Abstract
Pancreas divisum, the most common congenital malformation of the pancreas, occurs due to a failure of fusion of the ductal systems of the dorsal and ventral pancreatic buds in the seventh week of intra-uterine life. This leads to a dominant dorsal pancreatic duct draining though the minor papilla and a small ventral pancreatic duct draining through the major papilla. The prevalence in western populations is about 10% and more than 95% of these patients are without pancreatic symptoms, with the anomaly found incidentally on abdominal imaging for an unrelated indication. The etiological role and clinical significance of pancreas divisum in relation to pancreatic disease has not yet been clearly defined, but may predispose to pancreatic disease in co-existence with other factors. Secretin-enhanced Magnetic Resonance Cholangiopancreatography is the non-invasive imaging modality of choice to identify pancreas divisum. Patients may be offered minor papilla therapy when they present with recurrent acute pancreatitis, severe acute pancreatitis and can be considered for therapy in the setting of chronic pancreatitis and chronic abdominal pain of pancreatic origin. Minor papilla endotherapy (sphincterotomy and/or stenting) via Endoscopic Retrograde Cholangiopancreatography and minor papilla surgical therapy have comparable outcomes with endotherapy typically considered first-line due to a favorable adverse event profile. The response to therapy is variable with maximal benefit seen in patients with recurrent acute pancreatitis and least with chronic pancreatic-type abdominal pain. Data supporting either therapy are of low quality as they are predominantly retrospective with a sub-optimal follow up period. Surgical options including a pancreatojejunostomy (Puestow or Frey procedure) or a total pancreatectomy with auto-islet cell transplantation may be considered in a subset of patients.
Keywords: Recurrent Acute Pancreatitis, Chronic Pancreatitis, Idiopathic Pancreatitis, Pancreas Divisum, Dorsal Duct Syndrome, Endoscopic retrograde cholangiopancreatography, Magnetic Resonance Cholangiopancreatography
Introduction
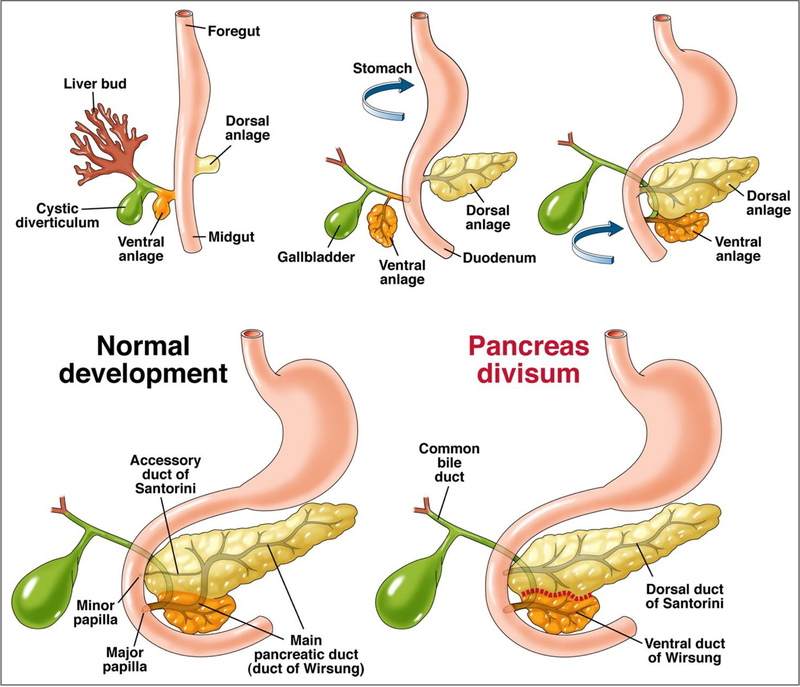
Pancreas Divisum (PD) is a fusional abnormality from failure of fusion of the ductal systems of the dorsal and ventral buds of the embryonic pancreas approximately in the seventh week of intrauterine life[1] [Figure 1]. It is a proposed etiology for Idiopathic Recurrent Acute Pancreatitis (I-RAP) and Chronic Pancreatitis (CP) but, thus far, the exact extent of its clinical significance has not been quantifiable.
Figure 1:
Rotation of the ventral pancreatic bud/anlage (from which originates the hepatobiliary system) and fusion with the dorsal pancreatic bud/anlage in the foregut. The failure of fusion of the ductal systems of the dorsal and ventral anlage results in pancreas divisum with the majority of the pancreas draining via the dorsal duct of Santorini through the minor papilla.
Epidemiology, Embryogenesis, Subtypes
PD is a the most common congenital pancreatic anatomic anomaly with a prevalence of about 6–10% based on autopsy data[2, 3], imaging[4] and endoscopic studies[5, 6], with most patients (more than 95%) being without pancreatic symptoms and found incidentally. The frequency is lower in Asians[6, 7] and Africans[2] at about 1–2%. It has been postulated that a small subset of patients with PD are predisposed to recurrent attacks of pancreatic-type abdominal pain, acute pancreatitis (AP) and associated complications such as duct disruption, duct disconnection, necrosis, pseudocysts, and eventually CP and its associated morbidity due to exocrine and endocrine insufficiency, metabolic derangements and chronic abdominal pain[8, 9, 10, 11]. Studies have shown that PD is prevalent in about 25–50% of the patients being evaluated for I-RAP[5, 12].
The normal pancreas is formed by rotation of the ventral pancreatic bud (from which originates the hepatobiliary system) and fusion with the dorsal pancreatic bud in the foregut [Figure 1]. The ventral bud forms a majority of the head of the pancreas as well as the uncinate process (<25% of pancreatic parenchyma), with the dorsal bud contributing a minority to the head and all of the body and tail of the pancreas. The ductal system of the ventral bud (duct of Wirsung) and the dorsal bud (duct of Santorini) fuse together to form the main pancreatic duct of Wirsung that opens at the major papilla along with the common bile duct. The accessory duct of Santorini often persists with varying patency and length, draining from the main duct to the minor papilla[13, 14] [Figure 1]. The etiology for PD is the failure of fusion of the two ductal systems to a varying degree leading to three known variants/subtypes.
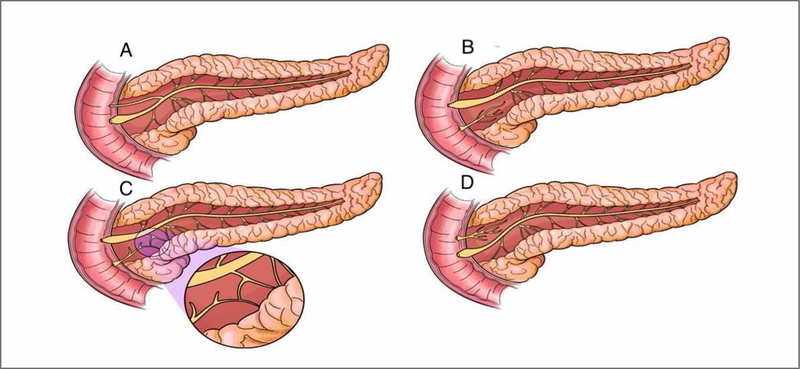
Complete (Classic) PD
The ventral duct system is short and opens into the major papilla, while the longer dorsal duct system opens into the minor papilla with no communication between the two ducts. It is the major variant with an approximate prevalence of 70%[3, 15]. [Figure 2B, Figure 3]
Figure 2:
A - Normal Pancreas; B - Classic Pancreas Divisum; C – Incomplete Pancreas Divisum (magnified image demonstrates the small communication between the dorsal and ventral pancreatic ductal systems); D – Reverse Pancreas Divisum
Figure 3:
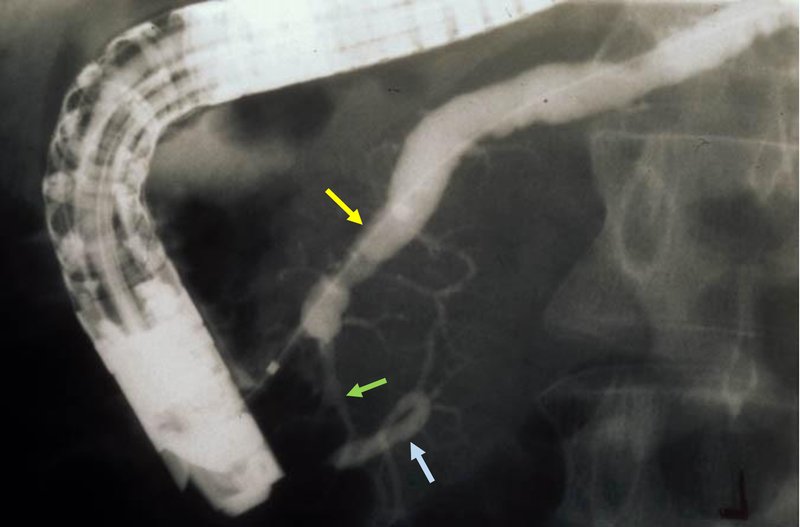
The dorsal pancreatic duct (yellow arrow) crosses the bile duct (blue arrow) and empties at a separate orifice/minor papilla (green arrow).
Incomplete PD
A similar anatomy to the classic PD except for a small branch of communication between the two systems. This can lead to opacification of the dorsal system during injection of contrast in the ventral duct during Endoscopic Retrograde Pancreatography (ERP). The prevalence of this variant is about 15%[16, 17, 18]. [Figure 2C, Figure 4]
Figure 4:
Incomplete Pancreas Divisum - Dorsal pancreatic duct (yellow arrow) is cannulated and opacified from the minor papilla with contrast passing into the ventral pancreatic duct (blue arrow) through a small communication between the two systems (green arrow).
Reverse PD
This is a rare variant wherein the accessory duct of Santorini does not communicate with the main pancreatic duct, leading to a small isolated component of the dorsal pancreas. The interruption in drainage system of the accessory duct in reverse PD can frequently be interpreted as an obstruction by a malignant or a benign stricture. [Figure 2D]
The clinical behavior of complete and incomplete PD is similar except for the variation at the time of ERP as noted [19, 20]. However, a reverse PD behaves more like a normal pancreas wherein the severity of gallstone pancreatitis (from impaction of a gallstone at the major papilla) can be much worse as a significant portion of the pancreas drains through the major papilla unlike the preceding two variants where only a minority drains through the major papilla.
Pseudo/False PD
An obstruction of the main pancreatic duct of Wirsung downstream to the origin of the accessory duct of Santorini in a normal pancreas from injury due to acute pancreatitis, a stricture due to chronic pancreatitis or a malignancy can give the appearance of true pancreas divisum. This obstruction of the ventral duct in the head can lead to a decrease in its size and compensatory increase in size of the accessory duct draining the body and tail mimicking the anatomic findings of PD on imaging and ERP. It is important to recognize this condition due to the possibility of malignancy.
Associated Pancreatic-Biliary Anomalies
PD has been found to be associated with other pancreatic and biliary anomalies. The most frequent association is with annular pancreas where there is an abnormal rotation of the ventral bud of the pancreas around the duodenum, leading to a ring or semi-ring of pancreatic tissue around the second part of the duodenum[21]. Prior studies suggested that there is about a 30–38% co-existence between the two conditions[21, 22, 23] but a recent larger study suggested the prevalence is as high as 50%[24]. There have reports scattered in the literature with PD co-existing with anomalous pancreatic-biliary junction (APBJ) and choledochal cysts[25, 26, 27, 28, 29]. However, there have been no large-scale studies to support any specific associations between these congenital anomalies. It has also been reported recently that there is a higher prevalence of pancreatic and biliary tumors in patients with PD than with a normal ductal system. (7.8% vs 3.5%)[30]. There have been reports of partial agenesis of the dorsal pancreas with PD with some debate as to whether the agenesis led to the PD or a consequence[14, 31].
Pathogenesis of Pancreatitis
The majority of patients with PD are without pancreas symptoms and therefore there is considerable debate as to whether it is causally associated with pancreatitis or pancreatic-type abdominal pain. It has been theorized that a larger and longer dominant dorsal duct opening through a relatively smaller or stenotic minor papilla could lead to inadequate drainage of pancreatic secretions with obstruction of flow. The resultant increase in intraductal pressure and distention of the dorsal duct could then potentially lead to abdominal pain and even pancreatitis. As a consequence of this imbalance, the term “dominant duct syndrome” has been coined in the literature[32, 33]. Manometry studies have shown an increased pressure in the dorsal duct and minor papilla when compared to the ventral duct and major papilla in PD suggestive of dorsal duct and minor papilla hypertension[34]. It has been suggested that this obstruction is transient and could be related to intermittent plugging of the minor papilla by proteinaceous material in the pancreatic secretions[9]. However, the large majority of patients with a dilated dorsal duct in PD are asymptomatic, suggestive of a poor correlation between obstruction and symptoms[35].
Some observational studies of patients with I-RAP and CP have shown that the frequency of pancreatitis was similar in patients with PD when compared to patients with normal duct variants[8, 36, 37]. However, other studies have shown an increased prevalence of PD (complete and incomplete) in adults[12, 38, 39, 40] and children[41] being evaluated for I-RAP and CP, again supporting PD as a potentially pathologic entity. Similar support for pancreas divisum as a potential pathologic entity is provided by studies that have shown pathological changes of pancreatitis limited to the dorsal pancreas (i.e normal ventral pancreas) in patients with PD[40, 42]. Studies have shown inconsistent and variable response to minor papilla and dorsal duct therapy in patients who present with pancreatic-type pain, with suboptimal results in the majority of patients[43, 44, 45, 46, 47, 48, 49, 50].
This heterogeneity suggests that PD may not be a primary driving force of pancreatic pathology but rather a co-factor which in association with other factors leads to pancreatic disease[51]. The basis for this line of thought comes from studies that show the prevalence of I-RAP and CP is as high as 50% in adults and children with PD when associated with certain genetic mutations of serine protease inhibitor Kazal type 1 gene (SPINK1), cystic fibrosis transmembrane conductance regulator gene (CFTR), chymotrypsin C gene (CTRC)[36, 41, 52, 53, 54]. These gene mutations or polymorphisms have been reported to have an independent but not proven causal association with pancreatic disease[55, 56, 57]. Additionally, it has also been postulated that the presence of PD reduces the threshold for pancreatic disease from other known primary factors of pancreatitis such as alcohol, medications and trauma[34].
Symptomatology and Diagnosis
The spectrum of symptoms in patients with PD is similar to what is encountered in patients with normal pancreatic ductal anatomy, ranging from recurrent pancreatic-type abdominal pain of varying frequency, chronic pancreatic-type abdominal pain, recurrent attacks of mild to severe acute pancreatitis to chronic pancreatitis and their associated respective complications.
PD is frequently identified incidentally on cross-sectional abdominal imaging for unrelated indications and symptoms and further evaluation is usually not indicated or pursued.
Contrast-enhanced computed tomography (CT) may help identify variations in pancreatic ductal anatomy but has a low sensitivity (50–60%)[58, 59] especially when the ductal system can become obscured by pancreatic disease like pancreatitis and its complications. When the ducts are visualized, the sensitivity does increase to about 83% and the anatomy is classically described as the dorsal duct crossing anteriorly and superiorly to the distal common bile duct in the pancreatic head and opening separately into the minor papilla with a small distinct ventral duct opening into the major papilla[60]. Dynamic CT with secretin provocation (S-CT) can increase the visibility of the ducts when not visualized on initial imaging with the sensitivity matching the previously described 83% with no immediate complications[59].
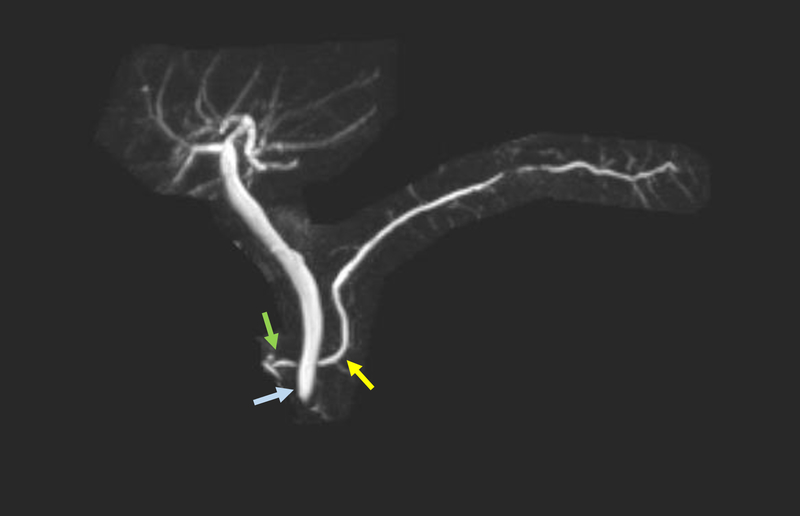
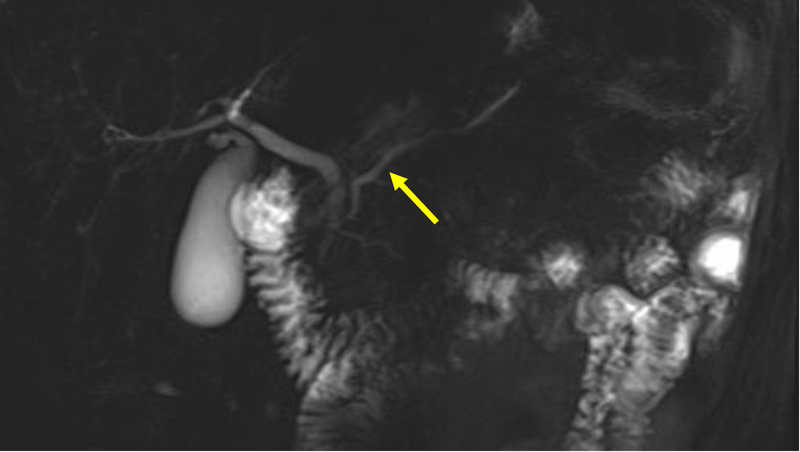
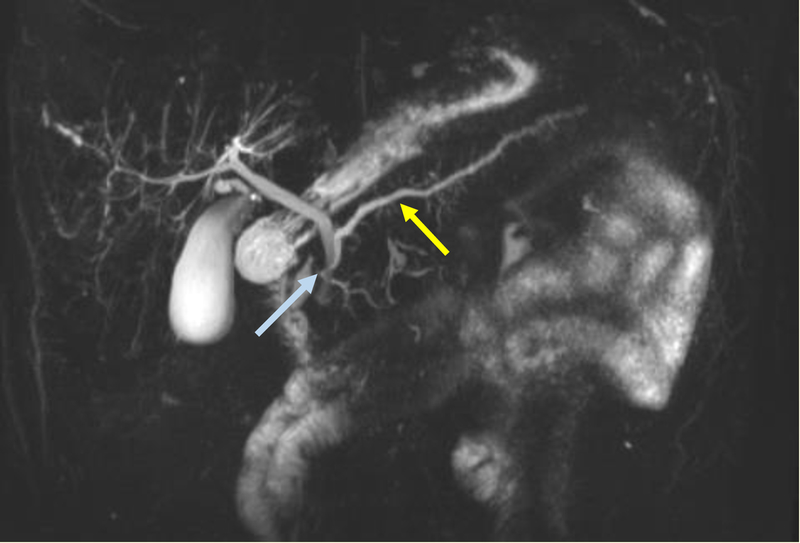
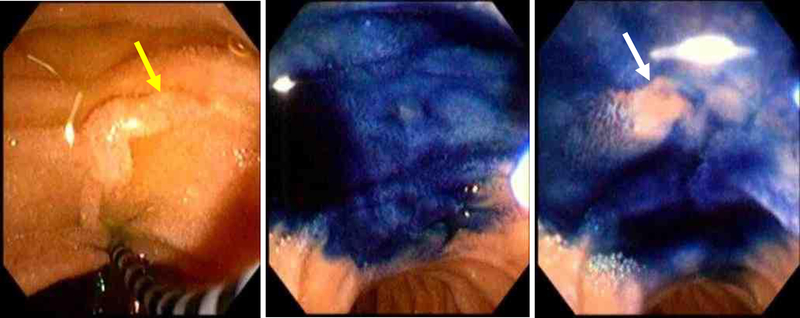
Contrast-enhanced magnetic resonance cholangiopancreatography (MRCP) [Figure 5] is reported to have sensitivity of about 50–70%[60, 61, 62, 63, 64, 65]. However, individual studies have shown the sensitivity increases to up to 83–86% with a specificity of 97–99% with secretin-enhanced MRCP (S-MRCP) [Figure 6][66, 67, 68, 69, 70, 71] with systematic reviews and meta-analyses validating the results[64, 65, 72]. Secretin has also been shown to be associated with minor side effects, but generally is well tolerated with no reported events of exacerbating or precipitating pancreatic disease[71]. The description of the ductal anatomy is similar to what is described on CT images[63]. Cystic dilation of the terminal dorsal duct near the opening of the minor papilla described as a “Santorinicele” can also be visualized on an S-MRCP[73, 74].
Figure 5:
This non-secretin stimulated MRCP shows an irregular dorsal main pancreatic duct (yellow arrow).
Figure 6:
This secretin-stimulated MRCP shows a dilated and irregular dorsal pancreatic duct (yellow arrow) crossing the bile duct and opening separately at the minor papilla (blue arrow). Irregular side branches are also seen and are more prominent than the non-secretin enhanced image (Figure 5).
Endoscopic Ultrasound (EUS) has also been reported to have a high diagnostic accuracy for PD with a sensitivity of 87%−95%[60, 75, 76] with secretin enhancement (S-EUS) offering marginal benefit[77]. The absence of a “stack sign” (distal common bile duct, ventral pancreatic duct and portal vein can be seen to run on a parallel axis in a normal pancreas), the presence of a “crossed duct sign” (dorsal pancreatic duct will appear to cross over the bile duct anteriorly and superiorly) are indicative of PD[78].
Systematic reviews suggest S-MRCP has a superior accuracy to EUS and is the preferred diagnostic modality of choice[65, 72] with one study suggesting no significant difference between S-MRCP and S-EUS[79].
Secretin-Enhanced Ultrasound (S-US) has been used in the past with studies showing significant heterogeneity in regards to dilation of the dorsal duct in response to secretin and the ability to predict the anatomy and response to treatment[80, 81, 82]. Factors such as body habitus and overlying bowel gas have also led to variable accuracy and, therefore, S-US has fallen out of favor.
Endoscopic retrograde cholangiopancreatography (ERCP) is considered the gold-standard for diagnosis of PD[83, 84] but is seldom used currently when therapy is not planned given the radiation exposure, procedure- and anesthesia-related complications and the high accuracy of S-MRCP.
Secretin augments the visualization of the pancreatic ductal system by stimulating the production of bicarbonate and water from the exocrine pancreas and thereby increasing the volume of pancreatic secretions[85]. While the accuracy of all secretin augmented studies is lower in patients with chronic pancreatitis and pancreatic exocrine insufficiency, secretin-enhanced images may still yield more information than non-secretin studies.
Management
Asymptomatic patients with PD found incidentally on imaging do not require any further diagnostic evaluation or therapeutic management.
In symptomatic patients, the choice of therapy and degree of intervention depends on the frequency, duration and intensity of symptoms and presence of complications. The approach and options for therapy of pancreatic disease remain the same irrespective of the variations in the pancreatic ductal system with a few exceptions/modifications for PD.
In patients with infrequent or mild symptoms of pancreatic-type abdominal pain and no radiological features to suggest chronic pancreatitis, a conservative approach directed towards symptom management would be preferred over an intervention of the minor papilla or the dorsal pancreatic duct. Options include a low-fat diet (50gms/day), non-narcotic analgesics, anti-spasmodic/anti-cholinergic medications and pancreatic enzyme supplementation.
In patients with acute pancreatitis and its complications, chronic pancreatitis and its complications leading to significant functional impairment, a comprehensive evaluation for the underlying etiology should be undertaken. This should include a detailed social and family history, medication history, genetic testing and evaluation of metabolic causes of pancreatic disease and direct therapy towards mitigation of the inciting etiology.
Therapeutic intervention is reserved for patients with recurrent attacks of acute pancreatitis regardless of severity and can be considered in patients with one attack of severe pancreatitis with no other identifiable cause for the disease. Therapy is also offered in chronic pancreatitis if a modifiable target such as a stone, stricture or dilated dorsal duct can be identified. More commonly, in the setting of PD and chronic pancreatitis, the entire gland is involved with changes of chronic pancreatitis and treatment should be recommended as it would in patients with normal pancreatic duct anatomy.
The primary management when the dorsal duct is normal, near normal or just dilated is directed towards relieving obstruction at the level of the minor papilla. Options for therapy include endoscopy (i.e. ERCP) and surgery. The choice of therapy depends on patient profile and preference as well as local institutional expertise. It is important not to ignore the standard management principles of acute pancreatitis as well.
Endoscopic Therapy
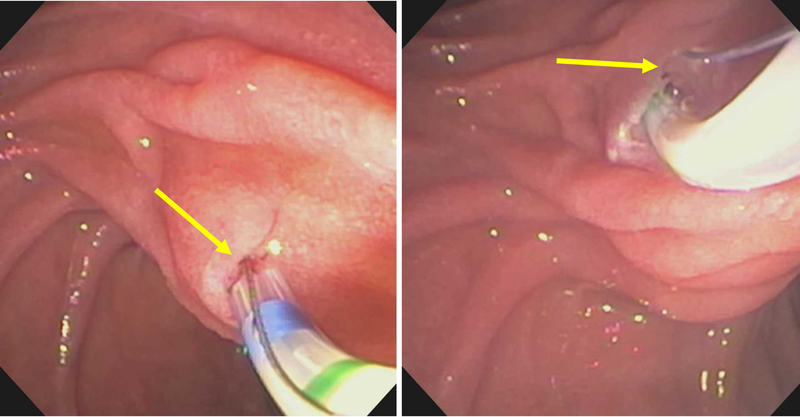
Endoscopic therapy includes minor papilla endoscopic sphincterotomy(mPES), minor papilla orifice balloon dilation (the term sphincteroplasty is present in the literature but should not be used in this setting) and trans minor papilla dorsal duct stenting. Techniques for cannulation of the minor papilla to achieve endotherapy have been well described[83, 84, 86, 87]. Cannulation is best achieved when the duodenoscope is pushed along the greater curvature of the stomach into what is described as a long position (80–90cms from incisors) [Figure 7]. Once deep minor papilla cannulation is achieved, the duodenoscope may be returned to the short position off the greater curve of the stomach (55–60cms from incisors) to improve stability and manipulation of instruments [Figure 8]. The minor papilla is almost always in the right upper quadrant of the visual field when facing the major papilla with a variable distance between the two orifices (10mm-30mm cephalad and anterior) [Figure 9]. In the rare instances when a diagnostic dorsal pancreatogram is desired, a tapered 5Fr catheter with a 23G-25G blunt needle tip protruding beyond the tip of the catheter is usually preferred. In the majority of situations when deep dorsal pancreatic duct cannulation is desired, a highly tapered 3–4–5Fr catheter with a 0.018–0.021inch guidewire is employed [Figure 10, Figure 11]. Once cannulation is achieved, the subsequent fluoroscopic and interventional techniques are similar to those used in conventional ERP. The success rate of minor papilla cannulation is variable with some studies suggesting 73–83% success[45, 88, 89] but this increases to 90–95% in the hands of an experienced interventionalist[37, 42]. Failures are usually related to an imperceptible or distorted minor papilla from inflammation (pancreatitis, duodenal peptic ulcer disease), duodenal diverticula or malignancy, or inability to achieve adequate angles of approach for cannulation.
Figure 7:
Placing the duodenoscope in the long position, along the greater curvature of the stomach (yellow arrow), provides the optimum angle for minor papilla cannulation and opacification of the dorsal pancreatic duct (blue arrow).
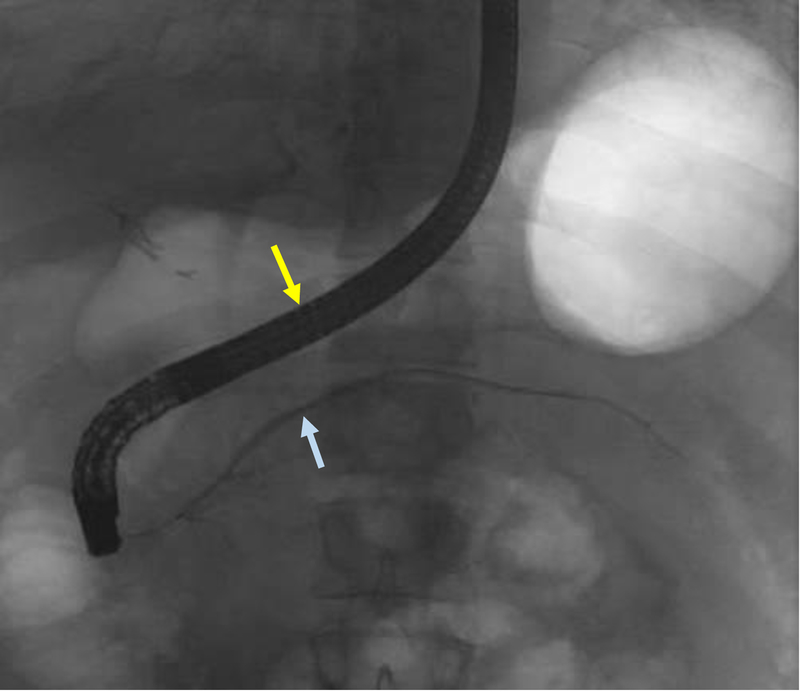
Figure 8:
Duodenoscope can be reduced to a short position along the lesser curvature of the stomach (yellow arrow) once deep minor papilla cannulation is achieved to allow for improved stability to interrogate and instrument the dorsal pancreatic duct (blue arrow).
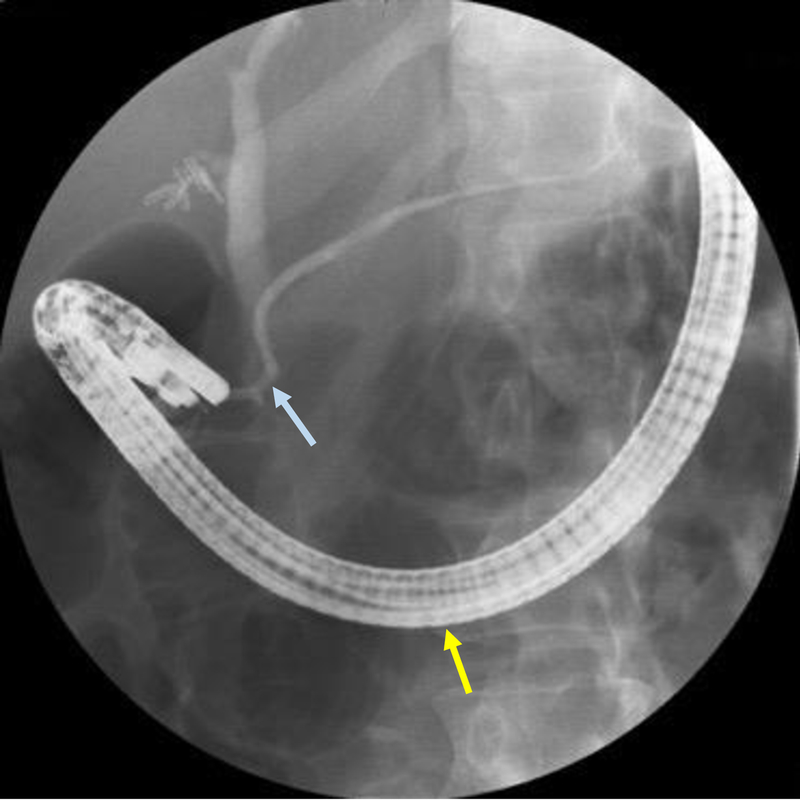
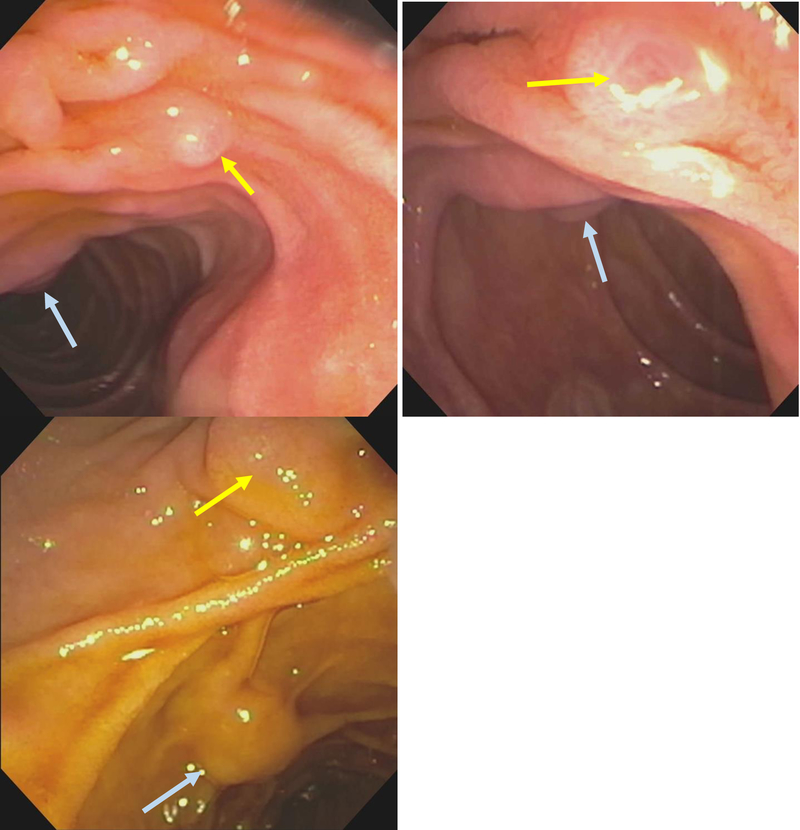
Figure 9:
Minor papilla (yellow arrow) anterior and to the right of the major papilla (blue arrow)
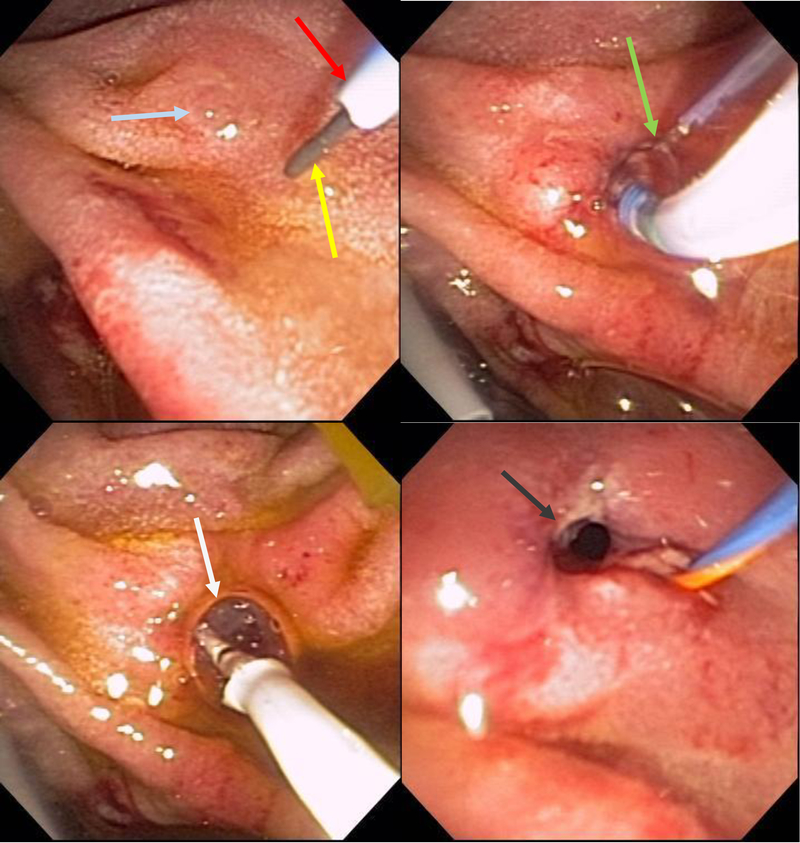
Figure 10:
Minor papilla (green arrow) identified with assistance of methylene blue and subsequently cannulated by a guide-wire (blue arrow) and a highly tapered catheter (yellow arrow). Deep cannulation of the minor papilla (grey arrow).
Figure 11:
Minor papilla (green arrow) cannulated by a guide-wire (blue arrow) and a highly tapered catheter (yellow arrow).
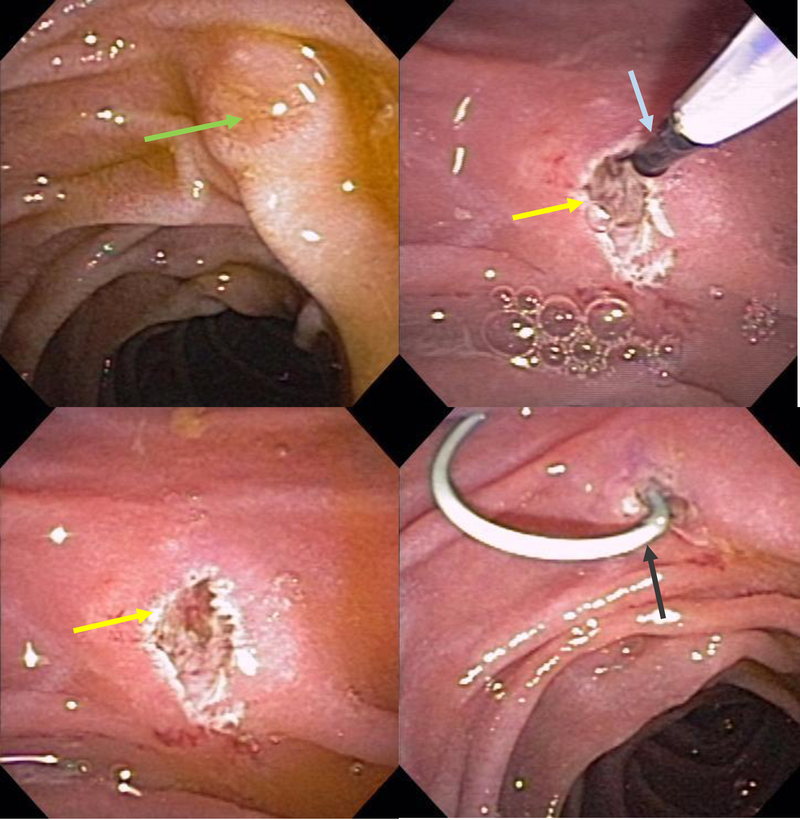
In the instance that the minor papilla and/or orifice cannot be clearly visualized, administration of secretin can stimulate pancreatic exocrine function and increase exit of pancreatic secretions from the minor papilla, making it more prominent and identifiable, enhancing the likelihood of cannulation [Figure 12, Figure 13]. The safety and efficacy of secretin for cannulation of the dorsal pancreatic duct has been demonstrated in a randomized double blinded study[90]. However, there is the potential of an increased risk of post-ERCP pancreatitis (PEP) when performing a dorsal duct pancreatogram due to the vigorous flow of pancreatic secretions and the need for high pressure contrast injection to visualize the pancreatic tail. Therefore, the use of secretin should be limited to scenarios where the minor papilla and/or orifice cannot be identified. Chromoendoscopy by spraying the duodenal mucosa and/or minor papilla with diluted methylene blue (1:10), particularly when used in conjunction with secretin, may also be helpful to identify the minor papilla orifice with clear pancreatic secretions washing away the dye [Figure 12, Figure 13]. If there is a communication between the dorsal and ventral pancreatic ducts (incomplete PD), injecting diluted methylene blue into the ventral duct and observing the dye exiting out of the minor papilla may also help identify the minor papilla orifice [91].
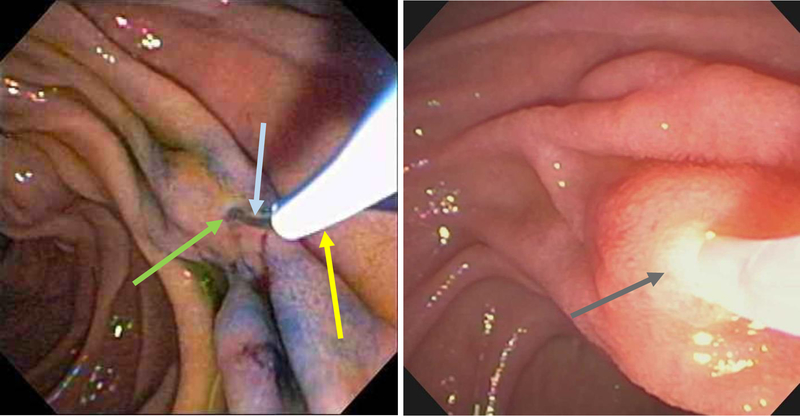
Figure 12:
Minor papilla (arrow) clearly identified after spraying duodenal mucosa with methylene blue and administration of secretin to enhance pancreatic secretion.
Figure 13:
The minor papilla (yellow arrow) is easily identified after spraying the duodenal mucosa with methylene blue and administration of intravenous secretin. The pancreatic juice flow results in a zone of central clearing (white arrow).
Minor papilla endoscopic sphincterotomy (mPES) has been studied and found to be an effective modality of minor papilla treatment in symptomatic patients with PD[44, 46, 48, 49, 92]. Techniques for mPES have also been described.
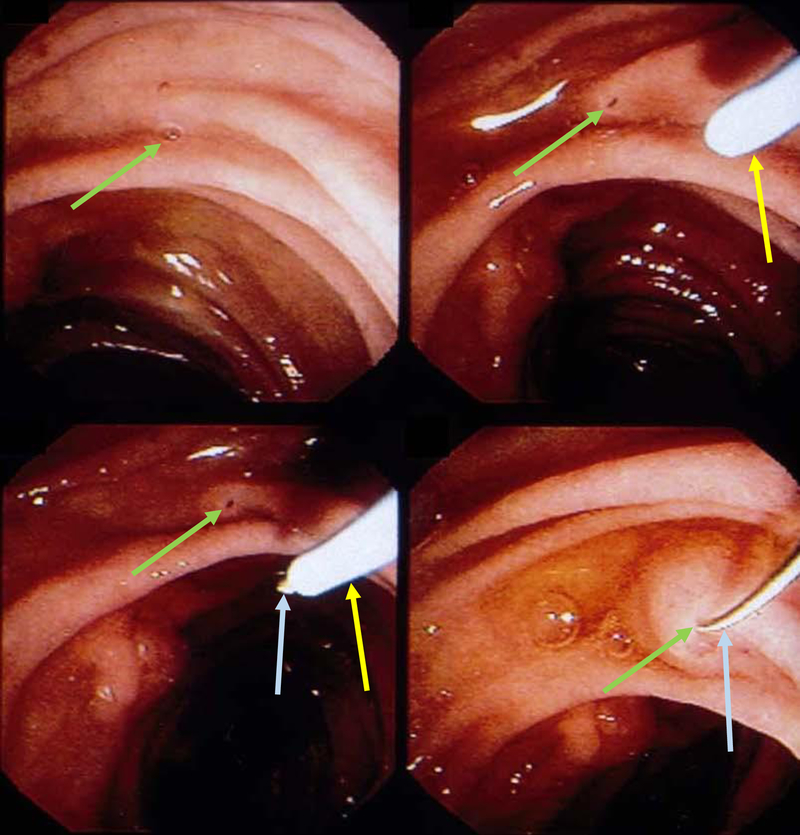
The wire-guided pull-type sphincterotomy maneuver [Figure 14] involves cannulation with a highly tapered catheter which does dilate the minor papilla orifice to 5 Fr. This allows for passage of either a mini-sphincterotome or a standard sphincterotome to make a 4–6 mm incision approximately in the 10–12 o’clock direction[84, 93].
Figure 14:
Minor papilla pull-type sphincterotomy.
The needle-knife technique over a pancreatic stent involves placement of a plastic stent (3–4Fr, 4–8 cm long) in the dorsal pancreatic duct followed by a 4–6 mm incision approximately in the 10–12 o’clock direction by a needle-knife sphincterotome[84, 93, 94]. The depth and height of the incision has not been standardized except in the case of a Santorinicele where the extent of incision is modified to achieve the goal of unroofing the dilated distal cystic segment of the dorsal duct[95].
Though it has been shown that the needle-knife technique is safer than the pull-type sphincterotome technique when performing a major papilla pancreatic sphincterotomy (PES) in high-risk patients[96], both techniques have been found to be safe and efficacious when employed at the minor papilla, with similar complication rates[97, 98, 99]. The complication profile of mPES is also similar to PES with the same traditional risk profile for PEP (younger age, female gender, prior PEP, absence of chronic pancreatitis)[100, 101].
A modification of the needle-knife technique is a wire-assisted access sphincterotomy. This involves deep dorsal duct cannulation with a guidewire, passing a needle-knife sphincterotome alongside the wire and incising the minor papilla by cutting away from the wire in the 10–12 o’clock direction. It has been shown to be equally efficacious as the other techniques and can be used when the orifice is stenotic and does not allow the passage of catheters and sphincterotomes.[102].
In situations described above where there is a failure of cannulation of the minor papilla, a free-hand pre-cut needle-knife [Figure 15] maneuver may be helpful in cannulating the minor papilla and gaining access to the dorsal duct to complete planned therapy. This technique involves inserting a needle-knife sphincterotome 1–2 mm into the minor papilla and making a 2–4 mm incision in the 10–12 o’clock direction. It has been shown to be efficacious and safe in a small series of patients[103]. However, this technique should be pursued with caution, as failed dorsal duct cannulation despite precut sphincterotomy may significantly increase the risk of PEP.
Figure 15:
Free hand needle-knife (blue arrow) sphincterotomy (yellow arrow) of minor papilla (green arrow) followed by placement of a plastic stent (grey arrow) in the dorsal pancreatic duct.
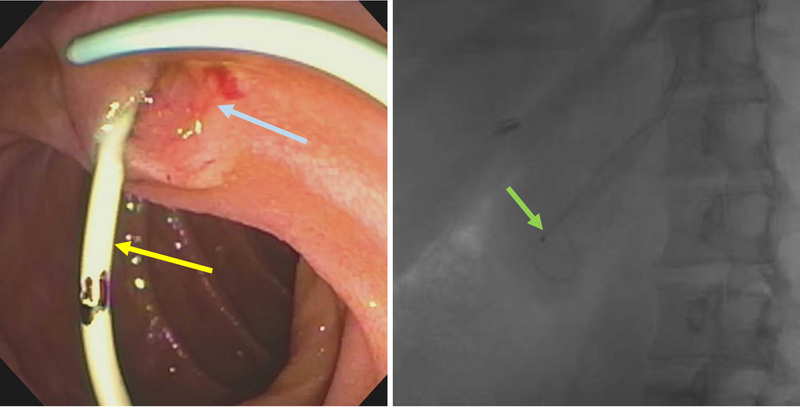
Given the high risk of PEP in mPES (similar to high risk patients undergoing PES)[104], a prophylactic temporary pancreatic stent can be placed after mPES to reduce the risk of PEP [Figure 16], based on data extrapolated from studies related to PES[98, 105, 106, 107]. These temporary stents typically migrate out of the pancreatic duct spontaneously, usually within 2 weeks[94, 108]. Migration can be confirmed on an upright abdominal X-ray (AXR/KUB) and if the stent remains intraductal, it can be removed by performing an upper endoscopy. On occasion, an urgent ERP may need to done to place a pancreatic stent or replace a migrated stent in an attempt to modify the course of an evolving PEP and hasten recovery[109]. Peri-procedure rectal indomethacin 100 mg[110, 111, 112, 113] and post procedure intravenous lactated Ringer’s solution[114] have also been shown to reduce the risk of PEP.
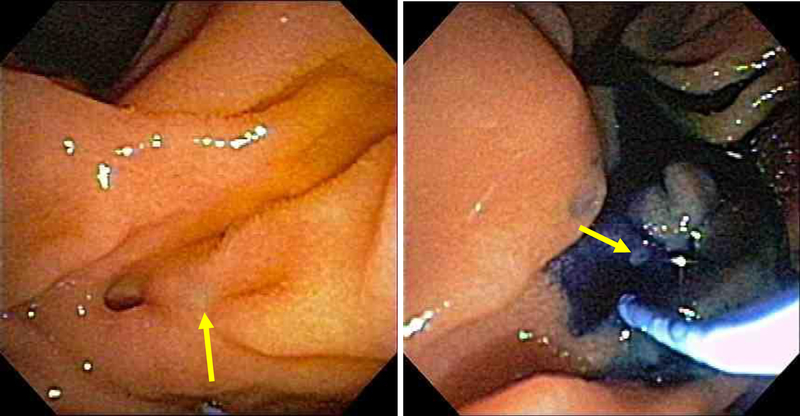
Figure 16:
Plastic stent (yellow arrow) placed in dorsal pancreatic duct after minor papilla endoscopic sphincterotomy (blue arrow). Stent in the dorsal pancreatic duct seen on fluoroscopy (green arrow)
Minor papilla endoscopic orifice balloon dilation [Figure 17] has also been studied and involves dilation of the minor papilla with comparable outcomes to mPES[115, 116]. As with balloon dilation of the major papilla, it carries a higher risk of PEP and therefore almost always is performed with placement of a protective pancreatic stent.
Figure 17:
Wire-guided (yellow arrow) cannulation of the minor papilla (blue arrow) with a highly tapered cannula (red arrow) followed by pull-type minor papilla sphincterotomy (green arrow) and a minor papilla endoscopic orifice dilation (white arrow). Widely open minor papilla orifice after endoscopic dilation (grey arrow).
“Long-term” dorsal pancreatic duct stent placement either independently or in conjunction with mPES or minor papilla orifice dilation has been shown to be an effective technique in offering relief of symptoms related to PD[44, 45, 47, 50, 88, 92]. However it is associated with complications such as occlusion, migration, ductal perforation, acute pancreatitis with pseudocyst formation [117, 118], ductal and parenchyma changes indicating chronic pancreatitis[119, 120, 121, 122].
The response and outcome to all pancreatic endotherapy from multiple studies over the years has been found to be highly variable and primarily determined by clinical presentation. Among them, the only randomized control trial by Lans et al [45] comparing endotherapy (dorsal pancreatic duct stenting with stent changes every 3 months for one year) to sham in 19 patients with idiopathic recurrent acute pancreatitis showed 90% of the patients in the intervention group reported >50% symptomatic improvement as compared to 11% in the placebo. The mean follow-up times in the stent and control groups were 28.6 and 31.5 months respectively. There was a lower incidence of recurrent pancreatitis in the stent group (1/10 vs 7/9), with 44% in the control group required stent placement due to uncontrolled symptoms. The patients in the control group who underwent stenting were subsequently followed up from 6 to 53 months and did not suffer from further episodes of pancreatitis or require hospitalization. This study demonstrated significant objective and subjective clinical improvement of symptoms with endotherapy compared with controls on short-term follow-up. Four systematic reviews and meta-analyses of these studies[6, 123, 124, 125] reporting a pooled response rate of 62–70%. The maximal improvement of symptoms was seen in patients with recurrent acute pancreatitis (76–80%), with a lower response rate in chronic pancreatitis (42–69%), and the least with chronic pancreatic-type abdominal pain (33–54%). The incidence of PEP was reported to be at 10% with the rate of minor papilla stenosis requiring re-intervention at 19%. The follow up period was between 14 to 64 months with most studies reporting a period of 36 months. In an effort to identify if endotherapy therapy is beneficial in PD, a sham-controlled, single blinded with a blinded outcome assessment, multi-center, international, randomized clinical trial (SHARP) of ERP with mPES for the treatment of RAP with PD is currently underway with planned total sample size of 234 subjects, and a planned maximum follow-up of 48 months (ClinicalTrials.gov Identifier: ).
Surgery
Surgical minor papilla sphincterotomy[48] and surgical minor papilla sphincteroplasty[33, 126] have both been described to provide comparable effective and durable relief of symptoms with maximal efficacy noted in patients with recurrent acute pancreatitis (RAP).
The response to all surgical minor papilla therapy has also been variable with one systematic review and meta-analysis[6] reporting a pooled response rate of 75%. Similar to endotherapy, maximal symptomatic improvement was seen in patients with RAP (83%), with a lower response rate in chronic pancreatitis (67%), and the least with chronic pancreatic-type abdominal pain (52%). The follow up period was between 6 to 120 months with most studies greater than 50 months.
The clinical presentation and response rates to endotherapy and surgery within the three types of pancreas divisum were not significantly different[6, 89].
Given the comparable outcomes between endotherapy and surgery of minor papilla in alleviating symptoms but with endotherapy having a favorable complication and mortality rate, endoscopy (i.e. ERCP) is usually considered the first line treatment, with surgery reserved for patients who fail minor papilla cannulation, endotherapy or have altered anatomy (e.g., gastric bypass).
In patients with RAP who unfortunately fail minor papilla therapy or have progressed to chronic pancreatitis, further therapeutic options are limited. These may include management of symptoms related to chronic pancreatitis, including periods of pancreas rest with small bowel nutrition, celiac plexus block, somatostatin analogues, and an intrathecal pain pump[127]. Patients with significantly dilated dorsal pancreatic ducts may benefit from a lateral pancreatojejunostomy (Puestow or Frey procedure)[128, 129]. Total dorsal pancreatectomy has been proposed for benign or low grade malignant pancreatic tumors limited to the dorsal pancreas as an alternative to total pancreatectomy and may be extrapolated to patients with changes of chronic pancreatitis limited to the dorsal pancreas[130]. Total pancreatectomy with auto-islet cell transplantation (TPAIT) is an emerging option for patients with disease refractory to endotherapy. Reports of outcomes of TPAIT data performed in patients with a normal anatomy suggest favorable outcomes and potentially can be extrapolated to patients with PD[131, 132, 133, 134].
Expert Commentary
5-year review/Speculation
Pancreas divisum is the most common congenital pancreatic anomaly and has been reported on autopsy, imaging and endoscopy for many years. As the majority of patients lack pancreatic symptoms, its association with pancreatic disease is debatable. However, there have been several studies that show a substantial association with pancreatic disease in the background of other factors that predispose to pancreatic pathology. Over the past few decades, several attempts have been made to understand the pathology of pancreatic disease in relation to pancreas divisum and to prove a definite association but none have been definitively successful. Numerous studies have been undertaken in an attempt to demonstrate a therapeutic benefit with various modalities of minor papilla therapy but, thus far, the results have been heterogenous and not definitive. Most of the data suggest a role for endoscopic intervention in the setting of acute recurrent pancreatitis. However, most studies have been of low quality, small patient sample, predominantly retrospective and without long-term follow up. Surgical and endoscopic modalities have also not been directly compared. With the current data available, it would not be prudent to routinely advise a definitive line of management for pancreatic disease associated with pancreas divisum. This should involve a comprehensive discussion with the individual patient to define expectations before embarking on any medical and/or interventional therapy.
Summary/Key-Issues.
Pancreas divisum is the most common congenital malformation of the pancreas and occurs due to a failure of fusion of the ductal systems of the dorsal and ventral pancreatic buds in the seventh week of intra-uterine life. This leads to a dominant dorsal pancreatic duct draining through the minor papilla and a small ventral pancreatic duct draining through the major papilla. The prevalence in the western population is about 10% with a lower frequency of 1–2% in Asians and Africans.
More than 95% of patients with pancreas divisum lack pancreatic symptoms, with the anomaly found incidentally on abdominal imaging (e.g., Computerized Tomography, Magnetic Resonance Cholangiopancreatography) performed for unrelated symptoms and indications.
The etiological role and clinical significance of pancreas divisum in pancreatic disease such as pancreatic-type abdominal pain (intermittent or chronic), acute pancreatitis or chronic pancreatitis has been suggested based on its increased incidence in the IRAP population, pathologic studies suggesting pancreatic disease limited to the dorsal pancreas and improvement in outcome following minor papilla interventions. However, this remains debatable with no clear causality identified.
It has been demonstrated to predispose to pancreatic disease in association with other factors such has genetic mutations of SPINK-1, CFTR, CTRC and therefore should be checked in pertinent clinical situations. It has also been shown to decrease the threshold for other known causes of pancreatic disease such as alcohol, medications, toxins, trauma.
Secretin augmented Magnetic Resonance Cholangiopancreatography is the non-invasive imaging modality of choice for diagnosing pancreas divisum.
The pathogenesis of pancreatic disease in pancreas divisum is not clearly defined but is believed to be due to an imbalance between the drainage of a larger and longer dominant dorsal pancreatic duct opening through a relatively smaller or stenotic minor papilla, causing inadequate drainage of pancreatic secretions and transient obstruction of flow. The resultant increase in intraductal pressure and distention of the dorsal duct may then lead to abdominal pain and pancreatitis.
No further pancreatic evaluation or therapy is needed or recommended in patients with asymptomatic pancreas divisum. In patients deemed to be symptomatic from pancreas divisum, after a comprehensive evaluation for causes of pancreatic disease is completed, therapy is offered for patients suffering from recurrent attacks of acute pancreatitis and can be considered for a severe attack of acute pancreatitis and its complications, chronic pancreatitis and its associated complications and chronic pancreatic-type abdominal pain causing significant functional impairment and debility. It is important not to ignore the standard management principles of acute and chronic pancreatitis.
In patients with mild or infrequent symptoms of pancreatic-type abdominal pain, conservative management is pursued and directed towards symptom control.
Endoscopic Retrograde Cholangiopancreatography with minor papilla endotherapy is the current first line of therapy. Minor papilla sphincterotomy is the predominant intervention due to the adverse event profile of balloon dilation of the minor papilla and long-term pancreatic stents. The procedure-related risk profile is similar to that of major papilla pancreatic sphincterotomy in high-risk patients.
Minor papilla surgical therapy has a comparable outcome with endotherapy but due to a less favorable adverse event profile is currently reserved for patients who fail endoscopic intervention. The choice of endoscopic or surgical therapy also depends on patient profile and preference as well as local institutional expertise.
The response to either endoscopic or surgical therapy is variable with maximal benefit seen in patients with recurrent acute pancreatitis, less with chronic pancreatitis and least with chronic pancreatic-type abdominal pain.
Data supporting either endoscopic or surgical therapy are of low quality as the studies are predominantly retrospective with a relatively short follow-up period.
In patients who fail minor papilla therapy, management is directed towards symptom control, with options including periods of pancreas rest with post-ligament of Treitz enteral feeding, celiac plexus block, somatostatin analogues and placement of an intrathecal pain pump.
Additional surgical options including a pancreatojejunostomy (Puestow or Frey procedure) or a total pancreatectomy with auto- islet cell transplantation may be an option for a subset of patients.
Contributor Information
Aditya Gutta, Advanced Endoscopy Gastroenterology Fellow, Indiana University School of Medicine, Division of Gastroenterology, 550 N. University Blvd, Indianapolis, IN 46202.
Evan Fogel, Professor of Medicine, Indiana University School of Medicine, Division of Gastroenterology, 550 N. University Blvd, Suite 1602, Indianapolis, IN 46202.
Stuart Sherman, Professor of Medicine, Glen Lehman Professor in Gastroenterology, Indiana University School of Medicine, Division of Gastroenterology, 550 N. University Blvd, Suite 1634, Indianapolis, IN 46202.
References
- 1.Kozu T, Suda K, Toki F. Pancreatic development and anatomical variation. Gastrointestinal endoscopy clinics of North America. 1995. January;5(1):1–30. [PubMed] [Google Scholar]
- 2.Smanio T Proposed nomenclature and classification of the human pancreatic ducts and duodenal papillae. Study based on 200 post mortems. International surgery. 1969. August;52(2):125–41. [PubMed] [Google Scholar]
- 3.Stimec B, Bulajic M, Korneti V, et al. Ductal morphometry of ventral pancreas in pancreas divisum. Comparison between clinical and anatomical results. The Italian journal of gastroenterology. 1996. Feb-Mar;28(2):76–80. [PubMed] [Google Scholar]
- 4.Bulow R, Simon P, Thiel R, et al. Anatomic variants of the pancreatic duct and their clinical relevance: an MR-guided study in the general population. European radiology. 2014. Dec;24(12):3142–9. doi: 10.1007/s00330-014-3359-7. [DOI] [PubMed] [Google Scholar]
- 5.Bernard JP, Sahel J, Giovannini M, et al. Pancreas divisum is a probable cause of acute pancreatitis: a report of 137 cases. Pancreas. 1990. May;5(3):248–54. [DOI] [PubMed] [Google Scholar]
- 6.Liao Z, Gao R, Wang W, et al. A systematic review on endoscopic detection rate, endotherapy, and surgery for pancreas divisum. Endoscopy. 2009. May;41(5):439–44. doi: 10.1055/s-0029-1214505. [DOI] [PubMed] [Google Scholar]
- 7.Suga T, Nakagawa T, Miyakawa H, et al. Clinical Features of Patients with Pancreas Divisum. 1994;6(1):80–86. doi: doi: 10.1111/j.1443-1661.1994.tb00668.x. [DOI] [Google Scholar]
- 8.Delhaye M, Cremer M. Clinical significance of pancreas divisum. Acta gastro-enterologica Belgica. 1992. May-Jun;55(3):306–13. [PubMed] [Google Scholar]
- 9.Cotton PB. Pancreas divisum--curiosity or culprit? Gastroenterology. 1985. December;89(6):1431–5. [DOI] [PubMed] [Google Scholar]
- 10.Brenner P, Duncombe V, Ham JM. Pancreatitis and pancreas divisum: aetiological and surgical considerations. The Australian and New Zealand journal of surgery. 1990. November;60(11):899–903. [DOI] [PubMed] [Google Scholar]
- 11.Richter JM, Schapiro RH, Mulley AG, et al. Association of pancreas divisum and pancreatitis, and its treatment by sphincteroplasty of the accessory ampulla. Gastroenterology. 1981. December;81(6):1104–10. [PubMed] [Google Scholar]
- 12.Takuma K, Kamisawa T, Hara S, et al. Etiology of recurrent acute pancreatitis, with special emphasis on pancreaticobiliary malformation. Advances in medical sciences. 2012;57(2):244–50. doi: 10.2478/v10039-012-0041-7. [DOI] [PubMed] [Google Scholar]
- 13.Kamisawa T, Koike M, Okamoto A. Embryology of the pancreatic duct system. Digestion. 1999. Mar-Apr;60(2):161–5. doi: 10.1159/000007642. [DOI] [PubMed] [Google Scholar]
- 14.Kamisawa T, Takuma K, Egawa N, et al. A new embryological theory of the pancreatic duct system. Digestive surgery. 2010;27(2):132–6. doi: 10.1159/000286906. [DOI] [PubMed] [Google Scholar]
- 15.MacCarty RL, Stephens DH, Brown AL Jr., et al. Retrograde pancreatography in autopsy specimens. The American journal of roentgenology, radium therapy, and nuclear medicine. 1975. February;123(2):359–66. [DOI] [PubMed] [Google Scholar]
- 16.Kamisawa T, Egawa N, Tu Y, et al. Pancreatographic investigation of embryology of complete and incomplete pancreas divisum. Pancreas. 2007. January;34(1):96–102. doi: 10.1097/01.mpa.0000240607.49183.7e. [DOI] [PubMed] [Google Scholar]
- 17.Moreira VF, Merono E, Ledo L, et al. Incomplete pancreas divisum. Gastrointestinal endoscopy. 1991. Jan-Feb;37(1):104–5. [DOI] [PubMed] [Google Scholar]
- 18.Tulassay Z, Papp J, Farkas IE. Diagnostic aspects of incomplete pancreas divisum. Gastrointestinal endoscopy. 1986. December;32(6):428. [DOI] [PubMed] [Google Scholar]
- 19.Kamisawa T, Tu Y, Egawa N, et al. Clinical implications of incomplete pancreas divisum. JOP : Journal of the pancreas. 2006. November 10;7(6):625–30. [PubMed] [Google Scholar]
- 20.Kim MH, Lee SS, Kim CD, et al. Incomplete pancreas divisum: is it merely a normal anatomic variant without clinical implications? Endoscopy. 2001. September;33(9):778–85. doi: 10.1055/s-2001-16521. [DOI] [PubMed] [Google Scholar]
- 21.Zyromski NJ, Sandoval JA, Pitt HA, et al. Annular pancreas: dramatic differences between children and adults. Journal of the American College of Surgeons. 2008. May;206(5):1019–25; discussion 1025–7. doi: 10.1016/j.jamcollsurg.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 22.Lehman GA, O’Connor KW. Coexistence of annular pancreas and pancreas divisum--ERCP diagnosis. Gastrointestinal endoscopy. 1985. February;31(1):25–8. [DOI] [PubMed] [Google Scholar]
- 23.Sandrasegaran K, Patel A, Fogel EL, et al. Annular pancreas in adults. AJR American journal of roentgenology. 2009. August;193(2):455–60. doi: 10.2214/ajr.08.1596. [DOI] [PubMed] [Google Scholar]
- 24.Gromski MA, Lehman GA, Zyromski NJ, et al. Annular pancreas: endoscopic and pancreatographic findings from a tertiary referral ERCP center. Gastrointestinal endoscopy. 2018. September 18. doi: 10.1016/j.gie.2018.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cao J, Wang L, Zou X. Coexistence of Complete Pancreas Divisum and Anomalous Pancreaticobiliary Junction in a Patient With Type IA Choledochal Cyst. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2018. August 2. doi: 10.1016/j.cgh.2018.07.036. [DOI] [PubMed] [Google Scholar]
- 26.Ng JW, Wong MK, Huang J, et al. Incomplete pancreas divisum associated with abnormal junction of pancreaticobiliary duct system. Gastrointestinal endoscopy. 1992. Jan-Feb;38(1):105–6. [DOI] [PubMed] [Google Scholar]
- 27.Patidar Y, Agarwal N, Gupta S, et al. Choledochocele with pancreas divisum: A rare co-occurrence diagnosed on magnetic resonance cholangiopancreatography. World journal of radiology. 2013. July 28;5(7):264–6. doi: 10.4329/wjr.v5.i7.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ransom-Rodriguez A, Blachman-Braun R, Sanchez-Garcia Ramos E, et al. A rare case of choledochal cyst with pancreas divisum: case presentation and literature review. Annals of hepato-biliary-pancreatic surgery. 2017. February;21(1):52–56. doi: 10.14701/ahbps.2017.21.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanada Y, Sakuma Y, Sata N. Embryological etiology of pancreaticobiliary system predicted from pancreaticobiliary maljunction with incomplete pancreatic divisum: a case report. BMC surgery. 2018. August 2;18(1):50. doi: 10.1186/s12893-018-0385-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adibelli ZH, Adatepe M, Isayeva L, et al. Pancreas divisum: A risk factor for pancreaticobiliary tumors - an analysis of 1628 MR cholangiography examinations. Diagnostic and interventional imaging. 2017. February;98(2):141–147. doi: 10.1016/j.diii.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 31.Lehman GA, Kopecky KK, Rogge JD. Partial pancreatic agenesis combined with pancreas divisum and duodenum reflexum. Gastrointestinal endoscopy. 1987. December;33(6):445–8. [DOI] [PubMed] [Google Scholar]
- 32.Madura JA. Pancreas divisum: stenosis of the dorsally dominant pancreatic duct. A surgically correctable lesion. American journal of surgery. 1986. June;151(6):742–5. [DOI] [PubMed] [Google Scholar]
- 33.Warshaw AL, Simeone JF, Schapiro RH, et al. Evaluation and treatment of the dominant dorsal duct syndrome (pancreas divisum redefined). American journal of surgery. 1990. January;159(1):59–64; [DOI] [PubMed] [Google Scholar]
- 34.Staritz M, Meyer zum Buschenfelde KH. Elevated pressure in the dorsal part of pancreas divisum: the cause of chronic pancreatitis? Pancreas. 1988;3(1):108–10. [DOI] [PubMed] [Google Scholar]
- 35.Cotton PB. Congenital anomaly of pancreas divisum as cause of obstructive pain and pancreatitis. 1980;21(2):105–114. doi: 10.1136/gut.21.2.105%JGut. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bertin C, Pelletier AL, Vullierme MP, et al. Pancreas divisum is not a cause of pancreatitis by itself but acts as a partner of genetic mutations. The American journal of gastroenterology. 2012. February;107(2):311–7. doi: 10.1038/ajg.2011.424. [DOI] [PubMed] [Google Scholar]
- 37.Delhaye M, Engelholm L, Cremer M. Pancreas divisum: congenital anatomic variant or anomaly? Contribution of endoscopic retrograde dorsal pancreatography. Gastroenterology. 1985. November;89(5):951–8. [DOI] [PubMed] [Google Scholar]
- 38.Dhar A, Goenka MK, Kochhar R, et al. Pancrease divisum: five years’ experience in a teaching hospital. Indian journal of gastroenterology : official journal of the Indian Society of Gastroenterology. 1996. January;15(1):7–9. [PubMed] [Google Scholar]
- 39.Morgan DE, Logan K, Baron TH, et al. Pancreas divisum: implications for diagnostic and therapeutic pancreatography. AJR American journal of roentgenology. 1999. July;173(1):193–8. doi: 10.2214/ajr.173.1.10397125. [DOI] [PubMed] [Google Scholar]
- 40.Wang DB, Yu J, Fulcher AS, et al. Pancreatitis in patients with pancreas divisum: imaging features at MRI and MRCP. World journal of gastroenterology. 2013. August 14;19(30):4907–16. doi: 10.3748/wjg.v19.i30.4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin TK, Abu-El-Haija M, Nathan JD, et al. Pancreas Divisum in Pediatric Acute Recurrent and Chronic Pancreatitis: Report From INSPPIRE. Journal of clinical gastroenterology. 2018. June 2. doi: 10.1097/mcg.0000000000001063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Benage D, McHenry R, Hawes RH, et al. Minor papilla cannulation and dorsal ductography in pancreas divisum. Gastrointestinal endoscopy. 1990. Nov-Dec;36(6):553–7. [DOI] [PubMed] [Google Scholar]
- 43.Borak GD, Romagnuolo J, Alsolaiman M, et al. Long-term clinical outcomes after endoscopic minor papilla therapy in symptomatic patients with pancreas divisum. Pancreas. 2009. November;38(8):903–6. doi: 10.1097/MPA.0b013e3181b2bc03. [DOI] [PubMed] [Google Scholar]
- 44.Chacko LN, Chen YK, Shah RJ. Clinical outcomes and nonendoscopic interventions after minor papilla endotherapy in patients with symptomatic pancreas divisum. Gastrointestinal endoscopy. 2008. October;68(4):667–73. doi: 10.1016/j.gie.2008.01.025. [DOI] [PubMed] [Google Scholar]
- 45.Lans JI, Geenen JE, Johanson JF, et al. Endoscopic therapy in patients with pancreas divisum and acute pancreatitis: a prospective, randomized, controlled clinical trial. Gastrointestinal endoscopy. 1992. Jul-Aug;38(4):430–4. [DOI] [PubMed] [Google Scholar]
- 46.Lehman GA, Sherman S, Nisi R, et al. Pancreas divisum: results of minor papilla sphincterotomy. Gastrointestinal endoscopy. 1993. Jan-Feb;39(1):1–8. [DOI] [PubMed] [Google Scholar]
- 47.Heyries L, Barthet M, Delvasto C, et al. Long-term results of endoscopic management of pancreas divisum with recurrent acute pancreatitis. Gastrointestinal endoscopy. 2002. March;55(3):376–81. doi: 10.1067/mge.2002.121602. [DOI] [PubMed] [Google Scholar]
- 48.Keith RG, Shapero TF, Saibil FG, et al. Dorsal duct sphincterotomy is effective long-term treatment of acute pancreatitis associated with pancreas divisum. Surgery. 1989. October;106(4):660–6; [PubMed] [Google Scholar]
- 49.Kwan V, Loh SM, Walsh PR, et al. Minor papilla sphincterotomy for pancreatitis due to pancreas divisum. ANZ journal of surgery. 2008. April;78(4):257–61. doi: 10.1111/j.1445-2197.2008.04431.x. [DOI] [PubMed] [Google Scholar]
- 50.Siegel JH, Ben-Zvi JS, Pullano W, et al. Effectiveness of endoscopic drainage for pancreas divisum: endoscopic and surgical results in 31 patients. Endoscopy. 1990. May;22(3):129–33. doi: 10.1055/s-2007-1010727. [DOI] [PubMed] [Google Scholar]
- 51.Klein SD, Affronti JP. Pancreas divisum, an evidence-based review: part I, pathophysiology. Gastrointestinal endoscopy. 2004. September;60(3):419–25. [DOI] [PubMed] [Google Scholar]
- 52.Garg PK, Khajuria R, Kabra M, et al. Association of SPINK1 gene mutation and CFTR gene polymorphisms in patients with pancreas divisum presenting with idiopathic pancreatitis. Journal of clinical gastroenterology. 2009. October;43(9):848–52. doi: 10.1097/MCG.0b013e3181a4e772. [DOI] [PubMed] [Google Scholar]
- 53.Gelrud A, Sheth S, Banerjee S, et al. Analysis of cystic fibrosis gener product (CFTR) function in patients with pancreas divisum and recurrent acute pancreatitis. The American journal of gastroenterology. 2004. August;99(8):1557–62. doi: 10.1111/j.1572-0241.2004.30834.x. [DOI] [PubMed] [Google Scholar]
- 54.Choudari CP, Imperiale TF, Sherman S, et al. Risk of Pancreatitis with Mutation of the Cystic Fibrosis Gene [Original Contribution]. American Journal Of Gastroenterology. 2004. 07/01/online;99:1358. doi: 10.1111/j.1572-0241.2004.30655.x. [DOI] [PubMed] [Google Scholar]
- 55.Bhatia E, Choudhuri G, Sikora SS, et al. Tropical calcific pancreatitis: strong association with SPINK1 trypsin inhibitor mutations. Gastroenterology. 2002. October;123(4):1020–5. [DOI] [PubMed] [Google Scholar]
- 56.Cohn JA, Friedman KJ, Noone PG, et al. Relation between mutations of the cystic fibrosis gene and idiopathic pancreatitis. The New England journal of medicine. 1998. September 3;339(10):653–8. doi: 10.1056/nejm199809033391002. [DOI] [PubMed] [Google Scholar]
- 57.Witt H, Luck W, Hennies HC, et al. Mutations in the gene encoding the serine protease inhibitor, Kazal type 1 are associated with chronic pancreatitis. Nature genetics. 2000. June;25(2):213–6. doi: 10.1038/76088. [DOI] [PubMed] [Google Scholar]
- 58.Asayama Y, Fang W, Stolpen A, et al. Detectability of pancreas divisum in patients with acute pancreatitis on multi-detector row computed tomography. Emergency radiology. 2012. April;19(2):121–5. doi: 10.1007/s10140-011-1008-x. [DOI] [PubMed] [Google Scholar]
- 59.Lindstrom E, Ihse I. Dynamic CT scanning of pancreatic duct after secretin provocation in pancreas divisum. Digestive diseases and sciences. 1990. November;35(11):1371–6. [DOI] [PubMed] [Google Scholar]
- 60.Kushnir VM, Wani SB, Fowler K, et al. Sensitivity of endoscopic ultrasound, multidetector computed tomography, and magnetic resonance cholangiopancreatography in the diagnosis of pancreas divisum: a tertiary center experience. Pancreas. 2013. April;42(3):436–41. doi: 10.1097/MPA.0b013e31826c711a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Carnes ML, Romagnuolo J, Cotton PB. Miss rate of pancreas divisum by magnetic resonance cholangiopancreatography in clinical practice. Pancreas. 2008. August;37(2):151–3. doi: 10.1097/MPA.0b013e318164cbaf. [DOI] [PubMed] [Google Scholar]
- 62.Chalazonitis NA, Lachanis BS, Laspas F, et al. Pancreas divisum: magnetic resonance cholangiopancreatography findings. Singapore medical journal. 2008. November;49(11):951–4; [PubMed] [Google Scholar]
- 63.Kamisawa T, Tu Y, Egawa N, et al. MRCP of congenital pancreaticobiliary malformation. Abdominal imaging. 2007. Jan-Feb;32(1):129–33. doi: 10.1007/s00261-006-9005-3. [DOI] [PubMed] [Google Scholar]
- 64.Rustagi T, Njei B. Magnetic resonance cholangiopancreatography in the diagnosis of pancreas divisum: a systematic review and meta-analysis. Pancreas. 2014. August;43(6):823–8. doi: 10.1097/mpa.0000000000000143. [DOI] [PubMed] [Google Scholar]
- 65.Shen Z, Munker S, Zhou B, et al. The Accuracies of Diagnosing Pancreas Divisum by Magnetic Resonance Cholangiopancreatography and Endoscopic Ultrasound: A Systematic Review and Meta-analysis. Scientific reports. 2016. October 13;6:35389. doi: 10.1038/srep35389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chamokova B, Bastati N, Poetter-Lang S, et al. The clinical value of secretin-enhanced MRCP in the functional and morphological assessment of pancreatic diseases. The British journal of radiology. 2018. April;91(1084):20170677. doi: 10.1259/bjr.20170677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Matos C, Metens T, Deviere J, et al. Pancreas divisum: evaluation with secretin-enhanced magnetic resonance cholangiopancreatography. Gastrointestinal endoscopy. 2001. June;53(7):728–33. doi: 10.1067/mge.2001.114784. [DOI] [PubMed] [Google Scholar]
- 68.Mosler P, Akisik F, Sandrasegaran K, et al. Accuracy of magnetic resonance cholangiopancreatography in the diagnosis of pancreas divisum. Digestive diseases and sciences. 2012. January;57(1):170–4. doi: 10.1007/s10620-011-1823-7. [DOI] [PubMed] [Google Scholar]
- 69.Sandrasegaran K, Cote GA, Tahir B, et al. The utility of secretin-enhanced MRCP in diagnosing congenital anomalies. Abdominal imaging. 2014. October;39(5):979–87. doi: 10.1007/s00261-014-0131-z. [DOI] [PubMed] [Google Scholar]
- 70.Sandrasegaran K, Tahir B, Barad U, et al. The Value of Secretin-Enhanced MRCP in Patients With Recurrent Acute Pancreatitis. AJR American journal of roentgenology. 2017. February;208(2):315–321. doi: 10.2214/ajr.16.16566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sherman S, Freeman ML, Tarnasky PR, et al. Administration of secretin (RG1068) increases the sensitivity of detection of duct abnormalities by magnetic resonance cholangiopancreatography in patients with pancreatitis. Gastroenterology. 2014. September;147(3):646–654.e2. doi: 10.1053/j.gastro.2014.05.035. [DOI] [PubMed] [Google Scholar]
- 72.Wan J, Ouyang Y, Yu C, et al. Comparison of EUS with MRCP in idiopathic acute pancreatitis: a systematic review and meta-analysis. Gastrointestinal endoscopy. 2018. May;87(5):1180–1188.e9. doi: 10.1016/j.gie.2017.11.028. [DOI] [PubMed] [Google Scholar]
- 73.Boninsegna E, Manfredi R, Ventriglia A, et al. Santorinicele: secretin-enhanced magnetic resonance cholangiopancreatography findings before and after minor papilla sphincterotomy. European radiology. 2015. August;25(8):2437–44. doi: 10.1007/s00330-015-3644-0. [DOI] [PubMed] [Google Scholar]
- 74.Manfredi R, Brizi MG, Costamagna G, et al. Pancreas divisum and santorinicele: assessment by dynamic magnetic resonance cholangiopancreatography during secretin stimulation. La Radiologia medica. 2002. Jan-Feb;103(1–2):55–64. [PubMed] [Google Scholar]
- 75.Lai R, Freeman ML, Cass OW, et al. Accurate diagnosis of pancreas divisum by linear-array endoscopic ultrasonography. Endoscopy. 2004. August;36(8):705–9. doi: 10.1055/s-2004-825663. [DOI] [PubMed] [Google Scholar]
- 76.Rana SS, Bhasin DK, Sharma V, et al. Role of endoscopic ultrasound in the diagnosis of pancreas divisum. Endoscopic ultrasound. 2013. Jan-Mar;2(1):7–10. doi: 10.7178/eus.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Catalano MF RM, Geenen JE, et al. Pancreatic endotherapy of pancreas divisum (PDIV): Response based on clinical presentation and results of secretin stimulated endoscopic ultrasound: (S-EUS). Gastrointestinal endoscopy. 2001;53(5):AB133. doi: 10.1016/S0016-5107(01)80267-4. [DOI] [Google Scholar]
- 78.Sharma M, Pathak A, Rameshbabu CS, et al. Imaging of pancreas divisum by linear-array endoscopic ultrasonography. Endoscopic ultrasound. 2016. Jan-Feb;5(1):21–9. doi: 10.4103/2303-9027.175878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mariani A, Arcidiacono PG, Curioni S, et al. Diagnostic yield of ERCP and secretin-enhanced MRCP and EUS in patients with acute recurrent pancreatitis of unknown aetiology. Digestive and liver disease : official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver. 2009. October;41(10):753–8. doi: 10.1016/j.dld.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 80.Glaser J, Stienecker K. Ultrasound secretin test in patients with pancreas divisum--an aid in the diagnosis of papillary or dorsal duct stenosis? Zeitschrift fur Gastroenterologie. 1999. July;37(7):585–8. [PubMed] [Google Scholar]
- 81.Lowes JR, Lees WR, Cotton PB. Pancreatic duct dilatation after secretin stimulation in patients with pancreas divisum. Pancreas. 1989;4(3):371–4. [DOI] [PubMed] [Google Scholar]
- 82.Tulassay Z, Jakab Z, Vadasz A, et al. Secretin provocation ultrasonography in the diagnosis of papillary obstruction in pancreas divisum. Gastroenterologisches Journal : Organ der Gesellschaft fur Gastroenterologie der DDR. 1991;51(1):47–50. [PubMed] [Google Scholar]
- 83.Lehman GA, Sherman S. Pancreas divisum. Diagnosis, clinical significance, and management alternatives. Gastrointestinal endoscopy clinics of North America. 1995. January;5(1):145–70. [PubMed] [Google Scholar]
- 84.Lehman GA, Sherman S. Diagnosis and therapy of pancreas divisum. Gastrointestinal endoscopy clinics of North America. 1998. January;8(1):55–77. [PubMed] [Google Scholar]
- 85.Chey WY, Chang TM. Secretin: historical perspective and current status. Pancreas. 2014. March;43(2):162–82. doi: 10.1097/01.mpa.0000437325.29728.d6. [DOI] [PubMed] [Google Scholar]
- 86.McCarthy J, Fumo D, Geenen JE. Pancreas divisum: a new method for cannulating the accessory papilla. Gastrointestinal endoscopy. 1987. December;33(6):440–2. [DOI] [PubMed] [Google Scholar]
- 87.O’Connor KW, Lehman GA. An improved technique for accessory papilla cannulation in pancreas divisum. Gastrointestinal endoscopy. 1985. February;31(1):13–7. [DOI] [PubMed] [Google Scholar]
- 88.Ertan A Long-term results after endoscopic pancreatic stent placement without pancreatic papillotomy in acute recurrent pancreatitis due to pancreas divisum. Gastrointestinal endoscopy. 2000. July;52(1):9–14. doi: 10.1067/mge.2000.106311. [DOI] [PubMed] [Google Scholar]
- 89.Jacob L, Geenen JE, Catalano MF, et al. Clinical presentation and short-term outcome of endoscopic therapy of patients with symptomatic incomplete pancreas divisum. Gastrointestinal endoscopy. 1999. January;49(1):53–7. [DOI] [PubMed] [Google Scholar]
- 90.Devereaux BM, Fein S, Purich E, et al. A new synthetic porcine secretin for facilitation of cannulation of the dorsal pancreatic duct at ERCP in patients with pancreas divisum: a multicenter, randomized, double-blind comparative study. Gastrointestinal endoscopy. 2003. May;57(6):643–7. doi: 10.1067/mge.2003.195. [DOI] [PubMed] [Google Scholar]
- 91.Park SH, de Bellis M, McHenry L, et al. Use of methylene blue to identify the minor papilla or its orifice in patients with pancreas divisum. Gastrointestinal endoscopy. 2003. March;57(3):358–63. doi: 10.1067/mge.2003.110. [DOI] [PubMed] [Google Scholar]
- 92.Gerke H, Byrne MF, Stiffler HL, et al. Outcome of endoscopic minor papillotomy in patients with symptomatic pancreas divisum. JOP : Journal of the pancreas. 2004. May;5(3):122–31. [PubMed] [Google Scholar]
- 93.Rustagi T, Golioto M. Diagnosis and therapy of pancreas divisum by ERCP: a single center experience. Journal of digestive diseases. 2013. February;14(2):93–9. doi: 10.1111/1751-2980.12004. [DOI] [PubMed] [Google Scholar]
- 94.Siegel JH, Cohen SA, Kasmin FE, et al. Stent-guided sphincterotomy. Gastrointestinal endoscopy. 1994. Sep-Oct;40(5):567–72. [DOI] [PubMed] [Google Scholar]
- 95.Crinò SF, Bernardoni L, Conti Bellocchi MC, et al. Efficacy of Endoscopic Minor Papilla Sphincterotomy for Symptomatic Santorinicele. Clinical Gastroenterology and Hepatology. 2017;15(2):303–306. doi: 10.1016/j.cgh.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 96.Varadarajulu S, Wilcox CM. Randomized trial comparing needle-knife and pull-sphincterotome techniques for pancreatic sphincterotomy in high-risk patients. Gastrointestinal endoscopy. 2006. November;64(5):716–22. doi: 10.1016/j.gie.2006.02.058. [DOI] [PubMed] [Google Scholar]
- 97.Attwell A, Borak G, Hawes R, et al. Endoscopic pancreatic sphincterotomy for pancreas divisum by using a needle-knife or standard pull-type technique: safety and reintervention rates. Gastrointestinal endoscopy. 2006. November;64(5):705–11. doi: 10.1016/j.gie.2006.02.057. [DOI] [PubMed] [Google Scholar]
- 98.Lawrence C, Romagnuolo J, Cotton PB, et al. Post-ERCP pancreatitis rates do not differ between needle-knife and pull-type pancreatic sphincterotomy techniques: a multiendoscopist 13-year experience. Gastrointestinal endoscopy. 2009. June;69(7):1271–5. doi: 10.1016/j.gie.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 99.Lee JK, Park JK, Yoon WJ, et al. Risk for post-ERCP pancreatitis after needle knife precut sphincterotomy following repeated cannulation attempts. Journal of clinical gastroenterology. 2007. April;41(4):427–31. doi: 10.1097/01.mcg.0000225695.46874.b5. [DOI] [PubMed] [Google Scholar]
- 100.Moffatt DC, Cote GA, Avula H, et al. Risk factors for ERCP-related complications in patients with pancreas divisum: a retrospective study. Gastrointestinal endoscopy. 2011. May;73(5):963–70. doi: 10.1016/j.gie.2010.12.035. [DOI] [PubMed] [Google Scholar]
- 101.Freeman ML, DiSario JA, Nelson DB, et al. Risk factors for post-ERCP pancreatitis: a prospective, multicenter study. Gastrointestinal endoscopy. 2001. October;54(4):425–34. [DOI] [PubMed] [Google Scholar]
- 102.Maple JT, Keswani RN, Edmundowicz SA, et al. Wire-assisted access sphincterotomy of the minor papilla. Gastrointestinal endoscopy. 2009. January;69(1):47–54. doi: 10.1016/j.gie.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 103.Sahin B, Parlak E, Cicek B, et al. Precutting of the minor papilla for pancreatic duct cannulation in pancreas divisum patients. Endoscopy. 2005. August;37(8):779. doi: 10.1055/s-2005-870133. [DOI] [PubMed] [Google Scholar]
- 104.Cheng CL, Sherman S, Watkins JL, et al. Risk factors for post-ERCP pancreatitis: a prospective multicenter study. The American journal of gastroenterology. 2006. January;101(1):139–47. doi: 10.1111/j.1572-0241.2006.00380.x. [DOI] [PubMed] [Google Scholar]
- 105.Sofuni A, Maguchi H, Itoi T, et al. Prophylaxis of post-endoscopic retrograde cholangiopancreatography pancreatitis by an endoscopic pancreatic spontaneous dislodgement stent. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2007. November;5(11):1339–46. doi: 10.1016/j.cgh.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 106.Sherman S, Eversman D, Fogel E, et al. Sphincter of oddi dysfunction (SOD): Needle-knife pancreatobiliary sphincterotomy over pancreatic stent (NKOPS) has a lower post-procedure pancreatitis rate than pull-type biliary sphincterotomy (BES). Gastrointestinal endoscopy. 1997;45(4):AB148. doi: 10.1016/S0016-5107(97)80496-8. [DOI] [Google Scholar]
- 107.Tarnasky PR. Mechanical prevention of post-ERCP pancreatitis by pancreatic stents: results, techniques, and indications. JOP : Journal of the pancreas. 2003. January;4(1):58–67. [PubMed] [Google Scholar]
- 108.Rashdan A, Fogel EL, McHenry L Jr., et al. Improved stent characteristics for prophylaxis of post-ERCP pancreatitis. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2004. April;2(4):322–9. [DOI] [PubMed] [Google Scholar]
- 109.Kerdsirichairat T, Attam R, Arain M, et al. Urgent ERCP with pancreatic stent placement or replacement for salvage of post-ERCP pancreatitis. Endoscopy. 2014. December;46(12):1085–94. doi: 10.1055/s-0034-1377750. [DOI] [PubMed] [Google Scholar]
- 110.Elmunzer BJ, Scheiman JM, Lehman GA, et al. A randomized trial of rectal indomethacin to prevent post-ERCP pancreatitis. The New England journal of medicine. 2012. April 12;366(15):1414–22. doi: 10.1056/NEJMoa1111103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Garg R, Mohan BP, Krishnamoorthi R, et al. Pre-endoscopic retrograde cholangiopancreatography (ERCP) administration of rectal indomethacin in unselected patients to reduce post-ERCP pancreatitis: A systematic review and meta-analysis. Indian journal of gastroenterology : official journal of the Indian Society of Gastroenterology. 2018. March;37(2):120–126. doi: 10.1007/s12664-018-0841-1. [DOI] [PubMed] [Google Scholar]
- 112.Inamdar S, Han D, Passi M, et al. Rectal indomethacin is protective against post-ERCP pancreatitis in high-risk patients but not average-risk patients: a systematic review and meta-analysis. Gastrointestinal endoscopy. 2017. January;85(1):67–75. doi: 10.1016/j.gie.2016.08.034. [DOI] [PubMed] [Google Scholar]
- 113.Lyu Y, Cheng Y, Wang B, et al. What is impact of nonsteroidal anti-inflammatory drugs in the prevention of post-endoscopic retrograde cholangiopancreatography pancreatitis: a meta-analysis of randomized controlled trials. BMC gastroenterology. 2018. July 4;18(1):106. doi: 10.1186/s12876-018-0837-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Buxbaum J, Yan A, Yeh K, et al. Aggressive hydration with lactated Ringer’s solution reduces pancreatitis after endoscopic retrograde cholangiopancreatography. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2014. February;12(2):303–7.e1. doi: 10.1016/j.cgh.2013.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Satterfield ST, McCarthy JH, Geenen JE, et al. Clinical experience in 82 patients with pancreas divisum: preliminary results of manometry and endoscopic therapy. Pancreas. 1988;3(3):248–53. [DOI] [PubMed] [Google Scholar]
- 116.Yamamoto N, Isayama H, Sasahira N, et al. Endoscopic minor papilla balloon dilation for the treatment of symptomatic pancreas divisum. Pancreas. 2014. August;43(6):927–30. doi: 10.1097/mpa.0000000000000148. [DOI] [PubMed] [Google Scholar]
- 117.Ikenberry SO, Sherman S, Hawes RH, et al. The occlusion rate of pancreatic stents. Gastrointestinal endoscopy. 1994. Sep-Oct;40(5):611–3. [DOI] [PubMed] [Google Scholar]
- 118.Johanson JF, Schmalz MJ, Geenen JE. Incidence and risk factors for biliary and pancreatic stent migration. Gastrointestinal endoscopy. 1992. May-Jun;38(3):341–6. [DOI] [PubMed] [Google Scholar]
- 119.Kozarek RA. Pancreatic stents can induce ductal changes consistent with chronic pancreatitis. Gastrointestinal endoscopy. 1990. Mar-Apr;36(2):93–5. [DOI] [PubMed] [Google Scholar]
- 120.Sherman S, Hawes RH, Savides TJ, et al. Stent-induced pancreatic ductal and parenchymal changes: correlation of endoscopic ultrasound with ERCP. Gastrointestinal endoscopy. 1996. September;44(3):276–82. [DOI] [PubMed] [Google Scholar]
- 121.Smith MT, Sherman S, Ikenberry SO, et al. Alterations in pancreatic ductal morphology following polyethylene pancreatic stent therapy. Gastrointestinal endoscopy. 1996. September;44(3):268–75. [DOI] [PubMed] [Google Scholar]
- 122.Mariani A, Di Leo M, Petrone MC, et al. Outcome of endotherapy for pancreas divisum in patients with acute recurrent pancreatitis. World journal of gastroenterology. 2014. December 14;20(46):17468–75. doi: 10.3748/wjg.v20.i46.17468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kanth R, Samji NS, Inaganti A, et al. Endotherapy in symptomatic pancreas divisum: a systematic review. Pancreatology : official journal of the International Association of Pancreatology (IAP) [et al]. 2014. Jul-Aug;14(4):244–50. doi: 10.1016/j.pan.2014.05.796. [DOI] [PubMed] [Google Scholar]
- 124.Michailidis L, Aslam B, Grigorian A, et al. The efficacy of endoscopic therapy for pancreas divisum: a meta-analysis. Annals of gastroenterology. 2017;30(5):550–558. doi: 10.20524/aog.2017.0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hafezi M, Mayschak B, Probst P, et al. A systematic review and quantitative analysis of different therapies for pancreas divisum. American journal of surgery. 2017. September;214(3):525–537. doi: 10.1016/j.amjsurg.2016.12.025. [DOI] [PubMed] [Google Scholar]
- 126.Bradley EL 3rd, Stephan RN. Accessory duct sphincteroplasty is preferred for long-term prevention of recurrent acute pancreatitis in patients with pancreas divisum. Journal of the American College of Surgeons. 1996. July;183(1):65–70. [PubMed] [Google Scholar]
- 127.Anderson MA, Akshintala V, Albers KM, et al. Mechanism, assessment and management of pain in chronic pancreatitis: Recommendations of a multidisciplinary study group. Pancreatology : official journal of the International Association of Pancreatology (IAP) [et al]. 2016. Jan-Feb;16(1):83–94. doi: 10.1016/j.pan.2015.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pappas SG, Pilgrim CH, Keim R, et al. The Frey procedure for chronic pancreatitis secondary to pancreas divisum. JAMA surgery. 2013. November;148(11):1057–62. doi: 10.1001/jamasurg.2013.3728. [DOI] [PubMed] [Google Scholar]
- 129.Skorzewska M, Romanowicz T, Mielko J, et al. Frey operation for chronic pancreatitis associated with pancreas divisum: case report and review of the literature. Przeglad gastroenterologiczny. 2014;9(3):175–8. doi: 10.5114/pg.2014.43581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Conci S, Ruzzenente A, Bertuzzo F, et al. Total Dorsal Pancreatectomy, an Alternative to Total Pancreatectomy: Report of a New Case and Literature Review. Digestive surgery. 2018. July 13:1–6. doi: 10.1159/000490198. [DOI] [PubMed] [Google Scholar]
- 131.Bellin MD, Abu-El-Haija M, Morgan K, et al. A multicenter study of total pancreatectomy with islet autotransplantation (TPIAT): POST (Prospective Observational Study of TPIAT). Pancreatology : official journal of the International Association of Pancreatology (IAP) [et al]. 2018. April;18(3):286–290. doi: 10.1016/j.pan.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bellin MD, Kerdsirichairat T, Beilman GJ, et al. Total Pancreatectomy With Islet Autotransplantation Improves Quality of Life in Patients With Refractory Recurrent Acute Pancreatitis. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2016. September;14(9):1317–23. doi: 10.1016/j.cgh.2016.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wilson GC, Sutton JM, Salehi M, et al. Surgical outcomes after total pancreatectomy and islet cell autotransplantation in pediatric patients. Surgery. 2013. October;154(4):777–83; discussion 783–4. doi: 10.1016/j.surg.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 134.Wilson GC, Sutton JM, Smith MT, et al. Completion pancreatectomy and islet cell autotransplantation as salvage therapy for patients failing previous operative interventions for chronic pancreatitis. Surgery. 2015. October;158(4):872–8; discussion 879–80. doi: 10.1016/j.surg.2015.04.045. [DOI] [PubMed] [Google Scholar]