Abstract
Background
Impairment in cognitive control in alcohol use disorder (AUD) contributes to difficulty controlling alcohol use and, in many populations, difficulties with emotion regulation. However, the most reliable and robust marker of clinically-relevant deficits in cognitive control in AUD is unclear. Our aims were to measure relationships between BOLD signal during a Stroop task and AUD severity and change in BOLD signal and change in drinking over three weeks. We also aimed to explore the relationships between BOLD signal and subjective negative affect.
Methods
Thirty-three individuals with AUD underwent a multisensory Stroop task during functional magnetic resonance imaging (fMRI), as well as a battery of neuropsychological tests and self-report assessments of negative affect and AUD severity.
Results
Greater activation in temporal gyrus and cerebellum during incongruent trials compared to congruent trials was observed, and percent signal change (incongruent minus congruent) in both clusters was positively correlated with AUD severity and self-reported negative affect. Neuropsychological task performance and self-reported impulsivity were not highly correlated with AUD severity. Hierarchical regression analyses indicated that percent signal change (incongruent minus congruent) in cerebellum was independently associated with negative affect after controlling for recent and chronic drinking. In a subset of individuals (n=23) reduction in cerebellar percent signal change (incongruent minus congruent) was correlated with increases in percent days abstinent over 3 weeks.
Conclusions
BOLD activation during this Stroop task may therefore be an important objective marker of AUD severity and negative affect. The potential importance of the cerebellum in emotion regulation and AUD severity is highlighted.
Keywords: cerebellum, Stroop, cognitive control, alcohol use disorder, negative affect
Introduction
Loss of control of alcohol use is a primary symptom of an alcohol use disorder (AUD) (American Psychiatric Association 2013) and may be partially mobilized by global impairments in cognitive control (Charney et al. 2010; Houben et al. 2011; Kwako et al. 2015; Mayer et al. 2013; Wilcox et al. 2014; Wilcox et al. 2015). Abberations in neural systems involved in regulating behavior and inhibiting prepotent responses can render someone more vulnerable to impulses to use alcohol, even when there is a desire to cut down or stop (Camchong et al. 2013; Houben et al. 2011; Kwako et al. 2015; Petit et al. 2014; Rupp et al. 2016; Schmidt et al. 2017; Wilcox et al. 2014). However, the measure of the integrity of these systems most strongly related to AUD severity is not definitively established.
Performance on tasks of response inhibition, selective attention/distractor interference control (i.e. Stroop) and working memory can be used as a probe for this vulnerability, being associated with AUD severity and diagnosis, but results are mixed regarding the relationships between AUD and many of these measures of cognitive control or with self-reported impulsivity (Joos et al. 2013a; Joos et al. 2013b; Wilcox et al. 2014). Inconsistency across studies may exist, in part, because self-report may be sensitive to daily mood fluctuations, and because such a wide variety of tasks are used within the cognitive control domains (Wilcox et al. 2014).
Because it measures brain function directly, and is objective, there is reason to believe that BOLD signal [obtained through functional magnetic resonance imaging (fMRI)] during tasks of cognitive control may be a more suitable measure than indirect measures, such as task performance and self-report, of this underlying vulnerability. FMRI during tasks of cognitive control have demonstrated varying results in AUD; during response inhibition tasks AUD demonstrate lower activation in the cognitive control network [prefrontal cortex, insula, striatum] during inhibition in AUD compared to controls, or in AUD with greater severity (Claus et al. 2013; Karch et al. 2008; Li et al. 2009; Schmaal et al. 2013), whereas Stroop-like tasks are associated with greater activation in AUD and other substance use disorders in a number of regions in the cognitive control network when all trials are combined (Mayer et al. 2013; Wilcox et al. 2015), and in a more limited network (PCC, precuneus, cerebellum) when group differences in incongruent versus congruent contrast maps are examined (Hatchard et al. 2015; Schulte et al. 2012); the directionality of associations are mixed during tasks of working memory (Desmond et al. 2003; Park et al. 2011; Wilcox et al. 2014).
Impaired emotion regulation can cause higher levels of negative affect, is associated with AUD severity, and likely contributes to loss of control of alcohol use through negative reinforcement (Wilcox et al. 2016). Impairments in cognitive control may contribute to dysregulated emotion: these constructs are associated (Hendricks and Buchanan 2015), and studies show that enhancement of cognitive control through training reduces anxiety (Sari et al. 2015). Third, the insula and fronto-parietal circuit, including lateral and dorsomedial prefrontal cortex, are believed play important roles in both cognitive control and emotion regulation in individuals with substance use disorder and without substance use disorder (Ochsner et al. 2012; Viviani 2014; Wilcox et al. 2014; Wilcox et al. 2016).
To our knowledge, only one study has explored relationships between negative affect and brain activation during a pure task of cognitive control (without emotional stimuli) in AUD (Karch et al. 2008), showing that anxious individuals with AUD have increased brain activation during a cognitive control task than non-anxious AUD. More work in this area is important to disambiguate the circuitry involved in processing emotional cues versus those involved in top-down control during tasks of emotion regulation (Crane et al. 2016). In addition, no study has examined the degree to which the relationships between impairment in cognitive control and AUD severity is driven by its association with negative affect.
In this study, which was a secondary analysis of a group of participants who were also undergoing a clinical trial of prazosin for the treatment of AUD (Wilcox et al. 2018b), our first aim was to examine the relationships between brain activation in the cognitive control network during a Stroop task (Mayer et al. 2013; Wilcox et al. 2015) and AUD severity at baseline. We hypothesized that greater activation during incongruent compared to congruent trials in the cognitive control network would be associated with greater AUD severity (Aim 1a). We also hypothesized that greater activation during incongruent compared to congruent trials would be more strongly correlated with (and a more sensitive predictor of) AUD severity than self-reported impulsivity and performance on neuropsychological tasks of cognitive control (Aim 1b). We also aimed to examine the relationships between brain activation and negative affect, and hypothesized that greater negative affect would be associated with greater activation in the cognitive control network during incongruent compared to congruent trials (Karch et al. 2008) (Aim 2). Our third aim was to explore whether prazosin, belonging to a class of medications (alpha-1 antagonists) showing efficacy for reducing drinking in this and other samples (Kenna et al. 2016; Simpson et al. 2015; Simpson et al. 2009; Wilcox et al. 2018b), affected brain activation in a subset of participants (n=23) who received a second scan 3 weeks later, and whether changes in activation was related to changes in drinking (Aim 3). We hypothesized that prazosin would reduce activation during incongruent relative to congruent trials, and that larger reductions in activation would be associated with larger reductions in drinking over time.
Material and Methods
Participants
Participants were 33 (63.6% male) individuals with AUD interested in cutting back or quitting drinking. Participants ranged in age from18 to 65 (39.7 ± 11.3) and were recruited through newspapers, online postings like craigslist, and flyers. 36 individuals were initially recruited and scanned, but one was excluded for unusable scan data, one for an anatomical abnormality and one for excessive motion. All participants were subsequently enrolled in a 6-week double-blind, placebo-controlled trial of prazosin for the treatment of AUD [https://clinicaltrials.gov/ct2/show/NCT01916941, (Wilcox et al. 2018b)], of whom 23 received a second brain scan. For inclusion, participants were required to have at least four drinking days during the past month, and to meet criteria for alcohol dependence (First et al. 2002) in the past three months. Participants were excluded if they were currently receiving alcohol treatment, taking daily medications that could influence BOLD signal or treatment outcome, suffering from significant medical problems or particular psychiatric diagnoses (PTSD, bipolar I, current dependence on another drug other than nicotine or cannabis). See supplementary information for more details on inclusion/exclusion and trial procedures.
Clinical Measures
General Measures
All participants completed the Fagerstrom Test for Nicotine Dependence (FTND) (Heatherton et al. 1991), and underwent a Structured Clinical Interview for DSM Disorders (SCID) (First et al. 2002) administered by a research assistant, a urine toxicology screen, and a Clinical Institute Withdrawal Assessment of Alcohol Scale (CIWA; excluded for score > 8) (Sullivan et al. 1989) prior to the imaging session.
Measures of Cognitive Control and Function
All participants performed a Stroop color and word test (Golden 1978) from which the Interference T score (standardized score with mean of 50 and standard deviation of 10) was calculated. In addition, all participants completed a Connors Continuous Performance Test (CPTII) (Conners 2000) in which participants are asked to click the space bar when they are presented with any letter other than “X”; they are to refrain from clicking if they see the letter “X”. Primary measures from the CPT were T scores for the errors of commission and hit reaction time scores (measures of inattentiveness), given their previously observed relationship with AUD or AUD severity (Wilcox et al. 2014). Secondary measures were errors of omission, CPTD’ (ability to discriminate between targets and non-targets), and hit reaction time block change (change in reaction time over the task) given their association with related pathologies like attention deficit hyperactivity disorder (Epstein et al. 2003; Miranda et al. 2012). All participants also completed a measure that estimates intelligence (Weschsler Test of Adult Reading; WTAR) (Whitney et al. 2010).
Measures of Alcohol Consumption, AUD Severity and Related Measures
The Timeline Follow-back calendar (Sobell et al. 1992) was used to determine alcohol and other drug use during the previous 90 days using percent days abstinent and drinks per week. Measures of drinking in the 90 days prior to the scan visit and the 7 days prior to the scan (recent drinks per week and recent percent days abstinent) were calculated. In addition, all participants completed measures of AUD severity including the Alcohol Use Disorders Identification Test (AUDIT) (Babor et al. 1992), a standard measure of AUD severity, and the Drinkers Inventory of Consequences (DRINC) (Forcehimes et al. 2007), which addresses consequences of heavy drinking over the past 3 months. In addition, all participants reported the number of years since onset of problem drinking during administration of the SCID.
Measures of Impulsivity
The Barratt Impulsiveness Scale total score (Patton et al. 1995) was our primary measure of impulsivity given its known relationship with AUD severity in previous work (Wilcox et al. 2014).
Measures of Negative Affect
We administered two measures of negative affect, the PROMIS Anxiety and PROMIS Depression Scales (Schalet et al. 2016). In addition, a measure of emotion regulation (Wilcox et al. 2016), the Affective Lability Scale (Look et al. 2010) was obtained.
Imaging Task
A multisensory numeric Stroop task was presented to all participants during fMRI scanning and was similar to previous published work (Mayer et al. 2011; Mayer et al. 2013; Wilcox et al. 2015). Each 10s block consisted of simultaneously presented multisensory (visual and auditory) congruent or incongruent numeric stimuli (targets) occurring at a low (0.33 Hz) frequency. Each block started with the cue word (exemplary visual angle = 7.69°) “LOOK” or “HEAR” followed by a stream of target numbers presented visually (one, two, or three; exemplary visual angle = 9.73°). If the cue word was “LOOK,” participants were instructed to press a button corresponding to the number for the visual stimuli and ignore the number that was simultaneously presented aurally. If the cue word was “HEAR,” subjects attended to the aural number stream while ignoring visual targets. There was a 1325 ms delay between the presentation of the cue (175 ms duration) and the presentation of the first target number (200 ms duration) to maximize attentional focus. The inter-block interval varied between 8, 10, and 12s to decrease temporal expectations and permit modeling of the baseline (visual fixation plus baseline gradient noise). There were 3 trials per block. A total of 24 trials per trial type (96 total trials), and 8 blocks of each type (32 total blocks), were presented across two separate imaging runs. Before being placed in the scanner, participants practiced the behavioral task until demonstrating competency.
Multisensory Stroop Task Performance
Two measures of task performance during the multisensory Stroop task were obtained during each of four trial types (auditory incongruent, auditory congruent, visual incongruent, visual congruent): an accuracy measure, and reaction time on the task.
MR Imaging and Analyses
A high-resolution MPRAGE T1 sequence and whole-brain echo-planar image were collected on a 3 Tesla Siemens Trio scanner (acquisition details in supplementary information). All participants were inspected for significant anatomic abnormalities (one subject was dropped for large ventricles) and then analyzed for excessive frame-wise head motion (greater than three times the interquartile range on two or more of six parameters) compared to the rest of their cohort (one was determined to have excessive head motion using these criteria and was excluded). No subjects were found to be behavioral outliers (accuracy or reaction time).
The Analysis of Functional Neuroimages (AFNI) software package (Version 16.0.15) (Cox 1996) was used to generate functional images using standard pre-processing techniques (time-slice correction, motion correction, 6 mm Gaussian full-width half-maximum spatial filter, and spatial normalization to Talairach space resampled to 3×3×3 mm). A voxel-wise deconvolution analysis was then performed to generate a single hemodynamic response function (HRF) that spanned the first 22s post-stimulus onset (to account for the fact that the HRF generally peaks at 4–6s and lasts 10–15 s) for each trial-type with error trials modeled separately (Mayer et al. 2011; Mayer et al. 2013; Wilcox et al. 2015). Paired t-tests were performed (Incongruent vs. Congruent trials) and corrected for multiple comparisons using 3dClustsim. Percent signal change estimates were calculated based on the beta coefficients for the images occurring 6 to 14s post-cue onset for each relevant trial type, and then divided by the average model intercept.
For all evoked task-associated findings, false positives were corrected at p <0.001 (input volume voxel-wise threshold) and alpha < 0.05 (p threshold for clusters, minimum cluster size of 896 μl based on based on 10,000 Monte-Carlo simulations and using spherical autocorrelation) within a white matter exclusion mask (mask details in supplementary information). In a subset of participants (n=23) a second scan (Scan2) was performed approximately 3 weeks later, for which preprocessing techniques were identical. Mean percent signal change (incongruent minus congruent) estimates were calculated from the Scan2 images in the significant clusters identified from the whole brain analysis during the baseline scan.
Correlations
For most but not all measures, variables were normally distributed. In the cases where they were not normally distributed [i.e. if either the Kolmogorov-Smirnov or Sharpiro Wilk test p value was less than 0.05], variables were transformed to create variables with normal distributions [drinks per week in the previous 90 days, and recent drinks per week in the 7 days prior to the scan, CPT errors of omission, WTAR, CPTD’, CPT hit reaction time, Affective Lability Scale score]. When transformations were performed but we were unable to create normally distributed variables [CIWA, multisensory Stroop task behavioral measures, percent days abstinent in the previous 90 days], non-parametric statistics were used.
Corrections for Multiple Comparisons
Given the high number of correlation analyses performed, we corrected for multiple comparisons using false discovery rate within each aim (Aim 1a, Aim 1b, Aim 2) for our primary measures, results from which are reported in the corresponding tables (Table 2,3,4).
Table 2a.
Correlations Between Percent Signal Change in Cerebellum/Temporal Gyrus, and AUD Severity/Drinking
Table 3.
Correlations Between Cognitive Control Task Performance/Impulsivity and AUD Severity/Drinking
| r/p | ||||
|---|---|---|---|---|
| Measure | AUDIT | DRINC | PDA Scan901 | DPW Scan90 |
| BIS | 0.2061 0.258 | 0.4681 0.006^ | 0.0391 0.831 | 0.2781 0.117 |
| StroopInt | 0.2551 0.159 | .02151 0.229 | 0.1411 0.435 | 0.0381 0.835 |
| CPTHRT | −0.2571 0.156 | −0.1331 0.461 | −0.0121 0.953 | 0.0391 0.830 |
| CPTCom | −0.0471 0.800 | 0.2291 0.200 | 0.0761 0.675 | −0.0961 0.596 |
| CPTOm | 0.1001 0.586 | −0.3181 0.071 | 0.3241 0.066 | 0.0981 0.587 |
| WTAR | −0.3231 0.071 | −0.2551 0.152 | −0.1131 0.531 | −0.2151 0.230 |
| CPTHRT BC | −0.0401 0.826 | 0.2221 0.214 | −0.1101 0.542 | 0.0171 0.925 |
| CPTD’ | 0.0421 0.820 | 0.2191 0.221 | 0.0061 0.984 | 0.0121 0.948 |
DPW during the previous 7 days was not related to any of the cognitive measures (ps >.2) and was not controlled for in any of above analyses.
All analyses with AUDIT were with n=32.
Column Key:
AUDIT = Alcohol Use Disorders Identification Test, DRINC = Drinker Inventory of Consequences Total Score, PDA = percent days abstinent, DPW = Drinks per week, Scan90 = 90 days prior to the scan visit.
Row Key:
CPTD’ = Continuous Performance Task (CPT) Detectability, CPTCom = CPT Errors of Commission, CPTOm = CPT Errors of Omission, StroopInt = Stroop Interference T Score, CPTHRT = CPT Hit Reaction Time, WTAR = Wechsler Test of Adult Reading, CPTHRTBC = CPT Hit Reaction Time Block Change, BIS = Barratt Impulsivity Scale
Correction for Multiple Comparisons:
Shaded boxes were primary variables and p-values entered into FDR correction; non-shaded boxes were not entered into FDR correction.
None were significant with FDR correction.
Trend with FDR correction (p<0.1)
Transformations:
WTAR = (WTAR)4, CPTOm = 1/(CPTOm)3, CPTD = (CPTD’)2, CPTHRT= √(CPTHRT), DPW Scan90 = ln(DPW Scan90)
Spearmans rho substituted for r.
Table 4a.
Correlations Between Percent Signal change in Cerebellum/Temporal Gyrus, and Negative Affect
Results
Sample Characteristics
All subjects met criteria for alcohol dependence (American Psychiatric Association 2013) within the last month except for one subject who met 2/7 criteria for alcohol dependence in the last month, but had 2/7 additional criteria for dependence and one abuse criteria in the past, prior to 1 month ago. Fourty-two percent (42.4%) were Hispanic/Latino and 51.5% were Caucasian, 18.2% were Native American, 18.1% were mixed/other, 15.1% were unemployed, and mean years of education for the sample was 14.2, standard deviation (SD)=1.92. Thirteen participants were smokers and 3 participants had current (past month) marijuana dependence. Table 1, supplementary information and the clinical trial manuscript provide further sample details (Wilcox et al. 2018b).
Table 1.
Means and SD for primary measures
| Mean (SD), n=33 | |
|---|---|
| AUDIT | 20.47 (7.14) |
| DRINC | 40.82 (22.23) |
| PDA (90 days before scan) | 36.50 (30.7l) |
| DPW (90 days before scan) | 32.20 (22.26) |
| DPW (7 days before scan) | 24.74 (20.92) |
| PROMIS Anxiety | 15.39 (6.18) |
| PROMIS Depression | 14.06 (6.28) |
| Affective Lability Scale | 28.27 (9.92) |
| CIWA | 0.85 (1.12) |
| CPT Errors of Commission T | 52.58 (9.97) |
| CPT Hit Reaction Time T | 53.28 (9.77) |
| Stroop Interference T | 52.94 (8.30) |
| Barratt Impulsivity Scale | 59.70 (11.76) |
Key: AUDIT = Alcohol Use Disorders Identification Test, DRINC = Drinker Inventory of Consequences Total Score, PDA = percent days abstinent, DPW = drinks per week, CIWA = Clinical Institute Withdrawal Assessment of Alcohol Scale, CPT = Connors Continuous Processing Test
At the time of the scan, all individuals had a breath alcohol level of <0.01 and a CIWA of less than 4 (mean=0.82, SD=1.11). Six individuals were positive for THC, and 3 for benzodiazepines but no participants reported using illicit drugs in the 24 hours before the scan. Six individuals reported drinking alcohol within 24 hours of the scan (1, 2, 3, 3.2, 5, and 12.7 standard drinks, respectively).
Behavioral Results During the fMRI Task
Behavioral results during the task were as expected (Mayer et al. 2017; Mayer et al. 2013; Wilcox et al. 2015), demonstrating greater accuracy and higher reaction times for congruent compared to incongruent trials, as well as a main effect of modality, with participants having significantly slower reaction times during auditory trials [mean=760.23, standard error (SE)=23.79] than visual trials (mean=717.29, SE=22.62) (details in supplementary information).
Imaging Results
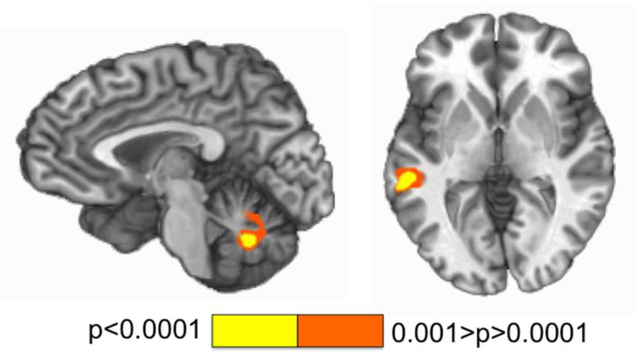
Greater activation was observed within 2 clusters: bilateral cerebellum (vermis; 3963 μl) (mean percent signal change incongruent=0.247, SD=0.213; congruent=0.130=SD 0.193) and left superior/middle temporal gyrus (BAs 21,22) (2941 μl) (mean percent signal change incongruent=0.33, SD=0.221; mean percent signal change congruent=0.243, SD=0.224) for incongruent compared to congruent trials (Figure 1). Two variables for use in the analyses that follow were subsequently created by extracting percent signal change for incongruent minus congruent stimuli from these 2 clusters: cerebellum (Cerebellum) and temporal gyrus (Temp).
Figure 1.
This figure depicts regions showing differences in activation during incongruent compared to congruent trials during a multisensory Stroop task (bilateral cerebellum, left, talairach x=−4; left temporal gyrus, right, talairach z=2). Activation maps are color-coded according to the magnitude of the voxelwise p threshold for a paired T test comparing incongruent to congruent stimuli.
Relationships Between Imaging Results and Possible Confounding Variables
None of the two imaging variables were related to smoking status, CIWA, age or gender [ps>0.1; rho (CIWA)/pearsons (age)<.2; T (gender, smoking)<0.2]. Brain activation during incongruent minus congruent stimuli was negatively correlated with recent drinks per week (Cerebellum r=−0.335, p=0.057; Temp r=0.38, p=0.03), so we control for this (to control for effects of recent drinking) in the relevant analyses that follow. Comparing those who drank 24h before the scan and those who did not, there were no significant differences between groups (ps>0.15).
Correlations Between Imaging Results and AUD Severity and Drinking (Aim 1a)
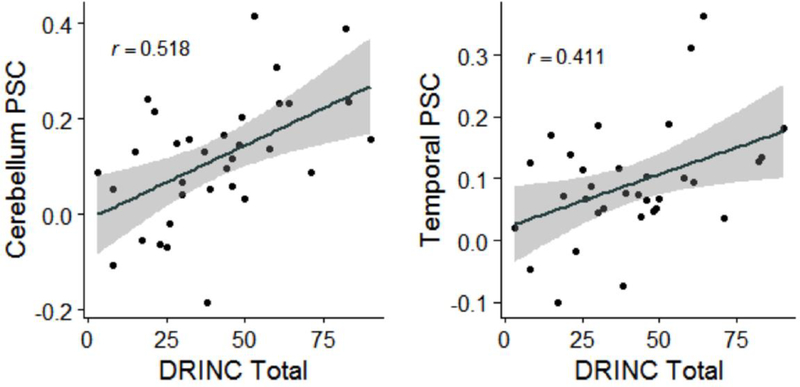
Our primary AUD severity measures were AUDIT and DRINC, and drinking measures (percent days abstinent and drinks per week) in the previous 90 days. Both clusters (Cerebellum>Temp) were positively correlated with AUD severity (AUDIT, DRINC) (Figure 2 for DRINC). These effects remained significant or were trending towards significance after adding recent (previous 7 days) drinks per week as a covariate (Table 2).
Figure 2.
Scatterplots with regression lines and 95% confidence bands graphing Drinkers Inventory of Consequences (DRINC) total score versus Cerebellum (left) and Temporal Gyrus (Temporal) (right) percent signal change (PSC) in incongruent minus congruent trials during the multisensory Stroop task.
Correlations Between Cognitive Control Task Performance/Impulsivity and AUD Severity/Drinking (Aim 1b)
Primary measures of cognitive control were the CPT errors of comission, CPT hit reaction time, and Stroop Interference T scores. For impulsivity, the Barratt Impulsivity Scale score was our primary measure. WTAR, CPTD’, CPT hit reaction time block change and CPT errors of omission T scores were secondary measures. Barratt Impulsivity Scale score was significantly positively correlated with DRINC but this did not meet criteria for significance after correcting for multiple comparisons. Cerebellum and Temp were therefore more robustly associated with AUD severity compared to the primary and secondary cognitive task performance measures (Table 3). Task performance on the multisensory Stroop task was not significantly associated with AUD severity measures (supplementary information).
Correlations Between Imaging Results and Negative Affect Measures (Aim 2)
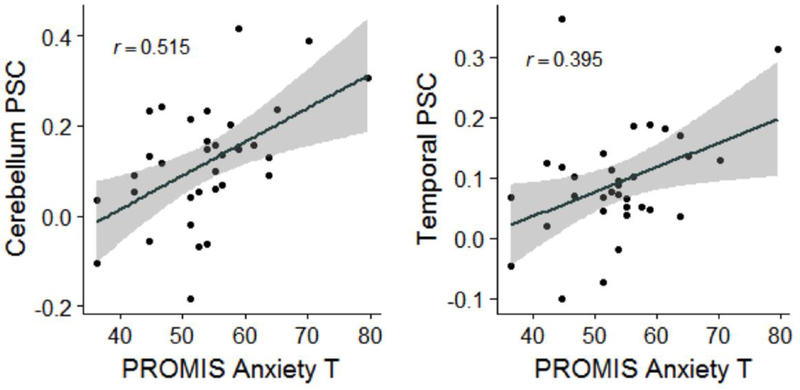
Negative affect measures were obtained with the Affective Lability Scale, and the PROMIS Anxiety and Depression Scales. Both clusters (Cerebellum>Temp) were positively correlated with the negative affect measures (0.001<ps<0.039) (Table 4, Figure 3 for PROMIS Anxiety). This association persisted upon correction for recent (previous 7 days) drinks per week (Table 4).
Figure 3.
Scatterplots with regression lines and 95% confidence bands graphing PROMIS Anxiety T-Scores (x axis) versus Cerebellum (left) and Temporal Gyrus (Temporal) (right) percent signal change (y axis) for incongruent minus congruent trials during the multisensory Stroop task.
Since Cerebellum and Temp were correlated with both AUD severity and negative affect, we performed a series of hierarchical regression analyses to clarify the nature of their relationships. In the first step, the negative affect measures (Affective Lability Scale, PROMIS Anxiety, PROMIS Depression) were entered as dependent variables and AUD severity (AUDIT or DRINC), number of years with a drinking problem, and recent drinks per week were entered as independent variables (total of 6 models run). In the second step the imaging variable (either Cerebellum or Temp) was added as a predictor (total of 12 models run). These analyses indicated that adding Cerebellum to the model significantly increased model fit in 5/6 of these models, whereas adding Temp to the model only significantly increased fit for one. By contrast, if analogous analyses were performed with AUD severity as a dependent variable, and negative affect measures, number of years with a drinking problem and recent drinks per week as predictors, neither Cerebellum nor Temp increased model fit.
Aim 3. Longitudinal analyses (Aim 3)
A repeated measures ANOVA with Time (baseline vs. Scan 2) as a within subjects factor and Condition (prazosin vs. placebo) as a between subjects factor indicated that prazosin treatment was not associated with significant changes in percent signal change (incongruent minus congruent) compared to placebo in Cerebellum or Temp [Condition*Time effect for Cerebellum: F=0.088, p=0.770; Condition*Time effect for Temp: F=0.190, p=0.667). However, change in Cerebellum from baseline to Scan2 (Scan2-baseline) was correlated with increases in percent days abstinent [recent percent days abstinent during the 7 days prior to the second scan minus percent days abstinent over the 90 days prior to the second scan] (rho=−0.554, p=0.006) and reductions in drinks per week (recent drinks per week in the 7 days prior to the second scan minus drinks per week over the 90 days prior to the second scan) (rho=0.369, p=0.084), indicating that greater reductions in percent signal change (incongruent minus congruent) were associated with greater reductions in drinking. Change in Temp was not correlated with changes in drinking, nor were changes in scores from the Barratt Impulsivity Scale, the Affective Lability Scale, or the PROMIS Anxiety or Depression Scales (rho<0.4, ps>0.1).
Discussion
In summary, greater activation during incongruent relative to congruent trials in cerebellum and temporal lobe during a Stroop task was positively correlated with AUD severity even when controlling for recent (previous 7 days) drinking. Moreover, reductions in drinking from baseline to a second scan at 3 weeks were correlated with reductions in cerebellar activation. Furthermore, greater activation in both regions to incongruent relative to congruent stimuli was positively correlated with self-reported negative affect. Finally, in cerebellum, this relationship was independent of the effects of current drinking, AUD severity and length of time with a drinking problem.
The finding that AUD severity was positively correlated with brain activation during a Stroop task in the cerebellum is in-line with previous work showing greater activation in fronto-cerebellar circuits during working memory (Desmond et al. 2003) in AUD relative to controls and with work showing greater activation to incongruent versus congruent stimuli in young alcohol-using adults (Hatchard et al. 2015). It also supports a growing literature on the importance of the cerebellum in drug craving (Froeliger et al. 2015; Moreno-Rius and Miquel), although there was not a relationship with craving in our sample, per se (data not reported). That AUD severity was correlated with greater activation in temporal lobe has not, to our knowledge, been highlighted in previous work, although temporal grey matter loss is noted in AUD for example (Chanraud et al. 2007) and greater activation in temporal lobe during a conflict task in individuals with a positive family history of AUD (Acheson et al. 2014) has been observed. Moreover, as stated, increases in percent days abstinent and reductions in drinks per week were associated with reductions in percent signal change (incongruent minus congruent) in cerebellum, which could either indicate that reductions in percent signal change were a mechanism of drinking reduction, and could be a treatment target, or that reductions were simply a consequence of drinking reduction.
That BOLD signal in cerebellum was robustly correlated with measures of negative affect was especially interesting. Furthermore, according to supplemental regression analyses, the cerebellar signal appeared to be independently associated with negative affect, correcting for AUD severity, number of years of alcohol drinking, and recent alcohol exposure. By contrast, BOLD signal in cerebellum did not add any predictive power above and beyond levels of negative affect when AUD severity was the dependent variable in a regression. This indicates that the relationship between AUD severity and BOLD signal during incongruent compared to congruent stimuli was probably being effected primarily through negative affect, rather than through the effects of chronic alcohol use (which we felt was best measured in our dataset with AUD severity). This is important because negative affect is believed to be a motivator for alcohol consumption in AUD (Koob and Le Moal 2008; Wilcox et al. 2016; Wilcox and Tonigan 2016) and provides support for the possibility that cerebellar activation during this task may be a treatment target.
Unfotunately, prazosin, which was associated with modest reductions in drinks per week in the clinical trial (Wilcox et al. 2018b), did not affect percent signal change in the cerebellum or temporal lobe relative to placebo. Prazosin is an alpha-1 antagonist, and this class of medications has shown some efficacy for AUD treatment in the sample examined in this manuscript (Wilcox et al. 2018b) and in other samples (Kenna et al. 2016; Simpson et al. 2015; Simpson et al. 2009). There is reason to believe that the noradrenergic system may also play an important role in both cognitive control, negative affect and emotion regulation (Arnsten et al. 1999; Arnsten et al. 2015; Fox et al. 2012; Le et al. 2011; Raskind et al. 2003). Therefore, although prazosin modestly reduced drinking in this sample (Wilcox et al. 2018b), our findings indicate that this treatment was likely not acting via effects on cerebellar or temporal lobe function.
In the absence of an intervention that affects cerebellar signal, it is impossible to parse whether the negative affect is causing the higher BOLD signal in cerebellum, perhaps by way of heightened adrenergic tone, as stress is known to affect cognitive function (Arnsten et al. 2015) or if the higher signal is a marker of poor cerebellar function which is driving the negative affect. Supporting the former, increased anxiety can trigger deterioration in cognitive control, as evidenced by studies showing that negative affective stimuli can impair performance on tasks requiring inhibition and cognitive control (Hobson et al. 2014; Roelofs et al. 2009; Wingenfeld et al. 2009), or that rumination is associated with difficulty inhibiting negative information when switching from negative to positive affective blocks on a Go No-Go task (Hilt et al. 2014). There is also support for the latter possibility, as evidenced by a growing appreciation in the literature of the role that the cerebellum, and especially the vermis (the region activated by our task, dubbed the limbic cerebellum), plays in emotion regulation, emotional processing (Adamaszek et al. 2017; Anderson et al. 2006; Frodl et al. 2011; Lupo et al. 2015; Schmahmann 2010) and impulsivity (Park et al. 2014). From a circuitry perspective, it is known that the cerebellar vermis projects to the medial prefrontal cortex and the limbic system (Adamaszek et al. 2017; Caligiore et al. 2017; De Vidovich et al. 2016; Roostaei et al. 2014). The principal output of the cerebellum to the prefrontal cortex is believed to be inhibitory (Casula et al. 2016; Daskalakis et al. 2005), and so hyperactivation in this area could lead to excessive inhibitory drive to the prefrontal cortex, taking important emotional-regulatory functions “off line”. That cerebellar dysfunction could contribute to dysregulated emotion deserves further exploration.
Although the relationships between activation in the temporal lobe and negative affect were no longer significant upon correcting for AUD severity, this still deserves further exploration as a potential biomarker of negative affect and emotion regulation, as it was not possible for us to isolate the effects of AUD severity from those of negative affect levels. Temporal lobe (middle and superior) is activated during similar versions of this Stroop task in cocaine use disorder (Mayer et al. 2013) and this area may also be related to emotion regulation during reappraisal (Morawetz et al. 2016) and may link prefrontal with subcortical regions involved in affect regulation (Morawetz et al. 2016).
There have been mixed results in previous work regarding the relationship between performance on tasks of cognitive control and AUD severity (Wilcox et al. 2014), but few studies have compared the degree to which fMRI signal versus self-report scales or task performance on widely-utilized neuropsychiatric tests is related to AUD severity, important in light of the fact that fMRI is more expensive. That the BOLD signal was more strongly correlated with AUD severity than performance on the cognitive neuropsychiatric tests is notable. The relative importance of fMRI in this sample was supported by the fact that changes in percent days abstinent and drinks per week over time were correlated with changes in cerebellar BOLD signal, but not with changes in self-reported impulsivity or negative affect, when measured with questionnaires that correlated with drinking at baseline.
Although these results indicate that fMRI has the potential outperform behavioral measures, and be a more robust biomarker of disease severity, there are several things that would need to be demonstrated prior to deployment of fMRI to obtain this signal as, say, a treatment-outcome predictor, into the clinical arena. Some of the more obvious barriers to the use of fMRI in the clinical setting, as opposed to neuropsychiatric testing, are higher cost, cross-scanner inconsistency, and difficulty of widespread implementation, particularly when cognitive tasks are utilized to evoke brain activity (resting state or anatomical data are generally more simple to acquire) (Milham et al. 2017; Wilcox et al. 2018a). Furthermore, one of the major limitations of these kinds of results and others like it, is the challenge of replication (Milham et al. 2017; Wilcox et al. 2018a); this finding has not, yet, been replicated in an independent sample. Before we will be able to utilize fMRI in clinical populations to identify individuals with more severe illness who might need increased support, or to identify subtypes more likely to respond to particular treatments (treatment matching), we will need more replication studies, and we will need to collect more large longitudinal datasets to permit utilization of more state of the art prediction analysis methods (i.e. machine learning with cross-validation) (Milham et al. 2017; Wilcox et al. 2018a). That said, these kinds of findings can still inform research and treatment development. A study is currently underway to investigate whether inhibitory repetitive transcranial magnetic stimulation to the cerebellum affects alcohol use and negative affect in treatment-seeking individuals with AUD (R21 AA026573). The idea for this study came from these results and other work demonstrating that targeting the cerebellum with neurostimulation may alter one’s capacity for emotion regulation and levels of negative affect (Adamaszek et al. 2017; Anderson et al. 2006; Frodl et al. 2011; Froeliger et al. 2015; Park et al. 2014).
There are a number of additional limitations to this work. As previously alluded to, it cannot be determined whether neural activation is causing negative affect and is a treatment target, or if negative affect is causing greater activation. Future work utilizing interventions in a prospective placebo-controlled design targeting brain activation in these regions and/or negative affect can better answer some of these questions. Second, we did not have a control group; however our sample had a range of AUD severity. Finally, we did not have other potentially important measures of impulsivity such as delay discounting and risky decision making which have both been demonstrated to predict alcohol use (Courtney et al. 2012), and which may be more robust than some of the measures of impulsivity and cognitive control employed in this sample which were not highly correlated with AUD severity.
In conclusion, our results indicate that imaging may provide a sensitive measure of AUD severity, and that cerebellar activation during a cognitive control task is independently related to negative affect, controlling for AUD severity. The potential importance of the cerebellum, a previously under-appreciated structure in the pathophysiology of psychiatric disorders, as a biomarker of negative affect and impaired emotion regulation in AUD is highlighted.
Supplementary Material
Table 2b.
Correlations Between Percent Signal change in Cerebellum/Temporal Gyrus, and AUD Severity/Drinking Controlling for Drinks Per Week in the Previous 7 Days
| r/p | ||||
|---|---|---|---|---|
| AUDIT | DRINC | PDA Scan90 | DPW Scan90 | |
| Cerebellum | 0.426/ 0.017* | 0.484/ 0.005* | −0.031/ 0.864 | 0.414/ 0.019* |
| Temporal Gyrus | 0.413/ 0.021* | 0.364/ 0.041^ | −0.014/ 0.939 | 0.538/ 0.001* |
All analyses with AUDIT were with n=32.
Key: Percent Signal Change = percent signal change during incongruent minus congruent stimuli, AUDIT = Alcohol Use Disorders Identification Test, DRINC = Drinker Inventory of Consequences Total Score, PDA = percent days abstinent, DPW = drinks per week, Scan90 = 90 days prior to the scan visit.
Correction for Multiple Comparisons:
Significant with FDR correction (p<0.05, only primary variables entered)
Trend with FDR correction (p<0.1)
Transformations:
DPW Scan90 = ln(DPW Scan90)
DPW in the previous 7 days = √(DPW in the previous 7 days)
Spearmans rho substituted for r.
Table 4b.
Correlations Between Percent Signal Change in Cerebellum/Temporal Gyrus, and Negative Affect Controlling for Drinks Per Week in the Previous 7 Days
| r/p | ||
|---|---|---|
| Measure | Cerebellum | Temporal Gyrus |
| PROMIS Anxiety | 0.488/0.005* | 0.357/0.045* |
| Affective Lability Scale | −0.573/0.001* | −0.354/0.047* |
| PROMIS Depression | 0.618/<0.001* | 0.499/0.004* |
Key: Percent Signal Change = percent signal change during incongruent minus congruent stimuli
Transformations:
Affective Lability Scale score = 1/(Affective Lability Scale score), Drinks Per Week in the Previous 7 Days = √(Drinks Per Week in the Previous 7 Days)
Correction for Multiple Comparisons:
Significant with FDR correction (p<0.05)
Acknowledgments
Funding: This work was supported by the National Institutes of Health grant number K23-AA021156 awarded to Claire Wilcox.
Footnotes
Compliance with Ethical Standards
Conflict of Interest: Dr. Claire Wilcox, Mr. Joshua Clifford, Mr. Josef Ling, Dr. Andrew R. Mayer, Ms. Rose Bigelow, Dr. Michael Bogenschutz and Dr. J. Scott Tonigan have no conflicts of interest to report.
Ethical Approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was obtained from all individual participants included in the study.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- Acheson A, Franklin C, Cohoon AJ, Glahn DC, Fox PT, Lovallo WR (2014) Anomalous temporoparietal activity in individuals with a family history of alcoholism: studies from the Oklahoma Family Health Patterns Project Alcohol Clin Exp Res 38:1639–1645 doi: 10.1111/acer.12420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamaszek M et al. (2017) Consensus Paper: Cerebellum and Emotion Cerebellum 16:552–576 doi: 10.1007/s12311-016-0815-8 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association (2013) Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition: DSM-V-TR®. Author, Washington, DC [Google Scholar]
- Anderson CM, Lowen SB, Renshaw PF (2006) Emotional task-dependent low-frequency fluctuations and methylphenidate: Wavelet scaling analysis of 1/f-type fluctuations in fMRI of the cerebellar vermis J Neurosci Methods 151:52–61 doi: 10.1016/j.jneumeth.2005.09.020 [DOI] [PubMed] [Google Scholar]
- Arnsten A, Mathew R, Ubriani R, Taylor J, Li B-M (1999) alpha-1 Noradrenergic receptor stimulation impairs prefrontal cortical cognitive function vol 45. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Raskind MA, Taylor FB, Connor DF (2015) The Effects of Stress Exposure on Prefrontal Cortex: Translating Basic Research into Successful Treatments for Post-Traumatic Stress Disorder Neurobiol Stress 1:89–99 doi: 10.1016/j.ynstr.2014.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babor T, de la Fuente J, Saunders J, Grant M (1992) The Alcohol Use Disorders Identification Test. Guidelines for use in primary health care. [Google Scholar]
- Caligiore D et al. (2017) Consensus Paper: Towards a Systems-Level View of Cerebellar Function: the Interplay Between Cerebellum, Basal Ganglia, and Cortex Cerebellum 16:203–229 doi: 10.1007/s12311-016-0763-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camchong J, Stenger A, Fein G (2013) Resting-state synchrony in long-term abstinent alcoholics Alcohol Clin Exp Res 37:75–85 doi: 10.1111/j.1530-0277.2012.01859.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casula EP, Pellicciari MC, Ponzo V, Stampanoni Bassi M, Veniero D, Caltagirone C, Koch G (2016) Cerebellar theta burst stimulation modulates the neural activity of interconnected parietal and motor areas Sci Rep 6:36191 doi: 10.1038/srep36191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanraud S et al. (2007) Brain morphometry and cognitive performance in detoxified alcohol-dependents with preserved psychosocial functioning Neuropsychopharmacology 32:429–438 doi: 10.1038/sj.npp.1301219 [DOI] [PubMed] [Google Scholar]
- Charney DA, Zikos E, Gill KJ (2010) Early recovery from alcohol dependence: factors that promote or impede abstinence J Subst Abuse Treat 38:42–50 doi:S0740-5472(09)00096-8 [pii] 10.1016/jjsat.2009.06.002 [DOI] [PubMed] [Google Scholar]
- Claus ED, Feldstein Ewing SW, Filbey FM, Hutchison KE (2013) Behavioral control in alcohol use disorders: relationships with severity J Stud Alcohol Drugs 74:141–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conners CK (2000) Conners’ Continuous Performance Test II: Computer Program for Windows Technical Guide and Software Manual. Multi-Health Systems, North Tonawanda, NY [Google Scholar]
- Courtney KE et al. (2012) The relationship between measures of impulsivity and alcohol misuse: an integrative structural equation modeling approach Alcohol Clin Exp Res 36:923–931 doi: 10.1111/j.1530-0277.2011.01635.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW (1996) AFNI: software for analysis and visualization of functional magnetic resonance neuroimages Comput Biomed Res 29:162–173 doi:S0010480996900142 [pii] [DOI] [PubMed] [Google Scholar]
- Crane NA et al. (2016) Comorbid anxiety increases cognitive control activation in Major Depressive Disorder Depress Anxiety 33:967–977 doi: 10.1002/da.22541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daskalakis ZJ, Christensen BK, Fitzgerald PB, Fountain SI, Chen R (2005) Reduced cerebellar inhibition in schizophrenia: a preliminary study Am J Psychiatry 162:1203–1205 doi: 10.1176/appi.ajp.162.6.1203 [DOI] [PubMed] [Google Scholar]
- De Vidovich GZ et al. (2016) Repetitive TMS on Left Cerebellum Affects Impulsivity in Borderline Personality Disorder: A Pilot Study Front Hum Neurosci 10:582 doi: 10.3389/fnhum.2016.00582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmond JE, Chen SH, DeRosa E, Pryor MR, Pfefferbaum A, Sullivan EV (2003) Increased frontocerebellar activation in alcoholics during verbal working memory: an fMRI study Neuroimage 19:1510–1520 [DOI] [PubMed] [Google Scholar]
- Epstein JN, Erkanli A, Conners CK, Klaric J, Costello JE, Angold A (2003) Relations between Continuous Performance Test performance measures and ADHD behaviors J Abnorm Child Psychol 31:543–554 [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW (2002) Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition With Psychotic Screen (SCID-I/P W/ PSY SCREEN). [Google Scholar]
- Forcehimes AA, Tonigan JS, Miller WR, Kenna GA, Baer JS (2007) Psychometrics of the Drinker Inventory of Consequences (DrInC) Addict Behav 32:1699–1704 doi: 10.1016/j.addbeh.2006.11.009 [DOI] [PubMed] [Google Scholar]
- Fox HC et al. (2012) Prazosin effects on stress- and cue-induced craving and stress response in alcohol-dependent individuals: preliminary findings Alcohol Clin Exp Res 36:351–360 doi: 10.1111/j.1530-0277.2011.01628.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frodl T et al. (2011) Different effects of mirtazapine and venlafaxine on brain activation: an open randomized controlled fMRI study J Clin Psychiatry 72:448–457 doi: 10.4088/JCP.09m05393blu [DOI] [PubMed] [Google Scholar]
- Froeliger B, McConnell PA, Stankeviciute N, McClure EA, Kalivas PW, Gray KM (2015) The effects of N-Acetylcysteine on frontostriatal resting-state functional connectivity, withdrawal symptoms and smoking abstinence: A double-blind, placebo-controlled fMRI pilot study Drug Alcohol Depend 156:234–242 doi: 10.1016/j.drugalcdep.2015.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden CJ (1978) Stroop Color and Word Test: A Manual for Clinical and Experimental Uses. Skoelting, Chicago, Illinois [Google Scholar]
- Hatchard T, Smith AM, Halchuk RE, Longo CA, Fried PA, Hogan MJ, Cameron I (2015) Effects of low-level alcohol use on cognitive interference: an fMRI study in young adults Alcohol 49:7–13 doi: 10.1016/j.alcohol.2014.07.020 [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO (1991) The Fagerstrom Test for Nicotine Dependence: A revision of the Fagerstrom Tolerance Questionnaire British Journal of Addictions 86:1119–1127 [DOI] [PubMed] [Google Scholar]
- Hendricks MA, Buchanan TW (2015) Individual differences in cognitive control processes and their relationship to emotion regulation Cognition & emotion:1–13 doi: 10.1080/02699931.2015.1032893 [DOI] [PubMed] [Google Scholar]
- Hilt LM, Leitzke BT, Pollak SD (2014) Cognitive Control and Rumination in Youth: The Importance of Emotion Journal of experimental psychopathology 5:302–313 doi: 10.5127/jep.038113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobson NM, Saunders B, Al-Khindi T, Inzlicht M (2014) Emotion down-regulation diminishes cognitive control: a neurophysiological investigation Emotion 14:1014–1026 doi: 10.1037/a0038028 [DOI] [PubMed] [Google Scholar]
- Houben K, Nederkoorn C, Wiers RW, Jansen A (2011) Resisting temptation: Decreasing alcohol-related affect and drinking behavior by training response inhibition Drug and Alcohol Dependence:1–5 [DOI] [PubMed] [Google Scholar]
- Joos L, Goudriaan AE, Schmaal L, Fransen E, van den Brink W, Sabbe BG, Dom G (2013a) Effect of modafinil on impulsivity and relapse in alcohol dependent patients: A randomized, placebo-controlled trial Eur Neuropsychopharmacol 23:948–955 doi: 10.1016/j.euroneuro.2012.10.004 [DOI] [PubMed] [Google Scholar]
- Joos L, Goudriaan AE, Schmaal L, van den Brink W, Sabbe BG, Dom G (2013b) Effect of modafinil on cognitive functions in alcohol dependent patients: a randomized, placebo-controlled trial J Psychopharmacol 27:998–1006 doi: 10.1177/0269881113503505 [DOI] [PubMed] [Google Scholar]
- Karch S et al. (2008) Influence of trait anxiety on inhibitory control in alcohol-dependent patients: simultaneous acquisition of ERPs and BOLD responses J Psychiatr Res 42:734–745 doi:S0022-3956(07)00126-4 [pii] 10.1016/j.jpsychires.2007.07.016 [DOI] [PubMed] [Google Scholar]
- Kenna GA, Haass-Koffler CL, Zywiak WH, Edwards SM, Brickley MB, Swift RM, Leggio L (2016) Role of the alpha1 blocker doxazosin in alcoholism: a proof-of-concept randomized controlled trial Addict Biol 21:904–914 doi: 10.1111/adb.12275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M (2008) Review. Neurobiological mechanisms for opponent motivational processes in addiction Philos Trans R Soc Lond B Biol Sci 363:3113–3123 doi:C54412M472226253 [pii] 10.1098/rstb.2008.0094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwako LE, Momenan R, Litten RZ, Koob GF, Goldman D (2015) Addictions Neuroclinical Assessment: A Neuroscience-Based Framework for Addictive Disorders Biol Psychiatry doi: 10.1016/j.biopsych.2015.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le AD, Funk D, Juzytsch W, Coen K, Navarre BM, Cifani C, Shaham Y (2011) Effect of prazosin and guanfacine on stress-induced reinstatement of alcohol and food seeking in rats Psychopharmacology (Berl) doi: 10.1007/s00213-011-2178-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CS, Luo X, Yan P, Bergquist K, Sinha R (2009) Altered impulse control in alcohol dependence: neural measures of stop signal performance Alcohol Clin Exp Res 33:740–750 doi:ACER891 [pii] 10.1111/j.1530-0277.2008.00891.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Look AE, Flory JD, Harvey PD, Siever LJ (2010) Psychometric properties of a short form of the Affective Lability Scale (ALS-18) Pers Individ Dif 49:187–191 doi: 10.1016/j.paid.2010.03.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupo M, Troisi E, Chiricozzi FR, Clausi S, Molinari M, Leggio M (2015) Inability to Process Negative Emotions in Cerebellar Damage: a Functional Transcranial Doppler Sonographic Study Cerebellum 14:663–669 doi: 10.1007/s12311-015-0662-z [DOI] [PubMed] [Google Scholar]
- Mayer AR, Ryman SG, Hanlon FM, Dodd AB, Ling JM (2017) Look Hear! The Prefrontal Cortex is Stratified by Modality of Sensory Input During Multisensory Cognitive Control Cereb Cortex 27:2831–2840 doi: 10.1093/cercor/bhw131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer AR, Teshiba TM, Franco AR, Ling J, Shane M, Stephen JM, Jung RE (2011) Modelling Conflict and Error in the Medial Frontal Cortex Hum Brain Mapp 33:2843–2855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer AR, Wilcox CE, Teshiba TM, Ling JM, Yang Z (2013) Hyperactivation of the cognitive control network in cocaine use disorders during a multisensory Stroop task Drug Alcohol Depend doi: 10.1016/j.drugalcdep.2013.04.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milham MP, Craddock RC, Klein A (2017) Clinically useful brain imaging for neuropsychiatry: How can we get there? Depress Anxiety 34:578–587 doi: 10.1002/da.22627 [DOI] [PubMed] [Google Scholar]
- Miranda MC et al. (2012) Performance patterns in Conners’ CPT among children with attention deficit hyperactivity disorder and dyslexia Arq Neuropsiquiatr 70:91–96 [DOI] [PubMed] [Google Scholar]
- Morawetz C, Bode S, Baudewig J, Jacobs AM, Heekeren HR (2016) Neural representation of emotion regulation goals Hum Brain Mapp 37:600–620 doi: 10.1002/hbm.23053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Rius J, Miquel M (2017) The cerebellum in drug craving Drug and Alcohol Dependence doi: 10.1016/j.drugalcdep.2016.12.028 [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Silvers JA, Buhle JT (2012) Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion Ann N Y Acad Sci 1251:E1–24 doi: 10.1111/j.1749-6632.2012.06751.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CA et al. (2014) The effects of caffeine ingestion on cortical areas: functional imaging study Magn Reson Imaging 32:366–371 doi: 10.1016/j.mri.2013.12.018 [DOI] [PubMed] [Google Scholar]
- Park MS, Sohn S, Park JE, Kim SH, Yu IK, Sohn JH (2011) Brain functions associated with verbal working memory tasks among young males with alcohol use disorders Scandinavian journal of psychology 52:1–7 doi: 10.1111/j.1467-9450.2010.00848.x [DOI] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES (1995) Factor structure of the Barratt impulsiveness scale J Clin Psychol 51:768–774 [DOI] [PubMed] [Google Scholar]
- Petit G, Cimochowska A, Kornreich C, Hanak C, Verbanck P, Campanella S (2014) Neurophysiological correlates of response inhibition predict relapse in detoxified alcoholic patients: some preliminary evidence from event-related potentials Neuropsychiatr Dis Treat 10:1025–1037 doi: 10.2147/NDT.S61475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskind MA et al. (2003) Reduction of nightmares and other PTSD symptoms in combat veterans by prazosin: a placebo-controlled study Am J Psychiatry 160:371–373 [DOI] [PubMed] [Google Scholar]
- Roelofs K, Minelli A, Mars RB, van Peer J, Toni I (2009) On the neural control of social emotional behavior Soc Cogn Affect Neurosci 4:50–58 doi:nsn036 [pii] 10.1093/scan/nsn036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roostaei T, Nazeri A, Sahraian MA, Minagar A (2014) The human cerebellum: a review of physiologic neuroanatomy Neurol Clin 32:859–869 doi: 10.1016/j.ncl.2014.07.013 [DOI] [PubMed] [Google Scholar]
- Rupp CI, Beck JK, Heinz A, Kemmler G, Manz S, Tempel K, Fleischhacker WW (2016) Impulsivity and Alcohol Dependence Treatment Completion: Is There a Neurocognitive Risk Factor at Treatment Entry? Alcohol Clin Exp Res 40:152–160 doi: 10.1111/acer.12924 [DOI] [PubMed] [Google Scholar]
- Sari BA, Koster EH, Pourtois G, Derakshan N (2015) Training working memory to improve attentional control in anxiety: A proof-of-principle study using behavioral and electrophysiological measures Biol Psychol doi: 10.1016/j.biopsycho.2015.09.008 [DOI] [PubMed] [Google Scholar]
- Schalet BD et al. (2016) Clinical validity of PROMIS Depression, Anxiety, and Anger across diverse clinical samples J Clin Epidemiol 73:119–127 doi: 10.1016/j.jclinepi.2015.08.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmaal L, Joos L, Koeleman M, Veltman DJ, van den Brink W, Goudriaan AE (2013) Effects of modafinil on neural correlates of response inhibition in alcohol-dependent patients Biol Psychiatry 73:211–218 doi: 10.1016/j.biopsych.2012.06.032 [DOI] [PubMed] [Google Scholar]
- Schmahmann JD (2010) The role of the cerebellum in cognition and emotion: personal reflections since 1982 on the dysmetria of thought hypothesis, and its historical evolution from theory to therapy Neuropsychol Rev 20:236–260 doi: 10.1007/s11065-010-9142-x [DOI] [PubMed] [Google Scholar]
- Schmidt TP, Pennington DL, Cardoos SL, Durazzo TC, Meyerhoff DJ (2017) Neurocognition and inhibitory control in polysubstance use disorders: Comparison with alcohol use disorders and changes with abstinence J Clin Exp Neuropsychol 39:22–34 doi: 10.1080/13803395.2016.1196165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte T, Muller-Oehring EM, Sullivan EV, Pfefferbaum A (2012) Synchrony of corticostriatal-midbrain activation enables normal inhibitory control and conflict processing in recovering alcoholic men Biol Psychiatry 71:269–278 doi: 10.1016/j.biopsych.2011.10.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson TL et al. (2015) A pilot trial of prazosin, an alpha-1 adrenergic antagonist, for comorbid alcohol dependence and posttraumatic stress disorder Alcohol Clin Exp Res 39:808–817 doi: 10.1111/acer.12703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson TL et al. (2009) A pilot trial of the alpha-1 adrenergic antagonist, prazosin, for alcohol dependence Alcohol Clin Exp Res 33:255–263 doi:ACER807 [pii] 10.1111/j.1530–0277.2008.00807.x [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB, Litten RZ, Allen JP (1992) Timeline follow-back: A technique for assessing self-reported alcohol consumption.
- Sullivan J, Sykora K, Schneiderman J, Naranjo C, Sellers E (1989) Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar) Br J Addict 84:1353–1357 [DOI] [PubMed] [Google Scholar]
- Viviani R (2014) Neural correlates of emotion regulation in the ventral prefrontal cortex and the encoding of subjective value and economic utility Front Psychiatry 5:123 doi: 10.3389/fpsyt.2014.00123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney KA, Shepard PH, Mariner J, Mossbarger B, Herman SM (2010) Validity of the Wechsler Test of Adult Reading (WTAR): effort considered in a clinical sample of U.S. military veterans Appl Neuropsychol 17:196–204 doi: 10.1080/09084282.2010.499787 [DOI] [PubMed] [Google Scholar]
- Wilcox CE, Abbott CC, Calhoun VD (2018a) Alterations in resting-state functional connectivity in substance use disorders and treatment implications Prog Neuropsychopharmacol Biol Psychiatry doi: 10.1016/j.pnpbp.2018.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox CE, Dekonenko CJ, Mayer AR, Bogenschutz MP, Turner JA (2014) Cognitive control in alcohol use disorder: deficits and clinical relevance Rev Neurosci:1–24 doi: 10.1515/revneuro-2013-0054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox CE, Mayer AR, Bogenschutz MP, Ling J, Dekonenko C, Cumbo H (2015) Cognitive control network function in alcohol use disorder before and during treatment with lorazepam Subst Use Misuse 50:40–52 doi: 10.3109/10826084.2014.957771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox CE, Pommy JM, Adinoff B (2016) Neural Circuitry of Impaired Emotion Regulation in Substance Use Disorders American Journal of Psychiatry [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox CE, Tonigan JS (2016) Changes in depression mediate the effects of AA attendance on alcohol use outcomes American Journal of Drug and Alcohol Abuse 44:103–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox CE, Tonigan JS, Bogenschutz MP, Clifford J, Bigelow RC, Simpson TL (2018b) A randomized, placebo-controlled, clinical trial of prazosin for the treatment of alcohol use disorder Journal of addiction medicine 12:339–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingenfeld K et al. (2009) Neural correlates of the individual emotional Stroop in borderline personality disorder Psychoneuroendocrinology 34:571–586 doi:S0306–4530(08)00294–1 [pii] 10.1016/j.psyneuen.2008.10.024 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.