Highlights
-
•
Conducted a review of the worldwide occurrence of aflatoxins in rice and associated health effects.
-
•
High variability in aflatoxin contamination and detection levels in rice has been observed.
-
•
In some sub-Saharan and Asian countries, humans are exposed to aflatoxins.
-
•
A good agricultural and manufacturing practice may help to reduce the aflatoxin level in rice.
-
•
Human biomonitoring can be an alternative approach to assess human exposure to aflatoxins.
Keywords: Aflatoxins, Rice, Occurrence, Biomarker, Exposure, Human health
Abstract
Aflatoxins are fungal secondary metabolites that contaminate dietary staples worldwide, including maize, rice and groundnuts. Dietary exposure to aflatoxins is a public health concern due to their carcinogenic, acute and chronic effects. Rice is an important staple food consumed widely and consists of a major part of the diets for half of the world population. Human exposure to these mycotoxins is a serious problem especially in developing countries where hot and humid climates favor the fungal growth and where food storage conditions are poor and lack of regulatory limits enforcement. The recent developments of biomarkers have provided opportunities in assessing aflatoxins exposure and related health effects in the high-risk population groups. This review describes the worldwide occurrence of aflatoxins in rice during the period from 1990 to 2015 and biomarkers-based evidence for human exposure to aflatoxins and their adverse health effects. Aflatoxin is a potent hepatocarcinogen and humans may expose to it at any stage of life. Epidemiological studies reported an association between aflatoxin intake and the incidence of hepatocellular carcinoma in some sub-Saharan and Asian countries. Even daily high intake of rice with a low level of contamination is of health concern. Thus, it is necessary to implement effective strategies to prevent contamination and fungal growth in rice. A good agricultural and manufacturing practice should be applied during handling, storage and distribution of rice to ensure that aflatoxins contamination level is lower in the final product. Moreover, a regular survey for aflatoxins occurrence in rice and biomarkers-based studies is recommended to prevent and reduce the adverse health effects in the world population.
1. Introduction
Aflatoxins are a family of toxins produced by Aspergillus species (mainly A. flavus and A. parasiticus) that contaminate cereals and dietary staples, including maize, rice and groundnuts [1,2]. These fungi are widely distributed in agriculture and highly prevalent in tropical regions specifically sub-Saharan Africa and South East Asia, where hot and humid climates favor fungal growth on food commodities [3]. The four major aflatoxins are aflatoxin B1 (AFB1), aflatoxin B2 (AFB2), aflatoxin G1 (AFG1), and aflatoxin G2 (AFG2). Aflatoxin M1 (AFM1) is a less toxic metabolite of AFB1 produced in farm animals that consume aflatoxins contaminated feed. AFB1 is the most occurring one and has been identified as the group-1 hepatocarcinogen in animals and humans [2]. The occurrence of aflatoxins and their metabolites in foodstuffs are a matter of concern in terms of human health and economic interest [4,5].
Rice is the dominant grain after wheat for half of the world population, provides more than 20% of their daily calories [6]. Asia is the leading continent for the production and consumption of rice. In general, rice is cultivated in subtropical environments with hot and humid climates that stimulate the fungal growth and production of secondary metabolites. Rice can be contaminated by aflatoxins producing fungi when the climatic conditions become favorable for their growth in the field, during harvest, handling and storage [7,8]. The occurrence of aflatoxins in rice has been reported in several studies with a high prevalence in Asian countries [8,9]. The high prevalence of aflatoxins contamination in rice and rice products underscore the importance of intensive monitoring of this dietary staple worldwide.
According to the World Health Organization (WHO), aflatoxin is a global food security concern [10]. In considering toxicity and carcinogenicity, the presence of aflatoxin in rice is a serious public health concern especially in developing countries where people are at risk of aflatoxin exposure [11]. Rice and other staple food are susceptible to aflatoxin contamination [12,13]. Low aflatoxin awareness, food insecurity and lack of regulatory limits enforcement are the significant contributors to high aflatoxin exposure of these populations [11].
Recent developments of aflatoxin biomarkers have provided opportunities in assessing aflatoxins exposure and its associated health effects in the high-risk population groups. Biomarkers analysis in human body fluids covers mycotoxin intake from all dietary sources and exposure by various routes [14,15]; thus human biomonitoring may provide valuable insights, especially in developing countries where AFB1 contaminant food data are scarce [16]. In this situation, biomarkers analysis in human body fluids might be important tool to estimate the impact of aflatoxins reduction intervention on public health [11]. Several biomarkers-based studies have been conducted in the last decades to assess aflatoxins exposure in humans [17,18]. The presence of aflatoxins in rice attracts worldwide attention because of the significant economic losses associated with their negative impacts on animal and human health and trade [15,19]. Hence, this review aimed to describe the worldwide occurrence of aflatoxins in rice during the period between 1990 and 2015 and biomarkers-based evidence for human exposure to aflatoxins and its associated health effects.
1.1. Toxicity of aflatoxins in animals and humans
AFB1 is the most prevalent aflatoxin and a potent hepatocarcinogen in various species, including humans and has been classified as a group 1 carcinogen [2]. In mycotoxins research, most of the research that has been conducted focused upon the study of aflatoxin B1 due to its strong carcinogenic effects on human beings. The main human cytochrome P450 (CYP) enzymes involved in human AFB1 metabolism in the liver are CYP3A4, 3A5 and 1A2 [20]. In AFB1 metabolism, diversity has been observed in different animal species [21] and the most critical reaction is bioactivation to (endo-, exo-) AFB1-8,9-epoxide, a highly reactive metabolite which covalently binds to DNA and induces mutations or forms adducts with proteins. A recent study indicated that residual AFB1 in the liver negatively affects the p53 and protein Rb pathways in hepatocellular carcinoma [22]. Hepatitis also affects aflatoxins exposure in humans. It has been demonstrated that AFB1 and hepatitis B virus (HBV) are synergistic causative agents of hepatocellular carcinoma [23]. Infection by HBV directly or indirectly sensitizes hepatocytes to the carcinogenic effects of AFB1 [23]. In an epidemiological study, a higher concentration of AFB1 adducts was found in chronically infected Gambian children and adolescents with HBV than uninfected individuals [24].
AFB1 possess toxic effects with a range of consequences; large doses cause acute toxicity and death whereas, chronic sublethal doses induce tumors and impair growth [25]. Acute toxicity of AFB1 has been well elucidated in animal experiments: the most susceptible species are ducks, and rabbits while chickens and rats have comparatively greater tolerance [25]. AFB1-induced hepatotoxicity occurs in a dose-dependent manner in a rat model [26]. The degree of AFB1 toxicity depends on age, sex, species, dose as well as nutritional status and length of exposure; young animals are being more sensitive than adults [25,27]. It is important to note that a number of the toxicological studies have been performed in non-realistic high doses and effects under the RLRS (real-life exposure scenario) approach of low doses and exposure of chemical mixtures were lacking [28,29].
There is limited information on acute aflatoxin toxicity in humans. Acute poisoning in humans has been reported in developing countries, for example, the severe acute aflatoxicosis outbreak in Kenya in 2004 with a mortality of 39.4% [30,31]. Abdominal pain, vomiting, fatty liver and necrosis are common acute poisoning in humans [27]. Other symptoms include depression, anorexia, diarrhea, jaundice, and photosensitivity [27]. On the other hand, acute aflatoxicosis is more common in animals, because of highly contaminated feed and the susceptibility of livestock species to this toxin [27]. Chronic aflatoxicosis in animals is associated with weight loss, lower feed conversion, decreased egg or milk production and increased susceptibility to infectious diseases [27]. In human beings, prolonged consumption of aflatoxin-contaminated food has been linked to liver cancer [32], impaired immune function, decreased reproductive functions, visceral encephalopathy and pulmonary interstitial fibrosis [27,33].
1.2. Worldwide rice production and consumption
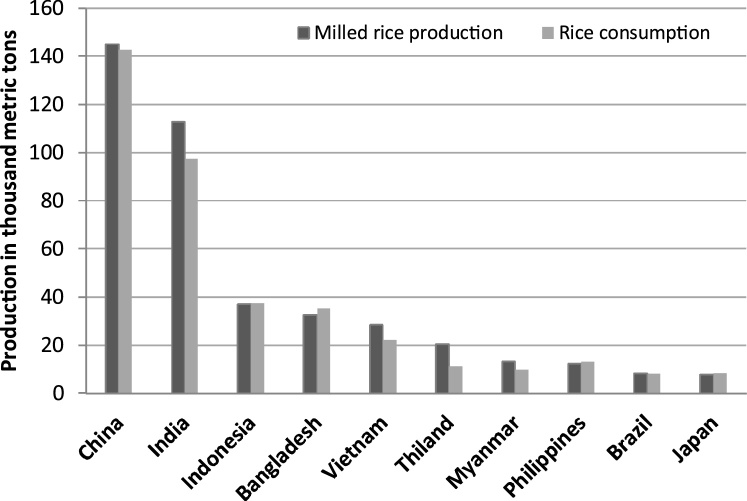
Rice is an important dietary staple that is largely consumed after wheat that consists of a significant part of the diets for half of the world population [34]. Rice is composed of 27% of the global diet and 20% of dietary protein intake in low and middle-income countries [35]. A diverse production system and consumption patterns have been observed for this important food commodity in the world. About 89% of the world’s rice is produced in Asia, with China and India leading the way accounting for 55% of the production [36]. However, it is not equally consumed throughout the country, with more urbanized nations such as Japan experiencing per capita consumption of 65 kg which is four times less than an overpopulated country like Bangladesh (258 kg) [36]. So far, rice is cultivated on 144 million hectares throughout the continent, with China and India dominating with over 50% of the total area harvested and the area under rice cultivation [36]. According to a recent report, nearly 487.5 million metric tons of milled rice were produced in 2017/2018 with a greater production volume in China and India (https://www.statista.com/topics/1443/rice/). The total global consumption of milled rice was approximately 485 million metric tons in that year. According to the above source, the rice consumption in China was about 143 million metric tons and the global use of rice per capita amounted to about 54 kg in that year. The worldwide top ten countries of rice production and consumption are depicted in Fig. 1. In America, maximum rice is produced in Brazil; in Africa, Egypt and Nigeria are the leading rice producer [37]; and in Europe, it is mainly produced in France and Spain [38].
Fig. 1.
Rice production and consumption in top ten countries in the world in 2017/2018. https://www.statista.com/topics/1443/rice/.
1.3. Worldwide occurrence of aflatoxins in rice
The worldwide occurrence of aflatoxins in rice is presented in Table 1. The presence of aflatoxins in rice is relatively high in tropical and subtropical regions in the world, where climatic conditions provide an optimal environment for fungal growth on food and feed [39]. Rice is generally cultivated in flooded irrigation conditions and high moisture levels that favor mold growth and subsequent mycotoxin contamination [34,40]. Among several mycotoxins, the most potent carcinogenic mycotoxins are aflatoxins which are mainly produced by Aspergillus flavus, Aspergillus parasiticus and the rare Aspaergillus nomius [41]. These fungi can grow on rice under favorable conditions such as floods and heavy rainfall during harvest and storage. Insufficient sun-drying and inappropriate storage make the rice prone to fungal attacks [40]. The contamination level of aflatoxins in rice varies from continent to continent. Several studies reported the occurrence of aflatoxins in rice from different continents.
Table 1.
Occurrence of aflatoxins in rice in Asia.from 1990 to 2015.
| Country, survey year | Type of rice | Origin of rice | Aflatoxins | Analytical Method | LOD/LOQ (μg/kg) | Incidence n (%) | Range (μg/kg) | Mean (μg/kg) | Reference |
|---|---|---|---|---|---|---|---|---|---|
| ASIA | |||||||||
| Bangladesh | Rice | Markets | AFB1 | HPLC | 0.2/0.5 | – | <LOD-0.9 | 0.3 | [131] |
| China | Rice | Stores, granaries, markets | AFB1 | DLLME-HPLC | 0.009/0.03 | 235/370 (63.5) | 0.03-20.0 | 0.06 ± 2.1 | [42] |
| AFB2 | 0.006/0.02 | 65/370 (18) | nd-1.6 | 0.15 ± 0.28 | |||||
| AFt | 235/370 (63.5) | 0.03-21.0 | 0.65 ± 2.3 | ||||||
| China | Rice | Household | AFB1 | ELISA | 0.01/- | 29/29 (100) | 0.1-1.4 | 0.5-0.6 | [43] |
| China | Rice | Local markets | AFB1 | HPLC-FD | 0.012/- | 16/84 (19) | 0.15-3.22 | – | [44] |
| India | Parboiled rice | Markets | AFB1 | HPTLC | 5.0/- | 581/1511 (38.5) | <LOD-361 | – | [45] |
| India | Paddy rice and milled rice | Markets | AFB1 | ELISA | 0.02ppt/- | 814/1200 (67.8) | 0.1-308 | – | [46] |
| Indonesia | Rice products | Supermarket, traditional market | AFB1 | ELISA | – | 2/2 (100) | 2.0-7.0 | – | [47] |
| Iran | Rice | Markets | AFB1 | IAC, HPLC-FD | 0.008/0.025 | 27/30 (90) | <LOQ-15.15 | 2.9 ± 4.4 | [48] |
| Iran | Rice | Local markets | AFB1 | LC-MS/MS | -/0.3 | 14/65 (21.5) | <LOQ-30.83 | 3.90 | [50] |
| AFB2 | -/0.6 | 2/65 (3.1) | 0.6-1.26 | 0.93 | |||||
| Iran, 2007-2008 | Polished rice | Retail markets | AFB1 | IAC, HPLC-FD | 0.01/0.03 | 251/256 (98) | nd-5.8 | 1.4 ± 1.0 | [51] |
| AFt | 0.1-6.3 | 1.6 ± 1.1 | |||||||
| Iran | Rice | Local area | AFB1 | IAC, HPLC-FD | 0.2/0.6 | 180/261 (69) | 0.2-4.3 | 0.72 ± 0.73 | [49] |
| Korea, 2002 | Polished rice | Grocery markets | AFB1 | ELISA | – | 5/88 (6) | 1.8-7.3 | 4.3 | [8] |
| Malaysia | Red Rice | Shops | AFt | IAC, ELISA | – | 46/50 (92) | 0.6-77.3 | 14.7 ± 16.2 | [52] |
| Malaysia | Rice based | Supermarkets | AFB1 | ELISA | 0.2/0.35 | 9/13 (69.2) | 0.68-3.79 | 1.75 | [54] |
| Malaysia | Rice | Retail markets | AFt | HPLC | – | – | 3.7-96.3 | – | [53] |
| Pakistan | Rice | Vendors | AFB1 | TLC | 1.0/- | 250/262 (95.4) | 10.07-24.6 | 3.80 | [56] |
| AFB2 | 0.5/- | 20/262 (7.6) | 0.52-2.62 | 0.09 | |||||
| AFt | 250/262 (95.4) | 10.07-27.27 | 3.89 | ||||||
| Pakistan | SK basmati rice | City areas | AFB1 | HPLC-UV | 0.05/0.10 | -/361 (13.3) | 1.1-32.9 | – | [57] |
| Basmati rice | AFB1 | -/585 (18.3) | 1.0-15.4 | – | |||||
| Parboiled rice | AFB1 | -/70 (42.9) | 1.1-9.2 | – | |||||
| Broken rice | AFB1 | -/11 (36.4) | 2.1-25.3 | – | |||||
| Pakistan | Brwon rice | Export areas | AFB1 | HPLC-UV | 0.10/- | 105/200 (52) | – | 0.56 | [58] |
| White rice | 80/200 (40) | – | 0.49 | ||||||
| Parboiled rice | 70/119 (59) | – | 0.73 | ||||||
| Pakistan | Rice | Retail markets, local industries | AFB1 | IAC, HPLC-FD | 0.05/- | 38/68 (56) | – | 8.23 | [60] |
| AFt | 38/68 (56) | – | 19.54 | ||||||
| Pakistan | Rice and rice products | Local markets, shops, super stores | AFB1 | IAC, HPLC-FD | 0.04/0.20 | 73/208 (35) | 0.04-7.4 | 2.40 ± 0.43 | [55] |
| AFt | 0.04-7.4 | 2.40 ± 0.43 | |||||||
| Pakistan, 2013 | Rice | Local markets | AFB1 | IAC, HPLC-FD | 0.03/0.12 | 100/120 (83) | 0.21-10.54 | 3.56 | [83] |
| AFt | 0.14/0.38 | 100/120 (83) | 0.21-11.89 | 3.79 | |||||
| AFB1 | LC-MS/MS | 0.02/0.06 | 104/120 (87) | 0.10-10.88 | 3.73 | ||||
| AFt | 0.09/0.24 | 104/120 (87) | 0.10-12.39 | 3.89 | |||||
| Pakistan, 2010 | Rice | Retail markets, agriculture fields | AFt | HPLC-FD | 0.04/0.12 | 185/413 (45) | LOD-68.3 | 11.2 ± 3.91 | [59] |
| Philippines, 2003 | Brown and polished rice | Rice mill | AFB1 | IAC, HPLC | – | 74/78 (95) | nd-8.55 | 1.48 | [34] |
| AFB2 | – | 74/78 (95) | nd-0.33 | 0.08 | |||||
| AFG1 | – | 74/78 (95) | nd-0.93 | 0.08 | |||||
| AFt | 0.025/- | 74/78 (95) | nd-8.66 | 1.53 | |||||
| SriLanka | Parboiled rice | Mills | AFB1 | TLC and UV-FD | – | -/485 | nd-185 | – | [62] |
| Thailand, 2012-2013 | Rice | Markets, retail shops | AFB1 | IAC, HPLC-FD | 0.09/- | 83/240 (35) | <LOD-26.61 | – | [63] |
| Turkey, 2006 | Rice | Fields | AFt | ELISA | 1.0/- | 56/100 (56) | nd-21.4 | – | [39] |
| AFB1 | 58/100 (58) | nd-17.2 | – | ||||||
| Vietnam | Rice | – | AFB1 | HPLC-FD | 0.07/0.22 | 51/100 (51) | nd-29.8 | 3.31 | [61] |
| United Arab Emirates | Grain rice | household | AFB1 | HPLC-FD | – | 241/500 (48) | 1.2-16.5 | – | [64] |
DLLME-HPLC: Dispersive liquid-liquid microextraction coupled to high performance liquid chromatography with fluorescence detection, IAC: Immunoaffinity column, SPE: Solid phase extraction, nd: not detectable, LOD: limit of detection, LOQ: limit of quantification.
AFt: Aflatoxins total (ie. sum of AFB1, AFB2, AFG1, AFG2), SK: Super Kernel.
1.3.1. Asia
In China, AFB1 was found in 235 of 370 samples with an average of 0.06 μg/kg [42]. In another study, AFB1 detected in all 29 samples with an average contamination level of around 0.5-0.6 μg/kg [43]. A previous study reported the presence of AFB1 in 16 out of 84 samples with a range between 0.15–3.22 μg/kg [44]. The presence of aflatoxins has been reported in rice from India. A study covering rice from 12 states of India reported that about 38.5% out of 1511 samples were contaminated by AFB1 [45]. Another survey covered 20 states of India, reported that AFB1 was present in 814 of 1200 samples ranging from 0.1 to 308 μg/kg [46]. In Indonesia, AFB1 was detected in 2 of 2 rice samples with a range between 2.0–7 0.0 μg/kg [47]. Some studies have been conducted in Iran to monitor aflatoxin in rice and rice products. A recent study reported the presence of AFB1 in 27 of 30 samples with an average level of 2.9 μg/kg (range < LOQ-15.15 μg/kg) in Iran [48]. AFB1 also detected in rice samples at different levels in Iran [[49], [50], [51]]. In South Korea, AFB1 was present in 5 of 88 samples at the range of 1.8–7.3 (mean 4.3 μg/kg) [8]. In Malaysia, rice samples were contaminated with total aflatoxins ie. the sum of AFB1, AFB2, AFG1 and AFG2, (ranging from 0.6 to 77.3 μg/kg [52]. The presence of aflatoxins in rice in Malaysia has also been reported in previous studies [53,54].
Several studies have been conducted in Pakistan to analyses the levels of aflatoxins in rice. In a recent study, contamination was found in 73 of 208 samples with AFB1 at the range of 0.04–7.4 μg/kg [55]. The contamination of rice with aflatoxins at different ranges has also been reported in previous studies in Pakistan [[56], [57], [58], [59], [60]]. In the Philippines, AFB1 was found in 74 of 78 samples with an average level of 1.48 μg/kg [34] and it was found to be comparatively higher (51 of 100) with an average of 3.31 μg/kg [61]. Aflatoxin contamination was found in rice from Sri Lanka [62]. In Turkey, 58 of the 100 samples were found to be contaminated with AFB1 at a range between non-detectable (nd) to 17.2 μg/kg [39]. In Thailand, AFB1 was detected in 83 of 240 samples at the range of < LOD-26.61 μg/kg [63]. AFB1 was detected in 241 of 500 samples from the United Arab Emirates with a range between 1.2–16.5 μg/kg [64].
1.3.2. Africa
In West Africa, AFB1 has been detected in all samples with an average level of 37.2 μg/kg (range 4.1–309.0 μg/kg) in Nigeria [37]. In the Ivory Coast, AFB1 has been detected in all rice samples at the range of <1.5–10.0 μg/kg [65]. AFB1 has also been reported in rice ranging from LOD to 11.0 μg/kg in Egypt [66].
1.3.3. Europe
Regulation for aflatoxins in food commodities is maintained in a better way in Europe than other continents. Up to now, a few studies have been conducted in Europe to analyse aflatoxins in rice. The occurrence of aflatoxins has been reported in rice from Austria, Scotland, Sweden and Spain. In Austria, aflatoxin B1 was detected in 24 samples out of 81 rice samples (range 0.45–9.86 μg/kg) imported mainly from Asian countries [67]. In Scotland, 1 out of 33 rice samples (Asian origin) has shown contamination with a mean 14.7 μg/kg of total aflatoxin [68]. Aflatoxin contamination in rice (collected from the Swedish retail market) has been reported in Sweden with a range between 0.1–50.7 μg/kg for total aflatoxin [69]. In Spain, the contamination level was higher in both local and imported samples; aflatoxin total was detected in almost all samples with an average concentration of 37.3 μg/kg (range 1.6–138.3 μg/kg) [38].
1.3.4. America
In Canada, AFB1 in rice (imported from Asia and the United States) was found in 99 of 200 samples with a mean level of 0.34–0.39 μg/kg [70]. The contamination was higher in Brazil, of 230 samples, 135 were contaminated with total aflatoxin with an average level of 13.3 μg/kg [71]. In Colombia, AFB1 was detected in 4 of 40 samples with a mean level of 7.1 μg/kg [72]. The detection frequency of AFB1 was lower (3 of 43) in paddy rice from Ecuador with a mean level of 20. 6 μg/kg [73]. In Mexico, aflatoxin total was found in rice imported from Asian countries with an average level of 16.9 μg/kg [38].
1.4. Methods and approaches in aflatoxins analysis
Aflatoxins in rice can be measured by applying different analytical methods (see Table 1, Table 2). The widely used methods are high-performance liquid chromatography with fluorescence detection (HPLC-FD) and enzyme-linked immunosorbent assay (ELISA) [39,74]. ELISA is a largely used technique over HPLC-FD and TLC methods due to its high throughput and requires low sample volumes, minimal sample extraction and clean-up process [39]. This method is rapid, simple and specific and can be used for the quantitative purpose for the detection of mycotoxins in food and feeds in the field [39]. However, this technique often overestimates the targeted analyte in the samples because of the cross-reactive nature of antibodies with compounds similar to mycotoxins. The ELISA test kit has been validated and applied for the detection of total aflatoxins in milled rice with the comparison of HPLC-FD [75]. This technique is often used for screening purposes in highly contaminated samples and also in biomarker-based epidemiological studies. On the other hand, sample clean-up and enrichment of the analyte by immunoaffinity columns and HPLC-FD analysis is more sensitive and reliable for measuring of aflatoxins in food and biological specimens. Although some studies validated the accuracy of ELISA against a reference method applying HPLC-FLD and reported a good correlation between HPLC and ELISA when the same extracts were used [39].
Table 2.
Occurrence of aflatoxins in rice in Africa, America and Europe.from 1990 to 2015.
| Country, survey year | Type of rice | Origin of rice | Aflatoxins | Analytical Method | LOD/LOQ (μg/kg) | Incidence n (%) | Range (μg/kg) | Mean (μg/kg) | Reference |
|---|---|---|---|---|---|---|---|---|---|
| AFRICA | |||||||||
| Egypt | Rice grains | Local markets | AFB1 | IAC, HPLC-FD | 0.01/- | -/40 | nd-11.0 | – | [66] |
| AFt | nd-21.7 | – | |||||||
| Nigeria | Rice | Fields, storage, markets | AFB1 | HPLC | 0.02 | 21/21 (100) | 4.1–309.0 | 37.2 ± 14.0 | [37] |
| AFB2 | 0.01 | 21/21 (100) | 1.3–24.2 | 8.3 ± 1.1 | |||||
| AFG1 | 0.01 | 21/21 (100) | 5.5–76.8 | 22.1 ± 3.4 | |||||
| AFG2 | 0.06 | 19/21 (90) | 3.6–44.4 | 14.7 ± 2.5 | |||||
| AFt | 27.7–371.9 | 82.5 ± 16.9 | |||||||
| Ivory Coast | Rice | Markets | AFB1 | ELISA | – | 10/10 (100) | <1.5-10.0 | – | [65] |
| AMERICA | |||||||||
| Brazil, 2007-2009 | Rice | Rice mills | AFB1 | IAC, HPLC-FD | 0.03/- | 128/230 (55.7) | 0.08-180.7 | 9.1 | [71] |
| AFt | 0.01-0.03/- | 135/230 (58.7) | 0.11-207 | 13.3 | |||||
| Canada, 2008-2009 | Rice | Markets | AFB1 | IAC, HPLC-FD | 0.002/0.05 | 99/199 (49.7) | nd-7.1 | 0.36 0.18 |
[70] |
| Colombia | Rice and rice products | Supermarkets, Retails stores, Stock centres | AFB1 | LC-FD | 1.0 | 4/40 (10) | nd-13.6 | 7.1 | [72] |
| Ecuador | Paddy rice | Rice mills | AFB1 | IAC, UHPLC/TOFMS | 4.0/8.0 | 3/43 (6.9) | 4.9-47.4 | 20.6 | [73] |
| AFG1 | 7.0/14.0 | 1/43 (2.3) | 63.7 | – | |||||
| AFG2 | 3.0/5.0 | 1/43 (2.3) | 3.3 | – | |||||
| Polished rice | AFG1 | 1.0/1.0 | 1/46 (2.2) | 2.0 | – | ||||
| Mexico, 2008-2009 | Rice | Local stores Supermarkets |
AFt | IAC, HPLC-FD | 0.4-0.6/ 1.2-1.9 |
-/67 | – | 16.9 | [38] |
| EUROPE | |||||||||
| Austria | Rice | Market | AFB1 | IAC, HPLC-FD | 0.1/0.5 | 24/81 (29.6) | 0.45-9.86 | – | [67] |
| AFB2 | 0.15/0.5 | 1.5 | – | ||||||
| Scotland | Rice | Retail market | AFt | SPE, HPLC-FD | – | 1/3 (33.3) | 0.4-14.7 | 14.7 | [68] |
| Spain | Rice | Local stores Supermarkets |
AFt | IAC, HPLC-FD | 0.4-0.6/ 1.2-1.9 |
-/67 | 1.6-138.3 | 37.3 | [38] |
| Sweden | Rice | Retail market | AFt | IAC, HPLC-FD | -/0.1 | -/99 | nd-50.7 | – | [69] |
| AFB1 | nd-46.2 | – | |||||||
| AFB2 | nd-4.5 | – |
DLLME-HPLC: Dispersive liquid-liquid microextraction coupled to high performance liquid chromatography with fluorescence detection, IAC: Immunoaffinity column, SPE: Solid phase extraction, nd: not detectable, LOD: limit of detection, LOQ: limit of quantification.
AFt: Aflatoxins total (ie. sum of AFB1, AFB2, AFG1, AFG2), SK: Super Kernel.
1.5. Dietary intake and consumer health
Dietary daily intake of aflatoxins depends on the levels in the food and the amount of food ingested [76]. Due to genotoxic and carcinogenic properties of aflatoxins, the tolerable daily intake (TDI) cannot be considered as a safety factor, so human exposure should be reduced to levels as low as possible [39]. However, a provisional maximum tolerable daily intake (PMTDI) of 1 ng AFB1 per kg body weight per day for adults and children without hepatitis B and 0.4 ng AFB1 per kg body weight per day for adults with hepatitis B may be used as a guide value in the risk assessment of AFB1 from food [77]. An association between dietary aflatoxins exposure and the incidence of human liver cancer has been reported in some African and Asian countries [78]. To make awareness, many countries have set the maximum levels of aflatoxins in foodstuffs as a safeguard of human health, as well as the economic interest of crop producers and traders [79]. The maximum tolerable limit of aflatoxin in rice set by different countries and regulatory bodies are presented in Table 3. In order to protect public health, the European Union has set a maximum level of aflatoxin B1 and total aflatoxins (2 μg/kg and 4 μg/kg, respectively) in rice desired for human intake [80], while [81] set maximum levels of aflatoxin B1 and total aflatoxins (5 μg/kg, 10 μg/kg, respectively) in rice before human consumption. A comparable regulatory limit for total aflatoxin has been reported in India (30 μg/kg), Brazil (30 μg/kg) Mexico (20 μg/kg), USA (20 μg/kg), Canada (15 μg/kg) Taiwan (10 μg/kg) [6]. In Japan, Korea and China the reported regulatory limit for AFB1 is 10 μg/kg [6]. The lowest regulatory limit for AFB1 (1 μg/kg in) has been reported in Bosnia and Herzegovina [82] and Switzerland [41].
Table 3.
Maximum residual limits (MRLs) of aflatoxin in rice in EU and other countries.
| Countries/ Organization | Aflatoxin | MRLs (μg/kg) | Reference |
|---|---|---|---|
| Bosnia and Herzegovina | AFB1 | 1 | [82] |
| Brazil | AFB1 | 30 | [6] |
| Canada | AFt | 15 | [6] |
| Chile | AFt | 5 | [82] |
| China | AFB1 | 10 | [6] |
| Egypt | AFt | 5 | [6] |
| EU | AFB1 | 2 | [132] |
| India | AFt | 30 | [6] |
| Iran | AFB1 | 5 | [49] |
| Japan | AFB1 | 10 | [6] |
| Korea | AFB1 | 10 | [6] |
| Malyasia | AFt | 5 | [52] |
| Mexico | AFt | 20 | [6] |
| Russia | AFB1 | 5 | [6] |
| Switzerland | AFB1 | 1 | [41] |
| Taiwan | Aft | 10 | [6] |
| Turkey | AFB1 | 2 | [6] |
| USA | Aft | 20 | [6] |
EU, European Union; AFt, Aflatoxin total.
1.6. Exceeding the regulatory limit in rice
Several studies reported the levels of aflatoxins in rice that exceeded the recommended limit value (Table 4). In a recent study in China, 5 of 370 samples (1.4%) exceeded the maximum regulatory limit for AFB1 [42]. In India, 256 of 1511 (17%) samples were found to exceed the AFB1 regulatory limit [45]. In another study, 24 of 1200 samples (2%) had AFB1 levels above the regulatory limit [46]. In Iran, 55 of 256 polished rice (21%) were found to exceed the maximum limit for AFB1 [51]. Another study in the same country showed that 3 of 63 (4.6%) samples had the AFB1 concentration above the regulatory limit [50]. In Malaysian red rice, 35 of 46 samples (70%) showed the total aflatoxin concentration above the maximum limit [52]. Several studies conducted in Pakistan reported the AFB1 contamination levels in rice above the regulatory limit. In a recent study, 19 out of 208 samples exceeded the recommended limit [55]. The other studies also reported the close percentage of samples that exceeded the maximum limit [57,58,60,83]. In Thailand, 12 of 240 samples (5%) had AFB1 levels above the regulatory limit [63]. In Vietnam, 10% rice (10 of 100) exceeded the maximum limit for AFB1 [61]. In Turkey, 32% of the rice exceeded the maximum tolerable limit for total aflatoxins and 14% of rice samples exceeded this limit for AFB1 [39]. In a study in Sri Lanka, aflatoxin levels in parboiled rice were found to be several times higher than TDI with the highest levels of AFB1 being 185 μg/kg [62]. In Austria, 3 of 81 samples (3.7%) imported mainly from Asian countries had the AFB1 levels that crossed the maximum limit [67].
Table 4.
Exceeded maximum tolerable limit of aflatoxin in rice of different countries.
| Countries | Type of rice | Sample (n) | Aflatoxin | Limit (μg/kg) | Exceeded, n (%) | Reference |
|---|---|---|---|---|---|---|
| China | Rice | 370 | AFB1 | 2 | 5 (1.4) | [42] |
| India | Parboiled rice | 1511 | AFB1 | 30 | 256 (17.0) | [45] |
| India | Paddy rice | 1200 | AFB1 | 30 | 24 (2.0) | [46] |
| Iran | Rice flour | 30 | AFB1 | 0.1a | 20 (67) | [48] |
| Iran | Polished rice | 256 | AFB1 | 2 | 55 (21) | [51] |
| AFt | 4 | 7 (2.7) | ||||
| Iran | Rice | 63 | AFB1 | 5 | 3 (4.6) | [50] |
| Malaysia | Red rice | 46 | AFt | 5 | 35 (70) | [52] |
| Pakistan | SK Basmati rice | 361 | AFB1 | 2 | (6.4) | [57] |
| Pakistan | Brown rice | 200 | AFB1 | 2 | (5.6) | [58] |
| Pakistan | Rice | 68 | AFB1 | 2 | 18 | [60] |
| Pakistan | Rice and rice products | 208 | AFB1 | 2 | 19 | [55] |
| Pakistan | Rice | 120 | AFB1 | 2 | 44 | [83] |
| Thailand | Rice | 240 | AFB1 | 2 | 12 (5) | [63] |
| Vietnam | Rice | 100 | AFB1 | 2 | 10 (10) | [61] |
| Turkey | Rice | 100 | AFt | 4 | 32 (32) | [39] |
| Austria | Rice | 81 | AFB1 | 2 | 3 (3.7) | [67] |
0.1 μg/kg: maximum established level of EU regulations for baby food. AFt, Aflatoxin total.
1.7. Aflatoxins exposure and health effects
Human exposure to aflatoxins occurs through the consumption of contaminated foodstuffs and such exposure can be happened throughout the life course, beginning in utero via transplacental exposure [84]. Human breast milk is one of the major pathways of aflatoxin exposure for young children during the breastfeeding period [85]. Consumption of AFB1- contaminated food might result in the secretion and presence of AFM1 (a metabolite of AFB1) in human breast milk. Therefore, children’s exposure to AFM1 through breastfeeding is at high risk for the life-threatening side effects of aflatoxins. Preliminary evidence suggests an interaction between chronic aflatoxin exposure and malnutrition, as reduced uptake of nutrients from the diet, may result in growth retardation in children [86]. An association between aflatoxin exposure in utero and growth faltering has been reported in Gambian children [87]. Chronic aflatoxin exposure is linked with kwashiorkor, a severe Protein Energy Malnutrition (PEM) disease [88]. Studies conducted in the last three decades have shown a higher aflatoxin concentration in the blood and urine of children with kwashiorkor compared to healthy children [89]. Besides growth impairment, chronic aflatoxin exposure also affects the immune system. A decreased IgA was found in the saliva of children who were highly exposed to AFB1 [90].
Acute aflatoxicosis in humans occurs during high exposure over a relatively short time. For example, in Kenya in 2004, 317 individuals were diagnosed with acute liver failure of which 37% subsequently died as a result of acute aflatoxicosis [91]. Chronic aflatoxicosis occurs because of low dose aflatoxin exposure over a long period which is more prevalent than acute aflatoxicosis. Liver cancer is the well-known health effects of chronic aflatoxicosis in human and it was the sixth most common cancer worldwide in 2012, with over 80% of cases in developing countries in Africa and Asia [11]. Both aflatoxin and hepatitis B exposure have been reported in epidemiological studies, resulting in an increased risk of hepatocellular carcinoma in these countries [92,93]. Aflatoxins have also been found to be linked with other liver diseases such as cirrhosis and hepatomegaly [11]. In Asia, an outbreak of hepatitis due to aflatoxin was reported in the states of Rajasthan and Gujrat in India, resulting in an approximate 106 deaths in 1974 [94]. Another outbreak of aflatoxin affecting both humans and dogs was reported in northwest India in 1974 [95]. Avoiding contaminated diet, agricultural reforms with changing more aflatoxins susceptible crops into less susceptible crops and implementation of the hepatitis B virus immunization program may have the potential in reducing and preventing hepatocellular carcinoma in humans.
1.8. Aflatoxin biomarkers measurement in biological fluids
Aflatoxin biomarkers have been established and validated in epidemiological studies that investigated the association between exposure and risk of diseases [96,97]. Various analytical techniques such as ELISA and HPLC-FD have been widely used in aflatoxins biomarkers analysis in human body fluids. LC–MS/MS multitoxin approach has also been applied in aflatoxin biomarker analysis in human urine [98,99]. Among these techniques, HPLC-FD was found to be a more specific and sensitive one for aflatoxin biomarker studies [16]. Such investigations have analyzed the levels of serum AF-alb or AFB1-lysine adduct in blood or AFB1-N7-guanine and AFM1 metabolite levels in urine as valid biomarkers of aflatoxins exposure. Human biomonitoring is an effective tool and may provide valuable insights, especially in developing countries where food contaminated data are scare or no regular surveillance of mycotoxins exists [16]. Mycotoxin biomarkers analysis in human body fluids covers mycotoxin intake from all dietary sources and exposure by several routes [100]. The concentration of AFB1-lysine albumin in serum indicates exposure over a period of several weeks or months because of its long half-life in blood, whilst AFB1-N7-guanine or AFM1 in urine reflects recent exposure and thus used as a short-term biomarker of exposure [18,101,102]. In the last decades, several studies reported the presence of aflatoxin biomarkers in human body fluids. High human exposure was found in rural subsistence farming communities in developing countries especially in Asia and Africa [11]. Aflatoxin exposure has been rarely reported in developed countries as strict regulation for aflatoxins in food is maintained there. An early study reported a correlation between daily intake of AFB1 and its urinary excretion of AFM1 [103].
1.8.1. AF-alb and AFB1-lysine adducts in blood
In East African countries, analysis of AF-alb biomarker in serum indicated a high aflatoxin exposure, where it was detected in 78% out of 597 serum samples (range ND-211 pg/mg) from Kenya [104], in Uganda, this biomarker was detected in 98% (192 of 196) samples (range ND-238 pg/mg) [105], in Tanzania, where AF-alb was detected in 67–99% of samples collected from children and adults [106]. In the North and South part of Africa, the prevalence of exposure was comparatively lower than the levels found in East and West Africa. AF-alb was found in 67% of 46 samples (range ND-32.8 pg/mg) from Egypt [107]. AF-alb was detected in 35% (34 of 98) of serum samples from pregnant women in Egypt [108]. In Asia, AFB1-lysine adduct was detected in 97% of 170 samples (range 0.2–23.3 pg/mg) from Malaysia [109]. A recent study [101] reported the presence of AFB1-lysine biomarkers in 94% of 141 samples (range 0.4–2939.3 pg/mg) from pregnant women in Nepal. In the same investigation, this biomarker was detected in 100% (63 of 63) samples from pregnant women from Bangladesh as well as in 100% (63 of 63) cord blood samples and in infants 100% (63 of 63) whose mothers were exposed to aflatoxin during pregnancy. In Brazil, AFB1-lysine adduct was found in 62% samples in a concentration ranging from ND to 57.3 pg AFB1-lysine/mg blood albumin [110].
1.8.2. AFM1 biomarkers in urine
Compared to blood, urine has widely used a matrix for AFM1 biomarker analysis because of its non-invasive sampling and better acceptance by the participants in field studies [16,111]. In low-income countries where food contaminated data are scarce, biomonitoring may be an effective tool to gain more insights into human exposure to aflatoxins. Recently, many have been conducted to analyse the AFM1 biomarkers in humans of different countries [16].
In Asia, AFM1 biomarker has been frequently detected in urines from Malaysia, China, and Bangladesh. AFM1 was detected in 98 of 160 urines (61%) in a concentration ranging from LOD-74.7 pg/mL (mean 23.4 pg/mL) in a Malaysian cohort [112]. A recent study in the Zhejiang province of China indicated the presence of AFM1 in urines from adult males (mean of 51.5 and range LOD–4900 pg/mL) and pregnant women cohort (mean of 50.3 and range LOD-3500 pg/mL) [113]. Another study in this country, reported far more frequent detection and higher AFM1 level in 1988 (mean 48 and range 5.7–243 pg/mL) than in 2000 (only one urine 9 pg/mL) [114]. In a recent study in Bangladesh, AFM1 was detected in 40% of urine samples (mean 13.6 and range of 1.7–104 pg/mL) in summer and 42% of samples (mean 27.7 and range of 1.8–190 pg/mL) in the winter season [16]. In the same study, AFM1 was detected in 17 of 54 (31%) urines (mean 13.9 and range 1.7–141.5 pg/mL) from pregnant women cohort in Bangladesh. AFM1 was present in 3 of 60 urines from Thailand, (range 160–550 pg/mL), [115].
In Africa, the highest AFM1 levels in urines were found in Ghanian adults (mean 1800, range LOD–11562 pg/mg creatinine; [116]. A recent study in Nigeria reported AFM1 occurrence in children and adolescents urines (mean 300 and range LOD–1500 pg/mL; [117] and urines from Guinean infants (mean 97 and range 8–801 pg/mL [118]; reveal that AFB1 exposure is a serious health concern in several sub-Saharan African countries. Another study detected urinary AFM1 in Ivory Coast [119] and Cameroon [120].
In Europe, urines from Germany (n = 30 and n = 50) and Belgium (n = 32) had no detectable levels of AFM1 in urines [98,121,122]. In Southern Italy, only 3 of 52 urines had detectable levels of AFM1 (mean 68 and range 20–146 pg/mL; [99].
There are a few reports from America. Three studies conducted in Brazil reported the presence of AFM1 in urines. A recent study detected AFM1 in 65% urine samples (range 0.37 to 1.70 pg/mg creatinine; [123]. Another study reported AFM1 occurrence in urines (mean 1.2 pg/mL, range 0.25–6.9 pg/mg creatinine; [124]. The other study conducted in Brazil detected urinary AFM1 (5.9 pg/mL range 1.8–39.9 pg/mL; [125]). In the USA, a study in adults with an elevated risk of liver cancer reported urinary AFM1 in 11.7% of 179 samples (mean 223.8 pg/mg creatinine, range 1.89–935.49 pg/mg creatinine; [126]. In a more recent study, AFM1 was found in 14% and 22% of urines from rural and urban Haitians (max. 700 pg/mL) [127].
1.8.3. AFM1 biomarker in human breast milk
Beside human blood and urine, breast milk has also been used for AFM1 biomarker analysis in some epidemiological studies. AFM1 was found in human breast milk (range 5–3400 pg/mL) of the Arab Emirates [128]. In Brazil, AFM1 was detected in 1 of 50 samples at a concentration of 0.02 ng/mL [129]. In a recent study in Iran, AFM1 was detected in 157 of 160 samples, with a concentration ranging from 0.3 to 26.7 ng/kg [130].
2. Conclusions and recommendations
High variability in aflatoxin contamination has been observed worldwide and the contamination is higher in developing countries where rice constitutes the major nutritional source of the diet. Several biomarker-based studies report human exposure to aflatoxins especially in some sub-Saharan and Asian countries. To minimize aflatoxin contamination, applying effective strategies can be the prevention against fungal growth in rice. Implementation of good agricultural and manufacturing practices during harvesting, storage, and distribution of rice may ensure the lower level of aflatoxin in the final product. Physical, chemical and microbiological approaches can be used to reduce the aflatoxin levels in the rice. Hazard Analysis and Critical Control Point (HACCP) approach may also be a useful food safety strategy to control contamination from field to consumer and so that the level does not cross the limit value recommended by the legislation. Biomarkers-based monitoring is recommended to integrate with conventional food analysis approach to assess aflatoxins exposure and related health effects in the population groups who are at high-risk. Finally, considering the importance of rice as a major part of the human diet, further research is needed to reduce the exposure effects of aflatoxins in humans.
Conflict of interest
The author declare no conflict of interest.
Acknowledgment
This work did not receive any external funding. It was supported by a small fund as internal.
References
- 1.IARC . Vol. 82. 2002. Some traditional herbal medicines, some mycotoxins, naphthalene and styrene. (IARC Monogr Eval Carcinog Risk Chem Hum). [PMC free article] [PubMed] [Google Scholar]
- 2.IARC . Vol. 56. 1993. Some naturally occurring substances: food items and constituents, heterocyclic aromatic amines and mycotoxins.https://www.cabdirect.org/cabdirect/abstract/19952006807 (IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans). [Accessed 24 November 2018] [Google Scholar]
- 3.Wild C.P., Gong Y.Y. Mycotoxins and human disease: a largely ignored global health issue. Carcinogenesis. 2010;31:71–82. doi: 10.1093/carcin/bgp264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elzupir A.O., Alamer A.S., Dutton M.F. The occurrence of aflatoxin in rice worldwide: a review. Toxin Rev. 2015;34:37–42. [Google Scholar]
- 5.Goumenou Marina, Axiotis D., Trantallidi M., Vynias D., Tsakiris I., Alegakis A., Dumanov J., Tsatsakis A. In: Toxicological Effects, Risk Assessment and Legislation for Aflatoxins” in Aflatoxins: Food Sources, Occurrence and Toxicological Effects. Faulkner Adina G., editor. Nova Publications; 2014. pp. 191–232. ISBN: 978-1-63117-298-4. [Google Scholar]
- 6.FAO . Food and Agriculture Organization; Rome: 2004. Worldwide Regulations for Mycotoxins in Food and Feed in 2003. FAO Food and Nutrition Paper no. 81; pp. 1–180. [Google Scholar]
- 7.Ali N. Co-occurrence of citrinin and ochratoxin A in rice in Asia and its implications for human health. J. Sci. Food Agric. 2018;98:2055–2059. doi: 10.1002/jsfa.8667. [DOI] [PubMed] [Google Scholar]
- 8.Park J.W., Choi S.-Y., Hwang H.-J., Kim Y.-B. Fungal mycoflora and mycotoxins in Korean polished rice destined for humans. Int. J. Food Microbiol. 2005;103:305–314. doi: 10.1016/j.ijfoodmicro.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Sun X.D., Su P., Shan H. Mycotoxin contamination of rice in China: mycotoxin contamination of rice in China…. J. Food Sci. 2017;82:573–584. doi: 10.1111/1750-3841.13631. [DOI] [PubMed] [Google Scholar]
- 10.WHO . 2015. WHO Estimates of the Global Burden of Foodborne Diseases: Foodborne Disease Burden Epidemiology Reference Group 2007–2015. [Google Scholar]
- 11.Gong Y.Y., Watson S., Routledge M.N. Aflatoxin exposure and associated human health effects, a review of epidemiological studies. Food Saf. 2016;4:14–27. doi: 10.14252/foodsafetyfscj.2015026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taghizadeh S.F., Rezaee R., Davarynejad G., Asili J., Nemati S.H., Goumenou M., Tsakiris I., Tsatsakis A.M., Shirani K., Karimi G. Risk assessment of exposure to aflatoxin B1 and ochratoxin A through consumption of different Pistachio (Pistacia vera L.) cultivars collected from four geographical regions of Iran. Environ. Toxicol. Pharmacol. 2018;61:61–66. doi: 10.1016/j.etap.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 13.Tsakiris I.N., Renieri E.M., Vlachou M., Theodoropoulou E., Goumenou M., Tsatsakis A.M. In: Food Sources and Occurrence of Aflatoxins: The Experience in Greece” in Aflatoxins: Food Sources, Occurrence and Toxicological Effects. Faulkner Adina G., editor. Nova Publications; 2014. pp. 233–258. ISBN: 978-1-63117-298-4. [Google Scholar]
- 14.Tsakiris I.N., Tzatzarakis M.N., Alegakis A.K., Vlachou M.I., Renieri E.A., Tsatsakis A.M. Risk assessment scenarios of children’s exposure to aflatoxin M1 residues in different milk types from the Greek market. Food Chem. Toxicol. 2013;56:261–265. doi: 10.1016/j.fct.2013.02.024. [DOI] [PubMed] [Google Scholar]
- 15.Wagacha J.M., Muthomi J.W. Mycotoxin problem in Africa: current status, implications to food safety and health and possible management strategies. Int. J. Food Microbiol. 2008;124:1–12. doi: 10.1016/j.ijfoodmicro.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 16.Ali N., Blaszkewicz M., Hossain K., Degen G.H. Determination of aflatoxin M1 in urine samples indicates frequent dietary exposure to aflatoxin B1 in the Bangladeshi population. Int. J. Hyg. Environ. Health. 2017;220:271–281. doi: 10.1016/j.ijheh.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 17.Groopman J.D., Wild C.P., Hasler J., Junshi C., Wogan G.N., Kensler T.W. Molecular epidemiology of aflatoxin exposures: validation of aflatoxin-N7-guanine levels in urine as a biomarker in experimental rat models and humans. Environ. Health Perspect. 1993;99:107–113. doi: 10.1289/ehp.9399107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wild C.P., Hudson G.J., Sabbioni G., Chapot B., Hall A.J., Wogan G.N., Whittle H., Montesano R., Groopman J.D. Dietary intake of aflatoxins and the level of albumin-bound aflatoxin in peripheral blood in the Gambia, West Africa. Cancer Epidemiol. Biomark. Prev. 1992;1:229–234. [PubMed] [Google Scholar]
- 19.Ostry V., Malir F., Toman J., Grosse Y. Mycotoxins as human carcinogens—the IARC Monographs classification. Mycotoxin Res. 2017;33:65–73. doi: 10.1007/s12550-016-0265-7. [DOI] [PubMed] [Google Scholar]
- 20.Wild C.P., Turner P.C. The toxicology of aflatoxins as a basis for public health decisions. Mutagenesis. 2002;17:471–481. doi: 10.1093/mutage/17.6.471. [DOI] [PubMed] [Google Scholar]
- 21.Wu Q., Jezkova A., Yuan Z., Pavlikova L., Dohnal V., Kuca K. Biological degradation of aflatoxins. Drug Metab. Rev. 2009;41:1–7. doi: 10.1080/03602530802563850. [DOI] [PubMed] [Google Scholar]
- 22.Ramalho L.N.Z., Porta L.D., Rosim R.E., Petta T., Augusto M.J., Silva D.M., Ramalho F.S., Oliveira C.A.F. Aflatoxin B1 residues in human livers and their relationship with markers of hepatic carcinogenesis in São Paulo, Brazil. Toxicol. Rep. 2018;5:777–784. doi: 10.1016/j.toxrep.2018.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kew M.C. Synergistic interaction between aflatoxin B1 and hepatitis B virus in hepatocarcinogenesis. Liver Int. 2003;23:405–409. doi: 10.1111/j.1478-3231.2003.00869.x. [DOI] [PubMed] [Google Scholar]
- 24.Turner P.C., Mendy M., Whittle H., Fortuin M., Hall A.J., Wild C.P. Hepatitis B infection and aflatoxin biomarker levels in Gambian children. Trop. Med. Int. Health. 2000;5:837–841. doi: 10.1046/j.1365-3156.2000.00664.x. [DOI] [PubMed] [Google Scholar]
- 25.EFSA Opinion of the scientific panel on contaminants in the food chain on a request from the commission related to the potential increase of consumer health risk by a possible increase of the existing maximum levels for aflatoxins in almonds, hazelnuts and pistachios and derived products. EFSA J. 2007;446:1–127. [Google Scholar]
- 26.Rotimi O.A., Rotimi S.O., Duru C.U., Ebebeinwe O.J., Abiodun A.O., Oyeniyi B.O., Faduyile F.A. Acute aflatoxin B1 – induced hepatotoxicity alters gene expression and disrupts lipid and lipoprotein metabolism in rats. Toxicol. Rep. 2017;4:408–414. doi: 10.1016/j.toxrep.2017.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marin S., Ramos A.J., Cano-Sancho G., Sanchis V. Mycotoxins: occurrence, toxicology, and exposure assessment. Food Chem. Toxicol. 2013;60:218–237. doi: 10.1016/j.fct.2013.07.047. [DOI] [PubMed] [Google Scholar]
- 28.Kostoff R.N., Goumenou M., Tsatsakis A. The role of toxic stimuli combinations in determining safe exposure limits. Toxicol. Rep. 2018;5:1169–1172. doi: 10.1016/j.toxrep.2018.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsatsakis A., Goumenou M., Liesivuori J., Dekant W., Hernández A.F. Toxicology for real-life risk simulation - Editorial preface to this special issue. Toxicol. Lett. 2019;309:33–34. doi: 10.1016/j.toxlet.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 30.Lewis L., Onsongo M., Njapau H., Schurz-Rogers H., Luber G., Kieszak S., Nyamongo J., Backer L., Dahiye A.M., Misore A. Aflatoxin contamination of commercial maize products during an outbreak of acute aflatoxicosis in eastern and central Kenya. Environ. Health Perspect. 2005;113:1763. doi: 10.1289/ehp.7998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Probst C., Njapau H., Cotty P.J. Outbreak of an acute aflatoxicosis in Kenya in 2004: identification of the causal agent. Appl. Environ. Microbiol. 2007;73:2762–2764. doi: 10.1128/AEM.02370-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu H.C., Santella R. The role of aflatoxins in hepatocellular carcinoma. Hepat. Mon. 2012;12 doi: 10.5812/hepatmon.7238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiang Y., Jolly P.E., Preko P., Wang J.-S., Ellis W.O., Phillips T.D., Williams J.H. Aflatoxin-related immune dysfunction in health and in human immunodeficiency virus disease. Clin. Dev. Immunol. 2008;2008:1–12. doi: 10.1155/2008/790309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sales A.C., Yoshizawa T. Updated profile of aflatoxin and Aspergillus section Flavi contamination in rice and its byproducts from the Philippines. Food Addit. Contam. 2005;22:429–436. doi: 10.1080/02652030500058387. [DOI] [PubMed] [Google Scholar]
- 35.Ok H.E., Kim D.M., Kim D., Chung S.H., Chung M.-S., Park K.H., Chun H.S. Mycobiota and natural occurrence of aflatoxin, deoxynivalenol, nivalenol and zearalenone in rice freshly harvested in South Korea. Food Control. 2014;37:284–291. [Google Scholar]
- 36.Milovanovic V., Smutka L. Asian countries in the global rice market. Acta Univ. Agric. Silvic. Mendel. Brun. 2017;65:679–688. [Google Scholar]
- 37.Makun H.A., Dutton M.F., Njobeh P.B., Mwanza M., Kabiru A.Y. Natural multi-occurrence of mycotoxins in rice from Niger State, Nigeria. Mycotoxin Res. 2011;27:97–104. doi: 10.1007/s12550-010-0080-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suárez-Bonnet E., Carvajal M., Méndez-Ramírez I., Castillo-Urueta P., Cortés-Eslava J., Gómez-Arroyo S., Melero-Vara J.M. Aflatoxin (B 1, B 2, G 1, and G 2) contamination in rice of Mexico and Spain, from local sources or imported: aflatoxins in rice …. J. Food Sci. 2013;78:T1822–T1829. doi: 10.1111/1750-3841.12291. [DOI] [PubMed] [Google Scholar]
- 39.Aydin A., Aksu H., Gunsen U. Mycotoxin levels and incidence of mould in Turkish rice. Environ. Monit. Assess. 2011;178:271–280. doi: 10.1007/s10661-010-1688-9. [DOI] [PubMed] [Google Scholar]
- 40.Majeed S., De Boevre M., De Saeger S., Rauf W., Tawab A., Fazal-e-Habib, Rahman M., Iqbal M. Multiple mycotoxins in rice: occurrence and health risk assessment in children and adults of Punjab, Pakistan. Toxins. 2018;10:77. doi: 10.3390/toxins10020077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Creppy E.E. Update of survey, regulation and toxic effects of mycotoxins in Europe. Toxicol. Lett. 2002;127:19–28. doi: 10.1016/s0378-4274(01)00479-9. [DOI] [PubMed] [Google Scholar]
- 42.Lai X., Liu R., Ruan C., Zhang H., Liu C. Occurrence of aflatoxins and ochratoxin A in rice samples from six provinces in China. Food Control. 2015;50:401–404. [Google Scholar]
- 43.Sun G., Wang S., Hu X., Su J., Zhang Y., Xie Y., Zhang H., Tang L., Wang J.-S. Co-contamination of aflatoxin B 1 and fumonisin B 1 in food and human dietary exposure in three areas of China. Food Addit. Contam. Part A. 2011;28:461–470. doi: 10.1080/19440049.2010.544678. [DOI] [PubMed] [Google Scholar]
- 44.Wang J., Liu X.-M. Contamination of aflatoxins in different kinds of foods in China. Biomed. Environ. Sci. 2007;20:483–487. [PubMed] [Google Scholar]
- 45.Toteja G.S., Mukherjee A., Diwakar S., Singh P., Saxena B.N., Sinha K.K., Sinha A.K., Kumar N., Nagaraja K.V., Bai G., Krishna Prasad C.A., Vanchinathan S., Roy R., Sarkar S. Aflatoxin B 1 contamination of parboiled rice samples collected from different states of India: a multi-centre study. Food Addit. Contam. 2006;23:411–414. doi: 10.1080/02652030500442490. [DOI] [PubMed] [Google Scholar]
- 46.Reddy K., Reddy C., Muralidharan K. Detection of Aspergillus spp. and aflatoxin B1 in rice in India. Food Microbiol. 2009;26:27–31. doi: 10.1016/j.fm.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 47.Noviandi C.T., Razzazi E., Agus A., Böhm J., Hulan H.W., Wedhastri S., Maryudhani Y.B., Leibetseder J. Natural occurrence of aflatoxin B 1 in some Indonesian food and feed products in Yogyakarta in year 1998–1999. Mycotoxin Res. 2001;17:174–177. doi: 10.1007/BF03036430. [DOI] [PubMed] [Google Scholar]
- 48.Mottaghianpour E., Nazari F., Mehrasbi M.R., Hosseini M.-J. Occurrence of aflatoxin B 1 in baby foods marketed in Iran: aflatoxin B 1 in baby foods in Iran. J. Sci. Food Agric. 2017;97:2690–2694. doi: 10.1002/jsfa.8092. [DOI] [PubMed] [Google Scholar]
- 49.Feizy J., Beheshti H.R., Fahim N.K., Janati S.S.F., Davari G. Survey of aflatoxins in rice from Iran using immunoaffinity column clean-up and HPLC with fluorescence detection. Food Addit. Contam.: Part B. 2010;3:263–267. doi: 10.1080/19440049.2010.516456. [DOI] [PubMed] [Google Scholar]
- 50.Nazari F., Sulyok M., Yazdanpanah H., Kobarfard F., Krska R. A survey of mycotoxins in domestic rice in Iran by liquid chromatography tandem mass spectrometry. Toxicol. Mech. Methods. 2014;24:37–41. doi: 10.3109/15376516.2013.844752. [DOI] [PubMed] [Google Scholar]
- 51.Rahmani A., Soleimany F., Hosseini H., Nateghi L. Survey on the occurrence of aflatoxins in rice from different provinces of Iran. Food Addit. Contam.: Part B. 2011;4:185–190. doi: 10.1080/19393210.2011.599865. [DOI] [PubMed] [Google Scholar]
- 52.Samsudin N.I.P., Abdullah N. A preliminary survey on the occurrence of mycotoxigenic fungi and mycotoxins contaminating red rice at consumer level in Selangor, Malaysia. Mycotoxin Res. 2013;29:89–96. doi: 10.1007/s12550-012-0154-7. [DOI] [PubMed] [Google Scholar]
- 53.Abdullah N., Nawawi A., Othman I. Survey of fungal counts and natural occurrence of aflatoxins in Malaysian starch-based foods. Mycopathologia. 1998;143:53–58. doi: 10.1023/a:1006945514876. [DOI] [PubMed] [Google Scholar]
- 54.Reddy K.R.N., Farhana N.I., Salleh B. Occurrence of Aspergillus spp. and aflatoxin B1 in Malaysian foods used for human consumption. J. Food Sci. 2011;76:T99–T104. doi: 10.1111/j.1750-3841.2011.02133.x. [DOI] [PubMed] [Google Scholar]
- 55.Iqbal S.Z., Asi M.R., Hanif U., Zuber M., Jinap S. The presence of aflatoxins and ochratoxin A in rice and rice products; and evaluation of dietary intake. Food Chem. 2016;210:135–140. doi: 10.1016/j.foodchem.2016.04.104. [DOI] [PubMed] [Google Scholar]
- 56.Asghar M.A., Iqbal J., Ahmed A., Khan M.A. Occurrence of aflatoxins contamination in brown rice from Pakistan. Iran. J. Public Health. 2014;43:291–299. [PMC free article] [PubMed] [Google Scholar]
- 57.Firdous S., Ashfaq A., Khan S.J., Khan N. Aflatoxins in corn and rice sold in Lahore, Pakistan. Food Addit. Contam.: Part B. 2014;7:95–98. doi: 10.1080/19393210.2013.851123. [DOI] [PubMed] [Google Scholar]
- 58.Firdous S., Ejaz N., Aman T., Khan N. Occurrence of aflatoxins in export-quality Pakistani rice. Food Addit. Contam.: Part B. 2012;5:121–125. doi: 10.1080/19393210.2012.675360. [DOI] [PubMed] [Google Scholar]
- 59.Iqbal S.Z., Asi M.R., Ariño A., Akram N., Zuber M. Aflatoxin contamination in different fractions of rice from Pakistan and estimation of dietary intakes. Mycotoxin Res. 2012;28:175–180. doi: 10.1007/s12550-012-0131-1. [DOI] [PubMed] [Google Scholar]
- 60.Majeed S., Iqbal M., Asi M.R., Iqbal S.Z. Aflatoxins and ochratoxin A contamination in rice, corn and corn products from Punjab, Pakistan. J. Cereal Sci. 2013;58:446–450. [Google Scholar]
- 61.Nguyen M., Tozlovanu M., Tran T., Pfohlleszkowicz A. Occurrence of aflatoxin B1, citrinin and ochratoxin A in rice in five provinces of the central region of Vietnam. Food Chem. 2007;105:42–47. [Google Scholar]
- 62.Bandara J., Vithanege A.K., Bean G.A. Occurrence of aflatoxins in parboiled rice in Sri Lanka. Mycopathologia. 1991;116:65–70. doi: 10.1007/BF00436366. [DOI] [PubMed] [Google Scholar]
- 63.Panrapee I., Phakpoom K., Thanapoom M., Nampeung A., Warapa M. Exposure to aflatoxin B1 in Thailand by consumption of brown and color rice. Mycotoxin Res. 2016;32:19–25. doi: 10.1007/s12550-015-0236-4. [DOI] [PubMed] [Google Scholar]
- 64.Osman N.A., Abdelgadir A.M., Moss M.O., Bener A. Aflatoxin contamination of rice in the United Arab Emirates. Mycotoxin Res. 1999;15:39–44. doi: 10.1007/BF02945213. [DOI] [PubMed] [Google Scholar]
- 65.Sangare-Tigori B., Moukha S., Kouadio H.J., Betbeder A.-M., Dano D.S., Creppy E.E. Co-occurrence of aflatoxin B 1, fumonisin B 1, ochratoxin A and zearalenone in cereals and peanuts from Côte d’Ivoire. Food Addit. Contam. 2006;23:1000–1007. doi: 10.1080/02652030500415686. [DOI] [PubMed] [Google Scholar]
- 66.Madbouly A.K., Ibrahim M.I.M., Sehab A.F., Abdel-Wahhab M.A. Co-occurrence of mycoflora, aflatoxins and fumonisins in maize and rice seeds from markets of different districts in Cairo, Egypt. Food Addit. Contam.: Part B. 2012;5:112–120. doi: 10.1080/19393210.2012.676078. [DOI] [PubMed] [Google Scholar]
- 67.Reiter E.V., Vouk F., Böhm J., Razzazi-Fazeli E. Aflatoxins in rice – a limited survey of products marketed in Austria. Food Control. 2010;21:988–991. [Google Scholar]
- 68.Ruadrew S., Craft J., Aidoo K. Occurrence of toxigenic Aspergillus spp. and aflatoxins in selected food commodities of Asian origin sourced in the West of Scotland. Food Chem. Toxicol. 2013;55:653–658. doi: 10.1016/j.fct.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 69.Fredlund E., Thim A.-M., Gidlund A., Brostedt S., Nyberg M., Olsen M. Moulds and mycotoxins in rice from the Swedish retail market. Food Addit. Contam.: Part A. 2009;26:527–533. doi: 10.1080/02652030802562912. [DOI] [PubMed] [Google Scholar]
- 70.Bansal J., Pantazopoulos P., Tam J., Cavlovic P., Kwong K., Turcotte A.-M., Lau B.P.-Y., Scott P.M. Surveys of rice sold in Canada for aflatoxins, ochratoxin A and fumonisins. Food Addit. Contam.: Part A. 2011;28:767–774. doi: 10.1080/19440049.2011.559279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Almeida M.I., Almeida N.G., Carvalho K.L., Gonçalves G.A.A., Silva C.N., Santos E.A., Garcia J.C., Vargas E.A. Co-occurrence of aflatoxins B 1, B 2, G 1 and G 2, ochratoxin A, zearalenone, deoxynivalenol, and citreoviridin in rice in Brazil. Food Addit. Contam.: Part A. 2012;29:694–703. doi: 10.1080/19440049.2011.651750. [DOI] [PubMed] [Google Scholar]
- 72.Diaz G., Perilla N., Rojas Y. Occurrence of aflatoxins in selected Colombian foods. Mycotoxin Res. 2001;17:1520. doi: 10.1007/BF02946113. [DOI] [PubMed] [Google Scholar]
- 73.Ortiz J., Van Camp J., Mestdagh F., Donoso S., De Meulenaer B. Mycotoxin co-occurrence in rice, oat flakes and wheat noodles used as staple foods in Ecuador. Food Addit. Contam.: Part A. 2013;30:2165–2176. doi: 10.1080/19440049.2013.853228. [DOI] [PubMed] [Google Scholar]
- 74.Lin L., Zhang J., Wang P., Wang Y., Chen J. Thin-layer chromatography of mycotoxins and comparison with other chromatographic methods. J. Chromatogr. A. 1998;815:3–20. doi: 10.1016/s0021-9673(98)00204-0. [DOI] [PubMed] [Google Scholar]
- 75.Zheng Z., Humphrey C.W., King R.S., Richard J.L. Validation of an ELISA test kit for the detection of total aflatoxins in grain and grain products by comparison with HPLC. Mycopathologia. 2005;159:255–263. doi: 10.1007/s11046-004-8666-0. [DOI] [PubMed] [Google Scholar]
- 76.Park J.W., Kim E.K., Kim Y.B. Estimation of the daily exposure of Koreans to aflatoxin B 1 through food consumption. Food Addit. Contam. 2004;21:70–75. doi: 10.1080/02652030310001622782. [DOI] [PubMed] [Google Scholar]
- 77.World Health Organisation (WHO) World Health Organisation; Geneva (Switzerland): 1998. Safety Evaluation of Certain Food Additives and Contaminants. Food Additives Series No. 40. [Google Scholar]
- 78.Hill J.E., Lomax L.G., Cole R.J., Dorner J.W. Toxicologic and immunologic effects of sublethal doses of cyclopiazonic acid in rats. Am. J. Vet. Res. 1986;47:1174–1177. [PubMed] [Google Scholar]
- 79.Mazaheri M. Determination of aflatoxins in imported rice to Iran. Food Chem. Toxicol. 2009;47:2064–2066. doi: 10.1016/j.fct.2009.05.027. [DOI] [PubMed] [Google Scholar]
- 80.Commission Regulation (EC) No. 1881/2006 of 19 December . 2006. Setting Maximum Levels for Certain Contaminants in Foodstuffs. [Google Scholar]
- 81.Commission Regulation (EC) No 165/2010 of 26 February . 2010. Amending Regulation (EC) No 1881/2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs As Regards Aflatoxins. [Google Scholar]
- 82.Jager A.V., Ramalho F.S., Zambelli L.N., Oliveira C.A.F. Aflatoxins-Biochemistry and Molecular Biology. InTech; 2011. Biomarkers of aflatoxin exposure and its relationship with the hepatocellular carcinoma. [Google Scholar]
- 83.Iqbal J., Asghar M.A., Ahmed A., Khan M.A., Jamil K. Aflatoxins contamination in Pakistani brown rice: a comparison of TLC, HPLC, LC–MS/MS and ELISA techniques. Toxicol. Mech. Methods. 2014;24:544–551. doi: 10.3109/15376516.2014.948247. [DOI] [PubMed] [Google Scholar]
- 84.Wild C.P., Rasheed F.N., Jawla M.F., Hall A.J., Jansen L.A., Montesano R. In-utero exposure to aflatoxin in West Africa. Lancet. 1991;337:1602. doi: 10.1016/0140-6736(91)93295-k. [DOI] [PubMed] [Google Scholar]
- 85.Magoha H., Kimanya M., De Meulenaer B., Roberfroid D., Lachat C., Kolsteren P. Association between aflatoxin M1 exposure through breast milk and growth impairment in infants from Northern Tanzania. World Mycotoxin J. 2014;7:277–284. [Google Scholar]
- 86.Kroker-Lobos M.F., Alvarez C.S., Rivera-Andrade A., Smith J.W., Egner P., Torres O., Lazo M., Freedman N.D., Guallar E., Graubard B.I., McGlynn K.A., Ramírez-Zea M., Groopman J.D. Association between aflatoxin-albumin adduct levels and tortilla consumption in Guatemalan adults. Toxicol. Rep. 2019;6:465–471. doi: 10.1016/j.toxrep.2019.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Turner P.C., Collinson A.C., Cheung Y.B., Gong Y., Hall A.J., Prentice A.M., Wild C.P. Aflatoxin exposure in utero causes growth faltering in Gambian infants. Int. J. Epidemiol. 2007;36:1119–1125. doi: 10.1093/ije/dym122. [DOI] [PubMed] [Google Scholar]
- 88.Mupunga I., Mngqawa P., Katerere D. Peanuts, aflatoxins and undernutrition in children in Sub-Saharan Africa. Nutrients. 2017;9:1287. doi: 10.3390/nu9121287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Khlangwiset P., Shephard G.S., Wu F. Aflatoxins and growth impairment: a review. Crit. Rev. Toxicol. 2011;41:740–755. doi: 10.3109/10408444.2011.575766. [DOI] [PubMed] [Google Scholar]
- 90.Turner P.C., Moore S.E., Hall A.J., Prentice A.M., Wild C.P. Modification of immune function through exposure to dietary aflatoxin in Gambian children. Environ. Health Perspect. 2003;111:217–220. doi: 10.1289/ehp.5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Azziz-Baumgartner E., Lindblade K., Gieseker K., Rogers H.S., Kieszak S., Njapau H., Schleicher R., McCoy L.F., Misore A., DeCock K. Case–control study of an acute aflatoxicosis outbreak, Kenya, 2004. Environ. Health Perspect. 2005;113:1779–1783. doi: 10.1289/ehp.8384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Qian G.-S., Ross R.K., Yu M.C., Yuan J.-M., Gao Y.-T., Henderson B.E., Wogan G.N., Groopman J.D. A follow-up study of urinary markers of aflatoxin exposure and liver cancer risk in Shanghai, People’s Republic of China. Cancer Epidemiol. Prevent. Biomark. 1994;3:3–10. [PubMed] [Google Scholar]
- 93.Wu H.-C., Wang Q., Yang H.-I., Ahsan H., Tsai W.-Y., Wang L.-Y., Chen S.-Y., Chen C.-J., Santella R.M. Aflatoxin B1 exposure, hepatitis B virus infection, and hepatocellular carcinoma in Taiwan. Cancer Epidemiol. Prevent. Biomark. 2009;18:846–853. doi: 10.1158/1055-9965.EPI-08-0697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Krishnamachari K.A.V.R., Nagarajan V., Bhat R., Tilak T.B.G. Hepatitis due to aflatoxicosis–an outbreak in Western India. Lancet. 1975;305:1061–1063. doi: 10.1016/s0140-6736(75)91829-2. [DOI] [PubMed] [Google Scholar]
- 95.Tandon B.N., Krishnamurthy L., Koshy A., Tandon H.D., Ramalingaswami V., Bhandari J.R., Mathur M.M., Mathur P.D. Study of an epidemic of jaundice, presumably due to toxic hepatitis, in northwest India. Gastroenterology. 1977;72:488–494. [PubMed] [Google Scholar]
- 96.Kensler T.W., Roebuck B.D., Wogan G.N., Groopman J.D. Aflatoxin: a 50-year odyssey of mechanistic and translational toxicology. Toxicol. Sci. 2010;120:S28–S48. doi: 10.1093/toxsci/kfq283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Turner P.C., Flannery B., Isitt C., Ali M., Pestka J. The role of biomarkers in evaluating human health concerns from fungal contaminants in food. Nutr. Res. Rev. 2012;25:162–179. doi: 10.1017/S095442241200008X. [DOI] [PubMed] [Google Scholar]
- 98.Gerding J., Ali N., Schwartzbord J., Cramer B., Brown D.L., Degen G.H., Humpf H.-U. A comparative study of the human urinary mycotoxin excretion patterns in Bangladesh, Germany, and Haiti using a rapid and sensitive LC-MS/MS approach. Mycotoxin Res. 2015;31:127–136. doi: 10.1007/s12550-015-0223-9. [DOI] [PubMed] [Google Scholar]
- 99.Solfrizzo M., Gambacorta L., Visconti A. Assessment of multi-mycotoxin exposure in southern Italy by urinary multi-biomarker determination. Toxins. 2014;6:523–538. doi: 10.3390/toxins6020523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Degen G. Tools for investigating workplace-related risks from mycotoxin exposure. World Mycotoxin J. 2011;4:315–327. [Google Scholar]
- 101.Groopman J.D., Egner P.A., Schulze K.J., Wu L.S.-F., Merrill R., Mehra S., Shamim A.A., Ali H., Shaikh S., Gernand A. Aflatoxin exposure during the first 1000 days of life in rural South Asia assessed by aflatoxin B 1-lysine albumin biomarkers. Food Chem. Toxicol. 2014;74:184–189. doi: 10.1016/j.fct.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mitchell N.J., Kumi J., Johnson N.M., Dotse E., Marroquin-Cardona A., Wang J.-S., Jolly P.E., Ankrah N.-A., Phillips T.D. Reduction in the urinary aflatoxin M1 biomarker as an early indicator of the efficacy of dietary interventions to reduce exposure to aflatoxins. Biomarkers. 2013;18:391–398. doi: 10.3109/1354750X.2013.798031. [DOI] [PubMed] [Google Scholar]
- 103.Zhu J., Zhang L., Hu X., Xiao Y., Chen J., Xu Y., Fremy J., Chu F.S. Correlation of dietary aflatoxin B1 levels with excretion of aflatoxin M1 in human urine. Cancer Res. 1987;47:1848–1852. [PubMed] [Google Scholar]
- 104.Yard E.E., Daniel J.H., Lewis L.S., Rybak M.E., Paliakov E.M., Kim A.A., Montgomery J.M., Bunnell R., Abudo M.U., Akhwale W. Human aflatoxin exposure in Kenya, 2007: a cross-sectional study. Food Addit. Contam.: Part A. 2013;30:1322–1331. doi: 10.1080/19440049.2013.789558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Asiki G., Seeley J., Srey C., Baisley K., Lightfoot T., Archileo K., Agol D., Abaasa A., Wakeham K., Routledge M.N. A pilot study to evaluate aflatoxin exposure in a rural Ugandan population. Trop. Med. Int. Health. 2014;19:592–599. doi: 10.1111/tmi.12283. [DOI] [PubMed] [Google Scholar]
- 106.Shirima C.P., Kimanya M.E., Kinabo J.L., Routledge M.N., Srey C., Wild C.P., Gong Y.Y. Dietary exposure to aflatoxin and fumonisin among T anzanian children as determined using biomarkers of exposure. Mol. Nutr. Food Res. 2013;57:1874–1881. doi: 10.1002/mnfr.201300116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Turner P.C., Loffredo C., Kafrawy S.E., Ezzat S., Eissa S.A.L., Daly M.E., Nada O., Abdel-Hamid M. Pilot survey of aflatoxin–albumin adducts in sera from Egypt. Food Addit. Contam. 2008;25:583–587. doi: 10.1080/02652030701713939. [DOI] [PubMed] [Google Scholar]
- 108.Piekkola S., Turner P.C., Abdel-Hamid M., Ezzat S., El-Daly M., El-Kafrawy S., Savchenko E., Poussa T., Woo J.C.S., Mykkänen H. Characterisation of aflatoxin and deoxynivalenol exposure among pregnant Egyptian women. Food Addit. Contam.: Part A. 2012;29:962–971. doi: 10.1080/19440049.2012.658442. [DOI] [PubMed] [Google Scholar]
- 109.Leong Y.-H., Rosma A., Latiff A.A., Izzah A.N. Associations of serum aflatoxin B1–lysine adduct level with socio-demographic factors and aflatoxins intake from nuts and related nut products in Malaysia. Int. J. Hyg. Environ. Health. 2012;215:368–372. doi: 10.1016/j.ijheh.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 110.Scussel V.M., Haas P., Gong Y.Y., Turner C.P., Wild C.P. Study of aflatoxin exposure in a Brazilian population using an aflatoxin-albumin biomarker, mycotoxins and phycotoxins: advances in determination. Toxicol. Expo. Manage. 2006:197–202. [Google Scholar]
- 111.Ali N., Hossain K., Blaszkewicz M., Rahman M., Mohanto N.C., Alim A., Degen G.H. Occurrence of aflatoxin M1 in urines from rural and urban adult cohorts in Bangladesh. Arch. Toxicol. 2016;90:1749–1755. doi: 10.1007/s00204-015-1601-y. [DOI] [PubMed] [Google Scholar]
- 112.Redzwan S.M., Rosita J., Sokhini A.M.M., Aqilah A.R.N. Association between aflatoxin M 1 excreted in human urine samples with the consumption of milk and dairy products. Bull. Environ. Contam. Toxicol. 2012;89:1115–1119. doi: 10.1007/s00128-012-0853-y. [DOI] [PubMed] [Google Scholar]
- 113.Lei Y., Fang L., Akash M.S.H., Rehman K., Liu Z., Shi W., Chen S. Estimation of urinary concentration of aflatoxin M1 in Chinese pregnant women. J. Food Sci. 2013;78:T1835–T1838. doi: 10.1111/1750-3841.12259. [DOI] [PubMed] [Google Scholar]
- 114.Sun Z., Chen T., Thorgeirsson S.S., Zhan Q., Chen J., Park J.-H., Lu P., Hsia C.C., Wang N., Xu L. Dramatic reduction of liver cancer incidence in young adults: 28 year follow-up of etiological interventions in an endemic area of China. Carcinogenesis. 2013;34:1800–1805. doi: 10.1093/carcin/bgt007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Warth B., Petchkongkaew A., Sulyok M., Krska R. Utilising an LC-MS/MS-based multi-biomarker approach to assess mycotoxin exposure in the Bangkok metropolitan area and surrounding provinces. Food Addit. Contam.: Part A. 2014;31:2040–2046. doi: 10.1080/19440049.2014.969329. [DOI] [PubMed] [Google Scholar]
- 116.Jolly P., Jiang Y., Ellis W., Awuah R., Nnedu O., Phillips T., Wang J.-S., Afriyie-Gyawu E., Tang L., Person S. Determinants of aflatoxin levels in Ghanaians: sociodemographic factors, knowledge of aflatoxin and food handling and consumption practices. Int. J. Hyg. Environ. Health. 2006;209:345–358. doi: 10.1016/j.ijheh.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 117.Ezekiel C.N., Warth B., Ogara I.M., Abia W.A., Ezekiel V.C., Atehnkeng J., Sulyok M., Turner P.C., Tayo G.O., Krska R. Mycotoxin exposure in rural residents in northern Nigeria: a pilot study using multi-urinary biomarkers. Environ. Int. 2014;66:138–145. doi: 10.1016/j.envint.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 118.Polychronaki N., Wild C.P., Mykkänen H., Amra H., Abdel-Wahhab M., Sylla A., Diallo M., El-Nezami H., Turner P.C. Urinary biomarkers of aflatoxin exposure in young children from Egypt and Guinea. Food Chem. Toxicol. 2008;46:519–526. doi: 10.1016/j.fct.2007.08.034. [DOI] [PubMed] [Google Scholar]
- 119.Kouadio J.H., Lattanzio V.M., Ouattara D., Kouakou B., Visconti A. Assessment of mycotoxin exposure in Côte d’ivoire (Ivory Coast) through multi-biomarker analysis and possible correlation with food consumption patterns. Toxicol. Int. 2014;21:248. doi: 10.4103/0971-6580.155336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Abia W.A., Warth B., Sulyok M., Krska R., Tchana A., Njobeh P.B., Turner P.C., Kouanfack C., Eyongetah M., Dutton M. Bio-monitoring of mycotoxin exposure in Cameroon using a urinary multi-biomarker approach. Food Chem. Toxicol. 2013;62:927–934. doi: 10.1016/j.fct.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 121.Föllmann W., Ali N., Blaszkewicz M., Degen G.H. Biomonitoring of mycotoxins in urine: pilot study in mill workers. J. Toxicol. Environ. Health Part A. 2016;79:1015–1025. doi: 10.1080/15287394.2016.1219540. [DOI] [PubMed] [Google Scholar]
- 122.Huybrechts B., Martins J.C., Debongnie P., Uhlig S., Callebaut A. Fast and sensitive LC–MS/MS method measuring human mycotoxin exposure using biomarkers in urine. Arch. Toxicol. 2015;89:1993–2005. doi: 10.1007/s00204-014-1358-8. [DOI] [PubMed] [Google Scholar]
- 123.Jager A.V., Tonin F.G., Baptista G.Z., Souto P.C., Oliveira C.A. Assessment of aflatoxin exposure using serum and urinary biomarkers in São Paulo, Brazil: a pilot study. Int. J. Hyg. Environ. Health. 2016;219:294–300. doi: 10.1016/j.ijheh.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 124.Jager A.V., Tonin F.G., Souto P.C., Privatti R.T., Oliveira C.A. Determination of urinary biomarkers for assessment of short-term human exposure to aflatoxins in São Paulo, Brazil. Toxins. 2014;6:1996–2007. doi: 10.3390/toxins6071996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.de Cássia Romero A., Ferreira T.R.B., dos Santos Dias C.T., Calori-Domingues M.A., da Gloria E.M. Occurrence of AFM1 in urine samples of a Brazilian population and association with food consumption. Food Control. 2010;21:554–558. [Google Scholar]
- 126.Johnson N.M., Qian G., Xu L., Tietze D., Marroquin-Cardona A., Robinson A., Rodriguez M., Kaufman L., Cunningham K., Wittmer J. Aflatoxin and PAH exposure biomarkers in a US population with a high incidence of hepatocellular carcinoma. Sci. Total Environ. 2010;408:6027–6031. doi: 10.1016/j.scitotenv.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Schwartzbord J.R., Leroy J.L., Severe L., Brown D.L. Urinary aflatoxin M1 in Port-au-Prince and a rural community in north-east Haiti. Food Addit. Contam.: Part A. 2016;33:1036–1042. doi: 10.1080/19440049.2016.1185899. [DOI] [PubMed] [Google Scholar]
- 128.Abdulrazzaq Y.M., Osman N., Yousif Z.M., Al-Falahi S. Aflatoxin M1 in breast-milk of UAE women. Ann. Trop. Paediatr. 2003;23:173–179. doi: 10.1179/027249303322296484. [DOI] [PubMed] [Google Scholar]
- 129.Navas S.A., Sabino M., Rodriguez-Amaya D.B. Aflatoxin M1 and ochratoxin A in a human milk bank in the city of Sao Paulo, Brazil. Food Addit. Contam. 2005;22:457–462. doi: 10.1080/02652030500110550. [DOI] [PubMed] [Google Scholar]
- 130.Sadeghi N., Oveisi M.R., Jannat B., Hajimahmoodi M., Bonyani H., Jannat F. Incidence of aflatoxin M1 in human breast milk in Tehran, Iran. Food Control. 2009;20:75–78. [Google Scholar]
- 131.Roy M., Harris J., Afreen S., Deak E., Gade L., Balajee S.A., Park B., Chiller T., Luby S. Aflatoxin contamination in food commodities in Bangladesh. Food Addit. Contam.: Part B. 2013;6:17–23. doi: 10.1080/19393210.2012.720617. [DOI] [PubMed] [Google Scholar]
- 132.European Commission (EC) Commission Regulation No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union. 2006;364:5–24. [Google Scholar]