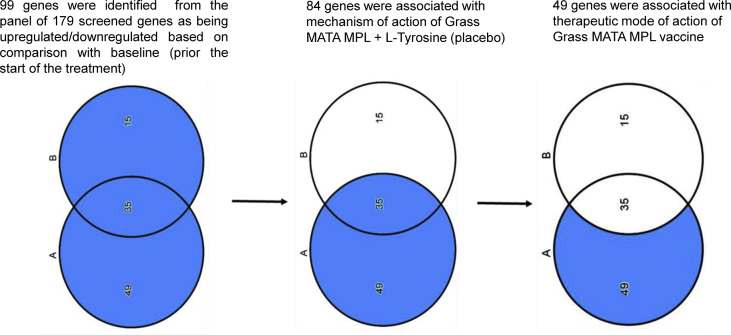
The causal analysis approach based on the Ingenuity Knowledge Database – a structured collection of gene functions sourced from the biomedical research publications23 - was used to analyze differentially expressed genes from the dataset. Causal analytics algorithms were implemented via the IPA software program. A Venn diagram was used for a stepwise selection of differentially expressed genes from the active treatment group (Fig. 3).
Fig. 3.
Venn diagram. A (35600 SU group) −14 subjects with seasonal allergic rhinoconjunctivitis. B (Placebo group) −16 subjects with seasonal allergic rhinoconjunctivitis (SAR)
From the 99 differentially expressed genes in both groups of subjects, 49 genes were putatively regulated by the mechanism of action of Grass MATA MPL.
Differentially expressed genes modulated in response to grass MATA MPL immunotherapy
A panel of 49 differentially expressed genes, which were associated with the mode of action of Grass MATA MPL immunotherapy, included key markers from the innate and adaptive immune response associated with an early stage of immune tolerance induction against the grass allergens: effector molecules, Th1/Th2 cellular markers and signature cytokines, T regulatory markers (Supplemental Table 1). The fold-change values for differentially expressed genes ranged between 1.87 and −1.07, which indicated changes to mRNA expression level following Grass MATA MPL immunotherapy.
Several differentially expressed genes associated with changes in expression levels of cytokines/chemokines and cellular markers correlated with potential biomarkers for AIT, which were suggested by the EAACI Immunotherapy Interest Group.21 In particular, changes in expression level of such cytokines/chemokines as CCL2, IL2, IL3, IL12, TNF, IL18, IL9, IL4, IL5, IL27 (Supplemental Table 1) corresponded to the potential immunotherapy biomarkers from the Cytokines and Chemokines domain.21 Upregulation of FOXP3 alongside other transcription factors and nuclear receptors (NR4A3, NR4A1, STAT5A, RUNX1), which are required for induction and activation of Treg cells, correlated with prospective biomarkers from the Cellular Biomarkers domain.21
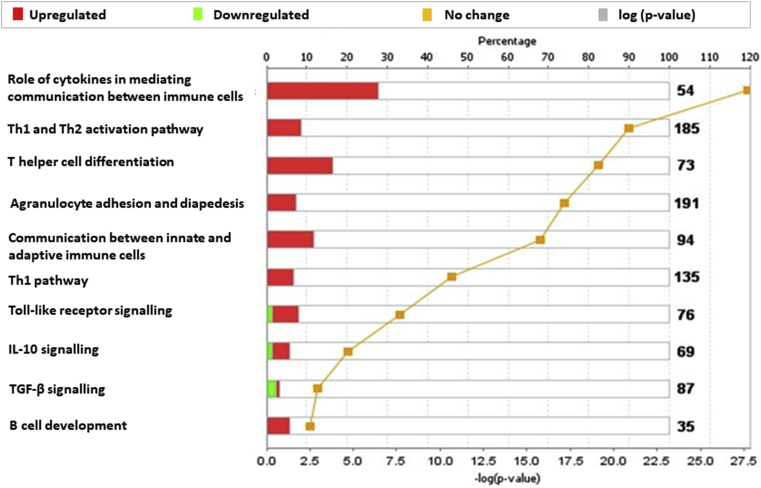
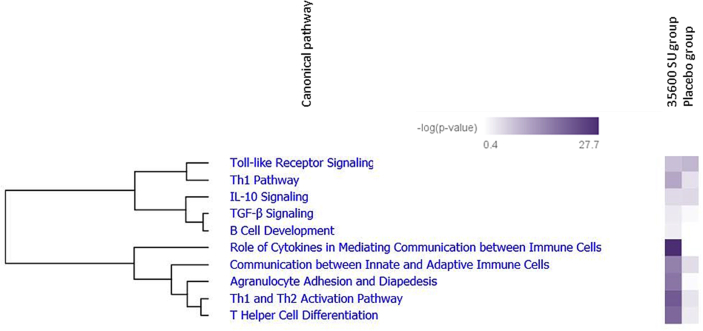
Canonical pathway analysis using IPA was employed to determine the functional relationship between differentially expressed genes from the 35600 SU group. The top 10 significantly enriched canonical pathways are presented in Fig. 4. The most enriched categories of canonical pathways included cytokine signalling (P = 1.91 × 10−28, 15 molecules), Th1 and Th2 activation pathway (P = 1.35 × 10−21, 16 molecules), T helper cell differentiation (P = 8.17 × 10−20, 12 molecules) and agranulocyte adhesion and diapedesis (P = 4.76 × 10−20, 15 molecules).
Fig. 4.
Canonical pathways enriched in 35600 SU subjects group one week post-treatment. A total of 49 genes associated with Grass MATA MPL mode of action were mapped onto canonical pathway using IPA. The stacked bars indicate overlap of the significantly expressed dataset genes with the canonical pathway. The yellow threshold indicates 95% CI.
It can be seen from the clustergram (Fig. 5) that these pathways were most significantly enriched in the active 35,600 SU group in comparison with placebo. Some of the upregulated canonical pathways are most likely the result of the specific mode of action of immunotherapy.
Fig. 5.
Hierarchical clustering heat map of the most significantly enriched pathways in 35600 SU group versus placebo one week post-treatment. Two clusters of co-regulated canonical pathways are displayed based on -log (p-value)>1.3: TLR signalling and Th1 pathways co-regulated with IL-10/TGFβ pathways, innate and adaptive immune responses co-regulated with T helper cells differentiation and proliferation
Upstream regulator analysis of T helper cells differentiation
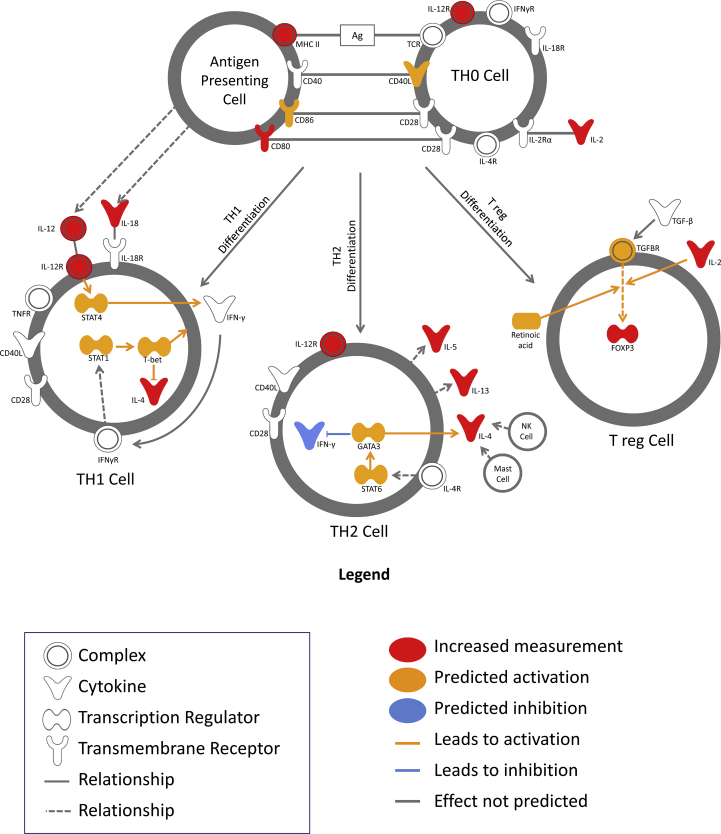
A significantly enriched canonical pathway of interest, associated with the gene dataset, was related to T cell differentiation (P = 8.17 × 10−20, 12 molecules). Based on the data set, Th0 cells upon allergen stimulation via antigen-MHCII complex on the surface of antigen presenting cells (APC) undergo further differentiation and polarization towards Th1 (IFNγ, IL-12), Th2 (IL-4, IL-5, IL-13) or Treg (IL-10, TGFβ) (Fig. 6).
Fig. 6.
T-helper cells differentiation canonical pathway. Each subset of T cells is defined as lineage, which express selective signature cytokines and transcription factors. IPA analysis identified upregulation of IL-12 and IL-18 Th1 signature cytokines, which induce Th1 differentiation pathway; upregulated Th2 related genes IL-4, IL-5 and IL-13 can activate Th2 differentiation pathway; T regulatory cell differentiation is modulated by upregulated IL-2 and FOXP3 transcription factor
Upstream Regulator Analysis (URA) was used to identify the molecules upstream of the differentially expressed genes that had a high probability to cause the observed gene expression changes. Two functions - an overlap P-value measuring enrichment of network-regulated genes in the dataset and an activation Z-score were employed in URA. Z-score measured a statistically significant pattern match between the observed gene expression changes and the predicted pattern derived from prior knowledge.23
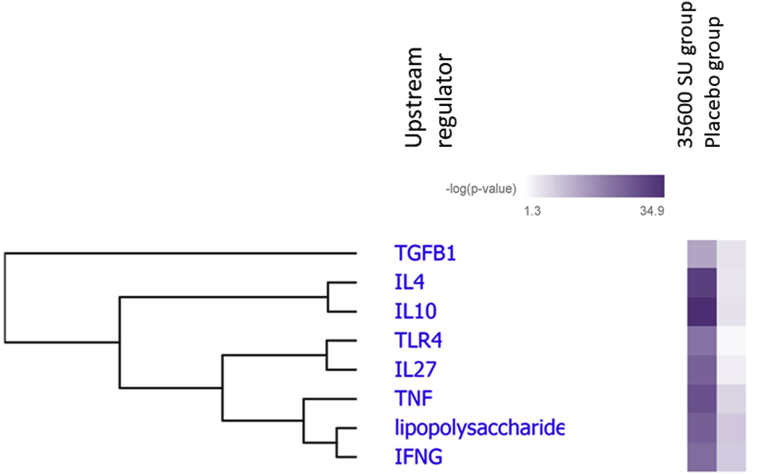
URA predicted several activated upstream regulators of T helper cell differentiation: IL-10 (P = 1.29 × 10−35), IL-4 (P = 1.89 × 10−32), TNF (P = 4.23 × 10−29), Lipopolysaccharide (P = 1.24 × 10−26), IL-27 (P = 3.33 × 10−26), IFNγ (P = 1.77 × 10−24), TGFβ (P = 3.73 × 10−18) and TLR4 (P = 4.07 × 10−23) (Fig. 7).
Fig. 7.
Hierarchical clustering heat map of the top predicted upstream regulators. The upstream regulators for T helper cell differentiation in the treated group were differentially expressed compared with placebo group based on the -log (p value). The p value calculated using the Fisher exact test
Discussion
One of the novel immunotherapeutic approaches, which has shown a significantly improved risk/benefit profile in clinical trials, is an adjuvant system-based allergoid vaccine.2,3 This clinical study has, for the first time, demonstrated an acceptable safety and tolerability profile for a high cumulative dose regimen of 35600 SU Grass MATA MPL vaccine for subcutaneous immunotherapy in adults with SAR. Increasing total cumulative dose approximately 7-fold to 35600 SU was safe and well tolerated. Local AEs such as injection site pain and site swelling were usually only of mild severity and resolved spontaneously without further treatment. No relevant changes of the clinical chemistry and hematology parameters between the screening visit and Visit 8/EoS were observed. For all vital sign parameters, the mean values were within normal ranges at all visits and individual measurements did not cause any alert.
Therapeutic efficacy of Grass MATA MPL immunotherapy has been indicated in several clinical studies and investigations to date,2,3,17,18 including recently completed Phase II dose finding study clinical trial.10 However, gene expression analysis following immunotherapy with Grass MATA MPL has not been previously explored. Transcriptome analysis of PBMCs from both groups of subjects was conducted one week post-treatment to identify immunological changes, which take place during the early phase of SIT. Ingenuity Pathway Analysis (IPA) - which allows us to determine differentially expressed genes and link individual genes to biological networks and pathways in order to build mechanistic models - identified 49 genes regulated by Grass MATA MPL immunotherapy. This set of genes appears to reflect early immunological changes observed in PBMCs after immunotherapy with Grass MATA MPL.
From the interrogated gene dataset there were a number of significantly upregulated molecules associated with innate and adaptive immune responses due to the presence and activation of eosinophils, basophils, mast cells, dendritic cells and T-helper lymphocytes (Supplementary Table 1).24,25 Increase of Th1 cytokines and chemokines such as eotaxin, IL-12, IL-27, IL-18 and TNF-α suggest early signs of immunodeviation from a Th2 type towards a Th1 type of immune response and correlates with similar findings from experimental in vitro studies using MPL.26,27
Canonical pathway analysis identified T-helper cell differentiation as one of the top significantly enriched canonical pathways with twelve molecules from the 49 gene dataset being upregulated (Fig. 4). A postulated immunological mechanism of successful immunotherapy is the formation of a long-term clinical tolerance based on shift of Th2 cellular response towards Th1 response and up-regulation of allergen-specific T regulatory cells.21 Differentiation of naïve CD4+ T helper cells (Th0) towards a specific T cell subset will depend on polarization cytokine milieu and signals from the antigen presenting cells.28 In this study it was shown that during the early phase of immunotherapy with Grass MATA MPL, polarization of cytokines towards a Th1 response (IL-12, IL-18, IL-27) co-existed with the polarization of the cytokine repertoire towards a Th2 (IL-4) and Treg (IL-2, IL-27) responses, based on a canonical pathway (Fig. 6) and upstream regulator analysis (Fig. 7).
It has been previously reported that during the early stages of allergen immunotherapy innate mechanisms in the form of dendritic cells (DCs) orchestrate cellular adaptive immune responses and T cell differentiation, thus predicting the clinical outcome of immunotherapy.29, 30, 31 Polarization and differentiation of T cells will depend on the type of DCs circulating in peripheral blood. DC1s will drive differentiation of Th1 cells, DC2s will promote pro-allergic Th2 response and DCreg will induce Treg differentiation (Table 3).21
Table 3.
Naïve CD4+ T cells polarization and differentiation
| T cells subtype (transcription factor) | Effector cytokines | Polarization milieu | Dendritic cells subtype |
|---|---|---|---|
| Th1 (Tbet) | IFN-γ, IL-2, TNF-α | IL-12, IFN-γ, IL-18, IL-27 | Type 1 DC (DC1s) |
| Th2 (GATA3) | IL-4, IL-5, IL-13 | IL-4 | Type 2 DC (DC2s) |
| Tregs (FOXP3) | IL-10, TGF-β | IL-2, TGF-β, IL-27 | Regulatory DC (DCreg) |
Recently, specific molecular markers were identified for certain subsets of polarized DCs: DC2s associated markers – CD141, GATA3 and OX40 ligand, DCreg associated markers – C1Q and Stabilin-1.29,30 Based on these molecular markers a study conducted among grass pollen allergic patients, who received 4 months SLIT, has shown upregulation of DCreg subtype in peripheral blood using quantitative PCR. It has correlated with improvement in rhinoconjunctivitis symptoms and was used to distinguish clinical responders from non-responders.29,30
Due to the evaluation of a single post-treatment time point analysis, the present study has not been able to show a distinct DC subtype molecular signature at gene expression level in PBMCs, which could predict differentiation of T cells towards a specific cell subset and clinical response. However, based on significantly upregulated level of IL-27 (Supplemental Table 1), it is plausible to suggest that DCreg cell subtypes were present in peripheral blood of subjects, who received 35600 SU of Grass MATA MPL. The main source of IL-27 synthesis are tolerogenic regulatory dendritic cells (DCregs), which express C1Q and Stabilin-1 cellular biomarkers.31 It was shown previously that IL-27 is the main factor, which induces iTreg cells and promotes their differentiation towards IL-10 producing iTreg alongside with TGFâ. Furthermore, IL-27 initiates T cell proliferation and differentiation towards Th1 subtype via STAT1 dependant T-bet transcription factor. It also inhibits clonal expansion and activation of pathogenic pro-allergic Th2 cell subset via decreased expression of GATA3 transcription factor.32 Thus, the subtype of DCs identified by use of quantitative PCR on PBMCs could become a potential biomarker of successful immunotherapy and be used to separate clinical responders and non-responders.
Profiling and monitoring the level of specific cytokines/chemokines associated with the specific T cells subsets would allow a greater understanding of the underlying immunological mechanisms following immunotherapy with Grass MATA MPL. Clinical efficacy of this immunotherapy may depend on induction of inducible Treg cell subset (iTregs) with increased level of regulatory cytokines – IL-10 and TGFβ.33,34 IPA gene expression analysis identified IL-10 and TGFβ canonical pathways significantly enriched from the gene dataset (Fig. 4). Furthermore, using Upstream Regulator Analysis, both regulatory cytokines were identified as predicted molecular drivers based on the observed immunological changes (Fig. 7).
As a standard approach in clinical practice, profiling changes in serum level of cytokines/chemokines is done via multiplex analysis with Meso Scale Discovery and Luminex platforms.32 However, the changes in serum cytokines/chemokines do not correlate with clinical outcome partially due to heterogeneous cell population in peripheral blood.21 Recently it is being reported that measurements of local cytokine levels in nasal fluid may provide a more sensitive approach.35,36 Despite these positive data the use of serum or local cytokines and chemokines as predictive biomarkers remains a topic of active research.
An alternative approach of cytokine/chemokine measurements at gene expression level (mRNA) could offer an advantage of the technological platform with high sensitivity and specificity and would allow to detect changes prior they will become evident at the protein level in serum or nasal fluid.
It is important to emphasize the significance of TLR-4 being identified as a predicted upstream regulator (Fig. 7). Currently no direct evidence exists between MPL immunomodulatory function and iTreg induction. However, taking into account a necessity of TGFß presence as a transcription factor for FoxP3 induction37,38 and high level of TGFß following MPL stimulation, one might suggest a possible link between MPL mechanism of action and iTreg induction, which is supported by upregulated level of FoxP3 in the current study (Supplemental Table 1). Further research is required to unravel the immunomodulatory potential of MPL-containing allergoid vaccines and their mode of action. However, the data presented herein provides a first step toward this, which will be taken into a larger-scale field study to verify and to explore gene-expression profiles combined with serum analysis to elucidate further mode-of-action of short-course pre-seasonal MPL adjuvanted SCIT.
Conclusion
In summary, the safety and tolerability profile of the 35600 SU dose regimen of Grass MATA MPL vaccine was found to be acceptable for further dose-finding study in Phase II clinical trials, which has been recently completed and the cumulative dose regimen of 35600 SU has been found to be optimal for future Phase III clinical study.10 Transcriptome analysis of PBMCs using the IPA algorithm approach identified 49 genes related to an early phase response during SIT, which were linked to T helper cell differentiation and cytokine signalling. Upstream Regulator Analysis predicted that IL-27, IL-10, IL-4, TNF, IFNγ, TGFβ and TLR4 were the main molecular drivers of observed gene expression changes following early stages of SIT with Grass MATA MPL immunotherapy. These markers will be further verified in extended larger scale field studies in order to explore their potential as predictive markers of successful immunotherapy.
Declaration
Consent for publication and ethics approval
The study was conducted in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki. Written informed consent was obtained from all subjects before the study commencement. The protocol of the study was approved by the relevant ethics and regulatory authorities.
Data availability statement
Safety clinical data and gene expression data used to support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of interest
S. Starchenka, Dr. M. Heath, A. Lineberry and Dr. M. Skinner are employees of Allergy Therapeutics (UK) Ltd. Prof. T. Higenbottam was an employee of Allergy Therapeutics (UK) Ltd at the time the study was conducted.
Author contributions
All authors participated in the conception, design and implementation of the study. All authors were involved in the interpretation of data and the decision to submit for publication. SS and MDH wrote the manuscript. All authors read and approved the final manuscript.
Funding statement
The study was funded by Allergy Therapeutics (UK) Ltd.
Footnotes
Full list of author information is available at the end of the article
Supplementary data to this article can be found online at https://doi.org/10.1016/j.waojou.2019.100087.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.van Cauwenberge P., Bachert C., Passalacqua G. Consensus statement on the treatment of allergic rhinitis. European academy of allergology and clinical immunology. Allergy. 2000;55(2):116–134. doi: 10.1034/j.1398-9995.2000.00526.x. [DOI] [PubMed] [Google Scholar]
- 2.DuBuske L.M., Frew A.J., Horak F. Ultra-short-specific immunotherapy successfully treats seasonal allergic rhinoconjunctivitis to grass pollen. Allergy Asthma Proc. 2011;32:239–247. doi: 10.2500/aap.2011.32.3453. [DOI] [PubMed] [Google Scholar]
- 3.Rosewich M., Lee D., Zielen S. Pollinex Quattro: an innovative four injections immunotherapy in allergic rhinitis. Hum Vaccines Immunother. 2013;9(7):1523–1531. doi: 10.4161/hv.24631. [DOI] [PubMed] [Google Scholar]
- 4.Drachenberg K.J., Prölla S., Urban E., Woroniecki S.R. Single-course specific immunotherapy with mixed pollen allergoids: results of a multi-centre study. Allergol Immunopathol. 2003;31(2):77–82. doi: 10.1016/s0301-0546(03)79172-1. [DOI] [PubMed] [Google Scholar]
- 5.Muraro A., Roberts G. 2017. (Immunotherapy Guidelines Part 1: Systematic Reviews. European Academy of Allergy and Clinical Immunology, Zurich, Switzerland). [Google Scholar]
- 6.Calderon M.A. PA et al. European Academy and Clinical Immunology task force report on 'dose-response relationship in allergen-specific immunotherapy. Allergy. 2011;66(10):1345–1359. doi: 10.1111/j.1398-9995.2011.02669.x. [DOI] [PubMed] [Google Scholar]
- 7.Didier A., Malling H.L., Worm M. Optimal dose, efficacy, and safety of once-daily sublingual immunotherapy with a 5-grass pollen tablet for seasonal allergic rhinitis. J Allergy Clin Immunol. 2007;120(6):1338–1345. doi: 10.1016/j.jaci.2007.07.046. [DOI] [PubMed] [Google Scholar]
- 8.Durham S.R., Yang W.H., Pedersen M.R., Johansen N., Rak S. Sublingual immunotherapy with once-daily grass allergen tablets: a randomised controlled trial in seasonal allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2006;117(4):802–809. doi: 10.1016/j.jaci.2005.12.1358. [DOI] [PubMed] [Google Scholar]
- 9.Frati F., Scurati S., Puccinelli P. Development of a sublingual allergy vaccine for grass pollinosis. Drug Des Dev Ther. 2010;4:99–105. doi: 10.2147/dddt.s10044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zielen S., Kuna P., Aberer W. Strong dose response after immunotherapy with PQ Grass using conjunctival provocation test. World Allergy Organ J. 2019;12(11):100075. doi: 10.1016/j.waojou.2019.100075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Starchenka S., Bell A., Mwange J., Skinner M., Heath M. Molecular fingerprinting of complex grass allergoids: size assessments reveal new insights in epitope repertoires and functional capacities. World Allergy Organ J. 2017;10(1):17. doi: 10.1186/s40413-017-0146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leuthard D.S., Duda A., Freiberger S.N. Microcrystalline tyrosine and aluminum as adjuvants in allergen-specific immunotherapy protect from IgE-mediated reactivity in mouse models and act independently of inflammasome and TLR signaling. J Immunol. 2018;200(9):3151–3159. doi: 10.4049/jimmunol.1800035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bell A.J., Heath M.D., Hewings S.J., Skinner M.A. The adsorption of allergoids and 3-O-desacyl-4′-monophosphoryl lipid A (MPL®) to microcrystalline tyrosine (MCT) in formulations for use in allergy immunotherapy. J Inorg Biochem. 2015;152:147–153. doi: 10.1016/j.jinorgbio.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 14.Klimek L., Schmidt-Weber C.B., Kramer M.F., Skinner M.A., Heath M.D. Clinical use of adjuvants in allergen-immunotherapy. Expert Rev Clin Immunol. 2017;13(6):599–610. doi: 10.1080/1744666X.2017.1292133. [DOI] [PubMed] [Google Scholar]
- 15.Pfaar O., Barth C., Jaschke C., Hörmann K., Klimek L. Sublingual allergen-specific immunotherapy adjuvanted with monophosphoryl lipid A: a phase I/IIa study. Int Arch Allergy Immunol. 2011;154(4):336–344. doi: 10.1159/000321826. [DOI] [PubMed] [Google Scholar]
- 16.Zielen S., Gabrielpillai J., Herrmann E., Schulze J., Schubert R., Rosewich M. Long-term effect of monophosphoryl lipid A adjuvanted specific immunotherapy in patients with grass pollen allergy. Immunotherapy. 2018;10(7):529–536. doi: 10.2217/imt-2018-0004. [DOI] [PubMed] [Google Scholar]
- 17.Rabe U., Altengarten J., Benke E. Long-term efficacy of specific subcutaneous, short-term MPL adjuvant immunotherapy over three treatment and three follow-up years, as measured by quality of life. Allergo J Int. 2017;26(5):147–154. [Google Scholar]
- 18.Drachenberg K.J., Heinzkill M., Urban E. Efficacy and tolerability of short-term specific immunotherapy with pollen allergoids adjuvanted by monophosphoryl lipid A (MPL®) for children and adolescents. Allergol Immunopathol. 2003;31(5):270–278. doi: 10.1016/s0301-0546(03)79195-2. [DOI] [PubMed] [Google Scholar]
- 19.Bachmann M.F., Kündig T.M. Allergen-specific immunotherapy: is it vaccination against toxins after all? Allergy. 2017;72(1):13–23. doi: 10.1111/all.12890. [DOI] [PubMed] [Google Scholar]
- 20.Burks A.W., Calderon M.A., Casale T. Update on allergy immunotherapy: American academy of allergy, asthma & immunology/european academy of allergy and clinical immunology/PRACTALL consensus report. J Allergy Clin Immunol. 2013;131(5):1288–1296. doi: 10.1016/j.jaci.2013.01.049. e3. [DOI] [PubMed] [Google Scholar]
- 21.Shamji M.H., Kappen J.H., Akdis M. Biomarkers for monitoring clinical efficacy of allergen immunotherapy for allergic rhinoconjunctivitis and allergic asthma: an EAACI Position Paper. Allergy. 2017;72(8):1156–1173. doi: 10.1111/all.13138. [DOI] [PubMed] [Google Scholar]
- 22.Hoffmann H.J., Valovirta E., Pfaar O. Novel approaches and perspectives in allergen immunotherapy. Allergy. 2017;72(7):1022–1034. doi: 10.1111/all.13135. [DOI] [PubMed] [Google Scholar]
- 23.Krämer A., Green J., Pollard J.J. Causal analysis approaches in ingenuity pathway analysis. Bioinformatics. 2013;30(4):523–530. doi: 10.1093/bioinformatics/btt703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kappen J.H., Durham S.R., Veen H.I.T. Applications and mechanisms of immunotherapy in allergic rhinitis and asthma. Ther Adv Respir Dis. 2017;11(1):73–86. doi: 10.1177/1753465816669662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davis L.S., Bhutani S., Barnett S.R. Early gene expression changes with rush immunotherapy”. Clin Mol Allergy. 2011;9(1):12. doi: 10.1186/1476-7961-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin M., Michalek S.M., Katz J. Role of innate immune factors in the adjuvant activity of monophosphoryl lipid A. Infect Immun. 2003;71(5):2498–2507. doi: 10.1128/IAI.71.5.2498-2507.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Puggioni F., Durham S.R., Francis J.N. Monophosphoryl lipid A (MPL®)* promotes allergen-induced immune deviation in favour of Th1 responses. Allergy. 2005;60(5):678–684. doi: 10.1111/j.1398-9995.2005.00762.x. [DOI] [PubMed] [Google Scholar]
- 28.Raphael I., Eagar T.N., Forsthuber T.G. T cell subsets and their signature cytokines in autoimmune and inflammatory diseases. Cytokine. 2015;74(1):5–17. doi: 10.1016/j.cyto.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gueguen C., Bouley J., Moussu H. Changes in markers associated with dendritic cells driving the differentiation of either TH2 cells or regulatory T cells correlate with clinical benefit during allergen immunotherapy. J Allergy Clin Immunol. 2017;137(2):545–558. doi: 10.1016/j.jaci.2015.09.015. [DOI] [PubMed] [Google Scholar]
- 30.Zimmer A., Bouley J., Le Mignon M. A regulatory dendritic cell signature correlates with the clinical efficacy of allergen-specific sublingual immunotherapy. J Allergy Clin Immunol. 2012;129(4):1020–1030. doi: 10.1016/j.jaci.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 31.O'mahony L., Akdis C.A., Eiwegger T. Innate mechanisms can predict successful allergy immunotherapy. J Allergy Clin Immunol. 2016;137(2):559–561. doi: 10.1016/j.jaci.2015.10.047. [DOI] [PubMed] [Google Scholar]
- 32.Shamji M.H., DurhamSR Mechanisms of allergen immunotherapy for inhaled allergens and predictive biomarkers. J Allergy Clin Immunol. 2017;140(6):1485–1498. doi: 10.1016/j.jaci.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 33.Möbs C., Slotosch C., Löffler H. Birch pollen immunotherapy leads to differential induction of regulatory T cells and delayed helper T cell immune deviation. J Immunol. 2010;184(4):2194–2203. doi: 10.4049/jimmunol.0901379. [DOI] [PubMed] [Google Scholar]
- 34.Akdis C.A., Akdis M. Mechanisms of allergen-specific immunotherapy and immune tolerance to allergens. World Allergy Organ J. 2015;8(1):1. doi: 10.1186/s40413-015-0063-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scadding G.W., Eifan A.O., Lao-Araya M. Effect of grass pollen immunotherapy on clinical and local immune response to nasal allergen challenge. Allergy. 2015;70(6):689–696. doi: 10.1111/all.12608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Renand A., Shamji M.H., Harris K.M. Synchronous immune alterations mirror clinical response during allergen immunotherapy. J Allergy Clin Immunol. 2018;141(5):1750–1760. doi: 10.1016/j.jaci.2017.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haiqi H., Yong Z., Yi L. Transcriptional regulation of Foxp3 in regulatory T cells. Immunobiology. 2011;216(6):678–685. doi: 10.1016/j.imbio.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 38.Passerini L., Santoni de Sio F.R., Roncarol MG Bacchetta R. Forkhead box P3: the peacekeeper of the immune system. Int Rev Immunol. 2014;33(2):129–145. doi: 10.3109/08830185.2013.863303. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Safety clinical data and gene expression data used to support the findings of this study are available from the corresponding author upon reasonable request.