SUMMARY
BACKGROUND:
Compared with smear microscopy, Xpert® MTB/RIF has the potential to reduce delays in tuberculosis (TB) diagnosis and treatment initiation, and improve treatment outcomes. We reviewed publications comparing treatment outcomes of drug-susceptible TB patients diagnosed using Xpert vs. smear.
METHODS:
Citations (2000–2016) reporting treatment outcomes of patients diagnosed using Xpert compared with smear were selected from PubMed, Scopus and conference abstracts. We conducted a systematic review and meta-analysis. Favorable (cured, completed) and unfavorable (failure, death, loss to follow-up) outcomes were pooled for meta-analysis; we also reviewed the number of TB cases diagnosed, time to treatment and empiric treatment. The Mantel-Haenszel method with a fixed-effect model was used; I2 was calculated to measure heterogeneity.
RESULTS:
From 13 citations, 43 594 TB patients were included and 4825 were with known TB treatment outcome. From the pooled analysis, an unfavorable outcomes among those diagnosed using Xpert compared with smear was 20.2%, 541/2675 vs. 21.9%, 470/2150 (risk ratio 0.92, 95%CI 0.82–1.02). Statistical heterogeneity was low (I2 = 0.0%, P = 0.910). Compared with smear, Xpert was reported to be superior in increasing the number of TB patients diagnosed (2/9 citations), increasing bacteriologically confirmed TB (7/9 citations), reducing empiric treatment (3/5 citations), reducing time to diagnosis (2/3 citations), and reducing time to treatment initiation (1/5 citations).
CONCLUSIONS:
Xpert implementation showed no discernible impact on treatment outcomes compared with conventional smear despite reduced time to diagnosis, time to treatment or reduced level of empiric treatment. Further research is required to learn more about gaps in the existing health system.
Keywords: patient-level, treatment outcome, Xpert, smear
RÉSUMÉ
CONTEXTE:
Comparé à la microscopie de frottis, l’Xpert® MTB/RIF a le potentiel de réduire les délais de diagnostic et de mise en route du traitement de la tuberculose (TB) et d’améliorer les résultats du traitement. Nous avons revu les publications comparant les résultats du traitement des patients atteints de TB pharmacosensible diagnostiquée par Xpert contre frottis.
MÉTHODE:
Les références (2000–2016) rapportant les résultats du traitement des patients, diagnostiqués par Xpert comparé au frottis, ont été sélectionnés sur PubMed, Scopus et dans des résumés de conférence. Nous avons conduit une revue systématique et une méta-analyse. Les résultats favorables (guéri, traitement achevé) et défavorables (échec, décès, perdus de vue) ont été regroupés pour une méta-analyse; nous avons revu les nombres de cas de TB diagnostiqués, le retard de traitement et la proportion de traitement empirique. Nous avons utilisé la méthode de Mantel-Haenszel avec un modèle à effet fixe ; I2 a été calculé afin de mesurer l’hétérogénéité.
RÉSULTATS:
A partir de 13 références, 43 594 patients TB ont été inclus et le résultat du traitement de TB a été connu pour 4825 d’entre eux. Selon l’analyse regroupée, les résultats défavorables parmi les patients diagnostiqués par Xpert comparés au frottis ont été de 20,2%, 541/2675 contre 21,9%, 470/2150 (ratio de risque de 0,92; IC95% 0,82–1,02). L’hétérogénéité statistique a été faible (I2 = 0,0%; P = 0,910). Comparé au frottis, l’Xpert s’est avéré supérieur en augmentant le nombre de patients TB diagnostiqués (2/9 références), en accroissant les TB confirmées par bactériologie (7/9 références), en réduisant le traitement empirique (3/5 références), en réduisant le retard de diagnostic (2/3 références) et en réduisant le retard de mise en route du traitement (1/5 références).
CONCLUSION:
La mise en œuvre de l’Xpert n’a pas misen évidence d’impact discernable sur les résultats du traitement comparé au frottis conventionnel, en dépit d’une diminution du délai de diagnostic, du délai de traitement ou de la réduction du traitement empirique. Il faut davantage de recherche pour en savoir plus sur les lacunes du système de santé existant.
RESUMEN
MARCO DE REFERENCIA:
En comparación con la baciloscopia del esputo, la prueba Xpert® MTB/RIF puede disminuir el retraso del diagnóstico de la tuberculosis (TB) y del inicio del tratamiento y mejorar los desenlaces terapéuticos. Se llevó a cabo una revision de las publicaciones que comparaban los desenlaces terapéuticos de pacientes con TB normosensible diagnosticada por la prueba Xpert y de los pacientes diagnosticados mediante baciloscopia.
MÉTODOS:
Se buscaron referencias de estudios que comparaban los desenlaces terapéuticos de pacientes diagnosticados mediante la prueba Xpert y la baciloscopia en las bases de datos de PubMed, Scopus y en resùmenes de conferencias (2000–2016). Se llevó a cabo una revisión sistemática y un metanálisis. Con fines del metanálisis se combinaron los desenlaces favorables (curados, tratamiento completo) y desfavorables (fracaso, muerte y pérdida durante el seguimiento); se analizó el nûmero de casos de TB diagnosticados, el lapso hasta el inicio del tratamiento y la prescripción de un tratamiento empírico. Se aplicó el método de Mantel-Haenszel con un modelo de efectos fijos y se midió la heterogeneidad mediante el estadístico I2.
RESULTADOS:
En las 13 citas encontradas, se incluían 43 594 pacientes con TB y en 4825 casos se conocía el desenlace del tratamiento antituberculoso. Segùn el análisis combinado, la proporción de desenlaces desfavorables en los pacientes diagnosticados con la prueba Xpert fue 20,2% (541/2675), comparada con 21,9% (470/2150) en los pacientes diagnosticados por baciloscopia (cociente de riesgos 0,92; IC95% 0,82–1,02). La heterogeneidad estadística fue baja (I2= 0,0%; P = 0,910). Los estudios comunicaban que, comparada con la baciloscopia, la prueba Xpert era superior para aumentar el nùmero de pacientes diagnosticados (2/9 citas), aumentar los casos de TB confirmados bacteriológicamente (7/9 citas), disminuir el tratamiento empírico (3/5 citas), acortar el lapso hasta el diagnóstico (2/3 citas) y para acortar el tiempo hasta el inicio del tratamiento (1/5 citas).
CONCLUSION:
La utilización de la prueba Xpert no reveló un impacto perceptible sobre el desenlace terapéutico, en comparación con la baciloscopia corriente, pese al acortamiento del tiempo hasta obtener el diagnóstico y el lapso hasta el inicio del tratamiento y a la disminución de los tratamientos empiricos. Las investigaciones futuras deberán aportar nuevas aclaraciones sobre las lagunas que existen en el sistema de salud vigente.
TUBERCULOSIS (TB) TREATMENT outcomes remain a concern among clinicians, particularly among TB patients co-infected with the human immunodeficiency virus (HIV), who have a high risk of mortality (~40%), even when receiving antiretroviral therapy (ART).1 Less sensitive, traditional TB diagnostic methods such as smear microscopy contribute to poor treatment outcomes; a missed diagnosis of TB can lead to late patient presentation and delayed TB treatment initiation.2,3 Smear has low sensitivity (~45 %) in diagnosing culture-positive disease among people living with HIV (PLHIV).4,5
The Xpert® MTB/RIF assay is a real-time, fully automated molecular test developed on the GeneXpert platform (Cepheid, Sunnyvale, CA, USA), which can detect both Mycobacterium tuberculosis complex (MTC) and rifampicin (RIF) resistance within 2 h.6,7 After endorsement by the World Health Organization (WHO) in 2010, over 145 countries had implemented the assay, as of December 2016.8
Recent evidence from meta-analysis suggests that in comparison with smear, Xpert shows a 23% increase in TB detection among culture-confirmed cases.9 Studies have indicated that Xpert has superior sensitivity (79–88%) than smear for TB diagnosis.9–11 In addition to diagnosing more TB cases, the shorter turnaround time of diagnosis using Xpert has the potential to reduce delays in TB treatment initiation and improve patient-level clinical outcomes.12 Since 2013, the WHO has been recommending the use of Xpert rather than conventional microscopy as the initial diagnostic test in all adults; however, we have yet to see an improvement in treatment outcomes, including mortality and morbidity.9 There is limited published evidence with detailed patient-level clinical outcomes on the impact of Xpert compared with smear.
From the limited emerging evidence comparing the use of Xpert vs. smear, varying results have been reported on the impact of Xpert on patient-level clinical outcomes. The majority of the reports agree that the inability to show improved clinical impact (treatment outcome) of Xpert over smear is potentially a result of a high level of empiric anti-tuberculosis treatment. The expectation is not that clinicians can eliminate use of empiric treatment, particularly in childhood TB and in smear-negative TB; however, compared with smear, Xpert has higher sensitivity to accurately diagnose TB and higher specificity to identify true-negatives, and this may be beneficial to patients, who would be spared unnecessary anti-tuberculosis treatment.13–17 Patients with non-tuberculous mycobacteria (NTM) disease who present with symptoms similar to TB are particularly at risk of avoidable empiric anti-tuberculosis treatment. Patients with NTM who receive anti-tuberculosis treatment may have unfavorable outcomes because NTMs do not respond, or partially respond (depending on the NTM species), to anti-tuberculosis treatment.18 We conducted a systematic review and meta-analysis to establish the effect of Xpert on treatment outcomes.
The aims of the present review were primarily to compare smear and Xpert assays among TB patients to determine if Xpert reduces: 1) unfavorable outcomes (failure, death, loss to follow-up [LTFU]); 2) time to diagnosis and time to treatment; 3) use of empiric treatment.
METHODS
Search strategy
We followed Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines.19 This systematic review and meta-analysis is registered at PROSPERO (http://www.crd.york.ac.uk/PROSPERO; number CRD42016050625).
As Xpert is a relatively new TB diagnostic technology, we searched PubMed and Scopus for all relevant articles published between January 2000 and December 2016. A sensitive search strategy was used, with Xpert as the key heading term used in combination with potentially relevant key factors: Xpert AND ‘tuberculosis’; or ‘impact’; or ‘effect’; or ‘treatment outcome’; or ‘time to diagnosis’; or ‘time to treatment’; or ‘empiric treatment’: or ‘loss to follow-up’; or ‘mortality’; or ‘morbidity’.
We also reviewed abstract books from the Union World Conference on Lung Health from 2012 to 2016 for studies that may have been completed but not yet published. In addition, we reviewed the reference lists of the primary studies to include additional references that might have been missed with our primary search strategy.
Review of studies
Studies designed as cohort studies or randomized clinical trials comparing the diagnosis and/or treatment outcomes of TB patients diagnosed using Xpert vs. smear were potentially eligible to be included in the review. Citations with patient-level treatment outcomes (favorable or unfavorable) comparing Xpert and smear microscopy were eligible for inclusion in the meta-analysis. The target study population was patients of any age or sex who presented with TB symptoms at any health care level.
The inclusion criteria that studies reported on were 1) treatment outcomes in drug-susceptible TB patients, diagnosed using Xpert compared with those diagnosed using smear; 2) the effect of Xpert on time to diagnosis, time to treatment, empiric treatment or mortality.
The exclusion criteria were 1) studies reporting on treatment outcomes for drug-resistant TB; and 2) studies not reporting on patient-level treatment outcomes or whose reporting was from modeling studies or population-level impact. Detailed descriptions of cohort selection as Xpert or smear groups are included as notes under each table.
Two reviewers (TA and RB) independently reviewed study titles and abstracts to determine eligibility. All studies that met the inclusion criteria received full-text reviews, and differences were resolved by consensus.
Data extraction and statistical analysis
For studies fulfilling the inclusion criteria, two independent reviewers selected eligible citations and compiled data on a pre-piloted Excel™ (MicroSoft, Redmond, WA, USA) spreadsheet. The following information was collected, if available: article author and title, year of publication, study setting, population (HIV-infected and/or non-infected), participant demographics and baseline characteristics, details of intervention and control conditions, study methodology, treatment outcomes, time to diagnosis, time to treatment, diagnostic test (smear and Xpert), incremental value (additional TB cases diagnosed) and description of empiric treatment practices.
Favorable TB treatment outcomes were defined as cured or completed treatment. Unfavorable treatment outcomes were defined as failed, death or LTFU. Empiric treatment was defined as initiation of treatment in the absence of a bacteriologically confirmed diagnosis. Diagnostic impact (reduced time to diagnosis, increased number of TB cases diagnoses and increased bacteriologically confirmed TB), therapeutic impacts (reduced time to treatment, reduced LTFU and reduced empirical treatment) and patient-level outcomes (improved treatment outcome, including mortality) were described based on Schumacher et al.’s framework.12
The data abstracted were aggregated and summarized as favorable and unfavorable outcomes for patients diagnosed and treated using smear- and Xpert-based algorithms. Pooled analysis was conducted using the Mantel-Haenszel method with a fixed-effect model and a forest plot created using STATA v14.0 (STATA Corporation, College Station, TX, USA). The statistical test, I2, was applied to measure heterogeneity. To support the results from the pooled analysis, a narrative analysis of articles that did not meet the criteria for meta-analysis was produced to identify factors potentially affecting treatment outcomes (time to diagnosis and time to treatment, empiric treatment and bacteriologically confirmed TB).
Quality assessment
While scales such as the Detsky et al. scale can be useful, many authors consider the scoring schemes to be imprecise, and cut-off points can be arbitrary; we therefore did not use any scale or scoring system.20,21 We applied a set of strict inclusion and exclusion criteria to identify as many comparable studies as possible. For consistency and to ensure high quality of data, two authors (TA and RB) independently reviewed methods used in potentially eligible articles and abstracts. The included citations provided information on how patient-level treatment outcomes were measured and reported results using risk ratio (RR), 95% confidence interval (CIs) or P values. Following review, any concern or uncertainty was resolved by consensus.
Ethics statement
Ethical approval was not required for this systematic review.
RESULTS
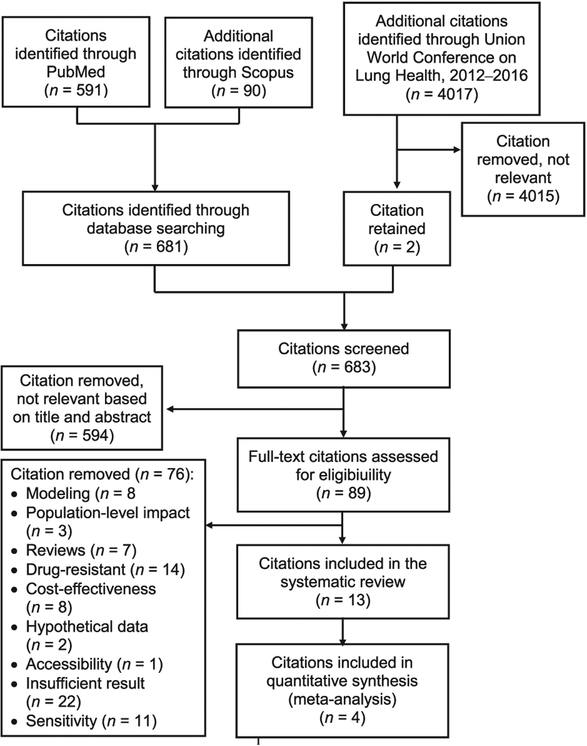
A total of 683 unique citations were identified, with 89 reporting on either outcome or impact (Figure 1). Of these, 13 citations on drug-susceptible pulmonary TB were eligible and were fully reviewed; respectively 43 594 patients and 4825 with known TB treatment outcomes were included for systematic review and meta-analysis.
Figure 1.
Flow chart of citations included for review and meta-analysis.
The designs of analyzed citations in the present study are included in Tables 1–4. Of the 13 citations included in this analysis, nine (including all four citations in the meta-analysis) reported results from randomized clinical trials (Tables 1–4).13–16,22–26 Citations reported on the following, comparing those diagnosed using Xpert vs. those diagnosed using smear: 1) patient-level treatment outcomes among TB patients were reported in 6/13 (46%) citations (Table 1),13–16,25,27 and among presumptive TB patients in 5/13 (31%) citations (Table 2);13,22,23,25,26 2) the total number of patients with a TB diagnosis was reported in 3/13 (23%) citations (Table 3),27–29 and in 6/13 (46%) citations which reported on TB patients diagnosed among presumptive TB patients (Table 4);13,22–26 3) the proportion of bacteriologically confirmed TB was reported in 6/13 (46%) citations among TB patients diagnosed (Table 3)14,15,22,27,29,30 and in 3/13 (23%) citations among presumptive TB patients (Table 4);13,24,28 4) the proportion of TB patients empirically treated was reported in 4/13 (31%) citations (Table 3)14,22,25,27 and in 1/13 (8%) citations among presumptive TB patients (Table 4);26 5) the proportion of LTFU among presumptive TB was reported in 2/13 (15%) citations (Table 4);23,25 and 6) time to diagnosis was reported in 3/13 (23%) citations,22,25,27 and time to treatment in 5/13 (39%) citations (Table 5).13,22,24,25,27
Table 1.
Treatment outcome measured among TB patients*
| Author, reference | Publication year | Country | Design | Sample size n | Settings | Treatment outcome† | TB patients diagnosed using smear n | n (%) | TB patients diagnosed using Xpert n | n (%) | RR (95%CI) | P value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yoon27 | 2012 | Uganda | PC | 477 | In-patients | Mortality at 2 months | 153 | 26 (17.0) | 99 | 14 (14.0) | — | 0.80 |
| Cox13 | 2014 | South Africa | CRT | 1985 | Out-patients, clinic | Unfavorable outcome‡ | 204 | 28 (13.7) | 249 | 31 (13.7) | — | NA |
| Mupfumi25 | 2014 | Zimbabwe | RCT | 424 | Out-patients, HIV clinic at hospital | Mortality at 3 months | 172 | 17 (10.0) | 182 | 11 (6.0) | — | 0.19 |
| Theron26 | 2014 | South Africa, Zambia, Tanzania, Zimbabwe | RCT | 1502 | Out-patients, TB clinic | Mortality at 2 months | 324 | 26 (8.0) | 321 | 14 (4.0) | — | 0.054 |
| Trajman14 | 2015 | Brazil | CRT | 4088 | In- and out-patients, clinic | Unfavorable outcome | 1676 | 409 (24.4) | 2015 | 444 (22.0) | — | NA |
| Agizew15 | 2015 | Botswana | CRT | 6041 | Out-patients, HIV clinic | Unfavorable outcome | 39 | 8 (21.0) | 162 | 32 (20.0) | 1.03 (0.63–1.71) | |
| Fielding16 | 2015 | South Africa | CRT | 4656 | Out-patients, clinic | Unfavorable outcome | 249 | 31 (12.5) | 213 | 25 (11.7) | 1.05 (0.70–1.59) | 0.8 |
The numerator and denominator of the number of TB patients for smear and Xpert groups were taken as published in the citations.
Favorable = cured, completed; and unfavorable = failure, death (mortality), LTFU.
Unfavorable = treatment failure, death, LTFU.
TB=tuberculosis; RR=risk ratio; CI=confidence interval; PC=prospective cohort; CRT=clustered randomized trial; NA=not available; RCT=randomized clinical trial; HIV=human immunodeficiency virus; LTFU=loss to follow-up.
Table 4.
TB cases diagnosed, bacteriologically confirmed TB, LTFU and empiric treatment among presumptive TB patients in reviewed citations*
| Author, reference | Publication year | Country | Design | Sample Size n | Settings | TB cases, bacteriologically confirmed TB, LTFU and empiric treatment | Presumptive TB patients tested using smear n | n (%) | Presumptive TB patients tested using Xpert n | n (%) or /100 000 | RR (95%CI) | P value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cox13 | 2014 | South Africa | CRT | 1 985 | Out-patients, HIV clinic | Bacteriologically confirmed TB† | 1 003 | 167 (17.0) | 982 | 257 (26.0) | 1.57 (1.32–1.87) | <0.001 |
| Initiated on treatment | 1 003 | 229 (23) | 982 | 277 (28) | — | 0.013 | ||||||
| Durovni24§ | 2014 | Brazil | CRT | 24 227 | In- and out-patients, clinic | Bacteriologically confirmed TB | 11 705 | 1 137 (9.7) | 12 522 | 1 777 (14.2) | — | 0.001 |
| Mupfumi25 | 2014 | Zimbabwe | RCT | 424 | Out-patient, HIV clinic at hospital | TB cases diagnosed | 210 | 45 (21.0) | 214 | 43 (20.0) | — | 0.800 |
| LTFU | 210 | 38 (18.0) | 214 | 32 (15.0) | — | 0.380 | ||||||
| Theron26 | 2014 | South Africa, Zambia, Tanzania, Zimbabwe | RCT | 1 502 | Out-patients, TB clinic | Empiric treatment‡ at 2 months | 758 | 197 (26.0) | 744 | 130 (17.0) | — | <0.001 |
| TB cases diagnosed | — | |||||||||||
| Day 1 | 758 | 115 (15.0) | 744 | 168 (23.0) | — | <0.001 | ||||||
| Day 2 | 758 | 24.0 | 744 | 30.0 | — | 0.009 | ||||||
| Day 3 | 758 | 27.0 | 744 | 32.0 | — | 0.041 | ||||||
| Day 14 | 758 | 39.0 | 744 | 41.0 | — | 0.383 | ||||||
| Day 28 | 758 | 40.0 | 744 | 42.0 | — | 0.409 | ||||||
| Day 56 | 758 | 42.0 | 744 | 43.0 | — | 0.641 | ||||||
| Calligaro22 | 2015 | South Africa | RCT | 341 | In-patients, hospitalized in intensive care unit | TB cases diagnosed | 115 | 16 (14.0) | 111 | 24 (22.0) | — | 0.129 |
| Churchyard23 | 2015 | South Africa | CRT | 4656 | Out-patients, clinic | TB cases diagnosed | 2 332 | 291 (12.5) | 2 324 | 250 (10.8) | 1.04 (0.76–1.43) | 0.79 |
| LTFU | 2 332 | 23 (0.99) | 2 324 | 25 (1.08) | — | NA | ||||||
| Sachdeva28 | 2015 | India | PC | 81 231 | Out-patients, clinic | Bacteriologically confirmed TB | 10 675 | 1 532 (14.4) | 70 556 | 14299 (20.3) | 1.33 (1.16–1.52) | NA |
The numerator and denominator of the number of presumptive TB patients for smear and Xpert groups were taken as published in the citations.
TB diagnosis based on bacteriological evidence of the presence of Mycobacterium tuberculosis complex.
Initiation of treatment in the absence of a bacteriologically confirmed diagnosis.17
Durovni et al. presented TB patients diagnosed/100 000 (79.6 vs. 91.7/100 000, smear and Xpert, respectively, P = 0.115) and not percentages.
TB=tuberculosis; LTFU=loss to follow-up; RR=risk ratio; CI=confidence interval; CRT=clustered randomized trial; HIV=human immunodeficiency virus; RCT=randomized clinical trial; PC=prospective cohort; NA=not available.
Table 2.
Mortality measured among presumptive TB patients*
| Author, reference | Publication year | Country | Design | Sample size n | Settings | Months at which mortality measured | Presumptive TB patients† tested using smear n | n (%) | Presumptive TB patients† tested using Xpert n | n (%) | RR (95%CI) | P value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cox13 | 2014 | South Africa | CRT | 1985 | Out-patients, clinic | 6 | 1003 | 38 (3.8) | 982 | 33 (3.4) | 0.89 (0.56–1.40) | 0.518 |
| Mupfumi25 | 2014 | Zimbabwe | RCT | 424 | Out-patients, HIV clinic at hospital | 3 | 172 | 17 (10.0) | 182 | 11 (6.0) | 0.61 (0.29–1.27) | 0.19 |
| Theron26 | 2014 | South Africa, Zambia, Tanzania, Zimbabwe | RCT | 1502 | Out-patients, TB clinic | 6 | 758 | 63 (8.0) | 744 | 58 (8.0) | — | 0.714 |
| Calligaro22 | 2015 | South Africa | RCT | 341 | In-patients, hospitalized in intensive care unit | 3 | 115 | 48 (42.0) | 111 | 36 (32.0) | — | 0.149 |
| Churchyard23 | 2015 | South Africa | CRT | 4656 | Out-patients, clinic | 6 | 2332 | 116 (5.0) | 2324 | 91 (3.9) | 1.1 (0.75–1.62) | 0.61 |
The numerator and denominator of the number of presumptive TB patients for smear and Xpert groups were taken as published in the citations.
Patients with any of the following four TB symptoms (cough, fever, night sweat or weight loss) among HIV-infected and >2 weeks TB symptoms among non-HIV-infected.
TB = tuberculosis; RR = risk ratio; CI = confidence interval; CRT=clustered randomized trial; RCT = randomized clinical trial; HIV = human immunodeficiency virus.
Table 3.
TB cases diagnosed, bacteriologically confirmed TB and empiric treatment in reviewed citations*
| Author, reference | Publication year | Country | Design | Sample Size n | Settings | TB cases diagnosed/ bacteriologically confirmed TB/ empiric treatment | TB patients diagnosed using smear n | n (%) | TB patients diagnosed using Xpert n | n (%) or /100 000 | RR (95%CI) | P value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yoon27 | 2012 | Uganda | PC | 477 | In-patients | Bacteriologically confirmed TB† | 178 | 107 (60.0) | 125 | 82 (65.0) | — | NA |
| Empiric treatment‡ | 178 | 27 (15.0) | 125 | 9 (7.0) | — | 0.047 | ||||||
| TB cases diagnosed | 178 | 134 (75.0) | 125 | 98 (78.0) | — | 0.51 | ||||||
| Mupfumi25 | 2014 | Zimbabwe | RCT | 424 | Out-patients, HIV clinic at hospital | Empiric treatment | 45 | 31 (69) | 43 | 23 (54) | — | 0.120 |
| Trajman14 | 2015 | Brazil | CRT | 4 088 | In- and out-patients, clinic | Bacteriologically confirmed TB | 1 856 | 1 262 (68.0) | 2 232 | 1 701 (76.2) | — | <0.05 |
| Empiric treatment | 1 856 | 594 (32.0) | 2 232 | 531 (23.0) | — | NA | ||||||
| Agizew15 | 2015 | Botswana | CRT | 6 041 | Out-patients, HIV clinic | Bacteriologically confirmed TB | 35 | 8 (23.0) | 149 | 86 (58.0) | — | <0.001 |
| Calligaro22 | 2015 | South Africa | RCT | 341 | In-patients, hospitalized in intensive care unit | Bacteriologically confirmed TB | 16 | 6 (37.5) | 24 | 20 (83.3) | — | NA |
| Empiric treatment | 16 | 9 (56.0) | 24 | 4 (17.0) | — | 0.015 | ||||||
| Creswell29§ | 2015 | Nepal | PC | 14 846 | In- and out-patients, clinic | Bacteriologically confirmed TB | 5 123 | 3 390 (66.2) | 9 375 | 7 812 (83.3) | — | 0.001 |
| Van Den Handel30 | 2015 | South Africa | PC | 1 449 | In- and out-patients, clinic | Bacteriologically confirmed TB | 75 | 48 (64.0) | 123 | 114 (92.0) | 0.69 (0.58–0.82) | <0.001 |
The numerator and denominator of the number of TB patients for smear and Xpert groups were taken as published in the citations.
TB diagnosis based on bacteriological evidence of the presence of Mycobacterium tuberculosis complex.
The initiation of treatment in the absence of a bacteriologically confirmed diagnosis.17
Creswell et al. reported an 8.5% non-significant, lower than expected annual pulmonary TB notifications in the second year of intervention (Xpert introduction) compared with baseline (pre-intervention) TB notifications (4 589 vs. 5 123).29 Sachdeva et al. reported TB patients diagnosed per 100 000 among Xpert and smear (respectively 134/100 000 vs. 116/100 000 population; RR 1.16, P, 0.001).28
TB = tuberculosis; RR = risk ratio; CI = confidence interval; PC = prospective cohort; NA = not available; RCT =randomized clinical trial; HIV = human immunodeficiency virus; CRT =clustered randomized trial.
Table 5.
Time to diagnosis and time to treatment*
| Author, reference | Publication year | Country | Design | Sample size n | Settings | Time to diagnosis or time to treatment days | TB patients diagnosed using smear n | Days median [IQR] | TB patients diagnosed using Xpert n | Days median [IQR] | P value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Yoon27 | 2012 | Uganda | PC | 477 | In-patients | Time to diagnosis† | 178 | 1 [0–26] | 125 | 0 [0–1] | <0.001 |
| Time to treatment† | 178 | 1 [0–5] | 125 | 0 [0–2] | <0.060 | ||||||
| Cox13 | 2014 | South Africa | CRT | 1 985 | Out-patients, clinic | Time to treatment | 229 | 8 [2–27] | 277 | 4 [2–8] | NA |
| Durovni24 | 2014 | Brazil | CRT | 24 227 | In- and out-patients, clinic | Time to treatment | 2 050 | 11.4 [8.5–14.5] | 2610 | 8.1 [5.4–9.3] | 0.04 |
| Mupfumi25 | 2014 | Zimbabwe | RCT | 424 | Out-patient HIV clinic at hospital | Time to diagnosis | 45 | 6 [1–25] | 43 | 2 [1–13] | 0.07 |
| Time to treatment | 45 | 8 [3–23] | 43 | 5 [3–13] | 0.26 | ||||||
| Calligaro22 | 2015 | South Africa | RCT | 341 | In-patients, hospitalized in intensive care unit | Time to diagnosis | 16 | 12.1 [0.3–22.2] | 24 | 0.2 [0.2–0.3] | <0.001 |
| Time to treatment | 16 | 0.7 [0.2–2.2] | 24 | 0.3 [0.2–1.2] | 0.479 |
The numerator and denominator of the number of TB patients for smear and Xpert groups were taken as published in the citations.
Time to diagnosis and time treatment defined as either time from enrollment to diagnosis,10,21 or from sputum collection to diagnosis.17
TB = tuberculosis; IQR = interquartile range; PC = prospective cohort; CRT =clustered randomized trial; NA = not available; RCT =randomized clinical trial; HIV = human immunodeficiency virus.
Meta-analysis
The four citations that fulfilled the criteria for meta-analysis were from Botswana (n = 1), Brazil (n = 1) and South Africa (n = 2). The data from these citations are summarized in Tables 1 and 6.13–16 Of a combined total of 4825 TB patients, 55% (2675/4825) were diagnosed using Xpert and 45% (2150/ 4825) using smear, were initiated on anti-tuberculosis treatment and had a known treatment outcome. Bivariate analysis showed similar treatment outcomes between TB patients diagnosed using Xpert and smear (Table 6). Unfavorable outcomes were reported for respectively 20.2% (541/2675) and 21.9% (470/2150) of patients diagnosed using Xpert and smear. The pooled analysis showed a RR of 0.92 (95%CI 0.82–1.02) (Figure 2). Statistical heterogeneity was low (I2 = 0.0%, P = 0.910).
Table 6.
Characteristics of studies included in the meta-analysis
| Author, country, reference | Year | Smear results | Xpert results | RR (95%CI) |
|---|---|---|---|---|
| Unfavorable† n/N (%) | Unfavorable n/N (%)‡ | |||
| Fielding, South Africa16 | 2015 | 31/249 (12) | 25/231 (11) | 1.05 (0.70–1.59) |
| Agizew, Botswana15 | 2015 | 8/39 (21) | 32/162 (20) | 1.04 (0.52–2.07) |
| Trajman, Brazil14 | 2015 | 409/1676 (24) | 444/2015 (22) | 1.12 (0.98–1.23) |
| Cox, South Africa13 | 2014 | 28/204 (14) | 34/249 (14) | 1.00 (0.63–1.56) |
TB patients who initiated treatment with known treatment outcome smear group.
Treatment failure, death, loss to follow-up.
TB patients initiated treatment with known treatment outcome Xpert group.
RR = risk ratio; CI = confidence interval.
Figure 2.
Forest plots of unfavorable treatment outcomes among tuberculosis patients diagnosed using Xpert vs. smear. RR = risk ratio; CI = confidence interval.
Tuberculosis treatment outcomes
Of the 13 reviewed citations, six reported on patient-level treatment outcomes among TB patients, four of which had sufficient data allowing for classification as favorable or unfavorable; treatment outcome was similar among those diagnosed using Xpert vs. those diagnosed with smear. Yoon et al. and Mupfumi et al. reported on mortality among TB patients at 2 and 3 months with no difference between smear and Xpert groups (Table 1).13–16,25,27 Another five citations, reporting among presumptive TB patients, showed no mortality benefit at 6 months,13,22,23,25,26 or at 3 months (Table 2).22 Cox et al. demonstrated superiority of Xpert to smear (21.9%, 215/982 vs. 17.5%, 176/1003; RR 1.25, P = 0.032) on treatment success (measured as favorable outcome) among presumptive TB patients.13
Reviewed diagnostic and therapeutic impact: Xpert vs. smear
TB patients diagnosed
Of the three citations reporting on the total number of TB patients diagnosed,27–29 only Sachdeva et al. indicated increased TB diagnosis (16%) among Xpert compared with smear (134/100 000 vs. 116/100 000 population, RR 1.16, P < 0.001). Creswell et al. reported lower than expected (8.5%, non-significant) annual pulmonary TB notifications at 4589 in the second year of intervention (Xpert introduction) compared with the baseline (pre-intervention) 5123 TB notifications.29 Of the six citations that reported on presumptive TB patients, only Cox et al. demonstrated a higher proportion of TB diagnosed in the Xpert arm than in the routine arm (smear) (28%, 277/98 vs. 23%, 299/1003; RR 1.44, P = 0.013); all other citations showed no incremental benefit (i.e., increased TB case detection), including Durovni et al., who reported results per 100 000 rather than proportions (79.6 vs. 91.7/100 000, smear and Xpert, respectively, P = 0.115).13,22–26 Theron et al. noted that the effect of Xpert, compared with smear, on incremental value was demonstrated only within the first 3 days after sputum collection, and the benefit was no longer seen at day 14 (41%, 303/744 vs. 39%, 292/758, P = 0.383) and thereafter at day 28 and day 56 (Table 4).26
Empiric treatment
Of the 5 citations that reported on empiric anti-tuberculosis treatment, 1 was among presumptive TB patients and 4 among TB patients.15,22,25–27 In 2/4 citations reporting on TB patients, Xpert use led to reduction in empiric treatment compared with smear (7%, 9/125 vs. 15%, 27/178 by Yoon et al. and 17%, 4/24 vs. 56%, 9/16 by Calligaro et al.) (Table 3).22,27 Theron et al. showed a decrease in empiric treatment compared with smear among presumptive TB patients (17%, 130/744 vs. 26%, 197/758) (Table 4).26
Bacteriologically confirmed TB
Of the 9 citations that reported the proportion of bacteriologically confirmed TB, 6 were among TB patients and 3 among presumptive TB patients. In 7/9 (78%) citations, Xpert was superior to smear in confirming TB bacteriologically.13,22–27 In studies by Yoon et al. and Calligaro et al., bacteriological evidence was similar between Xpert and smear; all TB patients included in these two analyses were hospitalized patients unlike the rest of the citations (Tables 3 and 4), in which Xpert was shown to be superior.
Time to diagnosis and time to treatment
Five citations reported on time to treatment, three of which also reported on time to diagnosis. Compared with smear, Xpert significantly reduced delay in diagnosis in 2 of the 3 citations that measured time to diagnosis,22,25,27 and reduced time to treatment in 1/5 citations (Table 5).13,22,24,25,27 One of the studies reporting on time to treatment (not time to diagnosis) was by Durovni et al., who reported that Xpert was useful in increasing the number of TB patients who initiated treatment early in a Brazilian setting, compared with smear (Table 4).13,24 Theron et al. reported the proportion of TB patients diagnosed and initiated on treatment but did not report the number of days to treatment initiation (Table 4); instead, they reported that Xpert increased the number of TB patients diagnosed and the number of patients who were subsequently treated in the first 3 days after sputum collection, but that there was little difference between Xpert and smear groups for those starting treatment at 2, 4 and 8 weeks after sputum collection. In this cohort, mortality at 2 and at 6 months was similar among patients diagnosed using Xpert and those diagnosed using smear, irrespective of time to treatment initiation.26 None of the citations showed that reduced time to diagnosis or treatment translated into an improvement in TB treatment outcome or mortality.
Loss to follow-up
Of the two citations reported on LTFU among presumptive TB patients,23,25 Xpert use had no effect on reducing LTFU. Comparing Xpert vs. smear, Mupfumi et al. reported that LTFU was 15% (32/ 214) vs. 18% (38/210), P = 0.380, and Churchyard et al. reported 0.99% (23/2332) vs. 1.08% (25/2324) (P value not available) (Table 4).
DISCUSSION
To our knowledge, this is the first systematic review and meta-analysis of the impact of using Xpert compared with smear on patient-level TB treatment outcomes (coded as favorable and unfavorable) in the diagnosis of drug-susceptible TB. Pooled results gathered from the four randomized trials included in the meta-analysis demonstrated similar TB treatment outcomes among patients diagnosed with Xpert compared with smear, suggesting that Xpert has not been observed to reduce unfavorable TB treatment outcomes. No previous meta-analysis has compared drug-susceptible TB treatment outcomes between cohorts using Xpert and smear. The report by Auld et al., who reviewed eight clinical trials, was the only one available; they concluded that Xpert showed no impact on the key patient-level outcomes, mortality and morbidity.31
Recent publications have demonstrated that Xpert has the potential to increase the number of TB cases diagnosed,9,13,28 and microbiologically confirmed TB cases, and reduce time to diagnosis and treatment initiation.9,13,22,24,27,28 Beohme et al. also demonstrated that shorter turnaround times resulted in substantially faster initiation of anti-tuberculosis treatment, which resulted in a significant reduction in LTFU.10 It remains unclear as to why Xpert did not result in a discernible impact in terms of improvement of clinical outcome despite such potential.
Theron et al. hypothesized that the potential benefits from Xpert are masked by high levels of rapid empiric treatment,17 and several reports agreed with this assumption.13,14,26 In contrast, Churchyard et al. reported that Xpert could minimize the proportion of patients receiving empiric treatment.23 Although clinicians cannot eliminate the use of empiric treatment, Xpert has a higher sensitivity than smear to accurately diagnose TB and a higher specificity to identify true-negatives, and this may be of benefit to patients, who would be spared unnecessary anti-tuberculosis treatment.9,18,23 Creswell et al. demonstrated an 8.5% reduction in empiric treatment in Nepal when Xpert was used in program settings.29 Churchyard et al., Creswell et al. and the present review do not support the assumption that the effects of Xpert are being masked by clinicians continuing a high level of empiric treatment. Nonetheless, the concern as to why treatment outcome has not been affected by reduced empiric treatment prevails. The majority of the reviewed citations in the present analysis indicated that the use of Xpert reduced empiric treatment, but no treatment outcome benefits were observed. It is also worth noting that empiric treatment may have some added value, particularly in TB in children and people with smear-negative TB, where Xpert sensitivity is lower than that in patients with smear-positive or culture-positive TB. In such cases, empiric treatment remains important. An editorial from Lawn et al., in support of Theron et al.’s hypotheses, concluded that the effect and extent of empiric treatment on treatment outcomes warranted further evaluations in real-world settings.17,32 An observational prospective study (ClinicalTrials.gov Identifier: ) was started in 2016 in Zambia to evaluate the effect of Xpert on patient health outcomes and empiric TB treatment among PLHIV. The study is expected to be completed in 2018, and the results will provide an opportunity to examine any association between Xpert use and empiric treatment.33
In the present analysis, we reviewed the diagnostic and therapeutic impact of Xpert, and their effect on patient-level treatment outcomes. In the majority of the citations (7/9), although Xpert increased bacteriologically confirmed TB, the effect did not translate into increased numbers of TB cases treated or improvement in treatment outcomes.13,15,22,24,27–29 We are in agreement with Auld et al. that merely replacing smear with Xpert is not enough to see the desired effect of highly sensitive new molecular testing.31 Another recent report from Auld et al. seems to support this hypothesis; their study showed a significant 12-month mortality reduction when enhanced care (defined as intensified case finding with additional staff support actively tracing patients who missed clinic appointments) was added to routine standard of care, rather than only replacing smear with Xpert. Auld et al. suggested that the intensified case finding and active tracing of TB patients to improve retention in care by health care workers were key factors for any mortality benefits.34
The need for enhanced care discussed by Auld et al. is an issue of health systems strengthening.31 An additional area of concern, which was not included in our systematic review but has a potential to negatively affect Xpert test results and thus treatment initiation, is sputum quality. Orina et al. from Kenya and Meyer et al. from Uganda reported concerns with a higher than expected proportion of salivary sputum samples collected for Xpert testing.35,36 The Xpert diagnostic test does not function in isolation, and considering other health system and pre- and post-diagnostic test-related factors in the TB diagnostic cascade is essential.37 There are three key intervention areas that are important to address for successful Xpert implementation. The first is the pre-diagnostic stage. Interventions may include training of Xpert operators, including proficiency tests, assessment of potential diagnostic delays, both patient-related (e.g., health care seeking) and health system (e.g., sample transport) and assessment of optimal placement of GeneXpert instruments to improve access to testing (high-burden areas, point-of-care peripheral clinics or even at the community level if GeneXpert Omni is available). The second is the diagnostic stage. GeneXpert instruments should be regularly maintained and should use the latest version of Xpert cartridges (currently G4 or Ultra). The third area is post-diagnostic stage. Attention should be placed on turnaround time, early treatment initiation, reduced empiric treatment, and reduced LTFU by actively tracking patients after treatment initiation.
While Xpert as a highly sensitive and rapid diagnostic test is appreciated in TB control efforts, patient-related factors, quality sputum samples and stronger health systems seem to matter more than the use of innovative technology alone. Population-level TB control success in high-income countries was achieved long before molecular testing was introduced, which was not necessarily due to the diagnostic technology used.38 Having said this, we are not by any means minimizing the need for new Xpert molecular technology, as it plays a very important role in the rapid diagnosis of drug-resistant TB, extra-pulmonary TB and childhood TB. Furthermore, the use of the Xpert Ultra assay in settings with limited infrastructure is promising, and should be further explored.
Our study had seven main limitations. First, of the 13 citations that reported on the impact or treatment outcomes, only four fulfilled the inclusion criteria for the meta-analysis. All of these citations were from randomized clinical trials but the covariate data on age, sex, HIV status and previous history of TB were insufficient to be included in the analysis. Second, TB treatment outcomes for meta-analysis reported by Agizew et al. and Fielding et al. were adjusted for clustering,15,16 whereas data from the trial reports from Cox et al. and Trajiman et al. were extracted and thus not adjusted for clustering.13,14 Third, studies that measured delays in time to diagnosis or time to treatment used inconsistent definitions. In some studies, delays were measured from the time of enrollment,13,25 while in others, this was measured from the time of sputum sample collection;26 some did not provide clear definitions.22,24 Fourth, no data were available on other potential factors that could influence patient care and outcomes, such as delays in sample transportation, efficiency of patient referrals, proficiency of staff in conducting diagnostic tests, and adherence of clinicians to standard guidelines for patient screening, diagnosis and treatment, all of which could have had an effect on time to diagnosis and time to treatment. Fifth, the population studied in the present analysis included a mix of HIV-infected and non-infected persons, with the exception of two citations that included only HIV-infected persons in the analysis. Due to their compromised immune status, HIV-infected individuals might possibly have affected treatment outcomes. However, the citations which included both did not report treatment outcomes by HIV status; it was therefore not possible to see any impact difference between the HIV-infected and non-infected groups. Sixth, in the present analysis, the microbiological status of TB patients may have affected treatment outcomes; however, none of the citations included reported treatment outcomes by microbiological status. Finally, although 9/13 citations reported in this analysis were from randomized clinical trials,14–16,22–26 the relative effect of the study design may have affected the impact of the interventions, as highlighted in a review by Auld et al.31
In conclusion, Xpert implementation showed no discernible impact on clinical outcomes compared with conventional smear. Despite improved time to TB diagnosis, treatment initiation and/or empiric treatment, patient-level outcomes for Xpert and smear were similar. With the available evidence, efforts towards TB control need more than just consideration of replacement of smear with Xpert. Further implementation research is required to better understand the gaps in existing health systems. Although improved clinical outcomes have not yet been observed with Xpert, identifying areas of intervention for health system reinforcement is a worthwhile exercise on an ongoing basis. Within a wider context, optimizing the use of Xpert to maximize benefits, such as early diagnosis and management of drug-resistant TB, and diagnosis of extra-pulmonary TB and pediatric TB, are also essential.
Acknowledgements
This work has been supported by the President’s Emergency Plan for AIDS Relief (PEPFAR) through the US Centers for Disease Control and Prevention (CDC) and Division of TB Elimination, CDC. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the US CDC.
The authors also thank Professor G Churchyard from The Aurum Institute, Johannesburg, and University of Witwatersrand, Johannesburg, South Africa, for support in a technical advisory role from inception of the design and analysis of the study.
Footnotes
Publisher's Disclaimer: Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the funding agencies or of the CDC. References in this manuscript to any specific commercial products, process, service, manufacturer, or company do not constitute their endorsement or recommendation by the US Government.
Conflicts of interest: none declared.
References
- 1.Gupta RK, Lucas SB, Fielding KL, Lawn SD. Prevalence of tuberculosis in post-mortem studies of HIV-infected adults and children in resource-limited settings: a systematic review and meta-analysis. AIDS 2015; 29: 1987–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dale D, Nega D, Yimam B, Ali E. Predictors of poor tuberculosis treatment outcome at Arba Minch General Hospital, Southern Ethiopia: a case-control study. J Tuberc Ther 2017; 2: 110. [Google Scholar]
- 3.Amante TD, Ahemed TA. Risk factors for unsuccessful tuberculosis treatment outcome (failure, default and death) in public health institutions, Eastern Ethiopia. Pan Afr Med J 2015; 20: 247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lawn SD, Wilkinson RJ, Lipman MC, Wood R. Immune reconstitution and ‘unmasking’ of tuberculosis during antiretroviral therapy. Am J Respir Crit Care Med 2008; 177: 680–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marais BJ, Graham SM, Cotton MF, Beyers N. Diagnostic and management challenges for childhood tuberculosis in the era of HIV. J Infect Dis 2007; 196 (Suppl 1): S76–S85. [DOI] [PubMed] [Google Scholar]
- 6.Banada P, Sivasubramani S, Blakemore R, et al. Containment of bioaerosol infection risk by the Xpert® MTB/RIF assay and its applicability to point-of-care settings. J Clin Microbiol 2010; 48: 3551–3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Helb D, Jones M, Story E, et al. Rapid detection of Mycobacterium tuberculosis and rifampin resistance by use of on-demand, near-patient technology. J Clin Microbiol 2010; 48: 229–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization. World Health Organization monitoring of Xpert MTB/RIF roll-out. Geneva, Switzerland: WHO, 2018. http://www.who.int/tb/areas-of-work/laboratory/mtb-rif-rollout/en/ Accessed September 2018. [Google Scholar]
- 9.World Health Organization. Xpert MTB/RIF assay for the diagnosis of pulmonary and extra-pulmonary TB in adults and children: policy update. WHO/HTM/TB/2013.16. Geneva, Switzerland: WHO, 2013. [Google Scholar]
- 10.Boehme CC, Nicol MP, Nabeta P, et al. Feasibility, diagnostic accuracy, and effectiveness of decentralised use of the Xpert MTB/RIF test for diagnosis of tuberculosis and multidrug resistance: a multicentre implementation study. Lancet 2011; 377: 1495–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steingart KR, Schiller I, Horne DJ, Pai M, Boehme CC, Dendukuri N. Xpert MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev 2014; 1: CD009593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schumacher SG, Sohn H, Qin ZZ, et al. Impact of molecular diagnostics for tuberculosis on patient important outcomes: a systematic review of study methodologies. PLOS ONE 2016. 11: e0151073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cox H, Mbhele S, Mohess N, et al. Impact of Xpert MTB/RIF for TB diagnosis in a primary care clinic with high TB and HIV prevalence in South Africa: a pragmatic randomised trial. PLOS Med 2014; 11: e1001760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trajman A, Durovni B, Saraceni V, et al. Impact on patients’ treatment outcomes of Xpert® MTB/RIF implementation for the diagnosis of tuberculosis: follow-up of a stepped-wedge randomized clinical trial. PLOS ONE 2015; 10: e0123252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agizew T, Nyirenda S, Mathoma A, et al. Comparison of pre-and post-Xpert clinical outcomes among patients in programme settings with high TB/HIV burden in Botswana. 46th Conference on Lung Health, Cape Town, South Africa, 2–6 December 2015. [Abstract PC-1124–06]. Int J Tuberc Lung Dis 2015: 19 (Suppl 2): S460. [Google Scholar]
- 16.Fielding K, Mccarthy K, Ginindza S, et al. Treatment outcome participant in the XTEND trial. 46th Conference on Lung Health, Cape Town, South Africa, 2–6 December 2015. [Abstract OA-386–05]. Int J Tuberc Lung Dis 2015: 19 (Suppl 2): S273. [Google Scholar]
- 17.Theron G, Peter J, Dowdy D, Langley I, Squire SB, Dheda K. Do high rates of empirical treatment undermine the potential effect of new diagnostic tests for tuberculosis in high-burden settings? Lancet Infect Dis 2014; 14: 527–532. [DOI] [PubMed] [Google Scholar]
- 18.Agizew T, Boyd R, Mathebula U, et al. Treatment outcome among people living with HIV: non-tuberculosis mycobactariun versus Mycobacterium tuberculosis in Botswana. 48th Conference on Lung Health, Guadalajara, Mexico, 11–14 October 2017 [Abstract OA 121–12]. [Google Scholar]
- 19.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and metaanalyses: the PRISMA statement. PLOS Med 2009; 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Detsky S, Naylor D, O’Rourke K, McGeer J, L’Abbe A. Incorporating variations in the quality of individual randomized trials into meta-analysis. J Clin Epidemiol 1992; 45: 255–265. [DOI] [PubMed] [Google Scholar]
- 21.Zlowodzki M, Poolman R, Kerkhoffs G, Tornetta III P, Bhandari M; International Evidence-Based Orthopedic Surgery Working Group. How to interpret a meta-analysis and judge its value as a guide for clinical practice. Acta Orthop 2007; 78: 598–609. [DOI] [PubMed] [Google Scholar]
- 22.Calligaro G, Theron G, Khalfey H, et al. Burden of tuberculosis in intensive care units in Cape Town, South Africa, and assessment of the accuracy and effect on patient outcomes of the Xpert MTB/RIF test on tracheal aspirate samples for diagnosis of pulmonary tuberculosis: a prospective burden of disease study with a nested randomised controlled trial. Lancet Resp Med 2015; 3: 621–630. [DOI] [PubMed] [Google Scholar]
- 23.Churchyard G, Stevens W, Mametja L, et al. Xpert MTB/RIF versus sputum microscopy as the initial diagnostic test for tuberculosis: a cluster-randomised trial embedded in South African roll-out of Xpert MTB/RIF. Lancet Glob Health 2015; 3: e450–457. [DOI] [PubMed] [Google Scholar]
- 24.Durovni B, Saraceni V, van den Hof S, et al. Impact of replacing smear microscopy with Xpert® MTB/RIF for diagnosing tuberculosis in Brazil: a stepped-wedge cluster-randomized trial. PLOS Med 2014; 11: e1001766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mupfumi L, Makamure B, Chirehwa M, et al. Impact of Xpert® MTB/RIF on antiretroviral therapy-associated tuberculosis and mortality: a pragmatic randomized controlled trial. Open Forum Infect Dis 2014; 1: ofu038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Theron G, Zijenah L, Chanda D, et al. Feasibility, accuracy, and clinical effect of point-of-care Xpert® MTB/RIF testing for tuberculosis in primary care settings in Africa: a multicentre, randomised, controlled trial. Lancet 2014; 383: 424–435. [DOI] [PubMed] [Google Scholar]
- 27.Yoon C, Cattamanchi A, Davis J, et al. Impact of Xpert® MTB/ RIF testing on tuberculosis management and outcomes in hospitalized patients in Uganda. PLOS ONE 2012; 7: e48599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sachdeva K, Raizada N, Sreenivas A, et al. Use of Xpert® MTB/ RIF in decentralized public health settings and its effect on pulmonary TB and DR-TB case finding in India. PLOS ONE 2015; 10: e0126065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Creswell J, Rai B, Wali R, et al. Introducing new tuberculosis diagnostics: the impact of Xpert® MTB/RIF testing on case notifications in Nepal. Int J Tuberc Lung Dis 2015; 19: 545–551. [DOI] [PubMed] [Google Scholar]
- 30.Van Den Handel T, Hampton K, Sanne I, Stevens W, Crous R, Van-Rie A. The impact of Xpert MTB/RIF in sparsely populated rural settings. Int J Tuberc Lung Dis 2015; 19: 392–398. [DOI] [PubMed] [Google Scholar]
- 31.Auld A, Fielding K, Gupta-Wright A, Lawn S. Xpert MTB/ RIF—why the lack of morbidity and mortality impact in intervention trials? Trans R Soc Trop Med Hyg 2016; 110: 432–444. [DOI] [PubMed] [Google Scholar]
- 32.Lawn S, Nicol M, Corbett E. Effect of empirical treatment on outcomes of clinical trials of diagnostic assays for TB. Lancet Infect Dis 2015; 15: 17–18. [DOI] [PubMed] [Google Scholar]
- 33.Herce M The effect of Xpert MTB/RIF on patient health outcomes and empirical TB treatment among persons living with HIV/AIDS: a parallel-group prospective cohort study under real-world conditions in Lusaka, Zambia ClinicalTrials. gov Identifier: NCT02729532. Bethesda, MD, USA: National Institutes of Health, 2016. https://clinicaltrials.gov/ct2/show/NCT02729532 Accessed September 2018. [Google Scholar]
- 34.Auld A, Agizew T, Mathoma A, et al. Effect of TB screening and retention interventions on early ART mortality in Botswana. 25th Conference on Retroviruses and Opportunistic Infections, Boston, Massachusetts, USA, 4–7 March 2018 [Oral Abstract 31.] [Google Scholar]
- 35.Orina F, Mwangi M, Githui A, et al. Effect of sputum quality on Xpert® MTB/RIF results in the detection of Mycobacterium tuberculosis from persons presumed to have tuberculosis in EAPHLN Project operational research study sites in Kenya. J Afr Health Sci 2014; 27: 446–456. [Google Scholar]
- 36.Meyer A, Atuheire C, Worodria W, et al. Sputum quality and diagnostic performance of GeneXpert MTB/RIF among smear-negative adults with presumed tuberculosis in Uganda. PLOS ONE 2017; 12: e0180572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun A, Denkinger C, Dowdy D. Understanding the diagnostic cascade of tuberculosis: insight from a transmission model. 45th Conference on Lung Health, Barcelona, Spain, 28 October-1 November 2014 [Abstract OAP-314–31]. [Google Scholar]
- 38.Cummings K Tuberculosis control: challenges of an ancient and ongoing epidemic Public Health Rep 2007; 122: 683–692. [DOI] [PMC free article] [PubMed] [Google Scholar]