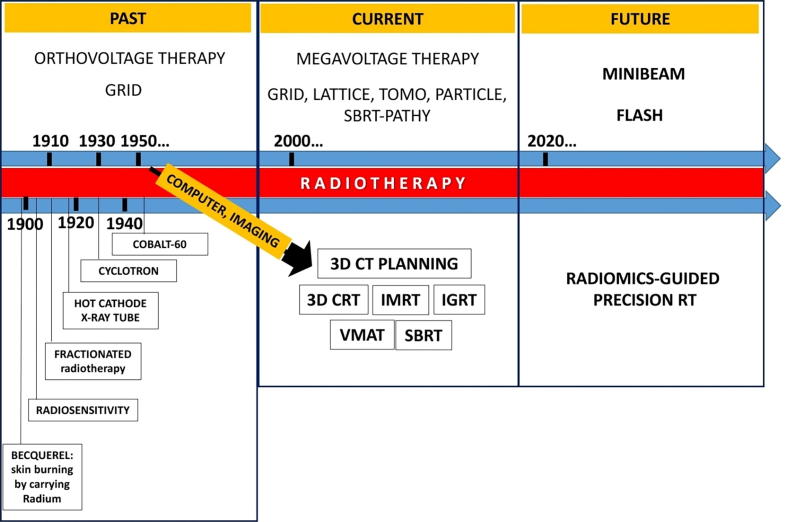
1. Introduction
Spatially Fractionated Radiation therapy (SFRT) has a history of over 100 years. The principle of SFRT is distinctive from the standard radiation approaches, as it treats the total tumor with a non-uniform dose, effectively treating the tumor while staying within normal tissue tolerance of the surrounding structures. Historically, SFRT is frequently used to treat bulky malignant tumors with a high radiation dose in the stereotactic radiosurgery (SRS)/stereotactic body radiotherapy (SBRT) dose range (10–20 Gy per fraction) using megavoltage x-ray beams. The application of SFRT, historically known as GRID therapy, has produced dramatic relief of severe symptoms, significant objective regression, above average local control rates and minimal toxicity in palliative settings [1]. The advancement of physics and technology has provided more techniques to deliver SFRT. Some understandings of radiobiology and immunology have been generated from studies of SFRT. These promising clinical results have generated a renewed interest in this technique at many centers in the United States and internationally. A series of novel application of SFRT in clinical trials are being anticipated in the near future. Here, we summarize the history, the present and the future of SFRT. As current reviews of SFRT are lacking, we present a comprehensive review of SFRT and its clinical implications.
2. Origination of SFRT with GRID therapy
The original technique to deliver SFRT was called GRID therapy, which was introduced in 1909 by Kohler and was commonly used through the 1930′s [2]. This type of therapy involved delivering a relatively high but heterogeneous radiation dose to the tumor through a perforated screen with blocked areas called a GRID. GRID was used to create a beam arrangement that is similar to an array of pencil beams. This delivery of SFRT, does not attempt to treat the whole tumor like the conventional approaches. Instead, this technique allows for the delivery of irradiation of SRS/SBRT dose level in areas within tumors, especially bulky tumors, avoiding producing prohibitive damage or detrimental toxicities to surrounding tissues. Thus, skin and subcutaneous tissues can tolerate much higher doses with SFRT than attempting to cover the whole tumor with radiation. SFRT was initially delivered via orthovoltage X-rays to treat advanced bulky or tumors that were deeply seated in the 1950s [3]. With the introduction of megavoltage radiation, skin sparing and better dosimetry can be obtained easier than orthovoltage X-rays. Thus, GRID radiotherapy has become less commonly used as a clinical delivery method. In addition, the use of more modern technology to deliver SFRT with superior dosimetry to GRID has not been well characterized. Hence, new investigations are warranted.
3. GRID’s application in megavoltage era
In the 1990s, GRID therapy was delivered with megavoltage photon beams to treat patients with massive or recurrent tumors who had underwent previous radiation. Published clinical results employing GRID therapy have most commonly focused on its use in the palliative setting. Many reports of patients with large or recurrent tumors treated with GRID therapy showed patients achieving good oncological outcomes [4], [5], [1], [6]. Placement of 10–15 Gy to Dmax was delivered with a single field and a GRID block with 50:50 (1:1) open to close areas ratio employed. No acute effects and no unusual late damage were observed in a follow-up time range of 1–18 months [4]. Subsequently, palliative GRID therapy showed a response rate of more than 90% and complete response (CR) rate of 27% [5]. Another study of 71 patients with bulky tumors (size > 8 cm) of varying histologies demonstrated a 78% response in pain improvement, a 59% partial response (PR) rate, and a 73% objective clinical response rate for mass effect after GRID therapy of 10–20 Gy with or without additional external beam radiation [1]. Neither of these studies demonstrated significant toxicity with GRID therapy. GRID therapy has also been applied in a definitive setting. Twenty-seven patients with stage IV localized head and neck cancers received a dose of 15–20 Gy with GRID therapy followed by conventional doses of external beam radiation [6]. GRID was used either as a definitive treatment or in the pre-operative setting [6]. Neck control was reported as 96%, a pathologic complete response rate of 85% was achieved in those patients that subsequently underwent neck dissection. There were no grade 4 toxicities. More recently, 14 patients with bulky head and neck squamous cell carcinomas received a single fraction of GRID therapy followed by standard concurrent chemoradiation. These patients experienced a similar toxicity profile to patients treated with standard chemoradiation alone, while achieving a gross tumor volume tumor control rate of 79% [7]. A Summary of publications about GRID therapy is shown in Table 1. Table 1 summarizes the representative clinical application studies of GRID therapy from 1990 to 2019. Among them, all patients treated with GRID therapy were at advanced stage. Clincal application studies of other SFRT methods are summarized in Table 2, this series of clinical reports demonstrated generally better than expected relief of severe symptoms, objective tumor regression, and local control rates with minimal toxicity.
Table 1.
Clinical application of GRID therapy.
| Study | Pt# | Stage | Pathology | Sites | GRID (Gy) | EBRT (Gy) | Outcomes | Toxicity |
|---|---|---|---|---|---|---|---|---|
| Mohiuddin et al. [4] | 22 | Advanced | Mixed | Mixed | 10–15 | N/A | 90% symptoms control | N/A |
| Mohiuddin et al. [5] | 61 | Advanced | Mixed | Pelvis | 10–15 | 8–78 | Overall CR 27% PR 64% |
N/A |
| Abdomen Extremities Thorax Head & neck |
15–25 | |||||||
| Mohiuddin et al. [1] | 71 | Advanced >8 cm | Mixed | Lung, & N, GI, sarcomas, GU, GYN, Skin, Breast, Liver | 10–20 Gy | 4000 cGy | Cr 0–24%, PR50-85% | 1 case developed carotid blowout |
| Huhn et al. [6] | 27 | Bulky N2- N3 | Head and neck | Head and neck | 15–20 Gy | 54–79 Gy | Group1 GRID + RT neck control rate 93% DSS 50%, LC 86%. Group 2 GRID + R T + Sx neck control rate 92%, DSS 85%, LC 92% |
No grade 4 toxicities, 3 pts had post-op wound healing complication |
| Peñagarícano et al. [7] | 14 | Locally advanced | Head and neck | Head and neck | 20 Gy | 66 Gy | 80% pCRin planned neck dissection or primary tumor biopsies of the GRID area | No patient developed Grade 4 acute toxicity. 1 case of carotid blow-in after neck dissection. may have not been attributable to GRID therapy. |
| Neuner et al. [59] | 79 | Bulky tumor s | Mixed | Mixed | 10–20 Gy | 9–70.2 Gy | Pain 74–75% Mass effect 67–73% |
12 pts had G3- G4 toxicities. |
| Edwards et al. [60] | 53 | Locally advanced | Head and Neck | Head and Neck | 15 Gy | 48–79.2 Gy | 81% LC, 9% DM | 4% G3+ toxicities |
Table 2.
Clinical Application of other SFRT methods.
| Study | Pt # | Stage | Pathology | Technique | GRID (Gy) | EBRT (Gy) | Clinical outcome s | Toxicity |
|---|---|---|---|---|---|---|---|---|
| Zhang et al. [10] | 10 | N/A | Mixed | Helical Tomotherapy And LINAC based GRID | Dmax 20 Gy Mean dose 8–12 Gy |
N/A | N/A | N/A |
| Amendola et al. [14] | 10 | Advanced stage | NSCLC | Lattice radiation therapy with LINAC | LRTX1 (18 Gy to vertices and 3 Gy to GTV), | EBRT 45- 66 Gy | Tumor Volume response 15–83% mean 52%. OS 4–86 months, mean 22 months |
Grade1 pneumonitis in all patients , no mortality |
| Tubin et al. [25] | 23 | N/A | mixed | SBRT to hypovascularized and hypometabolic tumor segment | 10–12 Gy X1-3 | None | LC 96%, Distant response rate (Abscopal effect) 52%. Tumor median shrinkage 70% |
Toxicity (grade 1–4: 0%) |
4. Implementation and improvement of GRID therapy
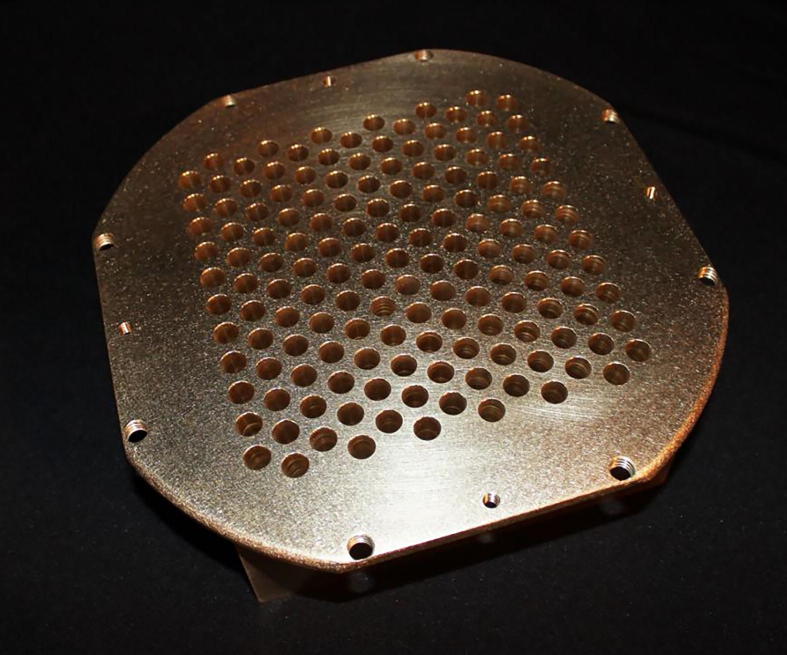
Initially, GRID therapy was generally delivered with a conventional linear accelerator with a GRID block that is either independently customized or commercially fabricated. Usually only one radiation field was used for GRID. A commercially available GRID block is shown in Fig. 1.
Fig. 1.
Clinical GRID block commercially available from decimal, LLC, photo provided by decimal, LLC.
Some institutes also use customized GRID block such as a block with holes created in a square lattice manner so that the passage will follow photon beam divergence. Alloy was used to fit into the spaces between the tubes forming the shielded areas of the GRID [5]. However, physical GRID blocks have some challenges in clinical practice, such as being heavy to use in the daily practice and technical challenges in following the divergence of beams. Due to the wide range of GRID usages and different methodology, the optimal arrangement of hole diameter and spacing in GRID blocks has not been defined yet. Monte Carlo simulation was performed to simulate 25 different patterns of Grid blocks. The results showed that the optimal range of hole diameters should be between 1.00 and 1.25 cm with spacing of 1.7 or 1.8 cm. GRID design has major impact on radio-resistant tumors (SF2 > 0.4), thus appropriate design of the hole diameter and hole spacing may lead to 40% higher clinical responses (i.e. hole size change from0.5 to 1.1 cm) [8].
With the advancement of modern linear accelerators, MLCs can be used to create GRID-equivalent dosimetry. MLC-based GRID therapy has several advantages: 1) LINAC head has pre-installed MLCs; 2) better dosimetry calculation can be easily achieved within the treatment planning system; and 3) the flexibility to change dosimetry accordingly such as hole size and separation. However, one major issue of MLC-based GRID therapy is the larger MLC leaf size to form the similar hole size compared to a physical GRID block and risk for dose spillage to the shielded area under MLCs generated by GRID blocks [9].
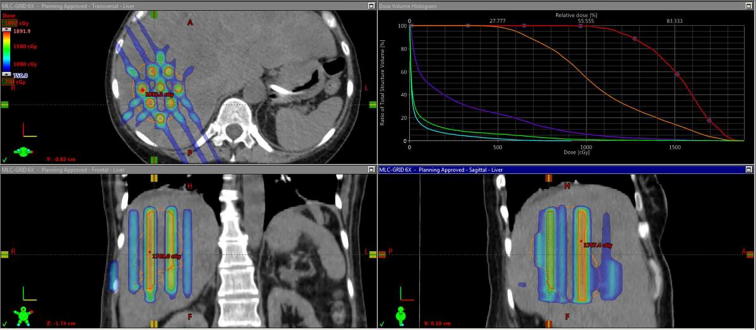
For deep seated bulky tumors, conventional GRID therapy faces a problem that the high dose falls into the normal tissue, given that only one single field or two adjacent (non-opposing) fields are typically used. The adjacent normal tissues usually receive very high doses when the dose drops off quickly because of larger skin-to-tumor distance. So, when deep-seated tumors with skin-to-tumor distances over 8 cm are treated with conventional GRID blocks with photons, tumor dose coverage posed a challenge. Since then, modern advanced GRID therapy techniques using Helical Tomotherapy or Volumetric Arc Therapy techniques have been developed. (Fig. 2, GRID therapy delivered by Volumetric Arc). Tomotherapy was used to create a radiation plan that is similar to the one obtained in an interstitial brachytherapy procedure. The advantage of Tomo-based GRID is lower normal tissue EUD (equivalent uniform doses) compared to LINAC-based GRID plans. In addition, Tomo-based GRID allows better conformity for tumors that are located in a complex structural relationships with avoidance structures [10].
Fig. 2.
GRID therapy delivered with Volumetric Arc, (photo credit Dr. Waleed Mourad University of Kentucky) DVH and GRID dosimetry on Axial, Coronal and Sagittal Planes demonstrated in treatment planning system.
5. SFRT in the modern 3D era
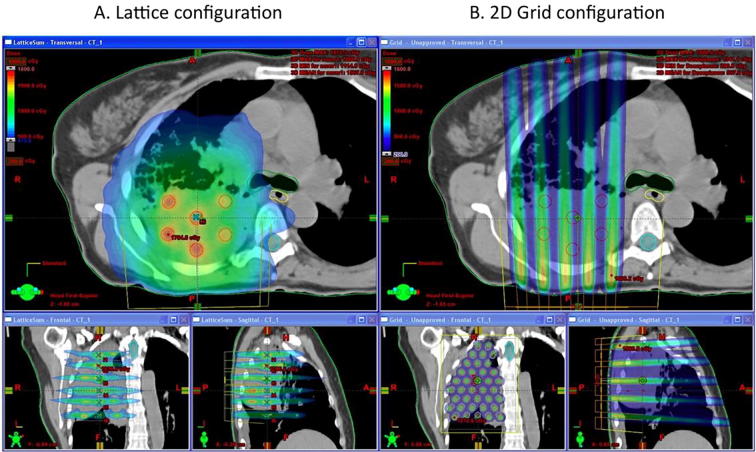
As described above, 2D GRID therapy often results in the high radiation dose outside of the radiation clinical target volume. Hence, Wu et al. [11] developed the concept of 3D LATTICE Radiotherapy (LRT). Its basic principle is to create multiple localized high-dose small spheres called vertices with a certain degree of separation within the tumor volume, while keeping the dose level lower in the periphery of the tumor to avoid related toxicity. LRT is the 3D technical extension of 2D GRID technique, the vertices are strictly contained within the gross tumor volume (Fig. 3). LRT is based on: 1) the proven clinical effectiveness of GRID therapy and 2) the new advances in physics and techniques that allow creating three dimensional (3D) high dose regions concentrated in small spheres called vertices inside the tumor volume possible [1], [11]. LRT has been utilized clinically in patients with bulky disease and has resulted in improved local control without added toxicity [12]. Amendola et al used LRT in the largest extent so far, they have treated cancers located in pelvic and chest [13]. They have safely delivered LRT to 10 patients with advanced stage NSCLC resulting in a statistically significant reduction in tumor size and in long overall survival in patients deemed hospice candidates. This was associated with no significant morbidity or mortality. LRT dose was 18 Gy in the vertices; the size of vertices range from 1 to 2 cm, the ratio of the dose between the vertices and the entire tumor volume is less than 3%. The average distance between vertices (center to center) was 3.6 cm [14].
Fig. 3.
Lattice vs GRID in same lung case (Photo Credit Dr. Xiaodong Wu) – (Lattice is a 3D way of delivering GRID and can decrease the dose in peripheral tissues compared to 2D GRID).
There is no systematic guidelines of vertices placement in LRT therapy so far.
6. SBRT with SFRT--SBRT-PATHY
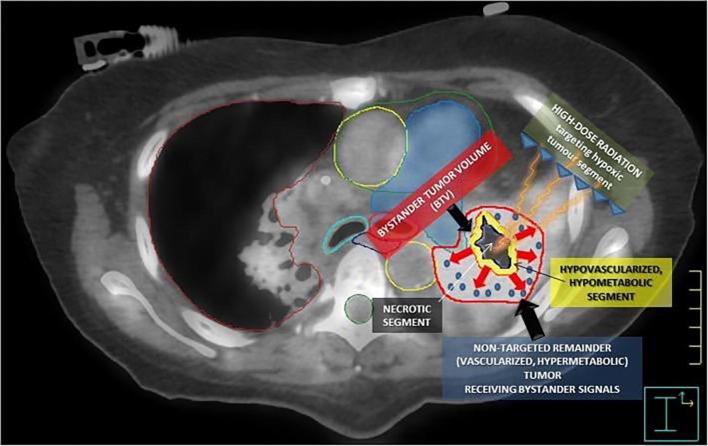
Stereotactic body radiation therapy [SBRT] uses specialized devices to immobilize and position patient to ensure precise delivery of high dose radiation to tumor using coordinates. For example, the results with SBRT/stereotactic ablative radiotherapy [SABR] in early-stage lung cancer patients have shown local control rates up to 86% [15], exceeding the control rates previously demonstrated with conventionally fractionated radiation. Recent attention to oxygen sensing research has suggested that intratumor hypoxia leads to increased Hypoxia-inducible factors (HIFs) activities, which leads to the activation of down-stream pathways for angiogenesis [16], cancer stem cell survival [17], immune evasion [18] and potential resistance to radiation therapy [19]. Thus, when hypoxic core of tumor is specifically targeted by SBRT, there might be novel radiation induced changes in the tumor environment that is yet to be studied and contributed to the improved outcomes. Radiomics method has been used to assess tumor-infiltrating CD8 cells and to predict the clinical outcomes of patients’ response to anti-PD-1 immunotherapy [20]. Tumor microenvironment has been suggested to be inhomogeneous, which suggested that given intra-tumor areas are more resistant/aggressive than others [21]. For example, severe hypoxic areas in tumor will need higher (ablative) doses compared to nonhypoxic areas, which could be cured with current dose level from conventional radiation therapy, as suggested by dose response models [22]. Thus, several intra-tumor boosting strategies have been proposed, which includes a micro-boost to the high risk/tumor harboring area in the prostate gland while sparing the entire gland from uniform dose-escalation to avoid excessive toxicity to bladder and rectum [23], [24]. These strategies matched with the concept of SFRT and can be applied to other tumor sites. A recent study using SFRT concept delivered stereotactic body radiation therapy of PArtial Tumor irradiation (SBRT-PATHY) targeting exclusively the HYpoxic segment of unresectable bulky tumors. The treatment triggered bystander effects (local) and abscopal effects (distant). SBRT-PATHY alone showed an overall response rate (96%) devoid of toxicity (grade 1–4: 0%) and 52% of patients presented abscopal effects [25]. Fig. 4 shows the radiobiology of the bystander effect-induction by SBRT-PATHY.
Fig. 4.
The radiobiology of the bystander effect-induction by SBRT-PATHY. (Photo Credit Dr. Slavisa Tubin). The figure summarizes the radiobiology of the bystander effect-induction by SBRT- PATHY. An 18F-FDG PET combined with a contrast-enhanced CT was used for the definition of BTV (smaller yellow contour), which corresponds to the junctional region between the central necrotic segment (black region) and the contrast- enhanced, hypermetabolic peripheral tumor (red contour, not targeted for irradiation). The red arrows represent “anti-angiogenic bystander signal” (blue pellets) released by the irradiated hypoxic tumor, inducing the regression of the non- targeted tumor. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
7. SFRT with particle therapy
GRID is being utilized with protons as well, since protons have the unique advantage of minimizing or even eliminating exit dose in normal tissues located beyond the tumor due to its inherent property (i.e. the Bragg peak) and potentially less scattering in normal tissues [26]. When proton is used instead of photon in GRID therapy, it successfully replicated the valley-to-peak ratios inside tumor. The depth-dose curve dropped quickly beyond the target and resulted in a more uniform beamlet dose within the tumor. Proton PBS has been tested in patients and can reduce the dose to proximal organs when treating a deep-seated tumor [26]. Carbon-ion beam has been evaluated for GRID treatment, GRIDs containing 0.5 or 3 mm wide carbon ion beam share similar characteristics with GRID therapy. If carbon ion beam grids are cross fired in a GRID manner, then it is feasible to produce homogenous dose distribution in the tumor while sparing normal tissue as well [27].
8. The evolution of SFRT in the smaller dimensions
Microbeam is a narrow beam of radiation with a beam size of micrometer or sub-micrometer dimensions. In 1960 s Woodley et al reported that mimicking heavy particles in space that is around 25 μm in diameter, a microbeam that is in that size range can increase the tolerance of mice brain to radiation significantly (4000 Gy vs 150 Gy) [28]. Consequently, Slatkin et al. studied the synchrotron-generated X-ray microbeams, they found that brain tissues are spared if high dose of radiation is given in a spatially spaced manner with micorobeams [29]. Hence, the European Synchrotron Radiation Facility (ESRF) in Grenoble, France, assembled a micro beamline for preclinical experiments and clinical trials [30]. Microbeam Radiation Therapy (MRT) uses X-ray microbeams (typically around 50–600 keV) in a collimated array manner. MRT has intrinsic characteristics like high dose rate and minimal beam divergence. High dose rates are necessary to deliver high therapeutic doses in microscopic volumes. The minimal beam divergence achieves a higher dose deposition in the target volume. Typically, MRT uses arrays of narrow microplanar. MRT can deliver peak entrance doses in the range of several hundred Grays which preferably kills tumors and does not lead to added toxicities in normal tissues. MRT does selectively ablates neurons in CNS but can outstandingly preserve normal brain tissues from necrosis. For example, when rats are exposed to MRT with peak/-valley doses of 357/12.7 Gy, only 2/10 developed late paresis. However, when single beam was used to deliver radiation, the ED50 for paralysis in rats was 130 Gy. Therapeutic ratios of MRT in small animal models have exceeded those from pre-clinical radiotherapy trying to cover tumor with conformal dose across a range of malignancies [31]. MRT improved survival in rat model. However, MRT has not been reported in large animals to date. The translation of MRT to clinical application faces 2 major challenges, accurate positioning of the beam and the patient; major transition of thinking and notions in radiation oncology field to accept inhomogeneous dose in treatment and prescribing/standardizing new MRT/SFRT treatment [30].
9. Radiobiology findings from GRID therapy
Current understanding about radiobiology behind GRID therapy may involve the bystander effect, vascular damage, and anti-tumor immune responses. The term bystander effect describes the ability of cells affected by irradiation to convey manifestations of damage to other cells not directly targeted for irradiation [32]
Bystander effect in GRID therapy is mostly referring to the changes happened in cells that are located in the valley (low dose) regions. Griffin et al. [33] demonstrated significant bystander killing in cells that are located next to high-dose radiated regions with GRID therapy. Plus, the killing of cells in the regions that was not directly radiated was found to be higher than those generated by background or scatter doses, which suggested that high dose GRID irradiation (10 Gy) triggered true cytotoxic bystander effects and it is possible that low doses of irradiation may also increase CD8 T cell recruitment to these areas whereas higher doses may lead to lethal effects within these newly recruited cells during radiotherapy. These bystander effects are not observed with conventionally fractionated radiation which encompass the entire region of the tumor. TNF-α [34] and TRA IL [35] have been suggested to play a role in bystander effects. These factors were induced by cells that received high dose under open areas of GRID [36], [37]. These factors could theoretically trigger bystander effects in the cells that were next to high-dose regions. These data suggest that GRID therapy can induce a different mechanism of tumor cell killing to that from conventional radiotherapy approaches, this mechanism is especially appealing in tumors that are bulky or has hypoxic cores. Work from Garcia-Barros et al. [38] has suggested that “high dose” radiation of 15 Gy causes an environment of “Potential Lethal Damage” that makes these cells sensitive to further doses of radiation, especially the endothelial cells of the tumor microvasculature. Ceramide is generated from sphingomyelin by SMase, it has been shown to be involved in sensitizing microvascular endothelial cells to radiation induced apoptosis.[38] When the activity of Secretory SMase and the concentration of ceramide were measured in patients who received SFRT using GRID therapy, an increase in SMase activity and ceramide levels was found only in patients with complete or partial response to the SFGRT, but not in non-responders.[39] Based on current knowledge, each endothelial cell in tumor vasculature supplies to a segment/unit of thousands of tumor cells. Thus, killing endothelial cell or occlusion of small capillaries inside of tumor would lead to an avalanche of death of tumor cells [40]. Data from mice model has suggested that > 10 Gy/fraction of radiation can lead to severe vascular damage and decreased blood supply. Also, delayed secondary tumor cell death has been shown to occur after 15–30 Gy of radiation in animals due to changes in intratumor microenvironment after radiation induced blood vessel obstruction. This would be a plausible explanation for the debulking effect from GRID therapy [41].
10. SFRT and immune responses
Radiation induced regression of cancer that was not directly radiated was called the abscopal effect. The abscopal effect has been attributed to activation of immune system. Today, the consensus is that combining radiotherapy with immunotherapy might increase abscopal response rates [42]. Current understanding of optimal radiation dose to elicit an immune response appears to be in the range of 8 and 10 Gy per fraction commonly delivered in 1– 3 fractions [42], suggesting that sufficient number of tumor lysates or extent of tumor necrosis and neoantigen release are needed to induce specific immune responses. Peters et al. reported that GRID/SFRT can trigger robust abscopal effect in tumors that are not directly irradiated: their mice model was treated with high dose SFGRT and showed increased cell death in the unirradiated tumor when compared to the mice that received open field radiation [43]. In addition, some preclinical data have shown SFRT can elicit both local and metastatic/distant tumor control through triggering immune responses. Single fraction SFRT significantly delayed un-irradiated distant tumor growth in a mice Xenograft tumor model. SFRT induced increased secretion of inflammatory cytokines and infiltration of T-cells increased significantly in the right sided un-irradiated tumor after irradiation with SFRT to 50% tumor volume in the left sided leg tumor [44]. This suggested that the cellular immunity might play a role in SFRT-triggered abscopal response. Clinical data have shown that partially irradiated tumor can become immunogenic. Abscopal effects have been triggered in patients by using a high, single dose of radiation to target the hypoxic segment of tumor with image-guided treatment planning and delivery [25]. A recent phase 2 randomized control trial in NSCLC patients demonstrated that SBRT can convert a cold tumor into a hot tumor. Patients that were PD-L1 low saw significant improvement when pembrolizumab and SBRT were combined compared with pembrolizumab alone [45].
11. Tumor types that could benefit from SFRT
Outcomes from tumors with different histological characteristics and treatment sites from historical clinical outcomes from GRID therapy have been summarized by Meigooni et al. [8]. The clinical total response rates with grid therapy were as following: Osteosarcoma (100%), Liposarcoma (50%), Leiomyosarcoma (100%), Colorectal (100%) (4); Sarcoma (94%), SCC (92%), Melanoma (83%), Adenocarcinoma (69%) [4]; Sarcoma (83%), SCC (94%), melanoma (50%) [5]; SCC of H&N (93%) [6]; Parotid (0%), Base of tongue (30%) Maxillary sinus (50%), Nasopharynx, Retromolar trigone, and Larynx (100%) Tonsil (25%) [7]. A few publications have highlighted that SFRT may be useful in radiation-resistant tumors, such as sarcoma. A case study used a total of 32 Gy by cEBRT following 18 Gy by SFGRT in a very large spindle cell sarcoma. The pre-treatment tumor volume is 631 cm3 and the postoperative pathology demonstrated 65 cm3 of residual tumor. Surgical specimen showed only 5–10% viable cells with extensive fibrosis and necrosis. Thus, a treatment response of 90% was seen in this case report, which was dramatic in comparison to studies with a 0– 0.5% radiological regression rate for 50 Gy EBRT alone [46]. A mechanism study of effectiveness of GRID therapy was done with The Monte Carlo simulation techniques to simulate the dose distribution profiles from GRID blocks. The therapeutic ratio (TR) and the equivalent uniform dose (EUD) for different types of tumors with respect to their radiation sensitivities were calculated using the simulated dose profiles and linear quadratic (LQ) and the Hug–Kellerer (H-K)models. Their results showed that TR of radioresistant tumor is known to increase with prescribed dose in grid therapy, but the TR of radio sensitive tumor does not change significantly with dose. Hence, grid therapy is expected to be biologically be more effective for radioresistant tumors [8].
12. Likely future Evolution of SFRT
12.1. Delivery techniques
Minibeam (MBRT) has beam widths around 500–7 00 μm (interbeam distance being the double of the width) which is wider than MRT (beam width 25 to 100 μm). The irradiation is performed by using an array of parallel thin beams spaced by 1 to 3 mm. Like MRT, MBRT is also typically delivered by synchrotron beams consisting of very intense kilovoltage x-rays. So far studies have indicated that beam dimensions beyond 0. 68 mm is a threshold for MBRT ‘s tissue-sparing effect [47]. MBRT has advantage in its thicker beam and dose profiles that they are not effected by cardiac pulsations like MRT [48]. It does not require very high dose rate like MRT and the implementation is technically easier than in MRT [49]. To bypass the high cost and geographical limitations of synchrotron, a small animal radiator has been developed to deliver minibeam patterns with peak-to-valley dose ratio (PVDR) values similar to those at synchrotrons and dose rate feasible for one fraction in a time compatible with rodent’s anesthesia. MBRT was delivered successfully (20 Gy in one fraction) to rodents with less severe skin damage and almost no brain damage compared to traditional radiation group [49]. Carbon ion minibeams with 525um beam thickness were used at the NASA Space Radiation Lab oratory (NSRL) in an “interlaced” (or “interleaved”) geometry to ab late a 6.5 mm target in the rabbit brain without major damage to the brain. While Proton MBRT was performed with 400-μm beam -wide slits and 3200-μm center-to- center spacing in glioma bearing rats. A 70 Gy peak dose in one fraction was delivered to whole brain, pMBRT leads to a significant tumor control and tumor eradication in 22% of the cases and no substantial brain damage [50]. The advantage of carbon minibeams over conventional carbon therapy is that the radiation impact on the non-targeted tissues, particularly the proximal tissues is lower. Which can allow dose escalation in treating radioresistant tumors. Similarly, proton minibeams save the skin and the proximal tissues better than conventional proton therapy. Both proton and carbon ion minibeams can be delivered with a collimator in beam path with current charged particles therapy facilities [51].
12.2. Precision medicine/radiotherapy
A point for SFRT and conventional radiation therapy to merge is precision radiotherapy, which is covering whole tumor with inhomogeneous dose. However, the inhomogeneous dose is designed/selected by the biological differences within the tumor, such as radiosensitivity, tumor aggressiveness or the nature of mutations in genes or pathways in a given intratumor loci. With development of radiomics, tumor homogeneity is being explored. Intratumoral subregions have been identified and could be used for prediction of tumor prognosis [52]. For example, one of the subregions in lung tumors (NSCLC) that is associated with the most metabolically active, heterogeneous component of the tumor was defined as the ‘high-risk’ subregion, the volume of which can predict distant metastasis and overall survival in patients treated with radiation therapy [52]. Precision radiotherapy in a different name, adaptive dose painting by number (DPbN), has been attempted in clinical treatment planning studies: FDG-PET/CT images was used to acquire tumor voxels, tumor voxel was then used to create a domain and used to calculate the probability of achieving local control with standard radiation 35X2Gy dose. The study showed that most human PPV negative tumors require a treatment dose > 100 Gy to certain local tumor regions and this is not likely achieved by 35 X 2 Gy but could be achieved by adaptive dose painting by number (DPbN) [53]. Thus, high-risk tumor sub regions, which harbors aggressive cancer cells, can be targeted with SFRT concepts/methods to improve local control and patient survival. The concept of SFRT can be incorporated into daily radiation planning as well. Fig. 5 summarized this article in a lively manner, SFRT was placed on top and traditional radiation therapy is on the bottom. It is interesting to see that SFRT and traditional radiation therapy grows together like yin and yang. And every major ways of delivery of tradition radiation can be creatively used to deliver SFRT. (Fig. 5. Evolution of SFRT along traditional radiation therapy)
Fig. 5.
Evolution of SFRT with traditional radiation therapy.
13. Advantages of SFRT
An easy analogy of SFRT is precision-guided munition in military, which is intended to be precisely delivered to a given target, inflict lethal damage to the target and minimize collateral damage other than the target [54]. The advantage of SFRT in medicine lies in increased firepower (high BED), increased precision and less toxicity. Increased firepower (high biological equivalent dose (BED)) is a key factor in the success of SBRT treating early stage lung cancers as Onishi et al. [55] demonstrated in a review that a better local control and survival rates are generated when a BED of 100 Gy or more is achieved. For SBRT or SABR, increased toxicity has been reported when targets are larger (>5–7 cm) or adjacent to critical normal structures, which limits the application of current practice of SBRT/SABR [56], [57]. As indicated in ASTRO guideline, application of SBRT in centrally located tumors is associated with unique and significant risks [58]. Hence, SBRT in lung cancer is, therefore, more commonly delivered for patients with small peripheral tumors; SFRT can easily achieve BED of > 100 Gy in a given tumor volume even in bulky or centralized tumor without the current limit of SBRT/SABR.
14. Conclusion
SFRT is important in its concept of covering whole or partial tumor with inhomogeneous radiation dose and gives the field more creativity and complimentary applications to supplement standard school of radiation therapy. While its clinical use to date has been limited to select centers, most commonly for palliative or recurrent cases, SFRT does have potential in treating primary cancers in a definitive manner such as head and neck, lung, breast, gynecologic and sarcoma cases. Clinical trials incorporating SFRT as a boost or as the primary radiotherapy component are warranted. The future application of the concept of SFRT will depend on technology advancement and more understandings of the biological mechanism in tumor or normal tissues radiated with SFRT.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Mohiuddin M., Fujita M., Regine W.F. High-dose spatially-fractionated radiation (GRID): a new paradigm in the management of advanced cancers. Int J Radiat Oncol Biol Phys. 1999;45:721–727. doi: 10.1016/s0360-3016(99)00170-4. [DOI] [PubMed] [Google Scholar]
- 2.Kohler H. Zur roentiefentherapie mit massendosen MMW. Fortschr Med. 1909;56:2314–2316. [Google Scholar]
- 3.Marks H. Clinical experience with irradiation therapy in grid. Radiology. 1952;58:338–342. doi: 10.1148/58.3.338. [DOI] [PubMed] [Google Scholar]
- 4.Mohiuddin M. Palliative treatment of advanced cancer using multiple nonconfluent pencil beam radiation. Cancer. 1990 Jul 1;66(1):114–118. doi: 10.1002/1097-0142(19900701)66:1<114::aid-cncr2820660121>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 5.Mohiuddin M., Stevens J.H., Reiff J.E. Spatially fraction- ated (GRID) radiation for palliative treatment of advanced cancer. Radiat Oncol Invest. 1996;4:41–47. [Google Scholar]
- 6.Huhn J.L., Regine W.F., Valentino J.P. Spatially fractionated GRID radiation treatment of advanced neck disease associated with head and neck cancer. Technol Cancer Res Treat. 2006 Dec;5(6):607–612. doi: 10.1177/153303460600500608. [DOI] [PubMed] [Google Scholar]
- 7.Peñagarícano J.A., Moros E.G., Ratanatharathorn V. Evaluation of spatially fractionated radiotherapy (GRID) and definitive chemoradiotherapy with curative intent for locally advanced squamous cell carcinoma of the head and neck: initial response rates and toxicity. Int J Radiat Oncol Biol Phys. 2010 Apr;76(5):1369–1375. doi: 10.1016/j.ijrobp.2009.03.030. [DOI] [PubMed] [Google Scholar]
- 8.Gholami S., Nedaie H.A., Longo F., Ay M.R., Wright S., Meigooni A.S. Is grid therapy useful for all tumors and every grid block design? J Appl Clin Med Phys. 2016 Mar 8;17(2):206–219. doi: 10.1120/jacmp.v17i2.6015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ha J.K., Zhang G., Naqvi S.A. Feasibility of delivering grid therapy using a multileaf collimator. Med Phys. 2006 Jan;33(1):76–82. doi: 10.1118/1.2140116. [DOI] [PubMed] [Google Scholar]
- 10.Zhang X., Penagaricano J., Yan Y. Spatially fractionated radiotherapy (GRID) using helical tomotherapy. J Appl Clin Med Phys. 2016 Jan 8;17(1):396–407. doi: 10.1120/jacmp.v17i1.5934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu X, Ahmed M, Wright J, et al. On Modern Technical Approaches of Three- Dimensional High-Dose Lattice Radiotherapy (LRT). Cureus 2(3): e9.
- 12.Blanco Suarez J.M., Amendola B.E., Perez N. The Use of lattice radiation therapy (LRT) in the treatment of bulky tumors: a case report of a large metastatic mixed mullerian ovarian tumor. Cureus. 2015;24(7) doi: 10.7759/cureus.389. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amendola B.E., Perez N.C., Wu X. Improved outcome of treating locally advanced lung cancer with the use of Lattice Radiotherapy (LRT): a case report. Clin Transl Radiat Oncol. 2018 Jan;12(9):68–71. doi: 10.1016/j.ctro.2018.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amendola B.E., Perez N.C., Wu X., Amendola M.A., Qureshi I.Z. Safety and efficacy of lattice radiotherapy in voluminous non-small cell lung cancer. Cureus. 2019 Mar 18;11(3) doi: 10.7759/cureus.4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shibamoto Y., Hashizume C., Baba F. Stereotactic body radiotherapy using a radiobiology-based regimen for stage I non-small cell lung cancer: a multicenter study. Cancer. 2012 Apr 15;118(8):2078–2084. doi: 10.1002/cncr.26470. [DOI] [PubMed] [Google Scholar]
- 16.Rapisarda A., Melillo G. Overcoming disappointing results with antiangiogenic therapy by targeting hypoxia. Nat Rev Clin Oncol. 2012;9:378–390. doi: 10.1038/nrclinonc.2012.64. [DOI] [PubMed] [Google Scholar]
- 17.Zhang H. HIF-1 regulates CD47 expression in breast cancer cells to promote evasion of phagocytosis and maintenance of cancer stem cells. Proc Natl Acad Sci USA. 2015;112:E6215–E6223. doi: 10.1073/pnas.1520032112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang C.-H. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell. 2015;162:1229–1241. doi: 10.1016/j.cell.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang M. HIF-1α regulates the response of primary sarcomas to radiation therapy through a cell autonomous mechanism. Radiat Res. 2015;183:594–609. doi: 10.1667/RR14016.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.E Sun R. A radiomics approach to assess tumour-infiltrating CD8 cells and response to anti-PD-1 or anti-PD-L1 immunotherapy: an imaging biomarker, retrospective multicohort study. Lancet Oncol. 2018 Sep;19(9):1180–1191. doi: 10.1016/S1470-2045(18)30413-3. [DOI] [PubMed] [Google Scholar]
- 21.Jia Q., Zhu B. Local mutational diversity drives intratumoral immune heterogeneity in non-small cell lung cancer. Nat Commun. 2018 Dec 18;9(1):5361. doi: 10.1038/s41467-018-07767-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nahum A., Movsas B., Horwitz E.M. Incorporating clinical measurements of hypoxia into tumor local control modeling of prostate cancer: implications for the alpha/beta ratio. Int J Radiat Oncol Biol Phys. 2003 Oct 1;57(2):391–401. doi: 10.1016/s0360-3016(03)00534-0. [DOI] [PubMed] [Google Scholar]
- 23.Lips I.M., van der Heide U.A., Haustermans K. Single blind randomized phase III trial to investigate the benefit of a focal lesion ablative microboost in prostate cancer (FLAME-trial): study protocol for a randomized controlled trial. Trials. 2011;5(12):255. doi: 10.1186/1745-6215-12-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miralbell R., Molla M., Rouzaud M. Hypofractionated boost to the dominant tumor region with intensity modulated stereotacticradiotherapy for prostate cancer: a sequential dose escalation pilotstudy. Int J Radiat Oncol Biol Phys. 2010;78(1):50–57. doi: 10.1016/j.ijrobp.2009.07.1689. [DOI] [PubMed] [Google Scholar]
- 25.Tubin S, Helmut P, Brcic L. Novel stereotactic body radiation therapy (SBRT)-based partial tumor irradiation targeting hypoxic segment of bulky tumors (SBRT-PATHY): improvement of the radiotherapy outcome by exploiting the bystander and abscopal effects. Radiat Oncol. 2019;14(21) doi: 10.1186/s13014-019-1227-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao M., Mohiuddin M.M., Hartsell W.F. Spatially fractionated (GRID) radiation therapy using proton pencil beam scanning (PBS): Feasibility study and clinical implementation. Med Phys. 2018 Apr;45(4):1645–1653. doi: 10.1002/mp.12807. [DOI] [PubMed] [Google Scholar]
- 27.Tsubouchi T., Henry T., Ureba A. A Quantitative evaluation of potential irradiation geometries for carbon-ion beam grid therapy. Med Phys. 2018 Mar;45(3):1210–1221. doi: 10.1002/mp.12749. [DOI] [PubMed] [Google Scholar]
- 28.Baker C.P., Curtis H.J., Zeman W., Woodley R.G. The design and calibration of a deuteron microbeam for biological studies. Radiat Res. 1961;15(4):489. [PubMed] [Google Scholar]
- 29.Slatkin D.N., Spanne P., Dilmanian F.A. Subacute neuropathological effects of microplanar beams of X-rays from a synchrotron wiggler. Proc Natl Acad Sci USA. 1995;92:8783–8787. doi: 10.1073/pnas.92.19.8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eling L., Bouchet A., Nemoz C. Ultra high dose rate Synchrotron Microbeam Radiation Therapy. Preclinical evidence in view of a clinical transfer. Radiother Oncol. 2019;S0167–8140(19) doi: 10.1016/j.radonc.2019.06.030. 32970–6.37. [DOI] [PubMed] [Google Scholar]
- 31.Grotzera M., Schültkeb E., Bräuer-Krischc J. Microbeam radiation therapy: clinical perspectives. Phys Med. 2015;31(6):P564–P567. doi: 10.1016/j.ejmp.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 32.Kausik Ray and Melissa Stick. 2015. Radiation and Health Effects. Handbook of Toxicology of Chemical Warfare Agents, 431–446.
- 33.Asur R., Butterworth K.T., Penagaricano J.A., Griffin R.J. High dose bystander effects in spatially fractionated radiation therapy. Cancer Lett. 2015 Jan 1;356(1):52–57. doi: 10.1016/j.canlet.2013.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharif S.F., Hariri R.J., Chang V.A. Human astrocyte production of tumor necrosis factor-alpha, interleukin-1 beta and interleukin-6 following exposure to lipopolysaccharide endotoxin. Neurol Res. 1993;15:109–116. doi: 10.1080/01616412.1993.11740119. [DOI] [PubMed] [Google Scholar]
- 35.Ivanov V.N., Hei T.K. A role for TRAIL/TRAIL-R2 in radiation-induced apoptosis and radiation-induced bystander response of human neural stem cells. Apoptosis. 2014 Mar;19(3):399–413. doi: 10.1007/s10495-013-0925-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sathishkumar S., Dey S., Meigooni A.S. The impact of TNF-alpha induction on therapeutic efficacy following high dose spatially fractionated (GRID) radiation. Technol Cancer Res Treat. 2002 Apr;1(2):141–147. doi: 10.1177/153303460200100207. [DOI] [PubMed] [Google Scholar]
- 37.Shareef M.M., Cui N., Burikhanov R. Role of tumor necrosis factor-alpha and TRAIL in high-dose radiation-induced bystander signaling in lung adenocarcinoma. Cancer Res. 2007;67:11811–11820. doi: 10.1158/0008-5472.CAN-07-0722. [DOI] [PubMed] [Google Scholar]
- 38.Garcia-Barros M., Paris F., Cordon-Cardo C. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science. 2003;300:1155–1159. doi: 10.1126/science.1082504. [DOI] [PubMed] [Google Scholar]
- 39.Sathishkumar S., Boyanovsky B., Karakashian A.A. Elevated sphingomyelinase activity and ceramide concentration in serum of patients undergoing high dose spatially fractionated radiation treatment: implications for endothelial apoptosis. Cancer Biol Ther. 2005 Sep;4(9):979–986. doi: 10.4161/cbt.4.9.1915. [DOI] [PubMed] [Google Scholar]
- 40.Denekamp J. Vascular endothelium as the vulnerable element in tumours. Acta Radiol Oncol. 1984;23(4):217–225. doi: 10.3109/02841868409136015. [DOI] [PubMed] [Google Scholar]
- 41.Song C.W., Lee Y.J., Cho L.C. Indirect Tumor Cell Death After High-Dose Hypofractionated Irradiation: implications for Stereotactic Body Radiation Therapy and Stereotactic Radiation Surgery. Int J Radiat Oncol Biol Phys. 2015 Sep 1;93(1):166–172. doi: 10.1016/j.ijrobp.2015.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Buchwald Z., Wynne J., Nasti T.H. Radiation, immune checkpoint blockade and the Abscopal effect: a critical review on timing. Dose Fract Front Onc. 2018 doi: 10.3389/fonc.2018.00612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peters M.E., Shareef M.M., Gupta S. Potential utilization of bystander/abscopal- mediated signal transduction events in the treatment of solid tumors. Curr Signal Transduct Ther. 2007;2:129–143. [Google Scholar]
- 44.Kanagavelu S., Gupta S., Wu X. In vivo effects of lattice radiation therapy on local and distant lung cancer: potential role of immunomodulation. Radiat Res. 2014 Aug;182(2):149–162. doi: 10.1667/RR3819.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Theelen W.S.M.E., Peulen H.M.U., Lalezari F. Effect of pembrolizumab after stereotactic body radiotherapy vs pembrolizumab alone on tumor response in patients with advanced non-small cell lung cancer: results of the PEMBRO-RT Phase 2 randomized clinical trial. JAMA Oncol. 2019 doi: 10.1001/jamaoncol.2019.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roberge D., Skamene T., Nahal A. Radiological and pathological response following pre-operative radiotherapy for soft-tissue sarcoma. Radiother Oncol. 2010;97(3):404–407. doi: 10.1016/j.radonc.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 47.Dilmanian F.A., Zhong Z., Bacarian T. Interlaced X-ray microplanar beams: a radiosurgery approach with clinical potential. Proc Natl Acad Sci USA. 2006;103(25):9709–9714. doi: 10.1073/pnas.0603567103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Poncelet B.P., Wedeen V.J., Weisskopf R.M. Brainparenchyma motion: measurement with cine echo-planar MR imaging. Radiology. 1992;185:645–651. doi: 10.1148/radiology.185.3.1438740. [DOI] [PubMed] [Google Scholar]
- 49.Prezado Y., Dos Santos M., Gonzalez W. Transfer of Minibeam Radiation Therapy into a cost-effective equipment for radiobiological studies: a proof of concept. Sci Rep. 2017 Dec 11;7(1):17295. doi: 10.1038/s41598-017-17543-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prezado Y., Jouvion G., Patriarca A. Proton minibeam radiation therapy widens the therapeutic index for high-grade gliomas. Sci Rep. 2018 Nov 7;8(1):16479. doi: 10.1038/s41598-018-34796-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dilmanian F.A., Eley J.G., Rusek A. Charged particle therapy with mini-segmented beams. Front Oncol. 2015 Dec;1(5):269. doi: 10.3389/fonc.2015.00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu J., Gensheimer M.F., Dong X. Robust intratumor partitioning to identify high-risk subregions in lung cancer: a pilot study. Int J Radiat Oncol Biol Phys. 2016 Aug 1;95(5):1504–1512. doi: 10.1016/j.ijrobp.2016.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yan D., Chen S., Krauss D.J. Tumor voxel dose-response matrix and dose prescription function derived using 18F-FDG PET/CT images for adaptive dose painting by number. Int J Radiat Oncol Biol Phys. 2019 May 1;104(1):207–218. doi: 10.1016/j.ijrobp.2019.01.077. [DOI] [PubMed] [Google Scholar]
- 54.Hamilton Richard. Air Power Studies Centre, Royal Australian Air Force; 1995. Precision guided munitions and the new era of warfare. [Google Scholar]
- 55.Onishi H., Shirato H., Nagata Y. Hypofractionated stereotactic radiotherapy (Hypo-FXSRT) for stage I non-small cell lung cancer: updated results of 257 patients in a Japanese multi-institutional study. J Thorac Oncol. 2007;2(7 Suppl 3):S94–S100. doi: 10.1097/JTO.0b013e318074de34. [DOI] [PubMed] [Google Scholar]
- 56.Kang K.H., Okoye C.C., Patel R.B. Complications from stereotactic body radiotherapy for lung cancer. Cancers (Basel) 2015 Jun 15;7(2):981–1004. doi: 10.3390/cancers7020820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Allibhai Z., Taremi M., Bezjak A. The impact of tumor size on outcomes after stereotactic body radiation therapy for medically inoperable early-stage non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2013 Dec 1;87(5):1064–1070. doi: 10.1016/j.ijrobp.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 58.Videtic G.M., Donington J., Giuliani M. Stereotactic body radiation therapy for early-stage non-small cell lung cancer: Executive Summary of an ASTRO Evidence- based guideline. Pract Radiat Oncol. 2017;7(5):295–301. doi: 10.1016/j.prro.2017.04.014. [DOI] [PubMed] [Google Scholar]
- 59.Neuner G. High-dose spatially fractionated GRID radiation therapy (SFGRT): a comparison of treatment outcomes with Cerrobend vs. MLC SFGRT. Int J Radiat Oncol Biol Phys. 2012;82(5):1642–1649. doi: 10.1016/j.ijrobp.2011.01.065. [DOI] [PubMed] [Google Scholar]
- 60.Edwards J. Definitive GRID and Fractionated Radiation in Bulky Head and Neck Cancer Associated With Low Rates of Distant Metastasis. Int J Radiat Oncol Biol Phys. 2015;93(3):E334. [Google Scholar]