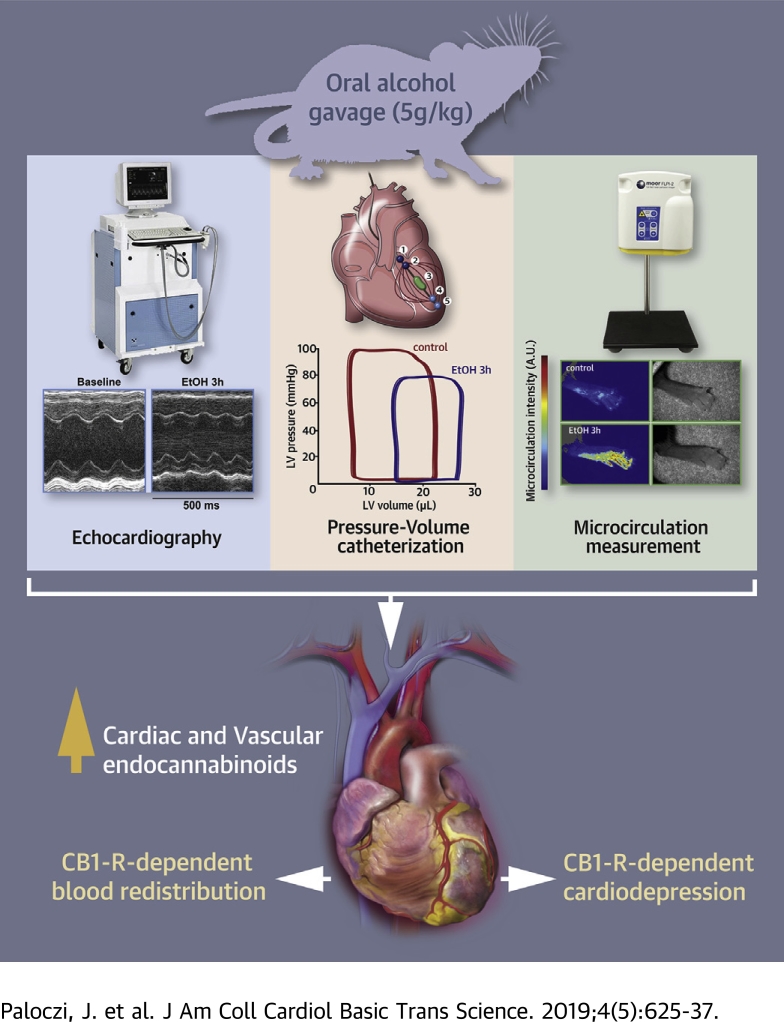
Visual Abstract
Key Words: binge alcohol drinking, cannabinoids, contractility, endocannabinoids
Abbreviations and Acronyms: 2-AG, 2-arachidonyl glycerol; AEA, anandamide; CB1-R (CB1), cannabinoid 1 receptor; CB2-R (CB2), cannabinoid 2 receptor; dP/dtmax, maximal slope of pressure increment; EF, ejection fraction; LV, left ventricle; MAP, mean arterial pressure; PRSW, preload recruitable stroke work; P-V, pressure-volume; TPR, total peripheral resistance
Highlights
-
•
Alcohol is one of the most frequently used intoxicants in the United States. Binge alcohol drinking is a major contributor of emergency department visits.
-
•
Binge alcohol drinking may adversely affect cardiovascular function.
-
•
Here we show that acute alcohol intoxication is associated with elevated levels of cardiac endocannabinoid anandamide and profound cardiovascular dysfunction and blood redistribution lasting for several hours.
-
•
The adverse cardiovascular effects of acute alcohol intoxication are attenuated by CB1-R antagonist or in CB1-R knockout mice.
-
•
A single alcohol binge has profound effect on the cardiovascular system, which involves endocannabinoid-CB1-R signaling.
Summary
Excessive binge alcohol drinking may adversely affect cardiovascular function. In this study we characterize the detailed hemodynamic effects of an acute alcohol binge in mice using multiple approaches and investigate the role of the endocannabinoid–cannabinoid 1 receptor (CB1-R) signaling in these effects. Acute alcohol binge was associated with elevated levels of cardiac endocannabinoid anandamide and profound cardiovascular dysfunction lasting for several hours and redistribution of circulation. These changes were attenuated by CB1-R antagonist or in CB1-R knockout mice. Our results suggest that a single alcohol binge has profound effects on the cardiovascular system, which involve endocannabinoid–CB1-R signaling.
Alcohol remains one of the most frequently used intoxicants in the United States, especially among younger individuals 1, 2. According to the National Epidemiologic Survey on alcoholism and related conditions, among 73% of human subjects aged 18 or older who drank, 46% consumed more than twice the number of drinks considered binge drinking at least once in the last year (1). Binge alcohol drinking, defined as having 4 or more drinks over 2 h on an occasion for women, or 5 or more drinks for men, is a major contributor of emergency department visits in the United States (1). There is also evidence that binge alcohol consumption can lead to development of life-threatening or fatal conditions (3). Apart from the severe indirect consequences, alcohol abuse has been shown to increase the risk of atrial fibrillation, acute myocardial infarction, and congestive heart failure to a similar degree as other well-known risk factors (4). Despite numerous studies investigating the effects of chronic moderate or excessive alcohol consumption on cardiovascular function and/or risk 4, 5, 6, 7, 8, 9, 10, relatively few reports have explored the cardiovascular effects of binge ethanol use and/or intoxication 11, 12, 13, 14, 15, 16, 17. Furthermore, these studies primarily focused on the determination of blood pressure or load- and/or heart rate–dependent indices of myocardial function measured by echocardiography (e.g., ejection fraction [EF]). Since alcohol is also known to time- and dose-dependently alter vascular function, heart rate, cardiac contractility, and peripheral resistance 10, 11, 12, 13, 14, 15, 16, 17, 18, reliance on load- and heart rate-dependent indices of contractile function may lead to inaccurate conclusions.
An increasing number of high school students and young adults have been reported to consume marijuana or synthetic cannabinoids [a mixture of potent cannabinoid 1 receptor (CB1-R) agonists 19, 20] for recreational purposes, which may induce deleterious cardiovascular effects via CB1-R–dependent mechanisms 21, 22, 23. Moreover, synthetic cannabinoids are often consumed together with alcoholic beverages, leading to more severe outcomes and longer hospital stays (24). In the cardiovascular system, CB1-R is expressed in cardiomyocytes, endothelial and smooth muscle cells, and fibroblasts 25, 26, 27, 28, as well as in the peripheral and central autonomic nervous systems (29). The effects of synthetic cannabinoids acting via CB1-R have been well documented and may have robust impact on the cardiovascular system, leading to abnormalities in cardiac inotropy, chronotropy, conduction, and vascular tone 21, 29, 30 [for review see Montecucco et al. (21) and Pacher et al. (29)]. Endocannabinoids do not appear to play a significant role in normal cardiovascular regulation, but when overproduced in various pathologic conditions (e.g., various forms of shock, cardiomyopathies, or heart failure and atherosclerosis) may contribute to hypotension, decreased cardiac contractility, and/or vascular inflammation and cell death through cardiovascular and macrophage CB1-R [for review see Montecucco et al. (21) and Pacher et al. (29)].
In this study, using multiple approaches and a well-established animal model, we aimed to identify and characterize the effects of acute alcohol intoxication on the cardiovascular system and to explore the potential role of the endocannabinoid–CB1-R signaling in mediating these effects. These results may have important implications for the management of the acute cardiovascular consequences of alcohol and mixed alcohol-synthetic cannabinoid intoxication.
Methods
Animals
Animal handling and usage were in accordance with the National Institutes of Health guidelines and experimental protocols were approved by our Institutional Animal Care and Use Committee of the National Institute on Alcohol Abuse and Alcoholism (Bethesda, Maryland). Eight- to 12-week-old male and female C57BL/6J mice were used (The Jackson Laboratory, Bar Harbor, Maine). NIAAA colony of male and female Cnr1−/− (CB1−/−) and Cnr2−/− (CB2−/−) mice on C57BL/6J background and their corresponding wild-type controls were also used in this study 31, 32. A total number of 198 mice were used for the described experiments.
Drugs
The CB1-R antagonist/inverse agonist rimonabant (SR141716) was purchased from Tocris (Tocris Bioscience, Bio-Techne, Minneapolis, Minnesota). Drugs were dissolved in dimethyl sulfoxide:Tocrisolve:saline solution at the ratio of 1:1:18 and vortexed to obtain a stable emulsion for bolus intravenous or intraperitoneal injections (26). Rimonabant was administered at the dose of 5 mg/kg.
Experimental design
In the first set of experiments, a single alcohol binge (31.5% vol/vol, given at the dose of 5 g/kg body weight) or isocaloric maltodextrin solution were given to mice following 12 h of fasting as previously described (33). Cardiac function and hemodynamics were assessed 3 or 12 h after alcohol binge.
In the second set of experiments, the CB1-R antagonist/inverse agonist rimonabant (5 mg/kg) or its vehicle were administered intravenously to both alcohol- and maltodextrin-binged mice 3 h after binge (peak of cardiovascular effects) and hemodynamics were assessed for at least 15 consecutive minutes until the drug response stabilized. In other cohorts, the acute hemodynamic effects of binge alcohol drinking in CB1−/− and CB2−/− mice or in their wild-type controls were also investigated 3 h after acute alcohol exposure.
Pressure-volume catheterization and echocardiography
Left ventricular (LV) performance was assessed by using pressure-volume (P-V) approach (34). Briefly, a P-V catheter equipped with a 1-F size microtip (PVR-1045, Millar Instruments, Houston, Texas) was inserted into the right carotid artery and advanced into the LV. Polyethylene cannulas were also introduced into the right femoral artery and left jugular vein to measure mean arterial pressure (MAP) and to administer drugs, respectively. After stabilization, P-V signals were continuously recorded by using the PowerLab data acquisition system (ADInstruments Inc, Colorado Springs, Colorado). Multiple indices of load-dependent and load-independent LV function, MAP and total peripheral resistance (TPR) were calculated as previously described (35). Echocardiography was performed as previously reported (36).
Arterial and microvascular blood flow measurements
Blood flow in both renal and superior mesenteric arteries were measured by using Transonic flow probes (MA0.5PSB and MA0.7PSB perivascular flowprobes, Transonic Systems Inc., Ithaca, New York) and were recorded and evaluated by using Powerlab LabChart (ADInstruments Inc., Colorado Springs, Colorado). Acral microcirculation was also assessed by using the laser speckle contrast imager moorFLPI-2 (Moor Instruments, Wilmington, Delaware).
Tissue endocannabinoid measurement
Myocardial levels of anandamide (AEA), 2-arachidonyl glycerol (2-AG) were quantified by liquid chromatography/in-line mass spectrometry, as previously described (26). Values are expressed as femtomoles or picomoles per milligram of wet tissue.
Statistical analysis
Results are expressed as mean ± SEM and plotted as bar graphs in all figures (see Figures 1, 2, 3, 4, 5, 6, 7, and 8 and Supplemental Figures 1 to 6, except for Supplemental Figures 2B [pre-load recruitable stroke work or PRSW], Supplemental Figure 3A [maximal slope of pressure increment or dP/dtmax], and Supplemental Figure 4C [TPR], whereas data are presented as median ± 25th, 75th percentiles). Statistical significance between groups was determined by Student's t test or in case of multiple groups by 1-way analysis of variance followed by Dunnett’s post hoc multiple comparison test to compare each group to control. Equality of variances was also examined by using either F-test of the equality of 2 variances or Brown-Forsythe test, respectively. If populations had different distribution, nonparametric tests (Mann-Whitney test for comparing 2 groups or Kruskal-Wallis test for multiple comparisons) were applied (Supplemental Figures 2B, 3A, and 4C) to analyze data. Statistical significance was assessed by using GraphPad Prism 7 software (San Diego, California). Probability values of p < 0.05 were considered significant.
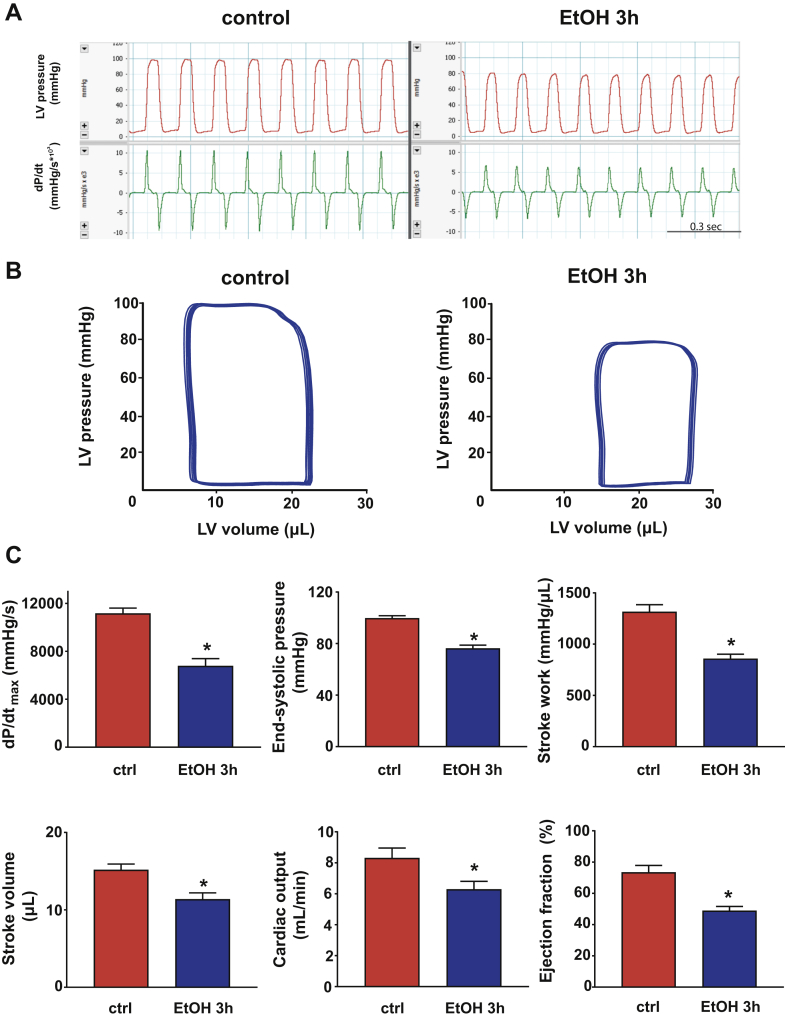
Figure 1.
Binge Alcohol Induces Left Ventricular Dysfunction
(A) Representative records of left ventricular (LV) pressure and its derivative (dP/dt) in control and alcohol-binged (EtOH 3h) mice, 3 h after maltodextrin or alcohol administration respectively. (B) Representative pressure-volume loops of control and alcohol-binged mice. (C) Systolic indices of LV performance [maximal slope of pressure increment (dP/dtmax), end-systolic pressure, stroke work, stroke volume, cardiac output and ejection fraction] in control (ctrl) and alcohol-binged (EtOH 3h) mice. Data are expressed as mean ± SEM, n = 7 to 8; *p < 0.05 vs. control group.
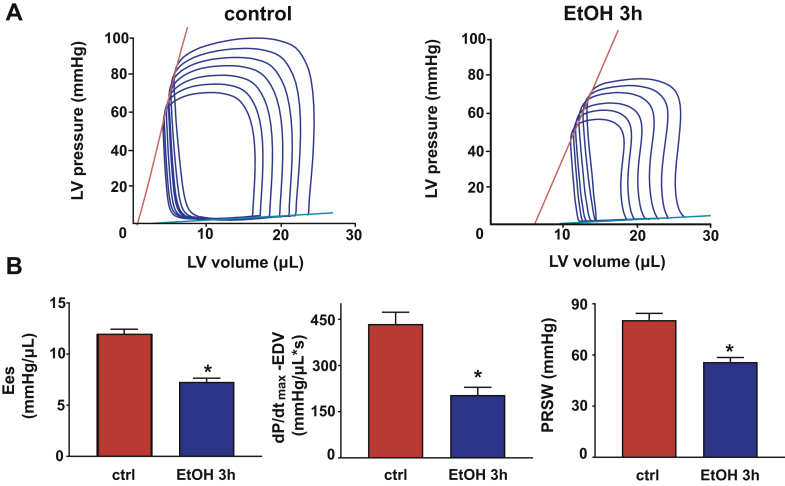
Figure 2.
Binge Alcohol Decreases Load-Independent Indices of Left Ventricular Function
(A) Pressure-volume (P-V) loops of control and alcohol-binged mice after gradual preload reduction obtained by vena cava inferior occlusion. Red lines indicate the slope of end-systolic P-V relationship; blue lines display the slope of end-diastolic P-V relationship. (B) Load-independent indices of LV performance: end-systolic elastance (Ees), the dP/dtmax-end-diastolic volume (EDV) relation and preload recruitable stroke work (PRSW). Data are expressed as mean ± SEM, n = 7 to 8; *p < 0.05 vs. control group. Abbreviation as in Figure 1.
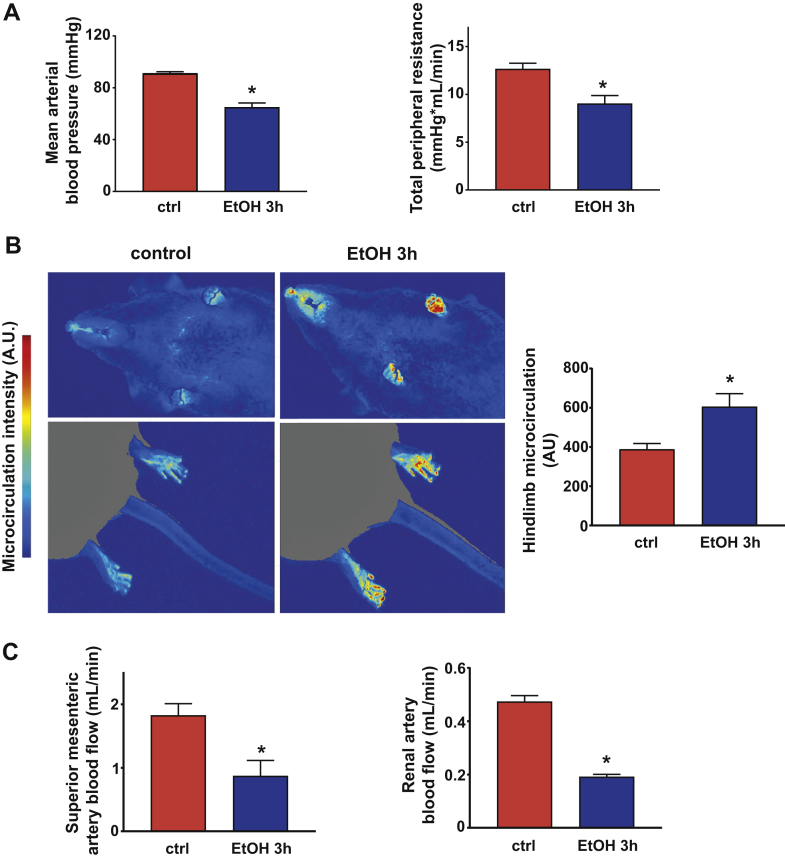
Figure 3.
Binge Alcohol Induces Vascular Dysfunction, Blood Redistribution, and Increased Cardiac Anandamide Levels
(A) Mean arterial blood pressure and total peripheral resistance measured in control and alcohol-binged (EtOH 3h) mice 3 h after maltodextrin or alcohol administration, respectively. (B) Representative images of the jowl and hindlimb microcirculation in control and alcohol-binged mice. Red color indicates more intense, whereas blue color represents lower microcirculation. Right panel shows data on hindlimb microcirculation in control and alcohol-binged mice. (C) Superior mesenteric artery and renal artery blood flow changes in control and alcohol-binged mice. Data are expressed as mean ± SEM, n = 6 to 8; *p < 0.05 vs. control group. Abbreviation as in Figure 1.
Figure 4.
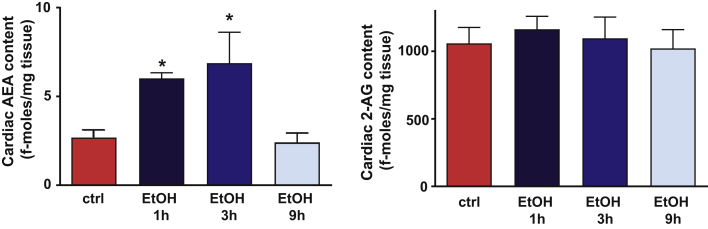
Binge Alcohol Increases Cardiac Anandamide Level
Cardiac levels of anandamide (AEA) and 2-arachidonyl glycerol (2-AG) measured in control and alcohol-binged mice. Data are expressed as mean ± SEM, n = 5 to 6; *p < 0.05 vs. control group. Abbreviations as in Figure 1.
Figure 5.
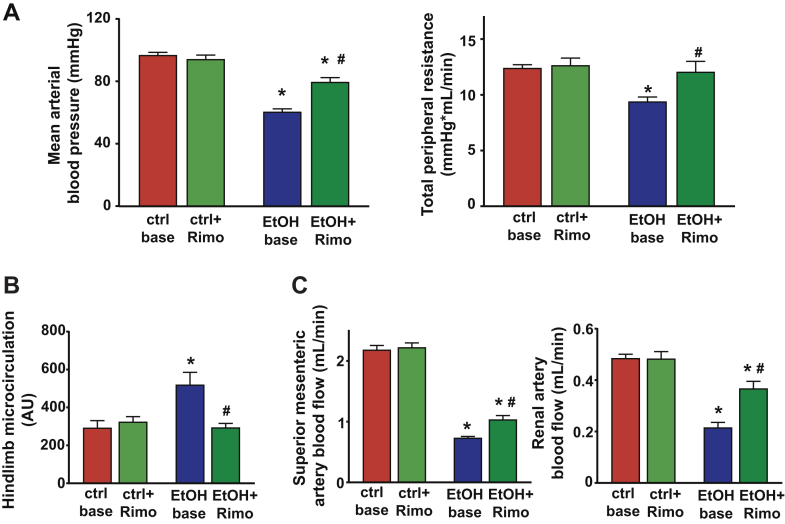
Cannabinoid 1 receptor Antagonist Attenuates the Binge Alcohol-Induced Vascular Changes
(A) Mean arterial blood pressure and total peripheral resistance measured after 3 h in control or in alcohol-binged mice before (ctrl base and EtOH base, respectively) and 15 min after intravenous rimonabant (Rimo) (ctlr + Rimo and EtOH + Rimo, respectively) administration. (B) Hindlimb microcirculation in control or in alcohol-binged mice before (ctrl base and EtOH base, respectively) and 15 min after intravenous Rimo. (C) Superior mesenteric artery and renal artery blood flow changes in control and in alcohol-binged mice before the treatment (ctrl base and EtOH base, respectively) and 15 min after intravenous Rimo (ctlr + Rimo and EtOH + Rimo, respectively) administration. Data are expressed as mean ± SEM, n = 5 to 7; *p < 0.05 vs. corresponding control group; #p < 0.05 vs. EtOH base group. CB1-R = cannabinoid 1 receptor; other abbreviations as in Figure 1.
Figure 6.
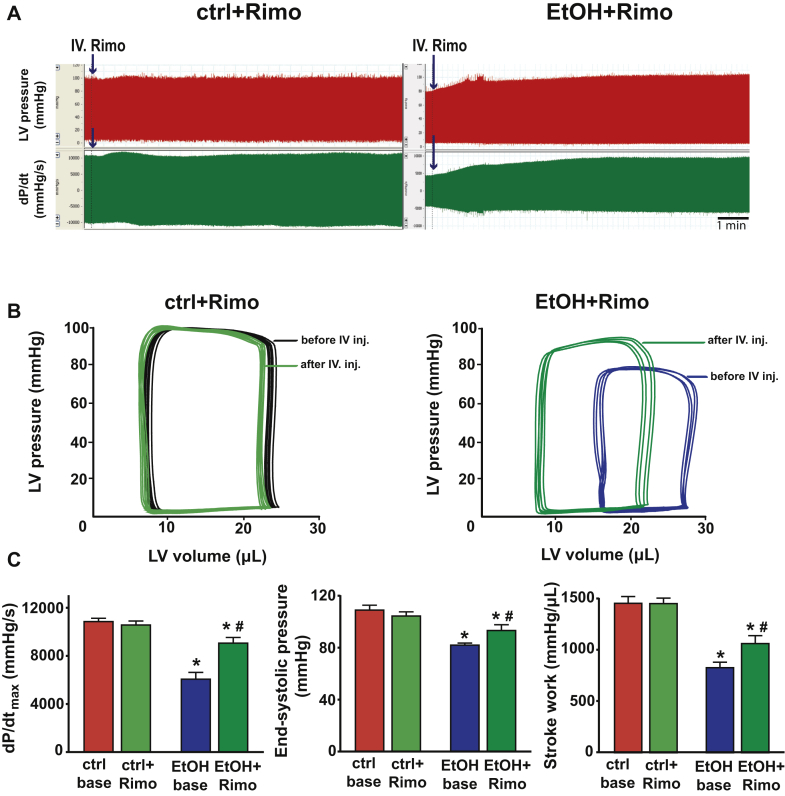
CB1-R Blockade Attenuates the Binge Alcohol-Induced Contractile Dysfunction
(A) Representative records of LV blood pressure changes and dP/dt in mice 3 h after either maltodextrin or alcohol binge alcohol binge following intravenous rimonabant (Rimo) (EtOH + Rimo) administration. (B) Representative pressure-volume (P-V) loops of maltodextrin and alcohol-binged mice before (red and dark blue, respectively) and 15 min after intravenous Rimo (ctrl + Rimo and EtOH + Rimo, light green and dark green loops, respectively) injection. (C) Systolic indices of LV performance [maximal slope of pressure increment (dP/dtmax), end-systolic pressure, stroke work] in control and in alcohol-binged mice before (ctrl base and EtOH base, respectively) and 15 min after intravenous Rimo (ctlr + Rimo and EtOH + Rimo, respectively) administration. Data are expressed as mean ± SEM, n = 6 to 8; *p < 0.05 vs. corresponding control group; #p < 0.05 vs. EtOH base group. Abbreviations as in Figures 1 and 5.
Figure 7.
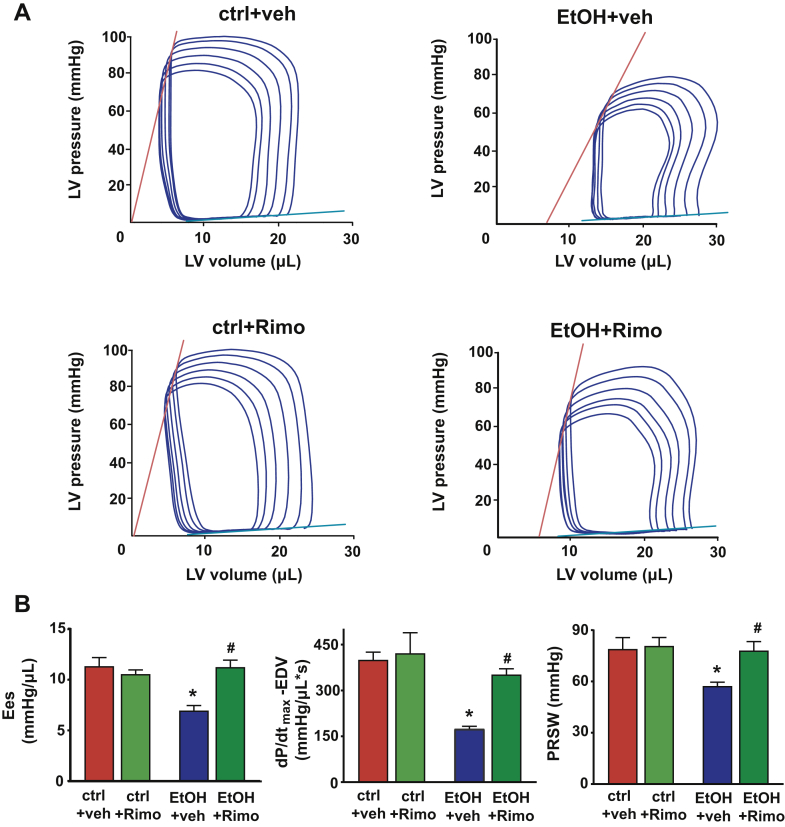
CB1-R blockade attenuates the binge alcohol induced depression of load-independent indices of left ventricular contractile function
(A) Representative P-V loops obtained by gradual preload reduction obtained by vena cava occlusion of vehicle (veh) (ctrl + veh and EtOH + veh) or Rimo (ctrl + Rimo and EtOH + Rimo) treated mice 3 h after either oral alcohol or maltodextrin administration, respectively. Red lines indicate the slope of end-systolic P-V relationship, whereas blue lines depict the slope of end-diastolic P-V relationship (B) Load-independent indices of LV performance (Ees, the dP/dtmax–end-diastolic volume (EDV) relation, and PRSW) in vehicle-treated control or alcohol-binged mice (ctrl base, or EtOH base, respectively); or in rimonabant (Rimo)-treated control or alcohol-binged mice (ctlr + Rimo, or EtOH + Rimo, respectively). Data are expressed as mean ± SEM, n = 5 to 6; *p < 0.05 vs. corresponding control group; #p < 0.05 vs. EtOH base group. Abbreviations as in Figures 1, 2, 5, and 6.
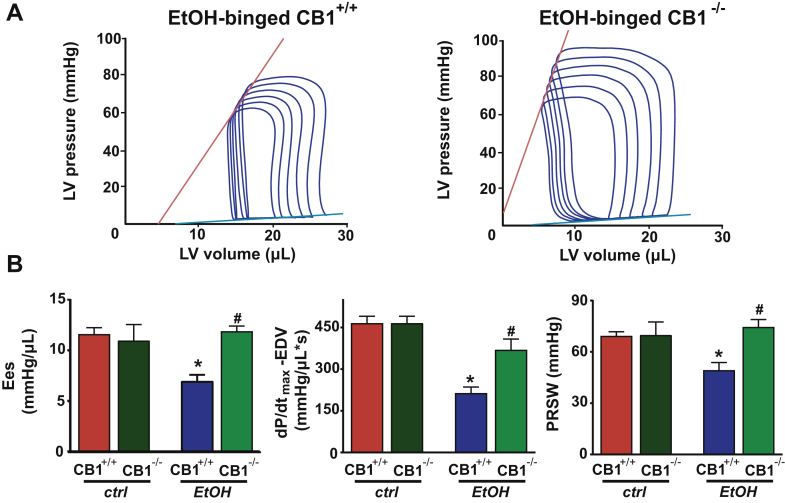
Figure 8.
CB1-R Deletion Attenuates the Binge Alcohol Intoxication-Induced Cardiac Dysfunction
(A) Representative P-V loops of alcohol-binged CB1+/+ and CB1-/- mice after gradual preload reduction obtained by vena cava occlusion. Red lines indicate the slope of end-systolic P-V relationship, whereas blue lines show the slope of end-diastolic P-V relationship. (B) Load-independent indices of LV performance (Ees, the dP/dtmax – EDV relation, and PRSW) in control or in alcohol-binged CB1+/+ and CB1-/- mice. Data are expressed as mean ± SEM, n = 5 to 6; *p < 0.05 vs. corresponding control group; #p < 0.05 vs. alcohol-binged CB1+/+ group. Abbreviations as in Figures 1, 2, 5, and 6.
Results
A single alcohol binge induces cardiac dysfunction and redistribution of circulation
Alcohol binge or intoxication may adversely impact both cardiac and vascular function (see introduction); therefore, we investigated the acute effects of alcohol binge on both load-dependent and load-independent indices of cardiac contractility (using echocardiography and P-V approaches) as well as on vascular blood flow in mesenteric and renal arteries and on skin microcirculation (using Transonic flow probes and/or laser speckle analysis) in vivo in mice.
In the first set of experiments, we attempted to characterize the time course of the effect of an acute alcohol binge on cardiovascular function and blood redistribution (Figures 1, 2, and 3; Supplemental Figures 1 to 3). First, we analyzed the effects of alcohol binge on cardiac function by echocardiography (Supplemental Figure 1). Alcohol-induced transient reduction of stroke volume and cardiac output peaked around 3 h following the binge and largely recovered after 12 h (Supplemental Figure 1). An acute binge had no significant effect on EF and fractional shortening measured by echocardiography. Since the latter parameters could be affected by loading conditions, which we hypothesized to change by alcohol binge, we further analyzed cardiac function using the P-V approach (35), allowing determination of load-independent indices of LV contractile function. In parallel, we also determined peripheral circulation by measuring blood flow in superior mesenteric and renal arteries of mice by using Transonic flow probes and acral area and hindlimb microcirculation by laser-speckle imaging; the MAP and TPR were also calculated.
P-V analysis revealed marked depression of both load-dependent (Figure 1) and load-independent (Figure 2) indices of LV contractile function 3 h following an acute alcohol binge, which largely recovered by 12 h (Supplemental Figure 2). Acute alcohol binge also induced decrease of the MAP and TPR (Figure 3A), a consequence of decreased cardiac function (Figures 1 and 2) and marked redistribution of circulation (acral area and hindlimb vasodilation) (Figure 3B), and decreased mesenteric and renal blood flow (Figure 3C). There was no difference in the hemodynamic response to acute alcohol binge in male and female mice (Supplemental Figure 3).
A single alcohol binge increases myocardial anandamide levels
Because numerous previous studies have shown that tissue injury and associated oxidative stress and inflammation can increase endocannabinoid levels in almost all organ systems and cell types (including myocardium, cardiomyocytes, activated endothelium, and inflammatory cells) and that endocannabinoid–CB1-R signaling promotes atherosclerosis and contributes to cardiovascular depressive state in various forms of shock, cardiomyopathies, heart failure, and liver cirrhosis 21, 29, we hypothesized that this signaling may also contribute to the acute cardiovascular effects of alcohol. We found that acute alcohol binge exposure time-dependently increased myocardial endocannabinoid AEA, but not 2-AG levels, peaking 3 h following acute alcohol administration (Figure 4) when the cardiac and vascular effects of alcohol were the most pronounced (Figures 1, 2, and 3). Myocardial AEA levels returned to baseline 9 h after alcohol ingestion (Figure 3D) in parallel with the recovery of alcohol-induced cardiovascular dysfunction.
CB1-R signaling contributes to alcohol-induced acute cardiovascular effects
Because AEA is the endocannabinoid primarily exerting its effects on CB1-R, next we investigated the role of CB1-R signaling in the alcohol-induced cardiovascular effects by using a CB1-R antagonist/inverse agonist rimonabant and CB1-R knockout mice. Intravenous administration of rimonabant rapidly improved the 3-h alcohol intoxication-induced decrease of MAP, TPR, and superior mesenteric and renal artery blood flow, and attenuated the alcohol-induced peripheral vasodilation (hindlimb microcirculation) (Figures 5A to 5C). Rimonabant also markedly improved the alcohol binge–induced depression of load-dependent (Figures 6A to 6C) and load-independent (Figures 7A and 7B) indices of LV systolic function without exerting any effect in controls. The vehicle used had no hemodynamic effects in control or alcohol-binged groups (Supplemental Figure 4). The marked alcohol-induced depression of load-independent indices of LV systolic function 3 h following the alcohol binge were also largely attenuated in CB1-R knockout mice (Figure 8). CB1-R deletion was also associated with improved load-dependent indices of LV contractile function as well as better preservation of blood pressure and TPR 3 h following an acute alcohol binge (Supplemental Figure 5). In contrast, CB2-R deletion had no significant effects on the alcohol intoxication-induced cardiovascular effects (Supplemental Figure 6). These results indicated that endocannabinoid–CB1-R, but not CB2-R signaling contributes to the acute hemodynamic effects of alcohol binge.
Discussion
In the present study, we characterized in detail the binge alcohol intoxication-induced hemodynamic alterations (cardiac and peripheral effects) in mice in vivo by using echocardiography, the state-of-the-art pressure-volume catheterization, ultrasound flow probes, and laser speckle contrast approach.
The results of the current investigation provide several unique findings worthy of consideration. At first, acute alcohol binge intoxication in mice induced a profound decrease in LV contractile function accompanied with marked redistribution of peripheral circulation leading to decreased TPR and MAP, which peaked around 3 h following acute alcohol exposure and recovered 12 h later. Secondly, these hemodynamic effects were paralleled by time-dependent increases in myocardial endocannabinoid AEA levels. Thirdly, the acute inhibition of CB1-R with rimonabant dramatically improved alcohol-induced cardiac dysfunction, whereas this effect was widely diminished in CB1-R, but not CB2-R, knockout mice. Such observations strongly suggest a critical role of CB1-R activation of the peripheral vessels and the myocardium in mediating a hypotensive and cardiodepressive response to acute alcohol binge intoxication.
Heavy episodic alcohol drinking (also known as binge drinking) is a serious health problem in the United States (2). According to the latest surveys (1) level I binge drinking is defined as 4 to 7 drinks for women, and 5 to 9 drinks for men; level II as 8 to 11 drinks for women and 10 to 14 drinks for men; and level III as 12 or more drinks for women and 15 or more drinks for men on a single occasion. Alcohol drinking at levels II and III is considered extreme binge drinking and is associated with increased number of alcohol-related emergency department visits as compared to non-binge drinkers (1).
In this study, we used a single oral administration of an established dose of alcohol (5 g/kg) to mimic excessive human binge drinking (33). Consistent with previous reports using echocardiography, we found an alcohol-induced transient reduction of stroke volume and cardiac output with minimal effect on EF and fractional shortening (Supplemental Figure 1). However, under altered loading conditions, heart rate assessment of cardiac function by using echocardiography is challenging and may lead to a variety of interpretation of the results (35). We used multiple additional approaches including measurements of renal and superior mesenteric arterial flow and acral microcirculation by flow probes and/or laser speckle imaging combined with P-V analysis and found that excessive alcohol binge led to a profound redistribution of peripheral circulation (decreased mesenteric and renal blood flow and increased acral microcirculation) as well as a decrease in myocardial contractile function. The alcohol-induced decrease of mesenteric and renal blood flow can be the consequence of either the activation of compensatory mechanisms (e.g., activation of the sympathetic nervous system) to counteract the peripheral vasodilation, decrease in blood pressure, and cardiac output; however, this can also be the consequence of markedly reduced cardiac output. Based on our results, the vasodilation induced by binge alcohol exposure may ultimately lead to alteration of loading conditions affecting cardiac output and macrovascular function, at least in part, via CB1-R–dependent mechanism. Furthermore, the alcohol-induced activation of myocardial AEA–CB1-R signaling may also decrease LV intrinsic contractility (determined by evaluation of load- and heart rate–independent indices of LV function) in vivo, further attenuating mean blood pressure and perhaps mesenteric and renal blood flow. These hemodynamic effects were peaking 3 h following the alcohol exposure (not different in male and female mice) and returned to baseline levels 12 h later. Similarly to our results, toxic doses of alcohol induced hypotension, decrease of TPR, and cardiac dysfunction in dogs (13). Human studies reported endothelial dysfunction and increases or biphasic effects on systolic blood pressure following binge alcohol consumption 14, 16, 37. In these studies, a relatively modest dose of alcohol was used.
Similar to the effects of binge alcohol drinking, increased endocannabinoid/CB1-R signaling has also been implicated in the impaired cardiac contractility associated with advanced liver cirrhosis 38, 39. As liver cirrhosis often develops on a background of chronic alcoholism, the impairment of cardiac contractility may be compounded in such cases. It has been also documented that binge alcohol drinking induces acute myocardial injury, and increases myocardial lipid peroxidation and protein carbonylation in mice (40). Oxidative stress has been implicated in triggering endocannabinoid production in parenchymal tissues 26, 32 contributing to depression of cardiac function, vasodilation, or vasoconstriction 26, 29, 41. In this study we show that acute alcohol exposure is associated with time-dependent increases in myocardial endocannabinoid AEA levels, which correlate with cardiac dysfunction. We also show that intravenous administration of CB1-R antagonist rimonabant 3 h after binge alcohol administration (at a peak of the cardiodepressive effects) significantly improves cardiac performance as well as redistribution of circulation, indicating an important role of the CB1-R signaling in these pathologic effects, whereas rimonabant had no effects in control mice (Figures 5, 6, and 7). This is further substantiated by the resistance of CB1-/- mice to alcohol intoxication-induced hemodynamic effects (Figure 8).
Besides alcohol, synthetic cannabinoid abuse is an emerging risk for youth with documented severe adverse cardiovascular consequences mediated by cardiovascular CB1-R (29). Synthetic cannabinoids are often consumed together with alcoholic beverages for recreational use, leading to more severe outcomes 24, 42.
Study limitations
The in vivo study was performed in mice and mice known to metabolize alcohol faster and less sensitive to toxic effects of ethanol compared to dogs or humans. The hemodynamic effects of alcohol intoxication are complex and most likely also confounded by the time-dependent activation of various compensatory mechanism (e.g., sympathetic nervous system). In our study we performed detailed hemodynamic analysis only 3 and 12 h following the acute alcohol intoxication.
Conclusions
Collectively, we show that excessive alcohol binge exerts complex and profound cardiovascular effects with transient (lasting for several hours) marked depression of myocardial contractile function. This may represent a problem in individuals with pre-existing cardiovascular disease, chronic alcohol consumption, or regular cannabis use (29). We also show that these hemodynamic effects involve endocannabinoid–CB1-R signaling, suggesting that repurposing CB1-R antagonists for combined alcohol-synthetic cannabinoid intoxication may save lives.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Excessive binge alcohol drinking has adverse effects on cardiovascular function. Activation of CB1-R signaling is a crucial step towards the development of acute alcohol intoxication-induced adverse cardiovascular events.
TRANSLATIONAL OUTLOOK: Since there is an increasing prevalence of alcohol ingestion alone, or in combination with synthetic cannabinoid use leading to adverse cardiovascular effects in adolescents, this emerging threat raises major health concerns, and targeting CB1-R signaling may evolve as an important therapeutic option in such conditions.
Footnotes
This study was supported by the Intramural Research Program of NIH/NIAAA to Dr. Pacher. The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
Appendix
References
- 1.Hingson R.W., Zha W., White A.M. Drinking beyond the binge threshold: predictors, consequences, and changes in the U.S. Am J Prev Med. 2017;52:717–727. doi: 10.1016/j.amepre.2017.02.014. [DOI] [PubMed] [Google Scholar]
- 2.Wechsler H., Nelson T.F. What we have learned from the Harvard School Of Public Health College Alcohol Study: focusing attention on college student alcohol consumption and the environmental conditions that promote it. J Stud Alcohol Drugs. 2008;69:481–490. doi: 10.15288/jsad.2008.69.481. [DOI] [PubMed] [Google Scholar]
- 3.Hingson R.W., Zha W., Weitzman E.R. Magnitude of and trends in alcohol-related mortality and morbidity among U.S. college students ages 18-24, 1998–2005. J Stud Alcohol Drugs Suppl. 2009:12–20. doi: 10.15288/jsads.2009.s16.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whitman I.R., Agarwal V., Nah G. Alcohol abuse and cardiac disease. J Am Coll Cardiol. 2017;69:13–24. doi: 10.1016/j.jacc.2016.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodrigues P., Santos-Ribeiro S., Teodoro T. Association between alcohol intake and cardiac remodeling. J Am Coll Cardiol. 2018;72:1452–1462. doi: 10.1016/j.jacc.2018.07.050. [DOI] [PubMed] [Google Scholar]
- 6.Leifer E.S. The risks and benefits of moderate alcohol consumption: there remains much to learn. J Am Coll Cardiol HF. 2017;5:845–847. doi: 10.1016/j.jchf.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 7.Collaborators GBDA Alcohol use and burden for 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2018;392:1015–1035. doi: 10.1016/S0140-6736(18)31310-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piano M.R. Alcohol's effects on the cardiovascular system. Alcohol Res. 2017;38:219–241. [PMC free article] [PubMed] [Google Scholar]
- 9.Xi B., Veeranki S.P., Zhao M., Ma C., Yan Y., Mi J. Relationship of alcohol consumption to all-cause, cardiovascular, and cancer-related mortality in U.S. adults. J Am Coll Cardiol. 2017;70:913–922. doi: 10.1016/j.jacc.2017.06.054. [DOI] [PubMed] [Google Scholar]
- 10.Voskoboinik A., Prabhu S., Ling L.H., Kalman J.M., Kistler P.M. Alcohol and atrial fibrillation: a sobering review. J Am Coll Cardiol. 2016;68:2567–2576. doi: 10.1016/j.jacc.2016.08.074. [DOI] [PubMed] [Google Scholar]
- 11.Bottoms G.D., Fessler J.F., Johnson M., Coatney R.W., Voorhees W. Effects of acute alcohol intake on tolerance to hypotension. Alcohol Clin Exp Res. 1990;14:776–780. doi: 10.1111/j.1530-0277.1990.tb01245.x. [DOI] [PubMed] [Google Scholar]
- 12.Kelbaek H., Gjorup T., Hartling O.J., Marving J., Christensen N.J., Godtfredsen J. Left ventricular function during alcohol intoxication and autonomic nervous blockade. Am J Cardiol. 1987;59:685–688. doi: 10.1016/0002-9149(87)91193-3. [DOI] [PubMed] [Google Scholar]
- 13.Webb W.R., Degerli I.U. Ethyl Alcohol and the cardiovascular system: effects on coronary blood flow. JAMA. 1965;191:1055–1058. doi: 10.1001/jama.1965.03080130015004. [DOI] [PubMed] [Google Scholar]
- 14.Rosito G.A., Fuchs F.D., Duncan B.B. Dose-dependent biphasic effect of ethanol on 24-h blood pressure in normotensive subjects. Am J Hypert. 1999;12:236–240. doi: 10.1016/s0895-7061(98)00237-4. [DOI] [PubMed] [Google Scholar]
- 15.Goslawski M., Piano M.R., Bian J.T., Church E.C., Szczurek M., Phillips S.A. Binge drinking impairs vascular function in young adults. J Am Coll Cardiol. 2013;62:201–207. doi: 10.1016/j.jacc.2013.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bau P.F., Bau C.H., Naujorks A.A., Rosito G.A. Early and late effects of alcohol ingestion on blood pressure and endothelial function. Alcohol. 2005;37:53–58. doi: 10.1016/j.alcohol.2005.10.034. [DOI] [PubMed] [Google Scholar]
- 17.Gardner J.D. Alcohol binge drinking: getting to the heart of it. Am J Physiol Heart Circ Physiol. 2016;310:H1606–H1607. doi: 10.1152/ajpheart.00368.2016. [DOI] [PubMed] [Google Scholar]
- 18.Tsutsui M., Matsuguchi T., Tsutsui H. Alcohol-induced sinus bradycardia and hypotension in patients with syncope. Jpn Heart J. 1992;33:875–879. doi: 10.1536/ihj.33.875. [DOI] [PubMed] [Google Scholar]
- 19.Patrick M.E., O'Malley P.M., Kloska D.D. Novel psychoactive substance use by US adolescents: characteristics associated with use of synthetic cannabinoids and synthetic cathinones. Drug Alcohol Rev. 2016;35:586–590. doi: 10.1111/dar.12372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McKeever R.G., Vearrier D., Jacobs D., LaSala G., Okaneku J., Greenberg M.I. K2—not the spice of life; synthetic cannabinoids and ST elevation myocardial infarction: a case report. J Med Toxicol. 2015;11:129–131. doi: 10.1007/s13181-014-0424-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Montecucco F., Di Marzo V. At the heart of the matter: the endocannabinoid system in cardiovascular function and dysfunction. Trends Pharmacol Sci. 2012;33:331–340. doi: 10.1016/j.tips.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 22.Andonian D.O., Seaman S.R., Josephson E.B. Profound hypotension and bradycardia in the setting of synthetic cannabinoid intoxication — a case series. Am J Emerg Med. 2017;35:940 e5–940 e6. doi: 10.1016/j.ajem.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 23.Kane E.M., Hinson J.S., Jordan C.D. Bradycardia and hypotension after synthetic cannabinoid use: a case series. Am J Emerg Med. 2016;34 doi: 10.1016/j.ajem.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 24.Palamar J.J., Acosta P. Synthetic cannabinoid use in a nationally representative sample of US high school seniors. Drug Alcohol Depend. 2015;149:194–202. doi: 10.1016/j.drugalcdep.2015.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Batkai S., Pacher P., Osei-Hyiaman D. Endocannabinoids acting at cannabinoid-1 receptors regulate cardiovascular function in hypertension. Circulation. 2004;110:1996–2002. doi: 10.1161/01.CIR.0000143230.23252.D2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mukhopadhyay P., Batkai S., Rajesh M. Pharmacological inhibition of CB1 cannabinoid receptor protects against doxorubicin-induced cardiotoxicity. J Am Coll Cardiol. 2007;50:528–536. doi: 10.1016/j.jacc.2007.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Valenta I., Varga Z.V., Valentine H. Feasibility evaluation of myocardial cannabinoid type 1 receptor imaging in obesity: a translational approach. J Am Coll Cardiol Img. 2018;11:320–332. doi: 10.1016/j.jcmg.2017.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rajesh M., Mukhopadhyay P., Hasko G., Liaudet L., Mackie K., Pacher P. Cannabinoid-1 receptor activation induces reactive oxygen species–dependent and –independent mitogen-activated protein kinase activation and cell death in human coronary artery endothelial cells. Br J Pharmacol. 2010;160:688–700. doi: 10.1111/j.1476-5381.2010.00712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pacher P., Steffens S., Haskó G., Schindler T.H., Kunos G. Cardiovascular effects of marijuana and synthetic cannabinoids: the good, the bad, and the ugly. Nat Rev Cardiol. 2017;15:151. doi: 10.1038/nrcardio.2017.130. [DOI] [PubMed] [Google Scholar]
- 30.Pacher P., Batkai S., Kunos G. Cardiovascular pharmacology of cannabinoids. Handb Exp Pharmacol. 2005:599–625. doi: 10.1007/3-540-26573-2_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mukhopadhyay P., Rajesh M., Batkai S. CB1 cannabinoid receptors promote oxidative stress and cell death in murine models of doxorubicin-induced cardiomyopathy and in human cardiomyocytes. Cardiovasc Res. 2010;85:773–784. doi: 10.1093/cvr/cvp369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Batkai S., Osei-Hyiaman D., Pan H. Cannabinoid-2 receptor mediates protection against hepatic ischemia/reperfusion injury. FASEB J. 2007;21:1788–1800. doi: 10.1096/fj.06-7451com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bertola A., Mathews S., Ki S.H., Wang H., Gao B. Mouse model of chronic and binge ethanol feeding (the NIAAA model) Nat Protoc. 2013;8:627–637. doi: 10.1038/nprot.2013.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pacher P., Batkai S., Osei-Hyiaman D. Hemodynamic profile, responsiveness to anandamide, and baroreflex sensitivity of mice lacking fatty acid amide hydrolase. Am J Physiol Heart Circ Physiol. 2005;289:H533–H541. doi: 10.1152/ajpheart.00107.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pacher P., Nagayama T., Mukhopadhyay P., Batkai S., Kass D.A. Measurement of cardiac function using pressure-volume conductance catheter technique in mice and rats. Nat Protoc. 2008;3:1422–1434. doi: 10.1038/nprot.2008.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matyas C., Varga Z.V., Mukhopadhyay P. Chronic plus binge ethanol feeding induces myocardial oxidative stress, mitochondrial and cardiovascular dysfunction, and steatosis. Am J Physiol Heart Circ Physiol. 2016;310:H1658–H1670. doi: 10.1152/ajpheart.00214.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hillbom M., Saloheimo P., Juvela S. Alcohol consumption, blood pressure, and the risk of stroke. Curr Hyperten Rep. 2011;13:208–213. doi: 10.1007/s11906-011-0194-y. [DOI] [PubMed] [Google Scholar]
- 38.Gaskari S.A., Liu H., Moezi L., Li Y., Baik S.K., Lee S.S. Role of endocannabinoids in the pathogenesis of cirrhotic cardiomyopathy in bile duct-ligated rats. Br J Pharmacol. 2005;146:315–323. doi: 10.1038/sj.bjp.0706331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Batkai S., Mukhopadhyay P., Harvey-White J., Kechrid R., Pacher P., Kunos G. Endocannabinoids acting at CB1 receptors mediate the cardiac contractile dysfunction in vivo in cirrhotic rats. Am J Physiol Heart Circ Physiol. 2007;293:H1689–H1695. doi: 10.1152/ajpheart.00538.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kannan M., Wang L., Kang Y.J. Myocardial oxidative stress and toxicity induced by acute ethanol exposure in mice. Exp Biol Med (Maywood) 2004;229:553–559. doi: 10.1177/153537020422900614. [DOI] [PubMed] [Google Scholar]
- 41.Pacher P., Batkai S., Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev. 2006;58:389–462. doi: 10.1124/pr.58.3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Castaneto M.S., Gorelick D.A., Desrosiers N.A., Hartman R.L., Pirard S., Huestis M.A. Synthetic cannabinoids: epidemiology, pharmacodynamics, and clinical implications. Drug Alcohol Depend. 2014;144:12–41. doi: 10.1016/j.drugalcdep.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.