A procedure called tissue expansion (TE) is used in reconstructive surgery to amend congenital or trauma-induced skin defects. During TE, a silicone expander is placed under the skin and gradually filled with saline to exert mechanical stretching (Zollner, Buganza Tepole, Gosain, & Kuhl, 2012; Zollner, Holland, Honda, Gosain, & Kuhl, 2013). Typically, TE takes about three months, and saline is added weekly. When moderately applied, TE stimulates skin growth, resulting in extra tissue that matches local characteristics. Although the procedure is commonly used in surgery, complications such as inflammation, necrosis, or scarring occur in up to 40% of the time (Bjornson, Bucevska, & Verchere, 2019; Karimi et al., 2019; LoGiudice & Gosain, 2003). TE is particularly difficult in anatomical regions such as the scalp and extremities (LoGiudice & Gosain, 2003; Tepole, Gosain, & Kuhl, 2012) suggesting that unknown factors limit mechanical stimulation, and necessitating more study. Porcine skin is similar to human skin, and these similarities are particularly evident in the epidermis, as both include undulated epidermal projections called rete ridges (RRs) (Penrose & Ohara, 1973; Rittie, 2016). The significance of RRs in epidermal maintenance is not well recognized, in humans, the appearance of RRs changes with age and health conditions; RRs are diminished in height in aging skin (Giangreco, Goldie, Failla, Saintigny, & Watt, 2010; Langton, Graham, Griffiths, & Watson, 2019), and are enlarged by diseases like psoriasis (Fraki, Briggaman, & Lazarus, 1983). Therefore, we employed a minipig model to study the effects of TE on epidermal structure. Our results indicate that acute skin stretching introduces morphogenetic changes in the epidermis. Mechanical force triggers a rapid proliferation of basal precursors leading to growth of the basal layer and enlargement of RRs. This mechanism protects the layered organization of the epidermis and increases the regenerative potential of expanded skin. Thus, brief mechanical stimulation could be considered as part of therapeutic procedures when an expansion of epidermal progenitors is needed.
We have previously developed a TE model using minipig (Purnell et al., 2018). All TE procedures were performed on a Yucatan strain of minipig. Three 5–6 week old females were fitted with two identical expanders placed subcutaneously on the back skin, one over the ribs, and the second over the abdomen. Expanders were placed under general anesthesia, the post-operative pain and distress were monitored and treated in accordance with IACUC protocol LCH14–006. After one week of recovery from the surgery, the expanders were inflated using remotely located external ports (LoGiudice & Gosain, 2003), each with 60 ml saline at 1 hour, 24 hours, 3 days, and 7 days before the animals’ sacrifice. In addition, a repeated stretch (TE-7+7 days), was developed by injecting the same volume of saline a second time, extending TE to 14 days. Punch biopsies from the apex of the expanded skin, and controls collected from the contralateral sites were preserved in paraffin, tissue sections (4 μm) were used for histology and immunostaining (Petridou & Skourides, 2016).
Maintenance of a healthy epidermis depends on a population of precursors that adhere to an underlying basement membrane (BM) through integrin receptors (Brakebusch et al., 2000). Moreover, mechanical force can activate β1 integrin at the lateral region of the cell cortex and change the orientation of the mitotic spindle and cell division (Petridou & Skourides, 2016). Accordingly, we hypothesized that during TE, β1 integrin will relocate dynamically, in order to adapt the basal cell to mechanical stimulation. If these changes promote symmetrical cell division than the basal layer would elongate, as evaluated on histological sections.
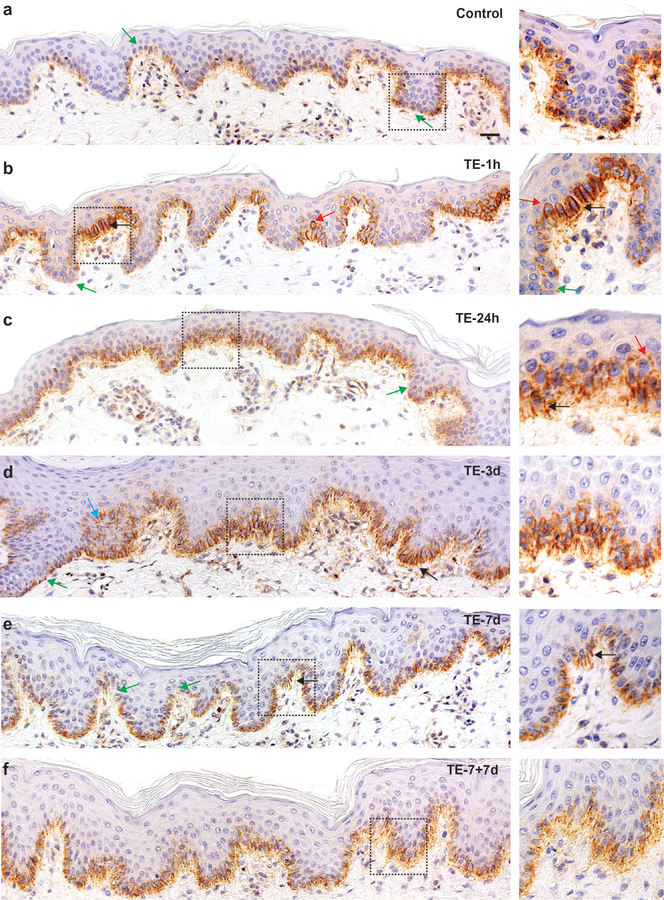
Consistent with the literature (Brakebusch et al., 2000; Hotchin, Gandarillas, & Watt, 1995; Jones, Harper, & Watt, 1995; Tan et al., 2013), we found that the normal porcine interfollicular epidermis (IFE) accumulates β1 integrin heterogeneously (Fig. 1 a). Cells at the tips of the RRs accumulated a relatively low level of the protein, but some basal cells covering the tips of the dermal papillae were strongly positive, suggesting that they may have been stem cells. However, after a one-hour stretch (TE-1h) larger clusters of negative cells were detected at RR tips (Fig. 1 b, green arrows), implying that the initial stretch caused detachment of some cells from the BM (Hotchin et al., 1995). In cells covering the tips of the dermal papillae, β1 integrin occurred in stripes between adjacent precursors (Fig. 1 b, black arrows) indicative of basolateral cortex accumulation. Furthermore, at TE-1h, we found β1 integrin at the apical site of mitotic precursors, with a frequency almost four times higher than in the control (p=0.0001) (Fig. 1 b, red arrows) suggesting reversed cell polarity (Clayton et al., 2007; Lechler & Fuchs, 2005). This effect gradually normalized, showing a 50±15% reduction by 24 hours (TE-24h, p=0.003). By 3 days of TE, β1 integrin almost disappeared from the apical localization (Fig. 1 c - d) and, became visible along the entire basal layer in a diffused manner (TE-7d and TE-7+7d). Stripes between cells were now rarely detected (Fig. 1 e, f, black arrows), confirming that basal cells were actively rearranging β1 integrin under continual force.
Fig. 1.
Changes in localization of β1 integrin induced in TE. Representative images of epidermis stained by IHC, nuclear counterstain with hematoxylin; the right panel represents electronically zoomed dashed squares; arrows indicate: green – negative/reduced β1 integrin, red – apical localization, black – stripes between adjacent cells, blue - suprabasal location; 20x magnification, scale bar = 20 μm. Images were captured using Axiovision Zeiss microscope and processed in Photoshop.
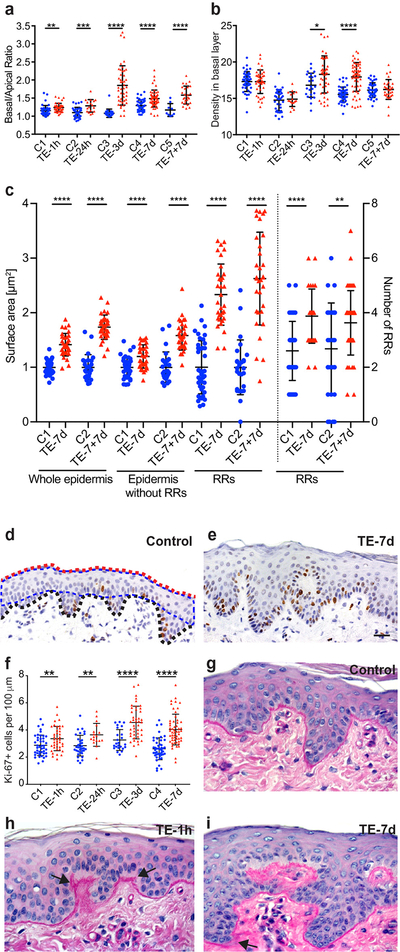
To farther evaluate epidermal responses to TE, we calculated the ratio of basal to apical length for each model (N≥3 section/model; ≥15 images/section) (Fig. 2 a). These data revealed progressively increased basal layer length with a maximum increase of 71±10% at TE-3d as compared to the control (p<0.0001). The comparison of TE-7d (35±8%) and TE-7+7d (39±4%) showed no statistically significant difference, suggesting that the elongation of basal layer slows down considerably in the second week of TE. Basal cell density, calculated as the number of nuclei per 100 μm of basal layer, was initially unchanged between control and treatment (TE-1h and TE-24h), but increased by 9±3% in TE-3d (p=0.01) and by 15±2% in TE-7d (p<0.0001), indicating that initial stretching favored symmetrical cell divisions (Fig. 2 b). By the 14th day of TE, cell density had normalized to the control level, reaching on average 16 nuclei per 100 μm of basal layer.
Fig. 2.
Quantification of morphological changes in epidermis under TE. (a) The basal/apical length ratio. (b) Cell density calculated as all nuclei per 100 μm of the basal layer. (c) Comparison of the surface area presented as a mean ± SD and number of RRs between TE-7d and TE-7+7d models. The mean of surface area for C1 was arbitrarily assigned as 1. (d, e) Representative images of IHC for Ki-67 and (f) number of Ki-67 positive cells per 100 μm of the basal layer. The red and black doted lines indicate apical and basal layers, respectively. The blue doted line indicates the surface area without RRs. (g-i) The representative images of PAS staining for BM. The measurements were collected and analyzed using ZEN Blue Zeiss software, and GraphPad Prism. Statistical significance was assessed by unpaired Student t-test *p≤0.05, **p≤0.01, ***p≤0.001, ****p<0.0001; 40x magnification, scale bar = 20 μm. A minimum of three tissue sections for each category and ten images for each section were examined for every model.
Morphological examination of histological sections indicated that the epidermis became more undulated under TE. Quantification of this phenomenon (N≥3 sections/model; N≥10 images per section) determined that the number of RRs doubled by TE-3d as compared to control (p<0.0001) and remained unchanged between TE-7d and TE-7+7d (Fig. 2 c). The surface area of RR (measured on histological sections as illustrated at Fig. 2 d), increased by 33±13% in Ex-7d and 62±19% in TE-7+7d, showing a significant enlargement of RRs in the second week of TE. Growth of the stratified epidermis, calculated as the surface area of the epidermis without RRs, increased by 19±5% (Ex-7d) and 58±7% (TE-7+7d). Total epidermal thickness increased as well, by 44±4% in TE-7d (p<0.0001) and by 77±6% in TE-7+7d (p<0.0001), as compared to the corresponding controls.
To correlate the above results with cell proliferation, we calculated the mitotic index for each model using IHC with Ki-67 marker (N ≥ 3 sections/model; ≥ 25 images per section) (Fig. 2 d–f). Relative to controls, mitotic indexes were increased by: 2.8±1% (p=0.004) TE-1h, 5.1±1.6% (p=0.003) TE-24h, 5.3±1.3% (p=0.0004) TE-3d, 5.6±1% (p<0.0001) TE-7d, and 5.7±1.1% (p<0.0001) for TE-7+7d. Thus, the highest increase in cell proliferation as a result of TE took place within the first 24 hours. Sporadic suprabasal location of Ki-67 and β1 integrin positive cells, indicative of local hyperproliferation, was observed in TE-3d at the expansion site over the ribs (Fig.1 d, blue arrow, and data not shown). Based on our previous work, we expect that these expanders exert higher mechanical stimuli than those placed over the abdomen, suggesting that stretching in this location was in the upper limits of epidermal tolerance to maintain overall healthy tissue.
As basal precursors are intimately connected to BM (Brakebusch et al., 2000; Raghavan, Bauer, Mundschau, Li, & Fuchs, 2000; Yang et al., 2016), we employed Periodic Acid-Schiff (PAS) REF staining to visualize the BM structure (Fig. 2 c - e). While stretches of disorganized BM and thickening areas were found, especially around growing RRs (black arrows), the BM was never obliterated.
Here, we present the effects of acute TE on epidermal structure and basal cells qualities. We found β1 integrin to be a very sensitive responder to mechanical stimulation instigated by TE procedure, able to dynamically relocate under external force. The basal cell is naturally polarized by attachment to the BM, and its location along the undulated basal layer dictates the cell’s orientation toward the epidermal surface. Thus, cells located at the slope of a RR as compared to those positioned parallel to the epidermal surface are exposed to different force trajectories upon the TE, so they shift β1 integrin differently. Based on literature and our data, we assume that β1 integrin accumulated at the lateral cortex of mitotic cells (stripes between cells in TE-1h), indicates the parallel orientation of the mitotic spindle, which favors symmetric cell divisions (Fink et al., 2011; Lechler & Fuchs, 2005; Petridou & Skourides, 2016). The apical location of β1 integrin indicates reverse cell polarity and likely impaired asymmetrical division further supports elongation of basal layer. However, we assume that the balance between these two types of cell division was promptly restored because in the second week of TE the accumulation of β1 integrin acquired a diffused pattern and this coincided with slower elongation of the basal layer. The expanded epidermis maintained stratified organization through all tested TE procedures, which further indicates the presence of asymmetric divisions required in epidermal homeostasis (Lechler & Fuchs, 2005).
In summary, morphogenetic changes initiated by the relocation of β1 integrin represent an adaptive response of the epidermis to mechanical stress. The basal layer elongation and RR growth absorbed the impact of the rapid proliferation in order to preserve the stratified organization and functionally of the tissue. Repeated mechanical stimulation with a seven-day interval generated healthy tissue without detrimental effects, suggesting a good approach for clinical settings.
ACKNOWLEDGMENTS,
We gratefully acknowledge the support by awards from NIH/NIBIB (EB021590) and Lurie Children’s Hospital Faculty Practice Plan.
Abbreviations:
- BM
basement membrane
- IFE
interfollicular epidermis
- IHC
immunohistochemistry
- KCs
keratinocytes
- RRs
rete ridges
- TE
tissue expansion
Footnotes
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
REFERENCES
- Brakebusch C, Grose R, Quondamatteo F, Ramirez A, Jorcano JL, Pirro A, … Fassler R (2000). Skin and hair follicle integrity is crucially dependent on beta 1 integrin expression on keratinocytes. EMBO J, 19(15), 3990–4003. doi: 10.1093/emboj/19.15.3990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton E, Doupe DP, Klein AM, Winton DJ, Simons BD, & Jones PH (2007). A single type of progenitor cell maintains normal epidermis. Nature, 446(7132), 185–189. doi: 10.1038/nature05574 [DOI] [PubMed] [Google Scholar]
- Fink J, Carpi N, Betz T, Betard A, Chebah M, Azioune A, … Piel M (2011). External forces control mitotic spindle positioning. Nat Cell Biol, 13(7), 771–778. doi: 10.1038/ncb2269 [DOI] [PubMed] [Google Scholar]
- Fraki JE, Briggaman RA, & Lazarus GS (1983). Transplantation of psoriatic skin onto nude mice. J Invest Dermatol, 80 Suppl, 31s–35s. [PubMed] [Google Scholar]
- Giangreco A, Goldie SJ, Failla V, Saintigny G, & Watt FM (2010). Human skin aging is associated with reduced expression of the stem cell markers beta1 integrin and MCSP. J Invest Dermatol, 130(2), 604–608. doi: 10.1038/jid.2009.297 [DOI] [PubMed] [Google Scholar]
- Hotchin NA, Gandarillas A, & Watt FM (1995). Regulation of cell surface beta 1 integrin levels during keratinocyte terminal differentiation. J Cell Biol, 128(6), 1209–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PH, Harper S, & Watt FM (1995). Stem cell patterning and fate in human epidermis. Cell, 80(1), 83–93. [DOI] [PubMed] [Google Scholar]
- Lechler T, & Fuchs E (2005). Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature, 437(7056), 275–280. doi: 10.1038/nature03922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann CG (1957). The expansion of an area of skin by progressive distention of a subcutaneous balloon; use of the method for securing skin for subtotal reconstruction of the ear. Plast Reconstr Surg (1946), 19(2), 124–130. [DOI] [PubMed] [Google Scholar]
- Penrose LS, & Ohara PT (1973). The development of the epidermal ridges. J Med Genet, 10(3), 201–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petridou NI, & Skourides PA (2016). A ligand-independent integrin beta1 mechanosensory complex guides spindle orientation. Nat Commun, 7, 10899. doi: 10.1038/ncomms10899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purnell CA, Gart MS, Buganza-Tepole A, Tomaszewski JP, Topczewska JM, Kuhl E, & Gosain AK (2018). Determining the Differential Effects of Stretch and Growth in Tissue-Expanded Skin: Combining Isogeometric Analysis and Continuum Mechanics in a Porcine Model. Dermatol Surg, 44(1), 48–52. doi: 10.1097/DSS.0000000000001228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavan S, Bauer C, Mundschau G, Li Q, & Fuchs E (2000). Conditional ablation of beta1 integrin in skin. Severe defects in epidermal proliferation, basement membrane formation, and hair follicle invagination. J Cell Biol, 150(5), 1149–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan DW, Jensen KB, Trotter MW, Connelly JT, Broad S, & Watt FM (2013). Single-cell gene expression profiling reveals functional heterogeneity of undifferentiated human epidermal cells. Development, 140(7), 1433–1444. doi: 10.1242/dev.087551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepole AB, Gosain AK, & Kuhl E (2012). Stretching skin: The physiological limit and beyond. Int J Non Linear Mech, 47(8), 938–949. doi: 10.1016/j.ijnonlinmec.2011.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]