Abstract
Mechanical power generated via triceps surae muscle-tendon interaction during walking is largely responsible for the total power needed to walk. This interaction is made complex by the biological architecture of the Achilles tendon, which consists of distinct bundles of tendon fascicles, known as “subtendons”, arising from the lateral and medial gastrocnemius (GAS) and soleus (SOL) muscles. Comparative data and our own in vivo evidence allude to a reduced capacity for sliding between adjacent subtendons compromising the Achilles tendon in old age. This is functionally important, as subtendon sliding could facilitate independent actuation between individual triceps surae muscles, perhaps augmenting contributions to trunk support and forward propulsion. Recently, we revealed that length change differences between the GAS and SOL of young adults positively correlated with non-uniform subtendon tissue displacement patterns. Here, we investigated aging effects on triceps surae muscle-subtendon interaction using dual-probe ultrasound imaging during a series of ramped isometric contractions. We hypothesized that, compared to young adults, older adults will have more uniform subtendon tissue displacements that are accompanied by anatomically consistent differences in GAS versus SOL muscle length change behavior. Our findings fully support our hypotheses, older adults have more uniform subtendon tissue displacements that extend to anatomically consistent and potentially unfavorable changes in muscle contractile behavior – evidenced by smaller differences between GAS and SOL peak shortening during isometric force generation. These findings provide an important biomechanical basis for previously reported correlations between more uniform Achilles subtendon behavior and reduced ankle moment generation during waking in older adults.
Keywords: Ultrasound, Biomechanics, Speckle Tracking, Interfascicle Adhesions, Plantarflexor
Introduction
The timing and magnitude of peak ankle moment, governed by the mechanical output of the lateral and medial gastrocnemius (GAS) and soleus (SOL) muscles (i.e., triceps surae) and their common series elastic structures, is an important determinant of walking performance1. However, despite sharing a common tendon, muscles comprising the triceps surae undergo different fascicle kinematics during walking and, biomechanically, contribute differently to forward propulsion and vertical support2. This differential behavior at the muscle level alludes to the possibility that these muscles act, at least in part, as functionally independent actuators. Building on this premise, more recent evidence shows that the architecturally complex Achilles tendon consists of distinct subtendons arising from each of the individual triceps surae muscles3,4. Comparative data and our own in vivo evidence allude to the prevalence of sliding between these adjacent subtendons that has the potential to facilitate independence between the GAS and SOL5–7. Unfortunately, animal models of the aging tendon present with a proliferation of collagen cross-linking and prominent reductions in interfascicle sliding8,9. Those observations also appear to be clinically meaningful; during walking, age-associated reductions in measures of subtendon sliding within the human Achilles tendon correlate with smaller peak ankle moments during push-off in older adults10. Although this alludes to a mechanistic link between Achilles subtendon tissue displacements and altered muscle contractile behavior due to aging, we are not aware of direct empirical data that would support such a conclusion.
There is strong evidence that a precipitous decline in peak ankle moment plays a vital role in age-related mobility deficits11,12. Reduced mechanical output from the triceps surae is likely multifactorial, and the functional consequences of, for example, sarcopenia and distal leg muscle weakness are well-documented. However, declines in muscle strength alone cannot fully explain age-related reductions in push-off intensity during walking, nor do simple strengthening exercises directly translate to improvements in ankle moment or walking speed11, 13. The current study is motivated by the overarching working hypothesis that changes in the interaction between triceps surae muscle behavior and Achilles subtendon tissue displacements contributes to a reduced capacity for ankle moment generation during walking in older versus young adults14. Finni et al. (2018) revealed that independent GAS versus SOL muscle stimulation elicits large differences in the Achilles subtendon strain patterns and mechanical output in rats15. Thus, as a logical and needed extension, we aim to determine the mechanistic link between these structures in humans.
Indirect evidence from young adults is consistent with the notion that subtendon-level changes can alter the contractile behavior of individual triceps surae muscles. For example, we recently investigated the relation between Achilles subtendon tissue displacement patterns and individual triceps surae muscle fascicle shortening using synchronized, dual-probe ultrasound imaging6. There, consistent with our conceptual premise, we revealed that differences between GAS and SOL subtendon displacements were accompanied by and correlated with anatomically consistent differences between GAS and SOL muscle shortening. In young adults, this implicates triceps surae muscle contractile behavior in precipitating sliding between adjacent subtendons comprising the Achilles tendon. By logical extension, this outcome also suggests that reduced interfascicle sliding at the tendon-level due to aging could unfavorably couple GAS and SOL behavior at the muscle-level. Length changes of GAS16 and SOL17 muscle fascicles have been measured separately during activities spanning isolated contractions to walking in young and older subjects. As one example, older adults walk with shorter GAS16 and SOL18 muscle fascicle lengths, most likely due to an age-related increase in Achilles tendon compliance19. However, only young adult studies have simultaneously measured fascicle behavior in both GAS and SOL2,20. Moreover, no study to date has compared simultaneous muscle- and tendon-level measurements between cohorts of young and older adults. Thus, we have an incomplete understanding of how age-related tendon-level changes influence muscle-level behavior.
As an important step toward establishing mechanistic causal links, the purpose of this study was to investigate aging effects on the relation between non-uniform Achilles subtendon displacement patterns and triceps surae muscle contractile behavior using dual-probe ultrasound imaging during a series of ramped maximum isometric voluntary contractions. We hypothesized that, compared to young adults, older adults would have (i) more uniform Achilles subtendon tissue displacements that (ii) would be accompanied by anatomically consistent (i.e., smaller differences, same sign) GAS versus SOL muscle length change behavior. Finally, based on evidence that the human AT becomes more compliant in old age, we tested the secondary hypothesis that older adult subtendons would be more sensitive than younger adult subtendons to changes in force transmission that follow from changes in ankle joint position (i.e., more displacement per unit force).
Materials and Methods
Subjects and protocol
We report data for nine younger subjects (age: 25.1 ± 5.6 years, mass: 69.8 ± 6.9 kg, height: 1.7 ± 0.1 m, four females) and ten older adult subjects (age: 74.3 ± 3.4 years, mass: 67.2 ± 9.0 kg, height: 1.7 ± 0.1 m, four females). Each subject provided written informed consent as per the University of North Carolina at Chapel Hill Internal Review Board (16–0379). All subjects did not require an assistive aid to walk, were free from any neurological disorder or disease, did not have a leg prosthesis, and have not had an orthopedic disorder within the last six months. To precondition their Achilles tendon and triceps surae, subjects first walked on a treadmill (Bertec Corporation, Columbus, OH) for six min at a self-selected walking speed (above 1.0 m/s)21. Thereafter, while sitting in a Biodex System 4-Pro (Biodex, Shirly, NY), subjects completed a series of three ramped maximum isometric voluntary contractions at four separate ankle angles (from a 0° neutral ankle angle to a 30° plantarflexion in 10° increments) with the knee flexed to replicate the push-off phase of walking (~20–30°). We instructed subjects to “push like a gas pedal” (i.e., attempt to isolate their triceps surae), and perform a four second, symmetric loading-unloading profile. To comply, subjects started at rest and increased ankle moment until they reached a voluntary maximum at two seconds, before steadily returning to rest at four seconds. Before the first recorded trial, subjects practiced the ramped contractions using a real-time display of their ankle moment. During data collection, we fully-randomized ankle angle and provided one minute of rest between contractions. Subjects were barefoot throughout the data collection to facilitate proper placement of the ultrasound transducers.
Measurements
We utilized the same methodological approach as previously reported in our younger adult data collection6. Two linear array 10 MHz ultrasound transducers simultaneously recorded GAS and SOL fascicle kinematics and tissue displacements in their associated tendinous structures (Supplementary Figure 1). The first transducer (LV7.5/60/128Z-2, UAB Telemed, Vilnius, Lithuania), placed over the GAS mid-belly of the subject’s right leg, recorded cine B-mode images at 61 frames/s through a longitudinal cross section using an image depth of 65 mm. The transducer placement and depth enabled synchronized assessment of GAS and SOL in the same image plane22. Simultaneously, a second ultrasound transducer (L14–5W/38, Ultrasonix Corporation, Richmond, BC) placed over the subjects’ right free AT, distal to the SOL muscle-tendon junction, recorded 128 lines of ultrasound radiofrequency data at 70 frames/s using an image depth of 20 mm. We secured the second ultrasound probe with a custom orthotic, secured just proximal to the malleoli to replicate the placement used in prior studies (i.e., ~ six cm proximal to the calcaneal insertion point)23.
Operating at 100 Hz, eight cameras (Motion Analysis Corporation, Santa Rosa, CA) recorded the three-dimensional positions of 14 retroreflective markers placed on the subjects’ lower right leg and each ultrasound transducer. We synchronized binary ultrasound signals (i.e., signals indicating the start and stop of collection) from each transducer at 1000 Hz using a wave form generator (SDG1025, SIGLENT, Shenzhen). The Biodex dynamometer (Biodex, Shirly, NY) recorded ankle moment at 1000 Hz. Post collection, we estimated ankle and knee joint angles using a custom inverse kinematics routine24. We co-registered ultrasound signals, ankle moment, and marker trajectories with GAS, SOL, and free Achilles tendon ultrasound data. Using a key-frame threshold of 5% peak ankle moment, we analyzed all co-registered data between key-frames at the beginning and end of each ramped contraction. Finally, we quantified time series of (i) triceps surae muscle kinematics (i.e., GAS and SOL muscle length change) and (ii) Achilles subtendon tissue kinematics (i.e., GAS and SOL subtendon displacements), each interpolated to 1000 data points per trial, described in detail below.
Muscle kinematics
We controlled for tracking limitations and inter-investigator variability by following the best practices outlined by Farris and Lichtwark (2016), with the same investigator interrogating and performing all triceps surae tracking25. First, we defined a static region of interest surrounding each muscle and their aponeuroses (Supplementary Figure 1). In the first key-frame of each trial, we defined one GAS and one SOL muscle fascicle, drawn from their superficial to deep aponeurosis. We selected a fascicle that was in the mid-region of the imaged plane and most represented the muscle belly. An open source MATLAB routine, UltraTrack25, based on an affine extension to an optic flow algorithm quantified time series of GAS and SOL fascicle length and pennation angle. For direct comparison of longitudinal tissue displacements, we multiplied muscle fascicle length by the cosine of pennation angle to compute longitudinal muscle length along the Achilles tendon line of action.
Tendon kinematics
Using previously published techniques26, we quantified localized displacements of Achilles subtendon tissue using a custom two-dimensional speckle tracking algorithm. Briefly, we created a rectangular region of interest (~15 × 3 mm grid of nodes with 0.83 × 0.42 mm spacing, encompassing only tendinous tissue) on a B-mode image of the free Achilles tendon reconstructed from the raw radiofrequency data at the first key-frame of each trial. Centered at each nodal position, a 2 mm × 1 mm kernel containing up-sampled (4×) radiofrequency data acted as a search window for successive two-dimensional normalized cross-correlation functions. Frame-to-frame nodal displacements that maximized the two-dimensional cross-correlations were regularized using second order polynomials. These cumulative displacements represented the average of forward and backward tracking results and quantified the longitudinal displacement originating from two equally sized tendon depths - superficial and deep - corresponding to tendon tissue thought to arise from GAS and SOL, respectively. This orientation (i.e., free Achilles tendon ~ six cm proximal to the calcaneal insertion point) exemplifies the most prevalent anatomical arrangement in cadaveric studies27–29, with the GAS subtendon and SOL subtendon each representing 50% of the longitudinal cross section of the Achilles tendon30. We report these average displacements as GAS and SOL subtendon tissue displacements. We report Achilles subtendon non-uniformity as the difference between peak GAS and peak SOL subtendon tissue displacements.
Statistics
For each outcome measure, we took the average of the three conditions for each ankle angle for statistical analysis in SPSS. First, a Shapiro-Wilks test assessed normal distributions for each outcome measure. Subsequently, a three-way mixed factorial analysis of variance (ANOVA) tested for main effects of and interactions between age, ankle angle, and muscle-tendon unit (i.e., MTU, GAS vs. SOL) on peak muscle shortening and peak subtendon tissue displacements using an alpha level of 0.05. Post-hoc comparisons identified the ankle angles at which the muscle-tendon units were significantly different. Specifically, we used independent samples and Mann-Whitney tests for outcome measures found normally and not normally distributed, respectively. A two-way repeated measures ANOVA tested for significant main effects of and interactions between age and ankle angle on peak ankle moment. Pearson’s correlation coefficients assessed the relation between muscle and subtendon kinematics (i.e., GAS muscle and GAS subtendon, SOL muscle and SOL subtendon) in young and in older adults. We then calculated Pearson’s correlation coefficients between tendon non-uniformity (i.e., difference between GAS subtendon displacement and SOL subtendon displacement) and muscle level differences (i.e., difference between GAS muscle shortening and SOL muscle shortening). Finally, we calculated Pearson’s correlation coefficients between GAS and SOL peak subtendon displacement and mass normalized peak ankle moment.
Results
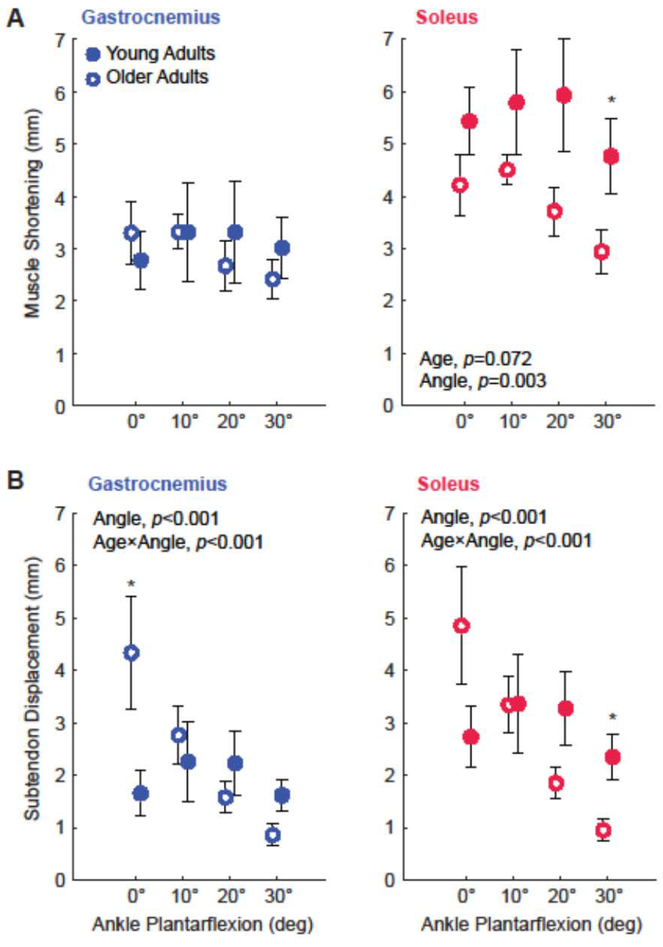
Across conditions, subjects’ knee flexion angle averaged 24.4 ± 5.1°. In young and older adults, peak ankle moment decreased progressively with increasing plantarflexion (p<0.001, Supplementary Figure 2). Compared to young adults, older adults’ peak ankle moment was significantly lower at each ankle angle (p-values≤0.024) and averaged 34% lower across all angles (Age, p<0.001). Average GAS and SOL muscle and subtendon profiles for each ankle angle are shown in Figure 1. We found significant age×MTU interactions for peak subtendon displacement and peak muscle shortening (p-values≤0.002). In young adults, peak SOL subtendon tissue displacements averaged 51% more than GAS subtendon tissue displacements (MTU, p<0.001), and peak SOL muscle shortening averaged 76% more than peak GAS muscle shortening (MTU, p=0.025, Figure 2). In older adults, peak SOL subtendon tissue displacements averaged 31% more than GAS subtendon tissue displacements (MTU, p=0.006, Figure 2). However, compared to young adults, those differences, indicative of tendon non-uniformity, averaged 63% smaller in older adults (Age, p<0.001, Figure 3). Also in older adults, peak SOL muscle shortening was indistinguishable from peak GAS muscle shortening (Age, p=0.502, Figure 2). Moreover, differences between GAS and SOL peak muscle shortening were 61% smaller in older adults (Age, p=0.002, Figure 3)
Figure 1:
(A) Group mean medial gastrocnemius (GAS) and soleus (SOL) peak muscle shortening for young and older adults. (B) Group mean GAS and SOL peak subtendon displacement. Asterisks (*) represent significant differences between young and older adults (p<0.05). Bars represent standard error.
Figure 2:
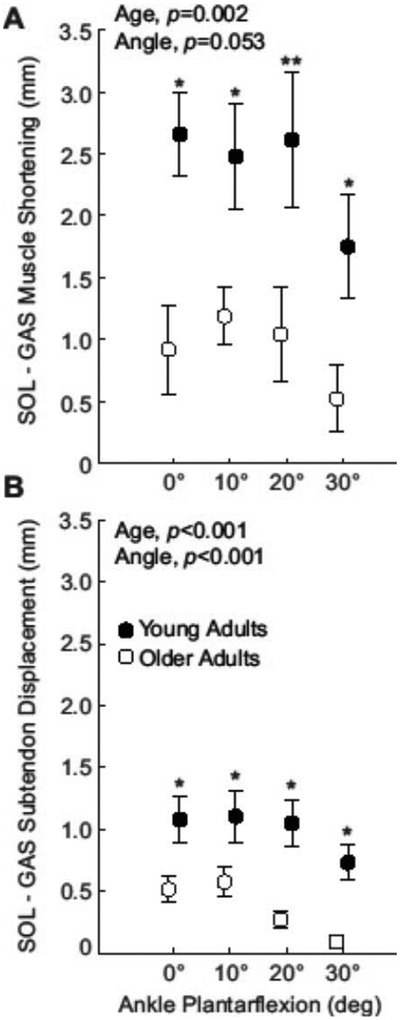
(A) Compared to young, differences between soleus (SOL) muscle shortening and medial gastrocnemius (GAS) muscle shortening decreased by 61% in older adults. (B) Achilles tendon non-uniformity (differences between peak SOL subtendon displacement and peak GAS subtendon displacement) decreased by 63%. Asterisks (*) represent significant differences between young and older adults (p<0.05). Double asterisk (**) indicates a Mann-Whitney comparison with p-value equal to 0.05. Bars represent standard error.
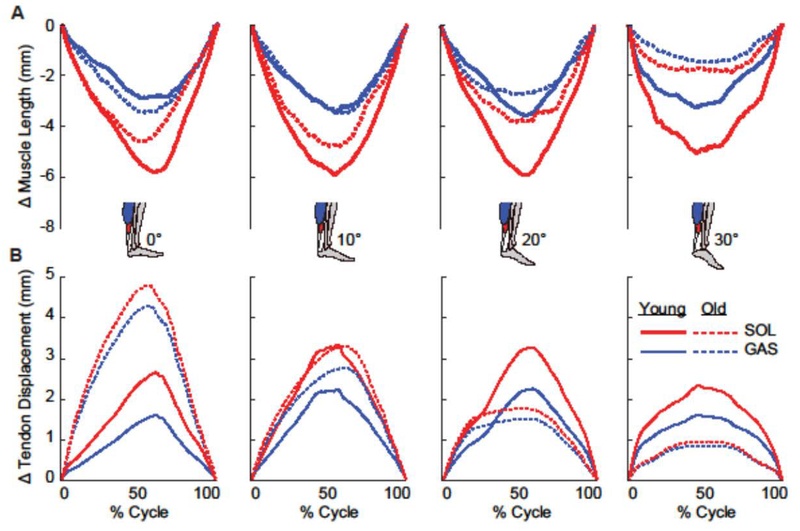
Figure 3:
Group mean (A) muscle shortening and (B) subtendon displacements across the range of ankle angles tested (plantarflexion, positive). Young adult (solid line) and older adult (dashed line) medial gastrocnemius (GAS) muscle and subtendon shown in blue and soleus (SOL) muscle and subtendon shown in red.
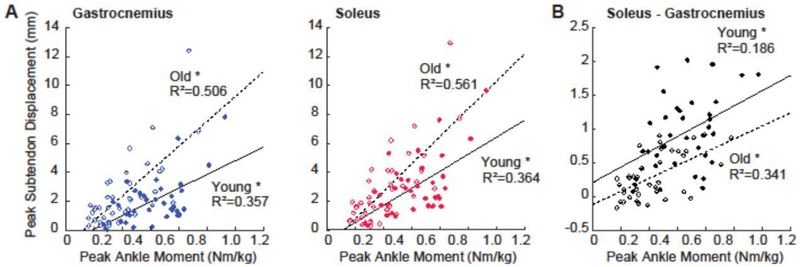
For young adults, the magnitude of peak GAS and peak SOL muscle shortening pooled across ankle angles positively correlated with peak displacements in their associated regions of the Achilles tendon (GAS: R2=0.486; SOL: R2=0.618; p-values<0.001, Figure 4A). In older adults, those correlations remained relatively strong and significant only for SOL (GAS: R2=0.089, p=0.061; SOL: R2=0.382, p<0.001). Only in young adults did tendon non-uniformity (i.e., differences between peak SOL subtendon displacement and peak GAS subtendon displacement) positively correlate with anatomically consistent differences between GAS and SOL muscle shortening (R2=0.310, p<0.001; Figure 4B). Indeed, we found no such relation in older adults (R2=0.008, p=0.579). Peak GAS subtendon displacement and peak SOL subtendon displacement positively correlated with peak ankle moment in young (GAS: R2=0.357; SOL: R2=0.364; p-values<0.001) and in older adults (GAS: R2=0.506; SOL: R2=0.561; p-values<0.001) (Figure 5). However, older adult GAS and SOL subtendons were significantly more sensitive to changes in ankle moment (Angle×Age interactions, p-values<0.001). Finally, peak ankle moment positively correlated with tendon non-uniformity (Young: R2=0.186; Older: R2=0.341; p-values≤0.009) but not with differences between GAS and SOL peak muscle shortening (Young: R2=0.036; Old: R2=0.077; p-values≥0.084).
Figure 4:
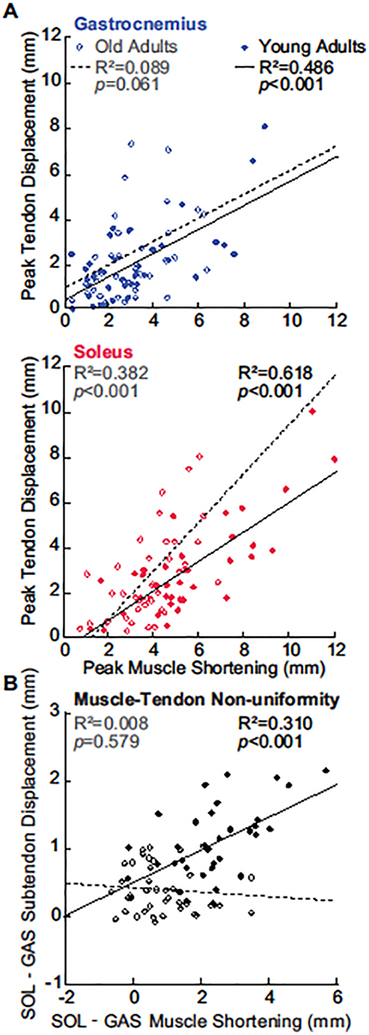
(A) Correlations between peak GAS or peak SOL muscle shortening and displacement in their associated Achilles subtendons. (B) Correlation between Achilles tendon non-uniformity and GAS versus SOL differences in muscle. All correlations are pooled across all conditions.
Figure 5:
(A) Correlations between peak GAS subtendon displacement or peak SOL subtendon displacement and normalized peak ankle moment for young (solid line) and older (dashed line) adults. (B) Correlations between Achilles tendon non-uniformity (differences between peak SOL subtendon displacement and peak GAS subtendon displacement) and normalized peak ankle moment. Asterisks (*) represent significant differences between young and older adults (p<0.05).
Discussion
In this study, we investigated the effect of aging on triceps surae muscle-subtendon interaction during a series of ramped maximum isometric voluntary contractions. Our data fully supported our primary hypothesis that age-related effects on Achilles subtendon tissue displacement patterns would be mirrored by anatomically consistent effects on triceps surae muscle contractile behavior. Specifically, compared to young adults, older adults had more uniform Achilles subtendon tissue displacements that extended to smaller differences between gastrocnemius and soleus muscle shortening. Likewise, our data supported our secondary hypothesis; compared to those in young adults, older adult subtendons were significantly more sensitive to changes in force transmission that followed from changes in ankle joint position. As we describe in more detail below, these results may provide a biomechanical basis for previously reported correlations between more uniform subtendon tissue displacements and reduced ankle moment generation during walking in older adults10.
Overall, our findings for peak GAS and peak SOL subtendon displacements, as well as the differences between the two, are consistent with prior studies5,31. Studies investigating the effect of aging on Achilles tendon non-uniformity have revealed more uniform free Achilles subtendon tissue displacements in animal models9, middle-aged humans32, and in older humans10. Moreover, our findings for GAS and SOL fascicle length, pennation angle, and longitudinal muscle length change are generally consistent with prior literature on isolated contractions33,34. Age-associated differences in triceps surae muscle contractile behavior have been extensively studied and have revealed lower triceps surae muscle volume35, force-generating capacity36, and specific tension (i.e., maximum voluntary force divided by cross-sectional area) with increasing age37. Consistent with these findings, our older adult subjects exerted, on average, 34% smaller peak ankle moments than young adults. However, we found no significant difference in peak GAS or peak SOL muscle shortening between young and older adults, consistent with some38 but not all36 studies. Indeed, some studies have reported an association between reduced muscle mass and smaller muscle length change during isolated triceps surae contractions in older adults36,39. Those studies also report an age-related decrease in pennation angle36,39, which we neither observed at rest (p’s≥0.080) nor at peak ankle moment (p’s≥0.205).
Compared to those in young adults, more uniform Achilles subtendon tissue displacements in older adults may reflect a proliferation of collagen cross-linking and interfascicle adhesions. Indeed, using equine models of the Achilles tendon, Thorpe et al. (2013) revealed an age-related reduction in interfascicular sliding9. They also suggest older tendon fascicles experience age-related alterations in viscoelastic and quasi-static properties (e.g., stress relaxation, stiffness, structural alignment). One plausible explanation for reduced interfascicular sliding in older tendon is variation in its underlying structure at the cellular level compared to younger tendon. The interfascicular matrix of the Achilles tendon contains a mixture of particular proteoglycans and glycoproteins that facilitate sliding and, compared to the collagen fibers themselves, is uniquely altered by aging40. However, we focus on non-uniform behavior at the subtendon level, and it is unclear how sliding at each hierarchical level of the Achilles tendon (e.g., subtendon, fascicle, fiber) manifests its influence on gross triceps surae muscle-subtendon interaction.
Age-related changes in the gross mechanical behavior of the Achilles tendon, and more specifically in sliding between adjacent subtendons, allude to the potential for functional consequences on triceps surae muscle contractile behavior in older adults. We previously posited that one such functional consequence could be coupling between individual muscles of the triceps surae, thereby compromising their ability to operate as independent actuators. Indeed, our findings may provide a biomechanical basis for the previously reported correlation between age-related decreases in Achilles subtendon non-uniformity and reduced peak ankle moment during walking10. Specifically, our findings are the first to suggest that a reduction in sliding between adjacent subtendons in the Achilles are accompanied by smaller differences in muscle shortening between GAS and SOL in older adults. It is unclear from our measurements if there are disparate functional effects between the individual triceps surae muscles. Here, aging disproportionately affected peak shortening of SOL across the range of motion tested, thereby contributing to its contractile behavior more resembling that of GAS in older than in young adults. Similarly, it is possible that coupling between the GAS and SOL subtendons, and thus interactions between the individual triceps surae muscles, explains the absence of a significant positive correlation in older adults between peak GAS muscle shortening and peak GAS subtendon displacement. However, the isolated contractions tested here may not be representative of the effects that would emerge during functional activity; incorporating age-associated subtendon adhesions in musculoskeletal simulations of walking revealed declines in muscle-tendon unit force output for GAS but not SOL41. Nevertheless, smaller differences between GAS and SOL length change in older adults could negatively affect those muscle’s ability to independently contribute to forward propulsion and trunk support – distinct biomechanical tasks attributed to these muscles during walking2.
Thus far, our position has been that age-related changes in Achilles subtendon tissue displacements negatively affect triceps surae muscle contractile behavior in older adults (i.e., ‘tendon-up’ theory). However, we cannot exclude the alternative hypothesis; aging may independently affect triceps surae muscle contractile behavior and thereby yield more uniform Achilles subtendon displacements (i.e., ‘muscle-down’ theory). Aging is widely known to deleteriously affect muscle contractile behavior through changes in myofilament protein biology, muscle coordination, and loss of viable motor units42. Reduced triceps surae muscle force generation in aging, even if homogenous across the triceps surae muscles, could itself diminish non-uniform Achilles subtendon tissue displacements. Indeed, peak ankle moment in this study positively correlated with tendon non-uniformity across our study cohort. Our mechanistic understanding of these causal relations may be further compounded by increased Achilles tendon tissue compliance due to aging43. Consistent with most human studies, our data revealed that older subtendons were significantly more sensitive to changes in tendon force than those in young adults. Similarly, peak GAS and peak SOL subtendon displacements were unaffected by age, despite lower ankle moments in older than young adults. We are currently trying to distinguish between these ‘tendon-up’ and ‘muscle-down’ theories through a combination of musculoskeletal modeling, dual-probe ultrasound imaging during walking, and electrical stimulation.
We previously outlined the limitations of the experimental techniques applied in this study6, but briefly: first, we interpreted our data using a generalized anatomical approximation of the muscles of the triceps surae and their associated subtendons4,28,29. Two-dimensional ultrasound imaging may not fully capture the complex architecture of three-dimensional structures44. Likewise, between-subject variability in anthropometrics may influence the precision of ultrasound probe placement. However, we note that there was no significant difference in subject height (p=0.312) nor leg length (p=0.192) between young and older adults. Second, UltraTrack uses semi-automated fascicle tracking that has inherent limitations in reliability and determination25. Third, two-dimensional speckle tracking of the Achilles tendon is subject to out of plane motion23. Fourth, we did not attempt to estimate Achilles tendon force transmission31. Finally, we report negligible but present ankle rotation that likely influences subtendon tissue displacements3. Here, we add limitations associated with uncovering aging effects. As previously noted, we cannot definitively attribute our observations to aging effects on sliding between adjacent subtendon versus those on muscle contractile behavior. Moreover, we only included self-reported healthy young and older adults, however, physical activity status most likely impacts the mechanical properties of the triceps surae muscle tendon unit45.
In summary, we reveal that more uniform Achilles subtendon tissue displacements in older versus young adults extend to anatomically consistent and potentially unfavorable changes in muscle contractile behavior – evidenced by smaller differences between GAS and SOL peak shortening during isometric force generation. Foremost, these findings provide an important biomechanical basis for previously reported correlations between more uniform Achilles subtendon behavior and reduced ankle moment generation during waking in older adults. Additional mechanistic insight into the causal relations underlying these changes are required before advocating for any specific clinical countermeasure. For example, conventional therapies prompting changes from the muscle down may be designed to promote independent actuation of individual triceps surae muscles. Alternatively, to promote changes from the tendon up, there is some support for the use of hyaluronic acid and/or lubricin injection to maintain and restore tendon homeostasis during the aging process46.
Supplementary Material
Acknowledgements
We thank Sam Vinogradov and Michael Browne for their assistance with data collection.
Funding
This work was supported by the NIH under Grant R01AG051748.
References
- 1.Sawicki GS, Lewis CL, and Ferris DP, It pays to have a spring in your step. Exerc Sport Sci Rev, 2009. 37(3): p. 130–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McGowan CP, Neptune RR, and Kram R, Independent effects of weight and mass on plantar flexor activity during walking: implications for their contributions to body support and forward propulsion. J Appl Physiol (1985), 2008. 105(2): p. 486–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Handsfield GG, et al. , A 3D model of the Achilles tendon to determine the mechanisms underlying nonuniform tendon displacements. J Biomech, 2017. 51: p. 17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edama M, et al. , The twisted structure of the human Achilles tendon. Scand J Med Sci Sports, 2015. 25(5): p. e497–503. [DOI] [PubMed] [Google Scholar]
- 5.Arndt A, et al. , Non-uniform displacement within the Achilles tendon during passive ankle joint motion. Knee Surg Sports Traumatol Arthrosc, 2012. 20(9): p. 1868–74. [DOI] [PubMed] [Google Scholar]
- 6.Clark WH and Franz JR, Do triceps surae muscle dynamics govern non-uniform Achilles tendon deformations? PeerJ, 2018. 6:e5182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Slane LC and Thelen DG, Non-uniform displacements within the Achilles tendon observed during passive and eccentric loading. J Biomech, 2014. 47(12): p. 2831–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thorpe CT, et al. , Specialization of tendon mechanical properties results from interfascicular differences. J R Soc Interface, 2012. 9(76): p. 3108–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thorpe CT, et al. , Capacity for sliding between tendon fascicles decreases with ageing in injury prone equine tendons: a possible mechanism for age-related tendinopathy? Eur Cell Mater, 2013. 25: p. 48–60. [DOI] [PubMed] [Google Scholar]
- 10.Franz JR and Thelen DG, Depth-dependent variations in Achilles tendon deformations with age are associated with reduced plantarflexor performance during walking. J Appl Physiol (1985), 2015. 119(3): p. 242–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beijersbergen CM, et al. , The biomechanical mechanism of how strength and power training improves walking speed in old adults remains unknown. Ageing Res Rev, 2013. 12(2): p. 618–27. [DOI] [PubMed] [Google Scholar]
- 12.DeVita P and Hortobagyi T, Age causes a redistribution of joint torques and powers during gait. J Appl Physiol (1985), 2000. 88(5): p. 1804–11. [DOI] [PubMed] [Google Scholar]
- 13.Foure A, et al. , Effects of plyometric training on both active and passive parts of the plantarflexors series elastic component stiffness of muscle-tendon complex. Eur J Appl Physiol, 2011. 111(3): p. 539–48. [DOI] [PubMed] [Google Scholar]
- 14.Browne MG and Franz JR, The independent effects of speed and propulsive force on joint power generation in walking. J Biomech, 2017. 55: p. 48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finni T, et al. , Non-uniform displacement and strain between the soleus and gastrocnemius subtendons of rat Achilles tendon. Scand J Med Sci Sports, 2018. 28(3): p. 1009–1017. [DOI] [PubMed] [Google Scholar]
- 16.Mian OS, et al. , Gastrocnemius muscle-tendon behaviour during walking in young and older adults. Acta Physiol (Oxf), 2007. 189(1): p. 57–65. [DOI] [PubMed] [Google Scholar]
- 17.Rubenson J, et al. , On the ascent: the soleus operating length is conserved to the ascending limb of the force-length curve across gait mechanics in humans. J Exp Biol, 2012. 215(Pt 20): p. 3539–51. [DOI] [PubMed] [Google Scholar]
- 18.Panizzolo FA, et al. , Soleus fascicle length changes are conserved between young and old adults at their preferred walking speed. Gait Posture, 2013. 38(4): p. 764–9. [DOI] [PubMed] [Google Scholar]
- 19.Onambele GL, Narici MV, and Maganaris CN, Calf muscle-tendon properties and postural balance in old age. J Appl Physiol (1985), 2006. 100(6): p. 2048–56. [DOI] [PubMed] [Google Scholar]
- 20.Neptune RR, Clark DJ, and Kautz SA, Modular control of human walking: a simulation study. J Biomech, 2009. 42(9): p. 1282–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hawkins D, et al. , Dynamic creep and pre-conditioning of the Achilles tendon in-vivo. J Biomech, 2009. 42(16): p. 2813–7. [DOI] [PubMed] [Google Scholar]
- 22.Tian M, et al. , Myofascial force transmission between the human soleus and gastrocnemius muscles during passive knee motion. J Appl Physiol (1985), 2012. 113(4): p. 517–23. [DOI] [PubMed] [Google Scholar]
- 23.Franz JR, et al. , Non-uniform in vivo deformations of the human Achilles tendon during walking. Gait Posture, 2015. 41(1): p. 192–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Silder A, Heiderscheit B, and Thelen DG, Active and passive contributions to joint kinetics during walking in older adults. J Biomech, 2008. 41(7): p. 1520–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farris DJ and Lichtwark GA, UltraTrack: Software for semi-automated tracking of muscle fascicles in sequences of B-mode ultrasound images. Comput Methods Programs Biomed, 2016. 128: p. 111–8. [DOI] [PubMed] [Google Scholar]
- 26.Chernak Slane L and Thelen DG, The use of 2D ultrasound elastography for measuring tendon motion and strain. J Biomech, 2014. 47(3): p. 750–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anson BJ and McVay CB, Surgical Anatomy. Leg (5th ed.). W.B. Saunders Company, Philadelphia, London, Toronto: (1971), 1971: p. 1186–1189. [Google Scholar]
- 28.Gils CC, Steed RH, and Page JC, Torsion of the human Achilles tendon. J Foot Ankle Surg, 1996. 35(1): p. 41–8. [DOI] [PubMed] [Google Scholar]
- 29.Szaro P, et al. , Fascicles of the adult human Achilles tendon - an anatomical study. Ann Anat, 2009. 191(6): p. 586–93. [DOI] [PubMed] [Google Scholar]
- 30.Doral MN, et al. , Functional anatomy of the Achilles tendon. Knee Surg Sports Traumatol Arthrosc, 2010. 18(5): p. 638–43. [DOI] [PubMed] [Google Scholar]
- 31.Bojsen-Moller J and Magnusson SP, Heterogeneous Loading of the Human Achilles Tendon In Vivo. Exerc Sport Sci Rev, 2015. 43(4): p. 190–7. [DOI] [PubMed] [Google Scholar]
- 32.Slane LC and Thelen DG, Achilles tendon displacement patterns during passive stretch and eccentric loading are altered in middle-aged adults. Med Eng Phys, 2015. 37(7): p. 712–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kawakami Y, Ichinose Y, and Fukunaga T, Architectural and functional features of human triceps surae muscles during contraction. J Appl Physiol (1985), 1998. 85(2): p. 398–404. [DOI] [PubMed] [Google Scholar]
- 34.Maganaris CN, Baltzopoulos V, and Sargeant AJ, In vivo measurements of the triceps surae complex architecture in man: implications for muscle function. J Physiol, 1998. 512 (Pt 2): p. 603–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Macaluso A, et al. , Contractile muscle volume and agonist-antagonist coactivation account for differences in torque between young and older women. Muscle Nerve, 2002. 25(6): p. 858–63. [DOI] [PubMed] [Google Scholar]
- 36.Narici MV, et al. , Effect of aging on human muscle architecture. J Appl Physiol (1985), 2003. 95(6): p. 2229–34. [DOI] [PubMed] [Google Scholar]
- 37.Kent-Braun JA and Ng AV, Specific strength and voluntary muscle activation in young and elderly women and men. J Appl Physiol (1985), 1999. 87(1): p. 22–9. [DOI] [PubMed] [Google Scholar]
- 38.Karamanidis K and Arampatzis A, Mechanical and morphological properties of human quadriceps femoris and triceps surae muscle-tendon unit in relation to aging and running. J Biomech, 2006. 39(3): p. 406–17. [DOI] [PubMed] [Google Scholar]
- 39.Morse CI, et al. , Changes in triceps surae muscle architecture with sarcopenia. Acta Physiol Scand, 2005. 183(3): p. 291–8. [DOI] [PubMed] [Google Scholar]
- 40.Thorpe CT, et al. , Fascicles and the interfascicular matrix show decreased fatigue life with ageing in energy storing tendons. Acta Biomater, 2017. 56: p. 58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Franz JR and Thelen DG, Imaging and simulation of Achilles tendon dynamics: Implications for walking performance in the elderly. J Biomech, 2016. 49(9): p. 1403–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miller MS, Callahan DM, and Toth MJ, Skeletal muscle myofilament adaptations to aging, disease, and disuse and their effects on whole muscle performance in older adult humans. Front Physiol, 2014. 5: p. 369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Narici MV, Maffulli N, and Maganaris CN, Ageing of human muscles and tendons. Disabil Rehabil, 2008. 30(20–22): p. 1548–54. [DOI] [PubMed] [Google Scholar]
- 44.Chow RS, et al. , Sonographic studies of human soleus and gastrocnemius muscle architecture: gender variability. Eur J Appl Physiol, 2000. 82(3): p. 236–44. [DOI] [PubMed] [Google Scholar]
- 45.Joseph MF, et al. , Achilles tendon biomechanics in response to acute intense exercise. J Strength Cond Res, 2014. 28(5): p. 1181–6. [DOI] [PubMed] [Google Scholar]
- 46.Kaux JF, Samson A, and Crielaard JM, Hyaluronic acid and tendon lesions. Muscles Ligaments Tendons J, 2015. 5(4): p. 264–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.