Abstract
Background
Epidemiologic knowledge regarding noncardiovascular and all‐cause mortality in apparently healthy cats (AH) and cats with preclinical hypertrophic cardiomyopathy (pHCM) is limited, hindering development of evidence‐based healthcare guidelines.
Objectives
To characterize/compare incidence rates, risk, and survival associated with noncardiovascular and all‐cause mortality in AH and pHCM cats.
Animals
A total of 1730 client‐owned cats (722 AH, 1008 pHCM) from 21 countries.
Methods
Retrospective, multicenter, longitudinal, cohort study. Long‐term health data were extracted by medical record review and owner/referring veterinarian interviews.
Results
Noncardiovascular death occurred in 534 (30.9%) of 1730 cats observed up to 15.2 years. Proportion of noncardiovascular death did not differ significantly between cats that at study enrollment were AH or had pHCM (P = .48). Cancer, chronic kidney disease, and conditions characterized by chronic weight‐loss‐vomiting‐diarrhea‐anorexia were the most frequently recorded noncardiovascular causes of death. Incidence rates/risk of noncardiac death increased with age in AH and pHCM. All‐cause death proportions were greater in pHCM than AH (65% versus 40%, respectively; P < .001) because of higher cardiovascular mortality in pHCM cats. Comparing AH with pHCM, median survival (study entry to noncardiovascular death) did not differ (AH, 9.8 years; pHCM, 8.6 years; P = .10), but all‐cause survival was significantly shorter in pHCM (P = .0001).
Conclusions and Clinical Importance
All‐cause mortality was significantly greater in pHCM cats due to disease burden contributed by increased cardiovascular death superimposed upon noncardiovascular death.
Keywords: cancer, chronic kidney disease, epidemiology, mortality, survival
Abbreviations
- AH
apparently healthy cats
- CKD
chronic kidney disease
- CWLVDA
conditions characterized by chronic weight loss, vomiting, diarrhea, and anorexia
- HCM
nonobstructive hypertrophic cardiomyopathy
- HCM/HOCM
combined HCM/HOCM cohort
- HOCM
obstructive hypertrophic cardiomyopathy
- IQR
interquartile range
- LV
left ventricular
- pHCM
preclinical hypertrophic cardiomyopathy
1. INTRODUCTION
Contemporary pet ownership surveys demonstrate substantial growth in feline pet populations. In the United States, there were approximately 74 million pet cats in 2012 (AVMA, US Pet Ownership & Demographics Sourcebook, 2012), and 94.2 million estimated for the 2017‐2018 time period (2017‐2018 APPA National Pet Owners Survey, http://americanpetproducts.org/pubs_survey.asp). Nevertheless, little has been published about long‐term health outcomes of domestic cats, constraining development of effective health‐monitoring strategies.
Most studies derive from veterinary insurance reports,1, 2, 3, 4, 5 cemetery6 and necropsy records,7 surveys,8, 9, 10 clinical registries,11 national research databases,12 health screening, and 13 medical record reviews from primary14, 15 and tertiary care16 practices. Information from these sources is generally limited to prevalence data, that is, the percentage of deaths due to a disease or condition identified at a point in time. However, insights regarding incidence, that is, the rate of new conditions arising in populations over time, are scarce; risk and survival comparisons for preclinical cohorts are largely unreported; and population effects related to age and cause‐specific mortality remain uncertain.
The REVEAL study reported cardiovascular morbidity and survival in 1730 apparently healthy cats (AH) and cats with preclinical hypertrophic cardiomyopathy (pHCM).17 To further our understanding of feline longevity, the present study aimed to identify the epidemiology of noncardiovascular and all‐cause death and assess its overall impact on health in the same population. Specific objectives were to determine from medical records, the major causes of death, contrast incidence rates, risk, and survival characteristics, and compare these observations between susceptible populations and age groups over a prolonged period of time.
2. MATERIALS AND METHODS
2.1. Study design
Data were derived from an analysis of medical and demographic data collected for the REVEAL Study17 project. Ethical review committee approval was obtained where required.
2.2. Cats
Analysis included 1730 cats: 722 AH and 1008 pHCM (430 nonobstructive hypertrophic cardiomyopathy [HCM] and 578 obstructive hypertrophic cardiomyopathy [HOCM]).17 Enrolled cats had normal physical examination except for the presence of heart murmurs in some cats. No other known serious illness or medical history abnormalities were detected. All had echocardiographic examinations performed at the time of study entry. The study cohort of AH received echocardiographic examinations for preanesthetic workups, to evaluate cardiac status when heart murmurs were detected, as part of case recruitment of AH without significant heart disease for the present study, and for breed screening in certain pedigrees.
2.2.1. Inclusion criteria
Each investigator had a searchable echocardiographic and medical record database permitting detailed review and long‐term health follow‐up. Medical records were examined for cats diagnosed with either HCM or HOCM, and AH without cardiomyopathy, whose health outcomes could be ascertained for at least 5 years after the date of study entry. Archived echocardiographic images were reviewed to confirm diagnosis. Study entry was recorded as the date when echocardiographic examination was first performed.17
2.2.2. Exclusion criteria
Any of the following conditions diagnosed at or before study entry resulted in study exclusion: congestive heart failure; arterial thromboembolism; syncope; heartworm disease; arterial hypertension (systolic arterial blood pressure ≥180 mm Hg); hyperthyroidism; anemia; chronic kidney disease (CKD), defined as any combination of serum creatinine concentration above laboratory reference interval, urine concentrating ability deemed to be inadequate or isosthenuria, proteinuria, and small, irregular kidneys; cardiomyopathy other than pHCM; congenital heart disease; cancer; any systemic, endocrine, hepatobiliary, pancreatic, or chronic gastrointestinal disease; and medical or surgical condition judged capable of limiting life expectancy.
2.3. Study sites
Investigators participated from 50 veterinary centers in 21 countries.17
2.4. Echocardiography
Cardiac diagnoses were confirmed from archived echocardiograms. Left ventricular (LV) hypertrophy represented end‐diastolic LV free wall and or interventricular wall thickness ≥6 mm.18 Hypertrophic obstructive cardiomyopathy was defined as LV hypertrophy with systolic anterior motion of the mitral valve, diffuse LV outflow tract turbulence, and peak systolic outflow velocity ≥2.5 m/s, whereas HCM represented hypertrophic cardiomyopathy without LV outflow tract obstruction.
2.5. Data collection and outcomes assessment
Medical records where the first echocardiographic examination was performed between November 2001 and January 2011 were reviewed to identify recorded death or permitted at least 5 years of follow‐up. Data collection extended to January 2016. Pertinent demographic and survival information was recorded. Serum thyroxine and creatinine concentrations and systolic arterial blood pressure results closest to the date of diagnosis were examined but were not available for every case. Date of death was designated by natural death or euthanasia. Cause of death was inferred to be the predominant condition or disease which, based on all available information including medical history and physical examination findings, diagnostic imaging, clinical pathology, ancillary testing, histopathology from biopsy, and or necropsy, contributed most substantially. When cause or date of death was unavailable, or if medical and clinical circumstances associated with death were uncertain, referral veterinarians or the pet owners were interviewed, aided by a standardized medical questionnaire.17 Cause of death was grouped into categories. Cancer death was supported by detection of a mass, masses, lymphadenopathy by physical examination or diagnostic imaging, or by confirmation from cytology, histology, or necropsy when performed. Supporting CKD death were history of polyuria and polydipsia, serum creatinine concentration exceeding laboratory reference interval, isosthenuria or inadequate urine concentrating ability, proteinuria, and small and irregular kidney size and shape by physical examination or abdominal ultrasound. Death was attributed to debilitation caused by 1 or more of the following: chronic weight loss, vomiting, diarrhea, and or anorexia (CWLVDA). Etiologies for CWLVDA mortality included exocrine pancreatic insufficiency and chronic, non‐neoplastic gastrointestinal disease including inflammatory bowel disease, and without clinical evidence for cancer, endocrine, metabolic diseases, or known conditions that could have caused these signs. Cancer or CKD death was the designated cause of death if criteria for these diagnoses were met, even when malaise, weight loss, vomiting, or diarrhea was present. A noncardiovascular death category denoted as “other causes” was designated when death resulted from anesthetic complications, trauma, endocrine diseases including diabetes mellitus and hyperthyroidism, hepatobiliary diseases including hepatic lipidosis and cholangiohepatitis, central nervous system diseases, toxicoses, and respiratory system diseases. Death was classified as “unknown cause” when there was insufficient or conflicting information or overlapping comorbidities.
2.6. Statistical analysis
Date of study entry was the time of echocardiographic examination and diagnosis. Data obtained at this time were evaluated in descriptive, baseline analyses, reported as mean and standard deviation for normally distributed variables, and median (interquartile range) for nonnormally distributed variables. Analysis of variance was used for between‐group analyses, as error residuals were normally distributed based on visual inspection. Analyses for proportions of categorical variables were evaluated using chi‐square or Fisher's exact test as appropriate. A generalized linear model was used to calculate incidence for the entire population and cohort level by age quartile expressed as rates per 1000 cat‐years, as goodness of fit assumptions were met. Kaplan‐Meier analysis was used to calculate proportion at‐risk and compare noncardiovascular and all‐cause survival within AH and HCM/HOCM cohorts. Survival time was further assessed at 1, 2.5, 5, 7.5, and 10‐year intervals after study entry, and these specified time points dictated the percentage of patients at‐risk as calculated by Kaplan‐Meier, permitting a cross‐sectional view of the respective time points. Additional analyses included stratification at age quartile determined by age at study entry. Univariate time‐to‐event survival analyses were performed using Kaplan‐Meier product limit estimates, where survival range was presented if median survival was not reached. Statistical differences between strata were determined by a log‐rank test. Time‐to‐event survival time analyses represented time from study entry to end‐date (death, date lost to follow‐up, or remaining alive at study termination). Cases lost to follow‐up or remaining alive were right‐censored. Analyses were performed with SAS 9.4 (Cary, North Carolina). P < .05 was deemed significant.
3. RESULTS
3.1. Population characteristics at time of diagnosis
Study population demographic data were recently reported.17 Briefly, median age of AH (4.9 years) was significantly younger than HCM (7.4 years; P < .001) and HOCM (5.7 years; P = .01) cohorts; HOCM were younger than HCM (P < .001) cohorts, and AH were also younger compared with the HCM/HOCM cohort (6.5 years; P < .001). Thirty‐four breeds comprised the total population. Domestic Shorthair, Maine Coon cat, Persian, Domestic Longhair, and Norwegian Forest cat breeds were most prevalent.
3.2. Overall mortality
Noncardiovascular death was recorded in 534 (30.9%) of 1730 cats. These comprised 230 (31.9%) of 722 AH, and 304 (30.2%) of 1008 HCM/HOCM (159 [37.0%] of 430 HCM and 145 [25.1%] of 578 HOCM; Table 1). Comparing AH with HCM/HOCM cats, there was no significant difference between the proportion of noncardiovascular mortality (P = .48). Cancer, CKD, and CWLVDA were the most commonly recorded causes of noncardiovascular death. Demographic characteristics were compared for cardiovascular and noncardiovascular mortalities (Tables 1 and 2). Approximate male:female ratio for AH and HCM/HOCM cohorts were for cancer, 1:1, 1.8:1; CKD, 0.6:1, 2.5:1; and CWLVDA, 1.5:1, 2.6:1, respectively).
Table 1.
Recorded deaths in cats that at study entry were diagnosed as apparently healthy or with preclinical HCM/HOCM
| Apparently healthy cats | HCM | HOCM | HCM/HOCM | |
|---|---|---|---|---|
| n = 722 | n = 430 | n = 578 | n = 1008 | |
| Cause of death | Number of events (%) | Number of events (%) | Number of events (%) | Number of events (%) |
| Cause‐specific mortality | ||||
| Cancer | 105 (14.5) | 52 (12.1) | 51 (8.8) | 103 (10.2) |
| Chronic kidney disease | 46 (6.4) | 41 (9.5) | 35 (6.1) | 76 (7.5) |
| Chronic weight loss‐vomiting‐diarrhea‐anorexia | 32 (4.4) | 24 (5.6) | 26 (4.5) | 50 (5.0) |
| Other diseases* | 47 (6.5) | 42 (9.8) | 33 (5.7) | 75 (7.4) |
| Unknown causes | 49 (6.8) | 25 (5.8) | 43 (7.4) | 68 (6.7) |
| Overall mortality | ||||
| Noncardiovascular causes | 230 (31.9) | 159 (37.0) | 145 (25.1) | 304 (30.2) |
| Cardiovascular causes | 7 (1.0) | 115 (26.7) | 166 (28.7) | 281 (27.9) |
| All causes** | 286 (39.6) | 299 (69.5) | 354 (61.2) | 653 (64.8) |
Note: Data are arranged by the cause of death.
Abbreviations: HCM, nonobstructive hypertrophic cardiomyopathy; HOCM, obstructive hypertrophic cardiomyopathy.
Death related to anesthesia, respiratory diseases, central nervous system diseases, hepatobiliary diseases, toxicoses, endocrine diseases, trauma.
Includes all cardiovascular, noncardiovascular, and unknown causes of death.
Table 2.
Demographic characteristics (age, body weight, sex) for feline populations that were diagnosed at study entry as apparently healthy or preclinical HCM/HOCM
| Study populations | Apparently healthy cats (n = 722) | HCM/HOCM (n = 1008) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Age, median (IQR), years | Body weight, median (IQR), kg | Sex | Age, median (IQR), years | Body weight, median (IQR), kg | Sex | P value | |||
| M | F | M | F | Apparently healthy versus HCM/HOCM cats | |||||
| Overall mortality | |||||||||
| Noncardiovascular | 9.0 (5.2‐12.6) | 4.2 (3.4‐5.1) | 109 | 121 | 9.8 (6.0‐12.2) | 5.0 (4.0‐5.9) | 210 | 94 | .48 (age) |
| <0.0001 (body weight) | |||||||||
| Cardiovascular | 10 (6.8‐11.0) | 4.8 (3.9‐7.2) | 5 | 2 | 6.4 (3.0‐9.6) | 5.0 (4.2‐6.1) | 214 | 67 | .03 (age) |
| .83 (body weight) | |||||||||
| All causesb | 9.0 (5.0‐12.2) | 4.3 (3.5‐5.3) | 138 | 148 | 8.0 (4.0‐11.0) | 5.0 (4.1‐6.0) | 475 | 178 | .006 (age) |
| <.0001 (body weight) | |||||||||
| Cause‐specific mortality | |||||||||
| Cancer | 10.5 (7.0‐13.7) | 4.6 (3.9‐5.7) | 53 | 52 | 9.0 (6.0‐12.6) | 4.9 (4.1‐5.9) | 66 | 37 | .09 (age) |
| .07 (body weight) | |||||||||
| Chronic kidney disease | 11.8 (7.0‐14.0) | 3.9 (3.2‐4.9) | 18 | 28 | 11.0 (8.2‐14.0) | 4.5 (3.8‐5.5) | 54 | 22 | .87 (age) |
| .03 (body weight) | |||||||||
| Chronic weight loss‐vomiting‐diarrhea‐anorexia | 6.0 (3.6‐11.0) | 3.9 (3.2‐4.7) | 19 | 13 | 10.0 (5.4‐11.8) | 5.3 (4.3‐6.1) | 36 | 14 | .02 (age) |
| <.0001 (body weight) | |||||||||
| Other noncardiovascular diseasesa | 6.5 (2.0‐10.3) | 4.0 (3.2‐4.6) | 19 | 28 | 9.7 (4.0‐12.0) | 4.1 (4.5‐5.6) | 54 | 21 | 0.02 (age) |
| <0.0001 (body weight) | |||||||||
| Unknown causes | 9.0 (3.0‐12.0) | 4.5 (3.9‐6.0) | 24 | 25 | 8.0 (4.0‐11.5) | 5.2 (4.2‐6.4) | 50 | 18 | .18 (age) |
| .80 (body weight) | |||||||||
Note: Data are arranged by cause of death.
Abbreviations: HCM, nonobstructive hypertrophic cardiomyopathy; HOCM, obstructive hypertrophic cardiomyopathy; IQR, interquartile range.
Death related to anesthesia, respiratory diseases, central nervous system diseases, hepatobiliary diseases, toxicoses, endocrine diseases, trauma.
Includes all cardiovascular, noncardiovascular, and unknown causes of death.
3.2.1. Incidence
Mortality rates per 1000 cat‐years were compared between cohorts defined by age quartile at the time of study entry and arranged by cause of death (Table 3). Noncardiovascular mortality was similar between AH and HCM/HOCM cats; however, all‐cause mortality was substantially higher in HCM/HOCM compared to AH due to cardiovascular death, which occurred frequently in HCM/HOCM cats but rarely in AH (Figure 1). Overall, noncardiovascular and all‐cause mortality rates were lowest in the first 2 age quartiles and increased with age (Table 3, Figure 2). Specifically, incidence rates for noncardiovascular death in AH were slightly more than twice higher in both the second compared with the first age quartile and in the third compared with the second quartile, and 2.4 times higher in the fourth compared with the third age quartile; in HCM/HOCM cats, noncardiovascular mortality was 2 times higher in the second compared with the first quartile, 1.8 times higher in the third compared with the second quartile, and 2.6 times higher in the fourth compared with the third quartile. For cause‐specific mortality rates in AH, cancer death was 23.3 times higher in cats >10 years than in cats <2.5 years old; CKD death was 51 times higher in cats >10 than for cats <2.5 years; and CWLVDA death was 3 times higher in cats >10 than <2.5 years. In HCM/HOCM cats, cancer, CKD, and CWLVDA death rates also increased with age. Comparing their mortality rates between cats older than 10 years with cats younger than 2.5 years, cancer death was 6.3 times higher, CKD death was 27.2 times higher, and CWLVDA death was 14.3 times higher, respectively. In both AH and HCM/HOCM cohorts, CKD death incidence rate increased substantially with age, especially in the third and fourth age quartiles (Table 3).
Table 3.
Comparison of incidence rates of cause‐specific death events per 1000 cat‐years
| Age | Population cohorts | Non‐ cardiovascular | Cardiovascular | All causes* | Cancer | Chronic kidney disease | Chronic weight loss‐vomiting‐diarrhea‐anorexia | Other causes** | Unknown causes |
|---|---|---|---|---|---|---|---|---|---|
| Total population | Apparently healthy | 60.9 | 1.9 | 75.4 | 27.8 | 12.7 | 8.7 | 11.1 | 12.7 |
| HCM/HOCM | 68.6 | 63.4 | 147.4 | 23.2 | 17.8 | 11.5 | 12.2 | 15.3 | |
| Age quartile 1 (<2.5 years) | Apparently healthy | 18.3 | 0 | 24.8 | 4.6 | 0 | 5.2 | 7.8 | 6.5 |
| HCM/HOCM | 19.4 | 57.1 | 87.7 | 8.2 | 2.0 | 2.0 | 3.1 | 11.2 | |
| Age quartile 2 (2.5‐5.6 years) | Apparently healthy | 39.3 | 1.12 | 47.2 | 15.7 | 6.7 | 9.0 | 7.9 | 6.7 |
| HCM/HOCM | 39.5 | 57.7 | 110.2 | 11.4 | 6.8 | 8.4 | 8.4 | 12.9 | |
| Age quartile 3 (>5.6‐10 years) | Apparently healthy | 80.4 | 4.7 | 101.7 | 34.3 | 18.9 | 10.6 | 13.0 | 16.6 |
| HCM/HOCM | 69.7 | 72.7 | 153.4 | 29.4 | 19.1 | 11.7 | 10.3 | 11 | |
| Age quartile 4 (>10 years) | Apparently healthy | 193.6 | 3.9 | 232.8 | 107.3 | 50.9 | 15.6 | 23.5 | 35.2 |
| HCM/HOCM | 178.5 | 64.7 | 275.6 | 51.8 | 54.3 | 28.5 | 34.9 | 33.7 |
Note: Feline populations are defined by age quartile at the time of study entry when they were diagnosed as apparently healthy or preclinical HCM/HOCM. Data are arranged by cause of death.
Abbreviations: HCM, nonobstructive hypertrophic cardiomyopathy; HOCM, obstructive hypertrophic cardiomyopathy.
Includes all cardiovascular, noncardiovascular, and unknown causes of death.
Death related to anesthesia, respiratory diseases, central nervous system diseases, hepatobiliary diseases, toxicoses, endocrine diseases, trauma.
Figure 1.
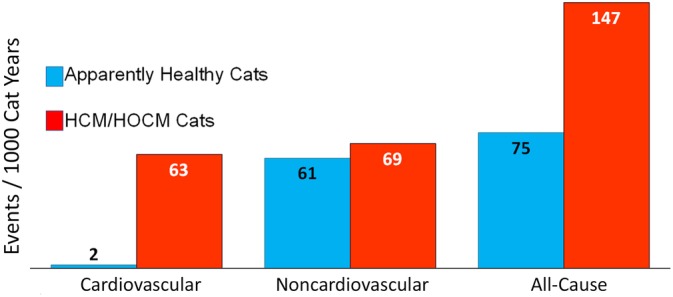
Comparison of cardiovascular, noncardiovascular, and all‐cause mortality rates per 1000 cat‐years recorded over the study duration for 1730 cats. Clinical status was designated at study entry for 722 apparently healthy cats or preclinical hypertrophic (HCM) and hypertrophic obstructive (HOCM) cardiomyopathy (HCM/HOCM, n = 1008)
Figure 2.

Mortality rates per 1000 cat‐years for noncardiovascular (upper graph) and all‐cause (lower graph) death compared by age quartile (Q) at the time of study entry when cats were diagnosed as apparently healthy cats (AH, n = 722) or preclinical hypertrophic (HCM) and hypertrophic obstructive (HOCM) cardiomyopathy (n = 1008)
3.2.2. Risk
Risk of death due to noncardiovascular and all‐cause mortalities increased variably at 1, 2.5, 5, 7.5, and 10‐year intervals following study entry (Table 4, Figure 3). For noncardiovascular mortality, the largest risk increments were recorded at 2.5 years compared with 1 year, and 5 years compared with 2.5 years following study entry (in AH 2.4 and 1.8 times higher, respectively; in HCM/HOCM cats 1.9 and 2.1 times higher, respectively). For all‐cause mortality, the largest risk increments were recorded at 2.5 years compared with 1 year and at 5 years compared with 2.5 years (in AH 2.7 and 1.9 times higher, respectively; in HCM/HOCM 2.0 and 1.9 times higher at each comparison, respectively). Noncardiovascular risk was 4.3 and 3.9 times higher at 5 years compared with 1 year following study entry for AH and HCM/HOCM cats, respectively. In AH, risk of cancer, CKD, and CWLVDA death was 3.9, 7.3, and 4.7 times higher at 5 years compared with 1 year following study entry, respectively; risks for the same mortalities in HCM/HOCM cats were 3.5, 7.0, and 4.9 times higher at 5 years compared with 1 year after study entry, respectively. The increment of risk for these mortalities was comparatively higher at 5 years compared with 10 years after study entry for AH and HCM/HOCM cats. Comparative risk of CKD death was greatest in cats that were in the fourth age quartile at study entry (Table 4).
Table 4.
Risk of mortality
| Noncardiovascular death | All‐cause death* | Cancer death | Chronic kidney disease death | Chronic weight loss‐vomiting‐diarrhea‐anorexia death | Other causes of death** | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Population remaining at risk | Affected | Population remaining at risk | Affected | Population remaining at risk | Affected | Population remaining at risk | Affected | Population remaining at risk | Affected | Population remaining at risk | Affected | ||
| 1 year after study entry | |||||||||||||
| Full population | AH | 94.5 | 5.5 | 94.2 | 5.8 | 97.2 | 2.8 | 99.4 | 0.6 | 99.3 | 0.7 | 98.3 | 1.7 |
| HCM/HOCM | 94.5 | 5.5 | 87.1 | 12.9 | 98.0 | 2.0 | 99.3 | 0.7 | 99.3 | 0.7 | 98.3 | 1.7 | |
| Age quartile1 | AH | 98.4 | 1.6 | 98.4 | 1.6 | 100.0 | 0.0 | 100.0 | 0.0 | 99.6 | 0.4 | 98.8 | 1.2 |
| HCM/HOCM | 97.9 | 2.1 | 92.1 | 7.9 | 99.5 | 0.5 | 99.5 | 0.5 | 100.0 | 0.0 | 98.9 | 1.1 | |
| Age quartile 2 | AH | 96.1 | 3.9 | 96.1 | 3.9 | 98.7 | 1.3 | 99.4 | 0.6 | 98.7 | 1.3 | 99.4 | 0.6 |
| HCM/HOCM | 95.6 | 4.4 | 87.5 | 12.5 | 98.9 | 1.1 | 99.3 | 0.7 | 98.9 | 1.1 | 99.3 | 0.7 | |
| Age quartile 3 | AH | 94.0 | 6.0 | 93.5 | 6.5 | 96.4 | 3.6 | 100.0 | 0.0 | 99.4 | 0.6 | 98.2 | 1.8 |
| HCM/HOCM | 96.5 | 3.5 | 89.2 | 10.8 | 98.1 | 1.9 | 100.0 | 0.0 | 99.7 | 0.3 | 99.4 | 0.6 | |
| Age quartile4 | AH | 86.4 | 13.6 | 85.7 | 14.3 | 91.8 | 8.2 | 98.0 | 2.0 | 99.3 | 0.7 | 96.6 | 3.4 |
| HCM/HOCM | 88.0 | 12.0 | 79.8 | 20.2 | 95.7 | 4.3 | 98.3 | 1.7 | 98.7 | 1.3 | 95.3 | 4.7 | |
| 2.5 years after study entry | |||||||||||||
| Full population | AH | 86.6 | 13.4 | 84.1 | 15.9 | 94.0 | 6.0 | 97.9 | 2.1 | 97.8 | 2.2 | 96.8 | 3.2 |
| HCM/HOCM | 89.8 | 10.2 | 74.2 | 25.8 | 96.3 | 3.7 | 98.3 | 1.7 | 98.2 | 1.8 | 97.5 | 2.5 | |
| Age quartile1 | AH | 96.8 | 3.2 | 95.2 | 4.8 | 99.2 | 0.8 | 100.0 | 0.0 | 99.2 | 0.8 | 98.4 | 1.6 |
| HCM/HOCM | 96.8 | 3.2 | 84.1 | 15.9 | 98.9 | 1.1 | 99.5 | 0.5 | 100.0 | 0.0 | 98.9 | 1.1 | |
| Age quartile 2 | AH | 91.6 | 8.4 | 90.3 | 9.7 | 98.1 | 1.9 | 98.7 | 1.3 | 97.4 | 2.6 | 97.4 | 2.6 |
| HCM/HOCM | 92.6 | 7.4 | 79.0 | 21.0 | 98.2 | 1.8 | 98.5 | 1.5 | 98.2 | 1.8 | 98.9 | 1.1 | |
| Age quartile 3 | AH | 83.9 | 16.1 | 81.0 | 19.0 | 92.9 | 7.1 | 98.2 | 1.8 | 97.6 | 2.4 | 95.8 | 4.2 |
| HCM/HOCM | 93.0 | 7.0 | 76.1 | 23.9 | 97.1 | 2.9 | 99.0 | 1.0 | 98.7 | 1.3 | 98.4 | 1.6 | |
| Age quartile 4 | AH | 66.7 | 33.3 | 61.9 | 38.1 | 82.3 | 17.7 | 93.2 | 6.8 | 95.9 | 4.1 | 94.6 | 5.4 |
| HCM/HOCM | 76.4 | 23.6 | 57.9 | 42.1 | 91.0 | 9.0 | 96.1 | 3.9 | 96.1 | 3.9 | 93.6 | 6.0 | |
| 5 years after study entry | |||||||||||||
| Full population | AH | 76.2 | 23.8 | 70.4 | 29.6 | 89.2 | 10.8 | 95.6 | 4.4 | 96.7 | 3.3 | 95.0 | 5.0 |
| HCM/HOCM | 78.8 | 21.2 | 51.7 | 48.3 | 93.0 | 7.0 | 95.1 | 4.9 | 96.6 | 3.4 | 95.4 | 4.6 | |
| Age quartile1 | AH | 91.7 | 8.3 | 88.1 | 11.9 | 98.0 | 2.0 | 100.0 | 0.0 | 97.6 | 2.4 | 96.8 | 3.2 |
| HCM/HOCM | 94.7 | 5.3 | 67.7 | 32.3 | 98.4 | 1.6 | 99.5 | 0.5 | 99.5 | 0.5 | 98.4 | 1.6 | |
| Age quartile 2 | AH | 87.1 | 12.9 | 85.2 | 14.8 | 96.1 | 3.9 | 98.7 | 1.3 | 96.8 | 3.2 | 95.5 | 4.5 |
| HCM/HOCM | 87.5 | 12.5 | 62.1 | 37.9 | 97.1 | 2.9 | 97.8 | 2.2 | 97.4 | 2.6 | 97.1 | 2.9 | |
| Age quartile 3 | AH | 71.4 | 28.6 | 63.7 | 36.3 | 86.3 | 13.7 | 95.2 | 4.8 | 96.4 | 3.6 | 94.6 | 5.4 |
| HCM/HOCM | 80.9 | 19.1 | 51.0 | 49.0 | 92.0 | 8.0 | 95.9 | 4.1 | 97.1 | 2.9 | 96.2 | 3.8 | |
| Age quartile 4 | AH | 43.5 | 56.5 | 32.0 | 68.0 | 70.1 | 29.9 | 85.0 | 15.0 | 95.2 | 4.8 | 91.8 | 8.2 |
| HCM/HOCM | 52.8 | 47.2 | 27.5 | 72.5 | 85.0 | 15.0 | 87.6 | 12.4 | 92.7 | 7.3 | 90.1 | 9.9 | |
| 7.5 years after study entry | |||||||||||||
| Full population | AH | 70.2 | 29.8 | 62.7 | 37.3 | 86.1 | 13.9 | 93.9 | 6.1 | 95.7 | 4.3 | 94.6 | 5.4 |
| HCM/HOCM | 71.9 | 28.1 | 38.4 | 61.6 | 90.8 | 9.2 | 92.8 | 7.2 | 95.2 | 4.8 | 94.7 | 5.3 | |
| Age quartile1 | AH | 90.5 | 9.5 | 86.5 | 13.5 | 97.6 | 2.4 | 100.0 | 0.0 | 97.2 | 2.8 | 96.4 | 3.6 |
| HCM/HOCM | 93.1 | 6.9 | 59.3 | 40.7 | 97.9 | 2.1 | 99.5 | 0.5 | 99.5 | 0.5 | 98.4 | 1.6 | |
| Age quartile 2 | AH | 80.6 | 19.4 | 76.1 | 23.9 | 92.3 | 7.7 | 97.4 | 2.6 | 95.5 | 4.5 | 95.5 | 4.5 |
| HCM/HOCM | 83.5 | 16.5 | 50.4 | 49.6 | 95.2 | 4.8 | 97.4 | 2.6 | 96.3 | 3.7 | 96.3 | 3.7 | |
| Age quartile 3 | AH | 61.9 | 38.1 | 52.4 | 47.6 | 83.3 | 16.7 | 91.7 | 8.3 | 94.6 | 5.4 | 93.5 | 6.5 |
| HCM/HOCM | 71.7 | 28.3 | 36.0 | 64.0 | 88.2 | 11.8 | 92.7 | 7.3 | 94.9 | 5.1 | 95.9 | 4.1 | |
| Age quartile 4 | AH | 34.0 | 66.0 | 19.7 | 80.3 | 63.3 | 36.7 | 82.3 | 17.7 | 94.6 | 5.4 | 91.8 | 8.2 |
| HCM/HOCM | 41.6 | 58.4 | 10.7 | 89.3 | 83.3 | 16.7 | 82.0 | 18.0 | 91.0 | 9.0 | 88.4 | 11.6 | |
| 10 years after study entry | |||||||||||||
| Full population | AH | 68.6 | 31.4 | 60.8 | 39.1 | 85.5 | 14.5 | 93.4 | 6.6 | 95.6 | 4.4 | 94.3 | 5.7 |
| HCM/HOCM | 70.2 | 29.8 | 35.8 | 64.1 | 89.9 | 10.1 | 92.4 | 7.6 | 95.0 | 5.0 | 94.6 | 5.4 | |
| Age quartile1 | AH | 89.3 | 10.7 | 85.3 | 14.6 | 97.2 | 2.8 | 100.0 | 0.0 | 97.2 | 2.8 | 95.6 | 4.4 |
| HCM/HOCM | 90.5 | 9.5 | 55.6 | 44.3 | 95.8 | 4.2 | 98.9 | 1.1 | 99.5 | 0.5 | 98.4 | 1.6 | |
| Age quartile 2 | AH | 77.4 | 22.6 | 72.9 | 27.0 | 91.0 | 9.0 | 96.1 | 3.9 | 94.8 | 5.2 | 95.5 | 4.5 |
| HCM/HOCM | 82.0 | 18.0 | 48.2 | 51.7 | 94.9 | 5.1 | 97.4 | 2.6 | 96.0 | 4.0 | 96.0 | 4.0 | |
| Age quartile 3 | AH | 60.7 | 39.3 | 50.0 | 49.9 | 82.7 | 17.3 | 91.1 | 8.9 | 94.6 | 5.4 | 93.5 | 6.5 |
| HCM/HOCM | 69.7 | 30.3 | 33.4 | 66.5 | 87.3 | 12.7 | 91.7 | 8.3 | 94.9 | 5.1 | 95.9 | 4.1 | |
| Age quartile 4 | AH | 32.7 | 67.3 | 18.4 | 81.5 | 62.6 | 37.4 | 81.6 | 18.4 | 94.6 | 5.4 | 91.8 | 8.2 |
| HCM/HOCM | 40.8 | 59.2 | 8.5 | 91.5 | 82.8 | 17.2 | 82.0 | 18.0 | 90.6 | 9.4 | 88.4 | 11.6 | |
Note: For each cause of death listed in the top row, the percentage of affected population and remaining population still at risk is designated for each age quartile recorded at study entry. Risk is further detailed at 1‐, 2.5‐, 5‐, 7.5‐, and 10‐year intervals after study entry.
Abbreviations: AH, apparently healthy cats; HCM, nonobstructive hypertrophic cardiomyopathy; HOCM, obstructive hypertrophic cardiomyopathy.
Includes all cardiovascular, noncardiovascular, and unknown causes of death.
Death related to anesthesia, respiratory diseases, central nervous system diseases, hepatobiliary diseases, toxicoses, endocrine diseases, trauma.
Figure 3.
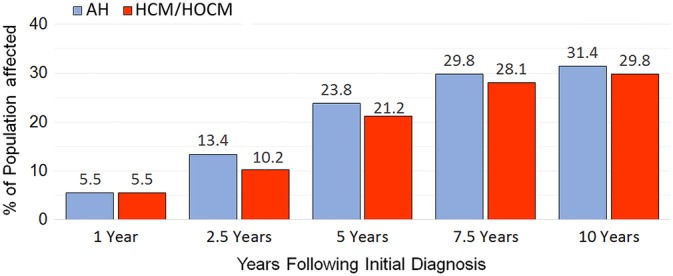
Risk of noncardiovascular death recorded at 1, 2.5, 5, 7.5, and 10‐year intervals after study entry in 1730 cats which, at the time of study entry, were diagnosed as apparently healthy cats (AH, n = 722) or preclinical hypertrophic (HCM) and hypertrophic obstructive (HOCM) cardiomyopathy (HCM/HOCM, n = 1008)
3.3. Survival analyses
Survival time from study entry to noncardiovascular death did not differ significantly in AH (median, 9.8 years; range, 6 days‐14.1 years) compared with HCM/HOCM cats (median, 8.6 years; range, 2 days‐15.2 years; P = .10; Figure 4). Survival times to all‐cause death were significantly shorter in HCM/HOCM cats (median, 5.1 years; range, 2 days‐15.2 years) compared to AH (median, 8.3 years; range, 6 days‐14.1 years; P = .0001; Figure 5). Survival curves for AH and HCM/HOCM cats stratified by age quartile at time of study entry diverged markedly for noncardiovascular (Figure 6) and all‐cause (Figure 7) death, most notably for the second and third, and the third and fourth age quartiles. This was largely caused by cancer and CKD deaths (Table 4). For example, at 2.5 years after study entry, risk of cancer death in AH was 7.1% in the third and 17.7% in the fourth age quartile. At 7.5 years after study entry, risk of cancer death was 16.7% in the third and 36.7% in the fourth age quartile.
Figure 4.
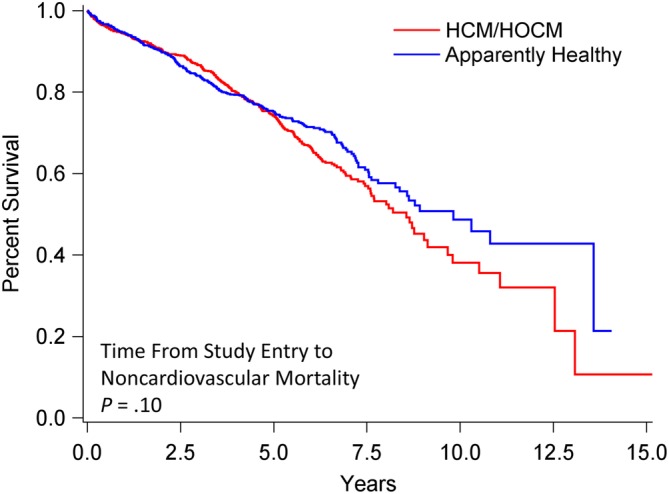
Survival analysis of 1730 cats that at study entry were diagnosed as apparently healthy (n = 722) or with preclinical HCM/HOCM (n = 1008). Percentage of cats that have not experienced death (y‐axis) is plotted against time from study entry to time of noncardiovascular death (x‐axis). Survival statistics include median and range (smallest and largest value): Apparently healthy cats, 9.8 years, 6 days‐14.1 years; HCM/HOCM cats, 8.6 years, 2 days‐15.2 years (P = .10). HCM, nonobstructive hypertrophic cardiomyopathy; HOCM, obstructive hypertrophic cardiomyopathy
Figure 5.

Survival analysis of 1730 cats that at study entry were diagnosed as apparently healthy (n = 722) or with preclinical HCM/HOCM (n = 1008). Percentage of cats that have not experienced death (y‐axis) is plotted against time from study entry to time to all‐cause death (x‐axis). Survival statistics include median and range (smallest and largest value): Apparently healthy cats, 8.3 years, 6 days‐14.1 years; HCM/HOCM cats, 5.1 years, 2 days‐15.2 years (P = .0001). HCM, nonobstructive hypertrophic cardiomyopathy; HOCM, obstructive hypertrophic cardiomyopathy
Figure 6.
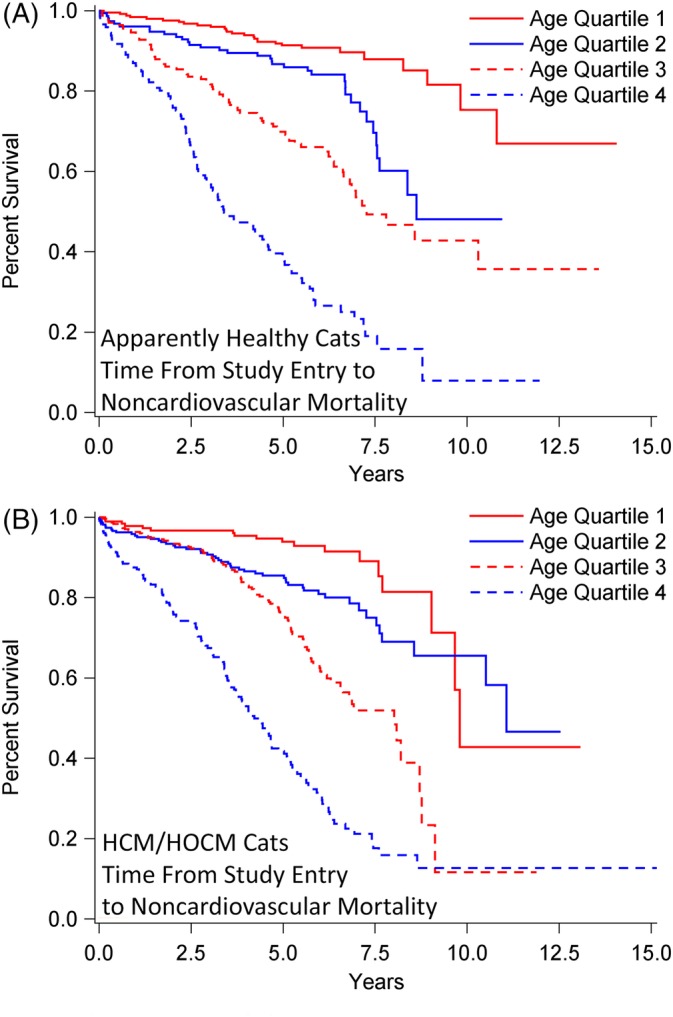
Survival analysis of 1730 cats stratified by age quartile (Q) at study entry when diagnosed as apparently healthy (n = 722; A) or with preclinical HCM/HOCM (n = 1008; B), and that died over time from noncardiovascular causes. Percentage of cats that have not experienced death (y‐axis) is plotted against time from study entry to time to noncardiovascular death (x‐axis). A, Q1: median not achieved, range 46 days‐14.1 years; Q2; median 8.6 years, range 15 days‐11.0 years; Q3: median 7.3 years, interquartile range (IQR) 3.8‐13.6 years; Q4: median 3.4 years, IQR 2.2‐6.9 years. Pairwise comparisons between age quartiles were P < .0001, except Q1 versus Q2 (P = .0009) and Q2 versus Q3 (P = .0007). B, All median, IQR. Q1: 9.8 years, 9.0‐13.1 years; Q2: 11.1 years, 7.5‐12.5 years; Q3: 8.0 years, 5.1‐8.8 years; Q4: 4.2 years, 2.2‐6.3 years. Pairwise comparisons between age quartiles were P < .0001, except Q1 versus Q2 (P = .006). HCM, nonobstructive hypertrophic cardiomyopathy; HOCM, obstructive hypertrophic cardiomyopathy
Figure 7.
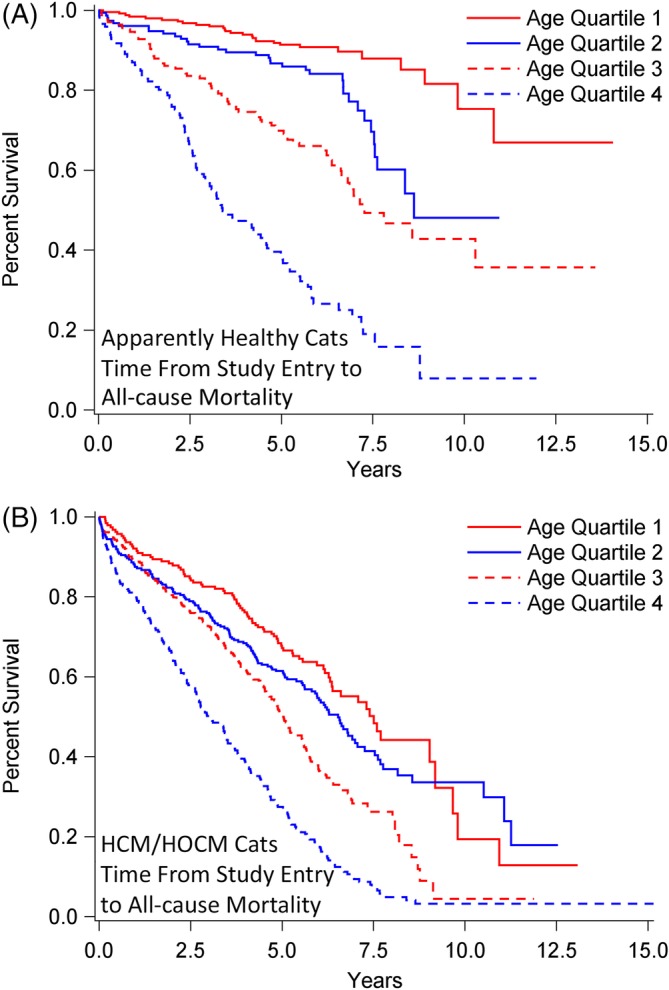
Survival analysis of 1730 cats stratified by age quartile (Q) at study entry when diagnosed as apparently healthy (n = 722; A) or with preclinical HCM/HOCM (n = 1008; B), and that died over time from all‐cause death. Percentage of cats that have not experienced death (y‐axis) is plotted against time from study entry to time to all‐cause death (x‐axis). A, Q1: Median not achieved, range 46 days‐14.1 years; Q2: median 8.6 years, range 15 days‐11.0 years; Q3: median 6.9 years, interquartile range (IQR) 3.3‐13.6 years; Q4: median 3.1 years, IQR 2.0‐5.5 years. Pairwise comparisons between age quartiles were P < .0001, except Q1 versus Q2 (P = .002). B, All median, IQR. Q1: 7.5 years, 4.1‐9.8 years; Q2: 6.5 years, 3.1‐11.1 years; Q3: 5.0 years, 2.7‐8.0 years; Q4: 3.0 years, 1.3‐5.2 years. Pairwise comparisons between age quartiles were P < 0001, except Q1 versus Q2 (P = .10) and Q2 versus Q3 (P = .0003). HCM, nonobstructive hypertrophic cardiomyopathy; HOCM, obstructive hypertrophic cardiomyopathy
Survival from study entry to recorded cancer death did not differ significantly between AH (median not achieved [NA]; range, 6 days‐14.1 years) and HCM/HOCM cats (NA; range, 14 days‐13.1 years; P = .28; Figure 8A). By contrast, survival to CKD death was significantly shorter in HCM/HOCM cats (NA; range, 16 days‐13.1 years) compared with AH (median NA; 28 days‐14.1 years; P = .04; Figure 8B). Survival to CWLVDA death did not differ significantly between AH (NA; range, 6 days‐14.1 years) compared with HCM/HOCM cats (median NA, range, 2 days‐15.2 years; P = .18; Figure 8C).
Figure 8.
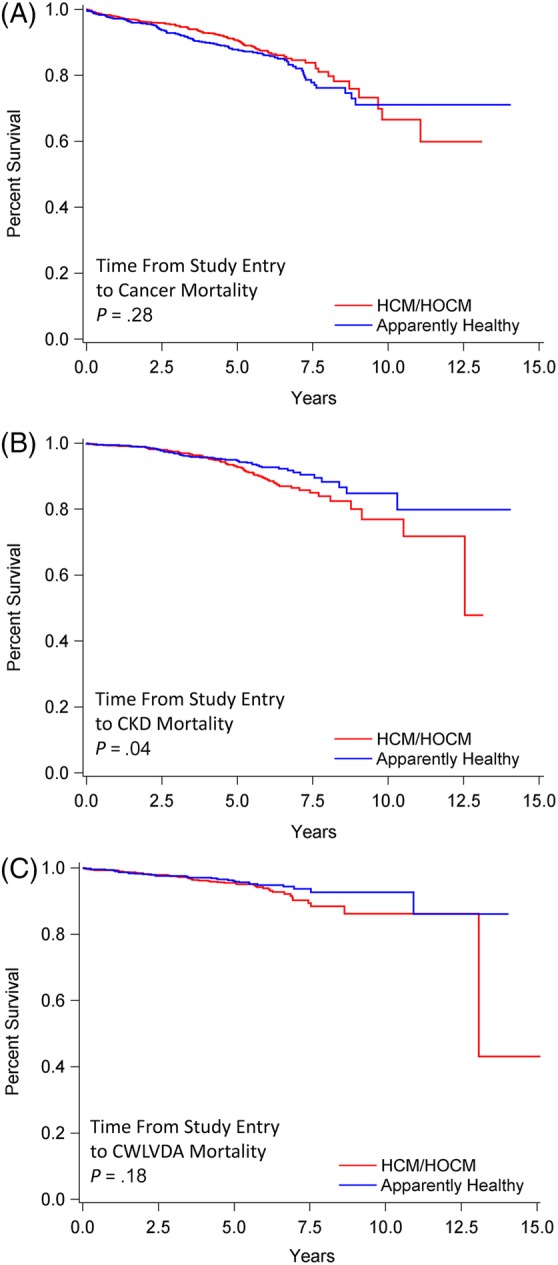
Survival analysis of 1730 cats that at study entry were diagnosed as apparently healthy (n = 722) or preclinical HCM/HOCM (n = 1008), and that died over time due to cancer, CKD, or CWLVDA. Percentage of cats that have not experienced death (y‐axis) is plotted against time from study entry to time to cause‐specific death (x‐axis). Survival statistics include range (smallest and largest value); median survival was not achieved. A, Death from cancer. AH, 6 days‐14.1 years; HCM/HOCM cats, 14 days‐13.1 years (P = .28). B, Death from CKD. AH, 28 days‐14.1 years; HCM/HOCM cats, 16 days‐13.1 years (P = .04). C, Death from CWLVDA. AH 6 days‐14.1 years; HCM/HOCM cats, 2 days‐15.2 years (P = .18). HCM, nonobstructive hypertrophic cardiomyopathy; HOCM, obstructive hypertrophic cardiomyopathy; CKD, chronic kidney disease; CWLVDA, chronic weight‐loss‐vomiting‐diarrhea‐anorexia
4. DISCUSSION
We identified noncardiovascular causes of death and examined all‐cause mortality, incidence rates, risk, and survival characteristics in 1730 cats. To the best of our knowledge, this represents the first long‐term comparison of causes of death between populations that at study entry were initially healthy or had preclinical HCM/HOCM. Death from cancer, CKD and CWLVDA represented 75%‐80% of noncardiovascular mortality in cats originating from around the world, confirming the major impact of these conditions on life expectancy.1, 2, 3, 4, 5, 6, 8, 11, 12, 13 These findings complement the epidemiology of cardiovascular mortality reported from the same population.17 In the present study, all‐cause mortality was significantly greater and survival duration was significantly shorter in HCM/HOCM cats compared to AH, due to cardiovascular death that was substantial in HCM/HOCM cats but rare in AH.
Cancer was the most frequently recorded cause of noncardiovascular death. Apparently healthy cats and HCM/HOCM cats with cancer death did not differ significantly for age at study entry or for survival time. In AH compared with HCM/HOCM cats, the increment of cancer death risk was substantial by 5 years (1 in 9 and 1 in 14, respectively) compared with 1 year (1 in 36 and 1 in 50, respectively) after study entry. By comparison at 10 years after study entry, risk was 1 in 7 and 1 in 10, respectively. Population‐based data describing epidemiology of feline neoplasia are scant. Direct comparison with our findings is problematic due to diverse study populations, disparate inclusion criteria, and dissimilar methods for disease estimation.19, 20, 21, 22, 23 Lymphoma is the most commonly reported feline neoplasm20 and most prevalent form of gastrointestinal cancer.24, 25 However, we did not attempt to document histological diagnoses in all cats whose deaths were attributed to cancer in our study.
Chronic kidney disease was the second most commonly recorded cause of noncardiovascular death. As with cancer, CKD death predominantly affected middle‐aged and older cats. Similar to cancer, in both AH and HCM/HOCM cats, the increment of CKD death risk was greatest at 5 years (1 in 23 and 1 in 20, respectfully) compared with 1 year (1 in 167 and 1 in 143, respectively) after study entry. By comparison, CKD death risk was 1 in 15 and 1 in 13 at 10 years, after study entry, respectively. Neutered male compared with spayed female status was reported to be a CKD risk factor.26 In our study, sex ratio with CKD death was approximately 1.6 to 1 female‐to‐male in AH and 2.5 to 1 male‐to‐female in HCM/HOCM cats. However, heart disease was excluded in AH, whereas HCM/HOCM is associated with strong male predilection, possibly explaining these differences.
Survival from study entry to CKD death was significantly shorter in HCM/HOCM cats compared with AH. Although age at study entry did not differ significantly between AH and HCM/HOCM cats with recorded CKD death, the HCM/HOCM cohort was significantly older than AH and might have introduced a population bias, because prevalence of CKD increases with age.13, 26, 27, 28, 29 Consequently, we cannot resolve whether pHCM predisposed to acute kidney injury as a hypothesis for their shorter survival time to CKD death, compared with AH. This merits further study.
Death associated with CWLVDA was the third most commonly recorded cause of noncardiovascular death. Compared with cancer death, incidence rates were approximately one‐third lower in AH and one‐half lower in HCM/HOCM cats, respectively. Consistent with cancer and CKD death, the increment of risk for CWLVDA death in AH and HCM/HOCM cats was greatest at 5 years (approximately 1 in 30 and 1 in 29 respectively) compared with 1 year (1 in 143) after study entry. Middle‐age predilection has been reported for feline inflammatory bowel disease, 1 of the most common feline enteropathies.30, 31 In our study, the cohort of AH with recorded CWLVDA death were middle‐aged at the time of study entry.
How best to apply epidemiology to promote disease detection and improve feline health remains a long‐standing topic of interest,13, 28, 32, 33, 34, 35, 36, 37 especially because current metrics to assess disease have limitations. Age categories are considerably subjective, as designated, for example, by middle‐aged, adult, mature, senior, or geriatric labels that are commonly applied. Indeed, interpreting age‐appropriate physical and physiologic aging changes may be ambiguous. Collectively, predicting risk based solely upon time from birth is indeterminate.38, 39, 40, 41 Nonetheless, increased health screening has been routinely advocated for “older” cats, emphasizing medical history, physical examination, and laboratory testing as footings to detect illness.28, 32, 33, 34, 35, 36, 42 Often, however, medical history and physical examination findings are vague or unremarkable. Furthermore, blood test variables might be unaffected, particularly in early disease stages and even when certain disease has progressed.43 Laboratory reference intervals that reflect normal aging improves test acuity,15 but available data are limited. Thus, screening clinical pathology test values frequently fall within normal reference intervals,13 and normal findings might not reflect the state of health nor assure the absence of disease.
The present study reports survival characteristics, incidence rates, and mortality risk in feline populations that at study entry were apparently healthy or had pHCM. These methods afford a constructive approach to support healthcare planning, and our findings provide informative considerations for future screening programs. We prioritized analysis of incidence over prevalence in order to compare noncardiovascular, all‐cause, and cause‐specific mortality across age quartiles and between‐study populations. Most feline reports emphasize prevalence,1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13 which describes the total number of cases in a study population at a single time point, but is affected by disease duration and occurrence, and is not well suited for monitoring disease trends.44 Our study revealed that cancer, CKD, and CWLVDA death were the major causes of noncardiovascular death. Although older cats had the highest noncardiovascular mortality event rates, substantial mortality was also recorded in the middle 2 age quartiles, ages not customarily targeted for enhanced wellness screening. We also observed that the greatest increment of risk for death caused by cancer, CKD, and CWLVDA occurred at 2.5 and 5 years compared with 1 year after study entry. The increment of risk for noncardiovascular death after study entry in AH and HCM/HOCM cats increased approximately 2.4 and 1.9 times at 2.5 years compared with 1 year, respectively, and increased further, by approximately 1.8 and 2.1 times at 5 years compared with 2.5 years after study entry, respectively. These findings might contribute to planning screening visits.
There were several important study limitations. As this was a retrospective study, it was not feasible to collect comprehensive test data, biopsy procedures, or necropsy results on every case, and we were not able to refine survival analyses according to histologic grading of cancer. Although exclusionary criteria were imposed to screen out cases with obvious clinical illness, some diseases may have been undetected. Attributing cause of death in cases associated with gastrointestinal signs of disease can be difficult. Indeed, well‐recognized challenges have been reported, including shifting and controversial classification systems, evolving terminology, unsettled diagnostic criteria, and uncertain test accuracy.30 Distinguishing between inflammatory bowel disease and lymphoma, the 2 principal diseases affecting the feline gastrointestinal system is especially problematic and comorbidities may exist.45, 46, 47, 48, 49, 50, 51, 52 Although cause of death was relegated to an “unknown category” whenever information was insufficient, misclassification of cause‐specific death was possible. However, the broader categories of noncardiovascular and all‐cause death still provide meaningful bases for comparing populations and age quartiles. Referral bias might have been present at some study sites. Moreover, some selection bias might have resulted because some AH were examined with echocardiography to assess a heart murmur for breeding examination or preanesthesia evaluation. It was not possible to assure that study populations represented endemic disease burdens in each geographic region. Thus, incidence rates might have been overrepresented or underrepresented at some sites. However, we are unaware of comprehensive survey data confirming geographic distribution of feline diseases. Our relatively large study population originating from varied geographical regions might have diminished these effects. We did not attempt to identify benefit or detriment of any drug treatment on the natural history of study populations or assess whether diet, genetic, or environmental factors influenced health and longevity19, 49, 53 Moreover, we could not control for possible regional attitudes towards euthanasia that might have influenced survival. Finally, cardiac status was based on 1 initial echocardiographic examination at the point of study entry. Limitations related to these factors have been detailed.17
5. CONCLUSIONS
Notwithstanding these limitations, our study contributes new epidemiologic information about noncardiovascular and all‐cause mortality, including incidence rates, risk, and survival outcomes in cats that at study entry were apparently healthy or had pHCM. The most commonly recorded noncardiovascular causes of death were cancer, followed by CKD, and then conditions characterized by CWLVDA. Although life spans of AH and pHCM cats that died from noncardiovascular causes were similar, pHCM conferred a substantial health burden over‐and‐above the risk for noncardiovascular death. All‐cause mortality was significantly greater in pHCM due to cardiovascular death which was common in this cohort but rare in AH.
Noncardiovascular death approximately doubled every successive age quartile in AH and pHCM cats. Moreover, the increment of risk of noncardiovascular death was highest at 2.5 years compared with 1 year after study entry and 5 years compared with 2.5 years after study entry, respectively, approximately doubling at each comparative time point. Furthermore, increment of noncardiovascular risk was approximately 4 times higher at 5 years than at 1 year after study entry. Comparatively, this increment was higher than the increment of risk recorded 10 years versus 5 years after study entry. Still, although noncardiovascular mortality increased with advancing age, it was also notably present in middle‐aged populations, cohorts that are often underemphasized in contemporary health screening guidelines.
Collectively, these findings add new epidemiologic information that may assist veterinarians anticipate potential health conditions, contribute to the future development of health care guidelines, and aid long‐term surveillance and wellness monitoring strategies.
CONFLICT OF INTEREST DECLARATION
The authors declare no conflicts of interest other than Dr. Masami Uechi who holds the position of chief executive officer at the Japan Animal Specialty Medical Institute Inc.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Where required by participating author institutions for this retrospective study.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Fox PR, Keene BW, Lamb K, et al. Long‐term incidence and risk of noncardiovascular and all‐cause mortality in apparently healthy cats and cats with preclinical hypertrophic cardiomyopathy. J Vet Intern Med. 2019;33:2572–2586. 10.1111/jvim.15609
Funding information Morris Animal Foundation, Grant/Award Number: D09FE‐026; Winn Feline Foundation, Grant/Award Number: W‐09‐017
REFERENCES
- 1. Inoue M, Hasegawa A, Sugiura K. Morbidity pattern by age, sex and breed in insured cats in Japan (2008‐2013). J Feline Med Surg. 2016;18:1013‐1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Egenvall A, Nødtvedt A, Häggström J, et al. Mortality of life‐insured Swedish cats during 1999‐2006: age, breed, sex, and diagnosis. J Vet Intern Med. 2009;23:1175‐1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bonnett BN, Egenvall A. Age patterns of disease and death in insured Swedish dogs, cats, and horses. J Comp Pathol. 2010;142:S33‐S38. [DOI] [PubMed] [Google Scholar]
- 4. Öhlund M, Fall T, Ström Holst B, Hansson‐Hamlin H, Bonnett B, Egenvall A. Incidence of diabetes mellitus in insured Swedish cats in relation to age, breed and sex. J Vet Intern Med. 2015;29:1342‐1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McCann TM, Simpson KE, Shaw DJ, et al. Feline diabetes mellitus in the UK: the prevalence within an insured cat population and a questionnaire‐based putative factor analysis. J Feline Med Surg. 2007;9:289‐299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hayashidani H, Omi Y, Ogawa M, Fukutomi K. Epidemiological studies on the expectation of life for cats computed from animal cemetery records. Nihon Juigaku Zasshi. 1989;51:905‐908. [DOI] [PubMed] [Google Scholar]
- 7. Olsen TF, Allen AL. Causes of sudden and unexpected death in cats: a 10 year retrospective study. Can Vet J. 2001;42:61‐62. [PMC free article] [PubMed] [Google Scholar]
- 8. Vapalahti K, Virtala AM, Joensuu TA, et al. Health and behavioural survey of over 8000 Finnish cats. Front Vet Sci. 2016;3:1‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. New JC Jr, Kelch WJ, Hutchison JM, et al. Birth and death rate estimates of cats and dogs in U.S. households and related factors. J Appl Anim Welf Sci. 2004;7:229‐241. [DOI] [PubMed] [Google Scholar]
- 10. Comfort A. Maximum ages reached by domestic cats. J Mammal. 1956;37:118‐119. [Google Scholar]
- 11. Vascellari M, Baioni E, Ru G, Carminato A, Mutinelli F. Animal tumour registry of two provinces in northern Italy: incidence of spontaneous tumours in dogs and cats. BMC Vet Res. 2009;5:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. O'Neill DG, Church DB, McGreevy PD, et al. Prevalence of disorders recorded in cats attending primary‐care veterinary practices in England. Vet J. 2014;202:286‐291. [DOI] [PubMed] [Google Scholar]
- 13. Paepe D, Verjans G, Duchateau L, Piron K, Ghys L, Daminet S. Routine health screening: findings in apparently healthy middle‐aged and older cats. J Feline Med Surg. 2013;15:8‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Doria‐Rose VP, Scarlett JM. Mortality rates and causes of death among emaciated cats. J Am Vet Med Assoc. 2000;216:347‐351. [DOI] [PubMed] [Google Scholar]
- 15. Lund EM, Armstrong PJ, Kirk CA, Kolar LM, Klausner JS. Health status and population characteristics of dogs and cats examined at private veterinary practices in the United States. J Am Vet Med Assoc. 1999;214:1336‐1341. [PubMed] [Google Scholar]
- 16. Hamilton JB, Hamilton RS, Mestler GE. Duration of life and causes of death in domestic cats: influence of sex, gonadectomy, and inbreeding. J Gerontol. 1969;24:427‐437. [DOI] [PubMed] [Google Scholar]
- 17. Fox PR, Keene BW, Lamb K, et al. International collaborative study to assess cardiovascular risk and evaluate long‐term health in cats with preclinical hypertrophic cardiomyopathy and apparently healthy cats: the REVEAL study. J Vet Intern Med. 2018;32:930‐943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fox PR, Liu SK, Maron BJ. Echocardiographic assessment of spontaneously occurring feline hypertrophic cardiomyopathy. An animal model of human disease. Circulation. 1995;92:2645‐2651. [DOI] [PubMed] [Google Scholar]
- 19. Cupp CJ, Jean‐Philippe C, Kerr WW, et al. Effect of nutritional interventions on longevity of senior cats. Int J Appl Res Vet Med. 2007;5:133‐149. [Google Scholar]
- 20. MacVean DW, Monlux AW, Anderson PS Jr, et al. Frequency of canine and feline tumors in a defined population. Vet Pathol. 1978;15:700‐715. [DOI] [PubMed] [Google Scholar]
- 21. Brønden LB, Flagstad A, Kristensen AT. Veterinary cancer registries in companion animal cancer: a review. Vet Comp Oncol. 2007;5:133‐144. [DOI] [PubMed] [Google Scholar]
- 22. Graf R, Grüntzig K, Boo G, et al. Swiss feline cancer registry 1965‐2008: the influence of sex, breed and age on tumour types and tumour locations. J Comp Pathol. 2016;154:195‐210. [DOI] [PubMed] [Google Scholar]
- 23. Graf R, Grüntzig K, Hässig M, et al. Swiss feline cancer registry: a retrospective study of the occurrence of tumours in cats in Switzerland from 1965 to 2008. J Comp Pathol. 2015;153:266‐277. [DOI] [PubMed] [Google Scholar]
- 24. Gieger T. Alimentary lymphoma in cats and dogs. Vet Clin North Am Small Anim Pract. 2011;41:419‐432. [DOI] [PubMed] [Google Scholar]
- 25. Malik R, Gabor LJ, Canfield PJ. Lymphoma in Australian cats—lessons for Europe? J Feline Med Surg. 2003;5:147‐150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Greene JP, Lefebvre SL, Wang M, Yang M, Lund EM, Polzin DJ. Risk factors associated with the development of chronic kidney disease in cats evaluated at primary care veterinary hospitals. J Am Vet Med Assoc. 2014;244:320‐327. [DOI] [PubMed] [Google Scholar]
- 27. Bartges JW. Chronic kidney disease in dogs and cats. Vet Clin North Am Small Anim Pract. 2012;42:669‐692. [DOI] [PubMed] [Google Scholar]
- 28. Paepe D, Daminet S. Feline CKD: diagnosis, staging and screening—what is recommended? J Feline Med Surg. 2013;15(Suppl 1):15‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. DiBartola SP, Rutgers HC, Zack PM, Tarr MJ. Clinicopathologic findings associated with chronic renal disease in cats: 74 cases (1973–1984). J Am Vet Med Assoc. 1987;190:1196‐1202. [PubMed] [Google Scholar]
- 30. Jergens AE. Feline idiopathic inflammatory bowel disease: what we know and what remains to be unraveled. J Feline Med Surg. 2012;14:445‐458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sabattini S, Bottero E, Turba ME, Vicchi F, Bo S, Bettini G. Differentiating feline inflammatory bowel disease from alimentary lymphoma in duodenal endoscopic biopsies. J Small Anim Pract. 2016;57:396‐401. [DOI] [PubMed] [Google Scholar]
- 32. Pittari J, Rodan I, Beekman G, et al. American association of feline practitioners. Senior care guidelines. J Feline Med Surg. 2009;11:763‐778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hoyumpa Vogt A, Rodan I, Brown M, et al. AAFP‐AAHA: feline life stage guidelines. J Feline Med Surg. 2010;12:43‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. American Animal Hospital Association—American Veterinary Medical Association Preventive Healthcare Guidelines Task Force . Development of new canine and feline preventive healthcare guidelines designed to improve pet health. J Am Vet Med Assoc. 2011;239:625‐629. [DOI] [PubMed] [Google Scholar]
- 35. Vogt AH, Rodan I, Brown M, et al. AAFP‐AAHA: Feline life stage guidelines. J Anim Hosp Assoc. 2010;46:70‐85. [DOI] [PubMed] [Google Scholar]
- 36. Fortney WD. Implementing a successful senior/geriatric health care program for veterinarians, veterinary technicians, and office managers. Vet Clin North Am Small Anim Pract. 2012;42:823‐834. [DOI] [PubMed] [Google Scholar]
- 37. Belshaw Z, Robinson NJ, Dean RS, Brennan ML. Owners and veterinary surgeons in the United Kingdom disagree about what should happen during a small animal vaccination consultation. Vet Sci. 2018;5:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Day MJ. Ageing, immunosenescence and inflammageing in the dog and cat. J Comp Pathol. 2010;142 Suppl 1:S60‐S609. [DOI] [PubMed] [Google Scholar]
- 39. Seward JB. Physiological aging: window of opportunity? J Am Coll Cardiol Cardiovasc Imaging. 2011;4:243‐245. [DOI] [PubMed] [Google Scholar]
- 40. Bellows J, Center S, Daristotle L, et al. Aging in cats: common physical and functional changes. J Feline Med Surg. 2016;18:533‐550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bellows J, Center S, Daristotle L, et al. Evaluating aging in cats: how to determine what is healthy and what is disease. J Feline Med Surg. 2016;18:551‐570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Metzger FL, Rebar AH. Clinical pathology interpretation in geriatric veterinary patients. Vet Clin North Am Small Anim Pract. 2012;42:615‐629. [DOI] [PubMed] [Google Scholar]
- 43. Dell'Osa D, Jaensch S. Prevalence of clinicopathological changes in healthy middle‐aged dogs and cats presenting to veterinary practices for routine procedures. Aust Vet J. 2016;94:317‐323. [DOI] [PubMed] [Google Scholar]
- 44. Ward MW. Estimating disease prevalence and incidence using administrative data: some assembly required. J Rheumatol. 2013;40:1241‐1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jugan MC, August JR. Serum cobalamin concentrations and small intestinal ultrasound changes in 75 cats with clinical signs of gastrointestinal disease: a retrospective study. J Feline Med Surg. 2017;19:48‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bertal M, Norman Carmel E, Diana A, Desquilbet L, Specchi S, Pey P. Association between ultrasonographic appearance of splenic parenchyma and cytology in cats. J Feline Med Surg. 2018;20:23‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Marolf AJ, Bachand AM, Sharber J, Twedt DC. Comparison of endoscopy and sonography findings in dogs and cats with histologically confirmed gastric neoplasia. J Small Anim Pract. 2015;56:339‐344. [DOI] [PubMed] [Google Scholar]
- 48. Daniaux LA, Laurenson MP, Marks SL, et al. Ultrasonographic thickening of the muscularis propria in feline small intestinal small cell T‐cell lymphoma and inflammatory bowel disease. J Feline Med Surg. 2014;16:89‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Moore PF, Woo JC, Vernau W, Kosten S, Graham PS. Characterization of feline T cell receptor gamma (TCRG) variable region genes for the molecular diagnosis of feline intestinal T cell lymphoma. Vet Immunol Immunopathol. 2005;106:167‐178. [DOI] [PubMed] [Google Scholar]
- 50. Kleinschmidt S, Harder J, Nolte I, Marsilio S, Hewicker‐Trautwein M. Chronic inflammatory and non‐inflammatory diseases of the gastrointestinal tract in cats: diagnostic advantages of full‐thickness intestinal and extraintestinal biopsies. J Feline Med Surg. 2010;12:97‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kiupel M, Smedley RC, Pfent C, et al. Diagnostic algorithm to differentiate lymphoma from inflammation in feline small intestinal biopsy samples. Vet Pathol. 2011;48:212‐222. [DOI] [PubMed] [Google Scholar]
- 52. Gaschen L. Ultrasonography of small intestinal inflammatory and neoplastic diseases in dogs and cats. Vet Clin North Am Small Anim Pract. 2011;41:329‐344. [DOI] [PubMed] [Google Scholar]
- 53. Cupp CJ, Kerr WW, Jean‐Philippe C, et al. The role of nutritional interventions in the longevity and maintenance of long‐term health in aging cats. Int J Appl Res Vet Med. 2008;6:69‐81. [Google Scholar]


