Summary
Sodium glucose cotransporter-2 (SGLT-2) inhibitors are approved to treat type 2 diabetes. These drugs provide multiple benefits – including glucose-lowering, weight loss, blood pressure lowering, and decreasing the risk of major adverse cardiovascular events. To address the high unmet medical need associated with type 1 diabetes, multiple clinical trials have evaluated the efficacy and safety of SGLT2 inhibitors in combination with insulin as therapy for type 1 diabetes. This review summarizes data from eight recent clinical trials of canagliflozin, dapagliflozin, empagliflozin, and sotagliflozin. Maximal HbA1c-lowering efficacy is observed early in the trials (at 8–12 weeks), but the magnitude of HbA1c-lowering wanes with longer duration of treatment (up to 52 weeks). Data are not yet available to determine how long glycemic efficacy will be sustained during chronic therapy in type 1 diabetic patients. Moreover, SGLT2 inhibitor therapy induces serious adverse events: a 5- to 10-fold increase in the risk of ketoacidosis. The US Food and Drug Administration estimated that one additional case of ketoacidosis will occur for every 26 type 1 diabetic patients under treatment (~4000 cases per year for every 100,000 patients under treatment). If one assumes a case mortality of 0.4%, this translates into 16 additional deaths each year. These considerations raise important questions about the benefit:risk profile of SGLT2 inhibitors when used as adjunctive therapy for type 1 diabetes.
Introduction
Type 1 diabetes markedly increases the risk of chronic complications including blindness, end-stage renal disease, and amputations. As demonstrated in the Diabetes Control and Complications Trial (DCCT)1, intensive insulin therapy decreases the risk of chronic microvascular complications of diabetes – a benefit believed to be mediated by improved glycemic control. Nevertheless, less than one third of type 1 diabetic patients achieve a glycemic target of HbA1c<7.0%2–4. Accordingly, there is keen interest to address this unmet medical need by improving the standard of care. Adjunctive therapies have been added to insulin to improve glycemic control5,6. SGLT2 inhibitors have been reported to provide multiple clinical benefits in the context of type 2 diabetes – including cardioprotection7,8 and renoprotection8–11. We review eight recent clinical trials evaluating efficacy and safety of SGLT2 inhibitors as adjunctive therapies of type 1 diabetes in combination with insulin12–19. All eight clinical trials observed dramatic increases in the risk of ketoacidosis – a life-threatening adverse event. The trials provided evidence of only modest HbA1c-lowering, which was maximal early in the trials (approximately eight weeks) but waned significantly over the duration of the trials14,17. The trials did not provide strong evidence that clinical benefits outweigh the substantial risk of serious side effects.
On February 1, 2019, the European Medicines Agency’s human medicines committee issued a positive opinion for dapagliflozin as an adjunctive treatment for type 1 diabetes – limited to type 1 diabetic patients with a BMI ≥27 kg/m2. They emphasized that insulin therapy should be continuously optimised to prevent ketosis and DKA, and that SGLT2 inhibitor therapy should be supervised by specialist doctors. Sotagliflozin is currently under review by the US Food and Drug Administration (FDA). An Advisory Committee meeting was held on January 17, 2019 at which eight Committee members voted to recommend approval and eight voted against approval.
Clinical Trial Design
This review includes eight randomized placebo-controlled clinical trials investigating safety and efficacy of SGLT2 inhibitors as adjunctive therapies to treat type 1 diabetes12–19 (Fig. S1). To be included in this review, studies were required to have a minimum duration of 12 weeks because this length of time is sufficient to achieve steady-state levels of HbA1c. Further, trials were required to have studied at least 100 research subjects per treatment arm – the minimum size to begin to obtain meaningful estimates of side effects occurring in 1–10% of patients. All eight clinical trials were sponsored by the pharmaceutical companies developing the drugs.
Most of the clinical trials included lead-in periods prior to administration of SGLT2 inhibitors. Patients’ baseline data were assessed at the end of the lead-in period (Table S1). The phase 2 canagliflozin trial12 included a short 2-week lead-in period. The DEPICT trials of dapagliflozin13–15 included 8-week lead-in periods for intensification of insulin therapy, during which time HbA1c was decreased by ~0.3%13,14. The EASE-2 and EASE-3 trials of empagliflozin19 as well as the inTandem1 and inTandem2 trials of sotagliflozin17,18 included 6-week lead-in periods. Intensification of insulin therapy substantially decreased HbA1c (~0.6–0.7%) during the lead-in periods. The inTandem3 clinical trial initiated sotagliflozin treatment without a lead-in period16 – leading to higher baseline HbA1c levels than in inTandem1 and inTandem2.
Prescribing Information describes pharmacodynamic drug-drug interactions whereby SGLT2 inhibitors increase susceptibility to hypoglycemia in insulin-treated type 2 diabetic patients. Accordingly, it is recommended to decrease the insulin dose when SGLT2 inhibitors are combined with insulin. Consistent with this recommendation, all eight clinical trials decreased insulin doses at the time treatment was initiated with study drug or placebo (Table S1): by 10% for the empagliflozin trials19, 10–20% for the canagliflozin trial12, 0–20% for the dapagliflozin trials13,14, and 30% for the sotagliflozin trials16–18.
After the initial decrease in insulin dose, physicians adjusted insulin regimens to optimize glycemic control. The studies of sotagliflozin16–18 and canagliflozin12 provided specific glycemic targets and algorithms to guide insulin therapy. The inTandem1 and inTandem2 trials of sotagliflozin established expert Insulin Dose Management Committees to oversee management of the insulin dose17,18. In contrast, clinical trials for dapagliflozin and empagliflozin relied on local guidelines for the best standard of care13,14,19. All patients were instructed to implement self-monitoring of glucose to guide adjustment of insulin doses.
Shortly after first approval of SGLT2 inhibitors to treat type 2 diabetes, regulatory agencies warned that these drugs have the potential to induce ketoacidosis20–22. The risk is greater in patients with type 1 compared to type 2 diabetes. All eight clinical trials excluded patients with recent episodes of ketoacidosis. With the exception of the canagliflozin trial12, the other seven trials provided patients with meters for self-monitoring of blood beta-hydroxybutyrate levels13–19. In addition, both patients and healthcare providers were trained in early detection and management of ketosis and ketoacidosis (Table S1). These precautions exceed what is routinely available to patients in the “real world”.
Patient populations in the eight trials were similar with respect to mean age (40–46 years), sex (41–57% female), and mean BMI (27.3–29.8) (Table S2). While most of the clinical trials reported primary outcomes after 24–26 weeks of SGLT2 inhibitor therapy13,15–19, the canagliflozin trial investigated the effect after treating for only 18 weeks12. Half of the clinical trials (inTandem1, inTandem2, DEPICT-1, and EASE-2) also reported secondary outcomes after 52 weeks of therapy14,17–19. While some patients followed multiple dose injection (MDI) regimens, other patients used continuous subcutaneous insulin infusion pumps (CSII) (Table S2). The percentage of patients using pumps ranged from 26% in the European inTandem2 clinical trial15 to 63% in the phase 2 trial of canagliflozin12.
Clinical Outcomes: Glycemic Efficacy
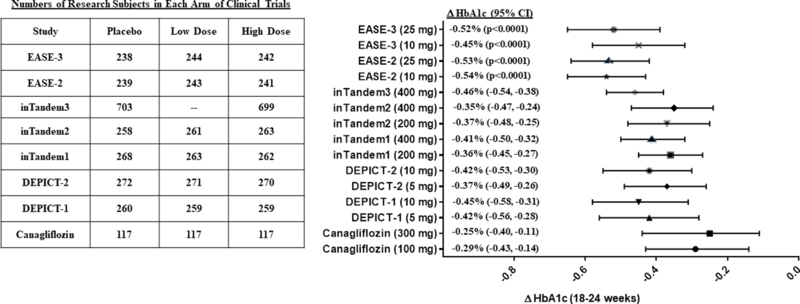
Baseline levels of HbA1c varied among the eight clinical trials. The highest levels were observed in the dapagliflozin clinical trials (mean baseline HbA1c ≈ 8.4–8.5%; 68.3–69.4 mmol/mol)13–15. The lowest levels were observed in the inTandem1 and inTandem2 trials with sotagliflozin (mean baseline HbA1c ≈ 7.5–7.8%; 58.5–61.7 mmol/mol)17,18. When added to insulin therapy, all four SGLT2 inhibitors decreased HbA1c. The magnitude of HbA1c lowering varied over a relatively narrow range for dapagliflozin, empagliflozin, and sotagliflozin – averaging 0.35–0.54% after 24–26 weeks of therapy13–19 (Fig. 1). Canagliflozin induced more modest HbA1c-lowering (0.25–0.29%)12. The means (±SEM) for HbA1c-lowering at 18–26 weeks were similar for both doses: 0.40% (±0.03%) for the low doses and 0.42% (±0.03%) for the high doses. Nevertheless, the various clinical trials differed with respect to management of insulin therapy, which could have had major impact upon glycemic efficacy. In any case, head-to-head comparisons would be required to draw rigorous conclusions about comparative efficacy.
Figure 1. HbA1c-lowering in eight clinical trials of SGLT2 inhibitors as adjunctive therapy in combination with insulin in patients with type 1 diabetes.
The right hand panel illustrates placebo-subtracted changes in HbA1c after 18 weeks of treatment with canagliflozin12, 24 weeks of dapagliflozin (DEPICT-113,14 and DEPICT-215), 24 weeks of sotagliflozin (inTandem117, inTandem218, inTandem316), or 26 weeks of empagliflozin (EASE-2 and EASE-319). With the exception of the EASE-2 and EASE-3 trials, 95% confidence intervals were explicitly stated in the publications. The 95% confidence intervals were estimated from error bars in Fig. 1B of the publication by Rosenstock et al.19. The left hand panel summarizes the numbers of research subjects in each arm of the eight clinical trials.
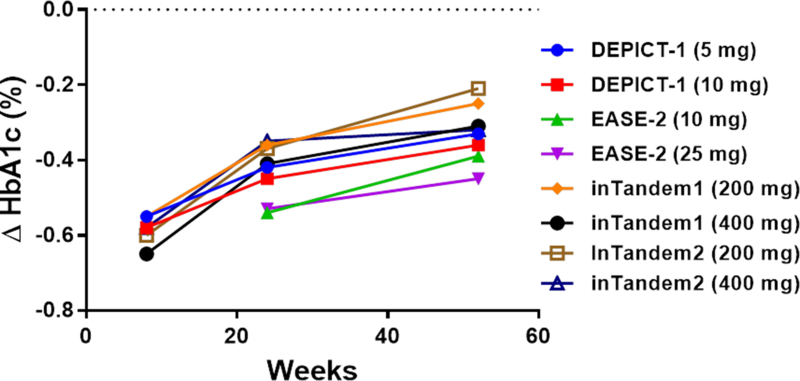
Four of the eight clinical trials included extensions providing outcome data at 52 weeks: DEPICT-114, EASE-219, inTandem117, and inTandem218. Maximal glycemic efficacy was observed at 8 weeks, and waned progressively over time (Fig. 2A). In all four studies, HbA1c-lowering was approximately 0.10–0.15% less at 52 weeks than at 24–26 weeks14,17–19. None of the studies reported data establishing whether glycemic efficacy would be sustained beyond one year of therapy. Long-term chronic therapy is likely required to have major impact on the risk of chronic diabetic complications. Thus, although it would have been more relevant to conduct longer duration studies (e.g., 3–5 years), DEPICT-215, EASE-319, and inTandem316 did not report outcomes beyond 24–26 weeks.
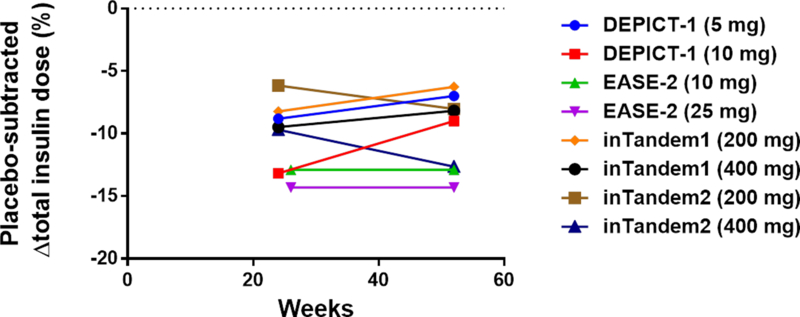
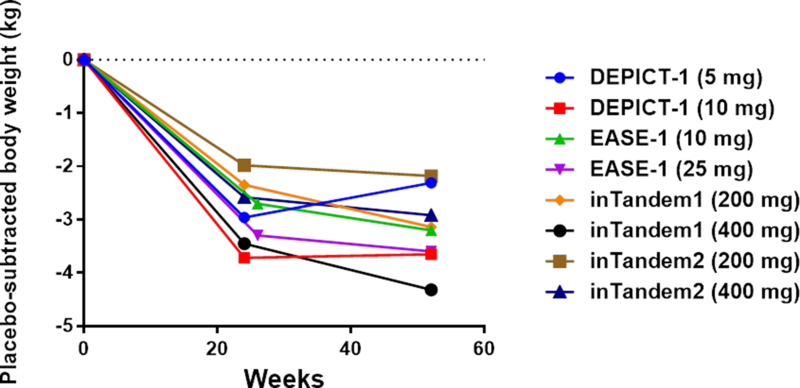
Figure 2. Progression of end-points over time in four clinical trials with 52 weeks of follow up.
Four clinical trials (DEPICT-114, inTandem117, inTandem218, and EASE-219) reported data after 52 weeks of follow-up. The graphs depict point estimates of placebo-subtracted changes in HbA1c (panel A), insulin doses (panel B), and body weight (panel C).
What were the underlying mechanisms driving the progressive loss of HbA1c-lowering efficacy? Based on the low drop-out rates in the trials, it seems unlikely that the observations are explained by lack of adherence to trial protocols. Furthermore, similar total doses of insulin were administered at 24–26 weeks and at 52 weeks (Fig. 2B). So, the waning efficacy cannot be accounted for by decreases in insulin dose. The literature suggests another possible mechanism. Classical symptoms of uncontrolled diabetes include polyuria, polydipsia, and polyphagia. Urinary loss of water triggers thirst, and urinary loss of glucose increases appetite. Ferrannini et al.23 investigated the effect of empagliflozin on energy balance in type 2 diabetic patients over the course of 90 weeks. Patients lost only 29% of the weight predicted based on the magnitude of calorie loss in the urine (−3.2 kg observed weight loss versus −11.3 kg predicted). The investigators concluded that mean calorie intake was increased by 269 kCal/day (~13% increase over baseline). Interestingly, the curve for the time course for observed weight loss begins to deviate from the curve for predicted weight loss at ~24 weeks – at which time observed body weight stabilizes23. Accordingly, a new steady-state is achieved after ~24 weeks – with increased food intake counterbalancing ongoing urinary calorie loss. This suggests that the drugs’ effects to promote net calorie loss might be transient. Thus, based on the work of Ferrannini et al.23, we propose that a drug-induced increase in calorie intake may explain the progressive loss of glycemic efficacy observed after 24 weeks of SGLT2 inhibitor therapy in type 1 diabetic patients. Interestingly, the weight loss observed at 24–26 weeks in type 1 diabetic patients is very similar to the magnitude of weight loss observed in type 2 diabetic patients23. The majority of the weight loss occurs within the first 24 weeks, with the average patient losing little if any additional weight between 24 and 52 weeks (Fig. 2C).
Clinical Outcomes: Severe Hypoglycemia and Adjudicated Ketoacidosis
To mitigate the risk of SGLT2 inhibitor-induced hypoglycemia, insulin doses were decreased by 10–30% when study drug (either placebo or SGLT2 inhibitor) was initiated. Subsequently, insulin doses were up-titrated with the objective of optimizing glycemic control. This approach did not entirely prevent hypoglycemia in the canagliflozin clinical trial12, which reported a dose-dependent increase in the risk of severe hypoglycemia. In contrast, SGLT2 inhibitor treatment was not associated with a consistent increase in the risk of severe hypoglycemia in the other seven clinical trials12–19 (Table 1).
Table 1.
Summary of selected outcomes in eight clinical trials of SGLT2 inhibitors as adjunctive therapy in combination with insulin in type 1 diabetes.
| Drug | Study Name | Severe Hypoglycemia | Adjudicated Ketoacidosis | Urinary Tract Infections | Genital Mycotic Infections (Male/Female) |
|---|---|---|---|---|---|
| Canagliflozin, 100 mg 300 mg placebo |
(18-wk Phase 2 study)12 | 2.6% 6.8% 1.7% |
4.3% 6.0% 0% |
4.3% 5.1% 1.7% |
0%/4.2% 0%/21.2% 0%/5.6% |
| Dapagliflozin, 5 mg 10 mg placebo |
DEPICT-113.14 (“Definite DKA”) |
10.5% 8.4% 11.5% |
4.0% 3.4% 1.9% |
11.6% 5.4% 8.1% |
7.6%/21.5% 8.6%/18.8% 0/6.3% |
| Dapagliflozin, 5 mg 10 mg placebo |
DEPICT-215 (“Definite DKA”) |
1.8% 0% 0.4% |
2.6% 2.2% 0% |
6.6% 3.7% 4.4% |
2.5%/15.7% 1.7%/12.8% 0%/3.3% |
| Empagliflozin, 10 mg 25 mg placebo |
EASE-2/3 pooled19 (“Certain DKA”) |
4.1% 2.7% 3.1% |
4.3% 3.3% 1.2% |
9.6% 8.4% 8.5% |
12.8% 14.3% 4.3% |
| Empagliflozin, 2.5 mg placebo |
EASE-319 (“Certain DKA”) |
1.2% 2.5% |
0.8% 1.2% |
5.4% 4.6% |
5.4% 2.5% |
| Sotagliflozin, 200 mg 400 mg placebo |
inTandem117 | 6.5% 6.5% 9.7% |
1.5% 1.5% 0% |
9.9% 4.2% 7.1% |
9.1% 13.0% 3.4% |
| Sotagliflozin, 200 mg 400 mg placebo |
inTandem218 | 5.0% 2.3% 5.0% |
2.7% 4.9% 0% |
4.2% 6.8% 5.0% |
9.2% 11.0% 2.3% |
| Sotagliflozin, 400 mg placebo |
inTandem316 | 3.0% 2.4% |
3.0% 0.6% |
3.6% 3.8% |
6.4% 2.1% |
All eight clinical trials demonstrated a dose-dependent increase in the risk of confirmed ketoacidosis (Table 1). The eight clinical trials included 2362 placebo-treated patients and 4076 SGLT2 inhibitor-treated patients. Among these, 3.5% of the SGLT2 inhibitor-treated patients developed ketoacidosis versus 0.6% of the placebo-treated patients – corresponding to a 5.8-fold increase in risk. Among the 143 patients who developed ketoacidosis, there was one death19. In contrast, the 2.5 mg dose of empagliflozin (fourfold lower than the lowest approved dose) did not increase the risk of ketoacidosis. While the 2.5 mg dose exhibited superior safety, it provided less glycemic efficacy: 0.28% HbA1c lowering as compared to 0.45% for the 10 mg dose19. Taken together, these data argue strongly for the value of investigating safety and efficacy of doses below the lowest approved (or proposed) doses of SGLT2 inhibitors: empagliflozin (<10 mg), sotagliflozin (<200 mg), dapagliflozin (<5 mg), <100 mg of canagliflozin (<300 mg), and ertugliflozin (<5 mg).
Other Secondary Outcomes
Table S3 summarizes data for two secondary measures of clinical efficacy: decreases in body weight and insulin dose. Placebo-subtracted mean weight losses were in the range of 3–5% during the clinical trials. Total daily doses of insulin were decreased in all the clinical trials (Table 2) – ranging from a decrease of ~5% for 200 mg of sotagliflozin in inTandem218 to a decrease of ~22% for 10 mg of dapagliflozin in DEPICT-113,14.
Table 1 summarizes risks of genitourinary infections. While these clinical trials did not demonstrate a consistent effect of SGLT2 inhibitors on the risk of urinary tract infections, the drugs caused a 3- to 5-fold increase the risk of mycotic genital infections in females – similar to risks reported in a meta-analysis of SGLT2 inhibitor-induced infectious complications in type 2 diabetic patients24.
Conclusions
Based on data with sotagliflozin, the FDA estimated an 8-fold increase in the risk of ketoacidosis with an additional case of ketoacidosis being observed for every 26 patient-years of adjunctive therapy [https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/UCM629782.pdf]. The data in the other five clinical trials are consistent with this estimate. If one assumes that 100,000 type 1 diabetic patients would be treated with SGLT2 inhibitors, this would translate into almost 4000 additional cases of ketoacidosis per year. Based on a case-fatality rate of 0.4%25, this would translate into ~16 deaths per year. If, as expected, the risk of ketoacidosis is greater when these drugs are used in real world clinical practice, it is possible that the risk of death would be even higher. These considerations raise critical questions about the benefit-risk of adjunctive therapy with SGLT2 inhibitors for patients with type 1 diabetes. Especially in the absence of proof that SGLT2 inhibitors will provide sustained glycemic efficacy over long periods of time in type 1 diabetic patients, it is unproven whether this approach provides sufficient protection from microvascular complications to outweigh the increased risk of death. Although some experts have described SGLT2 inhibitor induced ketoacidosis as “manageable” or “preventable”22,26, ketoacidosis ceases to be manageable if the patient dies.
It is instructive to place these data in the context of the tragic history of troglitazone. As reported in The New York Times (March 22, 2000), the FDA removed troglitazone from the market because of 63 deaths occurring during the three years it was on the market in the US. Based on our estimates, SGLT2 inhibitor-induced ketoacidosis would be predicted to result in death of ~64 deaths over the course of four years under the assumption that 100,000 type 1 diabetic patients received SGLT2 inhibitor therapy – a much smaller “at risk” population than the 1.9 million individuals who received troglitazone. In short, type 1 diabetic patients are predicted to be a far greater risk of death from SGLT2 inhibitors than the risk that led the FDA to remove troglitazone from the market.
When used in combination with insulin, SGLT2 inhibitors decreased mean HbA1c by approximately 0.4% at 18–26 weeks. However, the DCCT1 studied the benefit of sustained improvement in glycemic control for ten years. Indeed, intensive insulin therapy actually accelerated progression of retinopathy during the first two years of the DCCT. Moreover, there was a 3–5 year time lag before intensive insulin therapy decreased the rate of progression of diabetic kidney disease (albuminuria). Thus, it is not clear whether 24 weeks of improved glycemic control would actually slow the progression of microvascular complications. In this context, it is particularly concerning that the clinical trials demonstrated a consistent and substantial loss of HbA1c-lowering between 24–52 weeks. It is critical to investigate the glycemic efficacy of chronic adjunctive therapy with SGLT2 inhibitors (e.g., at least 3–5 years). Unfortunately, there are no published clinical trials demonstrating either sustained glycemic efficacy beyond a year or favorable impact on the risk of microvascular complications in type 1 diabetic patients.
Limitations
Insulin is among the most challenging drugs to use. Optimal therapy requires frequent adjustments of doses – guided by patients’ self-monitoring of glucose and influenced by changes in food intake and physical activity. In these studies of adjunctive therapy with SGLT2 inhibitors, all of the clinical end-points are greatly influenced by management of insulin dosing. This creates challenges in defining the impact of the SGLT2 inhibitor versus the effect of the concomitant insulin therapy. Some differences among the eight clinical trials may be due to variations in management of insulin regimens. For example, the canagliflozin trial was unique in observing a substantial increase in the risk of severe hypoglycemia in SGLT2 inhibitor-treated patients12. It is noteworthy that this trial had the earliest publication date. It is possible that later clinical trials benefited from experiences in early trials, which taught clinicians how to minimize hypoglycemia risk. Similarly, the EASE trials with empagliflozin were outliers with the lowest risk of SGLT2 inhibitor-induced ketoacidosis19. While all trials proactively decreased insulin doses to mitigate the risk of SGLT2 inhibitor-induced hypoglycemia, the EASE trials implemented the smallest decrease in insulin dose. This approach may have contributed to the lower risk of ketoacidosis when compared to trials that required larger decreases in insulin dose. Given the differences in study design among the eight trials, we have not attempted to conduct a quantitative meta-analysis combining data from the eight clinical trials. However, a recent meta-analysis has been published27, which included six publications included in our review12,13,15–18, but did not include the EASE-2 or EASE-3 trials19 or the 52 week follow up for the DEPICT-1 trial14. Also, that meta-analysis27 included several studies that were either too short or too small for inclusion in our review.
Our most novel insight is to draw attention to an exploratory analysis demonstrating a progressive decrease in glycemic efficacy (i.e., HbA1c-lowering). Further, we have provided a plausible hypothesis that progressive loss of efficacy is mediated by a compensatory increase in food intake triggered by drug-induced urinary loss of calories. This hypothesis is based on analogy to the study of Ferrannini et al.23 conducted in patients with type 2 diabetes. While those authors postulated an increase in food intake, their assessment of total daily energy expenditure was derived from a mathematical model rather than empirical measurements. Thus, it remains possible that drug-induced decreases in energy expenditure might also have contributed. For example, Leibel et al. have reported that weight loss induces a compensatory decrease in total energy expenditure28.
This review included sotagliflozin together with marketed SGLT2 inhibitors. Although sotagliflozin has been branded as a dual inhibitor29, it is reported to have 20-fold lower Ki for inhibition of SGLT2 versus SGLT130. Furthermore, because Km values for glucose are ~0.5 and ~5 mmol/L for SGLT1 and SGLT2, respectively31, sotagliflozin is predicted to achieve 50% inhibition of SGLT2 at drug levels that are ~100-fold lower than for SGLT1 at physiological levels of glucose (~5 mmol/L). While sotagliflozin is slightly less selective than marketed SGLT2 inhibitors, it seems appropriate to include sotagliflozin in the class of SGLT2 inhibitors.
Implications for Public Policy and Regulatory Science
Many questions need to be answered:
Will glycemic efficacy be sustained with chronic therapy? The maximum decrease in HbA1c was observed after approximately 8 weeks, but glycemic efficacy appeared to wane between eight and 52 weeks14,17–19. Longer duration studies (e.g., 3–5 years) will be required to determine whether adjunctive therapy with SGLT2 inhibitors will provide sustained HbA1c-lowering. Longer duration studies could also provide data on progression of retinopathy and diabetic kidney disease. Although the DCCT demonstrated that sustained HbA1c-lowering protects against chronic diabetic complications, there was a 3–5 year lag before clinical benefit was observed1. How long must SGLT2 inhibitor therapy be maintained to provide clinically significant protection against chronic complications of diabetes? At present, there is no convincing evidence demonstrating that chronic or lifelong therapy would provide a favorable benefit:risk profile.
What is the optimal dose of SGLT2 inhibitor? The approved (or proposed) doses of SGLT2 inhibitors dramatically increase the risk of ketoacidosis in type 1 diabetic patients12–19. In contrast, low dose empagliflozin (2.5 mg; fourfold less than the lowest approved 10 mg dose) did not increase the risk of ketoacidosis – albeit the 2.5 mg elicited 40–50% less HbA1c-lowering than the 10 mg and 25 mg doses, respectively19. If there is a desire to find a safe and effective dose, lower doses should be thoroughly investigated. Indeed, Danne et al.32 implicitly acknowledged that the proposed doses are too high. They explicitly recommend initiating therapy at the lowest approved dose, and raise the possibility of splitting tablets of currently marketed SGLT2 inhibitors. Rather than to promote splitting tablets, approval for lower dose tablets should be sought if and when a safe and effective dose is eventually established.
Will the use of SGLT2 inhibitors in real world practice provide a benefit:risk profile comparable to clinical trials? Given the challenges in managing insulin regimens when combined with SGLT2 inhibitors, it is likely that ketoacidosis risk might be higher and glycemic efficacy might be inferior to what was observed in clinical trials. An international consortium – including physicians who participated in pharmaceutical company-sponsored clinical trials – articulated consensus recommendations for risk management of ketoacidosis in SGLT2 inhibitor-treated type 1 diabetic patients32. Although their recommendations were based on approaches taken in those clinical trials (including monitoring of beta-hydroxybutyrate levels), the authors acknowledge that “currently there is no evidence to support specific testing regimens”. Further, they emphasize that “developing an evidence base and algorithms to support decisions on reducing insulin doses should be a priority for future research.” Ideally, this information should be available prior to use in real world clinical practice. Thus, we conclude it is premature to consider regulatory approval of these drugs as adjunctive therapy for type 1 diabetes.
-
Is it possible to identify sub-groups of patients with more (or less) favorable benefit:risk profiles? At present, there is no evidence-based precision medicine strategy to predict responses to SGLT2 inhibitors (e.g., based on pharmacogenomics). Nevertheless, rather than a “one size fits all” approach, it would likely be preferable to define a personalized medicine approach to stratify patients. For example, a therapeutic trial might begin with a lead-in period to optimize insulin therapy and establish a baseline level of HbA1c. Subsequently, a 24-week therapeutic trial of an SGLT2 inhibitor might be initiated. The SGLT2 inhibitor would only be continued if the patient experienced a substantial decrease in HbA1c (e.g., at least 0.5% of HbA1c-lowering from baseline). It is unlikely that patients with below average HbA1c-lowering (e.g., <0.2% at 52 weeks) would derive sufficient benefit to outweigh a dramatic increase in the risk of life-threatening ketoacidosis. Clearly, it would be especially desirable to identify patients at greatest risk to develop ketoacidosis (e.g., by monitoring beta-hydroxybutyrate levels). If these drugs are to be used in type 1 diabetes, it would be far preferable to focus the drugs on patients with the greatest probability of benefit and lowest risk of ketoacidosis.
Off-label prescription of approved SGLT2 inhibitors represents a major challenge for regulators. Even if they never approve a type 1 diabetes indication, some type 1 diabetic patients will continue to be exposed to risks associated with SGLT2 inhibitors. The current versions of FDA-approved Prescribing Information contains only limited information about type 1 diabetes – primarily stating that the drugs are not approved to treat type 1 diabetes. If regulatory agencies eventually approve one or more of these drugs, we strongly recommend that they implement a formal Risk Evaluation and Mitigation Strategy (REMS) with the following elements in order to provide a level of clinical care similar to that which was available in clinical trials:- Require certification of physicians who are eligible to prescribe the drugs.
- Provide patients with meters for self-monitoring of blood levels of beta-hydroxybutyrate (with costs covered by pharmaceutical companies if not reimbursed by payers).
- Establish a patient registry to provide high quality data to assess “real world” outcomes.
In the meantime, we are mindful of a central ethical precept that guides the practice of clinical medicine: Primum non nocere. First do no harm.
Supplementary Material
Acknowledgements
Drs. Beitelshees and Taylor acknowledge support from a grant (R01DK118942) from the National Institute of Diabetes and Digestive and Kidney Disease (NIDDK). Drs. Blau and Rother are US Government employees. The funding sources had no input into the manuscript. The views expressed in the manuscript are those of the authors, and do not necessarily reflect the views of the US Government.
SIT has received consulting fees from Ionis Pharmaceuticals. Regeneron Pharmaceuticals has provided a research grant in support of the Amish Research Clinic at the University of Maryland, but ALB and SIT do not receive financial support from that grant.
Footnotes
Disclosures
JEB and KIR have nothing to disclose.
REFERENCES
- 1.Diabetes C, Complications Trial Research G, Nathan DM, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993; 329(14): 977–86. [DOI] [PubMed] [Google Scholar]
- 2.Miller KM, Foster NC, Beck RW, et al. Current state of type 1 diabetes treatment in the U.S.: updated data from the T1D Exchange clinic registry. Diabetes Care 2015; 38(6): 971–8. [DOI] [PubMed] [Google Scholar]
- 3.McKnight JA, Wild SH, Lamb MJ, et al. Glycaemic control of Type 1 diabetes in clinical practice early in the 21st century: an international comparison. Diabet Med 2015; 32(8): 1036–50. [DOI] [PubMed] [Google Scholar]
- 4.Foster NC, Beck RW, Miller KM, et al. State of Type 1 Diabetes Management and Outcomes from the T1D Exchange in 2016–2018. Diabetes Technol Ther 2019. [DOI] [PMC free article] [PubMed]
- 5.Whitehouse F, Kruger DF, Fineman M, et al. A randomized study and open-label extension evaluating the long-term efficacy of pramlintide as an adjunct to insulin therapy in type 1 diabetes. Diabetes Care 2002; 25(4): 724–30. [DOI] [PubMed] [Google Scholar]
- 6.Mathieu C, Zinman B, Hemmingsson JU, et al. Efficacy and Safety of Liraglutide Added to Insulin Treatment in Type 1 Diabetes: The ADJUNCT ONE Treat-To-Target Randomized Trial. Diabetes Care 2016; 39(10): 1702–10. [DOI] [PubMed] [Google Scholar]
- 7.Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med 2015; 373(22): 2117–28. [DOI] [PubMed] [Google Scholar]
- 8.Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med 2017; 377(7): 644–57. [DOI] [PubMed] [Google Scholar]
- 9.Cherney DZ, Perkins BA, Soleymanlou N, et al. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation 2014; 129(5): 587–97. [DOI] [PubMed] [Google Scholar]
- 10.Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and Progression of Kidney Disease in Type 2 Diabetes. N Engl J Med 2016; 375(4): 323–34. [DOI] [PubMed] [Google Scholar]
- 11.Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med 2019; 380(4): 347–57. [DOI] [PubMed] [Google Scholar]
- 12.Henry RR, Thakkar P, Tong C, Polidori D, Alba M. Efficacy and Safety of Canagliflozin, a Sodium-Glucose Cotransporter 2 Inhibitor, as Add-on to Insulin in Patients With Type 1 Diabetes. Diabetes Care 2015; 38(12): 2258–65. [DOI] [PubMed] [Google Scholar]
- 13.Dandona P, Mathieu C, Phillip M, et al. Efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes (DEPICT-1): 24 week results from a multicentre, double-blind, phase 3, randomised controlled trial. Lancet Diabetes Endocrinol 2017; 5(11): 864–76. [DOI] [PubMed] [Google Scholar]
- 14.Dandona P, Mathieu C, Phillip M, et al. Efficacy and Safety of Dapagliflozin in Patients With Inadequately Controlled Type 1 Diabetes: The DEPICT-1 52-Week Study. Diabetes Care 2018; 41(12): 2552–9. [DOI] [PubMed] [Google Scholar]
- 15.Mathieu C, Dandona P, Gillard P, et al. Efficacy and Safety of Dapagliflozin in Patients With Inadequately Controlled Type 1 Diabetes (the DEPICT-2 Study): 24-Week Results From a Randomized Controlled Trial. Diabetes Care 2018; 41(9): 1938–46. [DOI] [PubMed] [Google Scholar]
- 16.Garg SK, Henry RR, Banks P, et al. Effects of Sotagliflozin Added to Insulin in Patients with Type 1 Diabetes. N Engl J Med 2017; 377(24): 2337–48. [DOI] [PubMed] [Google Scholar]
- 17.Buse JB, Garg SK, Rosenstock J, et al. Sotagliflozin in Combination With Optimized Insulin Therapy in Adults With Type 1 Diabetes: The North American inTandem1 Study. Diabetes Care 2018; 41(9): 1970–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Danne T, Cariou B, Banks P, et al. HbA1c and Hypoglycemia Reductions at 24 and 52 Weeks With Sotagliflozin in Combination With Insulin in Adults With Type 1 Diabetes: The European inTandem2 Study. Diabetes Care 2018; 41(9): 1981–90. [DOI] [PubMed] [Google Scholar]
- 19.Rosenstock J, Marquard J, Laffel LM, et al. Empagliflozin as Adjunctive to Insulin Therapy in Type 1 Diabetes: The EASE Trials. Diabetes Care 2018; 41(12): 2560–9. [DOI] [PubMed] [Google Scholar]
- 20.Taylor SI, Blau JE, Rother KI. SGLT2 Inhibitors May Predispose to Ketoacidosis. J Clin Endocrinol Metab 2015; 100(8): 2849–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peters AL, Buschur EO, Buse JB, Cohan P, Diner JC, Hirsch IB. Euglycemic Diabetic Ketoacidosis: A Potential Complication of Treatment With Sodium-Glucose Cotransporter 2 Inhibition. Diabetes Care 2015; 38(9): 1687–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosenstock J, Ferrannini E. Euglycemic Diabetic Ketoacidosis: A Predictable, Detectable, and Preventable Safety Concern With SGLT2 Inhibitors. Diabetes Care 2015; 38(9): 1638–42. [DOI] [PubMed] [Google Scholar]
- 23.Ferrannini G, Hach T, Crowe S, Sanghvi A, Hall KD, Ferrannini E. Energy Balance After Sodium-Glucose Cotransporter 2 Inhibition. Diabetes Care 2015; 38(9): 1730–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Puckrin R, Saltiel MP, Reynier P, Azoulay L, Yu OHY, Filion KB. SGLT-2 inhibitors and the risk of infections: a systematic review and meta-analysis of randomized controlled trials. Acta Diabetol 2018; 55(5): 503–14. [DOI] [PubMed] [Google Scholar]
- 25.Benoit SR, Zhang Y, Geiss LS, Gregg EW, Albright A. Trends in Diabetic Ketoacidosis Hospitalizations and In-Hospital Mortality - United States, 2000–2014. MMWR Morb Mortal Wkly Rep 2018; 67(12): 362–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferrannini E, Solini A. Therapy: SGLT inhibition in T1DM - definite benefit with manageable risk. Nat Rev Endocrinol 2017; 13(12): 698–9. [DOI] [PubMed] [Google Scholar]
- 27.Li K, Xu G. Safety and efficacy of sodium glucose co-transporter 2 inhibitors combined with insulin in adults with type 1 diabetes: A meta-analysis of randomized controlled trials. J Diabetes 2018. [DOI] [PubMed]
- 28.Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med 1995; 332(10): 621–8. [DOI] [PubMed] [Google Scholar]
- 29.Sands AT, Zambrowicz BP, Rosenstock J, et al. Sotagliflozin, a Dual SGLT1 and SGLT2 Inhibitor, as Adjunct Therapy to Insulin in Type 1 Diabetes. Diabetes Care 2015; 38(7): 1181–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zambrowicz B, Freiman J, Brown PM, et al. LX4211, a dual SGLT1/SGLT2 inhibitor, improved glycemic control in patients with type 2 diabetes in a randomized, placebo-controlled trial. Clin Pharmacol Ther 2012; 92(2): 158–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wright EM, Loo DD, Hirayama BA. Biology of human sodium glucose transporters. Physiol Rev 2011; 91(2): 733–94. [DOI] [PubMed] [Google Scholar]
- 32.Danne T, Garg S, Peters AL, et al. International Consensus on Risk Management of Diabetic Ketoacidosis in Patients with Type 1 Diabetes Treated with Sodium-Glucose Cotransporter (SGLT) Inhibitors. Diabetes Care 2019. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.