Abstract
Objective:
The California Lupus Surveillance Project (CLSP) is a population-based registry of individuals with SLE residing in San Francisco County, California from 2007–2009, with a special focus on Asians/Pacific Islanders (API) and Hispanics. We used retrospective CLSP data to analyze racial/ethnic differences in lupus manifestations and in the timing and risk of developing severe manifestations.
Methods:
724 patients with SLE were retrospectively identified. Prevalence ratios (PR) of SLE manifestations were calculated using Poisson regression models stratified by race/ethnicity and adjusted for sex, age at SLE diagnosis, and disease duration. We studied onset of severe SLE manifestations after SLE diagnosis using Kaplan-Meier methods to examine time-to-event and Cox proportional hazards regressions to estimate hazard ratios (HR). Whites were the referent group in all analyses.
Results:
Blacks, APIs, and Hispanics had increased prevalence of renal manifestations [PR 1.74 (95%CI: 1.40–2.16), PR 1.68 (95%CI: 1.38–2.05), PR 1.35 (95%CI: 1.05–1.74)], respectively. Furthermore, Blacks had increased prevalence of neurologic manifestations [PR 1.49 (95%CI: 1.12–1.98)] and both Blacks [PR 1.09 (95%CI: 1.04–1.15)] and APIs [PR 1.07 (95%CI: 1.01–1.13)] had increased prevalence of hematologic manifestations. Blacks, APIs, and Hispanics, respectively, had higher risk of developing lupus nephritis [HR 2.4 (95%CI: 1.6–3.8), HR 4.3 (95%CI: 2.9–6.4), HR 2.3 (95%CI: 1.4–3.8)] and thrombocytopenia [HR 2.3 (95%CI: 1.1–4.4), HR 2.3 (95%CI: 1.3–4.2), HR 2.2 (95%CI: 1.1–4.7)]. APIs and Hispanics had higher risk of developing antiphospholipid syndrome [HR 2.5 (95%CI: 1.4–4.4), HR 2.6 (95%CI: 1.3–5.1), respectively].
Conclusions:
This is the first epidemiologic study comparing lupus manifestations among four major racial/ethnic groups. We found 1) substantial differences in the prevalence of several clinical SLE manifestations among racial/ethnic groups, and 2) that Blacks, APIs, and Hispanics are at increased risk of developing several severe manifestations following SLE diagnosis.
Systemic lupus erythematosus (SLE) is a chronic, systemic autoimmune disease with a higher prevalence among women and racial/ethnic minority groups in the United States (U.S.). However, despite the growing numbers of Asians/Pacific Islanders (API) and Hispanics in the U.S., little is known about the epidemiology of SLE in these populations. To produce contemporary population-based estimates of incidence and prevalence among various racial/ethnic groups, the Centers for Disease Control and Prevention (CDC) funded four SLE registries across the U.S., including two registries in California and New York, which focused on Hispanics and Asians. Estimates from the registries showed, relative to whites, increased incidence and prevalence of SLE among Blacks (1–4), APIs (2,3), Hispanics (2,3), and American Indian/Alaskan Natives (5). Specifically, the California Lupus Surveillance Project (CLSP) reported a higher age-standardized incidence and prevalence of SLE among Black (15.5 per 100,000 person-years; 241.0 per 100,000 persons), API (4.1 per 100,000 person-years; 90.5 per 100,000 persons), and Hispanic populations (4.2 per 100,000 person-years; 94.7 per 100,000 persons) relative to whites (2.8 per 100,000 person-years; 55.2 per 100,000 persons) in San Francisco County during the period 2007–2009 (3).
There are currently few studies exploring racial/ethnic differences in the clinical presentation of SLE or in the development of severe disease manifestations subsequent to SLE diagnosis. Previous epidemiologic studies suggest that in comparison to whites, Blacks have a more severe presentation of symptoms at the time of diagnosis of SLE and a worse overall prognosis (6–8). However, no studies to date have analyzed racial/ethnic differences in manifestations of SLE across the four major racial/ethnic populations in the U.S., including among APIs and Hispanics. To address this gap, we examined data gathered in the CLSP to investigate racial/ethnic differences in the prevalence of SLE manifestations and in the risk and timing of development of severe SLE manifestations.
PATIENTS AND METHODS
California Lupus Surveillance Project (CLSP).
The California Department of Public Health (CDPH) collaborated with the University of California, San Francisco (UCSF) to conduct the CLSP, as described elsewhere (3). The State of California’s Institutional Review Board (IRB) granted a waiver for this public health surveillance activity. The project was reviewed and approved by the UCSF Committee on Human Research.
Source population/catchment area criteria.
The CLSP includes residents of San Francisco County within the period of 2007 to 2009. According to U.S. Census estimates during this time period, San Francisco County averaged 790,582 residents, with the following racial/ethnic composition: 56% white, 35% API, 7% Black, and 1% American Indian/Alaskan Native (9). Fifteen percent of the San Francisco population identified as Hispanic, which is reported separately from race in the Census.
Case definition.
In this analysis, patients were defined as having SLE if they met at least four of the 11 American College of Rheumatology (ACR) revised classification criteria defined in 1982 and updated in 1997 (10,11).
Case ascertainment.
Three primary sources within the catchment area provided possible cases of SLE: 1) community rheumatology and nephrology clinics, 2) community hospitals, and 3) integrated healthcare systems including UCSF, Kaiser Permanente, and the San Francisco Veterans Administration Medical Center (VAMC). Potential cases were identified using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnostic codes 710.0 (SLE), 695.4 (discoid lupus), 710.8 (other specified connective tissue disease), and 710.9 (unspecified connective tissue disease). Secondary sources of possible cases of SLE included a commercial laboratory, which was queried for a comprehensive panel of SLE-related serologic tests, as described elsewhere (3), and the California Office of Statewide Health Planning and Development (OSHPD) hospital discharge database, which was queried for patient discharges using similar ICD-9 codes listed above.
Clinical manifestations of SLE.
A defined number of SLE manifestations for each patient was ascertained from the medical records and grouped within the following ten categories: mucocutaneous, serositis, cardiovascular, pulmonary, gastrointestinal, renal, musculoskeletal, neurologic, hematologic, and serologic. The manifestations included under each of these categories are defined in Appendix 1.
Severe SLE Manifestations.
We evaluated the following four severe SLE manifestations: lupus nephritis, thrombocytopenia, neuropsychiatric lupus, and antiphospholipid syndrome (APS). Because the initial chart abstraction did not include the date of first appearance of lupus nephritis, we created a surrogate variable to define nephritis. Any one of the following three laboratory measurements were used to define onset of lupus nephritis: 24-hour proteinuria > 500mg, 24-hour urine protein/creatinine ratio > 0.5, or spot protein/creatinine ratio > 0.5. We chose this approach to increase the sensitivity of our definition and to ensure that barriers to access and timely diagnosis did not bias the results. A positive laboratory result nearest to the date of SLE diagnosis was used to define the time of initial lupus nephritis. Thrombocytopenia was defined as a documented platelet count below 100,000/mm3 or physician documentation of thrombocytopenia that was unexplained by medication effect or other causes. Neuropsychiatric manifestations included seizures, psychosis, and acute confusional state, as documented in the medical record. APS was diagnosed when there was physician documentation of APS. We also evaluated a combined outcome, i.e., development of any of lupus nephritis, thrombocytopenia, neuropsychiatric lupus, or APS.
Independent Variables.
Information regarding sex and race/ethnicity was abstracted from each medical chart. For race/ethnicity, patients were classified as non-Hispanic white, non-Hispanic Black, API, or Hispanic (any race). Non-Hispanic whites and non-Hispanic Blacks will be referred to as whites and Blacks from this point forward. American Indian/Alaskan natives were identified but excluded from this analysis due to small sample size (n = 4). Physician-documented age at SLE diagnosis was categorized into discrete age groups (≤18, 19–29, 30–39, 40–49, 50+ years) to account for the non-linear relationship between age of diagnosis and disease manifestations. At the time of chart abstraction, the number of years since physician-documented date of SLE diagnosis for each subject was calculated from the year of last reported clinic visit date; years elapsed since diagnosis were categorized as: ≤5, 6–10, 11–15, and 16+ years, which approximated quartiles of the distribution.
Statistical Analyses.
Baseline racial/ethnic differences in age at SLE diagnosis and years since SLE diagnosis were examined using ANOVA, after determining that the assumptions of normally distributed residuals and homoscedasticity were not violated. Racial/ethnic differences in sex and SLE manifestation were examined using a chi-square test. Race/ethnicity-stratified prevalence ratios (PR) for clinical manifestations of SLE were calculated using a Poisson regression model with robust error variances, including the covariates sex, age at SLE diagnosis, and years since SLE diagnosis. A Poisson regression model was chosen due to its appropriateness for analyzing count data and the rarity of individual manifestations. Kaplan-Meier survival methods were used to examine the time to onset of severe SLE manifestations. Individual survival curves representing separate race/ethnicities were compared using the log-rank test. For each severe SLE manifestation, we estimated the risk of manifestation onset for race/ethnicity, sex, and age at SLE diagnosis using multivariable Cox proportional hazards regression models that modelled the three characteristics simultaneously. Higher hazard ratios (HR) indicated a greater risk of developing specific manifestations over time. The proportional-hazards assumption based on Schoenfeld residuals was validated for appropriateness of use.
For the survival analysis, the baseline was defined as the physician-documented date of SLE diagnosis. Subjects who already had evidence of the reported outcome(s) at the time of SLE diagnosis were treated as having developed the outcome on day one. Patients were included in follow-up until they developed the outcome of interest or were censored at the date of the last recorded clinical visit through 2009. A chi-square test was used to evaluate racial/ethnic differences in the presence of severe SLE manifestations identified at the date of SLE diagnosis.
Given the use of a surrogate variable to define lupus nephritis onset, a sensitivity analysis was performed in which only those with subsequently physician-diagnosed lupus nephritis were analyzed. All statistical analyses were performed using STATA 13 (12).
RESULTS
Study population characteristics.
Seven hundred twenty-four patients with SLE residing in San Francisco County between 2007 and 2009 were retrospectively identified (Table 1). The distribution by race/ethnicity was as follows: white (26.2%), Black (18.8%), API (36.9%), and Hispanic (15.5%). API patients were predominantly Chinese (49.4%), followed by Filipino (15.4%) and Vietnamese (6.7%). Most Hispanic patients had no ethnic origin specified (50.9%), followed by South or Central American (except Brazilian) (31.3%) and Mexican (13.4%). Nineteen patients had missing race/ethnicity information (2.6%) and seven patients (1.0%) had a missing date of last clinic visit. All twenty-six (3.6%) of these patients were excluded from further analysis. Females comprised 89.5% of the patients. No statistically significant differences in age at SLE diagnosis (p = 0.341) or sex (p = 0.735) were observed by race/ethnicity. Conversely, there was a statistically significant difference in the duration of SLE among race/ethnicities, with whites more likely to have ≥16 years follow-up from diagnosis (44% compared to 15–30% for other groups, p < 0.001). With respect to the severe SLE manifestations studied, Blacks and APIs had higher prevalence of lupus nephritis (20% and 52%, respectively, compared to 13–14% among other groups, p < 0.001) and thrombocytopenia (24% and 39%, respectively, compared to 17–19% among other groups, p = 0.009). Neuropsychiatric lupus was less common among Hispanics (15% compared to 27–29% among other groups, p = 0.089), and APS was more common among APIs (43% compared to 18–20% among other groups, p = 0.205), although these differences did not meet statistical significance.
Table 1.
Baseline characteristics of prevalent cases of SLE in San Francisco County, 2007 – 2009
| Variable | Total (n = 724) | Non-Hispanic White (n =189) | Non-Hispanic Black (n = 135) | API (n = 265) | Hispanic (n = 109) | Missing (n = 26) | P value |
|---|---|---|---|---|---|---|---|
| Age, yr at SLE dx | 0.341 | ||||||
| ≤ 18 | 134 | 44 (23%) | 18 (13%) | 49 (18%) | 17 (16%) | 6 (23%) | |
| 19 – 29 | 205 | 52 (28%) | 35 (26%) | 76 (29%) | 39 (36%) | 3 (12%) | |
| 30 – 39 | 143 | 37 (20%) | 32 (24%) | 50 (19%) | 18 (17%) | 6 (23%) | |
| 40 – 49 | 106 | 19 (10%) | 24 (18%) | 38 (14%) | 21 (19%) | 4 (15%) | |
| 50+ | 136 | 37 (20%) | 26 (19%) | 52 (20%) | 14 (13%) | 7 (27%) | |
| Yrs since SLE dx diagnosis | < 0.001 | ||||||
| ≤ 5 | 231 | 47 (25%) | 45 (33%) | 78 (29%) | 44 (41%) | 17 (65%) | |
| 6 – 10 | 141 | 31 (16%) | 19 (14%) | 66 (25%) | 21 (19%) | 4 (15%) | |
| 11 – 15 | 125 | 28 (15%) | 29 (21%) | 45 (17%) | 22 (20%) | 1 (4%) | |
| ≥ 16 | 227 | 83 (44%) | 40 (30%) | 71 (27%) | 20 (20%) | 4 (15%) | |
| Sex | 0.735 | ||||||
| Male | 76 | 17 (9%) | 13 (10%) | 32 (12%) | 12 (11%) | 2 (8%) | |
| Female | 648 | 172 (91%) | 122 (90%) | 233 (88%) | 97 (89%) | 24 (92%) | |
| SLE manifestation | |||||||
| Lupus Nephritis* | 256 | 37 (14%) | 52 (20%) | 134 (52%) | 33 (13%) | 3 (1%) | < 0.001 |
| Thrombocytopenia | 210 | 40 (19%) | 51 (24%) | 81 (39%) | 36 (17%) | 2 (1%) | 0.009 |
| Neuropsychiatric** | 102 | 29 (28%) | 28 (27%) | 30 (29%) | 15 (15%) | 0 (0%) | 0.089 |
| APS | 111 | 22 (20%) | 20 (18%) | 48 (43%) | 21 (19%) | 0 (0%) | 0.205 |
P values calculated by ANOVA (age at SLE dx, yrs since SLE dx) and chi-square test (sex, SLE manifestation). API = Asian/Pacific Islander, SLE = systemic lupus erythematosus, APS = antiphospholipid antibody syndrome; Observations without recorded race/ethnicity or date of last clinic visit are grouped in the missing category.
Any of the following surrogate labs were used to define lupus nephritis: 24 hour urine for protein > 500mg, 24 hour urine protein/creatinine ratio > 0.5, or spot protein/creatinine ratio > 0.5.
Neuropsychiatric lupus manifestations studied include: seizures, psychosis, and acute confusional state.
Clinical manifestations of SLE.
In comparison to whites, Blacks, APIs, and Hispanics had increased prevalence of renal manifestations (PR 1.74, PR 1.68, PR 1.35, respectively; Table 2). Blacks had increased prevalence of neurologic manifestations (PR 1.49) and both Blacks and APIs had increased prevalence of hematologic manifestations (PR 1.09, PR 1.07, respectively). Because we were performing multiple statistical comparisons, we applied a Bonferroni correction (p < 0.005) when interpreting our p values to reduce the risk of Type 1 errors. After applying this correction, the higher prevalence of renal manifestations among Hispanics (p = 0.006), neurologic manifestations among Blacks (p = 0.007), and hematologic manifestations among APIs (p = 0.015) lost their statistical significance. There were no statistically significant differences in prevalence between racial/ethnic minority groups and whites for the mucocutaneous, serositis, cardiovascular, pulmonary, gastrointestinal, musculoskeletal, or serologic manifestation categories. Appendix 2 shows the prevalence of individual manifestations within each category, stratified by race/ethnicity.
Table 2.
Prevalence ratios of SLE manifestations stratified by race/ethnicity, among prevalent SLE cases in San Francisco County, 2007– 2009
| Characteristic | Non-Hispanic White | Non-Hispanic Black (n = 135) | API (n = 265) | Hispanic (n = 109) | |||
|---|---|---|---|---|---|---|---|
| (n = 189) | PR | (95% CI) | PR | (95% CI) | PR | (95% CI) | |
| Mucocutaneous | reference | 0.98 | (0.89 – 1.07) | 1.04 | (0.97 – 1.11) | 1.00 | (0.91 – 1.10) |
| Serositis | reference | 1.13 | (0.92 – 1.38) | 0.92 | (0.76 – 1.12) | 1.09 | (0.87 – 1.37) |
| Cardiovascular | reference | 1.38 | (0.95 – 2.01) | 1.09 | (0.77 – 1.55) | 1.34 | (0.88 – 2.04) |
| Pulmonary | reference | 1.09 | (0.87 – 1.36) | 0.96 | (0.78 – 1.17) | 1.13 | (0.89 – 1.44) |
| Gastrointestinal | reference | 1.64 | (0.84 – 3.18) | 1.30 | (0.71 – 2.37) | 1.71 | (0.85 – 3.44) |
| Renal | reference | ***1.74 | (1.40 – 2.16) | ***1.68 | (1.38 – 2.05) | *1.35 | (1.05 – 1.74) |
| Musculoskeletal | reference | 1.00 | (0.91 – 1.11) | 0.95 | (0.87 – 1.04) | 1.07 | (0.97 – 1.18) |
| Neurologic | reference | *1.49 | (1.12 – 1.98) | 0.76 | (0.56 – 1.04) | 0.98 | (0.67 – 1.44) |
| Hematologic | reference | ***1.09 | (1.04 – 1.15) | *1.07 | (1.01 – 1.13) | 1.06 | (1.00 – 1.13) |
| Serologic | reference | 1.00 | (1.00 – 1.01) | 1.00 | (1.00 – 1.01) | 1.00 | (1.00 – 1.01) |
Calculations based on Poisson regression model with robust error variances adjusting for sex, age at SLE diagnosis, and years since SLE diagnosis.
alpha < 0.05
alpha < 0.005
alpha < 0.001
API = Asian/Pacific Islander
Time to development of severe manifestations of SLE.
Figure 1 displays Kaplan-Meier curves illustrating time to incident severe SLE manifestations following SLE diagnosis, stratified by race/ethnicity. Mean and median follow-up times were 12.5 and 9.8 years, respectively. Log-rank tests demonstrated statistically significant differences between race/ethnicities in the time to development of lupus nephritis (p < 0.01), thrombocytopenia (p = 0.04), APS (p < 0.01), and the combined outcome (p < 0.01), but not for neuropsychiatric lupus (p = 0.59). For all racial/ethnic minorities, the risk in development of lupus nephritis, thrombocytopenia, APS, and the combined outcome was greatest in the first year after disease onset.
Figure 1.
Time to incident severe SLE manifestation following SLE diagnosis, stratified by race/ethnicity, among prevalent SLE cases in San Francisco County, 2007 – 2009
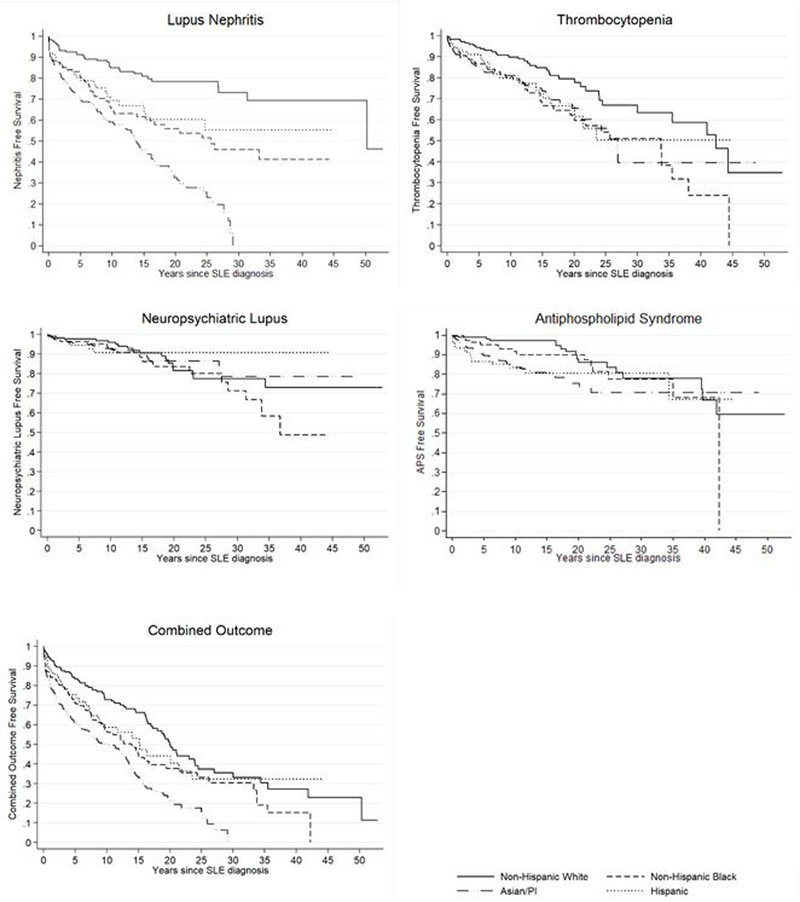
Combined outcome includes any of lupus nephritis, thrombocytopenia, neuropsychiatric lupus, or APS. Any of the following surrogate labs were used to define lupus nephritis: 24 hour urine for protein > 500mg, 24 hour urine protein/creatinine ratio > 0.5, or spot protein/creatinine ratio > 0.5. Neuropsychiatric lupus manifestations studied include: seizures, psychosis, and acute confusional state.
API = Asian/Pacific Islander, SLE = systemic lupus erythematosus, APS = antiphospholipid antibody syndrome
Cox proportional hazard regression results are displayed in Table 3. All racial/ethnic minorities with SLE had statistically significant increased hazard ratios relative to whites for lupus nephritis and thrombocytopenia. APIs and Hispanics had increased hazard ratios for APS, and Blacks and APIs had increased hazard ratios for the combined outcome relative to whites. There were no statistically significant differences in hazard ratios for neuropsychiatric lupus among the racial/ethnic groups. Relative to women, men with SLE had between 1.4 and 2.1 times the hazard ratio of lupus nephritis, thrombocytopenia, and the combined outcome. There were no statistically significant differences in hazard ratios for the severe SLE manifestations among the age at SLE diagnosis categories. Furthermore, there were no significant differences in the proportion of severe SLE manifestations identified at SLE diagnosis among race/ethnicities (data not shown).
Table 3.
Factors associated with severe manifestations of SLE in multivariable Cox proportional hazards regression, among prevalent SLE cases in San Francisco County, 2007 – 2009
| Lupus Nephritis* | Thrombocytopenia | Neuropsychiatric** | APS | Combined | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variables | HR | (95% CI) | HR | (95% CI) | HR | (95% CI) | HR | (95% CI) | HR | (95% CI) |
| Race/ethnicity | ||||||||||
| Non-Hispanic White | reference | reference | reference | reference | reference | |||||
| Non-Hispanic Black | 2.4 | (1.6 − 3.8) | 2.3 | (1.1 − 4.4) | 1.5 | (0.8 − 2.9) | 1.5 | (0.7 − 3.0) | 1.6 | (1.1 − 2.2) |
| API | 4.3 | (2.9 − 6.4) | 2.3 | (1.3 − 4.2) | 1.1 | (0.6 − 2.0) | 2.5 | (1.4 − 4.4) | 2.4 | (1.8 − 3.2) |
| Hispanic | 2.3 | (1.4 −3.8) | 2.2 | (1.1 − 4.7) | 1.0 | (0.4 − 2.5) | 2.6 | (1.3 − 5.1) | 1.4 | (0.9 − 2.1) |
| Sex | ||||||||||
| Female | reference | reference | reference | reference | reference | |||||
| Male | 1.6 | (1.1 − 2.4) | 2.1 | (1.2 − 3.6) | 1.8 | (0.9 − 3.6) | 1.4 | (0.7 − 2.6) | 1.8 | (1.3 − 2.5) |
| Age, yr at diagnosis | ||||||||||
| ≤18 | 1.1 | (0.8 − 1.5) | 1.0 | (0.6 − 1.8) | 1.1 | (0.6 − 2.2) | 1.1 | (0.6 − 1.8) | 0.9 | (0.7 − 1.2) |
| 19–29 | reference | reference | reference | reference | reference | |||||
| 30–39 | 0.8 | (0.5 − 1.2) | 0.7 | (0.4 − 1.4) | 0.8 | (0.4 − 1.8) | 0.7 | (0.4 − 1.4) | 0.8 | (0.5 − 1.1) |
| 40–49 | 1.0 | (0.6 − 1.5) | 0.5 | (0.2 − 1.1) | 1.2 | (0.5 − 2.8) | 0.7 | (0.4 − 1.6) | 0.9 | (0.7 − 1.4) |
| 50+ | 1.0 | (0.6 – 1.5) | 1.6 | (0.9 − 2.8) | 1.7 | (0.8 − 4.0) | 1.0 | (0.5 − 2.0) | 1.4 | (1.0 − 2.0) |
Calculations based on Cox proportional hazards model that contained each of race/ethnicity, sex, and age at diagnosis.
API = Asian/Pacific Islander, APS = antiphospholipid antibody syndrome, Combined outcome = any of lupus nephritis, thrombocytopenia, neuropsychiatric, or APS. HR = hazard ratio. Higher hazards indicate a shorter time to development of specific manifestations.
Any of the following surrogate labs were used to define lupus nephritis: 24 hour urine for protein > 500mg, 24 hour urine protein/creatinine ratio > 0.5, or spot protein/creatinine ratio > 0.5.
Neuropsychiatric lupus manifestations studied include: seizures, psychosis, and acute confusional state.
Of the patients identified with lupus nephritis based on the primary variable definition, 76% had a diagnosis of lupus nephritis by the treating physician in their medical charts. A sensitivity analysis examining only individuals diagnosed with lupus nephritis by the treating physician revealed slightly reduced hazard ratios with preserved statistical significance for all racial/ethnic minority groups, except Blacks (p = 0.054) (Appendix 3).
DISCUSSION
Using a large, racial/ethnically diverse population-based registry, we identified racial/ethnic differences in the prevalence of SLE manifestations and in the risk and timing of development of severe SLE manifestations. This analysis demonstrates substantial differences in the prevalence of several clinical SLE manifestations among race/ethnicities. Blacks, APIs, and Hispanics had a greater prevalence of renal abnormalities in comparison to whites. In addition, Blacks had increased neurologic manifestations and both Blacks and APIs had increased hematologic manifestations in comparison to whites. These findings are not explained by racial/ethnic differences in sex, age at SLE diagnosis or duration of SLE disease, as all of these risk factors were accounted for in this analysis. We also found that Blacks, APIs, and Hispanics are at increased risk of developing a number of severe manifestations—lupus nephritis, thrombocytopenia, and APS—earlier than whites following SLE diagnosis. Men also developed lupus nephritis and thrombocytopenia earlier than women. Our study represents the first comprehensive examination of differences in SLE manifestations in API and Hispanic patients in the U.S., two racial/ethnic groups that have been understudied in population-based epidemiologic investigations.
We found that for racial/ethnic minorities, the risk of lupus nephritis was greatest during the first year following diagnosis of SLE. Subsequently, the annual risk of lupus nephritis remained approximately constant, with the highest burden of risk experienced by APIs. Whites, on the other hand, had a nearly constant incidence rate. The hazard ratios calculated in our analyses are consistent with those of at least one previous study, which also found increased risks for Blacks (HR = 1.5), Asians (HR = 1.8), and Hispanics (HR = 1.5) in comparison to whites (6), although the hazard ratios in that study were not as large as discovered here.
There are several possible explanations for why racial/ethnic minority groups with SLE are at greater risk of developing lupus nephritis. Genetic factors have been proposed to explain why Blacks with SLE have a greater likelihood of renal disease, more severe disease presentation, and poorer prognosis relative to whites (13–16). A number of studies have also attributed worse outcomes among Black and Hispanic patients to socioeconomic factors, although no analysis has looked specifically at the association between socioeconomic factors and the onset of lupus nephritis (16–18). Surprisingly, the highest risk for lupus nephritis was observed among APIs, nearly double that of Blacks and Hispanics. This is despite the fact that APIs have the highest average levels of income and education and the best access to health care among racial/ethnic minority groups in San Francisco County (19), suggesting that there may be additional underlying factors that increase risk in this population. In Blacks, certain genotypes increase the risk of lupus nephritis, and although this is a potential mechanism among Asians, it remains understudied (20).
There are no previous studies describing the time to development of thrombocytopenia, neuropsychiatric lupus, or APS in patients with SLE. All racial/ethnic groups, including whites, were at greatest risk of thrombocytopenia and APS during the first year following SLE diagnosis, and continued to develop these manifestations throughout the follow-up period. It is plausible that genetic variation partially explains the observed racial/ethnic differences in the risk of APS. No defined racial/ethnic predominance for primary APS has been documented, but several studies support the increased risk conferred by various genetic variants, particularly human leukocyte antigen associations (21,22). Future studies investigating racial/ethnic group level differences in genetic variability are needed.
Numerous studies demonstrate that men with SLE are more likely to have organ damage, including renal disease and neuropsychiatric abnormalities, and have an increased mortality rate one year following initial SLE hospitalization (23–27). A previous analysis has shown that men develop lupus nephritis earlier than women, with a reported relative hazard of 1.7 (6). Our results show similar findings, and that men had the greatest risk of lupus nephritis during the first year following diagnosis of SLE (data not shown). Furthermore, our results show that men develop thrombocytopenia earlier following SLE diagnosis, a result not previously demonstrated. Although our analysis does not readily identify causes for these differences, several theories exist to explain gender differences in SLE presentation, including differences in sex hormones and decreased medical-seeking behavior among men, possibly leading to their delayed diagnosis (28).
A potential contributor to the more severe progression of lupus identified in men relative to women and in the studied minority groups relative to whites could be that SLE is diagnosed at a more advanced stage in these populations. This would explain both the relatively accelerated appearance of lupus nephritis and thrombocytopenia occurring within the first year and why racial/ethnic minorities appear to have greater risk for development of several manifestations of SLE after controlling for duration of disease. There is some preliminary support for this hypothesis (28,29). In one study, compared with women, men had higher risk of severe disease activity at the time of SLE diagnosis as determined by a SLEDAI score ≥ 12 independent of age, racial/ethnic group, anti-Ro positivity, or time to criteria accrual [OR 3.11 (95%CI: 1.09 – 8.92)] (29). Further work is needed to unravel the contribution of delayed access to diagnosis and treatment.
One of the major strengths of CLSP is the careful and systematic attention to case ascertainment using a variety of sources: university and community clinics, hospitals, regional laboratories, and state administrative databases. Asian and Hispanic patients were further identified through physicians focused on these populations (e.g. Chinese patients at San Francisco’s Chinese hospital) and through multilingual abstractors.
Several limitations to this study exist. There is always a potential for incomplete case ascertainment despite the efforts described above. Each clinic and hospital had to voluntarily agree to participate in CLSP. Unfortunately, two community hospitals in San Francisco chose not to participate in the program. Given the small number of cases identified solely through community-based hospitals, fewer than five cases were expected to be missing from this data (3). Incomplete case ascertainment might also have occurred because surveillance efforts were focused on rheumatology clinics and did not include primary care clinics. Capture-recapture analysis reported in a prior publication estimated an additional 147 patients, though this estimation had a wide confidence interval (3). A second limitation is that the quality of medical record documentation of SLE manifestations and criteria varied widely depending on the clinic or hospital setting. Older charts that may have documented early manifestations of disease, particularly serologic laboratory results, were difficult to obtain and may have been inadequately captured. Third, race and ethnicity were determined from the medical record, not through patient self-report. Race and ethnicity were sometimes poorly documented in the medical records, leading to missing data for race and ethnicity. Importantly, this study also did not account for variables that historically have been associated with severe disease manifestations including socioeconomic status, medications, access to care, and coexisting medical conditions. The analysis of genetic and other biologic data would have been useful if collected previously for this study population. A longitudinal cohort study called the California Lupus Epidemiology Surveillance Study (CLUES) has emerged from analysis of biologic specimens voluntarily provided by members of the CLSP cohort.
This study found important differences in the characteristics and progression of SLE between racial/ethnic minority groups and whites. It is the first study to use rigorous epidemiologic methods to compare SLE manifestations across four racial/ethnic groups, including APIs and Hispanics, two understudied populations. Data collected in this study support the importance of increased awareness of SLE and its accelerated progression by clinicians for these racial/ethnic groups. These data also advocate for greater resource allocation on early diagnosis and treatment in these populations. Future studies should attempt to collect and analyze data on additional risk factors, including socioeconomic status, access to care, medication and appointment adherence, coexisting medical conditions, and genetic variation and the relationship of these variables to the onset of disease manifestations.
Significance and Innovation.
Relative to whites, racial/ethnic minority groups (Blacks, Asian/Pacific Islanders, and Hispanics) develop more renal, neurologic, and hematologic manifestations. They also develop lupus nephritis, thrombocytopenia, and APS sooner.
It is the first study to use rigorous epidemiologic methods to compare SLE manifestations across four racial/ethnic groups, including APIs and Hispanics, two understudied populations.
Data collected in this study support the importance of increased awareness of SLE and its accelerated progression by clinicians for these racial/ethnic groups.
Acknowledgments
Disclosures: This work was supported by the Centers for Disease Control (grants A114297 and U01DP005120). Additional support came from NIH/NIAMS P30 AR070155. Dr. Yazdany is also supported by the Robert L. Kroc Chair in Rheumatic and Connective Tissue Diseases, and Drs. Yazdany and Dall’Era are supported by the Russell/Engleman Medical Research Center for Arthritis. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or the National Institutes of Health.
Appendix 1. Clinical manifestations of SLE, among prevalent SLE cases in San Francisco County, 2007 – 2009
| Mucocutaneous | Pulmonary hemorrhage | Hematologic |
| Malar rash | Shrinking lung syndrome | Vascular thrombosis |
| Discoid rash | Gastrointestinal | Hemolytic anemia |
| Subacute cutaneous LE | Peritonitis | Direct coomb’s test |
| Bullous skin lesions | Pancreatitis | Antiphospholipid syndrome (APS) |
| Panniculitis | Hepatitis | Leukopenia |
| Alopecia | Renal | Lymphopenia |
| Cutaneous vasculitis | Dialysis | Thrombocytopenia |
| Raynaud’s | Renal transplantation | Serologies |
| Livedo reticularis | Lupus nephritis | Lupus anticoagulant |
| Urticaria | 24 hour urine for protein (≥ 500mg) | Anticardiolipin antibodies |
| Photosensitivity | Urine protein/creatinine > 0.5 | Anti-beta 2 glycoprotein I |
| Mucosal ulcers | Urine protein/creatinine > 3 | Low C3 |
| Chilblain lupus | Urinalysis: protein | Low C4 |
| Lupus tumidus | Urinalysis: cellular casts | Low complement |
| LE mucosus | Musculoskeletal | ANA |
| Maculopapular rash | Arthritis | Anti-ds DNA |
| Toxic epidermal necrolysis | Jaccoud’s arthropathy | Anti-Smith |
| Serositis | Avascular necrosis | Anti-Smith/RNP |
| Serositis | Myositis | Anticardiolipin (aCL) antibodies (IgG) |
| Pleuritis | Neurological | Anticardiolipin (aCL) antibodies (IgM) |
| Pericarditis | Seizures | Anti-beta2 glycoprotein I-IgG |
| Peritonitis | Psychosis | Anti-beta2 glycoprotein I-IgM |
| Cardiovascular | Mononeuritis multiplex | Anticoagulant screen confirmatory test |
| Pericarditis | Cerebrovascular disease | Anti-RNP |
| Myocardial infarction | Transverse myelitis | Anti-Ro/SSA |
| Pulmonary | Aseptic meningitis | Anti-La/SSB |
| Pulmonary hypertension | Chorea | RF |
| Interstitial lung disease | Cranial and/or peripheral neuropathy | Antiphospholipid (aPL) antibodies |
| Pneumonitis | Acute confusional state | Lupus anticoagulant confirmatory |
Appendix 2. Prevalence ratios for SLE manifestations stratified by race/ethnicity, among prevalent SLE cases in San Francisco County, 2007 – 2009
| Characteristic | Non-Hispanic White (n = 189) | Non-Hispanic Black (n = 135) | API (n = 265) | Hispanic (n = 109) | |||
|---|---|---|---|---|---|---|---|
| PR | (95% CI) | PR | (95% CI) | PR | (95% CI) | ||
| Mucocutaneous | reference | 0.98 | (0.89 – 1.07) | 1.04 | (0.97 – 1.11) | 1.00 | (0.91 – 1.10) |
| Malar rash | reference | 0.80 | (0.62 – 1.03) | *1.23 | (1.04 – 1.46) | 1.02 | (0.81 – 1.29) |
| Raynaud’s | reference | 0.91 | (0.66 – 1.27) | 1.21 | (0.95 – 1.55) | 0.88 | (0.62 – 1.24) |
| Cutaneous vasculitis | reference | 1.09 | (0.54 – 2.18) | 1.66 | (0.97 – 2.84) | 0.78 | (0.34 – 1.80) |
| Photosensitivity | reference | 1.06 | (0.80 – 1.40) | 1.09 | (0.87 – 1.37) | 1.06 | (0.79 – 1.42) |
| Discoid rash | reference | ***2.13 | (1.39 – 3.26) | 0.90 | (0.57 – 1.43) | 0.58 | (0.28 – 1.21) |
| Alopecia | reference | *1.37 | (1.03 – 1.82) | *1.41 | (1.10 – 1.79) | 1.26 | (0.94 – 1.71) |
| Livedo reticularis | reference | **0.16 | (0.05 – 0.51) | 1.42 | (0.94 – 2.13) | 0.83 | (0.45 – 1.53) |
| Maculopapular rash | reference | 0.65 | (0.26 – 1.61) | 1.49 | (0.81 – 2.74) | 1.10 | (0.50 – 2.43) |
| Mucocutaneous ulcer | reference | 0.77 | (0.55 – 1.07) | 0.93 | (0.71 – 1.20) | 1.00 | (0.73 – 1.38) |
| Serositis | reference | 1.13 | (0.92 – 1.38) | 0.92 | (0.76 – 1.12) | 1.09 | (0.87 – 1.37) |
| Pleuritis | reference | 1.06 | (0.83 – 1.35) | 0.86 | (0.68 – 1.08) | 1.18 | (0.91 – 1.52) |
| Pericarditis | reference | 1.29 | (0.86 – 1.95) | 1.04 | (0.71 – 1.52) | 1.29 | (0.82 – 2.04) |
| Cardiovascular | reference | 1.38 | (0.95 – 2.01) | 1.09 | (0.77 – 1.55) | 1.34 | (0.88 – 2.04) |
| Myocardial infarction | reference | 2.17 | (0.92 – 5.14) | 1.33 | (0.58 – 3.09) | 1.83 | (0.62 – 5.42) |
| Pulmonary | reference | 1.09 | (0.87 – 1.36) | 0.96 | (0.78 – 1.17) | 1.13 | (0.89 – 1.44) |
| Pulmonary hypertension | reference | 2.12 | (0.96 – 4.71) | *2.60 | (1.32 – 5.11) | 2.04 | (0.87 – 4.77) |
| Interstitial lung disease | reference | 1.92 | (0.86 – 4.32) | 1.46 | (0.67 – 3.21) | 1.94 | (0.78 – 4.84) |
| Gastrointestinal | reference | 1.64 | (0.84 – 3.18) | 1.30 | (0.71 – 2.37) | 1.71 | (0.85 – 3.44) |
| Renal | reference | ***1.74 | (1.40 – 2.16) | ***1.68 | (1.38 – 2.05) | *1.35 | (1.05 – 1.74) |
| Lupus nephritis | reference | ***1.76 | (1.24 – 2.48) | ***2.11 | (1.56 – 2.85) | *1.50 | (1.01 – 2.21) |
| Dialysis | reference | **2.53 | (1.36 – 4.70) | *1.92 | (1.08 – 3.42) | 1.57 | (0.72 – 3.41) |
| Musculoskeletal | reference | 1.00 | (0.91 – 1.11) | 0.95 | (0.87 – 1.04) | 1.07 | (0.97 – 1.18) |
| Nonerosive arthritis | reference | 0.95 | (0.85 – 1.06) | 0.94 | (0.86 – 1.03) | 1.07 | (0.97 – 1.18) |
| Neurologic | reference | *1.49 | (1.12 – 1.98) | 0.76 | (0.56 – 1.04) | 0.98 | (0.67 – 1.44) |
| Seizures | reference | 1.44 | (0.85 – 2.44) | 0.75 | (0.43 – 1.28) | 0.99 | (0.51 – 1.94) |
| Psychosis | reference | 1.72 | (0.82 – 3.61) | 0.80 | (0.36 – 1.78) | 0.74 | (0.27 – 2.03) |
| Cerebrovascular disease | reference | *1.95 | (1.11 – 3.42) | 0.98 | (0.55 – 1.77) | 0.89 | (0.37 – 2.16) |
| Neuropathy | reference | 0.78 | (0.42 – 1.45) | *0.44 | (0.24 – 0.83) | 0.55 | (0.24 – 1.22) |
| Hematologic | reference | ***1.09 | (1.04 – 1.15) | *1.07 | (1.01 – 1.13) | 1.06 | (1.00 – 1.13) |
| Vascular thrombosis | reference | ***2.24 | (1.55 – 3.24) | 0.86 | (0.56 – 1.32) | 1.45 | (0.88 – 2.39) |
| Hemolytic anemia | reference | 2.24 | (0.97 – 5.17) | *2.19 | (1.04 – 4.64) | ***3.66 | (1.68 – 7.94) |
| Coombs | reference | *2.94 | (1.26 – 6.85) | 2.15 | (0.96 – 4.82) | **3.35 | (1.45 – 7.75) |
| APS | reference | 1.32 | (0.76 – 2.30) | 1.48 | (0.93 – 2.35) | 1.51 | (0.89 – 2.56) |
| Leukopenia | reference | **1.28 | (1.08 – 1.51) | ***1.29 | (1.11 – 1.49) | 1.20 | (1.00 – 1.45) |
| Lymphopenia | reference | *1.10 | (1.01 – 1.20) | 1.08 | (1.00 – 1.17) | 1.06 | (0.95 – 1.18) |
| Thrombocytopenia | reference | ***1.93 | (1.38 – 2.70) | *1.51 | (1.10 – 2.07) | **1.73 | (1.19 – 2.52) |
| Serologic | reference | 1.00 | (1.00 – 1.01) | 1.00 | (1.00 – 1.01) | 1.00 | (1.00 – 1.01) |
| ANA | reference | 1.04 | (0.98 – 1.10) | 1.00 | (0.94 – 1.06) | 1.00 | (0.93 – 1.08) |
| Anti-DNA/Anti-dsDNA | reference | 1.10 | (0.91 – 1.33) | ***1.37 | (1.19 – 1.59) | **1.27 | (1.06 – 1.51) |
| Lupus anticoagulant | reference | *1.46 | (1.07 – 2.00) | 1.05 | (0.78 – 1.42) | 1.09 | (0.75 – 1.60) |
| Anti-B2GP1 | reference | 0.60 | (0.18 – 1.96) | 0.62 | (0.24 – 1.58) | 2.24 | (0.95 – 5.23) |
| Low C3 | reference | 1.01 | (0.79 – 1.29) | ***1.58 | (1.32 – 1.88) | 1.24 | (0.99 – 1.56) |
| Low C4 | reference | 0.94 | (0.74 – 1.21) | *1.21 | (1.01 – 1.47) | *1.26 | (1.01 – 1.58) |
| Hypocomplementemia | reference | 0.85 | (0.70 – 1.04) | **1.23 | (1.08 – 1.41) | 1.08 | (0.91 – 1.29) |
| Anti-Smith | reference | ***3.14 | (1.94 – 5.09) | ***2.20 | (1.37 – 3.53) | *1.78 | (1.03 – 3.07) |
| Anti-SmRNP | reference | ***2.79 | (1.68 – 4.65) | **1.95 | (1.19 – 3.20) | 1.57 | (0.87 – 2.85) |
| Anti-RNP | reference | ***2.21 | (1.42 – 3.43) | ***2.18 | (1.44 – 3.28) | *1.84 | (1.14 – 2.97) |
| Anti-RoSSA | reference | 1.42 | (0.95 – 2.12) | ***1.94 | (1.39 – 2.71) | **1.70 | (1.14 – 2.53) |
| Anti-RoSSB | reference | 1.10 | (0.88 – 1.37) | *1.25 | (1.04 – 1.50) | 1.16 | (0.91 – 1.46) |
| RF | reference | 1.65 | (0.83 – 3.28) | *1.85 | (1.01 – 3.39) | ***3.14 | (1.66 – 5.95) |
| ACLAPL IgG | reference | 1.33 | (0.83 – 2.14) | 1.17 | (0.77 – 1.78) | 1.34 | (0.83 – 2.16) |
| ACLAPL IgM | reference | 0.82 | (0.43 – 1.57) | 0.97 | (0.59 – 1.59) | *1.84 | (1.10 – 3.08) |
| Antiphospholipid Ab | reference | 1.24 | (0.99 – 1.55) | 1.09 | (0.89 – 1.33) | 1.16 | (0.91 – 1.49) |
Calculations based on Poisson regression model with robust error variances using the following risk factors: sex, age at SLE diagnosis, and years since SLE diagnosis.
alpha < 0.05
alpha < 0.005
alpha < 0.001. Manifestations with less than 35 observations are not shown. API = Asian/Pacific Islander.
Appendix 3. Sensitivity analysis of physician-diagnosed lupus nephritis in multivariable Cox proportional hazards regression, among prevalent SLE cases in San Francisco County, 2007 – 2009
| Lupus Nephritis | ||
|---|---|---|
| Race/ethnicity | HR | (95% CI) |
| Non-Hispanic White | reference | |
| Non-Hispanic Black | 1.7 | (0.99 – 2.7) |
| API | 3.7 | (2.5 – 5.7) |
| Hispanic | 1.8 | (1.03 –3.2) |
Calculations based on Cox proportional hazards model that contained each of race/ethnicity, sex, and age at diagnosis.
API = Asian/Pacific Islander. HR = hazard ratio. Higher hazards indicate a shorter time to development of specific manifestations.
References
- 1.Somers EC, Marder W, Cagnoli P, Lewis EE, DeGuire P, Gordon C, et al. Population-based incidence and prevalence of systemic lupus erythematosus: the Michigan Lupus Epidemiology and Surveillance program. Arthritis Rheumatol (Hoboken, NJ) 2014;66:369–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Izmirly PM, Wan I, Sahl S, Buyon JP, Belmont HM, Salmon JE, et al. The Incidence and Prevalence of Systemic Lupus Erythematosus in New York County (Manhattan), New York: The Manhattan Lupus Surveillance Program. Arthritis Rheumatol 2017;69:2006–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dall’Era M, Cisternas MG, Snipes K, Herrinton LJ, Gordon C, Helmick CG. The Incidence and Prevalence of Systemic Lupus Erythematosus in San Francisco County, California: The California Lupus Surveillance Project. Arthritis Rheumatol (Hoboken, NJ) 2017;69:1996–2005. Available at: http://www.ncbi.nlm.nih.gov/pubmed/28891237. [DOI] [PubMed] [Google Scholar]
- 4.Lim SS, Bayakly AR, Helmick CG, Gordon C, Easley KA, Drenkard C. The incidence and prevalence of systemic lupus erythematosus, 2002–2004: The Georgia lupus registry. Arthritis Rheumatol 2014;66:357–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferucci ED, Johnston JM, Gaddy JR, Sumner L, Posever JO, Choromanski TL, et al. Prevalence and incidence of systemic lupus erythematosus in a population-based registry of American Indian and Alaska Native people, 2007–2009. Arthritis Rheumatol (Hoboken, NJ) 2014;66:2494–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seligman VA, Lum RF, Olson JL, Li H, Criswell LA. Demographic Differences in the Development of Lupus Nephritis: A Retrospective Analysis [DOI] [PubMed] [Google Scholar]
- 7.Alarcon GS, McGwin GJ, Bartolucci AA, Roseman J, Lisse J, Fessler BJ, et al. Systemic lupus erythematosus in three ethnic groups. IX. Differences in damage accrual. Arthritis Rheum 2001;44:2797–2806. [DOI] [PubMed] [Google Scholar]
- 8.Contreras G, Lenz O, Pardo V, Borja E, Cely C, Iqbal K, et al. Outcomes in African Americans and Hispanics with lupus nephritis. Kidney Int 2006;69:1846–1851. Available at: 10.1038/sj.ki.5000243. [DOI] [PubMed] [Google Scholar]
- 9.Anon. Bridged-Race Population Estimates Data Files and Documentation Available at: http://www.cdc.gov/nchs/nvss/bridged_race/data_documentation.htm. Accessed January 3, 2016.
- 10.Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1982;25:1271–1277. [DOI] [PubMed] [Google Scholar]
- 11.Hochberg MC. Updating the American College of Rheumatology Revised criteria for the classification of Systemic Lupus Erythematosus. Arthritis Rheum 1997;40:1997 Available at: http://scholar.google.com/scholar?hl=en&btnG=Search&q=intitle:Updating+the+American+College+of+Rheumatology#7%5Cnhttp://www.ncbi.nlm.nih.gov/pubmed/9324032. [DOI] [PubMed] [Google Scholar]
- 12.Anon. StataCorp. 2013. Stata Statistical Software: Release 13 College Station, TX: StataCorp LP. [Google Scholar]
- 13.Reveille JD, Moulds JM, Ahn C, Friedman AW, Baethge B, Roseman J, et al. Systemic lupus erythematosus in three ethnic groups: I. The effects of HLA class II, C4, and CR1 alleles, socioeconomic factors, and ethnicity at disease onset. LUMINA Study Group. Lupus in minority populations, nature versus nurture. Arthritis Rheum 1998;41:1161–1172. [DOI] [PubMed] [Google Scholar]
- 14.Alarcon GS, Bastian HM, Beasley TM, Roseman JM, Tan FK, Fessler BJ, et al. Systemic lupus erythematosus in a multi-ethnic cohort (LUMINA) XXXII: [corrected] contributions of admixture and socioeconomic status to renal involvement. Lupus 2006;15:26–31. [DOI] [PubMed] [Google Scholar]
- 15.Salmon JE, Millard S, Schachter LA, Arnett FC, Ginzler EM, Gourley MF, et al. Fc gamma RIIA alleles are heritable risk factors for lupus nephritis in African Americans. J Clin Invest 1996;97:1348–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Korbet SM, Schwartz MM, Evans J, Lewis EJ. Severe lupus nephritis: racial differences in presentation and outcome. J Am Soc Nephrol 2007;18:244–254. [DOI] [PubMed] [Google Scholar]
- 17.Reveille JD, Bartolucci A, Alarcon GS. Prognosis in systemic lupus erythematosus. Negative impact of increasing age at onset, black race, and thrombocytopenia, as well as causes of death. Arthritis Rheum 1990;33:37–48. [DOI] [PubMed] [Google Scholar]
- 18.Pons-Estel BA, Catoggio LJ, Cardiel MH, Soriano ER, Gentiletti S, Villa AR, et al. The GLADEL multinational Latin American prospective inception cohort of 1,214 patients with systemic lupus erythematosus: ethnic and disease heterogeneity among “Hispanics”. Medicine (Baltimore) 2004;83:1–17. [DOI] [PubMed] [Google Scholar]
- 19.Tseng W, Cook WK, & Chung C Demographic and Socioeconomic Profiles of Asian Americans, Native Hawaiians, and Pacific Islanders in the United States 2011. Available at: http://www.apiahf.org/sites/default/files/Demographic_Socioeconomic_Profiles_AANHPI.pdf. Accessed December 1, 2015.
- 20.Lanata CM, Nititham J, Taylor KE, Chung SA, Torgerson G, Seldin MF, et al. Genetic contributions to lupus nephritis in a multi-ethnic cohort of systemic lupus erythematous patients 2018:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horita T, Merrill JT. Genetics of antiphospholipid syndrome. Curr Rheumatol Rep 2004;6:458–462. [DOI] [PubMed] [Google Scholar]
- 22.Namjou B Antiphospholipid syndrome: genetic review. Curr Rheumatol Rep 2003;5:391–394. [DOI] [PubMed] [Google Scholar]
- 23.Prete PE, Majlessi A, Gilman S, Hamideh F. Systemic lupus erythematosus in men: a retrospective analysis in a Veterans Administration Healthcare System population. J Clin Rheumatol 2001;7:142–150. [DOI] [PubMed] [Google Scholar]
- 24.Tan TC, Fang H, Magder LS, Petri MA. Differences between male and female systemic lupus erythematosus in a multiethnic population. J Rheumatol 2012;39:759–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaufman LD, Gomez-Reino JJ, Heinicke MH, Gorevic PD. Male lupus: retrospective analysis of the clinical and laboratory features of 52 patients, with a review of the literature. Semin Arthritis Rheum 1989;18:189–197. [DOI] [PubMed] [Google Scholar]
- 26.Ward MM, Studenski S. Systemic lupus erythematosus in men: a multivariate analysis of gender differences in clinical manifestations. J Rheumatol 1990;17:220–224. [PubMed] [Google Scholar]
- 27.Resende AL, Titan SM, Barros RT, Woronik V. Worse renal outcome of lupus nephritis in male patients: a case-control study. Lupus 2011;20:561–567. [DOI] [PubMed] [Google Scholar]
- 28.Lu L-J, Wallace DJ, Ishimori ML, Scofield RH, Weisman MH. Review: Male systemic lupus erythematosus: a review of sex disparities in this disease. Lupus 2010;19:119–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Munoz-Grajales C, Gonzalez LA, Alarcon GS, Acosta-Reyes J. Gender differences in disease activity and clinical features in newly diagnosed systemic lupus erythematosus patients. Lupus 2016. [DOI] [PubMed] [Google Scholar]


