Summary
Background
Although undifferentiated MSCs and MSCs differentiated into Schwann-like cells have been extensively compared in vitro and in vivo, studies on the ability and efficiency of differentiated MSCs for delivery to nerve allografts are lacking. As this is essential for their clinical potential, the purpose of this study was to determine the ability of MSCs differentiated into Schwann-like cells to be dynamically seeded on decellularized nerve allografts and to compare their seeding potential to that of undifferentiated MSCs.
Methods
Fifty-six sciatic nerve segments from Sprague-Dawley rats were decellularized and MSCs were harvested from Lewis rat adipose tissue. Control and differentiated MSCs were dynamically seeded on the surface of decellularized allografts. Cell viability, seeding efficiencies, cell adhesion, distribution and migration were evaluated.
Results
The viability of both cell types was not influenced by the processed nerve allograft. Both cell types achieved maximal seeding efficiency after 12 hours of dynamic seeding, albeit that differentiated MSCs had a significantly higher mean seeding efficiency than control MSCs. Dynamic seeding resulted in a uniform distribution of cells among the surface of the nerve allograft. No cells were located inside the nerve allograft after seeding.
Conclusion
Differentiated MSCs can be dynamically seeded on the surface of a processed nerve allograft, in a similar fashion as undifferentiated MSCs. Schwann-like differentiated MSCs have a significantly higher seeding efficiency after 12 hours of dynamic seeding. We conclude that differentiation of MSCs into Schwan-like cells may improve the seeding strategy and the ability of nerve allografts to support axon regeneration.
Keywords: MSCs, differentiation, Schwann-like cells, Processed nerve allograft, Seeding
INTRODUCTION
When it is impossible to repair an injured peripheral nerve by direct coaptation of the nerve ends, there are several options that can be used to restore the nerve’s function. These include interposition nerve autografts, processed nerve allografts or bio-absorbable hollow conduits. While many studies have compared these options, the autologous nerve remains the gold standard in clinical practice, especially for restoration of major motor nerves.1, 2 The limited availability and length of autografts requires the development of a replacement nerve that results in equal functional outcomes.3 Although their regenerating potency still needs to be improved, processed allografts have shown to be a promising option.2, 4–6
Stem cells are believed to be an important control element in tissue regeneration by producing proteins and molecules that enhance angiogenesis, inhibit scar formation, stimulate tissue regeneration and have immunomodulatory effects.7, 8 Their use has demonstrated potential to provide the needed extra biological support to processed nerve allografts.9–13 Adipose tissue is a valuable source for mesenchymal stem cells (MSCs) from the stromal vascular fraction as it is easily accessible and contains relatively large amounts of rapidly proliferating MSCs.14–18
Schwann cells, the original facilitators of nerve regeneration, have been confirmed as even better providers of biological support to processed nerve allografts than MSCs.19 However, their harvest requires the sacrifice of autologous nerve tissue. Another option to obtain Schwann-like cells is to chemically differentiate MSCs into Schwann-like cells.20–23 Several in-vitro studies have demonstrated the potential of differentiated Schwann-like MSCs in peripheral nerve repair, resulting in increased neurite outgrowth of motor neurons compared to undifferentiated MSCs.19, 24, 25
The trophic function of MSCs that results in enhanced gene expression and production of neurotrophic growth factors24, 26, does not require delivery of MSCs inside the nerve allograft. Because microinjection and soaking of MSCs damage both the cells and the allograft while reducing the number of uniformly attached cells, dynamic seeding offers a promising cell delivery strategy that has not been evaluated for differentiated MSCs.27–29
The purpose of this study was to determine the ability of MSCs differentiated into Schwann-like cells to be dynamically seeded on decellularized nerve allografts and to compare their seeding potential to that of control MSCs. This was investigated by three different sub-studies that aimed to evaluate (I) the influence of processed nerve grafts on the viability of differentiated MSCs, (II) the seeding potential of Schwann-like MSCs on processed nerve grafts and temporal optimization of seeding duration, and (III) the survivability, distribution and migration of differentiated MSCs after seeding.
MATERIALS AND METHODS
This study was approved by the IACUC institutional review committee and the Institutional Review Board (IACUC protocol A3053-16).
Mesenchymal stem cell collection and characterization
Rat MSCs were obtained from the inguinal fat pad from Lewis rats and derived as previously published.18 The obtained MSCs were previously characterized by plastic adherence and pluripotency toward mesodermal lineages.29 For flow cytometric assessment of stem cell surface markers, MSCs in passage five were used. The expression of MSC surface markers (CD29 and CD90) and hemapoetic cell surface markers (CD34 and CD45) were tested and compared with three control samples. Cell suspensions were pre-incubated with Fc block (Purified Mouse Anti-Rat CD32, 0.5uG per 500uL; BD Pharmingen™, CA, USA) to avoid unspecific binding. Thereafter, CD29 antibody (CD29 antibody | HM beta 1–10; Bio-rad-antibodies, CA, USA), CD90 antibody (CD90 Antibody | OX-7; Bio-rad-antibodies, CA, USA), CD45 antibody (CD45 antibody | OX-1; Bio-rad-antibodies, CA, USA) and CD34 (Mouse/Rat CD34 antibody; R&D systems Inc, MN, USA) were introduced to different samples. For the CD34-sample, the appropriate secondary reagent was added as well (Donkey Anti-Sheep IgG Fluorescein-conjugated antibody; R&D systems Inc, MN, USA). Cells were washed twice in flow cytometry buffer and centrifuged at 300g for five minutes after each wash. Finally, 7-AAD staining was added to each sample to exclude dead cells. Flow cytometry was performed with a BD FACScan flow cytometer.
Mesenchymal Stem Cell differentiation into Schwann-like cells
MSC differentiation into Schwann-like cells was accomplished according to the extensively tested protocol of Kingham and colleagues.18 Briefly, after two preparatory steps with ß-mercaptoethanol (Sigma-Aldrich corp., MO, USA) and all-trans-retinoic acid (Sigma-Aldrich Corp., MO, USA), the growth medium was replaced by differentiation medium containing Forskolin (Sigma-Aldrich corp., MO, USA), basic fibroblast growth factor (bFGF; PeproTech, NJ, USA), platelet-derived growth factor (PDGF-AA; PeproTech, NJ, USA) and Neuregulin-1 ß1 (NRG1-b1; R&D systems Inc, MN, USA). Successful MSC differentiation was verified by immunocytochemistry for Schwann cell marker S100 (Rabbit anti-S100; ThermoFisher Scientific, MA, USA), glial cell marker GFAP (Glial fibrillary acidic protein, mouse anti-GFAP; ThermoFisher Scientific, MA, USA) and Neurotrophin Receptor p75 (p75 NTR, rabbit anti-p75 NTR; ThermoFisher Scientific, MA, USA). Goat anti-rabbit FITC and goat anti-mouse Cyanine-3 (CY3) (both ThermoFisher Scientific, MA, USA) were used as secondary antibodies. Cell nuclei were labeled with DAPI (4’,6-diamindino-2-phenylindole, ThermoFisher Scientific, MA, USA). The fluorescent expression of the differentiated MSCs was compared to the expression of undifferentiated MSCs and Schwann cells.
Nerve allograft processing
Fifty-six sciatic nerves were obtained from 28 Sprague-Dawley rats weighing 250–350 grams and were decellularized according to the protocol of Hundepool and colleagues.30 In this 5-day protocol, the nerve allografts are exposed to elastase, resulting in less cellular debris with preservation of the ultrastructure of the nerve. Sprague-Dawley rats were specifically selected as there is a known histocompatibility mismatch to Lewis rats.31, 32 The nerves were sterilized using γ-irradiation and stored at 4γ Celsius after processing.
Analysis of Cell viability
To assess the influence of chemical products used to process the allografts on the MSC-viability and to compare the vulnerability of differentiated MSCs and control MSCs, (3-(4,5-dimethylthiazol-2yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) (MTS) assays (CellTiter 96® Aqueous One Solution Cell Proliferation Assay; Promega Corporation, WI, USA) were carried out according to the manufacturer’s protocol.33 This assay is a colorimetric method to determine the number of viable cells. Living cells in culture have metabolic activity and will convert the added reagent in formazan. Formazan quantity is directly correlated to the amount of 490nm absorbance and can therefore be measured with an Infinite® 200 Pro TECAN Reader (Tecan Trading AG, Switzerland).
Undifferentiated and differentiated MSCs (5,000 in 100μL growth medium per well) were transferred to a p-HEMA (Poly 2-hydroxyethyl methacrylate; Sigma-Aldrich Corp., MO, USA) coated 96-well plate, to prevent cells from migrating to the plastic surface of the well. After soaking in growth medium for two hours, 48 2mm-segments of processed nerve allografts were divided among the wells containing the cells. The medium was changed every 72 hours; undifferentiated MSCs were cultured in normal growth medium, differentiated MSCs were cultured in differentiation medium. The metabolic activity of undifferentiated MSCs (undifferentiated MSCs + pHEMA + allograft) was compared to differentiated MSCs (differentiated MSCs + pHEMA + allograft) on four estimated time points (T = 1, 2, 3 and 7 days). The remaining groups represented normal cell viability (pHEMA + undifferentiated MSCs and pHEMA + differentiated MSCs) and negative controls (no cells). For each group, the metabolic activity of three replicates per sample was tested twice on each time point. Colorimetric assays were performed with the Infinite® 200 Pro TECAN Reader (Tecan Trading AG, Switzerland) at an absorbance wavelength of 490nm. The metabolic activity of undifferentiated MSCs and differentiated MSCs in the vicinity of an allograft was expressed as a ratio of the metabolic activity of undifferentiated MSCs and differentiated MSCs without an allograft. The experimental design is shown in table 1.
Table 1.
Experimental design experiment 1.
| Group | Description | Time points | N | Outcome measurements |
|---|---|---|---|---|
| I | Undifferentiated MSCs + | T1 = 1 day | 3 samples | - Metabolic activity |
| allograft | T2 = 2 days | per group | (MTS assay) | |
| II | Differentiated MSCs + | T3 = 3 days | for each | |
| allograft | T4 = 7 days | time point | ||
| III | Undifferentiated MSCs | |||
| IV | Differentiated MSCs | |||
MSCs: Mesenchymal Stem Cells
MTS assay: (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H- tetrazolium) assay
Seeding efficiency, cell adhesion, distribution and migration
A dynamic seeding strategy that was previously described for undifferentiated MSCs was used in this study.29 In total, 36 processed nerve graft segments of 10mm in length were soaked in growth medium for two hours to remove any toxic decellularization agents and divided among conical tubes containing 10mL growth medium with one million undifferentiated MSCs or differentiated MSCs per nerve. These tubes were placed on a rotating system which was rotated for six, 12 or 24 hours at 37°C (n = 6 per group per seeding duration). Seeding efficiency on each time point was determined by cell counts in the cell supernatant of all the different samples; this provided the average number of free floating cells in the tubes and the average number of cells that were adherent to the nerve. Two supernatant samples (10μL) were taken out of each of the conical tubes after the seeding duration had passed. Subsequently, one investigator performed three cell counts on each of the supernatant samples. So for each nerve sample (n=6 per group per time point), 6 cell counts were averaged for final analysis in order to minimize potential error.
The viability and distribution of the cells seeded on the nerve grafts was studied by live/dead Cell Viability Assays (Invitrogen, Life Technologies Corporation, NY, USA) and Hoechst stain (Hoechst stain solution; Sigma-Aldrich Corp., MO, USA) and visualized using a confocal microscope (Zeiss LSM 780 confocal microscope). The live/dead and Hoechst stain assays were prepared by one investigator. Both the Live/dead stain and the Hoechst stain were obtained according to standardized protocols; incubation of the samples with live/dead stain (Invitrogen, Life Technologies Corporation, NY, USA) or Hoechst stain mixture (Hoechst stain solution; Sigma-Aldrich Corp., MO, USA) for 20 minutes after which the samples were washed with PBS. Both stains were performed on three samples per group per seeding duration.
Cell shape and distribution was evaluated by Scanning Electron Microscopy (SEM) of the three remaining seeded samples per group per time point. To obtain SEM images, the samples were transferred to 2% Trump’s fixative solution (37% formaldehyde and 25% glutaraldehyde) overnight, washed in phosphate buffer, and rinsed in water and dehydrated through a graded series of ethanol. Subsequently, the samples were critical point dried using carbon dioxide, mounted on an aluminum stub and sputter-coated for 60 seconds using gold-palladium. The samples were imaged in a Hitachi S-4700 cold field emission scanning electron microscope (Hitachi High Technologies America, Inc., IL, USA) at 5kV accelerating voltage. The preparation and the imaging of the SEM samples were performed by the Microscopy and Cell analysis core lab of the Mayo Clinic. The distribution of cells on the outer surface was only assessed and described subjectively and were therefore not blinded. An overview of the experimental design is depicted in table 2.
Table 2.
Experimental design experiment 2.
| Group | Description | Time points (seeding duration) | Number of Outcome measurements samples | |
|---|---|---|---|---|
| I | Undifferentiated | T1 = 6 hours | 6 samples per | - Cell counts (n=6) |
| MSCs + nerve | T2 = 12 hours | group per | - Live/dead + | |
| allograft | T3 = 24 hours | time point | Hoechst stains (n=3) | |
| II | Differentiated | - SEM (n=3) | ||
| MSCs + nerve | ||||
| allograft | ||||
SEM: Scanning Electron Microscopy
MSCs: Mesenchymal Stem Cells
Two extra processed nerves per group were seeded with the previously estimated optimal seeding duration and transferred to 10% formalin and processed and embedded in paraffin to evaluate the migration of cells into the nerve grafts. Three 5μM sections of the proximal- and mid-nerve segment were sectioned and Hoechst stained. The Hoechst-stained cross-sectional segments of the nerves were blinded for the objective assessment of present cells on the inner surface of the samples (present versus absent). The cells were visualized with a confocal microscope (Zeiss LSM 780 confocal microscope; Zeiss, Germany).
Statistical analysis
Seeding efficiencies are displayed ± Standard Error of the Mean. Significance of the interaction between cell type, time and outcome were analyzed with two-way ANOVA. If the interaction was significant, the within and between group comparisons were analyzed with the Kruskal-Wallis test, followed by pairwise comparisons using Wilcoxon rank-sum tests with Bonferroni correction. A value of p < 0.05 was considered statistically significant.
RESULTS
Mesenchymal stem cell collection
Flow cytometric analysis showed that the cultured rat MSCs were positive for mesenchymal stem cell markers CD29 (88.2%) and CD90 (88.3%) and negative for the hematopoetic cell markers CD34 (91.1%) and CD45 (86.0%), demonstrating that MSCs were definitively the cell lineage utilized in this study.
Mesenchymal stem cell differentiation into Schwann-like cells
The morphology of differentiated MSCs changed in vitro in a more spindle-like shape, typical for Schwann cells. In contrast to undifferentiated MSCs, Schwann cells and differentiated MSCs showed positive immunofluorescence for Schwann cell surface markers S100 Calcium Binding Protein B (S100B), Glial Fibrillary Acidic Protein (GFAP) and Nerve Growth Factor Receptor (NGFR/P75NTR), which verified successful differentiation into Schwann-like cells.18
Cell viability
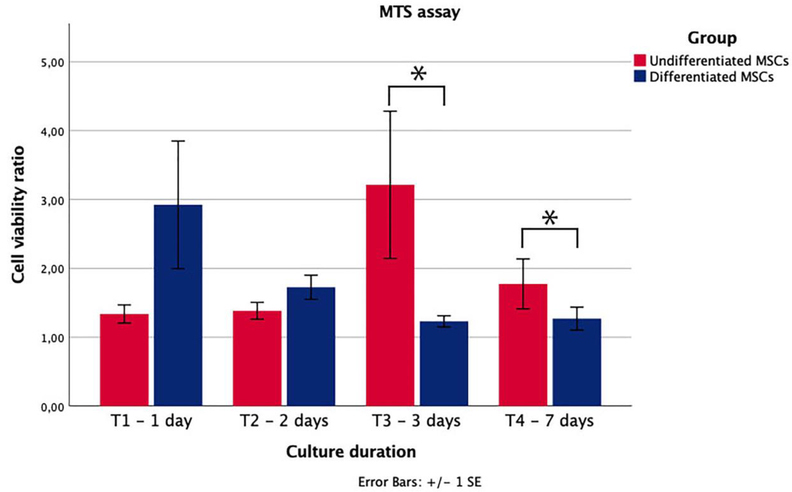
The cell viability of undifferentiated and differentiated MSCs were equal and remained constant during the first three time points, after which the viability of both cell types slightly decreased. No decrease in cell viability was observed upon co-culture of MSCs with the processed nerve allograft. The viability of differentiated MSCs in the vicinity of an allograft approaches the viability of differentiated MSCs alone over time (p=0.270), while the viability of undifferentiated MSCs with the allograft increased over time compared to undifferentiated MSCs alone; the increased viability ratio between 2 and 3 days of culture was statistically significant (p=0.025).
The differences between both cell-group ratios after 3 and 7 days of culture were statistically significant as well (p=0.009 and p=0.026). These results imply that chemical processing of nerve allografts does not generate a surface that decreases cell-viability. Interestingly, the demonstrated ratios suggest that the processed allograft stimulates the viability of undifferentiated MSCs, while this effect is not as obvious for differentiated MSCs. The absorbance ratios, and thus the viability ratios of the different cell cultures over time is shown in Figure 1.
Figure 1.
Metabolic activity of undifferentiated and differentiated MSCs after a processed nerve allograft was introduced to their well. The activity is expressed as a ratio of the metabolic activity of MSCs without a processed nerve allograft. Undifferentiated MSCs had a significantly higher metabolic ratio after 3 (p=0.009) and 7 (p=0.026) days of culture. Error bars = SEM. *=statistical significance, p<0.05
Cell adhesion
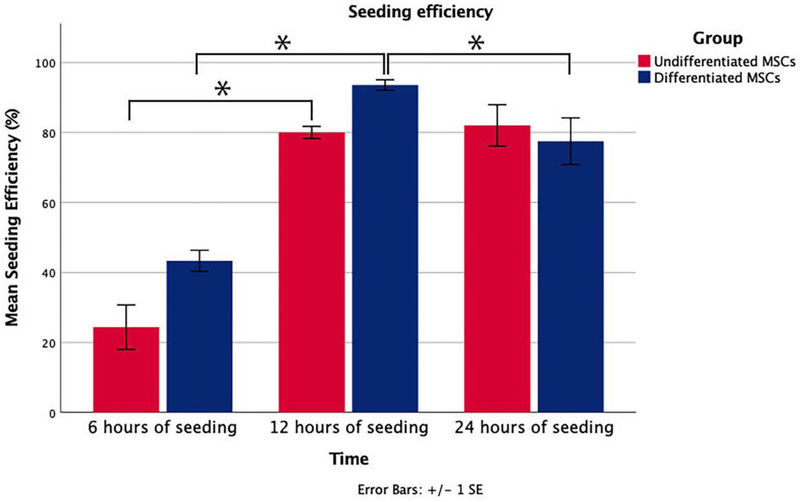
After six hours of seeding, only 24.38% (±6.34) of undifferentiated MSCs and 43.33% (±3.02) of differentiated MSCs were attached to the processed nerve allografts. Subsequently, these percentages increased to 80.00% (±1.73) (undifferentiated MSCs) and 94.54% (±1.50) (differentiated MSCs) after 12 hours of dynamic seeding and changed to 82.00% (±5.92) (undifferentiated MSCs) and 77.50% (±6.67) (differentiated MSCs) after 24 hours. ‘Between group’ analyses did not show any significant differences. ‘Within group’ analyses showed a significant interaction between seeding efficiency and seeding duration for undifferentiated MSCs (p=0.021) and differentiated MSCs (p=0.007). Pairwise comparisons revealed that the increase between 6 and 12 hours of seeding (p=0.029) undifferentiated MSCs was statistically significant, but not between 12 and 24 hours (p=0.486). The increased seeding efficiency between 6 and 12 hours and the decrease between 12 and 24 hours of seeding of differentiated MSCs were statistically significant (both p=0.029). Considering a shorter seeding duration is more cost effective and time efficient, the optimal dynamic seeding duration was determined to be 12 hours for both groups. The seeding efficiencies at different time points are shown in Figure 2.
Figure 2.
Seeding efficiency of three different seeding durations. The efficiency is expressed as a percentage of the cells provided per nerve (1 million cells). Error bars = SEM. *=statistical significance, p<0.05. The increase between 6 and 12 hours of seeding (p=0.029) undifferentiated MSCs was statistically significant. The increased seeding efficiency of differentiated MSCs between 6 and 12 hours and the decrease between 12 and 24 hours were also statistically significant (both p=0.029). Error bars = SEM. *=statistical significance, p<0.05
Cell distribution and migration
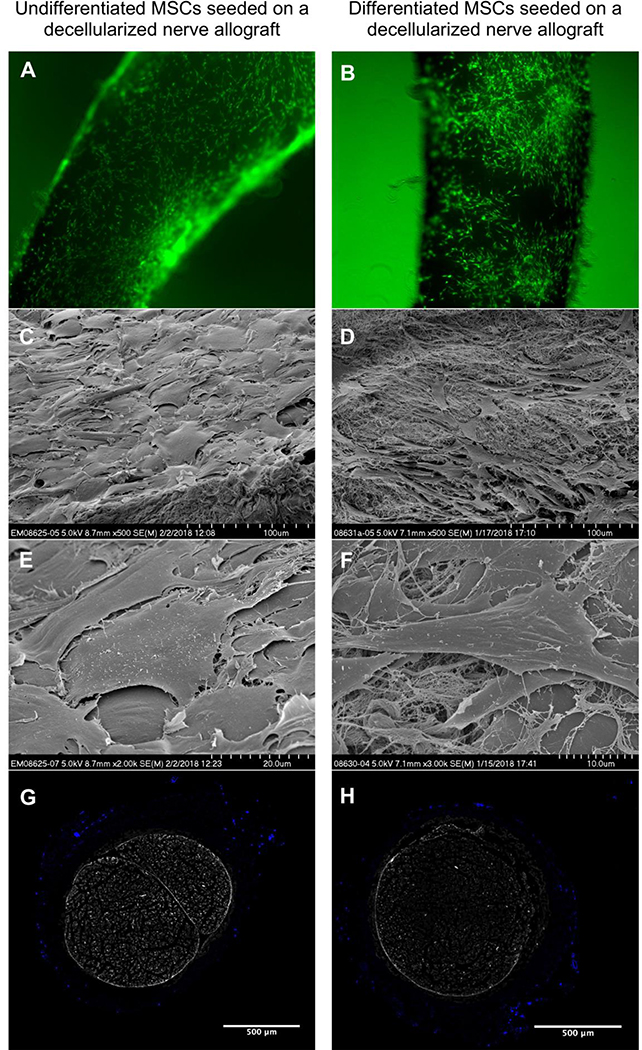
Dynamic seeding resulted in a layer of viable cells on the surface of the processed nerve allograft with no major differences in distribution between the cell-types and different seeding durations. Virtually no dead cells were apparent on the processed nerve allograft during dynamic seeding, which suggests that only viable cells are able to attach to the nerve. Although subjective, the differentiated MSCs seem to seed in a more clustered fashion than undifferentiated MSCs (Figure 3a–b). Hoechst stain (not shown) and SEM of the surface of the seeded nerve grafts showed a similar distribution and illustrated that the cells retain their typical shapes during dynamic seeding (Figure 3c–f). None of the cross-sectional segments of the proximal and mid-portion of the nerves showed migration of both undifferentiated MSCs and differentiated MSCs into the nerve graft (Figure 3g–h).
Figure 3.
Undifferentiated MSCs (left) and differentiated MSCs (right) seeded on a nerve allograft. 4a-b. Viable cells are visualized in green, dead cells in red (not present). 4c-f. Scanning Electron Microscopy images of undifferentiated MSCs seeded onto a nerve allograft (left) and differentiated MSCs seeded on a nerve allograft (right) in multiple magnifications (500X, 2000X and 3000X), showing the different morphology of the cells when seeded on the nerve allograft. 4g-h. Crosssectional images of the mid-portions of processed nerve allografts seeded with undifferentiated (left) and differentiated (right) MSCs. Cell nuclei are Hoechst-stained (bright blue). The inner ultrastructure of the nerve does not contain any cells.
DISCUSSION
The overall purpose of this study was to determine (I) the influence of processed nerve grafts on the viability of differentiated MSCs, (II) the seeding potential and optimal seeding duration of differentiated MSCs on processed nerve grafts, and (III) the survivability, distribution and migration of differentiated MSCs after seeding. The reagents used to process the nerve allografts in this study30 did not influence the metabolic activity of either differentiated MSCs or undifferentiated MSCs. Similar to undifferentiated MSCs, the differentiated MSCs were successfully seeded on a processed nerve allograft using a previously reported dynamic seeding strategy29, without compromising the quality of the inner nerve ultrastructure. The optimal dynamic seeding time for both groups was 12 hours, which led to the attachment of viable undifferentiated MSCs and differentiated MSCs to the surface of the processed nerve allograft. Both types of MSCs distributed among the nerve allograft in a uniform manner and did not migrate into the ultrastructure of the nerve allograft.
In vitro, Schwann-like MSCs support superior neurite outgrowth when co-cultured with motorneurons and express higher levels of neurotrophic and angiogenic genes compared to undifferentiated MSCs.18, 19, 24, 25 The potential for uncontrolled proliferation or differentiation of MSCs into non-neural lineages is another argument to differentiate MSCs before implementation. The pluripotency of undifferentiated MSCs makes them difficult to control in vivo. The concern that their potential to promote neoangiogenesis and to regulate the immune response may lead to tumor growth or metastasis has been described, but has not been confirmed yet.34, 35 The disadvantages of differentiating MSCs into Schwann-like cells include the additional effort and extended preparation time which may delay the period between nerve-injury and surgery. In vivo studies demonstrating no differences in functional outcomes compared to undifferentiated MSCs also favor the use of undifferentiated MSCs in the clinical setting.36, 37 The absence of in vivo differences is potentially caused by the hypothesized limited survivability of differentiated cells10 or an inefficient delivery of differentiated MSCs. Published delivery methods vary widely, while none of the delivery methods has specifically been tested on differentiated MSCs. Thus, to date there is no compelling evidence for the use of either undifferentiated MSCs or differentiated MSCs for seeded nerve allografts used for segmental motor nerve reconstruction.
Cell-injection and nerve-soaking in cell-solutions are used as delivery methods of MSCs with the rationale that they have a structural function as Schwann cells that need to be delivered within the nerve allograft itself. Injection may be traumatic to both MSCs and the ultrastructure of the nerve allograft and results in an unequal distribution of the delivered cells.27, 38–40 The average diameter of MSCs exceeds the calibers of myelinated axon fibers, suggesting that MSCs can block axon ingrowth when delivered inside the nerve graft.41–43 Soaking techniques deliver lower number of cells in a nonuniform distribution. 28
It has been recently reported that growth factors and cytokines produced by MSCs may enhance nerve regeneration, while not necessitating intraneural placement of the MSCs.7, 44 The straightforward dynamic seeding strategy of Rbia and colleagues successfully attaches large numbers of undifferentiated MSCs to the surface of a processed nerve allograft without harming the inner ultra-structure of the allograft.29 The same technique resulted in the same optimal seeding duration for undifferentiated MSCs in this study, indicating a high reproducibility of the Rbia method. The seeding of differentiated MSCs is less well established and differentiation of cells may decrease their potential to attach to processed nerve allografts, possibly due to their changes in cellular morphology (e.g., spindle-like shape).10 This study demonstrated there was no decrease in attachment efficiency when MSCs are differentiated. Based on our experience, dynamic seeding of differentiated MSCs onto a processed nerve allograft is possible and results in a uniform distribution of large amounts of both differentiated MSCs (and undifferentiated MSCs) on the surface of the nerve allograft which has not been accomplished by other previously described methods.
Both cell types have previously shown to produce neurotrophic and angiogenic factors and have been allocated an immunomodulatory role. 7, 18, 19, 24, 25, 44 The porous epineurium of the processed nerve allografts (demonstrated in figure 3) allows for these factors to both regulate the immune response and angiogenesis in the surroundings of the regenerating nerve, and stimulate nerve regeneration inside the nerve allograft, while the cells remain on the outer surface of the graft. The seeded MSCs form an addition to the circulating stem cells normally attracted to the regenerating nerve, also functioning from outside the epineurium.
A limitation of this study is the in vitro setting, which may not translate into results expected for an in vivo setting. In vitro studies permit testing of the seeding potential of undifferentiated MSCs and differentiated MSCs without having to sacrifice extra animals. With the described strategy, it would approximately take up two to five weeks after nerve injury to obtain a processed allograft seeded with patient’s own (undifferentiated or differentiated) MSCs. Although peripheral nerve injuries are ideally repaired as soon as possible after injury, a two- or five-week delay that eventually leads to the desirable improved nerve regeneration would be clinically applicable. Furthermore, the utility of methods to deliver undifferentiated MSCs and differentiated MSCs to the surface of a nerve graft relies on the idea that MSCs at least have a partly trophic function and that growth factors and cytokines produced by them will migrate through the epineurium and enhance nerve regeneration. This hypothesis needs to be confirmed both in vitro and in vivo. The current study is the essential first step in testing this hypothesis.
CONCLUSION
We successfully differentiated MSCs into Schwann-like cells and dynamically seeded them onto a processed nerve allograft. The viability of undifferentiated MSCs and differentiated MSCs was not influenced by the processed nerve allograft. Both cell types distributed equally among the nerve allograft, remained on the surface of the allograft and did not migrate into the graft. Thus, seeding of nerve allografts with Schwann-like MSCs can be further considered for in vivo studies for nerve repair.
Supplementary Material
Acknowledgements
We thank Patricia F. Friedrich for assistance with the preparations of the experiments.
Conflict of interest statement
None of the authors has a conflict of interest. This study was funded by the NIH R01, ‘Bridging the gap: angiogenesis and stem cell seeding of processed nerve allograft’. 1 RO1 NS102360-01A1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Rbia N, Shin AY. The Role of Nerve Graft Substitutes in Motor and Mixed Motor/Sensory Peripheral Nerve Injuries. J Hand Surg Am 2017;42;367–77. [DOI] [PubMed] [Google Scholar]
- 2.Giusti G, Willems WF, Kremer T, et al. Return of motor function after segmental nerve loss in a rat model: comparison of autogenous nerve graft, collagen conduit, and processed allograft (AxoGen). J Bone Joint Surg Am 2012;94;410–7. [DOI] [PubMed] [Google Scholar]
- 3.FF IJ, Nicolai JP, Meek MF. Sural nerve donor-site morbidity: thirty-four years of follow-up. Ann Plast Surg 2006;57;391–5. [DOI] [PubMed] [Google Scholar]
- 4.Cho MS, Rinker BD, Weber RV, et al. Functional outcome following nerve repair in the upper extremity using processed nerve allograft. J Hand Surg Am 2012;37;2340–9. [DOI] [PubMed] [Google Scholar]
- 5.Moore AM, MacEwan M, Santosa KB, et al. Acellular nerve allografts in peripheral nerve regeneration: A comparative study. MUSCLE NERVE 2011;44;221–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whitlock EL, Tuffaha SH, Luciano JP, et al. Processed allografts and type I collagen conduits for repair of peripheral nerve gaps. MUSCLE NERVE 2009;39;787–99. [DOI] [PubMed] [Google Scholar]
- 7.Caplan AI. Adult Mesenchymal Stem Cells: When, Where, and How. Stem Cells Int 2015;2015;628767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao F, Liu T, Xu Y, Xu D, Feng S. Culture and properties of adipose-derived mesenchymal stem cells: characteristics in vitro and immunosuppression in vivo. Int J Clin Exp Pathol 2015;8;7694–709. [PMC free article] [PubMed] [Google Scholar]
- 9.Moattari M, Kouchesfehani HM, Kaka G, et al. Chitosan-film associated with mesenchymal stem cells enhanced regeneration of peripheral nerves: A rat sciatic nerve model. J Chem Neuroanat 2017;88;46–54. [DOI] [PubMed] [Google Scholar]
- 10.Fairbairn NG, Meppelink AM, Ng-Glazier J, Randolph MA, Winograd JM. Augmenting peripheral nerve regeneration using stem cells: A review of current opinion. World J Stem Cells 2015;7;11–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao Z, Wang Y, Peng J, et al. Repair of nerve defect with acellular nerve graft supplemented by bone marrow stromal cells in mice. Microsurgery 2011;31;388–94. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, Zhao Z, Ren Z, et al. Recellularized nerve allografts with differentiated mesenchymal stem cells promote peripheral nerve regeneration. Neurosci Lett 2012;514;96–101. [DOI] [PubMed] [Google Scholar]
- 13.Hundepool CA, Nijhuis TH, Mohseny B, Selles RW, Hovius SE. The effect of stem cells in bridging peripheral nerve defects: a meta-analysis. J Neurosurg 2014;121;195–209. [DOI] [PubMed] [Google Scholar]
- 14.Strioga M, Viswanathan S, Darinskas A, Slaby O, Michalek J. Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells. Stem Cells Dev 2012;21;2724–52. [DOI] [PubMed] [Google Scholar]
- 15.Mahmoudifar N, Doran PM. Mesenchymal Stem Cells Derived from Human Adipose Tissue. Methods Mol Biol 2015;1340;53–64. [DOI] [PubMed] [Google Scholar]
- 16.Yoshimura H, Muneta T, Nimura A, et al. Comparison of rat mesenchymal stem cells derived from bone marrow, synovium, periosteum, adipose tissue, and muscle. Cell Tissue Res 2007;327;449–62. [DOI] [PubMed] [Google Scholar]
- 17.Safford KM, Hicok KC, Safford SD, et al. Neurogenic differentiation of murine and human adipose-derived stromal cells. Biochem Biophys Res Commun 2002;294;371–9. [DOI] [PubMed] [Google Scholar]
- 18.Kingham PJ, Kalbermatten DF, Mahay D, et al. Adipose-derived stem cells differentiate into a Schwann cell phenotype and promote neurite outgrowth in vitro. Exp Neurol 2007;207;267–74. [DOI] [PubMed] [Google Scholar]
- 19.Ladak A, Olson J, Tredget EE, Gordon T. Differentiation of mesenchymal stem cells to support peripheral nerve regeneration in a rat model. Exp Neurol 2011;228;242–52. [DOI] [PubMed] [Google Scholar]
- 20.di Summa PG, Kingham PJ, Raffoul W, et al. Adipose-derived stem cells enhance peripheral nerve regeneration. J Plast Reconstr Aesthet Surg 2010;63;1544–52. [DOI] [PubMed] [Google Scholar]
- 21.Ao Q, Fung CK, Tsui AY, et al. The regeneration of transected sciatic nerves of adult rats using chitosan nerve conduits seeded with bone marrow stromal cell-derived Schwann cells. Biomaterials 2011;32;787–96. [DOI] [PubMed] [Google Scholar]
- 22.Chen X, Wang XD, Chen G, et al. Study of in vivo differentiation of rat bone marrow stromal cells into schwann cell-like cells. Microsurgery 2006;26;111–15. [DOI] [PubMed] [Google Scholar]
- 23.Tomita K, Madura T, Mantovani C, Terenghi G. Differentiated adipose-derived stem cells promote myelination and enhance functional recovery in a rat model of chronic denervation. J Neurosci Res 2012;90;1392–402. [DOI] [PubMed] [Google Scholar]
- 24.Kingham PJ, Kolar MK, Novikova LN, Novikov LN, Wiberg M. Stimulating the neurotrophic and angiogenic properties of human adipose-derived stem cells enhances nerve repair. Stem Cells Dev 2014;23;741–54. [DOI] [PubMed] [Google Scholar]
- 25.Tomita K, Madura T, Sakai Y, et al. Glial differentiation of human adipose-derived stem cells: implications for cell-based transplantation therapy. Neuroscience 2013;236;55–65. [DOI] [PubMed] [Google Scholar]
- 26.Liu Y, Zhang Z, Qin Y, et al. A new method for Schwann-like cell differentiation of adipose derived stem cells. Neurosci Lett 2013;551;79–83. [DOI] [PubMed] [Google Scholar]
- 27.Jesuraj NJ, Santosa KB, Newton P, et al. A systematic evaluation of Schwann cell injection into acellular cold-preserved nerve grafts. J Neurosci Methods 2011;197;209–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thompson MJ, Patel G, Isaacs J, et al. Introduction of neurosupportive cells into processed acellular nerve allografts results in greater number and more even distribution when injected compared to soaking techniques. Neurol Res 2017;39;189–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rbia N, Bulstra LF, Bishop AT, van Wijnen AJ, Shin AY. A simple dynamic strategy to deliver stem cells to decellularized nerve allografts. Plast Reconstr Surg 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hundepool CA, Nijhuis TH, Kotsougiani D, et al. Optimizing decellularization techniques to create a new nerve allograft: an in vitro study using rodent nerve segments. Neurosurg Focus 2017;42. [DOI] [PubMed] [Google Scholar]
- 31.Hudson TW, Zawko S, Deister C, et al. Optimized acellular nerve graft is immunologically tolerated and supports regeneration. Tissue Eng 2004;10;1641–51. [DOI] [PubMed] [Google Scholar]
- 32.Kumta S, Yip K, Roy N, Lee SK, Leung PC. Revascularisation of bone allografts following vascular bundle implantation: an experimental study in rats. Arch Orthop Trauma Surg 1996;115;206–10. [DOI] [PubMed] [Google Scholar]
- 33.Cory AH, Owen TC, Barltrop JA, Cory JG. Use of an aqueous soluble tetrazolium/formazan assay for cell growth assays in culture. Cancer Commun 1991;3;207–12. [DOI] [PubMed] [Google Scholar]
- 34.Volarevic V, Markovic BS, Gazdic M, et al. Ethical and Safety Issues of Stem Cell-Based Therapy. Int J Med Sci 2018;15;36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lazennec G, Jorgensen C. Concise review: adult multipotent stromal cells and cancer: risk or benefit? Stem Cells 2008;26;1387–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Orbay H, Uysal AC, Hyakusoku H, Mizuno H. Differentiated and undifferentiated adiposederived stem cells improve function in rats with peripheral nerve gaps. J Plast Reconstr Aesthet Surg 2012;65;657–64. [DOI] [PubMed] [Google Scholar]
- 37.Watanabe Y, Sasaki R, Matsumine H, Yamato M, Okano T. Undifferentiated and differentiated adipose-derived stem cells improve nerve regeneration in a rat model of facial nerve defect. J Tissue Eng Regen Med 2017;11;362–74. [DOI] [PubMed] [Google Scholar]
- 38.Garvican ER, Cree S, Bull L, Smith RK, Dudhia J. Viability of equine mesenchymal stem cells during transport and implantation. Stem Cell Res Ther 2014;5;94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Agashi K, Chau DY, Shakesheff KM. The effect of delivery via narrow-bore needles on mesenchymal cells. Regen Med 2009;4;49–64. [DOI] [PubMed] [Google Scholar]
- 40.Mamidi MK, Singh G, Husin JM, et al. Impact of passing mesenchymal stem cells through smaller bore size needles for subsequent use in patients for clinical or cosmetic indications. J Transl Med 2012;10;229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sunderland S, Lavarack JO, Ray LJ. The caliber of nerve fibers in human cutaneous nerves. J COMP NEUROL 1949;91;87–101. [DOI] [PubMed] [Google Scholar]
- 42.Ryu YJ, Cho TJ, Lee DS, Choi JY, Cho J. Phenotypic characterization and in vivo localization of human adipose-derived mesenchymal stem cells. Mol Cells 2013;35;557–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ge J, Guo L, Wang S, et al. The size of mesenchymal stem cells is a significant cause of vascular obstructions and stroke. Stem Cell Rev 2014;10;295–303. [DOI] [PubMed] [Google Scholar]
- 44.Caplan AI, Hariri R. Body Management: Mesenchymal Stem Cells Control the Internal Regenerator. Stem Cells Transl Med 2015;4;695–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.