Abstract
Objective:
We investigated the relationship between early life growth patterns and blood telomere length (TL) in adulthood using conditional measures of lean and fat mass growth to evaluate potentially sensitive periods of early life growth.
Methods:
This study included data from 1,562 individuals (53% male; age 20–22 years) participating in the Cebu Longitudinal Health and Nutrition Survey, located in metropolitan Cebu, Philippines. Primary exposures included length- and weight-for-age (HAZ and WAZ) at birth and conditional measures of linear growth and weight gain during four postnatal periods: 0–6 months, 6–12 months, 12–24 months, 24 months–8.5 years. TL was measured at ~21 years of age. We estimated associations using linear regression.
Results:
The study sample had an average gestational age (38.5±2 weeks) and birth size (HAZ=−0.2±1.1, WAZ=−0.7±1.0), but by age 8.5 years had stunted linear growth (HAZ=−2.1±0.9) and borderline low weight (WAZ=−1.9±1.0) relative to WHO references. Heavier birth weight was associated with longer TL in early adulthood (p=0.03), but this association was attenuated when maternal age at birth was included in the model (p=0.07). Accelerated linear growth between 6–12 months was associated with longer TL in adulthood (p=0.006), whereas weight gain between 12–24 months was associated with shorter TL in adulthood (p=0.047).
Conclusions:
In Cebu, individuals who were born heavier have longer TL in early adulthood, but that birthweight itself may not explain the association. Findings suggest that childhood growth is associated with the cellular senescence process in adulthood, implying early life well-being may be linked to adult health.
Keywords: telomere, growth, birth weight, weight gain, developmental origins of health and disease
Introduction
Accelerated postnatal growth has been linked to increased body fat and risk for cardiometabolic disease in adulthood. The mechanisms underlying these associations may include postnatal growth rates influencing or reflecting traits like altered organ growth, metabolic function, insulin resistance, and lipid metabolism (Adair, Martorell, & Stein, 2009; Adair et al., 2013; Barker, 1998; Cameron, 2002; Cameron & Demerath, 2002; Eriksson et al., 1999; Gluckman & Hanson, 2006; Kuzawa, 2007; Kuzawa et al., 2012; Metcalfe & Monaghan, 2001; Norris et al., 2012; Victora et al., 2008a, 2008b). An emerging mechanism that may link postnatal cellular level developmental processes and later metabolic disease risk is telomere length (TL), a genetic determinant of cellular senescence (Cameron & Demerath, 2002).
Telomeres are variable length repetitive DNA sequences that cap the ends of linear chromosomes (Blackburn & Gall, 1978). They shorten with each cell division, so tend to decrease with age in most proliferating human tissues (Allsopp et al., 1992; Harley, Futcher, & Greider, 1990; Kimura et al., 2008). In addition to age, TL is influenced by sex, heredity and environmental factors (Allsopp et al., 1992; Aviv, Valdes, & Spector, 2006; Epel et al., 2004; Harley et al., 1990; Hunt et al., 2008; Kimura et al., 2008). When telomeres get too short, the cell is no longer able to replicate, and this process of cellular senescence is implicated as a potential cause of aging (Aviv & Shay, 2018). Telomerase, the enzyme responsible for extending telomere length (TL) (Greider & Blackburn, 1985), is usually not active in somatic cells, but is active in gametes, across a range of tissue during prenatal life, and in a highly-regulated fashion in somatic stem cells (Hiyama & Hiyama, 2007; Plunkett et al., 2001).
Consistent with telomerase being expressed at high levels prenatally, recent findings suggest that birth size (as an indicator of fetal growth) does not predict adult TL (Kajantie et al., 2012). Given the reduced postnatal telomerase activity compared to during the prenatal period, it is possible that rapid growth in childhood is a primary contributor to telomere shortening (Cameron & Demerath, 2002; Demerath, Cameron, Gillman, Towne, & Siervogel, 2004). Mechanisms that may link postnatal growth rates with TL include inflammation, oxidative stress and increases in blood volume, which are thought to lead to accelerated TL shortening due to increased DNA damage to telomeres and the accelerating cellular proliferation rates which can accompany these physiological processes (Allsopp et al., 1992; Aviv et al., 2006; Eisenberg, Borja, Hayes, & Kuzawa, 2017; Epel et al., 2004; Harley et al., 1990; Hunt et al., 2008; Kimura et al., 2008; Shalev, 2012; Von Zglinicki, 2002). Since body fat has inflammatory and oxidative stress properties, fat accumulation during childhood could also lead to shortening of TL (Reichert & Stier, 2017). Increases in lean body mass necessitate a corresponding increase in blood volume to support these tissues, while fat mass is relatively inert and requires minimal innervation (Raes, Van Aken, Craen, Donckerwolcke, & Vande Walle, 2006). Higher blood volumes entail higher blood cell counts, the production of which, via mitosis, is expected to cause accelerated telomere shortening. TL shortening generally commences at a rapid pace after birth, corresponding with ages when children have rapid growth rates. The rate of telomere attrition slows in childhood and adolescence, and telomeres then continue to shorten at a slower rate across adulthood. Because of these developmental patterns in the pace of telomere shortening, early life factors could be particularly salient in predicting adult TL (Eisenberg, 2011; Frenck, Blackburn, & Shannon, 1998; Unryn, Cook, & Riabowol, 2005).
Studies in non-humans and humans have found general support for the hypothesis that rapid post-natal growth shortens TL (Geiger et al., 2012; Guzzardi, Iozzo, Salonen, Kajantie, & Eriksson, 2016; Jennings, Ozanne, Dorling, & Hales, 1999; Näslund, Pauliny, Blomqvist, & Johnsson, 2015; Pauliny, Devlin, Johnsson, & Blomqvist, 2015; Ringsby et al., 2015; Tarry-Adkins et al., 2009). However, the present study is the first to evaluate the influence of sensitive periods of postnatal growth (weight gain and linear growth) on adult TL using conditional measures, a method which better enables us to disentangle the independent roles of weight and height gain at different ages. Studies relying on conditional measures of growth have observed an association between faster linear growth in childhood and taller height, lean body mass, higher attainment in school, employment and higher earnings in adulthood (Adair et al., 2009; Adair et al., 2013; Kuzawa et al., 2012; Martorell et al., 2010). However, the relationship between conditional growth measures and TL in adulthood have not been evaluated.
This analysis sought to evaluate the relationship between early life growth and adult TL among participants enrolled in a longitudinal birth cohort study living in metropolitan Cebu, Philippines. Using these data, we evaluated the hypotheses that accelerated linear growth and accelerated weight gain during early life are associated with shorter TL in adulthood.
Methods
Study Design and Sample
Data are from the Cebu Longitudinal Health and Nutrition Survey (CLHNS), a prospective birth cohort study which began in 1983–1984 and is still ongoing (Adair et al., 2011). The study sample for the present analysis included 1,562 individuals and is a complete case analysis utilizing data collected at birth, 6, 12 and 24 months, 8.5 years and in adulthood (~21 years) (Soediono, 1989). Cohort members of the original telomere study who were lost to follow up are described as a limitation in the Discussion section. The original Cebu cohort’s profile is described in detail elsewhere (Adair et al., 2011).
Outcome Measure
Full description of the methods used for measuring blood TL have been published previously (Eisenberg, Hayes, & Kuzawa, 2012; Eisenberg, Kuzawa, & Hayes, 2015). Briefly, TL was analyzed using a modified version of the monochrome multiplex quantitative polymerase chain reaction (MMqPCR) method and results have good internal and external validity. (Cawthon, 2009; Eisenberg et al., 2012, 2015).
Exposure Measures
The exposures of interest in the primary analysis were linear growth and weight gain during early life, including birthweight (WAZ and in kg) and length at birth (HAZ and in cm) relative to World Health Organization (WHO) reference medians. Postnatal growth was determined by conditional measures employed in previous studies (Adair et al., 2009; Adair et al., 2013; Kuzawa et al., 2012) to parse out the influences of particular intervals of postnatal linear growth (as a proxy measure of lean mass) and weight gain (as a proxy measure of fat mass) on adult TL (Adair et al., 2013). This approach was necessary because weight gain and linear growth are in the same causal pathway with regard to our outcome of interest. We measured conditional growth for the following intervals: 0–6 months, 6–12 months, 12–24 months, 24 months–8.5 years. Because conditional growth measures are not correlated, they are included in models simultaneously without concern for collinearity. This analytic approach enabled us to investigate the independent contribution of particular intervals of linear growth and weight gain on adult TL to identify sensitive periods of growth in relation to adult TL.
Conditional growth measures were calculated using a multivariate regression model approach (Keijzer-Veen et al., 2005). All weight and length/height measures were first converted to standard deviation scores (z-scores) based on WHO reference means. Standard deviation scores for current size were then regressed on all previous size measures to produce unexplained residuals that reflect the difference between the value of the current measure (e.g., weight at 24 months) and the expected value of this measure given all prior measures (e.g., birthweight, weight at 6 months, weight at 12 months). Conditional relative weight (CRW) and conditional height (CH) are therefore interpreted as follows: CRW reflects weight gain independent of height and prior inter-individual linear growth and weight gain, and CH reflects linear growth independent of prior inter-individual linear growth and weight gain. Positive CRW measures represent greater weight gain and positive CH represents faster linear growth than expected based on each child’s individual growth trajectory and on that of other children in the cohort (Adair et al., 2009). Further details on how CRW and CH were calculated may be found elsewhere (Adair et al., 2013; Keijzer-Veen et al., 2005).
Covariates
Covariates included sex, age at TL measurement (months), BMI (kg/m2) at the time of TL measurement in adulthood, and socioeconomic status (SES) measured by maternal education (highest grade completed) and a household asset index based on household possessions at birth. Additional covariates of interest included birth measures: gestational age (weeks), maternal age at delivery (years), paternal age at delivery (years), and maternal BMI during the first trimester of pregnancy (kg/m2).
Statistical Analyses
Study sample demographic characteristics, birth and childhood size, early life growth, and TL are described. To control for potential population genetic structure, correlated effects principal components (PCs) of genome-wide genetic variation were included in all models. The derivation of these principal components have been described previously (Croteau-Chonka et al., 2011; Croteau-chonka et al., 2012; Wu et al., 2012). As in previous analyses (Bethancourt et al., 2017; D. Eisenberg et al., 2017; Ryan et al., 2018), the bivariate association between the first ten principal components and TL were tested. The top principal components up to and including the last one showing a significant bivariate association with TL (10 total) were retained as control variables. There is evidence that TL varies across populations, at least in part due to natural selection (Hansen et al., 2016). Since cultural inheritance and treatment by others (e.g., racism) may parallel genetic inheritance, socio-demographic and environmental effects may parallel genetic ancestry indexed by these PCs as well. Thus, the genetic structure PC controls are intended to control for potential confounding genetic and ancestry correlated factors.
We carried out several preliminary analyses. First, based on the established knowledge that TL declines more rapidly in men than women (Gardner et al., 2014), we evaluated the role of sex in the growth-TL relationship using multivariate linear regression to determine whether to adjust for sex or include a growth*sex interaction term in our analyses. Next, we assessed bivariate associations between exposures and covariates with TL. Finally, to consider the role of birth factors in the growth-adult TL relationship, we evaluated birth measures (length, weight, maternal age at delivery, paternal age at delivery, gestational age at birth, maternal BMI during the first trimester of pregnancy) in relation to TL using multivariate linear regression.
The primary analysis evaluated postnatal growth and TL. Specifically, we assessed the relationships between HAZ and WAZ at birth and CH and CRW during four childhood growth intervals and adult TL (at approximately 21 years of age). Multivariate linear regression analysis with robust standard errors was used to detect statistical associations. We considered three models in our analyses to determine the relationship between early life growth and TL: (1) adjusted for population genetic structure, age at TL measurement and sex for statistical precision based on the established association between these measures and TL, (2) additionally adjusted for two SES measures as potential confounders selected a priori based on their presumed association with both early life growth and adult TL, and (3) additionally adjusted for birth factors that seem to confound the early life growth and TL relationship in this dataset (determined in preliminary analyses described above). Since it is likely that paternal age at delivery is associated with longer offspring TL (Eisenberg, Lee, Rej, Hayes, & Kuzawa, in press), paternal age should be included as a covariate to improve statistical precision in all models. However, given the correlation between maternal and paternal age and the consideration that maternal age influences TL differently than paternal age, we show the results of each of the above three models with and without adjustment for paternal age.
Analyses were completed with the PROC SURVEYREG function with the individual study id specified as the cluster variable in SAS Version 9.4 for Windows (SAS Institute Inc., Cary, NC, USA). Mean differences in T/S ratio, corresponding 95% confidence intervals and exact p-values are reported.
Written informed consent was obtained from all participants and data, and sample collection was conducted with approval and oversight from the Institutional Review Boards of University of North Carolina and Northwestern University. Telomere measurement and analysis of de-identified samples and data was not considered human subjects research by Northwestern University’s Institutional Review Board.
Results
The study sample is described by sex and overall in Table 1. This complete case analysis included 1,562 individuals.
Table 1.
Description of the study sample (n=1,562 individuals), by sex and overall
| Females n= 729 (46.7%) | Males n= 833 (53.3%) | t-test | Overall n= 1,562 | |
|---|---|---|---|---|
| mean ± SD | mean ± SD | p-value | mean ± SD | |
| Adult Characteristics | ||||
| telomere length, T/S ratio | 0.79 ± 0.16 | 0.76 ± 0.15 | 0.0007 ** | 0.77 ± 0.16 |
| age, months | 260.2 ± 4.2 | 260.0 ± 4.1 | 0.31 | 260.1 ± 4.1 |
| BMI, kg/m2 | 20.3 ± 3.3 | 21.1 ± 3.1 | < 0.001** | 20.7 ± 3.2 |
| Socioeconomic Status | ||||
| household assets index, 0–10 | 3.6 ± 2.4 | 3.7 ± 2.5 | 0.52 | 3.7 ± 2.5 |
| maternal education, years | 6.8 ± 3.2 | 7.0 ± 3.3 | 0.37 | 6.9 ± 3.2 |
| Birth Measures | ||||
| length-for-age, z-score | −0.15 ± 1.07 | −0.29 ± 1.06 | 0.0086** | −0.2 ± 1.1 |
| length, cm | 48.9 ± 2.0 | 49.3 ± 2.0 | < 0.0001** | 49.1 ± 2.0 |
| weight-for-age, z-score | −0.60 ± 0.96 | −0.71 ± 0.97 | 0.02* | −0.7 ± 1.0 |
| weight, kg | 2.99 ± 0.4 | 3.03 ± 0.4 | 0.03* | 3.0 ± 0.4 |
| gestational age, weeks | 38.6 ± 2.1 | 38.4 ± 2.1 | 0.06 | 38.5 ± 2.1 |
| maternal age, years | 26.8 ± 5.9 | 26.6 ± 6.1 | 0.64 | 26.7 ± 6.0 |
| Paternal age, years | 29.3 ± 6.6 | 29.4 ± 6.8 | 0.82 | 29.3 ± 6.7 |
| maternal BMI, kg/m2 | 21.0 ± 2.3 | 21.0 ± 2.4 | 0.90 | 21.0 ± 2.3 |
| Postnatal Size | ||||
| 6 months: height-for-age, z-score | −1.00 ± 1.02 | −1.26 ± 1.15 | <0.0001** | −1.1 ± 1.1 |
| 6 months: weight-for-age, z-score | −0.93 ± 0.99 | −1.03 ± 1.09 | 0.0478* | −1.0 ± 1.0 |
| 12 months: height-for-age, z-score | −1.61 ± 1.05 | −1.83 ± 1.17 | 0.0001 ** | −1.7 ± 1.1 |
| 12 months: weight-for-age, z-score | −1.39 ± 1.01 | −1.48 ± 1.07 | 0.11 | −1.4 ± 1.0 |
| 24 months: height-for-age, z-score | −2.4 ± 1.08 | −2.4 ± 1.12 | 0.38 | −2.4 ± 1.1 |
| 24months: weight-for-age, z-score | −1.71 ± 0.99 | −1.67 ± 0.97 | 0.42 | −1.7 ± 1.0 |
| 8.5 years: height-for-age, z-score | −2.03 ± 0.91 | −2.10 ± 0.93 | 0.10 | −2.1 ± 0.9 |
| 8.5 years: weight-for-age, z-score | −1.87 ± 0.92 | −2.00 ± 1.06 | 0.0096** | −1.9 ± 1.0 |
| Postnatal Growth | ||||
| 6 months: conditional height | 0.017 ± 0.953 | 0.025 ± 0.978 | 0.87 | 0.02 ± 0.98 |
| 6 months: conditional relative weight | −0.009 ± 0.973 | 0.021 ± 0.985 | 0.54 | 0.007 ± 0.98 |
| 12 months: conditional height | 0.011 ±0.993 | 0.016 ± 0.982 | 0.93 | 0.01 ± 0.98 |
| 12 months: conditional relative weight | 0.015 ± 0.958 | 0.038 ± 0.935 | 0.64 | 0.03 ± 0.95 |
| 24 months: conditional height | −0.036 ±0.978 | 0.002 ± 0.990 | 0.45 | −0.02 ± 0.98 |
| 24 months: conditional relative weight | 0.038 ± 0.969 | 0.031 ± 0.953 | 0.89 | 0.03 ± 0.96 |
| 8.5 years: conditional height | 0.008 ± 0.997 | 0.009 ± 0.976 | 0.97 | 0.008 ± 0.99 |
| 8.5 years: conditional relative weight | −0.02 ±0.98 | −0.016 ± 0.995 | 0.94 | −0.02 ± 0.99 |
P < 0.05 indicated in bold font with single asterisk
double asterisk indicates P < 0.01
Preliminary Analysis of Sex as a Covariate
Sex was associated with TL at 21 years of age and with age- and sex-specific size during certain ages of early postnatal life (birth, 6, 12 and 24 months, and 8.5 years; see Table 1). However, we did not detect an association between early life growth, indicated by the mean conditional measures, and sex (Table 1). Furthermore, estimates for the mean difference in TL for each postnatal growth interval did not differ after adjusting for sex (not shown), providing no evidence that sex confounds the relationship between early life growth and adult TL. The sex-specific strata estimates did not differ in direction or magnitude. Similarly, after adjusting for age at TL measurement and population genetic structure genome wide genetic variation, sex*postnatal growth interval interaction terms did not provide evidence that sex modifies the effect of postnatal growth on TL in early adulthood (not shown). Given the association between sex and adult TL, however, we included sex as a covariate in all subsequent analyses to improve the statistical precision of our estimates.
Preliminary Analyses of Bivariate Associations Between Exposures and Covariates with TL
We evaluated the bivariate relationships between demographic characteristics of the study sample, birth measures, and postnatal size and growth with TL (Table 2).
Table 2.
Bivariate associations between birth measures/factors and postnatal size/growth with TL
| Telomere Length (T/S ratio) | |||
|---|---|---|---|
| Mean difference | 95% CI | p-value | |
| Adult Characteristics | |||
| male v. female | −0.03** | (−0.04, −0.01) | 0.0008 |
| age at TL measurement, months | −0.005** | (−0.007, −0.003) | < 0.0001 |
| BMI, kg/m2 | 0.0007 | (−0.002, 0.003) | 0.60 |
| Socioeconomic Status | |||
| household assets index | −0.002 | (−0.005, 0.001) | 0.20 |
| maternal education, years | −0.0009 | (−0.003, 0.002) | 0.42 |
| Birth Measures/Factors | |||
| length-for-age, z-score | 0.003 | (−0.004, 0.01) | 0.36 |
| length, cm | 0.0006 | (−0.003, 0.004) | 0.76 |
| weight-for-age, z-score | 0.01* | (0.002, 0.02) | 0.02 |
| weight, kg | 0.02 | (−0.0002, 0.04) | 0.05 |
| gestational age, weeks | −0.002 | (−0.006, 0.002) | 0.30 |
| maternal age, years | 0.002** | (0.0006, 0.003) | 0.006 |
| Paternal age, years | 0.003** | (0.001, 0.004) | 0.0001 |
| maternal BMI, kg/m2 | 0.01 | (−0.02, 0.05) | 0.42 |
| Postnatal Size | |||
| 6 months: height-for-age, z-score | 0.0008 | (−0.006, 0.008) | 0.82 |
| 6 months: weight-for-age, z-score | 0.004 | (−0.003, 0.01) | 0.24 |
| 12 months: height-for-age, z-score | 0.007 | (−0.0003, 0.01) | 0.06 |
| 12 months: weight-for-age, z-score | 0.005 | (−0.003, 0.01) | 0.24 |
| 24 months: height-for-age, z-score | 0.004 | (−0.003, 0.01) | 0.30 |
| 24 months: weight-for-age, z-score | 0.001 | (−0.007, 0.009) | 0.77 |
| 8.5 years: height-for-age, z-score | 0.004 | (−0.004, 0.01) | 0.30 |
| 8.5 years: weight-for-age, z-score | 0.004 | (−0.004, 0.01) | 0.33 |
| Postnatal Growth | |||
| linear growth (CH)6m | −0.004 | (−0.01, 0.004) | 0.35 |
| weight gain (CRW)6m | 0.005 | (−0.003, 0.01) | 0.25 |
| linear growth (CH)12m | 0.009* | (0.001, 0.02) | 0.03 |
| weight gain (CRW)12m | −0.003 | (−0.01, 0.006) | 0.53 |
| linear growth (CH)24m | −0.0003 | (−0.008, 0.008) | 0.93 |
| weight gain (CRW)24m | −0.006 | (−0.01, 0.002) | 0.14 |
| linear growth (CH)8.5yr | 0.003 | (−0.005, 0.01) | 0.41 |
| weight gain (CRW)8.5yr | −0.0003 | (−0.008, 0.008) | 0.93 |
P < 0.05 indicated in bold font with single asterisk
double asterisk indicates P < 0.01
Preliminary Analyses of Correlated Birth Measures
We evaluated birth measures (length, weight, maternal age at delivery, paternal age at delivery, gestational age at birth, maternal BMI during the first trimester of pregnancy) in relation to TL (Table 3), to each other, and to early life growth (Table A1), using multivariate linear regression. We did not observe an association between birth length, gestational age at birth, or maternal BMI during the first trimester of pregnancy and TL (Table 3). Heavier birthweight (p<0.05), older maternal age at delivery (p<0.01), and older paternal age at delivery (p<0.01) were associated with longer adult TL, adjusting for sex and SES (Table 3, Model 2). In Model 2, a one SD increase in WAZ at birth corresponds to an interpolated 28.4 base pair (bp) longer TL in early adulthood (Eisenberg et al., 2015). This is equivalent to 2.1 fewer years of telomeric aging in middle age in this population (Eisenberg et al., 2012). A one-year increase in maternal or paternal age at delivery corresponds to an approximately 6.3 bp increase in TL in early adulthood, or 0.5 fewer years of telomeric aging.
Table 3.
Associations Between Birth Measures and TL – each birth measure included in independent regression model without including other birth measures. (mean difference in T/S ratio, 95% CI, p-value)
| Model 1± | Model 2† | Model 3‡ | |
|---|---|---|---|
| Birth Length, z-score | 0.003(−0.004, 0.01) | 0.003(−0.004, 0.01) | 0.002(−0.005, 0.01) |
| Birth Weight, z-score | 0.009*(0.0007, 0.02) | 0.009*(0.0007, 0.02) | 0.008(−0.0006, 0.02) |
| Gestational age at birth, weeks | −0.003(−0.007, 0.001) | −0.003(−0.007, 0.001) | −0.003(−0.007, 0.001) |
| Maternal age at delivery, years | 0.002**(0.0006, 0.003) | 0.002**(0.0006, 0.003) | ------- |
| Paternal age at delivery, years | 0.003**(0.001, 0.004) | 0.003**(0.001, 0.004) | 0.003**(0.001, 0.005) |
| Maternal BMI during pregnancy, kg/m2 | 0.01(−0.02, 0.05) | 0.01(−0.02, 0.05) | 0.006(−0.03, 0.04) |
adjusts for genome wide genetic variation, age at TL measurement and sex (as precision variables)
additionally adjusts for two SES measures: household asset index and maternal education at birth (confounders selected a priori)
additionally adjusts for maternal age at delivery (confounder or precision variable)
P < 0.05 indicated in bold font with single asterisk
double asterisk indicates P < 0.01
Since older maternal age was associated with longer TL in adult children, heavier birthweight, and greater postnatal weight gain and slower linear growth during the first year after birth (Table A1), we adjusted for maternal age at delivery as a confounder in a third set of models. When adjusted for maternal age at delivery (Table 3, Model 3), birthweight was no longer associated with TL at 21 years of age, suggesting maternal age may confound the growth-TL relationship. However, maternal and paternal age are strongly correlated (r=0.79, p<0.001), making it impossible to disentangle their effects on TL with the present approach. Despite this, the association between older paternal age and longer TL in adult children persisted after adjusting for maternal age.
Final Models
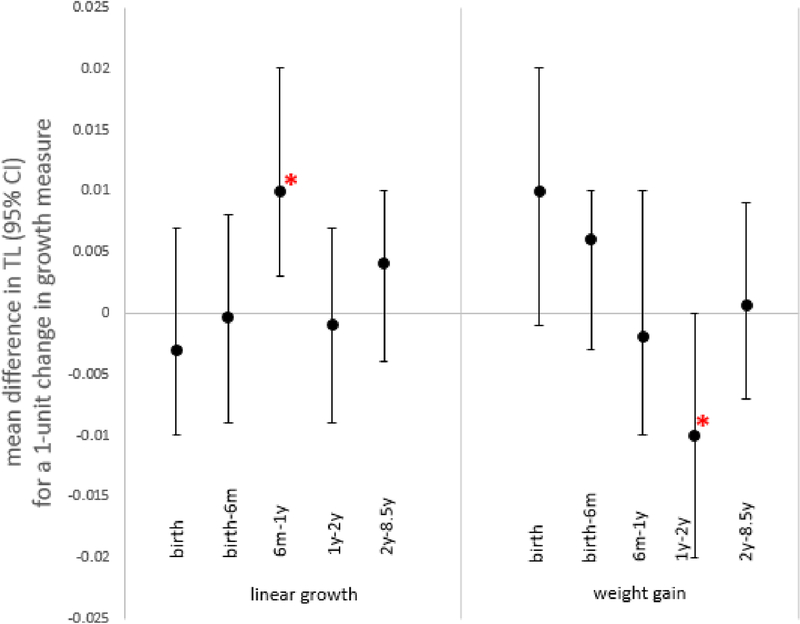
In our final models, accounting for current height and prior weight and height, we observed an association between heavier birthweight and accelerated linear growth from 6–12 months and longer adult TL and an association between accelerated weight gain between 12–24 months and shorter adult TL (Table 4, Figure 1). Adjustment for paternal or maternal age attenuated the observed birthweight-TL relationship (Table A2). The relationship between weight gain from 12–24 months and TL only emerged as statistically significant after adjusting for maternal age. In Model 3, a one SD increase in conditional relative weight between 12–24 months corresponds to a 25.28 bp shortening in TL in early adulthood, equivalent to 1.9 years of adult telomeric aging in this population. The observed association between accelerated linear growth from 6–12 months persisted despite adjustment for maternal and paternal age. In Model 3, a one SD increase in conditional height between 6–12 months corresponds to a 31.6 bp longer TL in adulthood and 2.3 fewer years of telomeric aging. Although we observed associations between birthweight, weight gain from 12–24 months, and linear growth from 6–12 months with TL length in adulthood, it is important to note the joint effect of all CH (p= 0.10) and all CRW (p=0.11) variables on TL in Model 3 were not associated with TL (not shown).
Table 4.
Multivariate linear regression results for associations between growth and TL (N=1,562) Mean difference in in T/S ratio per one-unit change in growth measure (95% CI, p-value)
| Model 1± | Model 2† | Model 3‡ | |
|---|---|---|---|
| Weight (WAZ)birth | 0.01*(0.0006, 0.02) | 0.01*(0.0004, 0.02) | 0.01(−0.001, 0.02) |
| Weight gain (CRW)6m | 0.004(−0.004, 0.01) | 0.004(−0.004, 0.01) | 0.006(−0.003, 0.01) |
| Weight gain (CRW)12m | −0.001(−0.01, 0.007) | −0.0008(−0.009, 0.008) | −0.002(−0.01, 0.01) |
| Weight gain (CRW)24m | −0.008(−0.02, 0.0002) | −0.008(−0.02, 0.0004) | −0.008*(−0.02, −0.0001) |
| Weight gain (CRW)8.5yr | 0.001(−0.007, 0.009) | 0.001(−0.007, 0.009) | 0.0006(−0.007, 0.009) |
| Length (HAZ)birth | −0.003(−0.01, 0.007) | −0.003(−0.01, 0.007) | −0.003(−0.01, 0.007) |
| Linear growth (CH)6m | −0.001(−0.01, 0.007) | −0.001(−0.01, 0.007) | −0.0004(−0.009, 0.008) |
| Linear growth (CH)12m | 0.01*(0.002, 0.02) | 0.01**(0.003, 0.02) | 0.01**(0.003, 0.02) |
| Linear growth (CH)24m | −0.001(−0.009, 0.006) | −0.001(−0.009, 0.007) | −0.001(−0.009, 0.007) |
| Linear growth (CH)8.5yr | 0.004(−0.004, 0.01) | 0.004(−0.004, 0.01) | 0.004(−0.004, 0.01) |
adjusts for genome wide genetic variation, age at TL measurement and sex (as precision variables)
additionally adjusts for two SES measures: household asset index and maternal education at birth (confounders selected a priori)
additionally adjusts for maternal age at delivery (confounder or precision variable)
P < 0.05 indicated in bold font with single asterisk
double asterisk indicates P < 0.01
Figure 1.
Linear growth and weight gain in relation to adult TL, overall (Table 4 – model 3)
Discussion
The present study is the first to evaluate the influence of sensitive periods of postnatal growth (weight gain and linear growth) on adult TL using conditional measures, which allow better discernment of the independent effects of weight and height gain at different ages. Results indicate that certain growth periods may be particularly influential on long-term TL. Accelerated linear growth, a proxy for lean body mass gain, between 6–12 months was associated with longer TL, the opposite of what we hypothesized. However, in line with our hypothesis, accelerated weight gain, as an indicator of fat mass gain, between 12–24 months of age was associated with shorter TL in adulthood, a marker of biological aging. Our findings are in line with other studies that evaluated postnatal weight gain using slightly different approaches. One study found that postnatal weight at 12 months as well as weight gain between 6–9 months and during the first year of life (0–12 months) were inversely associated with TL at 70 years of age in women (Guzzardi et al., 2016). Similarly, a birth cohort study from Finland found that, among women, BMI increase between early childhood and adulthood was associated with shorter TL at 31 years of age (Buxton, Das, Rodriguez, Kaakinen, & Alves, 2014). In our data, this finding only emerged as statistically significant after adjustment for maternal age, which may indicate inclusion of maternal age as a covariate increases statistical precision. Alternatively, it may be this was a spurious finding. These findings provide clues to the cellular underpinnings of complex growth patterns and their implications for the developing individual. Furthermore, given that TL is a marker of senescence, a clearer understanding of the influence of sensitive growth periods during early life on adult TL may help to inform on the importance of early life environment for health throughout the life course.
Our unexpected finding that slower linear growth was associated with shorter TL may point to associated factors, such as infection, as explanations (Eisenberg et al., 2017; Tennyson et al., 2018). As a common cause of stunted linear growth and shorter TL, infection may confound the association between postnatal linear growth and adult TL (Lin et al., 2017). Given that diarrheal disease in infancy predicts shorter adult TL (Eisenberg et al., 2017), the most robust finding of accelerated linear growth from 6–12 months may be driven by lowered infectious disease morbidity. Less infection would allow accelerated growth and reduced blood TL attrition due to decreased immune cell proliferation. To appropriately evaluate the roles of growth and infection over time simultaneously, a marginal structural model that can account for infection as a time-varying confounder measured during each growth interval of interest would be a necessary approach (Robins, Hernán, & Brumback, 2000). We recommend this as a next step to disentangling the influence of growth and infection on TL. Our accelerated weight gain finding is worthy of consideration given the conventional thinking that more rapid weight gain is an important goal of pediatric care in low resource settings like Cebu, where poor growth is associated with poor cognitive development, morbidity and mortality. Based on its association with adult BMI, early life weight gain may also play a role in explaining the inverse associations between adult BMI and TL observed by other studies (Gielen et al., 2018; Müezzinler, Zaineddin, & Brenner, 2014).
Our approach to measuring sensitive periods of growth has been used to study health outcomes other than TL in this cohort (Adair et al., 2013). Findings indicate that accelerated linear growth from birth to 8.5 years of age is related to a lesser likelihood of stunted growth in adulthood, but both accelerated linear growth and weight gain between birth and 8.5 years of age are related to an elevated risk of overweight and high blood pressure in adulthood (Adair et al., 2013). Studies in other populations relying on conditional measures of growth have observed an association between faster linear growth in childhood and taller height, lean body mass, higher attainment in school, employment and higher earnings in adulthood (Adair et al., 2009; Adair et al., 2013; Kuzawa et al., 2012; Martorell et al., 2010). Our finding that accelerated linear growth during early life is associated with longer TL corresponds with these findings. A positive relationship has also been observed between faster weight gain during the first two years of life and overweight and elevated blood pressure in adulthood in low- and middle-income countries (Adair et al., 2009; Kuzawa et al., 2012). Our finding that accelerated weight gain during early life is associated with shorter TL also coincide with these findings. Overall, this suggests an important but complicated interrelatedness of early life growth, TL and health outcomes that deserves more attention.
This study critically evaluated the roles of certain covariates in relation to growth and TL that are included in analyses inconsistently across the literature. To mimic prior studies’ findings that the effects of growth on TL vary by sex (Buxton et al., 2014; Guzzardi et al., 2016; Pearce et al., 2012), in a posthoc analysis we observed that females showed nominally stronger growth-TL associations than males (Figures A1, A2). When postnatal growth was evaluated by sex, only linear growth between 6–12 months in females was associated with TL. However, sex*growth interaction terms did not provide evidence for an association with TL and the growth-TL relationship observed did not differ by sex, so our findings do not convincingly support the idea that postnatal growth influences TL in adulthood more strongly in females than males. While it is clearly important to account for sex in analyses, sex-specific analyses should be more clearly justified and empirically evaluated in future work.
Like this analysis, other studies have observed an association between maternal age at delivery and TL (Kajantie et al., 2012). This relationship may be explained in part by maternal age serving as a proxy measure for paternal age, which has an established effect on offspring TL (Eisenberg & Kuzawa, 2018; Eisenberg et al., in press). When we additionally included paternal age in Table 4, Model 3, the maternal age association with TL was attenuated (from 0.002, p=0.004 to −0.0002, p=0.85) and paternal age was more strongly associated with TL (0.003, p=0.005). Some studies have not considered this important factor in analyses of early life growth and TL. Since maternal age predicts birth measures, including birthweight, it is possible that this explains some studies’ observed associations between both gestational age and birthweight with shorter TL (Friedrich, Schwab, Griese, Fritz, & Klotz, 2001; Raqib et al., 2007; Smeets, Codd, Samani, & Hokken-Koelega, 2015; Strohmaier et al., 2015). A better understanding of how and why maternal age is related to both birth measures and adult TL in offspring is needed to appropriately interpret findings relating early life growth and TL. We observed a positive association between birthweight and TL, but this was attenuated after adjustment for maternal age at delivery in our analyses. Similar to the omission of confounding factors leading to biased conclusions, including birth measures that are not associated with the outcome (TL) may over-adjust a model, resulting in misleading conclusions. However, given that maternal and paternal age have different roles in TL etiology, it is important to look to more advanced methods to disentangle the effects of these highly-correlated covariates.
This study had some limitations. First, 214 members of the original telomere study subset of the cohort study were excluded due to partially missing exposure and/or covariate data. We observed no differences in TL (p=0.17) between those included (n=1,562) and excluded (n=214) from the complete case analysis and few differences in early life measures: on average, excluded individuals were born to younger mothers (p=0.02), and gained less weight between 6–12 months (p=0.03) and between 12–24 months (p<0.01). Also, we evaluated blood TL. TL from different organs may be influenced by early life growth differently. It is possible that the sensitive periods of growth we evaluated were not sufficiently narrow to detect certain associations. Evaluating broad exposure periods may have reduced our study sensitivity, making it less likely to have detected true associations with particular, narrow times of growth. For example, given the longer timespan between the 2- to 8.5-year interval, we may have been more likely to miss a sensitive period within this period than the 6-month long periods we evaluated closer to birth, but existing evidence points to younger postnatal ages as the most critical for the developmental origins of health and disease. Our findings reinforce the evidence supporting the idea that subsequent analyses should focus on narrower growth intervals during the first two years of postnatal growth as potentially critical periods influencing adult TL.
In conclusion, we find evidence that conditional weight gain between 12–24 months predicts shorter adult TL in our maximally controlled model and that conditional linear growth between 6–12 months predicts longer TL in adulthood. Although we observed evidence that higher birthweight may predict longer adult TL, this association diminished when controlling for maternal age, a commonly-overlooked potential confounder in the literature, or paternal age, a predictor of offspring adult TL and a strong correlate of maternal age. These findings suggest that childhood growth is associated with the cellular senescence process in adulthood, implying early life well-being may be linked to adult health. Research priorities should include further investigation into underlying mechanisms and variation in the growth-adult TL-health relationships across populations. They should also focus on the influence of infectious disease burden and public health infrastructure on these relationships.
Supplementary Material
Acknowledgments
This work was supported by the National Science Foundation [BCS-0962282 and BCS-1519110]; National Institutes of Health [NIDCR T90DE021884, NICHD R24 HD042828, TW05596, DK078150, RR20649, ES10126, and DK056350]; the Wenner-Gren Foundation [Gr. 8111]; and institutional support from Northwestern University.
We thank Karen Mohlke for sharing aliquots of extracted DNA and genetic information. We especially thank the many researchers at the Office of Population Studies, University of San Carlos, Cebu, the Philippines, for their central role in study design and data collection, and the Filipino participants, who provided their time and samples for this study. We also thank the COHORTS consortium for their work developing the conditional growth variables used in this analysis.
Footnotes
Authors have no conflict of interest to declare.
References
- Adair L, Martorell R, & Stein A (2009). Size at birth, weight gain in infancy and childhood, and adult blood pressure in 5 low-and middle-income-country cohorts: when does weight gain matter? The American Journal of Clinical Nutrition, 89(13), 1383–1392. 10.3945/ajcn.2008.27139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adair LS, Fall CHD, Osmond C, Stein AD, Martorell R, Ramirez-Zea M, … Victora CG (2013). Associations of linear growth and relative weight gain during early life with adult health and human capital in countries of low and middle income: findings from five birth cohort studies. Lancet, 382(9891), 525–534. 10.1016/S0140-6736(13)60103-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adair LS, Popkin BM, Akin JS, Guilkey DK, Gultiano S, Borja J, … Hindin MJ (2011). Cohort profile: the Cebu longitudinal health and nutrition survey. International Journal of Epidemiology, 40(3), 619–625. 10.1093/ije/dyq085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allsopp RC, Vaziri H, Patterson C, Goldstein S, Younglai EV, Futcher AB, … Harley CB (1992). Telomere length predicts replicative capacity of human fibroblasts. Proceedings of the National Academy of Sciences of the United States of America, 89, 10114–10118. 10.1073/pnas.89.21.10114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv A, & Shay JW (2018). Reflections on telomere dynamics and ageing-related diseases in humans. Phil. Trans. R. Soc. B, 373(20160436). 10.1098/rstb.2016.0436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv A, Valdes AM, & Spector TD (2006). Human telomere biology: Pitfalls of moving from the laboratory to epidemiology. International Journal of Epidemiology. 10.1093/ije/dyl169 [DOI] [PubMed] [Google Scholar]
- Barker D (1998). Mothers, babies, and health later in life. Edinburgh, New York: Churchhill Livingstone. [Google Scholar]
- Bethancourt HJ, Kratz M, Beresford SAA, Hayes MG, Kuzawa CW, Duazo PL, … Eisenberg DTA (2017). No Association between Blood Telomere Length and Longitudinally-Assessed Diet or Adiposity in a Young Adult Filipino Population HHS Public Access. Eur J Nutr, 56(1), 295–308. 10.1007/s00394-015-1080-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn EH, & Gall JG (1978). A tandemly repeated sequence at the termini of the extrachromosomal ribosomal RNA genes in Tetrahymena. Journal of Molecular Biology, 120, 33–53. 10.1016/0022-2836(78)90294-2 [DOI] [PubMed] [Google Scholar]
- Buxton JL, Das S, Rodriguez A, Kaakinen M, & Alves C (2014). Multiple Measures of Adiposity Are Associated with Mean Leukocyte Telomere Length in the Northern Finland Birth Cohort. PLoS ONE, 9(6), 99133 10.1371/journal.pone.0099133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron Noël. (2002). Human Growth Curve, Canalization, andCatch-Up Growth. In Human Growth and Development (pp. 1–20). [Google Scholar]
- Cameron Noël, & Demerath EW (2002). Critical periods in human growth and their relationship to diseases of aging. American Journal of Physical Anthropology, 119(S35), 159–184. 10.1002/ajpa.10183 [DOI] [PubMed] [Google Scholar]
- Cawthon RM (2009). Telomere length measurement by a novel monochrome multiplex quantitative PCR method. Nucleic Acids Research, 37 10.1093/nar/gkn1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croteau-Chonka DC, Marvelle AF, Lange EM, Lee NR, Adair LS, Lange LA, & Mohlke KL (2011). Genome-wide association study of anthropometric traits and evidence of interactions with age and study year in Filipino women. Obesity (Silver Spring, Md.), 19, 1019–1027. 10.1038/oby.2010.256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croteau-chonka DC, Wu Y, Li Y, Fogarty MP, Lange LA, Kuzawa CW, … Mohlke KL (2012). Population-specific coding variant underlies genome-wide association with adiponectin level. Human Molecular Genetics, 21, 463–471. 10.1093/hmg/ddr480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demerath EW, Cameron N, Gillman MW, Towne B, & Siervogal RM (2004). Telomeres and Telomerase in the Fetal Origins of Cardiovascular Disease: A Review. Hum Biol, 76(1), 127–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg D, Borja J, Hayes M, & Kuzawa C (2017). Early life infection, but not breastfeeding, predicts adult blood telomere lengths in the Philippines. American Journal of Human Biology, 29(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg DTA, Hayes MG, & Kuzawa CW (2012). Delayed paternal age of reproduction in humans is associated with longer telomeres across two generations of descendants. Proceedings of the National Academy of Sciences, 109(26), 10251–10256. 10.1073/pnas.1202092109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg DTA, & Kuzawa CW (2018). The paternal age at conception effect on offspring telomere length: mechanistic, comparative and adaptive perspectives. Phil. Trans. R. Soc. B, 373(1741), 20160442 10.1098/RSTB.2016.0442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg DTA, Kuzawa CW, & Hayes MG (2015). Improving qPCR telomere length assays: Controlling for well position effects increases statistical power. American Journal of Human Biology, 27(4), 570–575. 10.1002/ajhb.22690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg DTA, Lee NR, Rej PH, Hayes MG, & Kuzawa CW (in press). Older paternal ages and grandpaternal ages at conception predict longer telomeres in human descendants. Proc. R. Soc. B [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg DTA (2011). An evolutionary review of human telomere biology: the thrifty telomere hypothesis and notes on potential adaptive paternal effects. American Journal of Human Biology, 23(2), 149–167. 10.1002/ajhb.21127 [DOI] [PubMed] [Google Scholar]
- Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, & Cawthon RM (2004). Accelerated telomere shortening in response to life stress. Proceedings of the National Academy of Sciences of the United States of America, 101, 17312–17315. 10.1073/pnas.0407162101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson JG, Forsén T, Tuomilehto J, Winter PD, Osmond C, & Barker DJ (1999). Catch-up growth in childhood and death from coronary heart disease: longitudinal study. BMJ, 318, 427–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenck R, Blackburn E, & Shannon K (1998). The rate of telomere sequence loss in human leukocytes varies with age. Proceedings of the National Academy of Sciences, 95(May), 5607–5610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich U, Schwab M, Griese EU, Fritz P, & Klotz U (2001). Telomeres in neonates: new insights in fetal hematopoiesis. Pediatric Research, 49(2), 252–256. 10.1203/00006450-200102000-00020 [DOI] [PubMed] [Google Scholar]
- Gardner M, Bann D, Wiley L, Cooper R, Hardy R, Nitsch D, … Ben-Shlomo Y (2014). Gender and telomere length: systematic review and meta-analysis. Experimental Gerontology, 51, 15–27. 10.1016/j.exger.2013.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger S, Le Vaillant M, Lebard T, Reichert S, Stier A, LE Maho Y, & Criscuolo F (2012). Catching-up but telomere loss: half-opening the black box of growth and ageing trade-off in wild king penguin chicks. Molecular Ecology, 21(6), 1500–1510. 10.1111/j.1365-294X.2011.05331.x [DOI] [PubMed] [Google Scholar]
- Gielen M, Hageman GJ, Antoniou EE, Nordfjall K, Mangino M, Balasubramanyam M, … Zeegers MP (2018). Body mass index is negatively associated with telomere length: a collaborative cross-sectional meta-analysis of 87 observational studies. The American Journal of Clinical Nutrition, 108(3), 453–475. 10.1093/ajcn/nqy107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman PD, & Hanson MA (2006). The conceptual basis for the developmental origins of health and disease. Developmental Origins of Health and Disease. [DOI] [PubMed] [Google Scholar]
- Greider CW, & Blackburn EH (1985). Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell, 43, 405–413. 10.1016/0092-8674(85)90170-9 [DOI] [PubMed] [Google Scholar]
- Guzzardi MA, Iozzo P, Salonen MK, Kajantie E, & Eriksson JG (2016). Maternal adiposity and infancy growth predict later telomere length: A longitudinal cohort study. International Journal of Obesity, (April), 1–28. 10.1038/ijo.2016.58 [DOI] [PubMed] [Google Scholar]
- Hansen MEB, Hunt SC, Stone RC, Horvath K, Herbig U, Ranciaro A, … Aviv A (2016). Shorter telomere length in Europeans than in Africans due to polygenetic adaptation. Human Molecular Genetics, 25(11). 10.1093/hmg/ddw070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley CB, Futcher AB, & Greider CW (1990). Telomeres shorten during ageing of human fibroblasts. Nature, 345, 458–460. 10.1038/345458a0 [DOI] [PubMed] [Google Scholar]
- Hiyama E Hiyama K (2007). Telomere and telomerase in stem cells. British Journal of Cancer, 96, 1020–1024. 10.1038/sj.bjc.6603671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt SC, Chen W, Gardner JP, Kimura M, Srinivasan SR, Eckfeldt JH, … Aviv A (2008). Leukocyte telomeres are longer in African Americans than in whites: The National Heart, Lung, and Blood Institute Family Heart Study and the Bogalusa Heart Study. Aging Cell, 7, 451–458. 10.1111/j.1474-9726.2008.00397.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings BJ, Ozanne SE, Dorling MW, & Hales CN (1999). Early growth determines longevity in male rats and may be related to telomere shortening in the kidney. FEBS Lett, 448(1), 4–8. [DOI] [PubMed] [Google Scholar]
- Kajantie E, Pietiläinen KH, Wehkalampi K, Kananen L, Räikkönen K, Rissanen A, … Hovatta I (2012). No association between body size at birth and leucocyte telomere length in adult life--evidence from three cohort studies. International Journal of Epidemiology, 41(5), 1400–1408. 10.1093/ije/dys127 [DOI] [PubMed] [Google Scholar]
- Keijzer-Veen MG, Margriet Euser A, Van Montfoort N, Dekker FW, Vandenbroucke JP, & Van Houwelingen HC (2005). A regression model with unexplained residuals was preferred in the analysis of the fetal origins of adult diseases hypothesis. Journal of Clinical Epidemiology, 58, 1320–1324. 10.1016/j.jclinepi.2005.04.004 [DOI] [PubMed] [Google Scholar]
- Kimura M, Cherkas LF, Kato BS, Demissie S, Hjelmborg JB, Brimacombe M, … Aviv A (2008). Offspring’s leukocyte telomere length, paternal age, and telomere elongation in sperm. PLoS Genetics, 4 10.1371/journal.pgen.0040037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzawa CW (2007). Developmental Origins of Life History : Growth, Productivity, and Reproduction. Am J Hum Biol, 661(February), 654–661. 10.1002/ajhb [DOI] [PubMed] [Google Scholar]
- Kuzawa CW, Hallal PC, Adair L, Bhargava SK, Fall CHD, Lee N, … Victora CG (2012). Birth weight, postnatal weight gain, and adult body composition in five low and middle income countries. American Journal of Human Biology, 24(November 2011), 5–13. 10.1002/ajhb.21227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin A, Arnold BF, Mertens AN, Lin J, Benjamin-Chung J, Ali S, … Luby SP (2017). Effects of water, sanitation, handwashing, and nutritional interventions on telomere length among children in a cluster-randomized controlled trial in rural Bangladesh. Elife October 5(6), 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martorell R, Horta BL, Adair LS, Stein AD, Richter L, Fall CHD, … Victora CG (2010). Weight gain in the first two years of life is an important predictor of schooling outcomes in pooled analyses from five birth cohorts from low- and middle-income countries. The Journal of Nutrition, 140, 348–354. 10.3945/jn.109.112300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe NB, & Monaghan P (2001). Compensation for a bad start: Grow now, pay later? Trends in Ecology and Evolution. 10.1016/S0169-5347(01)02124-3 [DOI] [PubMed] [Google Scholar]
- Müezzinler a, Zaineddin a K., & Brenner H (2014). Body mass index and leukocyte telomere length in adults: a systematic review and meta-analysis. Obesity Reviews, 15(3), 192–201. 10.1111/obr.12126 [DOI] [PubMed] [Google Scholar]
- Näslund J, Pauliny A, Blomqvist D, & Johnsson JI (2015). Telomere dynamics in wild brown trout: effects of compensatory growth and early growth investment. Oecologia, 1221–1230. 10.1007/s00442-015-3263-0 [DOI] [PubMed] [Google Scholar]
- Norris S. a., Osmond C, Gigante D, Kuzawa CW, Ramakrishnan L, Lee NR, … Mainwaring M (2012). Size at birth, weight gain in infancy and childhood, and adult diabetes risk in five low- or middle-income country birth cohorts. Diabetes Care, 35, 72–79. 10.2337/dc11-0456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauliny A, Devlin RH, Johnsson JI, & Blomqvist D (2015). Rapid growth accelerates telomere attrition in a transgenic fish. BMC Evolutionary Biology, 15(1), 159 10.1186/s12862-015-0436-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce MS, Mann KD, Martin-Ruiz C, Parker L, White M, von Zglinicki T, & Adams J (2012). Childhood growth, IQ and education as predictors of white blood cell telomere length at age 49–51 years: the Newcastle Thousand Families Study. PloS One, 7(7), e40116 10.1371/journal.pone.0040116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plunkett FJ, Soares MV, Annels N, Hislop A, Ivory K, Lowdell M, … Akbar AN (2001). The flow cytometric analysis of telomere length in antigen-specific CD8+ T cells during acute Epstein-Barr virus infection. Blood, 97, 700–707. 10.1182/blood.V97.3.700 [DOI] [PubMed] [Google Scholar]
- Raes A, Van Aken S, Craen M, Donckerwolcke R, & Vande Walle J (2006). A reference frame for blood volume in children and adolescents. BMC Pediatrics, 6, 3 10.1186/1471-2431-6-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raqib R, Alam DS, Sarker P, Ahmad SM, Ara G, Yunus M, … Fuchs G (2007). Low birth weight is associated with altered immune function in rural Bangladeshi children : a birth cohort study 1 – 3. Amer J Clinical NutritionClinical Nutrition, 85(3), 845–852. [DOI] [PubMed] [Google Scholar]
- Reichert S, & Stier A (2017). Does oxidative stress shorten telomeres in vivo ? A review. Biology Letters, 13(12), 20170463 10.1098/rsbl.2017.0463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringsby TH, Jensen H, Pärn H, Kvalnes T, Boner W, Gillespie R, … Monaghan P (2015). On being the right size: increased body size is associated with reduced telomere length under natural conditions. Proc. R. Soc. B, 282(1820), 20152331 10.1098/rspb.2015.2331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins JM, Hernán MA, & Brumback B (2000). Marginal structural models and causal inference in epidemiology. Epidemiology (Cambridge, Mass.), 11(5), 550–560. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10955408 [DOI] [PubMed] [Google Scholar]
- Ryan CP, Hayes MG, Lee NR, Mcdade TW, Jones MJ, Kobor MS, … Eisenberg DTA (2018). Reproduction predicts shorter telomeres and epigenetic age acceleration among young adult women OPEN. SCIEntIfIC REPoRTS |, 8, 11100 10.1038/s41598-018-29486-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev I (2012). Early life stress and telomere length: investigating the connection and possible mechanisms: a critical survey of the evidence base, research methodology and basic biology. BioEssays: News and Reviews in Molecular, Cellular and Developmental Biology, 34(11), 943–952. 10.1002/bies.201200084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeets CCJ, Codd V, Samani NJ, & Hokken-Koelega ACS (2015). Leukocyte Telomere Length in Young Adults Born Preterm: Support for Accelerated Biological Ageing. Plos One, 10(11), e0143951 10.1371/journal.pone.0143951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soediono B (1989). Attrition in the Cebu Longitudinal Health and Nutrition Survey. Journal of Chemical Information and Modeling, 53(1), 160 10.1017/CBO9781107415324.004 [DOI] [Google Scholar]
- Strohmaier J, van Dongen J, Willemsen G, Nyholt DR, Zhu G, Codd V, … Martin NG (2015). Low Birth Weight in MZ Twins Discordant for Birth Weight is Associated with Shorter Telomere Length and lower IQ, but not Anxiety/Depression in Later Life. Twin Research and Human Genetics, 18(02), 198–209. 10.1017/thg.2015.3 [DOI] [PubMed] [Google Scholar]
- Tarry-Adkins JL, Chen JH, Smith NS, Jones RH, Cherif H, & Ozanne SE (2009). Poor maternal nutrition followed by accelerated postnatal growth leads to telomere shortening and increased markers of cell senescence in rat islets. FASEB Journal: Of ficial Publication of the Federation of American Societies for Experimental Biology, 23(5), 1521–1528. 10.1096/fj.08-122796 [DOI] [PubMed] [Google Scholar]
- Tennyson RL, Gettler LT, Kuzawa CW, Hayes MG, Agustin SS, & Eisenberg DTA (2018). Lifetime socioeconomic status and early life microbial environments predict adult blood telomere length in the Philippines. American Journal of Human Biology, 30(5), e23145 10.1002/ajhb.23145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unryn BM, Cook LS, & Riabowol KT (2005). Paternal age is positively linked to telomere length of children. Aging Cell, 4, 97–101. 10.1111/j.1474-9728.2005.00144.x [DOI] [PubMed] [Google Scholar]
- Victora CG, Adair L, Fall C, Hallal PC, Martorell R, Richter L, & Sachdev HS (2008a). Maternal and child undernutrition: consequences for adult health and human capital. Lancet, 371(9609), 340–357. 10.1016/S0140-6736(07)61692-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victora CG, Adair L, Fall C, Hallal PC, Martorell R, Richter L, & Sachdev HS (2008b). Maternal and child undernutrition: consequences for adult health and human capital. The Lancet, 371(9609), 340–357. 10.1016/S0140-6736(07)61692-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Zglinicki T (2002). Oxidative stress shortens telomeres. Trends in Biochemical Sciences. 10.1016/S0968-0004(02)02110-2 [DOI] [PubMed] [Google Scholar]
- Wu Y, McDade TW, Kuzawa CW, Borja J, Li Y, Adair LS, … Lange LA (2012). Genome-wide association with C-reactive protein levels in CLHNS: Evidence for the CRP and HNF1A loci and their interaction with exposure to a pathogenic environment. Inflammation, 35, 574–583. 10.1007/s10753-011-9348-y [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.