Summary
Improvement in survival in Light chain (AL) amyloidosis has been seen over recent decades, enabling more patients to achieve long-term survival. Patients with AL amyloidosis who survived ≥10 years from time of diagnosis (n=186) were the subject of this study. Ten-year survivors represented 22% of the total population. These patients were characterized by favourable patient, organ and plasma cell features. Of note, trisomies were less common among 10-year survivors compared to those who did not survive to 10 years. Overall best response was complete response in 67%, very good partial response in 30%, partial response in 2% and no response in 1%, with 11% having received a consolidative strategy for inadequate response to first line therapy. The overall organ response rate to first-line therapy was 76%, which increased to 86% when considering subsequent line(s) of therapy. Forty-seven percent of the 10-year survivors did not require a second-line therapy. The median treatment-free survival (TFS) among the 10-year survivors was 10.5 years (interquartile range 7.4–12.2). On multivariate analysis independent predictors for TFS were the achievement of complete haematological response and lack of cardiac involvement. Long-term survivors are increasingly seen in AL amyloidosis and present distinct patient, organ and clonal disease features.
Keywords: prognosis, treatment, response, trend, outcome, mortality
Introduction
Light chain (AL) amyloidosis is a heterogeneous disease characterized by extracellular amyloid deposits, with an ultra-structure of β-sheeted fibrillary proteins.(Merlini, et al 2018) Disease heterogeneity, exceeding other types of amyloidosis syndromes, stems from a wide array of organ involvement. Moreover, AL amyloidosis originates from a clonal plasma cell disorder (or rarely a clonal B-cell disorder) which can range from a low tumour burden disorder to multiple myeloma, an incurable cancer, which adds to the complexity of the disease and its management. Heart involvement, seen in 60–80% of patients (Muchtar, et al 2016a), is the leading cause of death, with a significant proportion of patients who succumb to their disease early in its course. With increased availability of effective therapies for AL amyloidosis, we undertook this study to explore the factors associated with long-term survival. Such efforts may help in designing new avenues to increase survival in this disease.
Patients and Methods
The study was approved by the institutional review board. Patients with biopsy-proven systemic AL amyloidosis, who were seen in our institution within 90 days of the confirmed diagnosis, were considered for this study. Long-term survival was defined as patients who survived ≥10 years from the time of diagnosis. For this purpose, we screened all patients diagnosed between 1 January 2000 and 31 May 2008 (allowing a minimum of 10-year follow-up; n=833). The median follow-up was 12.3 years (interquartile range [IQR] 11–13.9).
First line treatment was defined as the first regimen patients received, regardless of subsequent modifications. Treatment was categorized as autologous stem cell transplantation (ASCT) (upfront or following induction treatment); melphalan-dexamethasone (MDex); bortezomib-based regimen; Immunomodulatory drug (IMiD)-based regimen; and melphalan-prednisone (MP) or dexamethasone alone. The haematological response assessment was based on consensus criteria.(Palladini, et al 2012) Organ response was assessed for heart, kidney and liver involvement and was based on consensus criteria (Gertz, et al 2005) with modifications for cardiac (Palladini, et al 2012) and renal response.(Palladini, et al 2014).
The χ2 test was used to compare differences between continuous variables, and the Wilcoxon signed-rank test was used for nonparametric group comparisons. Time to next therapy was defined as the time from diagnosis until second-line therapy, while patients who did not receive second-line therapy were censored. Treatment-free survival (TFS) was defined as the time from diagnosis until second-line of therapy or death; patients alive and treatment-free at the end of follow-up were censored. Multivariate analysis was performed using the Cox Proportional Hazards model and included all variables with P<0.1 on a univariate analysis. All statistical analyses were performed on JMP software (SAS Institute, Cary, NC).
Results
Baseline patients’ characteristics
One hundred and eighty-six of the 833 screened patients survived 10 years or more from the time of diagnosis (22% of the screening cohort). The proportion of patients achieving long-term survival increased over the study period. The 10-year survival rate increased from 19% to 25% between 2000–2003 and 2004–2008 (P=0.03).
Ten-year survivors had more favourable baseline characteristics, including younger age, female sex, renal only presentation, lower Mayo stage and higher systolic blood pressure (Table I). They were less likely to be seen within 30 days of diagnosis compared to their counterparts (47% vs 65%; P<0.001). This is largely explained by the fact that those seen within 30 days of diagnosis had higher Mayo stage compared to those seen more than 30 days after diagnosis (Mayo 2004 stage III 50% vs 41% respectively, P=0.04; Mayo 2012 stage III-IV 36% vs 25% respectively, P=0.007).
Table I.
Baseline characteristics and therapies of the study population
| 10-year survivors N=186 | 10-year non-survivors N=647 | P | |
|---|---|---|---|
| Age, years; median (range) | 57 (49–64) | 64 (57–71) | <0.001 |
| Male sex | 52% | 64% | 0.003 |
| Single organ | 47% | 27% | <0.001 |
| Heart involvement | 46% | 86% | <0.001 |
| Kidney involvement | 75% | 57% | <0.001 |
| Liver involvement | 18% | 26% | 0.02 |
| GI involvement | 15% | 18% | 0.31 |
| Nerve involvement | 10% | 20% | <0.001 |
| Mayo 2004 stage I/II/III/IIIB (%) | 35/46/15/4 | 11/33/30/26 | <0.001 |
| Mayo 2012 stage I/II/III/IV (%) | 41/37/13/9 | 12/19/30/39 | <0.001 |
| SBP <100 mmHg | 12% | 25% | <0.001 |
| Seen within 30 days from diagnosis | 47% | 65% | <0.001 |
| Lambda light chain | 76% | 76% | 0.98 |
| BMPC | 7 (5–10) | 10 (6–18) | <0.001 |
| dFLC | 13 (7–33) | 29 (14–73) | <0.001 |
| S-phase greater than 0%# | 33% | 44% | 0.01 |
| Significant immunoparesis^ (negative ARD) | 27% | 42% | <0.001 |
| FISH abnormalities* | |||
| First-line therapy& |
Available for 69% of the patients
Available for 84% of the patients. ARD is a quantifiable measure of immunoparesis, where a negative result is consistent with more pronounced immunoparesis.(Muchtar, et al 2017b)
Available for 19% of the patients
In the 10-year non-survival cohort 78 patients did not receive therapy, 27 patients had unconfirmed treatment and 4 received other forms of therapy than listed in the table.
Abbreviations: ARD: average relative difference; ASCT: autologous stem cell transplantation; BMPC: bone marrow plasma cells; dFLC: difference between involved to uninvolved light chains; FISH: fluorescence in situ hybridisation; GI: gastrointestinal; IMiD: immunomodulatory drug; MDex: melphalan-dexamethasone; MP: melphalan-prednisone; SBP: systolic blood pressure.
At the time of diagnosis, long-term survivors had lower tumour burden, as measured by lower bone marrow plasma cell percentage and the difference between involved to uninvolved light chain (dFLC) – and better lower proliferative cell indices (S-phase) (Table I). Fluorescence in situ hybridisation (FISH) abnormalities were comparable between long-term and non-long term survival groups with regard to t(11;14) and 13q abnormalities. However, trisomies were far less common among 10-year survivors (8% vs 27%, respectively; P=0.003).
Patients who had ASCT as first line treatment were more likely to be long-term survivors. Of all patients who underwent ASCT, 50% survived more than 10 years. In contrast, 10-year survival rates for MDex, bortezomib-based regimens, IMiD-based regimens and single agent dexamethasone/MP were 16%, 27%, 21% and 5%, respectively, which can be partly explained by their advanced stage at diagnosis.
Haematological and Organ Response among long-term survivors
Of the 186 patients who survived 10 years or more, data on haematological response to first line therapy was available for 168 patients (90%) (Table II). Complete response (CR), 62%; very good partial response (VGPR), 25%; partial response (PR), 8%; and 5% no response. All but one of the patients who attained a PR (n=13) or no response (n=8) to first line therapy and survived ≥10 years received additional consolidative therapy. Of these 20 patients, subsequent best haematological response was CR in 45%, VGPR in 40%, PR in 10% and no response in 1 patient. Overall best response for the 10-year survivors was CR in 67%, VGPR in 30% and PR in 2%.
Table II.
Haematological and organ response among the 10-year survivors
| 1st line therapy N (%) | Best response all lines of therapies N (%) | |
|---|---|---|
| Haematological response (n=168) | ||
| Complete response | 104 (62%) | 113 (67%) |
| Very good partial response | 43 (25%) | 51 (30%) |
| Partial response | 13 (8%) | 3 (2%) |
| No response | 8 (5%) | 1 (1%) |
| Organ response (n=165) | ||
| Any organ response | 125 (76%) | 142 (86%) |
| No organ response | 40 (24%) | 23 (14%) |
| Cardiac (n=63) | ||
| Response | 48 (76%) | 51 (81%) |
| No response | 15 (24%) | 12 (19%) |
| Not evaluable (n=22) | ||
| Renal (n=117) | ||
| Response | 92 (79%) | 106 (91%) |
| No response | 25 (21%) | 11 (9%) |
| Not evaluable (n=22) | ||
| Hepatic (n=33) | ||
| Response | 29 (88%) | 31(94%) |
| No response | 4 (12%) | 2 (6%) |
| Not evaluable (n=1) |
Overall, 125/165 (76%) of the long-term survivors who were evaluable for organ response achieved organ response to first line therapy, a proportion which increased to 86% of the evaluable patients when considering all lines of therapy (Table II). Cardiac response was available for assessment in 63/85 (74%) of patients with cardiac involvement. Of the evaluable 63 patients, cardiac response was seen in 76% of patients following first line therapy. When considering all lines of therapy this rate increased to 81%. The median time from treatment initiation to cardiac response was 14 months (range 2–78 months). The median time from treatment initiation to maximal cardiac response was 42 months (range 7–117 months). Of the 139 patients with renal involvement, 117(84%) were evaluable for renal response. Of these, renal response to first line therapy occurred in 79% of patients, a rate that increased to 91% when considering all-time renal response. The median time to renal response was 7 months (range 1–120 months), and the median time to maximal renal response was 47 months (range 2–174 months). Renal replacement therapy (RRT) was performed in 6 patients at diagnosis and in 24 patients during their follow-up (2 patients were renal stage I, 13 patients were renal stage II and 9 patients were renal stage III). Overall, this resulted in RRT requirement in 22% of the patients with renal involvement who survived 10 years or more. The median time from diagnosis to RRT among the 24 patients who required RRT in their follow-up was 5.6 years (IQR 1.6–8.6). Hepatic response was available for assessment in 97% of patients with liver involvement. Hepatic response to first line was seen in 88% of patients and increased to 94% for all-time hepatic response. The median time to hepatic response (50% reduction in serum alkaline phosphatase) was 15 months (range 1–40 months), while the median time to maximal hepatic response was 49 months (range 12–140 months).
Time to treatment failure and subsequent therapies among 10-year survivors
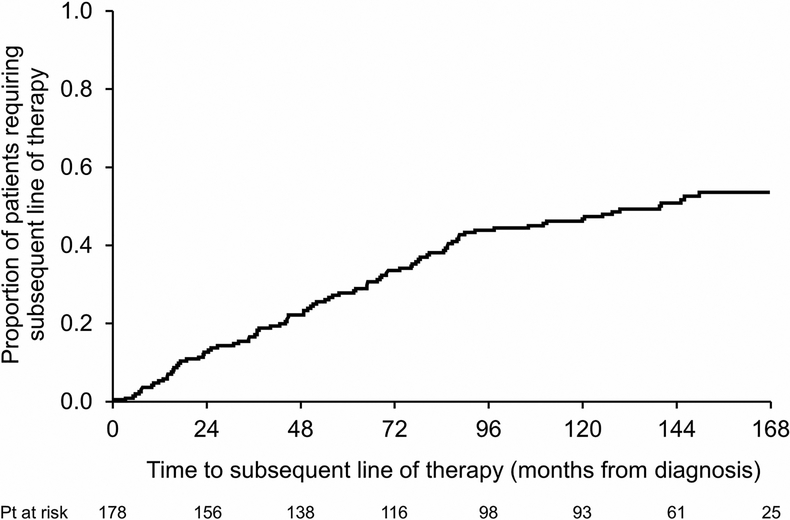
Details on subsequent therapies were available for 174 patients (94%). The time to next therapy curve is depicted in Figure 1. Eighty-two patients (47%) did not receive subsequent therapy throughout their entire follow-up. In the first 5 years from diagnosis, 28% received additional clone-directed therapy. The other 25% required therapy beyond the 5-year mark (range 5.2–14.4 years). Of those requiring a second line of therapy or more (n=92, 53% of the cohort), 40% received one subsequent line of therapy, 29% two subsequent lines of therapies and 31% more than two subsequent lines of therapy (range 3–7). By the end of follow-up, 30% of all patients who required second line therapy or more remained on anti-plasma cell treatment. Of the 219 subsequent lines of therapy given to 92 patients, the most commonly used regimen was a proteasome inhibitor-based therapy (mainly with bortezomib) given to 44% of patients, followed by IMiD-based (27%), Melphalan-based (10%), other (8%), ASCT (6%) and daratumumab-based (5%).
Figure 1:
Time to next therapy
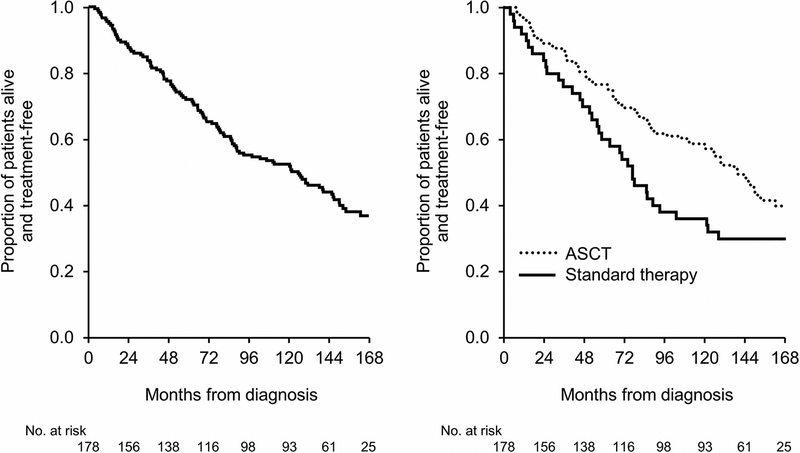
For the long-term survivors, the median TFS was 10.5 years (IQR 7.4–12.2) (Figure 2a). Separated by treatment (Figure 2b), TFS was 11.7 years for the ASCT group and 6.4 years for the non-ASCT group (P=0.02). Correspondingly, the 1-year, 2-year, 5-year and 10-year TFS rates among ASCT and non-ASCT groups were 96%, 88%, 76% and 58% versus 89%, 81%, 57% and 36%, respectively.
Figure 2:
Treatment-free survival: A. Entire Cohort. B. By autologous stem cell transplantation (ASCT) and standard therapy sub-categorization
On multivariate analysis, the achievement of complete haematological response and lack of cardiac involvement predicted for TFS (Table III).
Table III.
Predictors of treatment-free survival among 186 10-year survivors in a uni- and multivariate analyses
| Variable | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Male sex | 1.6 (1.1–2.4) | 0.01 | 1.3 (0.8–2.0) | 0.25 |
| Age ≥65 years | 1.2 (0.8–1.9) | 0.35 | Not included | |
| >1 organ | 1.2 (0.8–1.8) | 0.33 | Not included | |
| Cardiac involvement | 1.4 (0.95–2.1) | 0.07 | 1.6 (1.0–2.5) | 0.05 |
| Renal involvement | 0.7 (0.5–1.0) | 0.06 | 0.9 (0.6–1.5) | 0.71 |
| >10% BMPCs | 1.4 (0.9–2.1) | 0.1 | 1.1 (0.7–1.8) | 0.71 |
| dFLC >18 | 1.1 (0.7–1.7) | 0.63 | Not included | |
| ASCT | 0.6 (0.4–0.9) | 0.02 | 1.1 (0.7–1.7) | 0.81 |
| Complete response | 0.3 (0.2–0.4) | <0.001 | 0.3 (0.2–0.5) | <0.001 |
Abbreviations: ASCT: autologous stem cell transplantation; BMPC: bone marrow plasma cells; CI: confidence interval; dFLC: difference between involved to uninvolved light chains; HR: hazard ratio.
Discussion
In this study we explored the characteristics of AL amyloidosis patients achieving long-term survival, defined in this study as 10 years or more from the time of diagnosis. Not only does this study reinforce the value of known prognostic factors, including cardiac stage, age, tumour burden, haematological and organ response, but it also clarifies treatment patterns among these exceptional 22% of patients. It is worth noting that a prior Mayo study from the years 1966 to 1987 reported that only 5% of patients reached the 10-year survival mark.(Kyle, et al 1999) This represents a marked improvement in prognosis, driven by better therapies and increase in disease awareness and recognition. Despite improvement in disease management, early diagnosis remains a paramount goal in order to achieve a long-term survival.(Merlini, et al 2018) As patients were selected based on year of diagnosis to allow 10-year follow-up, we anticipate these figures to increase with the improvement in survival in recent years. (Muchtar, et al 2017a)
It was notable that one-third of these long-term survivors never achieved a CR; a VGPR was sufficient in 30%. Moreover, 11% required a consolidative strategy for inadequate response to first-line therapy. In addition, 14% did not achieve an organ response and renal replacement therapy was required in 22% of the patients with renal involvement. Also notable is that 53% of these exceptional patients required a second-line therapy at a median of 10.5 years (IQR 7.4–12.2). Re-treatment may be needed even after years of uneventful monitoring. Therefore, AL patients require continuous monitoring for disease recurrence which should not be limited by time.
Patients who were referred within 30 days of their diagnosis were more likely to succumb to their disease and to not achieve long-term survival. A similar finding was observed in our institution 3 decades ago.(Kyle and Gertz 1995) These early referrals were a sicker population with advanced stage at the time of diagnosis and their early referral was probably due to the gravity of their symptoms. However, available interventions cannot easily salvage those with advanced disease, thus emphasizing that early referral cannot substitute for early disease recognition.
Long-term survivors had more limited organ involvement and were less likely to have cardiac, liver and nerve involvement, emphasizing the impact of organ involvement on survival. However, it is also important to note that long-term survivors also have distinct plasma cell clone features. Bone marrow plasma cell burden and the proliferation index were lower and dFLC was smaller in size. Significant immunoparesis, an adverse factor in AL amyloidosis,(Muchtar, et al 2017b, Muchtar, et al 2016b, Muchtar, et al 2016c, Rodriguez-Lobato, et al 2017, Sachchithanantham, et al 2017) was also less frequent among long term survivors compared to those who did not. This observation emphasizes that long-term survival is influenced by the characteristics of the clonal process, whereas short-term survival is more influenced by organ involvement, mainly the heart. We recently demonstrated that the European modification of the Mayo 2004 model, which relies solely on cardiac biomarkers, has the best prediction for short-term survival, whereas Mayo 2012 model, which includes the involved light chain concentration in addition to cardiac biomarkers, has better prediction for long-term survival.(Muchtar, et al 2019) Therefore, the clonal plasma cell process seems to interact with prognosis mainly for the long-term outcome. This interplay between the systemic amyloid deposition and the bone marrow clonal process increases the complexity of AL amyloidosis and challenges our attempts to improve outcomes.
FISH findings are an important tool for prediction of progression and survival in plasma cell disorders. However, their role in prognosis in AL amyloidosis is less clear, partially due to the interplay between the clonal plasma cell process and the organ dysfunction. For example, t(11;14), a FISH abnormality suggested to pose inferior outcomes in bortezomib-treated patients (Bochtler, et al 2015), has an adverse impact mainly among favourable prognosis patients.(Muchtar, et al 2017c) In this study, we could not demonstrate a difference in long-term survival with regard to the presence or absence of t(11;14). As our population was selected based on era of therapy, it is unclear whether the growing use of bortezomib in the past years will have an impact on long-term outcomes. In contrast, the presence of trisomies has prognostic impact on long-term survival. AL patients with trisomies have higher baseline clonal burden, which makes disease recurrence more likely.(Warsame, et al 2015) This finding is in line with the impact of trisomies in monoclonal gammopathy of undetermined significance(Lakshman, et al 2018) and smouldering myeloma (Neben, et al 2013, Rajkumar, et al 2013) disorders, in which trisomies confer a higher risk for progression to myeloma.
Limitations of this study include its retrospective design and lack of complete data for all patients. By definition, the most modern patients were excluded to allow for 10-year follow-up, which may thereby underrepresent the anticipated 10-year survivorship of patients diagnosed in 2018. Finally, we could not control for referral bias, which limits the generalization of this study to other referral and non-referral centres.
In conclusion, long-term survival in AL amyloidosis patients is better than before and rates are anticipated to further increase. Nearly half of 10-year survivors did not require further chemotherapy during their follow-up. The features of the underlying clonal plasma cell disorder become meaningful for long-term survival, particularly in regard to a lower proportion of patients with trisomies, which should be a subject for further investigation.
Acknowledgements
The study was supported in part by the Jabbs Foundation (Birmingham, United Kingdom), the Henry J. Predolin Foundation (USA), and National Institutes of Health National Cancer Institute grant P50 CA186781.
Footnotes
Conflict-of-interest disclosure:
Eli Muchtar: None; Morie A. Gertz: Consultancy (Milleniu) and honoraria (Celgene, Millenium, Onyx, Novartis, Smith Kline, Prothena, Ionis). Martha Q. Lacy: Research funding (Celgene); Ronald S. Go: None; Francis Buadi: None; David Dingli: Research funding (Karyopharm Therapeutics, Amgen, Millenium Pharmaceuticals); Martha Grogan: None; Omar F. Abou Ezzeddine: None; Suzanne R. Hayman: None; Prashant Kapoor: Research funding (Takeda, Celgene, Amgen); Nelson Leung: None; Amie Fonder: None; Miriam Hobbs: None; Yi Lisa Hwa: None; Wilson Gonsalves: none; Rahma Warsame: None; Taxiarchis v. Kourelis: None; Stephen Russel: None; John A. Lust: None; Yi Lin: None; Steven Zeldenrust: None; Robert A. Kyle: None; Vincent Rajkumar: None; Shaji Kumar: Consultancy (Celgene, Millennium, Onyx, Janssen, BMS); and research funding (Celgene, Millennium, Novartis, Onyx AbbVie, Janssen, BMS). Angela Dispenzieri: Research funding (Celgene, Millennium, Pfizer, Janssen), Travel grant (Pfizer)
References:
- Bochtler T, Hegenbart U, Kunz C, Granzow M, Benner A, Seckinger A, Kimmich C, Goldschmidt H, Ho AD, Hose D, Jauch A & Schonland SO (2015) Translocation t(11;14) is associated with adverse outcome in patients with newly diagnosed AL amyloidosis when treated with bortezomib-based regimens. Journal of clinical oncology : official journal of the American Society of Clinical Oncology, 33, 1371–1378. [DOI] [PubMed] [Google Scholar]
- Gertz MA, Comenzo R, Falk RH, Fermand JP, Hazenberg BP, Hawkins PN, Merlini G, Moreau P, Ronco P, Sanchorawala V, Sezer O, Solomon A & Grateau G (2005) Definition of organ involvement and treatment response in immunoglobulin light chain amyloidosis (AL): a consensus opinion from the 10th International Symposium on Amyloid and Amyloidosis, Tours, France, 18–22 April 2004. American journal of hematology, 79, 319–328. [DOI] [PubMed] [Google Scholar]
- Kyle RA & Gertz MA (1995) Primary systemic amyloidosis: clinical and laboratory features in 474 cases. Semin Hematol, 32, 45–59. [PubMed] [Google Scholar]
- Kyle RA, Gertz MA, Greipp PR, Witzig TE, Lust JA, Lacy MQ & Therneau TM (1999) Long-term survival (10 years or more) in 30 patients with primary amyloidosis. Blood, 93, 1062–1066. [PubMed] [Google Scholar]
- Lakshman A, Paul S, Rajkumar SV, Ketterling RP, Greipp PT, Dispenzieri A, Gertz MA, Buadi FK, Lacy MQ, Dingli D, Fonder AL, Hayman SR, Hobbs MA, Gonsalves WI, Hwa YL, Kapoor P, Leung N, Go RS, Lin Y, Kourelis TV, Warsame R, Lust JA, Russell SJ, Zeldenrust SR, Kyle RA & Kumar SK (2018) Prognostic significance of interphase FISH in monoclonal gammopathy of undetermined significance. Leukemia, 32, 1811–1815. [DOI] [PubMed] [Google Scholar]
- Merlini G, Dispenzieri A, Sanchorawala V, Schonland SO, Palladini G, Hawkins PN & Gertz MA (2018) Systemic immunoglobulin light chain amyloidosis. Nat Rev Dis Primers, 4, 38. [DOI] [PubMed] [Google Scholar]
- Muchtar E, Buadi FK, Dispenzieri A & Gertz MA (2016a) Immunoglobulin Light-Chain Amyloidosis: From Basics to New Developments in Diagnosis, Prognosis and Therapy. Acta haematologica, 135, 172–190. [DOI] [PubMed] [Google Scholar]
- Muchtar E, Dispenzieri A, Kumar SK, Dingli D, Lacy MQ, Buadi FK, Hayman SR, Kapoor P, Leung N, Chakraborty R, Russell S, Lust JA, Lin Y, Go RS, Zeldenrust S, Kyle RA, Rajkumar SV & Gertz MA (2016b) Immunoparesis status in immunoglobulin light chain amyloidosis at diagnosis affects response and survival by regimen type. Haematologica, 101, 1102–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muchtar E, Magen H, Itchaki G, Cohen A, Rosenfeld R, Shochat T, Kornowski R, Iakobishvili Z & Raanani P (2016c) Uninvolved immunoglobulins predicting hematological response in newly diagnosed AL amyloidosis. Leuk Res, 41, 56–61. [DOI] [PubMed] [Google Scholar]
- Muchtar E, Gertz MA, Kumar SK, Lacy MQ, Dingli D, Buadi FK, Grogan M, Hayman SR, Kapoor P, Leung N, Fonder A, Hobbs M, Hwa YL, Gonsalves W, Warsame R, Kourelis TV, Russell S, Lust JA, Lin Y, Go RS, Zeldenrust S, Kyle RA, Rajkumar SV & Dispenzieri A (2017a) Improved outcomes for newly diagnosed AL amyloidosis between 2000 and 2014: cracking the glass ceiling of early death. Blood, 129, 2111–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muchtar E, Dispenzieri A, Kumar SK, Buadi FK, Lacy MQ, Zeldenrust S, Hayman SR, Leung N, Kourelis TV, Gonsalves W, Chakraborty R, Russell S, Dingli D, Lust JA, Lin Y, Kapoor P, Go R, Kyle RA, Rajkumar SV & Gertz MA (2017b) Immunoparesis in newly diagnosed AL amyloidosis is a marker for response and survival. Leukemia, 31, 92–99 [DOI] [PubMed] [Google Scholar]
- Muchtar E, Dispenzieri A, Kumar SK, Ketterling RP, Dingli D, Lacy MQ, Buadi FK, Hayman SR, Kapoor P, Leung N, Chakraborty R, Gonsalves W, Warsame R, Kourelis TV, Russell S, Lust JA, Lin Y, Go RS, Zeldenrust S, Kyle RA, Rajkumar SV & Gertz MA (2017c) Interphase fluorescence in-situ hybridization (iFISH) in untreated AL amyloidosis has an independent prognostic impact by abnormality type and treatment category. Leukemia. 31, 1562–1569. [DOI] [PubMed] [Google Scholar]
- Muchtar E, Therneau TM, Larson DR, Gertz MA, Lacy MQ, Buadi FK, Dingli D, Hayman SR, Kapoor P, Gonsalves W, Kourelis TV, Warsame R, Fonder A, Hobbs M, Hwa YL, Leung N, Russell S, Lust JA, Lin Y, Go RS, Zeldenrust S, Kyle RA, Rajkumar SV, Kumar SK & Dispenzieri A (2019) Comparative analysis of staging systems in AL amyloidosis. Leukemia, 33, 811–814. [DOI] [PubMed] [Google Scholar]
- Neben K, Jauch A, Hielscher T, Hillengass J, Lehners N, Seckinger A, Granzow M, Raab MS, Ho AD, Goldschmidt H & Hose D (2013) Progression in smoldering myeloma is independently determined by the chromosomal abnormalities del(17p), t(4;14), gain 1q, hyperdiploidy, and tumor load. Journal of clinical oncology : official journal of the American Society of Clinical Oncology, 31, 4325–4332. [DOI] [PubMed] [Google Scholar]
- Palladini G, Dispenzieri A, Gertz MA, Kumar S, Wechalekar A, Hawkins PN, Schonland S, Hegenbart U, Comenzo R, Kastritis E, Dimopoulos MA, Jaccard A, Klersy C & Merlini G (2012) New criteria for response to treatment in immunoglobulin light chain amyloidosis based on free light chain measurement and cardiac biomarkers: impact on survival outcomes. Journal of clinical oncology : official journal of the American Society of Clinical Oncology, 30, 4541–4549. [DOI] [PubMed] [Google Scholar]
- Palladini G, Hegenbart U, Milani P, Kimmich C, Foli A, Ho AD, Vidus Rosin M, Albertini R, Moratti R, Merlini G & Schonland S (2014) A staging system for renal outcome and early markers of renal response to chemotherapy in AL amyloidosis. Blood, 124, 2325–2332. [DOI] [PubMed] [Google Scholar]
- Rajkumar SV, Gupta V, Fonseca R, Dispenzieri A, Gonsalves WI, Larson D, Ketterling RP, Lust JA, Kyle RA & Kumar SK (2013) Impact of primary molecular cytogenetic abnormalities and risk of progression in smoldering multiple myeloma. Leukemia, 27, 1738–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Lobato LG, Fernandez de Larrea C, Cibeira MT, Tovar N, Isola I, Arostegui JI, Rosinol L, Diaz T, Lozano E, Yague J & Blade J (2017) Prognostic impact of immunoparesis at diagnosis and after treatment onset in patients with light-chain amyloidosis. Amyloid : the international journal of experimental and clinical investigation : the official journal of the International Society of Amyloidosis, 24, 245–252. [DOI] [PubMed] [Google Scholar]
- Sachchithanantham S, Berlanga O, Alvi A, Mahmood SA, Lachmann HJ, Gillmore JD, Hawkins PN, Harding S & Wechalekar AD (2017) Immunoparesis defined by heavy+light chain suppression is a novel marker of long-term outcomes in cardiac AL amyloidosis. British journal of haematology, 179, 575–585. [DOI] [PubMed] [Google Scholar]
- Warsame R, Kumar SK, Gertz MA, Lacy MQ, Buadi FK, Hayman SR, Leung N, Dingli D, Lust JA, Ketterling RP, Lin Y, Russell S, Hwa L, Kapoor P, Go RS, Zeldenrust SR, Kyle RA, Rajkumar SV & Dispenzieri A (2015) Abnormal FISH in patients with immunoglobulin light chain amyloidosis is a risk factor for cardiac involvement and for death. Blood cancer journal, 5, e310. [DOI] [PMC free article] [PubMed] [Google Scholar]