Abstract
Purpose:
Axicabtagene ciloleucel (axi-cel) is a CD19-directed chimeric antigen receptor (CAR) T-cell therapy for relapsed or refractory diffuse large B-cell lymphoma. Bridging therapy may be required for lymphoma control during the manufacturing interval between collection of autologous T cells and final CAR T product administration. The optimal bridging therapy is not known and patients are often chemorefractory. We present a case series of patients receiving radiation as a bridge to axi-cel.
Methods and Materials:
Between December 2017 and October 2018, 12 patients were intended to receive bridging radiation before axi-cel. The group was characterized by highly aggressive disease including 6 of 12 with “double hit” lymphoma and 6 of 12 with may be required for lymphoma control during manufacture. In our case series, no significant toxicities were identified during bridging radiation and no patient experienced in-field progression of disease before CAR T infusion. These data suggest radiation is a well-tolerated and effective bridging therapy, warranting further prospective study for optimization. disease ≥10 cm in diameter. All patients received 2 to 4 Gy/fraction to a median dose of 20 Gy (range, 6–36.5 Gy). Half of patients received either 30 Gy in 10 fractions or 20 Gy in 5 fractions. Seven patients received concurrent chemotherapy. Eleven patients underwent axi-cel infusion and one did not. Median follow-up was 3.3 months (range, 1.1–12.0 months).
Results:
No significant toxicities were identified during bridging radiation, and no patient experienced in-field progression of disease before axi-cel infusion. One patient experienced abdominal pain, which resolved after dose reduction. Two patients had out-of-field progression of disease during the bridging period. After axi-cel infusion, 3 of 11 patients (27%) experienced severe cytokine release syndrome or neurotoxicity. At 30 days, the objective response rate was 81.8% (11 of 12 evaluable; 1 stable disease, 1 out-of-field progression), with complete response in 27% (3 of 11). At last follow-up, the best objective response rate was 81.8%, with a complete response attained in 45% (5 of 11). Lymphocyte counts decreased slightly in 10 of 12 patients during radiation (median, 0.25 k/uL).
Conclusions:
Radiation (with or without concurrent chemotherapy) can be safely administered as a bridge to axi-cel in high-risk lymphoma. Caution should be taken if irradiation is started before apheresis, and lymphocyte counts should be monitored closely throughout. Future investigation is warranted to optimize the use of bridging radiation before CAR T therapy.
Summary
Although CD19-directed chimeric antigen receptor (CAR) T-cell therapies like axicabtagene ciloleucel are effective for relapsed or refractory diffuse large B-cell lymphoma, bridging therapy may be required for lymphoma control during manufacture. In our case series, no significant toxicities were identified during bridging radiation and no patient experienced in-field progression of disease before CAR T infusion. These data suggest radiation is a well-tolerated and effective bridging therapy, warranting further prospective study for optimization.
Introduction
Diffuse large B-cell lymphoma (DLBCL) and variants such as transformed follicular lymphoma represent the most common non-Hodgkin lymphoma, at 30% to 40% of all cases.1 Approximately 85% to 90% of patients attain initial remission after first-line therapy with rituximab plus anthracycline-containing chemotherapy (ie, R-CHOP); however, 20% to 25% of patients will subsequently relapse.2 Unfortunately, prognoses remain grim for primary refractory and relapsed and refractory DLBCL (R/R DLBCL). In the salvage setting, high-dose chemotherapy and autologous stem cell transplant is the standard of care.3 However, in a recent multi-institutional patient level meta-analysis of refractory DLBCL, patients not responding to their last line of therapy or relapsing within a year of autologous stem cell transplant obtained only a 26% objective response rate (ORR) and a complete response (CR) rate of 7%.4 Furthermore, median overall survival (OS) was 6.3 months and a 2-year OS was 20%. Prognosis is especially poor in “double hit” patients with concurrent translocations in MYC and BCL2 or BCL6, with 5-year progression-free survival of 18% and OS of 27% after primary therapy with R-CHOP.5
CD19-directed chimeric antigen receptor (CAR) T-cell therapy can lead to durable remissions in DLBCL. In the pivotal phase II portion of the ZUMA-1 trial testing axicabtagene ciloleucel in refractory DLBCL, ORR was 82% and CR rate was 54%.6 After a median follow-up of 27.1 months, the median duration of response was 11.1 months, with 39% of patients maintaining a complete response, causing median OS not to be reached.7 The JULIET trial, another pivotal phase II study of a different CD19-directed CAR T-cell therapy (tisagenlecleucel), reported an ORR of 52% with a CR rate of 40%, which led to a 12-month rate of relapse-free survival of 65% for responders.8 In addition, in a prior phase II trial of tisagenlecleucel, 4 patients received radiation as part of their therapy for lymphodepletion before CAR T infusion.9 The manufacture of autologous CAR T cells requires roughly 3 to 4 weeks between the collection of a patient’s T cells via apheresis and CAR T-cell infusion, during which disease may continue to progress. The goals of “bridging” therapy are to debulk tumor burden and maintain performance status until CAR T-cell therapy can be delivered. Radiation therapy provides effective local tumor control in DLBCL and has important roles as consolidative therapy, peritransplant therapy, or palliation.2,10,11 Here we describe another role for radiation therapy in DLBCL with a case series of patients who received bridging radiation therapy before CAR T infusion.
Methods and Materials
The institutional review boards of Moffitt Cancer Center and University of Maryland approved this retrospective review of patients diagnosed with DLBCL/transformed follicular lymphoma who failed at least 2 lines of prior therapy and who were intended to receive radiation as bridging therapy between T-cell collection and standard of care axi-cel infusion. Imaging was reviewed for each patient to evaluate responses using Lugano criteria12 at the following time points: before radiation therapy; after radiation therapy and before fludarabine/cyclophosphamide (flu/cy) conditioning; 30 days after CAR T infusion; 90 days after CAR T; and 180 days after CAR T. Radiation treatment plans and contours were also accessed to confirm treatment location and to determine in-field versus out-of field responses. Bulky disease was defined as a maximum diameter ≥ 10 cm.
Results
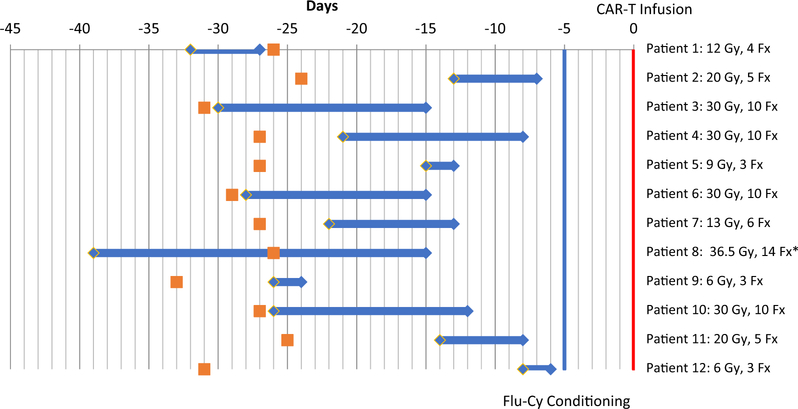
Immune cell therapy databases at [Institution 1] and [Institution 2] were searched to identify patients intended to receive standard of care CAR T-cell therapy with axi-cel for lymphoma. Patients were included if they were intended to receive radiation therapy between T cell apheresis and axi-cel infusion as of October 31, 2018. Twelve patients met the study criteria, of whom 8 were refractory to their last line of chemotherapy and 4 had newly identified relapsed disease (Table 1). All patients had a median product of perpendicular diameters of 102.7 cm2 and 8 patients presented with bulky disease (lesions >10 cm). Eleven patients out of 12 evaluable presented with pain, which was improved in 5 patients after radiation; other patients did not experience amelioration in pain levels per the medical record. The targets for radiation included the bulkiest site of disease (n = 5, one of whom was stage I/II), only site(s) of disease (n = 5, 3 of whom were stage I/II), and one site that was growing rapidly. Patient 10 received radiation to their only 2 sites of disease in the left lower extremity and left groin. Radiation was completed before apheresis in 1 patient, occurred before and after apheresis in 1 patient, and started after apheresis in 10 patients (Fig. 1). All patients received 2 to 4 Gy per fraction to a total median dose of 20 Gy (range, 6–30 Gy). The most common regimen was 30 Gy in 10 fractions (given in 4 patients). The first treated patient was intended to receive 30 Gy in 10 fractions that would continue after apheresis (patient 1). However, the course was reduced to 12 Gy and stopped immediately before apheresis because of theoretical concerns about radiation being given close to the time of CAR T-cell infusion. After this patient did well, subsequent patients were treated at the discretion of the treating radiation oncologist. Patient 7 was initially planned to receive 18 Gy in 6 fractions but developed abdominal pain after the first fraction and was then given 2 Gy fractions for a total dose of 13 Gy in 6 fractions. Patient 8 received a single fraction of 4 Gy and then received 13 fractions of 2.5 Gy for a total dose of 36.5 Gy. Radiation therapy ended a median of 13 days (range, 6–27 days) before CAR T infusion. The most common target for radiation therapy was within the abdomen (6 patients); other targets included the hip and lower extremity (4 patients), neck (1 patient), and chest wall (1 patient). A representative patient is shown in Figure 2 (patient 6). Seven patients also received concurrent systemic bridging therapy: one received one infusion of rituximab with dexamethasone; one received one infusion of obinutuzumab; 2 received rituximab, gemcitabine, and oxaliplatin; and 3 received oral cyclophosphamide with dexamethasone.
Table 1.
Patient characteristics
| Disease type | Disease status | Stage | IPI | Prior lines of therapy | Double/triple hit by FISH | Target | Max diameter (cm) | Ortho gonal diameter (cm) | Cross product (cm2) | Total dose (Gy) | Fra ctions | Concurrent systemic bridging therapy | Radiation completed days before CAR-T | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient 1 | DLBCL | Primary re fractory | III/IV | 5 | 2 | No | Abdomen | 26.2 | 16.4 | 429.68 | 12 | 4 | Oral cyclo-phosphamide, dexamethasone | −27 |
| Patient 2 | DLBCL | Relapsed re fractory | I/II | 3 | 5 | Yes | LLE/groin | 18.1 | 14.0 | 253.4 | 20 | 5 | Oral cyclo-phosphamide, dexamethasone | −7 |
| Patient 3 | TFL | Primary re fractory | III/IV | 4 | 5 | No | Right neck | 3.7 | 23 | 8.51 | 30 | 10 | rituximab, dexamethasone | −15 |
| Patient 4 | TFL | Relapsed re fractory | I/II | 2 | 3 | Yes | Abdomen | 17.7 | 16.5 | 292.05 | 30 | 10 | Dexamethasone | −8 |
| Patient 5 | DLBCL | Primary re fractory | I/II | 2 | 2 | Yes | Abdomen | 5.9 | 3.1 | 18.29 | 9 | 3 | Rituximab, gemcitabine, oxaliplatin | −13 |
| Patient 6 | TFL | Primary re fractory | III/IV | 3 | 3 | Yes | Left hip | 4.3 | 2.1 | 9.03 | 30 | 10 | None | −15 |
| Patient 7 | DLBCL | Primary re fractory | I/II | 1 | 2 | Yes | Abdomen | 12.4 | 9.3 | 115.32 | 13 | 6 | None | −13 |
| Patient 8 | DLBCL | Relapsed re fractory | III/IV | 4 | 2 | Yes | Left hip | 29.4 | 10.8 | 317.52 | 36.5 | 14 | Rituximab, gemcitabine, oxaliplatin | N/A |
| Patient 9 | DLBCL | Relapsed re fractory | III | 2 | 2 | No | Abdomen | 10.0 | 9.0 | 90 | 6 | 3 | None | −24 |
| Patient 10 | DLBCL | Primary re fractory | III/IV | 2 | 3 | No | LLE/groin | 5.5 | 2.5 | 13.75 | 30 | 10 | Oral cyclo phosphamide, dexamethasone | −12 |
| Patient 11 | DLBCL | Primary re fractory | III/IV | 3 | 2 | Unknown | Abdomen | 7.0 | 5.1 | 35.7 | 20 | 5 | None | −8 |
| Patient 12 | DLBCL | Primary re fractory | III | 3 | 3 | No | Left chest wall | 13.5 | 10.0 | 135 | 6 | 3 | Obinutuzumab | −6 |
| Median | 3 | 2 | 102.7 | 20 | −13 |
Abbreviations: DLBCL = diffuse large B-cell lymphoma; IPI = international prognostic index for lymphoma; LLE = left lower extremity; N/A = not applicable; TFL = transformed follicular lymphoma.
All patients were refractory to their most recently received line of chemotherapy. Primary refractory indicates disease that did not respond to any prior chemotherapy. Relapsed refractory indicates disease that responded to prior therapy but was refractory to the most recent line of therapy.
Fig. 1.
Timeline of radiation therapy. Chimeric antigen receptor (CAR) T-cell therapy consists of T-cell collection (apheresis; orange squares) followed by a bridging period while awaiting CAR T cell manufacturing. Conditioning chemotherapy (fludarabine plus cyclophosphamide; Flu-Cy) is given 5 days before (–5) and 3 days before (–3) infusion of CAR-T cells (day 0; vertical red line). Blue diamonds and horizontal blue lines indicate the period during which radiation therapy was given. The dose in gray (Gy) and fractions (Fx) is indicated on the right. *Patient 8 had out-of-field progression and a declining performance status and elected for hospice before CAR T cells were infused. CAR T cells were infused in the remainder of the patients.
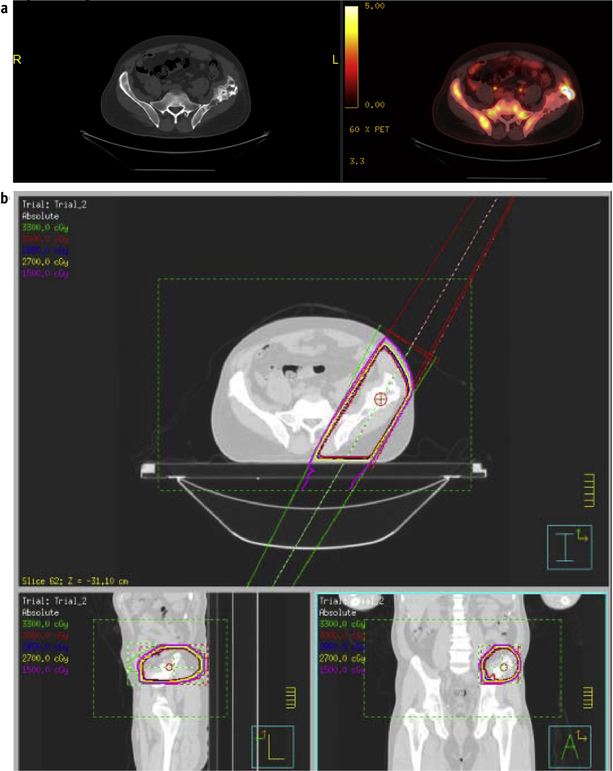
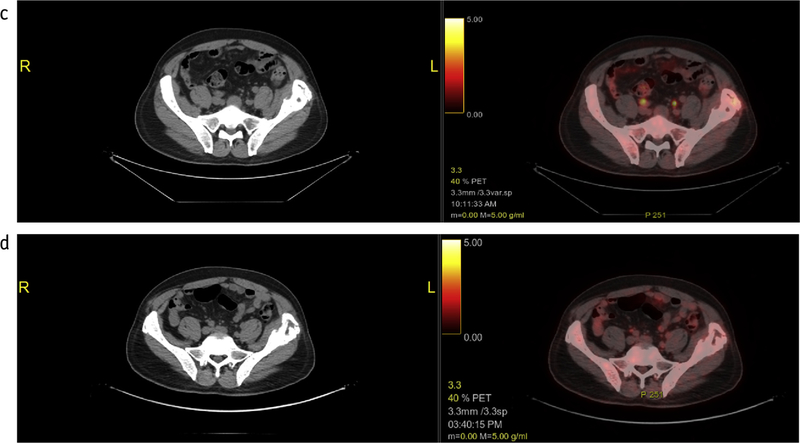
Fig. 2.
Bridging radiation therapy in a representative patient. (a) Positron emission tomography/computed tomography (PET/CT) showing disease at the time of T-cell collection before bridging and chimeric antigen receptor (CAR) T therapy. (b) Radiation treatment plan. (c) PET/CT showing disease response at the end of radiation therapy but before CAR T infusion. (d) PET/CT showing disease response at 30 days after CAR T therapy.
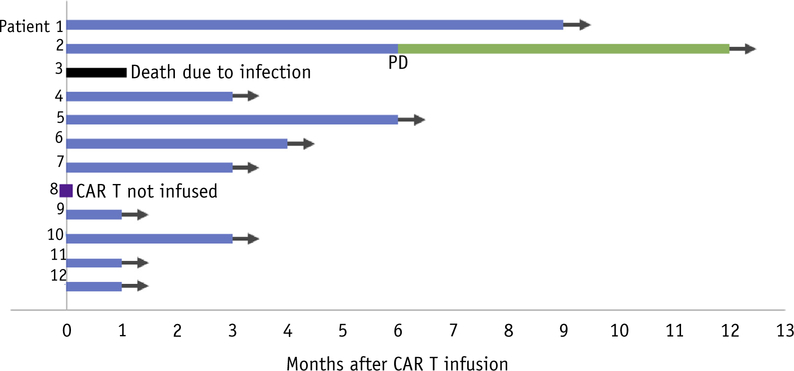
One patient who underwent apheresis and radiation therapy ultimately did not proceed with CAR T infusion but was still included in our analysis (patient 8). He also had out-of-field progression of disease (PD) after apheresis and radiation therapy. Because of declining performance status, he decided not to receive axi-cel infusion and elected to proceed with hospice. Patient 3 had out-of-field progression of disease (PD) after bridging radiation and had a complicated course after CAR T, eventually dying as a result of candidemia. Patient 8 experienced abdominal pain after the delivery of the first fraction, which resolved after dose reduction. This patient had a partial response (PR) 30 days after CAR T and eventually had a CR after 3 months. No other patients experienced any significant adverse effects after radiation therapy. After CAR T, one patient experienced grade 4 cytokine release syndrome and grade 4 CAR T-cellerelated encephalopathy syndrome.13 Two additional patients experienced severe CAR T-cellerelated encephalopathy syndrome, one grade 3 and one grade 4. Otherwise, all CAR T related toxicities were grades 0 to 2.
After bridging radiation therapy and before CAR T infusion, imaging was obtained for 10 patients (all patients except patients 9 and 12). Lugano assessment of response to radiation therapy is optimal when performed weeks after the conclusion of treatment.12 Within this limitation (assessment 0–21 days after completion of therapy), 7 patients had stable disease, 2 patients had PD (both out of field), and one patient had a PR.
Median follow-up after axi-cel infusion was 3.3 months (range, 1.1–12.0 months). Responses after axi-cel infusion are shown in Figure 3. Eleven patients were evaluable for response at 30 days after axi-cel. Three patients had CR, 6 patients had PR (3 both in and out of field, 2 at a single in-field site of disease, and 1 with an in-field CR/out-of-field PR), 1 had stable disease (at a single in-field site of disease), and 1 had PD (in-field CR but out-of-field PD). At last follow-up, 2 patients’ responses had improved from a PR to a CR and another patient initially with a partial response had an in-field PD after 4.3 months. Of the 11 patients who actually received axi-cel, best ORR was 81.8% with a CR rate of 45.5%. On intention to treat analysis, best ORR was 75.0% with a CR rate of 41.7%.
Fig. 3.
Swimmer plot of responses to chimeric antigen receptor (CAR) T therapy (axicabtagene ciloleucel [axi-cel]) in patients receiving bridging radiation. Blue lines indicate patients with stable disease, partial response, or complete response after CAR T therapy. The green line indicates a patient who progressed after CAR T (PD) but remains alive on subsequent therapy. The black line indicates a patient who died of infection on day + 40 after CAR T therapy. The purple square indicates a patient who had out-of-field relapse during bridging radiation and did not go on to CAR T-cell infusion because of declining performance status.
White blood cell counts (WBCs), hemoglobin levels (Hgb), platelet counts (Plt), absolute neutrophils counts (ANCs), and absolute lymphocyte counts (ALCs) were also tracked during therapy and evaluated using the Common Terminology Criteria for Adverse Events version 5.0 (11 of 12 patients evaluable after CAR T). A third of patients experienced neutropenia after radiation, and all evaluable patients experienced neutropenia after fludarabine and cyclophosphamide conditioning chemotherapy and CAR T. Lymphocyte counts followed a similar trend with a median absolute decrease of 0.25 k/μL, but 58% of patients had lymphopenia at baseline before any therapy. The majority of patients also experienced worsening anemia, but 75% of patients had preexisting anemia. Lastly, rates of thrombocytopenia had similar trends to a lesser degree, and only 17% of patients had preexisting thrombocytopenia (Table 2). Of the patients experiencing any form of cytopenia after radiation but before CAR T infusion, 58% were also receiving some form of concurrent bridging chemotherapy before the start of conditioning chemotherapy. WBCs and ALCs both tended to downtrend slightly with radiation therapy and continued to downtrend through flu/cy conditioning and appeared to reach their nadir at CAR T infusion. Of note, 7 patients in our series also received concurrent chemotherapy with bridging radiation. ANCs and Plt followed a similar trend, but the nadir appeared to be delayed to 7 days after CAR T infusion. WBCs, ANCs, and Plt generally started to recover by day 14 after CAR T infusion. ALC recovery appeared to be delayed to 21 days after CAR T infusion (Fig. E1; available online at https://doi.org/10.1016/j.ijrobp.2019.05.065). Hemoglobin levels stayed relatively consistent throughout radiation but started to downtrend with flu/cy conditioning with an apparent nadir at day 7 after CAR T infusion and recovery by day 14 (Fig. E1; available online at https://doi.org/10.1016/j.ijrobp.2019.05.065). We did not note increases in ferritin or C-reactive protein attributable to bridging radiation before CAR T (Fig. E2; available online at https://doi.org/10.1016/j.ijrobp.2019.05.065).
Table 2.
Toxicity data
| Baseline neutro penia | Neutropenia max grade after radiation | Neutropenia max grade after CART | Baseline lymp hopenia | Lymp hopenia max grade after radiation | Lymp hopenia max grade after CART | Baseline anemia | Anemia max grade after radiation | Anemia max grade after CART | Baseline thrombo cytopenia | Thrombo cytopenia max grade after radiation | Thrombo cytopenia max grade after CART | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient 1 | None | None | 3 | None | 2 | 3 | 2 | 2 | 3 | None | None | 2 |
| Patient 2 | None | 1 | 3 | None | 2 | 2 | 1 | None | 3 | None | None | 1 |
| Patient 3 | None | None | 3 | 2 | 3 | 3 | 2 | 3 | 2 | 1 | 1 | 3 |
| Patient 4 | None | None | 3 | 2 | 3 | 3 | 2 | 3 | 3 | None | None | 3 |
| Patient 5 | None | 2 | 3 | 1 | 2 | 3 | 2 | 3 | 3 | None | 1 | 1 |
| Patient 6 | 1 | 2 | 3 | 1 | 2 | 3 | None | None | 3 | None | None | 1 |
| Patient 7 | None | None | 3 | 2 | 3 | 3 | 2 | 2 | 3 | None | None | 2 |
| Patient 8 | None | 1 | N/A | 2 | 3 | N/A | 2 | 2 | N/A | 2 | 3 | N/A |
| Patient 9 | None | None | 2 | None | None | 3 | 2 | 2 | 1 | None | None | None |
| Patient 10 | None | None | 3 | 1 | 2 | 3 | 2 | 1 | 2 | none | none | none |
| Patient 11 | None | None | 3 | None | 1 | 3 | none | none | 2 | none | none | none |
| Patient 12 | None | None | 3 | None | None | 3 | none | 1 | 2 | none | none | 1 |
Abbreviations: CAR = chimeric antigen receptor; N/A = not applicable.
Values reported “after Radiation” were recorded after completion of bridging radiation therapy and before flu/cy conditioning. All toxicities were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events Version 5.0. Patient 8 did not proceed to CAR T infusion and therefore was only evaluable after radiation
Discussion
This report, we believe, is the first description of the use of radiation as a bridging therapy to control symptomatic and locally aggressive disease in patients undergoing axi-cel, a Food and Drug Administration-approved CD19 CAR T-cell therapy with CD28 costimulation for R/R DLBCL. In this cohort of patients in whom no effective systemic therapy was available, bridging radiation was able to safely bridge patients to axi-cel therapy with outcomes after CAR T comparable to patients treated in recent clinical trials. No significant radiation-related toxicity was identified, and infield disease control was excellent using standard palliative radiation therapy regimens. Patients in this study were relapsed or refractory to their latest line of systemic therapy, had at least 2 lines of prior therapy, and as a group had highly aggressive disease that would have been likely fatal in the absence of CAR T therapy. Our results suggest that radiation is a well-tolerated and effective bridging therapy before axi-cel infusion in R/R DLBCL patients with rapidly progressive, symptomatic disease.
Significant time typically elapses between identifying recurrent or refractory disease and CAR T infusion. This can include the time needed to refer to a CAR T treatment center for evaluation, approval by insurance, manufacturing of CAR T cells after collection (3–4 weeks), shipping, and conditioning chemotherapy. As a result, effective bridging strategies may be needed to provide patients with aggressive disease access to CAR T therapy. On the pivotal ZUMA-1 clinical trial that led to Food and Drug Administration approval of axi-cel, bridging therapy with chemotherapy or radiation was not permitted.14 However, in a recent real-world analysis of 293 axi-cel patients treated outside of clinical trial, 55% of patients were treated with bridging therapy.15 Despite the use of bridging therapy, 12 of 293 patients (4.1%) died as a result of lymphoma without having received CAR T. Moreover, covariates associated with a lower 3-month CR rate to axi-cel therapy (which correlates to 2-year progression-free survival7) included bulky disease ≥10 cm and an Eastern Cooperative Oncology Group performance status of 2 or worse. In a separate real-world analysis of axi-cel encompassing 104 patients, 52% received bridging therapy and 5.7% of patients died without receiving CAR T.16 Once again, poor performance status and tumor bulk was associated with inferior outcomes. For 4–1BB costimulated CAR T cells, radiation has been used in selected patients. For example, radiation was used as part of the conditioning chemo-therapy for CAR T in 4 patients with DLBCL or follicular lymphoma.9 Similarly, 5 patients have been reported to receive bridging radiation therapy for various lymphomas within 30 days of 4–1BB costimulated CAR T-cell infusion; it was reported that radiation did not have notable effects on CAR T expansion and no safety issues were identified.17 However, on the pivotal JULIET trial of tisagenlecleucel, 92% of patients received bridging therapy, typically with chemotherapy, because radiation was not allowed within 2 weeks of infusion per protocol.8 Bridging radiation has the potential to debulk disease and preserve performance status, and further study is needed to determine whether this results in improved CAR T outcomes.
In our study standard palliative radiation regimens were used with conventional or moderately hypofractionated treatment schedules at 2 to 4 Gy per fraction. The most commonly used treatment regimens were 30 Gy in 10 fractions and 20 Gy in 5 fractions, which are standard solid tumor palliative radiation regimens. Patients were not treated with the conventionally fractionated, higher total doses typically used for refractory non-Hodgkin lymphoma.18 Although not formally prespecified, in practice modest hypofractionation was commonly used for several reasons: (1) it helped minimize logistical challenges between T-cell collection and infusion (and avoid delays in CAR T therapy), (2) it provided shorter treatment courses for symptomatic patients, and (3) long-term disease control did not necessarily require permanent eradication of the target lesion, only that it provide an adequate temporizing response to allow effective bridging to CAR T therapy. With this approach we achieved approximately 80% control of targeted lesions at 30 days from CAR T infusion, suggesting that the standard palliative radiation doses used were able to provide adequate local control for the majority of patients.
Radiation therapy was well tolerated in our cohort. The only potentially radiation-related toxicity came from one patient with abdominal pain after his first fraction, which resolved after a dose reduction for the second and subsequent fractions. The majority of patients had bulky tumors (≥ 10 cm), requiring corresponding large radiation treatment volumes, and received concurrent systemic therapy, both which increase the risk for unanticipated adverse interactions between radiation and CAR T therapy. It is therefore notable that no significant acute toxicity was identified. With a median follow-up of 3.3 months, we do not have sufficient follow-up to evaluate long-term toxicities.
In our small cohort of patients, radiation before CAR T-cell infusion was associated with cytopenias in a significant proportion of patients. However, interpretation of these data requires careful consideration of additional factors that may contribute to cytopenia. First, 58% of the patients received some form of bridging systemic cytotoxic therapy in conjunction with radiation. Second, progressive lymphoma itself can be associated with cytopenias; therefore the absence of bridging therapy might be a risk factor itself for worsening cytopenias during CAR T manufacture. This is suggested by the presence of pre-existing cytopenias before bridging therapy. Finally, some cytopenias occurred after the start of bridging conditioning chemotherapy with fludarabine and cyclophosphamide, which is known to be associated with myelosuppression. In other published series, lymphopenia after radiation for lymphoma has been found to be associated with worse survival in other patients with solid tumors after radiation and chemotherapy.19 Certainly if radiation is to be administered before apheresis collection, close monitoring of lymphopenia and careful consideration of radiation targets and dose are required. With respect to neutropenia, anemia, and thrombocytopenia, these toxicities are common after flu/cy and CAR T-cell therapy. It is unclear to what degree radiation contributed, if at all, to these posttreatment cytopenias, and this should be evaluated in a larger cohort of patients.14 Given the ability of radiation to potentially contribute to cytopenias, this additional toxicity should prompt close monitoring of cell counts.
Given the small study size we did not attempt to infer the impact of radiation on overall CAR T response or efficacy. However, several potential benefits of bridging radiation have been proposed. Aside from staving off progression during the production of CAR T cells and debulking a high tumor burden, radiation therapy has also been put forth as a mechanism to trigger immune responses and amplify the effects of immunomodulatory therapies via exposure of neoantigens, potentially inducing an abscopal effect. To date, data supporting the synergistic effects of radiation and CAR T are preclinical.20–22 We were unable to thoroughly investigate such phenomena given a small sample size and retrospective analysis. Although ferritin and C-reactive protein are inflammatory biomarkers that may predict for worse toxicity,23 we did not note increases in ferritin or C-reactive protein attributable to bridging radiation before CAR T (Fig. E2 [available online at https://doi.org/10.1016/j.ijrobp.2019.05.065] and [*****]24). Further study is needed to determine whether radiation influences CAR T toxicity or efficacy through immunologic mechanisms. We believe the primary value in this report is to determine the safety profile and feasibility of bridging radiation and provide a stepping-stone to pursue greater investigations into radiation as a bona fide adjunct to CART.
Limitations
Although we found that the use of bridging radiation was well tolerated and effective, there are limitations to our study and several questions remain to be answered. First, given the retrospective nature of the study, radiation dose was not standardized with respect to dose and fractionation. Field design was relatively homogeneous in that we used involved site radiation therapy as part of our standard practice to target the fluorodeoxyglucose avid tumor volume with relatively modest additional margins (1–2 cm). The optimal or sufficient dose and fractionation likely vary by patient and remain unclear in this setting. Second, the exact timing of radiation varied with respect to CAR T, and it is unknown whether this had an impact on treatment efficacy. Third, patients were typically only treated at the site of symptomatic or concerning disease progression. The ideal treatment volumes (all sites of disease, only sites of initial bulky disease, only sites of persistent positron emission tomography—avid disease) remain unknown. Last, we did not systematically control the use of concurrent chemotherapy, and it is unclear which patients require chemotherapy, and if so, which regimen would be optimal.
Conclusions
In our case series of R/R DLBCL patients treated with CAR T, we found that bridging radiation therapy (with or without chemotherapy) could safely be administered, providing adequate local disease control until CAR T infusion while causing minimal acute toxicity. However, bridging radiation, particularly when given in conjunction with bridging chemotherapy, was associated with cytopenias, especially lymphopenia, which should prompt close monitoring and consideration of radiation dose, volumes, and timing, especially if started before apheresis. Much in the way that radiation has been found to be a valuable adjunct to previous treatment paradigms for R/R DLBCL, we believe that radiation could also serve this role in the CAR T paradigm by preventing progression with minimal toxicity. Future work to prospectively assess the value of bridging radiation is warranted.
Supplementary Material
Disclosures:
M.D.J.: Consultant for Kite/Gilead. J.C.C.: Advisory for Kite/Gilead, Novartis, Bayer, Genetech, and Karyopharm; Speaker’s Bureau for Genetech and AstraZeneca; institutional research support from Merck. B.D.S.: Research funding from Incyte and Jazz; honoraria from Pharmacyclics, Astra Zeneca, and Spectrum; Speaker’s Bureau for Amgen; Advisory Board for Celgene/Juno, Novartis. M.L.D.: Research funding from Celgene, Novartis, and Atara; other financial support from Novartis, Precision Biosciences, Celyad, Bellicum, and GlaxoSmithKline; stock options from Precision Biosciences, Adaptive Biotechnologies, and Anixa Biosciences. S.D.: Advisory board for Kite/Gilead. F.L.L.: Consultant for Cellular Biomedicine Group, Inc.; scientific advisor for Kite/Gilead and Novartis.
Footnotes
Supplementary material for this article can be found at https://doi.org/10.1016/j.ijrobp.2019.05.065.
References
- 1.Sehn LH, Gascoyne RD. Diffuse large B-cell lymphoma: Optimizing outcome in the context of clinical and biologic heterogeneity. Blood 2015;125:22–32. [DOI] [PubMed] [Google Scholar]
- 2.Ng AK, Yahalom J, Goda JS, et al. Role of radiation therapy in patients with relapsed/refractory diffuse large B-cell lymphoma: Guidelines from the International Lymphoma Radiation Oncology Group. Int J Radiat Oncol Biol Phys 2018;100:652–669. [DOI] [PubMed] [Google Scholar]
- 3.Philip T, Guglielmi C, Hagenbeek A, et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin’s lymphoma. N Engl J Med 1995;333:1540–1545. [DOI] [PubMed] [Google Scholar]
- 4.Crump M, Neelapu SS, Farooq U, et al. Outcomes in refractory diffuse large B-cell lymphoma: Results from the international SCHOLAR-1 study. Blood 2017;130:1800–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson NA, Slack GW, Savage KJ, et al. Concurrent expression of MYC and BCL2 in diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol 2012;30:3452–3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Locke FL, Rossi J, Neelapu SS, et al. Product characteristics associated with in vivo expansion of anti-CD19 CAR T cells in patients treated with axicabtagene ciloleucel (axi-cel). J Clin Oncol 2017; 35(suppl):3023. [Google Scholar]
- 7.Locke FL, Ghobadi A, Jacobson CA, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): A single-arm, multicentre, phase 1–2 trial. Lancet Oncol 2019;20:31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med 2018;380:45–56. [DOI] [PubMed] [Google Scholar]
- 9.Schuster SJ, Svoboda J, Chong EA, et al. Chimeric antigen receptor T cells in refractory B-cell lymphomas. N Engl J Med 2017;377:2545–2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoppe BS, Moskowitz CH, Filippa DA, et al. Involved-field radio-therapy before high-dose therapy and autologous stem-cell rescue in diffuse large-cell lymphoma: long-term disease control and toxicity. J Clin Oncol 2008;26:1858–1864. [DOI] [PubMed] [Google Scholar]
- 11.Biswas T, Dhakal S, Chen R, et al. Involved field radiation after autologous stem cell transplant for diffuse large B-cell lymphoma in the rituximab era. Int J Radiat Oncol Biol Phys 2010;77:79–85. [DOI] [PubMed] [Google Scholar]
- 12.Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: The Lugano classification. J Clin Oncol 1999;17:1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neelapu SS, Tummala S, Kebriaei P, et al. Chimeric antigen receptor T-cell therapydassessment and management of toxicities. Nat Rev Clin Oncol 2018;15:47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med 2017;377:2531–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nastoupil LJ, Jain MD, Spiegel JY, et al. Axicabtagene ciloleucel (Axi-cel) CD19 chimeric antigen receptor (CAR) T-cell therapy for relapsed/refractory large B-cell lymphoma: Real world experience. Blood 2018;132(Suppl 1):91. [Google Scholar]
- 16.Jacobson CA, Hunter B, Armand P, et al. Axicabtagene ciloleucel in the real world: Outcomes and predictors of response, resistance and toxicity. Blood 2018;132(Suppl 1):92. [Google Scholar]
- 17.Arscott WT, Miller D, Jones JA, Winchell N, Schuster S, Plastaras JP. Tandem induction radiation and chimeric antigen receptor T cell therapy in patients with relapsed or refractory non-hodgkin lymphoma. Int J Radiat Oncol Biol Phys 2018;102:S122. [Google Scholar]
- 18.Illidge T, Specht L, Yahalom J, et al. Modern radiation therapy for nodal non-Hodgkin lymphoma-target definition and dose guidelines from the International Lymphoma Radiation Oncology Group. Int J Radiat Oncol Biol Phys 2014;89:49–58. [DOI] [PubMed] [Google Scholar]
- 19.Grossman SA, Ellsworth S, Campian J, et al. Survival in patients with severe lymphopenia following treatment with radiation and chemo-therapy for newly diagnosed solid tumors. J Natl Comp Cancer Netw 2015;13:1225–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flynn JP, O’Hara MH, Gandhi SJ. Preclinical rationale for combining radiation therapy and immunotherapy beyond checkpoint inhibitors (i.e., CART). Transl Lung Cancer Res 2017;6:159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weiss T, Weller M, Guckenberger M, Sentman CL, Roth P. NKG2D-Based CAR T cells and radiotherapy exert synergistic efficacy in glioblastoma. Cancer Res 2018;78:1031–1043. [DOI] [PubMed] [Google Scholar]
- 22.DeSelm C, Palomba ML, Yahalom J, et al. Low-dose radiation conditioning enables CAR T cells to mitigate antigen escape. Mol Ther 2018;26:2542–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Locke FL, Neelapu SS, Bartlett NL, et al. Phase 1 Results of ZUMA-1: A multicenter study of KTE-C19 anti-CD19 CAR T cell therapy in refractory aggressive lymphoma. Mol Ther 2017;25: 285–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jain MD, Chavez JC, Shah BD, et al. Radiation therapy as a bridging strategy for refractory diffuse large B cell lymphoma patients awaiting CAR T manufacturing of axicabtagene ciloleucel. Blood 2018; 132(Suppl 1):4220. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.