Abstract
Tremor is the most common movement disorder; however, we are just beginning to understand the brain circuitry that generates tremor. Various neuroimaging, neuropathological, and physiological studies in human tremor disorders have been performed to further our knowledge of tremor. But, the causal relationship between these observations and tremor is usually difficult to establish and detailed mechanisms are not sufficiently studied. To overcome these obstacles, animal models can provide an important means to look into human tremor disorders. In this manuscript, we will discuss the use of different species of animals (mice, rats, fruit flies, pigs, and monkeys) to model human tremor disorders. Several ways to manipulate the brain circuitry and physiology in these animal models (pharmacology, genetics, and lesioning) will also be discussed. Finally, we will discuss how these animal models can help us to gain knowledge of the pathophysiology of human tremor disorders, which could serve as a platform towards developing novel therapies for tremor.
Keywords: tremor, animal models, cerebellum, Purkinje cells, pathology, physiology
Introduction (Elan D. Louis, Phyllis L. Faust)
Tremor is one of the most common forms of involuntary movement and may be defined by abnormal, rhythmic oscillations.1 Because of its features of frequency, amplitude, and phase, it is also one of the most measurable movement disorders in humans. A range of different diseases may manifest tremor, with the most common of these being essential tremor (ET), which is characterized by kinetic as well as other types of tremor.2 Indeed, it is estimated that in the United States alone, 7 million people are affected with ET (i.e., 2.2% of the United States population),3 and with age, this proportion increases, so that as many as 20% or more of 90-year-old may be affected.4 Tremors are also a feature of a range of other neurological diseases, including Parkinson disease (PD), dystonia, and spinocerebellar ataxia (SCA). Furthermore, tremor is a strikingly common and limiting side effect of numerous medications.5 Hence, the public health impact of research on ET and other tremor disorders is sizable. Despite the high prevalence of these disorders, the number and efficacy of pharmacotherapeutic options for these disorders remain severely limited, leaving a large population of patients symptomatic and untreated. Surgical treatments for some of these disorders, such as thalamic deep brain stimulation and focused ultrasound thalamotomy, can provide significant relief; however, surgical and post-surgical risks as well as tachyphylaxis remain issues of concern.6 One roadblock to more successful therapies is the relative lack of understanding of the mechanisms that underlie tremor disorders. It is only through additional research that this landscape can change.
Studies of tremor among humans have used a variety of approaches, including neuroimaging,7 neurophysiology,8 and neuropathology.9 However, approaches that observe humans are limited. Animal models allow investigators to manipulate the system, providing a fertile experimental avenue for understanding disease mechanisms.
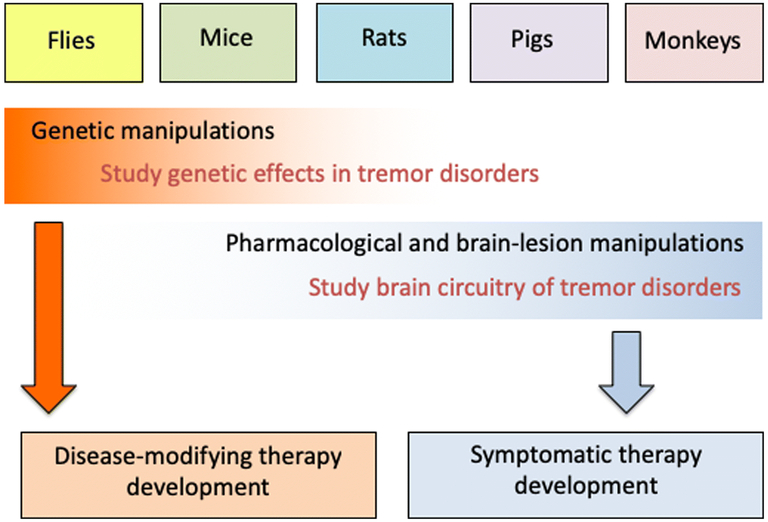
Tremor is a symptom or a sign rather than a disease; therefore, animal models may be roughly divided into those that reproduce this sign (e.g., toxin-based models) versus those that attempt to reproduce the underlying disease pathophysiology (e.g., genetic models). Put another way, some animal models are useful for studying the alterations in brain circuitry, particularly the cerebello-thalamo-cortical circuit, that drive the symptom of tremor whereas others are useful for understanding the underlying disease patho-mechanisms that cause ET and other tremor disorders. The downstream therapeutic products of these models are symptomatic therapy and disease modifying therapy, respectively.
The goal of this consensus paper is to bring together experts who have used animal models to study tremor disorders. Each comes from a different perspective. Specifically, we will first review pharmacological tremor models: rodent harmaline models (Section 1), a swine harmaline model (Section 2), and how the basic science learned from these models can help us understand tremor generation (Section 3). We will then discuss tremor models with structural changes in the cerebellar circuitry, in terms of molecular changes (i.e. Car8)(Section 4), synaptic alterations (Section 5), cerebellar degeneration (Section 6), and alterations of the inhibitory neural transmission (Section 7). Next, we will review the roles of ion channels in tremor models (Section 8 and 9). Previous tremor models have mostly focused on rodents; recently, fly models have been created to study tremor, for which we will review (Section 9 and 10). Finally, we will discuss lesion models of tremor in monkeys (Section 11). The investigators use a variety of animals for their studies – rats, mice, flies, pigs, monkeys – with each approach offering both advantages as well as disadvantages. Their work serves to highlight not only what is currently known, but just as important, areas of uncertainty and gaps in knowledge. Collectively, these experts provide a comprehensive overview of the field and ideas for future research directions.
The harmaline tremor model (Adrian Handforth)
Harmaline is a β-carboline that induces action tremor in mammals, with a frequency that ranges from 8-10 Hz in monkeys to 11-14 Hz in mice,10 likely reflecting brain size. In rodents, harmaline tremor involves the limbs, tail, trunk, head, and whiskers. Tremor cannot be reliably elicited on repeat administration, a phenomenon known as tolerance.
Harmaline promotes 6-12 Hz burst-firing in inferior olive (IO) neurons by increasing the cyclical post-hyperpolarization depolarizing rebound.11-13 In cats, harmaline selectively induces rhythmic, synchronous firing in the medial and dorsal accessory olive subnuclei14 that is propagated via climbing fibers (CFs) to Purkinje cells (PCs), where synchronous 6-12 Hz complex spikes (CSs) occur within parasagittal bands of cerebellar cortex.15-17 Convergent harmaline-induced rhythmic PC CS activity results in marked hyperpolarization of deep cerebellar nucleus (DCN) neurons, which often triggers rebound depolarization.18 In addition, metabolic and Fos mapping have shown activation of the molecular and granule cell layers of vermis and paravermis in the cerebellum.17, 19, 20
The behavioral expression of this rhythmic olivocerebellar oscillation is action tremor at the same frequency. Spinal motoneuron recordings reveal that the tremor is linked to IO-PC CS rhythmic activity.16 The requirement for IO coupling is shown by loss of the tremor response after IO destruction,21 administration of gap junction blockers,22 or after IO cells have been rendered tolerant by repeated harmaline administration.23 Lesions of CFs abolish tremor.16 The requirement for intact PCs and normal CSs is shown by marked reduction or loss of harmaline tremor in PC degeneration mice (pcd),24 and in Kv3.3 knockout (KO) mice that have defective CSs.25, 26 Thalamic stimulation suppresses harmaline tremor, indicating the requirement for the DCN-thalamus outflow.27
The degree of IO coupling, and hence PC CS synchrony, is controlled by GABAergic afferents, mainly from the DCN, and by glutamate release on α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors within the IO.28 AMPA receptor antagonists suppress harmaline tremor,29, 30 whereas the allosteric AMPA receptor agonist, PF-4778574, causes tremor in several species, including primates.31 5-HT2A receptor activation increases IO coupling and PC CS synchrony,32 potentiates harmaline tremor,32 and can itself induce tremor.33, 34 Systemically administered T-type calcium channel antagonists or intra-IO microinjection of mibefradil suppress harmaline tremor,35, 36 likely by interfering with the IO oscillation.
Much like how the maximal electroshock seizure (MES) model in mice has been used to screen drugs for epilepsy treatment although it models seizures, a symptom of epilepsy, rather than the disease epilepsy itself, the harmaline model may similarly offer utility for screening drugs to treat tremor, the main symptom of ET. The potential usefulness as a predictive model depends on the extent of anatomical, pharmacological, and physiological overlap between the tremor of ET and that of harmaline. In illustration, the pharmacologic overlap of MES seizures with that of clinical epilepsy is rooted in similar anatomic circuitry and physiology, so that it provides a platform for therapeutic drug development. Similarly, the harmaline model cannot be a model of ET the disease, but offers utility for screening drugs to treat tremor, the main symptom of ET.
Concerning the anatomy, the circuits activated by harmaline overlap substantially those implicated in ET. Positron emission tomography (PET) blood flow scans show activation of the DCN and medial cerebellar cortex in ET.37 In the harmaline model metabolic and Fos mapping shows similar cerebellar activation, including activation of the DCN neurons, PCs, and granule cell neurons.19, 20, 38 PCs are required for harmaline tremor as indicated in pcd mice.24 The requirement for PCs in ET is inferred by the loss of tremor when the afferent pathway to PCs is interrupted by pontine lesions.39 In ET, the DCN-thalamus outflow is critical for tremor expression as indicated by loss of tremor when the DCN-thalamic relay site is subjected to deep brain stimulation, which blocks oscillatory transmission, or to thalamotomy by various techniques. Similarly, thalamic stimulation suppresses harmaline tremor,27 and implies PCs are critical for tremor in both conditions. However, there are two caveats concerning the anatomy: (1) The term “overlap” is used, recognizing possible partial non-overlap. The utility for drug screening will depend on the extent of overlap, which we suspect is substantial, but non-overlapping tremor circuitry might lead to divergent results. (2) Harmaline is administered to animals with intact brains, whereas the cerebellum of ET patients displays pathology. Pathological changes in ET might affect the predictive value of harmaline tremor as a drug screening model.
The pharmacologic profile of harmaline tremor has considerable overlap with that of ET, responding to propranolol, primidone, alcohol, benzodiazepines, gabapentin, gamma-hydroxybutyrate, 1-octanol, and zonisamide, while being exacerbated by drugs that worsen ET, such as tricyclics and caffeine.40 As an action tremor, harmaline tremor is easily suppressed by anything that reduces activity, such as sedation, fatigue, fear, pain, ataxia, malaise, nausea, etc. At least in some cases, these factors likely led to false positives, i.e. drugs that suppressed harmaline tremor but did not show efficacy in clinical trials.
The physiological basis of harmaline tremor is enhanced, rhythmic synchronized IO and PC CS firing, with propagation of rhythmic synchronized activity at the IO oscillator frequency to the DCN, thalamus, and eventually spinal motoneurons. The use of this symptomatic model does not require any inference that the IO is pathologically or functionally compromised in ET. Indeed, normal functioning of the IO-DCN pathway has been inferred in ET patients receiving deep brain stimulation, as indicated by eyeblink conditioning;41 and imaging and pathologic studies have been negative for IO abnormalities.42 On the other hand, it is uncertain whether increased PC CS synchrony occurs in ET. This is a reasonable supposition, however, given the evidence that motor cortex outflow is gated by the IO PC CS oscillation, and movement amplitude is affected by the degree of PC CS synchrony.43 This oscillation could be the basis of the rhythmic 8-10 Hz EMG rhythmic firing that underlies ~10 Hz submovements during slow movements in humans44 (that also is responsible for 8-12 Hz physiological tremor), and has been mapped to an oscillatory circuit involving the cerebellum, thalamus, primary motor and premotor cortex;45 a circuit very similar to that mapped in ET.46
In summary, the venerable harmaline model does not offer insights into the etiology of ET but should be regarded as a potentially useful vehicle for screening drugs for the main symptom of ET, tremor. Care should be taken in dose selection to avoid confounding effects. Any drug dose that is tested in the harmaline model should be shown in sensitive tests not to compromise psychomotor functioning; otherwise the prediction of anti-tremor efficacy may not be valid. There may be potential for pharmacologic non-overlap with ET due to its lack of pathology, and possible partial non-overlap of tremor circuitry and physiology. Nonetheless, it is noteworthy that all drugs that suppress tremor in ET also suppress tremor in the harmaline model, suggesting a high degree of overlap. Based on the above discussion of clinical and model evidence, it may be inferred that logical targets for tremor therapy are overactive cerebellar granule cells and PCs, and excessive synchrony among IO neurons and among PCs. From this insights, the model has had predictive success for 1-octanol,47 and the results of a clinical trial of a T-type calcium channel antagonist, predicted by the model to be effective, were encouraging.48
Swine model of tremor (Su-youne Chang)
In the biomedical and translational research fields, it is important to perform physical fidelity tests with animals which are compatible with humans in size. Large animal models such as pigs, dogs, sheep and non-human primates have been used for this purpose. Recently, pigs have been increasingly used in the neuroscience field due to the similarity of their anatomy and neurodevelopment to the human brain, their compatible brain size and volume of the brain to nonhuman primates, and the relatively mild economic and ethical concerns surrounding their use compared to that of non-human primates.49, 50 In addition, the pig brain is gyrencephalic and more closely resembles the human brain in thalamic cellular population and function compared to the rodent brain.51-53 Swine models can become a reasonable preclinical animal model to investigate whole brain network mechanisms using multi-modal recording and neuromodulation systems.54, 55 However, due to the lifespan and difficulty in maintaining an inbreeding colony, genetic and/or molecular engineering approaches are currently limited.
For these reasons, harmaline-based tremor model has been developed and evaluated in pigs before genetic modification-based model development. Like other species, harmaline can induce visible and significant whole-body tremor in pigs.56 In the case of pigs, relatively low concentrations of harmaline have been administered (2.5- 6 mg/kg) compared to rodents. The tremor frequency is 10-16 Hz, similar to human ET and harmaline-induced tremor in rodents. Tremor is apparent during resting and walking; however, during walking, the tremor becomes more prominent than during lying or resting. The tremor amplitude is dose dependent, but the frequency is not. Harmaline tolerance has also been observed in the pig model. The underlying cellular and/or neural network mechanisms of tremor generation have not been fully investigated in the swine model. However, according to its peak tremor frequency, the olivary-cerebellar network might be expected to be involved.13, 57 Since it is hard to perform histological analyses with a large animal, functional magnetic resonance imaging (fMRI) or other whole brain imaging methodologies will be needed to identify the involvement of cerebellar and other brain areas.
Another interesting observation in the pig tremor model is that there are several other behavioral and physiological changes that occur. These changes have not been described in rodent models. ET is known to be a clinically heterogeneous disorders involving many other non-motor symptoms, such as cognitive, psychiatric, sensory impairment, and dementia.58 In clinical research, an fMRI study presented of patients with ET showed a greater magnitude of brain response in the dorsolateral prefrontal cortex and in the inferior parietal cortex as compared to controls, when patient performance was similar to the control group.59 Structural abnormalities in the prefrontal cortex, parietal and temporal lobe (fronto-temporal cortex) have been shown to be implicated in ET;60 therefore, involvement of non-motor functional changes have been well documented in clinical research.
Harmaline has been known to have a stimulant effect on the central nervous system.61 In the harmaline-induced pig model, not only tremor but also anxiety- and depression-like behavior have been reported.56 This phenomenon may not be directly translated into the clinical presentation or psychological comorbidity of ET,62 since these mood changes could be caused by side effects of cardiovascular changes because some pigs showed agitation, barking, falling and hyperthermia at the first harmaline (6 mg/kg) administration session. However, the possibility that harmaline can induce psychiatric behaviour, such as anxiety and depressive-like behavior, cannot be ruled out.
In pigs, congenital tremor (myoclonia congenita) has been reported as spontaneous and sporadic tremor. Its peak frequency of tremor is 14- to 15 Hz. Affected pigs showed a coarse tremor of the extremities when standing and walking, so they tended to remain recumbent, during which the tremor was suppressed.63 The underlying mechanism of this tremor is thought to be caused by viral infection.63 Since the congenital tremor doesn’t share neurodegenerative characteristics with clinical human tremor, this tremor in pigs is hard to be used as a clinically relevant model of tremor.
It is not easy to study genetic background or to pinpoint a specific molecule of tremor generation in pigs due to the difficulties in inbreeding the animals; However, pigs can be used to investigate whole brain networks and in neuromodulation studies, since a large volume of the brain can be accessible to implanting multiple sensing probes in multiple brain areas, and its anatomical similarities to humans helps accelerate translation from the bench to clinic. Recent development of molecular investigational tools, such as optogenetics64 and viral infection approaches for cellular calcium imaging,65 can be used in pigs to overcome some of these experimental limitations. Thus, a pig model of tremor could become a valuable preclinical model to understand the underlying neurophysiology contributing to ET and other action tremors, as well as the mechanism by which different therapeutic interventions can ameliorate tremor.
In search of mechanism of ET: Is there a role for the olivocerebellar system? (Billur Avlar and Eric J. Lang)
Harmaline tremor with its focus on the olivocerebellar system has been the major animal model of ET for many decades. Yet, evidence for dysfunction of the IO in ET is limited, and thus far, no pathological changes in the IO have been observed.66 Moreover, pathological changes in the cerebellum exist, including a modest loss of PCs, PC axonal abnormalities, and ‘hairy’ baskets.67-69 Decreases in GABA receptors in the DCN have also been reported.70 These changes raise the possibility that the cerebellum, and not the IO, is the primary site of pathogenesis, and provide the basis for the cerebellar degeneration hypothesis of ET.71
The cerebellar degeneration hypothesis, however, also faces challenges. In particular, the physiological basis by which this pathology could result in tremorigenic activity is not apparent (providing such a basis is one of the strengths of the harmaline model). With this issue in mind, we review the properties of the olivocerebellar system and the cerebellar circuitry (both intrinsic and the projections to and from the IO), and then discuss whether the pathological changes in PCs are likely to lead to tremor, or whether dysfunction of the olivocerebellar system may still be needed, as suggested by the harmaline model.
ET requires the rhythmic synchronous activation of motor circuits. The organization of the olivocerebellar system provides a clear mechanism for accomplishing this. IO neurons are electrically coupled,11, 72 and this coupling underlies CS synchrony.73, 74 Although the level and distribution of synchrony is normally controlled by IO afferents, loss of this control causes widespread synchrony,28, 75 which can drive a tremor with characteristics similar to ET.15, 16 Furthermore, bilateral synchronization of CS activity occurs,75-77 and could underlie the bilateral synchronization of the tremor in ET.
Nevertheless, these findings are not proof that the olivocerebellar system is involved in ET. So, it is worth considering how the cerebellum might generate synchronized 4-12 Hz activity independently of the olivocerebellar system as a result of the pathology mentioned above, all of which would likely reduce the inhibitory control of DCN neurons. One possibility is that because DCN cells or PCs (the main synaptic input to the DCN) are spontaneously active, they could become pacemakers for driving a tremor. However, most PCs and DCN neurons fire tonically at average rates well above the frequencies characteristic of ET, with no predilection to fire at those frequencies.78-82 An exception is glutamic acid decarboxylase (GAD)+ DCN cells, which, at least in vitro, firing phasically and at lower average firing rates (~10 Hz);79 however, many of these cells project back to the IO (some are local interneurons), and thus do not project to downstream, motor-related regions.
Could the tonic decrease in inhibition that would be expected to result from a decrease in PCs or PC axonal dysfunction lead to 4-12 Hz activity? This seems unlikely, given that cooling of the cerebellar cortex increases DCN firing rates,83, 84 and that lower simple spike rates are associated with increased DCN activity.85
Synchronization of DCN activity is also needed for cerebellar output to drive tremor. In theory, synchronized simple spike activity would lead to synchronous modulation of DCN cells. However, correlation of simple spikes among PCs is weak, and is limited to PCs that are located within a few hundred microns of each other.85-87 Moreover, there is no obvious mechanism by which intracerebellar circuitry could generate widespread simple spike synchrony. Indeed, the most obvious mechanism for synchronizing simple spikes is shared parallel fiber input, but parallel fibers are only a few millimeters in length, a small fraction of the human cerebellum. In sum, intracerebellar cortical networks are highly local in nature and not designed to generate widespread synchrony.
Given the local nature of intracerebellar networks, widespread synchrony likely needs to be imposed by cerebellar afferents. Interestingly, the cerebellum forms closed loop circuits with many of its targets, suggesting that aberrant cerebellar output could be fed back to the cerebellum in such a way as to generate widespread synchronized and rhythmic activity. Here, we consider whether the pathological changes in the cerebellum could alter input to the IO such that the olivocerebellar system would drive synchronized rhythmic output from the DCN, because this system is known to have the potential to synchronize widespread regions of the cerebellum.75
The reported pathology in ET (PC loss, axonal abnormalities, decreased GABA receptors in the DCN)67-70 would be expected to produce a general decrease in the PC inhibition of DCN neurons. This decrease in inhibitory control would cause a tonic increase in DCN-IO projection cell activity, which, in turn, would lead to a decrease in the synchronization of CS activity, rather than the increase needed to drive tremor.88 It should be mentioned that PC axons show increased branching in the cortex in ET,68 and this has been suggested to occur in the DCN as well.89 Increased PC branching in the DCN could, at least partly, compensate for the other pathological changes, so that tonic inhibitory drive to the DCN is closer to normal. Nevertheless, the tonic changes in DCN activity that would likely result from the ET pathology as a whole do not appear to provide an obvious mechanism for producing tremor.
It is possible, however, that ET pathology might produce changes in phasic activity could underlie tremor. In particular, the decrease in PCs, implies that each DCN cell would receive input from fewer PCs. As a result, each DCN cell could experience wider variations in the level of inhibition it receives (i.e., with fewer cells it is easier to have periods of highly synchronized input or ones free from inhibition). Such patterning of PC into periods of synchronous activity interspersed with silences has been shown to produce phase-locked DCN bursting.90
While this hypothesis by which ET pathology could cause bursting activity in individual DCN neurons, and perhaps even synchronous bursting among local clusters of DCN neurons (as a result of increased PC branching), may be part of the mechanism for generating tremor, it is likely not sufficient. In particular, it is unlikely to produce the widespread synchronization and 4-12 Hz rhythmicity needed to generate tremor because expansion of PC axonal territory in the setting of PC loss appears to be limited. For example, in the cortex, there is no significant increase in PC axonal territory in ET.68 Furthermore, in both the cortex and DCN, PC axons are normally restricted to specific regions based on their origin,91-93 and this compartmentalization seems to be obeyed in other instances of PC loss.89
Thus, the question how the observed pathological changes in ET lead to activity that could produce tremor remains? One possibility suggested by the closed loop nature of cerebellar-IO connections is that the locally synchronized modulation of DCN activity (resulting from the pathology and compensatory changes in the cerebellum) could be expanded and made more rhythmic, via the loop circuits with the IO. That is, the coordinated modulation of small clusters of DCN cells could entrain and couple the activity of clusters of IO neurons whose activity could serve as foci for triggering more globally synchronized CS because of the electrical coupling of IO neurons. Indeed, consistent with this idea, CS activity can rapidly sweep across bands of PCs,73 suggesting that activity in one region of the IO can trigger activity in connected regions, even in the presence of GABAergic input from the DCN that would tend to suppress coupling between IO regions. Such widespread CS activity would then synchronize large numbers of DCN cells, leading to tremor. While this suggestion is speculative, it may help to bridge the gap between the pathological findings in ET and the mechanism by which they lead to tremor.
Car8wdl mutant mouse as a model for cerebellar-related tremor (Lauren N. Miterko, Amanda M. Brown, Roy V. Sillitoe)
Tremor disorders have a neurological basis, which implies that specific brain oscillations drive the body to oscillate at the same frequency. However, it is still not clear where in the central nervous system the oscillations begin, and the processes that lead to oscillations in the connected brain regions remain unknown. In ET, structural and functional defects in the cerebellum have been heavily implicated as the major source of abnormal behavior. But how abnormal cerebellar activity leads to oscillating motions has been challenging to test. This is largely because of the lack of an appropriate animal model. To address this problem, we have identified a genetic mouse model that exhibits the core features of ET.94 We showed that the loss of a PC gene, carbonic anhydrase 8 (Car8), causes an ET-like tremor that mimics the human condition in its frequency of oscillation, progression with age, and responsiveness to alcohol.94 Over the past two decades, a number of animal models of tremor have been generated, each with their own benefits and problems. However, we found that the spontaneous mutant mouse, waddles, may be ideal for several reasons. Waddles (wdl) mice contain a deletion in exon 8 of the Car8 gene, creating a null allele with no protein.95 In the brain, CAR8 protein is expressed predominantly in PCs (Fig. 1). Its expression is initiated during embryogenesis and is maintained into adulthood.96, 97 CAR8 belongs to a family of zinc metalloenzymes that catalyze the reversible hydration of CO2,98 although it lacks the catalytic domain that would make it an active carbonic anhydrase.96 It does, however, bind to inositol 1,4,5-triphosphate receptor type 1, with the effect of decreasing the affinity of inositol 1,4,5-triphosphate for its receptor.99 Although the Car8wdl mice were originally reported to have ataxia and appendicular dystonia, in our recent study we reported that the mice also have a severe tremor.94 The most notable findings are that the peak of the tremor occurs at ~8-12Hz in adult Car8wdl mice and the tremor progresses with age (Fig. 2). In humans, mutations in the homologous gene, CA8, also cause tremor in addition to ataxia.100, 101 Based on the phenotypic outcomes of CA8 and tremor diseases in general, we propose that the Car8wdl mouse could be a powerful model for studying tremor because they allow for cellular, molecular, anatomical, and neural analyses in brain circuits that do not exhibit gross degeneration and therefore remain experimentally accessible at all stages of life.102
Fig. 1:
CAR8 is expressed predominantly by cerebellar PCs. a. Low power sagittal view of the cerebellum stained using a CAR8 antibody showing the localization of CAR8 in the cerebellum. b. Higher power view of the cerebellar cortex stained with a CAR8 antibody demonstrating the restriction of CAR8 to PCs. Scale bar = 500 μm in a and 50 μm in b. Used with permission from data published in White et al. (2016) Neurobiology of Disease.
Fig. 2:
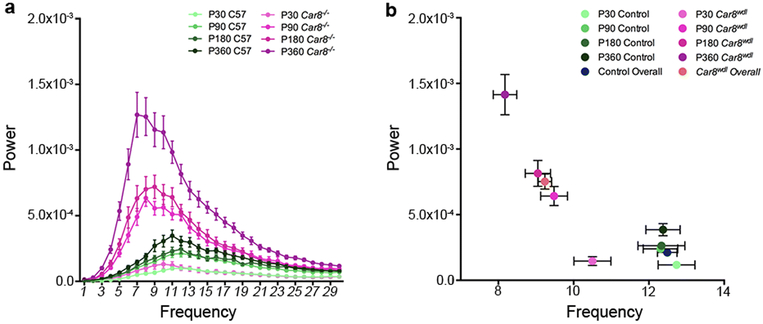
Car8wdl mice have a severe ~8-12Hz tremor that progresses with age. Tremor was analyzed using a Tremor Monitor (San Diego Instruments) from postnatal (P) day P30 through to P360. Note the normal level of physiological tremor observed in control mice. a The amplitude (power) of tremor in Car8wdl mice becomes more pronounced with age. b Car8wdl mice display a tremor that increases in amplitude, but decreases in frequency with age. Control physiological tremor also increases with age, but does not change in frequency. Used with permission from data published in White et al. (2016) Neurobiology of Disease.
Slice physiology showed that Car8wdl mice have a decrease in the frequency of mini excitatory postsynaptic currents.103 To test whether these defects are translated into circuit level problems, we used an extracellular recording approach to measure PC activity in vivo in anesthetized mice.104-106 PCs exhibit a specific firing profile that consists of simple spike action potentials, which are generated intrinsically and modulated by mossy fiber inputs, and CS action potentials, which are triggered by CFs. We found that simple spike firing frequency is not significantly different between control and Car8wdl mice. However, the pattern of firing is significantly altered. Compared to the controls, Car8wdl PCs fire with long pauses. The long simple spike pauses cause a significant increase in the coefficient of variation (CV) of the inter-spike interval (ISI) in Car8wdl. CV is a measure of firing regularity, with higher values indicating abnormal irregular firing. Interestingly, analysis of simple spike firing without the long pauses revealed periods of high frequency firing in Car8wdl PCs. We predicted that the abnormal properties of simple spike firing should lead to abnormal CS firing because the circuit operates within a closed loop involving PCs, DCN neurons, IO neurons, and finally back to their target, the PCs for both firing rates and CS synchrony.107, 108 Indeed, the CS firing frequency is significantly decreased in Car8wdl mice, and the regularity of CS firing is altered, as measured by CV2. CV2 is also a measure of regularity, although it provides a way of narrowing in on spike-to-spike variations. These data show that PC firing is irregular in Car8wdl mice.94
The electrophysiological properties and the tremor profile in Car8wdl mice present an interesting discussion. It is becoming well-accepted that cerebellar motor abnormalities may be dependent on erratic activity in the PCs and also in their downstream targets, the DCN neurons.104, 109-111 For example, there is an increased CV in PC spiking without a change in firing frequency112 in different mouse models of ataxia (e.g. ducky and leaner), which is reminiscent of the firing characteristics observed in Car8wdl mice. Similarly, the tottering mouse, which presents with episodic ataxia and dystonia, has an increased CV in DCN spiking activity, likely as a result of irregular PC signals.106, 111, 113, 114 Similarly, we have suggested that an altered functional connectivity between the PCs and DCN may underlie severe tremor.94 It is interesting to speculate that the similarities in cerebellar circuit defects offer common mechanisms for therapeutic targeting. When PC or DCN firing is normalized with drugs such as 4-aminopyridine (4-AP) or chlorzoxazone, motor behavior is improved in mouse models of cerebellar disease (e.g. Car8wdl, tottering, and SCA6 (84Q/84Q)).94, 115, 116 Interestingly, the benefits of 4-AP in alleviating various cerebellar symptoms in human motor conditions.117-121 may also extend to patients that have tremor.122
From analyses based on animal models and human studies, we can conclude with some degree of confidence that PCs are critical for cerebellum-related disorders. Tremor in particular could involve alterations in PC structure, connectivity, and function and therefore multiple types of pathology may play a role in generating tremor during motion. However, common distortions in output signals may be at the heart of the well-known motor diseases. A working model of cerebellar dysfunction across multiple diseases may involve one or more alterations in firing, such as an increased CV in PCs and DCN neurons, as well as in additional properties such as firing rate, CV2, connectivity, or synchrony. Still, despite the convergence of neural pathways and mechanisms, it should be considered that the combination of these firing properties determines why the efficacy of the same pharmacological intervention can vary across diseases. Each motor disease phenotype is distinct, yet the cerebellum is involved in all of them. This begs the question: what kind of abnormal activity is associated with one condition versus the other? Does abnormal cerebellar activity, in general, drive similar neuronal firing problems in structures to which it projects? Or, are certain structures sensitive to specific cerebellar deficits? The fact that the cerebellum communicates with so many different structures in the brain and spinal cord adds another level of questions since each of these structures, in each condition, might respond differently to the cerebellum, and there is no reason to assume that ataxia, dystonia, and tremor involve all the same extra-cerebellar circuits, although there is likely overlap.
The Car8wdl mouse exhibits a number of core motor features – clinically-relevant tremor, ataxia, and dystonia – and therefore it may indeed be an important model for cerebellum-related disorders. Although we are enthusiastic about the Car8wdl mice specifically as a model for tremor, and perhaps for ET, our future studies must proceed with caution in order to avoid the hurdles other models have run into. But, many models before Car8wdl exhibit phenotypes that have taught us a great deal about the cerebellum and tremor. And the shortcomings in previous models are distinct from Car8wdl, including that the tremor frequencies in other genetic models do not match the human phenotypes and that the spatial and temporal qualities of tremor disorders are difficult to reproduce in drug-induced models. In this regard, it would be ideal to eventually generate a conditional allele at the Car8 locus. It would also be exciting to examine human genetic databases for possible patients with mutations in CA8 that report tremor, but in particular to see if there are patients that lack the more severe gait deficits seen in the Turkish cohorts.100
CF-PC synaptic pathology: a potential implication in tremor (Ming-Kai Pan, Sheng-Han Kuo)
Although ET is a very common neurological disorder, the cause of tremor is not entirely clear, probably due to the fact that ET might be a heterogeneous group of diseases with complex genetic and environmental etiologies.123 For example, ET patients might have different pharmacological responses124, 125 and can be divided into subgroups based on age of tremor onset126 or a family history of tremor.127 Several genetic loci have been identified in genome-wide association studies128 and some environmental factors interacting with geneitc composition are likely to contribute to ET.123
Despite this complexity, the core clinical feature of kinetic tremor is the hallmark of ET, which implies that there are commonalities in the underlying brain circuitry that drives tremor across ET etiologies. Understanding the brain circuitry alterations and how these alterations will result in abnormal movements are the first steps for developing symptomatic therapy for tremor, and this approach has been used to study other movement disorders as well, such as PD. For example, the identification of dopaminergic neuronal loss in the postmortem PD brains provided the important scientific basis for dopamine replacement therapy.127, 129 In addition, dopamine blockade or depletion in animal models further demonstrated the causal relationship between dopamine deficiency and parkinsonian symptoms.130 Importantly, understanding the pathological substrate of neurological disorders also provide important framework for the future functional studies. For instance, the knowledge how dopamine neurons carrying PD-linked genetic mutations, such as GBA1 or LRRK2, can lead to cellular dysfunctions and neuronal death is critical for the development therapies to reverse these pathological events in PD.131, 132
To search for the pathological underpinnings of ET, extensive studies in the postmortem brain have been performed. Several pathological features of the ET cerebellum have been identified, including moderate PC loss,67, 133 increased number of PC axonal torpedoes67 and heterotopic PCs,134, 135 complex and elongated basket cell processes surrounding PC axonal initial segments,69, 136 and abnormal CF-PC synaptic connections, specifically, CFs forming synaptic connections with the thin, distal PC dendritic branchlets, which should have been the parallel fiber innervation territory.137, 138 Among these pathological alterations, abnormal CF-PC synapses are of major interest. We first identified this CF-PC synaptic pathology in an initial cohort of ET cases and controls139 and subsequently confirmed it in a replicate cohort.140 Interestingly, the observation that CF synapses in the parallel fiber synaptic territory along PC dendrites occurs in ET but not in other classical cerebellar degenerative disorders such as SCA1 or multiple system atrophy, demonstrates the specificity of this particular pathology.140 Furthermore, we also found the CF-PC synaptic pathology across ET cases with different clinical characteristics in terms of age of tremor onset or the family history of tremor,141 which indicates this pathology could be related to the core feature (i.e. kinetic tremor) for ET.
There are clues suggesting that abnormal CF-PC synaptic pathology might contribute to tremor. The upstream origin of CF-PC synapse is IO, which has intrinsic oscillatory rhythm. Enhanced IO coupling by harmaline administration in animals can induce PC CS synchrony and tremor13 and also intra-IO injections of picrotoxin to block GABAergic transmission also can induce tremor-like activities in rodents.75 These studies suggest that the olivocerebellar system is capable of generating oscillatory activities and tremor. To further explore the relationship between CF-PC synaptic pathology and tremor, an animal model of such CF-PC synaptic pathology needs to be studied. The molecular mechanisms of CF-PC synaptic wiring have been relatively well-understood and could be useful to establish such model in mice using genetic manipulations of key molecules controlling CF-PC synaptic formation and pruing. For example, Cav2.1, mGluR1, GluRδ2, Cbln1, and DAB2IP142 are all candidate molecules for CF-PC synaptic manipulation in mice.143, 144
In summary, human pathological observations provide important insight into the brain circuitry alterations in tremor; however, animal models will further allow us to investigate how the pathological observations made in postmortem human brains might result in tremor. Animal models of tremor with relevant brain circuitry alterations can be used as a tool to study pathophysiology of tremor and also to screen medications for tremor suppression, which will greatly accelerate the advancement of therapy development for ET.
Shaker rats and SCA2 as potential models to study tremor (Collin J. Anderson, Stefan M. Pulst)
Postural and intention tremor are observed in numerous SCAs, but they are particularly common in SCA2, with intention tremor presenting in nearly all patients that exhibit postural tremor.145, 146 Widespread PC loss has been observed in the cerebella of postmortem human brains in the context of both ET and most forms of SCAs, implicating PC loss in the generation of tremor. SCA2 is inherited in an autosomal dominant fashion and is caused by a CAG repeat expansion of the ATXN2 gene: 33+ repeats, compared to a normal allele, most typically 22 repeats in length.147 The ATXN2 gene encodes a polyglutamine expansion in the ATXN2 protein, leading to toxic gains of function.148, 149 CAG repeat length is a major factor in the age-of-onset variance, with greater expansion tied to earlier symptom onset and faster disease progression.150, 151 After determining that the mouse SCA2 cDNA contains no extended polyglutamine tract,152 we created transgenic153 and BAC-transgenic154 mouse models of SCA2, with Q58, Q72, and Q127 mice demonstrating cellular and motor phenotypes, while mice with wild-type human ATXN2, BAC-Q22, were indistinguishable from control mice.
SCA2 mice feature progressive PC degeneration,153, 154 and changes in PC firing precede their loss.155 PCs spontaneously fire at a fast, regular rate,156-158 but SCA2 mice show marked slowing of PC firing prior to their loss.155, 159, 160 Further, some studies have shown increased irregularity in PCs in SCA2 mice prior to their loss.159, 161 Interestingly, while the silencing of PCs on an acute timescale through optogenetic methods leads to rapid forelimb movements162 – which is unsurprising, given the inhibitory effects of PCs on the dentatothalamocortical pathway – the gradual degradation of PCs seen in SCAs is not typically associated with dyskinetic movements, but ataxia, tremor, and other motor phenotypes are more common. It has been proposed that downstream neurons, such as those in the dentatothalamocortical pathway, compensate for PC loss.163 Still, the specifics of how PC degradation, slowing, or irregularity leads to tremor and ataxia in SCA2 rather than ectopic movements is not fully understood.
In the context of ET, it has been debated whether the disorder is neurodegenerative in nature. There has been a long-standing idea that ET is largely monosymptomatic, stemming from a central oscillator driving tremor, typically thought to be the IO nucleus. However, there is growing acceptance of the idea that ET is a slowly progressing neurodegenerative disorder with a complex set of symptoms centered around cerebellar degeneration.42, 134, 135, 164-166 PC loss in ET has been shown to be more moderate compared to that observed in most SCAs, leading to the idea that ET could represent an intermediate form on a larger spectrum of spinocerebellar degeneration in comparison to SCAs.135 In fact, ET patients have been found to have similar tandem walk deficits consistent with ataxic gait indistinguishable from a cohort of patients with cerebellar disease, the deficits especially pronounced in the group described as being at an “advanced” stage of ET.167
The idea that ET may represent an intermediate form of spinocerebellar degeneration is captured well by the shaker rat, a genetic model of tremor and ataxia. The shaker mutant arose spontaneously in an outbred Sprague Dawley strain, first described as a hereditary model of PC degeneration and cerebellar ataxia in 1992.168 The mutation was later transferred to an inbred Wistar Furth strain segregating in an x-linked recessive fashion. We have characterized the shaker rat in the context of genetic analysis,169 phenotype, and as a model for therapeutic development, having recently described a quantitative analysis of its symptoms and the use of low-frequency deep cerebellar stimulation to reduce these symptoms.170 Shaker rats first present with a full-body tremor (Fig. 3a), evolving to a shaking ataxia (Fig. 3b) as PCs degenerate. With load cell-based center of mass tracking, we can track the progression of tremor, incoordination of gait, and additional motor symptoms and quantify their severity with respect to wild-type animals. Given the strong, quantifiable symptoms, the shaker rat is a good model in which to study the degree of PC loss and its relationship to tremor and other symptoms.
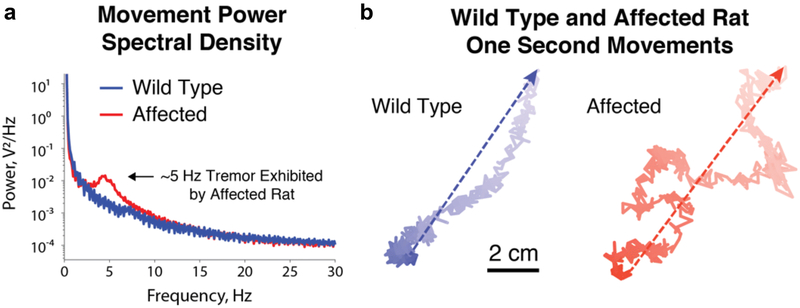
Fig. 3:
a. Power spectral densities of center of mass tracking data for 11-week-old shaker (red) and wild type (blue) Wistar Furth rats, recorded under awake and behaving conditions. Note the substantial peak at 4-5 Hz in the shaker rat, corresponding to a strong, full-body 4-5 Hz tremor. b. Representative example center of mass traces during rapid movements made by 14-week-old wild type (blue) and shaker (red) Wistar Furth rats. Change in color represents change in time, dark to light indicating the passage of one second, while dashed arrows indicate net displacement. Note that while the wild type rat moves in a coordinated fashion, the shaker rat’s movement demonstrates a great deal of incoordination, with truncal sway immediately obvious across the numerous changes in direction over the span of one second.
Using mouse and rat ataxia models allows electrophysiological studies that can determine both correlation and causation in the generation of tremor and other symptoms. Slice recordings have confirmed the slowing of PCs prior to their loss,155 and therapeutic development has confirmed that these firing rates are restored in conjunction with effective treatment of motor symptoms.171 Ongoing work employing in vivo cellular and field recordings is focused on the role of the dentate nucleus in symptom generation in shaker rats under awake and behaving conditions. Thus, we are testing several hypotheses, including that tremor coheres with oscillations in the dentate nucleus and that the progressive disinhibition of the dentate nucleus, observed through progressively increased firing rates corresponds to the progression of ataxia. Further, these metrics will likely generate electrophysiological biomarkers for study as we continue to work on novel therapeutic developments in the shaker rat.
Indeed, coupling rodent models with electrophysiology allows us to test several hypotheses regarding the generation of tremor. The loss of PCs disinhibits the dentatothamocortical pathway, and this appears to allow the propagation of oscillations through the pathway, perhaps continuing in a loop back to the deep cerebellum through pontine connectivity. Several possibilities as to how dentatothalamocortical disinhibition leads to tremor are worth discussing. First, it has been suggested that SCA2 patients may have more consistent thalamic degeneration than patients with other forms of SCAs.172 Included in this degeneration is the ventral lateral thalamus, which relays information to the cortex from the dentate nucleus in the dentatothalamocortical pathway. Interestingly, it has been further shown that pathologically increased thalamic local field potential oscillations at the tremor frequency band cohere with tremor and contribute heavily to tremor generation, at least in the context of parkinsonism.173 Furthermore, deep cerebellar local field potential oscillations cohere strongly with finger acceleration, specifically in the tremor frequency band.174 Thus, one possible explanation for the increased prevalence of tremor in SCA2 compared to other SCAs lies in the possibility that thalamic damage could lead to local field potential changes that propagate through the dentatothalamocortical pathway, contributing to tremor generation upon reaching the cortex. The loss or slowing of PCs, through disinhibition of the dentatothalamocortical pathway, may ultimately exaggerate the propagation of tremor frequency oscillations, leading to the tremor phenotype observed in SCA2. A second possibility for tremor generation borrows from recent ideas:42 with oscillatory activity generated in the IO nucleus, thalamus, DCN, PCs, sensorimotor cortex, and others, it is possible that there is no single central tremor generator. Perhaps all regions within the pathway generate oscillatory activity, but the disinhibition of the pathway through PC loss or altered firing allows for the propagation of these oscillations. However, both of these ideas fail to explain how a partial loss of PCs, as in ET or in the early symptomatic stages of the progression seen in shaker rats, typically leads to tremor, while a more complete loss tends to lead to a more ataxic phenotype. It is possible that the answer lies in the altered firing rather than in the complete loss of PCs. Given a low CV in PC ISI,11 perhaps the pacing of the dentate nucleus at a very specific rate is important for not only for coordination,35 but also for tremor. The observed slowed firing of PCs may mis-pace the DCN in a way that allows for the propagation of a low-frequency oscillation through the dentatothalamocortical pathway, leading to tremor, while their more complete loss does not. Any analyses performed must consider the differences between local field potentials and single unit activity: while cerebellar local field potential oscillations have been found to be phase-locked to PC firing in some contexts,175 it is important to remember that local field potentials do not necessarily predict spiking activity.176 However, if decreased inhibition from PCs in the form of slower firing rates still enable oscillatory propagation through the cerebellothalamocortical pathway, this will be a measuable change.
Beyond furthering our understanding of disease, the use of animal models has already contributed to the development of novel therapeutic strategies for ataxia and tremor. We generated and characterized a SCA2 knockout mice, determining that ATXN2 is not necessary in development or adulthood,36 ultimately leading to the use of an antisense oligonucleotide to downregulate ATXN2 mRNA and protein, delaying SCA2 phenotype onset and improving the motor phenotype of BAC-Q72 and BAC-Q127 SCA2 mice.31 Finally, we used the shaker rat to demonstrate low frequency deep brain stimulation in the deep cerebellar nuclei as a potential new therapeutic strategy ro reduce tremor and ataxia.170 Further work is ongoing, and rodent models will continue to play a major role in therapeutic development, both in antisense oligonucleotides and other treatment strategies.
GABAergic signaling and ET: Insight from epilepsy models (Martin J. Gallagher)
Because GABAA receptors (GABAAR) are the predominant ligand-gated inhibitory neurotransmitter receptors in mammalian brains,177 one might expect that altered GABAA-mediated neurotransmission contributes to hyperkinetic, hyper-oscillatory movement disorders such as ET. This idea is supported by the observations that ET symptoms are improved by GABAAR positive modulators,178 that GABAAR α1 subunit knockout mice exhibit ET-like tremor, and that GABAAR expression is altered within elements in the cortico-olivo-cerebello-thalamic circuitry in ET patients. Here, we will review the latter two results and draw parallels to findings observed in epilepsy models.
Two GABAAR α1 subunit (α-/-) knockout mice exhibit handling-induced tremor.179-181 Extensive phenotypic characterization of one of these α1−/− mice181 revealed a limb tremor present during ambulation and suspension that ceases during rest. Similar to human ET, the α−/− mouse tremor oscillates at a lower frequency and with a higher amplitude than the normal mouse physiological tremor. Also consistent with human ET, the α−/− mouse action tremor is substantially reduced by ethanol, primidone, propranolol, and gabapentin.181
Despite the face validity of the α1−/− model, genetic studies did not find any GABAAR subunit variation associated with human ET.128, 182, 183 Now, with results from further experiments using α1 subunit deficient mice and from genetic studies of epilepsy patients, we do not expect genetic disruption of GABAAR subunits to cause ET. Although the homozygous α−/− mice were initially reported to be viable and fertile,179, 180 breeding them from their mixed background into congenic lines and performing continuous electroencephalogram (EEG) monitoring revealed that mouse strain markedly affects homozygous α1−/− and heterozygous α1+/− mouse viability and that heterozygous mice experience absence and myoclonic seizures.184, 185 In addition, human genetic studies found a number of heterozygous loss-of-function α1 subunit mutations associated with moderate and severe forms of epilepsy.186-189 Given that heterozygous α1+/− mice did not have the tremor phenotype181 and thus presumably need a large reduction in GABAA signaling to produce the phenotype, it would be very unlikely that a patient would have a sufficiently large genetic disruption of GABAAR subunit function to cause tremor without also producing other severe neurological sequela that would prevent the diagnosis of ET.
Although widespread genetic disruption of the α1 subunit is unlikely to produce ET in humans, selective alteration of α1 subunit-containing GABAARs restricted to one or more elements of the cortico-olivo-cerebello-thalamic circuitry could potentially produce the tremor without reducing viability or producing seizures. In fact, [11C]flumazenil PET and [3H]flunitrazepam autoradiography studies did suggest that GABAAR expression is altered in the ET network. However, while the PET study found increased [11C]flumazenil signal in the dentate nuclei and other key elements of the ET circuitry, the autoradiography experiments showed decreased benzodiazepine binding within the dentate nuclei. Although, it is beyond the scope of this consensus to explore the myriad of possible reasons for these seemingly-discordant findings, we will briefly mention three of them to highlight the mechanisms by which GABAA signaling has been found to be altered in pathological circuits (in particular, epilepsy circuits) and could potentially appear differently in autoradiography and PET experiments. First, because fixed tissue used in autoradiography experiments allows greater access of a radioligand to intracellular receptors than intact tissue studied with in vivo PET, the two techniques will likely have different sensitivities for total and surface-expressed GABAAR. This underscores the observation that neurological diseases may be associated with changes GABAAR cell surface trafficking/endocytosis/recycling that alter the intracellular/surface distribution of receptors.190, 191 Second, endogenous GABA present in the in vivo PET experiments, but not the ex vivo autoradiography experiments, affects benzodiazepine/flumazenil binding.192 This highlights another possible pathological component in GABAA signaling, dysfunction of the presynaptic GABAergic neurons.193-195 Although recent magnetic resonance spectroscopy studies did not detect altered GABA concentrations in the dentate nuclei of ET patients,196 it is possible that degenerating GABAergic PCs133 alter the GABA concentration/release frequency at the presynaptic terminals. Finally, the PET experiment detected signal from a benzodiazepine antagonist ([11C]flumazenil) while the autoradiography experiment used a benzodiazepine ([3H]flunitrazepam). GABAARs composed of different subunits/subunit isoforms have unique physiological properties as well as different affinities for benzodiazepines and different relative affinities for benzodiazepine agonists and antagonists.177, 197-199 Alterations in GABAAR subunit/subunit isoform composition have been identified in key regions of epilepsy networks and have resulted in modified GABAA transmission.190, 200-204
Although genomic-wide loss-of-function mutations of GABAAR subunits are unlikely to cause ET, pharmacological and biochemical studies as well as the face validity of the α−/− knockout mice suggest a role for altered GABAA signaling in this disease. Similar to investigations being conducted in epilepsy networks, future experiments that target α1 deletion and rescue to specific areas of the cortico-olivo-cerebello-thalamic circuitry and that measure surface and synaptic GABAAR expression and composition as well as the presynaptic function within these regions will identify mechanisms by which GABAA transmission is disrupted. Importantly, causally manipulating inhibitory transmission in these regions will elucidate the basic mechanisms that initiate and sustain tremor oscillations and can thus be therapeutically targeted.
A clinical hypothesis for HCN channels in ET (Kyle A. Lyman, Dane Chetkovich)
Despite the prevalence of ET,205 relatively little is known about its pathogenesis which has limited the development of new therapeutics. One strategy for developing new hypotheses for the disease is to consider the mechanism of propranolol, a nonspecific β-blocker that readily crosses the blood-brain barrier and is currently used as a first line treatment for ET. Outside the central nervous system, propranolol is used to slow the heart rate through its effect on hyperpolarization-activated cyclic nucleotide-gated (HCN) channels in cardiac tissue. In this consensus, we discuss the clinical, theoretical, and empirical evidence suggesting a role for HCN channels in the pathophysiology of ET.
HCN channels are nonspecific cation channels in the voltage-gated potassium channel superfamily.206 These non-inactivating channels open in response to hyperpolarization and contribute a depolarizing influence because their reversal potential lies between that of Na+ and K+ (Erev ~ −30mV).207 Four genes encode HCN subunits (Hcn1-4) and the properties of heterotetrameric complexes found in vivo depend on the subunits involved, although HCN1 and HCN2 predominate in the mammalian central nervous system.208 These two isoforms are distinguished by differences in their activation kinetics and response to second messengers, but both isoforms are bound by auxiliary subunits such as TRIP8b that further alter their electrophysiologic properties.209-213 Collectively, the current mediated by HCN channels is referred to as ‘Ih’ (alternatively: ‘If’, or ‘Iq’) given that it is not possible to distinguish the molecular isoforms electrophysiologically.
The biophysical properties of HCN channels make them a natural candidate for the production of cellular rhythms in the heart and CNS.207, 214, 215 The IO provides a relevant example of this motif, where Ih interacts with a low-threshold voltage-gated calcium current (IT), and voltage-gated sodium and potassium currents (INa and IK). Ih initiates the cycle by bringing the membrane potential to the threshold for IT activation (Fig. 4A). IT further depolarizes the cell (deactivating Ih) and allows for a burst of action potentials until IK hyperpolarizes the cell and the cycle begins again.
Fig. 4:
The role of Ih in autonomous pacemaking. a. Recording from IO neuron showing a role for Ih in pacemaking, reproduced from Bal and McCormick 1997.225 b. Schematic showing noradrenergic signaling through β2-receptors leading to an increase in cAMP. c. Channel opening probability as a function of voltage. Note that in the presence of cAMP (in blue) the curve depolarizes so that more channels are open at a given voltage while less cAMP (in red) has the opposite effect. d. The effect of cAMP on the cardiac action potential in a sinoatrial node cell. cAMP mediated agonization of HCN channels increases the firing rate. Color scheme adopted in panel c corresponds to the conditions in panel d. Panels b-d based on Fig. 4 from DiFrancesco and Borer 2008,215 reprinted with modifications and permission from Springer.
In addition to their regulation by voltage,216 HCN channels are also influenced by the intracellular second messengers cAMP and cGMP (Fig. 4B). Binding of cyclic nucleotides to the intracellular cyclic-nucleotide binding-domain leads to more rapid channel opening and augments Ih (Fig. 4C). Increasing the intracellular cyclic nucleotide concentration increases the firing rate of the cell as Ih more rapidly brings the cell to threshold potential (Fig. 4D). Clinically, this effect is responsible for the increase in heart rate brought on by adrenergic signaling in the heart and for the bradycardic effect of β-blockers like propranolol.
Clinical evidence raises the possibility that augmenting HCN channel function leads to the production of tremors while inhibiting Ih limits them. Sympathomimetic compounds (ie salmeterol, cocaine, isoproterenol, etc) that increase adrenergic signaling and augment intracellular cAMP (and increase Ih), as well as drugs that directly stimulate cAMP signaling (ie caffeine) all lead to an increase in tremor.217, 218 Conversely, β-adrenergic antagonists like propranolol are first line therapies for ET. Work to identify other β-blockers with utility in treating ET has revealed that antagonizing β2-signaling is crucial for efficacy.219 Some studies have suggested a peripheral mechanism of action for β-blockers based on the efficacy of poorly lipid-soluble compounds, but it is generally thought that all β-blockers penetrate the central nervous system to some degree.220, 221 Within the CNS, β2-signaling is canonically linked to the production of cAMP, including in the thalamus, brainstem, and cerebellum.222, 223 These observations all suggest that increasing HCN function will promote ET, but the ubiquity of HCN channel expression in the brain makes it unclear where precisely this may occur.
Much of our understanding of the role of the IO in ET is based on the harmaline model (reviewed above in this manuscript). A compelling account of ET based on this model has focused on the role of synchronization within the IO.8, 89 The IO sends glutamatergic CFs that synapse onto PCs of the cerebellar cortex, and synchrony within the gap-junction coupled neurons of the IO produces synchrony in PCs.73, 74, 224 HCN1-3 are expressed by cells of the IO,208 and the interaction between Ih and IT is a key determinant of synchronized oscillations and autonomous pacemaking in these cells (Fig.4 A).225, 226 Knockdown of IT within the IO prevents the emergence of pathological 4-10Hz oscillations in the IO as well as tremor in response to harmaline administration.36 These results suggest that manipulations that interrupt oscillatory behavior within the IO may be sufficient to prevent ET. Recent work on the role of Ih in the physiology of the IO has also suggested an important role for the conductance in mediating resonance to synaptic inputs.227 This observation raises the possibility that inhibiting Ih may alter the response of the IO to pathologically synchronous inputs, again with the effect of limiting ET. Interestingly, harmaline administration has been shown to increase cGMP signaling within the cerebellar cortex raising the possibility that it may directly augment HCN channel function and lead to aberrant synchronization.228
In addition to the IO, the thalamus also plays an important role in ET, highlighted by its use as a target for neurosurgical intervention to control pharmacotherapy resistant ET.229 HCN channels play a key role in generating both autonomous activity and network level oscillations between reciprocally connected thalamic nuclei, hence antagonism of thalamic Ih may also limit ET.210, 226, 230
To date, there has been little evidence directly linking HCN channels to ET with the exception of a series of studies performed in rats by Kuramoto and colleagues.231, 232 They established that a loss of function mutation in Hcn1 produced a tremulous phenotype in the context of white matter abnormalities,232 which are not thought to occur in ET. Given the differences in pathology, it is unlikely that the model of Kuramoto et al will directly enhance our understanding of ET. In order to link Ih to the mechanism of ET, it will be necessary to experimentally block HCN channel function in adult animals in a cell type specific manner in an animal model of ET (ie through use of 4-hydroxytamoxifen dependent cre recombinase technology). This approach would circumvent the severe developmental consequences (seen in mice and humans233) of global loss of HCN channel function while permitting an examination of whether or not antagonizing HCN is sufficient to disrupt the pathological synchrony of the relevant cell types.
ET is a complex phenomenon that likely represents several pathological mechanisms with a convergent clinical phenotype.234 We speculate that the therapeutic utility of propranolol in treating ET is that it blocks activation of β2-receptors within the cerebellum and thalamus, limiting the production of cAMP and thus inhibiting HCN channels. This reduction in Ih may in turn limit the pathological synchrony that occurs at several nodes of the motor network and interrupt the pathogenesis of ET.8, 89 Refining our understanding of the role of HCN channels in these circuits may ultimately lead to better treatments for ET.
ET channelopathies: The role of ion channel mutations (Lorraine Clark)
Emerging evidence suggests that ET is a disease or a family of diseases in which the cerebellum plays an important, if not a central role, because there are changes in the PC population and altered (i.e. reduced) brain GABA tone. However, this is far from established. Genetic factors are thought to contribute significantly to disease etiology.235 However, the presence of substantial phenotypic and genotypic heterogeneity has hindered gene identification.236 Recent whole exome and whole genome sequencing studies in ET families have identified a number of interesting candidate genes that may play a role in the pathophysiology of ET. The identification of mutations in the voltage gated potassium (K+) channel genes KCNS2 (Kv9.2)127 and more recently in the T type calcium channel gene CACNA1G (Cav3.1) in two ET families237 (discussed in more detail below) in addition to mutations in SCN4A238 and SCN11A239 in ET families, suggests problems in the regulation of membrane excitability and synaptic transmission, which are functions important more broadly for motor control and other neuronal network functions. These channels may also have additional cellular functions distinct from the regulation of excitability that may contribute to the ET phenotype. The identification of rare monogenic forms of ET, coupled with functional studies and the generation of animal models, will continue to provide important clues about gene pathways impacted in ET.
We previously reported the identification of a mutation in the KCNS2 gene (Kv9.2, c.1137 T>A, p.D379E), encoding an electrically silent voltage-gated K+ channel α-subunit, Kv9.2, in a single family with early-onset ET.240 The KCNS2 mutation cosegregated with ET in this family and was present in all four affected individuals and absent in an unaffected family member. KCNS2 encodes for an electrically silent voltage-gated K+ channel α-subunit, Kv9.2, that is highly and selectively expressed in the brain and modulates the activity of the Kv2.1 and Kv2.2 channels by heteromulterization. Kv2.1 and Kv2.2 are also highly expressed in mammalian brain neurons and cluster on cell bodies, proximal dendrites and the axon initial segment.241 Kv2.1 is responsible for the majority of delayed rectifier current in many neurons. A similar localization of expression of Kv9.2 with Kv2.1 and Kv2.2 has been observed in the PCs and granule cells of the mouse cerebellum.242
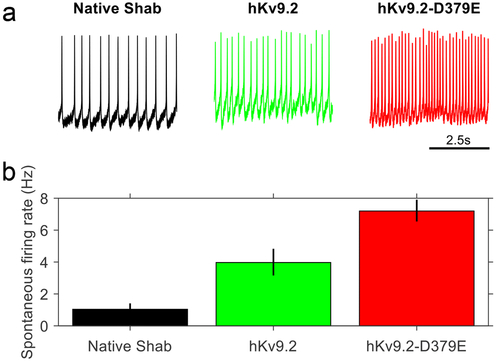
To determine how the mutation that we identified in Kv9.2 (p.D379E) could lead to an ET phenotype, we created a model of ET by expressing the human Kv9.2 channel subunit in Drosophila.243 In the KCNS2-associated ET Drosophila model, we show that the hKv9.2 subunit can modulate the endogenous Shab (Kv2 subfamily) channel activity. Behavioral manifestations of nervous system dysfunction consistent with a hyperexcitable phenotype were observed in flies expressing either the hKv9.2 or hKv9.2-D379E subunit compared to control flies and included significantly faster climbing, significantly reduced lifespan (post-developmentally), wing posture and motility deficits (post developmentally) and abnormal leg shaking under ether anesthetization.243 Through studying the effects of hKv9.2 and hKv9.2-D379E on CNS neuronal activity, we showed that the mutant hKv9.2-D379E subunit had significantly higher levels of inactivation (Fig. 5) than the wild-type hKv9.2 subunit and a significantly higher spontaneous firing rate of action potentials consistent with neuronal hyperexcitibility (Fig. 6). A biophysical model of the electrical activity of the cell, combining the individual ionic currents in flies expressing hKv9.2 subunits, was in agreement with the experimental data. In addition to locomotor and electrophysiology defects, characterization of circadian behavior in flies expressing hKv9.2 and hKv9.2-D379E channels showed that while rhythmicity was unaffected, significant differences were observed for night time activity and night time sleep, also consistent with neuronal hyperexcitability.243
Fig. 5:
a. Examples of the current evoked from Drosophila Shab when depolarized from −133mV to between −93mV and −3mV in flies expressing only native Shab (left panel), native Shab with the human Kv9.2 (middle panel), or native Shab with hKv9.2-D379E (right panel). The non-inactivating Shab becomes inactivating in the presence of either hKv9.2 subunit. b. Diagram of the voltage step protocol used. c. The I-V relationships for Shab determined from the three given genotypes. The peak current (left panel, n = 3) shows that expression of either Kv9.2 subunit causes a shift in activation to more negative voltages. The sustained current (middle panel, n = 3) shows that, at similar voltages, flies expressing either hKv9.2 subunit show reduced Shab current after 200ms of depolarization. When the sustained current after 200ms of depolarization to −3mV is expressed as a percentage of the peak current (right panel, n = 3) the flies expressing only native Shab show high percentages, indicating very low levels of inactivation. When tested by two-way ANOVA, flies expressing either the wild-type hKv9.2 subunit (p < 0.001) or the ET mutant hKv9.2-D379E subunit (p < 0.001) were significantly different to flies expressing only native Shab. The mutant hKv9.2-D379E subunit shows much higher levels of inactivation than the wild-type hKv9.2 subunit (p=0.0462).
Fig. 6:
a. Representative example of 5 seconds of spontaneous activity in flies between ZT1 and ZT7 (where ZT0 is lights-on and ZT12 is lights-off) expressing only native Shab (left panel), native Shab with hKv9.2 (middle panel), or native Shab with hKv9.2-D379E (right panel). b. The spontaneous firing rate of action potentials in flies expressing either hKv9.2 subunit (data are mean ± S.D., n = 3) are significantly different with the mutant subunit having a higher frequency (t(4) = 5.3806, p = 0.01).
While there are limitations to the Drosophila model that we have developed due to the lack of a clear Drosophila ortholog for Kv9.2, our data is consistent with previous studies that have demonstrated that Kv9.2 modulates the activity of Kv2 channels2 (such as Drosophila Shab), as well as that have observed significant differences in the phenotype (inactivation and spontaneous firing rate) between flies expressing the wild-type and mutant hKv9.2 channels.
Moreover, the T-type calcium channel, Cav3, has been implicated in neuronal autorhythmicity244, 245 and is thought to underlie tremors seen in PD,246 enhanced physiological tremor, and in ET.8 T-type calcium channel antagonists have been shown to reduce tremor in mouse models of ET.40, 247 In a whole genome sequencing study in eight families with early onset ET, we identified one family with a non-synonymous variant in CACNA1G (c.1367G>A (NM_018896.4), p.(Arg456Gln)) that co-segregated with ET and that was predicted to be deleterious or damaging by several in silico tools. Retrospective analysis of a whole exome sequencing dataset from a prior published analysis identified a second family with a non-synonymous variant in CACNA1G (c.3635G>A (NM_018896.4), p.(Arg1235Gln)), rs150972562) that is highly conserved evolutionarily and is predicted to be deleterious or damaging by several in silico tools and that co-segregated with ET (Fig. 7).
Fig. 7:
The variants (p.Arg456Gln and p.Arg1235Gln) identified in the Cav3.1 channel in ET families are indicated (Figure adapted from Huc et al., Biochimica et Biophysica Acta 2009; 1793:947-952).
Electrophysiology studies by whole cell patch clamp recordings were performed in HEK293 cells expressing the Cav3.1 p.Arg1235Gln mutant channel (unpublished data). Significant differences in the gating of the mutant Cav3.1 channel were observed compared to the wild-type channel. Although the effects on activation and inactivation kinetics were subtle, a shift of steady state inactivation towards more positive voltages (+3.4mV) was observed for the mutant Cav3.1 channel. A shift of activation toward positive voltages has also been reported for the Cav3.1 p.Arg1715His mutation identified in a family with SCA42.248 Computer simulation modeling for the Cav3.1 p.Arg1235Gln mutant channel (unpublished data) or Cav3.1 p.Arg1715His mutation248 suggests that both mutations result in decreased neuronal excitability. In contrast, epilepsy-associated T-type channel variants are classically associated with a gain of function through faster activation, a negative shift of steady state activation, and inactivation properties or increased protein amounts.249, 250 In addition to a role in neuronal excitability, recent studies suggest that Cav3 subunits of T channels are modulated by endogenous ligands such as anandamide, zinc, redox and oxidizing agents, as well as G-protein and protein kinase pathways.251
In summary, many neurological disorders are associated with altered activity, function or expression of ion channels and mutations in these channels can cause epilepsy or cerebellar dysfunction including motor deficits, tremor, dystonia and ataxia.252 Currently, it is not clear why mutations in specific channels that result in increased excitability and epileptic seizures are not associated with neurodegeneration and why different mutations in the same channel can lead to different disease phenotypes (e.g. ataxia, dystonia or tremor).253 These observations suggest that many ion channels may have additional cellular functions distinct from neuronal excitability253 and may modulate cell signaling and apoptotic pathways, which in ET may be accompanied by changes in the PC population and altered (i.e. reduced) brain GABA tone.
Furthermore, ET may represent a family of disorders of neurological channelopathies, with mutations identified in a voltage-gated K+ channel α subunit in a family with pure ET,240 in voltage-gated sodium channel α subunits in a family with epilepsy and ET (SCN4A)254 and a family with familial episodic pain and ET (SCN11A),239 and in the T-type Ca2+ channel (CACNA1G) in two families with pure ET. Nonetheless, the genetic basis for ET remains incomplete. Given the clinical and genetic heterogeneity observed in ET, further evaluation of ion channels as candidate genes for ET is warranted.
Drosophila model of FUS in ET: Challenges and limitations (Murni Tio and Eng-King Tan)
Despite strong genetic links, few pathogenic mutations have been identified in ET and there is a lack of in vivo genetic ET models. Due to high functional conservations in genes, genetic pathways, and neurological properties between Drosophila and humans, the fruit flies have been widely used to model human neurodegenerative diseases.255 The use of the GAL4/UAS system,256 which specifically targets the expression of human diseased genes to particular tissues and organs of interest, has successfully unravelled many mechanisms linked to those diseases.257
The FUS-Q290X is a nonsense mutation resulting in the truncation of the FUS protein and had been found to segregate in a large Canadian family with ET.258 We recently developed a Drosophila model by targeting the expression of FUS-Q290X in the dopaminergic and serotonergic neurons and characterized its phenotype. We studied the involvement of the GABAergic and serotonergic systems and the IIS/TOR signaling pathway, and we conducted a therapeutic challenge with gabapentin.
By targeting the expression of hFUS-Q290X in the dopaminergic and the serotonergic systems in Drosophila, we found that these flies exhibited progressive locomotor dysfunction, although in the absence of obvious neuronal degeneration. It is to be noted that unlike in PD, whereby the dopaminergic neurons are found to be degenerated, neuronal degeneration of neurons is not obvious in ET. Rather, neuropathological evidence mostly pointed to abnormalities in the cerebellum and the GABAergic PCs. Such abnormalities have been postulated to result in aberrant cerebellar cortical circuits and outputs and hence the observed tremor characteristics.9 In concordance with the observation of decreased GABA-R levels in postmortem samples from ET individuals,70 the hFUS-Q290X expressing flies also had impairment of the GABAergic system as shown by downregulation of the neurotransmitters GABA-R and N-methyl-D-aspartate (NMDA)-R levels. The impairment of the GABAergic system was shown to be associated with the observed locomotor impairment, since gabapentin (a drug currently used in the clinic to reduce symptoms in ET patients) was able to rescue the locomotion phenotype caused by overexpression of hFUS-Q290X in Drosophila. Interestingly, expression of hFUS-Q290X in Drosophila also resulted in prolonged lifespan, a phenomenon that has been associated with patients having early onset ET, as is in the case of the ET individuals carrying hFUS-Q290X mutation. We further showed that the increased longevity in the Drosophila was accompanied by a downregulation of the IIS/TOR signaling pathway, which has been strongly associated with longevity in humans.259 Serotonin, which is known for its roles in controlling the GABAergic and the insulin pathways, was also found to be significantly increased.
We demonstrated that our in vivo model of Drosophila expressing hFUS-Q290X could recapitulate some characteristics seen in patients with ET, such as increased motor dysfunction with age, but without obvious neuronal loss. Our model also shows increased lifespan, as has been reported to be associated with ET patients having early onset ET. These phenotypes were accompanied by molecular changes such as reduced expression of GABA-R and NMDA-R subunits, a downregulation of the IIS/TOR signaling pathway, and an increase in serotonin levels. The drug gabapentin was also able to improve the locomotion phenotype in our model. Therefore, the transgenic Drosophila model has shed some mechanistic insights on ET pathogenesis, which will provide impetus for further research on target identification and potential new therapeutics.
Although approximately 75% of human disease genes have functional orthologs in Drosophila,260 its use as a model organism has certain limitations. One major limitation is that there are significant anatomical differences between human and fly brains. For example, there is no known equivalent of PCs or cerebellum in the fly brain, making it impossible to link the cerebellar impairment to the motor phenotype seen in ET patients. There are also technical limitations in regards to good assays for analyzing tremor in flies as the methods are still currently unavailable and locomotion in the adult flies can only be monitored using negative geotaxis/climbing assay. In addition, ET is also known to be a clinically heterogeneous disorder with many non-motor (i.e. cognitive, psychiatric, dementia and sensory) impairments.58 As such, multiple systems are likely to be affected and the lack of or difference in certain cellular and molecular processes in flies may make understanding these other aspects of the disease difficult. Future studies to better quantitate tremor or the development of an automated system to evaluate tremor patterns in flies can further enhance the utility of this model in ET.
Primate model of action tremor (Rodger J. Elble)
Gordon Holmes described the tremor caused by injuries to the cerebellum in humans as “sometimes apparent during the whole range of movement” but “is usually more prominent towards its end.”261 He also observed that “the irregular oscillations in the intended direction result from failure of uniform deceleration, but this is complicated by secondary or correcting jerks when the object has not been accurately reached in the first attempt.”261 These seminal observations illustrate that humans with cerebellar action tremor have impaired feedforward motor control, causing a “failure of uniform deceleration” and an excessive reliance on feedback motor control, producing multiple “secondary or correcting jerks when the object has not been accurately reached in the first attempt”.261
The observations by Holmes have been confirmed and elucidated by subsequent studies in monkeys. Action tremor in the ipsilateral upper extremity occurs in monkeys with lesions in the DCN or in the outflow tracts of these nuclei in the brachium conjunctivum (superior cerebellar peduncle). This tremor is often referred to as cerebellar outflow tremor or intention tremor. Bilateral lesions in the superior cerebellar peduncles produce bilateral extremity tremor and irregular tremor of the head and trunk (titubation). These tremors occur immediately following the lesion(s) and therefore result from the loss of cerebellar influence in motor control.262
Studies of stereotactic cooling, destructive lesions, and muscimol injections in the cerebellar nuclei revealed that impairment of interpositus263 or both dentate and interpositus264-267 is necessary to produce a sustained tremor. Inactivation of dentate alone is probably not sufficient to produce tremor268 nor are lesions in cerebellar cortex.263, 267, 268 Tremor-related activity has been recorded from interpositus, but not from dentate,269 which receives no somatosensory feedback.270, 271 Tremor frequency is influenced by reflex arc length, joint stiffness and inertia,264, 266, 269, 272 and tremor-related neuronal activity has been recorded from sensorimotor cortex, the interpositus nucleus and somatosensory afferents.269 These results are consistent with the notion that cerebellar tremor is an abnormal mechanical-reflex oscillation that emerges from sensorimotor loops involving the cerebral cortex and the cerebellum. This mechanical-reflex tremor differs from ET, which has a frequency that is independent of reflex arc length, joint stiffness and inertia.273
Sudden joint perturbations (e.g., rapid passive elbow flexion) produce a normal stretch-reflex response in the stretched (agonist) muscle(s), but the subsequent burst of antagonist muscle activity is abnormally sized and delayed in monkeys with inactivated interpositus and dentate nuclei.272 Similar patterns of activation occur in agonist and antagonist neurons of motor cortex.274 Muscle spindle sensitivity is essentially normal.275 The delayed antagonist activation results in target overshoot and terminal oscillation. Delayed antagonist activation has been observed in patients with ET, and thalamic deep brain stimulation suppresses tremor without correcting the antagonist delay.276 In fact, excessive stimulation may increase the delay276 and produce cerebellar dysmetria.277
Tremor-related interpositus bursts in a rhesus monkey with nuclear damage occurred an average 12.5 milliseconds after the agonist EMG bursts.269 Therefore, the interpositus bursts occurred too late to drive the agonist muscle. Agonist pyramidal tract neurons in the cat are inhibited by the interpositus-ventrolateral thalamocortical pathway, but antagonist pyramidal tract neurons are either excited or excited and then inhibited.278 Thus, the timing of interpositus activity is consistent with feedforward facilitation of the antagonist muscles, using inputs (efferent copy) from agonist neurons in motor cortex. Cerebellar outflow lesions produce a loss of feedforward control, resulting in an excessive reliance on feedback control that produces abnormal mechanical-reflex oscillation in long-loop sensorimotor pathways (Fig. 8). The timing of cortical activity in cerebellar tremor is consistent with this hypothesis.274
Fig. 8:
Schematic diagram of the principal pathways involved in cerebellar outflow tremor. Tremor is initiated by cortical activation of the agonist muscles. The descending cortical command is also transmitted to the interpositus (IP) and associated spinocerebellar cortex via the pontine nuclei. IP nucleus projects back to the cerebral cortex via the ventrolateral thalamus. This corticobulbocerebellothalamocortical loop ② is an important avenue for feedforward motor control. Absent feedforward control, the nervous system relies excessively of feedback control ① based on sensory feedback from the musculoskeletal system. Interpositus and spinothalamic fibers have overlapping projections to the ventrolateral thalamus (VL).
Ventrolateral thalamotomy reduces cerebellar outflow tremor.279 The ventrolateral thalamic nucleus receives input from dentate, interpositus and spinothalamic pathway.280, 281 Therefore, thalamotomy could interrupt oscillation in the transcortical stretch reflex loop (loop ① in Fig. 8), the cerebellothalamic pathway, and reverberating thalamocortical loops.281 The corticobulbocerebellothalamocortical loop (loop ② in Fig. 8) is involved in virtually all forms of tremor in humans.282 However, the precise role of this loop in each form of tremor is unknown.
The Guillain-Mollaret triangle probably plays little or no role in cerebellar outflow tremor. This triangle consists of the dentate nucleus, the contralateral parvocellular red nucleus, the contralateral IO and their interconnecting fiber tracts.283 If this triangle played a role in cerebellar outflow tremor, one would expect to find tremor-related oscillation in dentate and olivary CF activity, and neither was found in the studies by Thach and coworkers.269, 284, 285 Lesions in the parvocellular red nucleus do not cause tremor,286 and as previously discussed, it unlikely that isolated lesions in dentate cause cerebellar outflow tremor.
Interpositus projects to the magnocellular red nucleus, which projects to the spinal cord in monkeys.283 However, this pathway appears to be vestigial in man,287, 288 and lesions in bulbospinal pathways do not suppress cerebellar outflow tremor in monkeys.289 The corticospinal tract is the principal descending pathway in cerebellar action tremor.289
In summary, cerebellar action tremor in the monkey is produced by impairment of the cerebellothalamic pathway. Action tremor is initiated by corticospinal activation of the agonist muscles. An efferent copy of this descending neuronal activity is sent to the cerebellum via corticobulbocerebellar pathways. This efferent copy is used by interpositus to direct the activation of antagonist muscles via the cerebello-thalamo-cortical pathway so as to decelerate the limb precisely at the desired target. This activation of the antagonist muscles is done in anticipation of the desired movement, based on prior motor learning, a process known as feedforward motor control. Impairment of the cerebello-thalamo-cortical pathway leads to a delay in antagonist muscle activation, resulting in dysmetria. Lacking feedforward control, the motor system relies excessively on feedback control, which is not sufficient to prevent transcortical and transcerebellar mechanical-reflex oscillation of the limb (i.e., action tremor). Lesions in the ventrolateral thalamus disrupt these long-loop mechanical-reflex oscillations and may also reduce tremor by preventing resonance in the excitatory reciprocal connections between sensorimotor cortex and ventrolateral thalamus.
There has been a dearth of tremor research in nonhuman primates since 2003. It is clear that cerebellar outflow lesions produce intention tremor. However, it is unknown whether the cerebellum normally functions to suppress or resist tremorogenic oscillations generated outside the cerebellum.285 The IO and DCN do not oscillate under normal circumstances in monkeys,290-292 and olivary discharges does not drive normal or abnormal mechanical-reflelx tremor in monkeys.285 However, monkey studies by Thach and coworkers suggest that one role of the cerebellum is to limit normal mechanical-reflex oscillation that is potentially tremorogenic.285
Consensus summary
Animal models present a unique opportunity for us to understand the pathophysiology and patho-mechanism of tremor. We have 12 groups of experts contributing opinions to this consensus paper, which collectively provide a comprehensive overview of the field and future directions.
The animal models of tremor can be divided by the animals used: rats (Eric Lang, Stefan Pulst, Dane Chetkovich), mice (Roy Sillitoe, Ming-Kai Pan, Sheng-Han Kuo, Martin Gallagher), flies (Lorraine Clark, Eng-King Tan), pigs (Su-youne Chang), and monkeys (Rodger Elble). Each animal species presents its own unique strengths and weaknesses to understand tremor (Fig. 9). For example, rats and monkeys are very useful to understand the abnormal cerebellar circuitry that generates tremor, whereas genetic manipulations in mice and flies can help to advance our knowledge of the genetic functions, anatomical alterations, and physiological changes that underlie tremor. Flies do not have defined olivocerebellar circuitry but are very useful to study overall neuronal hyperexcitability and the molecular consequences of genetic mutations (Lorraine Clark, Eng-King Tan). On the other hand, mouse models provide an important platform to study the effects of genetics on the cerebellar circuitry that drives tremor (Roy Sillitoe, Ming-Kai Pan, Sheng-Han Kuo, Stefan Pulst). However, flies can be used for large-scaled screening while mice are more suitable for detailed mechanistic studies.
Fig 9:
Schematic diagram about the relative utility of animal models for tremor research.
Another important point of consensus is that animal models can be used to study how brain circuitry alterations drive tremor or to elucidate the underlying disease patho-mechanism that causes ET and other tremor disorders. For example, several animal models help us to understand the brain circuitry that underlies different types of tremor. Abnormal zonal organization within the cerebellum and abnormal PC firing (Roy Sillitoe), abnormal PC synaptic organization (Ming-Kai Pan, Sheng-Han Kuo), and PC degenerative processes (Stefan Pulst) are all alterations made in the brain that may contribute to the pathogenesis of tremor and that might be modeled in animals. Despite the important roles these animal models play in our understanding of tremor, there is no direct evidence that Car8 or Shaker mutations can cause ET. Rather, these genetic mutations cause abnormal wiring of circuitry that generates tremor and in some cases, ataxia too. In such instances, these animal models, whose tremor is co-morbid with ataxia, can be used to study the interactions between tremor and ataxia, two symptoms that can come from a dysfunctional cerebellum. Aside from studying alterations in brain circuitry, genetic mutations identified in human tremor patients, such as FUS, KCNS2, and CACNA1G, can be introduced into animal models to understand disease patho-mechanisms (Lorraine Clark, Eng-King Tan). These genetic mutations might not be directly related to tremor but to other neurological symptoms since the generated animal models can exhibit some other non-motor symptoms that have also been identified in ET patients.293 Regardless, each animal model will be useful to the future development of either symptomatic therapy for tremor by understanding the brain circuitry or disease modifying therapy by advancing the knowledge of disease patho-mechanisms.
The field is in agreement that tremor is one of the most measurable movement disorders in both humans and animal models with a distinct phase, amplitude, and frequency. Because of these unique features of tremor as a movement disorder, tremor also can provide an opportunity to correlate real-time neuronal activities with movements in animal models. However, while mice and rats have conserved olivocerebellar circuitry, similar to humans, the tremor in these rodent models does not necessarily share the same frequency as human tremor. For example, harmaline can induce a different tremor frequency in mice than in rats (mice: 10-16Hz, rats: 8-12Hz), which might be due to the difference in size of the olivocerebellar circuitry. Alternatively, other circuitry dynamics might differ among species. Note that individuals intoxicated with P. harmala, which contains harmaline, could also have action tremor, but the frequency of tremor was not reported.294 Human ET is around 4-12Hz.295 Therefore, the circuitry control mechanisms that are common and differ between species and their influence on determining the frequency (range) of tremor will require further study.
ET is likely a group of heterogeneous disorders. Different brain circuitry alterations have been proposed in ET patients with different ages of onset and different pharmacological responses to alcohol or propranolol (i.e. only half of ET patients respond).124, 125 Individuals with ET can often have dystonic features or co-existing parkinsonism.1, 296 Therefore, it is very unlikely that studying a single animal model will reveal the complete picture of tremor disorders. It is more likely that each animal model we study will represent a subset of tremor patients and therefore, will advance our understanding of the heterogeneity of tremor disorders. In addition, investigators will also need to pay special attention to how results obtained from the study of tremor animal models may be complicated by the co-morbidity of symptoms such as by those associated with dystonia, parkinsonism, and ataxia.
The future directions for animal models of tremor research are (1) to establish quantitative methods for tremor measurement and standardized methods for testing pharmacological responses to enhance the translatability to human tremor disorders; (2) to characterize the anatomical brain circuitry alterations and corresponding physiological changes that drive tremor; (3) to study the links between human tremor disorders and animal models at the levels of pathology, physiology, and genetics. With these considerations in mind, the pathophysiology learned from different animal models will likely to guide us towards developing physiologic biomarkers to solve the heterogeneity of tremor disorders by stratifying tremor patients for personalized treatment and/or selection for clinical trials.
Acknowledgments
Funding: Sheng-Han Kuo has received funding from the National Institutes of Health: NINDS #R01 NS104423 (principal investigator), NINDS #K08 NS083738 (principal investigator), and the Louis V. Gerstner Jr. Scholar Award, Parkinson’s Foundation, and International Essential Tremor Foundation. Elan D. Louis has received research support from the National Institutes of Health: NINDS #R01 NS094607 (principal investigator), NINDS #R01 NS085136 (principal investigator), NINDS #R01 NS073872 (principal investigator), NINDS #R01 NS085136 (principal investigator) and NINDS #R01 NS088257 (principal investigator). He has also received support from the Claire O’Neil Essential Tremor Research Fund (Yale University). Phyllis L. Faust has received funding from the National Institutes of Health: NINDS #R01 NS088257 (principal investigator) and NINDS #R01 NS085136 (principal investigator). Adrian Handforth has received support from Veterans Affairs and the International Essential Tremor Foundation. Su-youne Chang has received NINDS #R01 NS088260 (principal investigator). Ming-Kai Pan has received research support from Ministry of Science and Technology in Taiwan: MOST #104-2314-B-002-076-MY3 and 107-2321-B-002-020 (principal investigator). Eric J. Lang has received funding from the National Institutes of Health: NINDS #R21 NS101386 (principal investigator). Stefan M. Pulst has received funding from the National Institutes of Health: NINDS #R37 NS033123 (principal investigator), NINDS #R01 NS097903 (principal investigator), NINDS #U01 NS103883 (principal investigator), NINDS #R21 NS103009 (principal investigator), NINDS #R21 NS104799 (principal investigator), NINDS #R21 NS079852 (principal investigator), and Utah Neuroscience Initiative Collaborative Pilot Project Award. Collin J. Anderson has received funding from National Ataxia Foundation Postdoc Fellowship. Roy V. Sillitoe has received funds from BCM IDDRC grant U54HD083092 (Neurovisualization Core), the National Institutes of Health NINDS #R01NS089664 (principal investigator) and #R01NS100874 (principal investigator), and the Hamill Foundation. Amanda M. Brown is supported by the National Institutes of Health NINDS #F31 NS101891. Dane M. Chetkovich is supported by the National Institutes of Health NINDS #R01NS059934 (principal investigator), NIMH #R01MH106511 (principal investigator), and NIMH #R21MH113262 (principal investigator). Lorriane Clark recieves support from the National Institutes of Health NINDS #R01 NS073872 (principal investigator) and NINDS #R21 NS0988930 (principal investigator). Rodger J. Elble receives support from the Neuroscience Research Foundation of Kiwanis International Illinois-Eastern Iowa District.
Abbreviations:
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- CF
climbing fiber
- CS
complex spike
- CV
coefficient of variation
- DCN
deep cerebellar nucleus
- EEG
electroencephalogram
- ET
essential tremor
- fMRI
functional magnetic resonance imaging
- KO
knockout
- GABAAR
GABAA receptor
- HCN channel
hyperpolarization-activated cyclic nucleotide-gated channel
- IO
inferior olive
- ISI
inter-spike interval
- MES
maximal electroshock seizure
- NMDA
N-methyl-D-aspartate
- PC
Purkinje cells
- pcd
Purkinje cell degeneration
- PD
Parkinson disease
- PET
positron emission tomography
- SCA
spinocerebellar ataxia
Footnotes
Disclosure: The authors report no conflicts of interest.
References
- 1.Bhatia KP, Bain P, Bajaj N, et al. Consensus Statement on the classification of tremors. from the task force on tremor of the International Parkinson and Movement Disorder Society. Mov Disord 2018;33:75–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Louis ED, Ferreira JJ. How common is the most common adult movement disorder? Update on the worldwide prevalence of essential tremor. Mov Disord 2010;25:534–541. [DOI] [PubMed] [Google Scholar]
- 3.Louis ED, Ottman R. How many people in the USA have essential tremor? Deriving a population estimate based on epidemiological data. Tremor Other Hyperkinet Mov 2014;4:259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Louis ED, Thawani SP, Andrews HF. Prevalence of essential tremor in a multiethnic, community-based study in northern Manhattan, New York, N.Y. Neuroepidemiology 2009;32:208–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morgan JC, Kurek JA, Davis JL, Sethi KD. Insights into pathophysiology from medication-induced tremor. Tremor Other Hyperkinet Mov 2017;7:442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Louis ED. Treatment of medically refractory essential tremor. N Engl J Med 2016;375:792–793. [DOI] [PubMed] [Google Scholar]
- 7.Louis ED, Huang CC, Dyke JP, Long Z, Dydak U. Neuroimaging studies of essential tremor: how well do these studies support/refute the neurodegenerative hypothesis? Tremor Other Hyperkinet Mov 2014;4:235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Filip P, Lungu OV, Manto MU, Bares M. Linking essential tremor to the cerebellum: Phys Evid. Cerebellum 2016;15:774–780. [DOI] [PubMed] [Google Scholar]
- 9.Louis ED. Linking essential tremor to the cerebellum: neuropathological evidence. Cerebellum 2016;15:235–242. [DOI] [PubMed] [Google Scholar]
- 10.Miwa H Rodent models of tremor. Cerebellum 2007;6:66–72. [DOI] [PubMed] [Google Scholar]
- 11.Llinas R, Yarom Y. Electrophysiology of mammalian inferior olivary neurones in vitro. Different types of voltage-dependent ionic conductances. J Physiol 1981;315:549–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Llinas R, Yarom Y. Properties and distribution of ionic conductances generating electroresponsiveness of mammalian inferior olivary neurones in vitro. J Physiol 1981;315:569–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Llinas R, Yarom Y. Oscillatory properties of guinea-pig inferior olivary neurones and their pharmacological modulation: an in vitro study. J Physiol 1986;376:163–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Montigny C, Lamarre Y. Effects produced by local applications of harmaline in the inferior olive. Can J Physiol Pharmacol 1975;53:845–849. [DOI] [PubMed] [Google Scholar]
- 15.de Montigny C, Lamarre Y. Rhythmic activity induced by harmaline in the olivo-cerebello-bulbar system of the cat. Brain Res 1973;53:81–95. [DOI] [PubMed] [Google Scholar]
- 16.Llinas R, Volkind RA. The olivo-cerebellar system: functional properties as revealed by harmaline-induced tremor. Exp Brain Res 1973;18:69–87. [DOI] [PubMed] [Google Scholar]
- 17.Beitz AJ, Saxon D. Harmaline-induced climbing fiber activation causes amino acid and peptide release in the rodent cerebellar cortex and a unique temporal pattern of Fos expression in the olivo-cerebellar pathway. J Neurocytol 2004;33:49–74. [DOI] [PubMed] [Google Scholar]
- 18.Llinas R, Muhlethaler M. An electrophysiological study of the in vitro, perfused brain stem-cerebellum of adult guinea-pig. J Physiol 1988;404:215–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Batini C, Bernard JF, Buisseret-Delmas C, Conrath-Verrier M, Horcholle-Bossavit G. Harmaline-induced tremor. II. Unit activity correlation in the interposito-rubral and oculomotor systems of cat. Exp Brain Res 1981;42:383–391. [DOI] [PubMed] [Google Scholar]
- 20.Tian JB, Bishop GA. Stimulus-dependent activation of c-Fos in neurons and glia in the rat cerebellum. J Chem Neuroanat 2002;23:157–170. [DOI] [PubMed] [Google Scholar]
- 21.Simantov R, Snyder SH, Oster-Granite ML. Harmaline-induced tremor in the rat: abolition by 3-acetylpyridine destruction of cerebellar climbing fibers. Brain Res 1976;114:144–151. [DOI] [PubMed] [Google Scholar]
- 22.Martin FC, Handforth A. Carbenoxolone and mefloquine suppress tremor in the harmaline mouse model of essential tremor. Mov Disord 2006;21:1641–1649. [DOI] [PubMed] [Google Scholar]
- 23.Lorden JF, Stratton SE, Mays LE, Oltmans GA. Purkinje cell activity in rats following chronic treatment with harmaline. Neuroscience 1988;27:465–472. [DOI] [PubMed] [Google Scholar]
- 24.Milner TE, Cadoret G, Lessard L, Smith AM. EMG analysis of harmaline-induced tremor in normal and three strains of mutant mice with Purkinje cell degeneration and the role of the inferior olive. J Neurophysiol 1995;73:2568–2577. [DOI] [PubMed] [Google Scholar]
- 25.McMahon A, Fowler SC, Perney TM, Akemann W, Knopfel T, Joho RH. Allele-dependent changes of olivocerebellar circuit properties in the absence of the voltage-gated potassium channels Kv3.1 and Kv3.3. Eur J Neurosci 2004;19:3317–3327. [DOI] [PubMed] [Google Scholar]
- 26.Hurlock EC, McMahon A, Joho RH. Purkinje-cell-restricted restoration of Kv3.3 function restores complex spikes and rescues motor coordination in Kcnc3 mutants. J Neurosci 2008;28:4640–4648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bekar L, Libionka W, Tian GF, et al. Adenosine is crucial for deep brain stimulation-mediated attenuation of tremor. Nat Med 2008;14:75–80. [DOI] [PubMed] [Google Scholar]
- 28.Lang EJ. GABAergic and glutamatergic modulation of spontaneous and motor-cortex-evoked complex spike activity. J Neurophysiol 2002;87:1993–2008. [DOI] [PubMed] [Google Scholar]
- 29.Mignani S, Bohme GA, Birraux G, et al. 9-Carboxymethyl-5H,10H-imidazo[1,2-a]indeno[1,2-e]pyrazin-4-one-2-carbocylic acid (RPR117824): selective anticonvulsive and neuroprotective AMPA antagonist. Bioorg Med Chem 2002;10:1627–1637. [DOI] [PubMed] [Google Scholar]
- 30.Paterson NE, Malekiani SA, Foreman MM, Olivier B, Hanania T. Pharmacological characterization of harmaline-induced tremor activity in mice. Eur J Pharmacol 2009;616:73–80. [DOI] [PubMed] [Google Scholar]
- 31.Shaffer CL, Hurst RS, Scialis RJ, et al. Positive allosteric modulation of AMPA receptors from efficacy to toxicity: the interspecies exposure-response continuum of the novel potentiator PF-4778574. J Pharmacol Exp Ther 2013;347:212–224. [DOI] [PubMed] [Google Scholar]
- 32.Sugihara I, Lang EJ, Llinas R. Serotonin modulation of inferior olivary oscillations and synchronicity: a multiple-electrode study in the rat cerebellum. Eur J Neurosci 1995;7:521–534. [DOI] [PubMed] [Google Scholar]
- 33.Wiklund L, Sjolund B, Bjorklund A. Morphological and functional studies on the serotoninergic innervation of the inferior olive. J Physiol 1981;77:183–186. [PubMed] [Google Scholar]
- 34.Barragan LA, Delhaye-Bouchaud N, Laget P. Drug-induced activation of the inferior olivary nucleus in young rabbits. Differential effects of harmaline and quipazine. Neuropharmacology 1985;24:645–654. [DOI] [PubMed] [Google Scholar]
- 35.Handforth A, Homanics GE, Covey DF, et al. T-type calcium channel antagonists suppress tremor in two mouse models of essential tremor. Neuropharmacology 2010;59:380–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park YG, Park HY, Lee CJ, et al. Ca(V)3.1 is a tremor rhythm pacemaker in the inferior olive. PNAS 2010;107:10731–10736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boecker H, Wills AJ, Ceballos-Baumann A, et al. The effect of ethanol on alcohol-responsive essential tremor: a positron emission tomography study. Ann Neurol 1996;39:650–658. [DOI] [PubMed] [Google Scholar]
- 38.Miwa H, Nishi K, Fuwa T, Mizuno Y. Differential expression of c-fos following administration of two tremorgenic agents: harmaline and oxotremorine. Neuroreport 2000;11:2385–2390. [DOI] [PubMed] [Google Scholar]
- 39.Dupuis MJ-M, Evrard FLA, Jacquerye PG, Picard GR, Lermen OG. Disappearance of essential tremor after stroke. Mov Disord 2010;25:2884–2887. [DOI] [PubMed] [Google Scholar]
- 40.Handforth A Harmaline tremor: underlying mechanisms in a potential animal model of essential tremor. Tremor Other Hyperkinet Mov 2012;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kronenbuerger M, Tronnier VM, Gerwig M, et al. Thalamic deep brain stimulation improves eyeblink conditioning deficits in essential tremor. Exp Neurol 2008;211:387–396. [DOI] [PubMed] [Google Scholar]
- 42.Louis ED, Lenka A. The Olivary Hypothesis of Essential Tremor: Time to Lay this Model to Rest? Tremor Other Hyperkinet Mov 2017;7:473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lang EJ, Sugihara I, Llinas R. Olivocerebellar modulation of motor cortex ability to generate vibrissal movements in rat. J Physiol 2006;571:101–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vallbo AB, Wessberg J. Organization of motor output in slow finger movements in man. J Physiol 1993;469:673–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schnitzler A, Timmermann L, Gross J. Physiological and pathological oscillatory networks in the human motor system. J Physiol 2006;99:3–7. [DOI] [PubMed] [Google Scholar]
- 46.Schnitzler A, Münks C, Butz M, Timmermann L, Gross J. Synchronized brain network associated with essential tremor as revealed by magnetoencephalography. Mov Disord 2009;24:1629–1635. [DOI] [PubMed] [Google Scholar]
- 47.Nahab FB, Wittevrongel L, Ippolito D, et al. An open-label, single-dose, crossover study of the pharmacokinetics and metabolism of two oral formulations of 1-octanol in patients with essential tremor. Neurotherapeutics 2011;8:753–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Papapetropoulos S, Lee MS, Boyer S. Proof-of-concept, double-blind, placebo-controlled study for CX-8998, a state-dependent T-Type calcium (Cav3) channel antagonist, in essential tremor patients (T-CALM): efficacy and safety results. 2018. Abstract in Movement Disorders Society Meeting [Google Scholar]
- 49.Sauleau P, Lapouble E, Val-Laillet D, Malbert CH. The pig model in brain imaging and neurosurgery. Animal 2009;3:1138–1151. [DOI] [PubMed] [Google Scholar]
- 50.Saikali S, Meurice P, Sauleau P, et al. A three-dimensional digital segmented and deformable brain atlas of the domestic pig. J Neurosci Methods 2010;192:102–109. [DOI] [PubMed] [Google Scholar]
- 51.Rose JE. The thalamus of the sheep: cellular and fibrous structure and comparison with pig, rabbit and cat. J Comp Neurol 1942;77:469–523. [Google Scholar]
- 52.Wakeman DR, Crain AM, Snyder EY. Large animal models are critical for rationally advancing regenerative therapies. Regen Med 2006;1:405–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Felix B, Leger ME, Albe-Fessard D, Marcilloux JC, Rampin O, Laplace JP. Stereotaxic atlas of the pig brain. Brain Res Bull 1999;49:1–137. [DOI] [PubMed] [Google Scholar]
- 54.Shon YM, Lee KH, Goerss SJ, et al. High frequency stimulation of the subthalamic nucleus evokes striatal dopamine release in a large animal model of human DBS neurosurgery. Neurosci Lett 2010;475:136–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Paek SB, Min HK, Kim I, et al. Frequency-dependent functional neuromodulatory effects on the motor network by ventral lateral thalamic deep brain stimulation in swine. Neuroimage 2015;105:181–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee J, Kim I, Lee J, et al. Development of harmaline-induced tremor in a swine model. Tremor Other Hyperkinet Mov 2018;8:532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Raethjen J, Deuschl G. The oscillating central network of essential tremor. Clin Neurophysiol 2012;123:61–64. [DOI] [PubMed] [Google Scholar]
- 58.Chandran V, Pal PK. Essential tremor: beyond the motor features. Parkinsonism Relat Disord 2012;18:407–413. [DOI] [PubMed] [Google Scholar]
- 59.Cerasa A, Passamonti L, Novellino F, et al. Fronto-parietal overactivation in patients with essential tremor during Stroop task. Neuroreport 2010;21:148–151. [DOI] [PubMed] [Google Scholar]
- 60.Cerasa A, Quattrone A. Linking essential tremor to the cerebellum-neuroimaging evidence. Cerebellum 2016;15:263–275. [DOI] [PubMed] [Google Scholar]
- 61.Moloudizargari M, Mikaili P, Aghajanshakeri S, Asghari MH, Shayegh J. Pharmacological and therapeutic effects of Peganum harmala and its main alkaloids. Pharmacogn Rev 2013;7:199–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Louis ED. Non-motor symptoms in essential tremor: A review of the current data and state of the field. Parkinsonism Relat Disord 2016;22:S115–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schwarz L, Riedel C, Hogler S, et al. Congenital infection with atypical porcine pestivirus (APPV) is associated with disease and viral persistence. Vet Res 2017;48:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yizhar O, Fenno LE, Davidson TJ, Mogri M, Deisseroth K. Optogenetics in neural systems. Neuron 2011;71:9–34. [DOI] [PubMed] [Google Scholar]
- 65.Nakai J, Ohkura M, Imoto K. A high signal-to-noise Ca(2+) probe composed of a single green fluorescent protein. Nat Biotechnol 2001;19:137–141. [DOI] [PubMed] [Google Scholar]
- 66.Louis ED, Babij R, Cortes E, Vonsattel J-PG, Faust PL. The inferior olivary nucleus: a postmortem study of essential tremor cases versus controls. Mov Disord 2013;28:779–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Louis ED, Faust PL, Vonsattel JPG, et al. Neuropathological changes in essential tremor: 33 cases compared with 21 controls. Brain 2007;130:3297–3307. [DOI] [PubMed] [Google Scholar]
- 68.Babij R, Lee M, Cortes E, Vonsattel J-PG, Faust PL, Louis ED. Purkinje cell axonal anatomy: quantifying morphometric changes in essential tremor versus control brains. Brain 2013;136:3051–3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Erickson-Davis CR, Faust PL, Vonsattel JPG, Gupta S, Honig LS, Louis ED. Hairy baskets associated with degenerative Purkinje cell changes in essential tremor. J Neuropathol Exp Neurol 2010;69:262–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Paris-Robidas S, Brochu E, Sintes M, et al. Defective dentate nucleus GABA receptors in essential tremor. Brain 2012;135:105–116. [DOI] [PubMed] [Google Scholar]
- 71.Louis ED. Re-thinking the biology of essential tremor: from models to morphology. Parkinsonism Relat Disord 2014;20:S88–93. [DOI] [PubMed] [Google Scholar]
- 72.Llinas R, Baker R, Sotelo C. Electrotonic coupling between neurons in cat inferior olive. J Neurophysiol 1974;37:560–571. [DOI] [PubMed] [Google Scholar]
- 73.Blenkinsop TA, Lang EJ. Block of inferior olive gap junctional coupling decreases Purkinje cell complex spike synchrony and rhythmicity. J Neurosci 2006;26:1739–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Marshall SP, van der Giessen RS, de Zeeuw CI, Lang EJ. Altered olivocerebellar activity patterns in the connexin36 knockout mouse. Cerebellum 2007;6:287–299. [DOI] [PubMed] [Google Scholar]
- 75.Lang EJ, Sugihara I, Llinas R. GABAergic modulation of complex spike activity by the cerebellar nucleoolivary pathway in rat. J Neurophysiol 1996;76:255–275. [DOI] [PubMed] [Google Scholar]
- 76.De Zeeuw CI, Lang EJ, Sugihara I, et al. Morphological correlates of bilateral synchrony in the rat cerebellar cortex. J Neurosci 1996;16:3412–3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yamamoto T, Fukuda M, Llinas R. Bilaterally synchronous complex spike Purkinje cell activity in the mammalian cerebellum. Eur J Neurosci 2001;13:327–339. [DOI] [PubMed] [Google Scholar]
- 78.Aizenman CD, Linden DJ. Regulation of the rebound depolarization and spontaneous firing patterns of deep nuclear neurons in slices of rat cerebellum. J Neurophysiol 1999;82:1697–1709. [DOI] [PubMed] [Google Scholar]
- 79.Uusisaari M, Obata K, Knopfel T. Morphological and electrophysiological properties of GABAergic and non-GABAergic cells in the deep cerebellar nuclei. J Neurophysiol 2007;97:901–911. [DOI] [PubMed] [Google Scholar]
- 80.Blenkinsop TA, Lang EJ. Synaptic action of the olivocerebellar system on cerebellar nuclear spike activity. J Neurosci 2011;31:14708–14720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rowland NC, Jaeger D. Coding of tactile response properties in the rat deep cerebellar nuclei. J Neurophysiol 2005;94:1236–1251. [DOI] [PubMed] [Google Scholar]
- 82.Cerminara NL, Rawson JA. Evidence that climbing fibers control an intrinsic spike generator in cerebellar Purkinje cells. J Neurosci 2004;24:4510–4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rosen I, Scheid P. Cerebellar surface cooling influencing evoked activity in cortex and in interpositus nucleus. Brain Res 1972;45:580–584. [DOI] [PubMed] [Google Scholar]
- 84.Andersson G, Hesslow G. Activity of Purkinje cells and interpositus neurones during and after periods of high frequency climbing fibre activation in the cat. Exp Brain Res 1987;67:533–542. [DOI] [PubMed] [Google Scholar]
- 85.Wise AK, Cerminara NL, Marple-Horvat DE, Apps R. Mechanisms of synchronous activity in cerebellar Purkinje cells. J Physiol 2010;588:2373–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Heck DH, Thach WT, Keating JG. On-beam synchrony in the cerebellum as the mechanism for the timing and coordination of movement. PNAS 2007;104:7658–7663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bell CC, Grimm RJ. Discharge properties of Purkinje cells recorded on single and double microelectrodes. J Neurophysiol 1969;32:1044–1055. [DOI] [PubMed] [Google Scholar]
- 88.Marshall SP, Lang EJ. Local changes in the excitability of the cerebellar cortex produce spatially restricted changes in complex spike synchrony. J Neurosci 2009;29:14352–14362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Handforth A Linking essential tremor to the cerebellum-animal model evidence. Cerebellum 2016;15:285–298. [DOI] [PubMed] [Google Scholar]
- 90.Person AL, Raman IM. Purkinje neuron synchrony elicits time-locked spiking in the cerebellar nuclei. Nature 2011;481:502–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hawkes R, Leclerc N. Purkinje cell axon collateral distributions reflect the chemical compartmentation of the rat cerebellar cortex. Brain Res 1989;476:279–290. [DOI] [PubMed] [Google Scholar]
- 92.Sugihara I, Shinoda Y. Molecular, topographic, and functional organization of the cerebellar nuclei: analysis by three-dimensional mapping of the olivonuclear projection and aldolase C labeling. J Neurosci 2007;27:9696–9710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chung SH, Marzban H, Hawkes R. Compartmentation of the cerebellar nuclei of the mouse. Neuroscience 2009;161:123–138. [DOI] [PubMed] [Google Scholar]
- 94.White JJ, Arancillo M, King A, et al. Pathogenesis of severe ataxia and tremor without the typical signs of neurodegeneration. Neurobiol Dis 2016;86:86–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jiao Y, Yan J, Zhao Y, et al. Carbonic anhydrase-related protein VIII deficiency is associated with a distinctive lifelong gait disorder in waddles mice. Genetics 2005;171:1239–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kato K Sequence of a novel carbonic anhydrase-related polypeptide and its exclusive presence in Purkinje cells. FEBS Lett 1990;271:137–140. [DOI] [PubMed] [Google Scholar]
- 97.Taniuchi K, Nishimori I, Takeuchi T, Ohtsuki Y, Onishi S. cDNA cloning and developmental expression of murine carbonic anhydrase-related proteins VIII, X, and XI. Brain Res Mol Brain Res 2002;109:207–215. [DOI] [PubMed] [Google Scholar]
- 98.Tripp BC, Smith K, Ferry JG. Carbonic anhydrase: new insights for an ancient enzyme. J Biol Chem 2001;276:48615–48618. [DOI] [PubMed] [Google Scholar]
- 99.Hirota J, Ando H, Hamada K, Mikoshiba K. Carbonic anhydrase-related protein is a novel binding protein for inositol 1,4,5-trisphosphate receptor type 1. Biochem J 2003;372:435–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Turkmen S, Guo G, Garshasbi M, et al. CA8 mutations cause a novel syndrome characterized by ataxia and mild mental retardation with predisposition to quadrupedal gait. PLoS Genet 2009;5:e1000487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kaya N, Aldhalaan H, Al-Younes B, et al. Phenotypical spectrum of cerebellar ataxia associated with a novel mutation in the CA8 gene, encoding carbonic anhydrase (CA) VIII. Am J Med Genet B Neuropsychiatr Genet 2011;156b:826–834. [DOI] [PubMed] [Google Scholar]
- 102.Miterko LN, White JJ, Lin T, Brown AM, O’Donovan KJ, Sillitoe RV. Persistent motor dysfunction despite homeostatic rescue of cerebellar morphogenesis in the Car8 waddles mutant mouse. Neural Dev. 2019. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hirasawa M, Xu X, Trask RB, et al. Carbonic anhydrase related protein 8 mutation results in aberrant synaptic morphology and excitatory synaptic function in the cerebellum. Mol Cell Neurosci 2007;35:161–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.White JJ, Arancillo M, Stay TL, et al. Cerebellar zonal patterning relies on Purkinje cell neurotransmission. J Neurosci 2014;34:8231–8245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Arancillo M, White JJ, Lin T, Stay TL, Sillitoe RV. In vivo analysis of Purkinje cell firing properties during postnatal mouse development. J Neurophysiol 2015;113:578–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.White JJ, Sillitoe RV. Genetic silencing of olivocerebellar synapses causes dystonia-like behaviour in mice. Nat Commun 2017;8:14912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chaumont J, Guyon N, Valera AM, et al. Clusters of cerebellar Purkinje cells control their afferent climbing fiber discharge. PNAS 2013;110:16223–16228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Witter L, Canto CB, Hoogland TM, de Gruijl JR, De Zeeuw CI. Strength and timing of motor responses mediated by rebound firing in the cerebellar nuclei after Purkinje cell activation. Front Neural Circuits 2013;7:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Luna-Cancalon K, Sikora KM, Pappas SS, et al. Alterations in cerebellar physiology are associated with a stiff-legged gait in Atcay(ji-hes) mice. Neurobiol Dis 2014;67:140–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Calderon DP, Fremont R, Kraenzlin F, Khodakhah K. The neural substrates of rapid-onset Dystonia-Parkinsonism. Nat Neurosci 2011;14:357–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fremont R, Tewari A, Angueyra C, Khodakhah K. A role for cerebellum in the hereditary dystonia DYT1. Elife 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Walter JT, Alvina K, Womack MD, Chevez C, Khodakhah K. Decreases in the precision of Purkinje cell pacemaking cause cerebellar dysfunction and ataxia. Nat Neurosci 2006;9:389–397. [DOI] [PubMed] [Google Scholar]
- 113.Fremont R, Calderon DP, Maleki S, Khodakhah K. Abnormal high-frequency burst firing of cerebellar neurons in rapid-onset dystonia-parkinsonism. J Neurosci 2014;34:11723–11732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hoebeek FE, Stahl JS, van Alphen AM, et al. Increased noise level of purkinje cell activities minimizes impact of their modulation during sensorimotor control. Neuron 2005;45:953–965. [DOI] [PubMed] [Google Scholar]
- 115.Alvina K, Khodakhah K. The therapeutic mode of action of 4-aminopyridine in cerebellar ataxia. J Neurosci 2010;30:7258–7268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jayabal S, Chang HH, Cullen KE, Watt AJ. 4-aminopyridine reverses ataxia and cerebellar firing deficiency in a mouse model of spinocerebellar ataxia type 6. Sci Rep 2016;6:29489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Glasauer S, Kalla R, Buttner U, Strupp M, Brandt T. 4-aminopyridine restores visual ocular motor function in upbeat nystagmus. J Neurol Neurosurg Psychiatry 2005;76:451–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kalla R, Glasauer S, Buttner U, Brandt T, Strupp M. 4-aminopyridine restores vertical and horizontal neural integrator function in downbeat nystagmus. Brain 2007;130:2441–2451. [DOI] [PubMed] [Google Scholar]
- 119.Lohle M, Schrempf W, Wolz M, Reichmann H, Storch A. Potassium channel blocker 4-aminopyridine is effective in interictal cerebellar symptoms in episodic ataxia type 2--a video case report. Mov Disord 2008;23:1314–1316. [DOI] [PubMed] [Google Scholar]
- 120.Strupp M, Kalla R, Dichgans M, Freilinger T, Glasauer S, Brandt T. Treatment of episodic ataxia type 2 with the potassium channel blocker 4-aminopyridine. Neurology 2004;62:1623–1625. [DOI] [PubMed] [Google Scholar]
- 121.Schniepp R, Wuehr M, Ackl N, et al. 4-Aminopyridine improves gait variability in cerebellar ataxia due to CACNA1A mutation. J Neurol 2011;258:1708–1711. [DOI] [PubMed] [Google Scholar]
- 122.Schniepp R, Jakl V, Wuehr M, et al. Treatment with 4-aminopyridine improves upper limb tremor of a patient with multiple sclerosis: a video case report. Mult Scler 2013;19:506–508. [DOI] [PubMed] [Google Scholar]
- 123.Hopfner F, Helmich RC. The etiology of essential tremor: Genes versus environment. Parkinsonism Relat Disord 2018;46:S92–s96. [DOI] [PubMed] [Google Scholar]
- 124.Lou JS, Jankovic J. Essential tremor: clinical correlates in 350 patients. Neurology 1991;41:234–238. [DOI] [PubMed] [Google Scholar]
- 125.Louis ED. Clinical practice. Essential tremor. N Engl J Med 2001;345:887–891. [DOI] [PubMed] [Google Scholar]
- 126.Deuschl G, Petersen I, Lorenz D, Christensen K. Tremor in the elderly: essential and aging-related tremor. Mov Disord 2015;30:1327–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Liu X, Hernandez N, Kisselev S, et al. Identification of candidate genes for familial early-onset essential tremor. Eur J Hum Genet 2016;24:1009–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kuhlenbaumer G, Hopfner F, Deuschl G. Genetics of essential tremor: meta-analysis and review. Neurology 2014;82:1000–1007. [DOI] [PubMed] [Google Scholar]
- 129.Fahn S. The 200-year journey of Parkinson disease: Reflecting on the past and looking towards the future. Parkinsonism Relat Disord 2018;46 Suppl 1:S1–s5. [DOI] [PubMed] [Google Scholar]
- 130.Schober A Classic toxin-induced animal models of Parkinson’s disease: 6-OHDA and MPTP. Cell Tissue Res 2004;318:215–224. [DOI] [PubMed] [Google Scholar]
- 131.Yao C, El Khoury R, Wang W, et al. LRRK2-mediated neurodegeneration and dysfunction of dopaminergic neurons in a Caenorhabditis elegans model of Parkinson’s disease. Neurobiol Dis 2010;40:73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rocha EM, Smith GA, Park E, et al. Glucocerebrosidase gene therapy prevents alpha-synucleinopathy of midbrain dopamine neurons. Neurobiol Dis 2015;82:495–503. [DOI] [PubMed] [Google Scholar]
- 133.Choe M, Cortes E, Vonsattel JP, Kuo SH, Faust PL, Louis ED. Purkinje cell loss in essential tremor: Random sampling quantification and nearest neighbor analysis. Mov Disord 2016;31:393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kuo SH, Erickson-Davis C, Gillman A, Faust PL, Vonsattel JPG, Louis ED. Increased number of heterotopic Purkinje cells in essential tremor. J Neurol Neurosurg Psychiatry 2011;82:1038–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Louis ED, Kuo SH, Tate WJ, et al. Heterotopic Purkinje cells: a comparative postmortem study of essential tremor and spinocerebellar ataxias 1, 2, 3, and 6. Cerebellum 2018;17:104–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kuo SH, Tang G, Louis ED, et al. Lingo-1 expression is increased in essential tremor cerebellum and is present in the basket cell pinceau. Acta Neuropathol 2013;125:879–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Miyazaki T, Yamasaki M, Takeuchi T, Sakimura K, Mishina M, Watanabe M. Ablation of glutamate receptor GluRdelta2 in adult Purkinje cells causes multiple innervation of climbing fibers by inducing aberrant invasion to parallel fiber innervation territory. J Neurosci 2010;30:15196–15209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Watanabe M Molecular mechanisms governing competitive synaptic wiring in cerebellar Purkinje cells. Tohoku J Exp Med 2008;214:175–190. [DOI] [PubMed] [Google Scholar]
- 139.Lin CY, Louis ED, Faust PL, Koeppen AH, Vonsattel JPG, Kuo SH. Abnormal climbing fibre-Purkinje cell synaptic connections in the essential tremor cerebellum. Brain 2014;137:3149–3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kuo SH, Lin CY, Wang J, et al. Climbing fiber-Purkinje cell synaptic pathology in tremor and cerebellar degenerative diseases. Acta Neuropathol 2017;133:121–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lee D, Gan SR, Faust PL, Louis ED, Kuo SH. Climbing fiber-Purkinje cell synaptic pathology across essential tremor subtypes. Parkinsonism Relat Disord 2018. 51:24–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Qiao S, Kim SH, Heck D, Goldowitz D, LeDoux MS, Homayouni R. Dab2IP GTPase activating protein regulates dendrite development and synapse number in cerebellum. PLoS One 2013;8:e53635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yuzaki M. New (but old) molecules regulating synapse integrity and plasticity: Cbln1 and the delta2 glutamate receptor. Neuroscience 2009;162:633–643. [DOI] [PubMed] [Google Scholar]
- 144.Yuzaki M Cbln1 and its family proteins in synapse formation and maintenance. Curr Opin Neurobiol 2011;21:215–220. [DOI] [PubMed] [Google Scholar]
- 145.Schols L, Peters S, Szymanski S, et al. Extrapyramidal motor signs in degenerative ataxias. Arch Neurol 2000;57:1495–1500. [DOI] [PubMed] [Google Scholar]
- 146.Gan SR, Wang J, Figueroa KP, et al. Postural tremor and ataxia progression in spinocerebellar ataxias. Tremor Other Hyperkinet Mov 2017;7:492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Pulst SM, Nechiporuk A, Nechiporuk T, et al. Moderate expansion of a normally biallelic trinucleotide repeat in spinocerebellar ataxia type 2. Nat Genet 1996;14:269–276. [DOI] [PubMed] [Google Scholar]
- 148.Scoles DR, Pulst SM. Spinocerebellar ataxia type 2. Adv Exp Med Biol 2018;1049:175–195. [DOI] [PubMed] [Google Scholar]
- 149.Pulst SM. Degenerative ataxias, from genes to therapies: The 2015 Cotzias Lecture. Neurology 2016;86:2284–2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hayes S, Turecki G, Brisebois K, et al. CAG repeat length in RAI1 is associated with age at onset variability in spinocerebellar ataxia type 2 (SCA2). Hum Mol Genet 2000;9:1753–1758. [DOI] [PubMed] [Google Scholar]
- 151.Figueroa KP, Coon H, Santos N, Velazquez L, Mederos LA, Pulst SM. Genetic analysis of age at onset variation in spinocerebellar ataxia type 2. Neurol Genet 2017;3:e155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Nechiporuk T, Huynh DP, Figueroa K, Sahba S, Nechiporuk A, Pulst SM. The mouse SCA2 gene: cDNA sequence, alternative splicing and protein expression. Hum Mol Genet 1998;7:1301–1309. [DOI] [PubMed] [Google Scholar]
- 153.Huynh DP, Figueroa K, Hoang N, Pulst SM. Nuclear localization or inclusion body formation of ataxin-2 are not necessary for SCA2 pathogenesis in mouse or human. Nat Genet 2000;26:44–50. [DOI] [PubMed] [Google Scholar]
- 154.Dansithong W, Paul S, Figueroa KP, et al. Ataxin-2 regulates RGS8 translation in a new BAC-SCA2 transgenic mouse model. PLoS Genet 2015;11:e1005182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hansen ST, Meera P, Otis TS, Pulst SM. Changes in Purkinje cell firing and gene expression precede behavioral pathology in a mouse model of SCA2. Hum Mol Genet 2013;22:271–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hausser M, Clark BA. Tonic synaptic inhibition modulates neuronal output pattern and spatiotemporal synaptic integration. Neuron 1997;19:665–678. [DOI] [PubMed] [Google Scholar]
- 157.Raman IM, Bean BP. Ionic currents underlying spontaneous action potentials in isolated cerebellar Purkinje neurons. J Neurosci 1999;19:1663–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Smith SL, Otis TS. Persistent changes in spontaneous firing of Purkinje neurons triggered by the nitric oxide signaling cascade. J Neurosci 2003;23:367–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kasumu AW, Hougaard C, Rode F, et al. Selective positive modulator of calcium-activated potassium channels exerts beneficial effects in a mouse model of spinocerebellar ataxia type 2. Chem Biol 2012;19:1340–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kasumu AW, Liang X, Egorova P, Vorontsova D, Bezprozvanny I. Chronic suppression of inositol 1,4,5-triphosphate receptor-mediated calcium signaling in cerebellar purkinje cells alleviates pathological phenotype in spinocerebellar ataxia 2 mice. J Neurosci 2012;32:12786–12796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Egorova P, Popugaeva E, Bezprozvanny I. Disturbed calcium signaling in spinocerebellar ataxias and Alzheimer’s disease. Semin Cell Dev Biol 2015;40:127–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lee KH, Mathews PJ, Reeves AM, et al. Circuit mechanisms underlying motor memory formation in the cerebellum. Neuron 2015;86:529–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Meera P, Pulst SM, Otis TS. Cellular and circuit mechanisms underlying spinocerebellar ataxias. J Physiol 2016;594:4653–4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Beliveau E, Tremblay C, Aubry-Lafontaine E, et al. Accumulation of amyloid-beta in the cerebellar cortex of essential tremor patients. Neurobiol Dis 2015;82:397–408. [DOI] [PubMed] [Google Scholar]
- 165.Shin H, Lee DK, Lee JM, et al. Atrophy of the Cerebellar Vermis in Essential Tremor: Segmental Volumetric MRI Analysis. Cerebellum 2016;15:174–181. [DOI] [PubMed] [Google Scholar]
- 166.Benito-Leon J Essential tremor: a neurodegenerative disease? Tremor Other Hyperkinet Mov 2014;4:252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Stolze H, Petersen G, Raethjen J, Wenzelburger R, Deuschl G. The gait disorder of advanced essential tremor. Brain 2001;124:2278–2286. [DOI] [PubMed] [Google Scholar]
- 168.La Regina MC, Yates-Siilata K, Woods L, Tolbert D. Preliminary characterization of hereditary cerebellar ataxia in rats. Lab Anim Sci 1992;42:19–26. [PubMed] [Google Scholar]
- 169.Figueroa KP, Paul S, Cali T, et al. Spontaneous shaker rat mutant - a new model for X-linked tremor/ataxia. Dis Model Mech 2016;9:553–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Anderson CJ, Figueroa KP, Dorval AD, Pulst SM. Deep cerebellar stimulation reduces ataxic motor symptoms in the shaker rat. Ann Neurol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Scoles DR, Meera P, Schneider MD, et al. Antisense oligonucleotide therapy for spinocerebellar ataxia type 2. Nature 2017;544:362–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Rub U, Del Turco D, Burk K, et al. Extended pathoanatomical studies point to a consistent affection of the thalamus in spinocerebellar ataxia type 2. Neuropathol Appl Neurobiol 2005;31:127–140. [DOI] [PubMed] [Google Scholar]
- 173.Tass P, Smirnov D, Karavaev A, et al. The causal relationship between subcortical local field potential oscillations and Parkinsonian resting tremor. J Neural Eng 2010;7:16009. [DOI] [PubMed] [Google Scholar]
- 174.Williams ER, Soteropoulos DS, Baker SN. Spinal interneuron circuits reduce approximately 10-Hz movement discontinuities by phase cancellation. PNAS 2010;107:11098–11103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Servais L, Hourez R, Bearzatto B, Gall D, Schiffmann SN, Cheron G. Purkinje cell dysfunction and alteration of long-term synaptic plasticity in fetal alcohol syndrome. PNAS 2007;104:9858–9863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Rasch MJ, Gretton A, Murayama Y, Maass W, Logothetis NK. Inferring spike trains from local field potentials. J Neurophysiol 2008;99:1461–1476. [DOI] [PubMed] [Google Scholar]
- 177.Sieghart W. Structure and pharmacology of gamma-aminobutyric acidA receptor subtypes. Pharmacol Rev 1995;47:181–234. [PubMed] [Google Scholar]
- 178.Deuschl G, Raethjen J, Hellriegel H, Elble R. Treatment of patients with essential tremor. Lancet Neurol 2011;10:14–14. [DOI] [PubMed] [Google Scholar]
- 179.Vicini S, Ferguson C, Prybylowski K, Kralic J, Morrow AL, Homanics GE. GABA(A) receptor alpha1 subunit deletion prevents developmental changes of inhibitory synaptic currents in cerebellar neurons. J Neurosci 2001;21:3009–3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Sur C, Wafford KA, Reynolds DS, et al. Loss of the major GABA(A) receptor subtype in the brain is not lethal in mice. J Neurosci 2001;21:3409–3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kralic JE, Criswell HE, Osterman JL, et al. Genetic essential tremor in gamma-aminobutyric acid A receptor alpha1 subunit knockout mice. J Clin Invest 2005;115:774–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Thier S, Kuhlenbaumer G, Lorenz D, et al. GABA(A) receptor- and GABA transporter polymorphisms and risk for essential tremor. Eur J Neurol 2011;18:1098–1100. [DOI] [PubMed] [Google Scholar]
- 183.Deng H, Xie WJ, Le WD, Huang MS, Jankovic J. Genetic analysis of the GABRA1 gene in patients with essential tremor. Neurosci Lett 2006;401:16–19. [DOI] [PubMed] [Google Scholar]
- 184.Arain F, Zhou C, Ding L, Zaidi S, Gallagher MJ. The developmental evolution of the seizure phenotype and cortical inhibition in mouse models of juvenile myoclonic epilepsy. Neurobiol Dis 2015;82:164–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Arain FM, Boyd KL, Gallagher MJ. Decreased viability and absence-like epilepsy in mice lacking or deficient in the GABAA receptor alpha1 subunit. Epilepsia 2012;53:e161–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Cossette P, Liu L, Brisebois K, et al. Mutation of GABRA1 in an autosomal dominant form of juvenile myoclonic epilepsy. Nat Genet 2002;31:184–189. [DOI] [PubMed] [Google Scholar]
- 187.Allen AS, Berkovic SF, Cossette P, et al. De novo mutations in epileptic encephalopathies. Nature 2013;501:217–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Carvill GL, Weckhuysen S, McMahon JM, et al. GABRA1 and STXBP1: novel genetic causes of Dravet syndrome. Neurology 2014;82:1245–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Lachance-Touchette P, Brown P, Meloche C, et al. Novel alpha1 and gamma2 GABAA receptor subunit mutations in families with idiopathic generalized epilepsy. Eur J Neurosci 2011;34:237–249. [DOI] [PubMed] [Google Scholar]
- 190.Zhou C, Huang Z, Ding L, et al. Altered cortical GABAA receptor composition, physiology, and endocytosis in a mouse model of a human genetic absence epilepsy syndrome. J Biol Chem 2013;288:21458–21472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Goodkin HP, Joshi S, Mtchedlishvili Z, Brar J, Kapur J. Subunit-specific trafficking of GABA(A) receptors during status epilepticus. J Neurosci 2008;28:2527–2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Frankle WG, Cho RY, Mason NS, et al. [11C]flumazenil binding is increased in a dose-dependent manner with tiagabine-induced elevations in GABA levels. PLoS One 2012;7:e32443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Zhou C, Ding L, Deel ME, Ferrick EA, Emeson RB, Gallagher MJ. Altered intrathalamic GABAA neurotransmission in a mouse model of a human genetic absence epilepsy syndrome. Neurobiol Dis 2015;73:407–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.De Stasi AM, Farisello P, Marcon I, et al. Unaltered network activity and interneuronal firing during spontaneous cortical dynamics in vivo in a mouse model of severe myoclonic epilepsy of infancy. Cereb Cortex 2016;26:1778–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Hedrich UB, Liautard C, Kirschenbaum D, et al. Impaired action potential initiation in GABAergic interneurons causes hyperexcitable networks in an epileptic mouse model carrying a human Na(V)1.1 mutation. J Neurosci 2014;34:14874–14889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Louis ED, Hernandez N, Dyke JP, Ma RE, Dydak U. In vivo dentate nucleus gamma-aminobutyric acid concentration in essential tremor vs. controls. Cerebellum 2018;17:165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Wisden W, Herb A, Wieland H, Keinanen K, Luddens H, Seeburg PH. Cloning, pharmacological characteristics and expression pattern of the rat GABAA receptor alpha 4 subunit. FEBS Lett 1991;289:227–230. [DOI] [PubMed] [Google Scholar]
- 198.Olsen RW, Sieghart W. GABA A receptors: subtypes provide diversity of function and pharmacology. Neuropharmacology 2009;56:141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Pym LJ, Cook SM, Rosahl T, McKernan RM, Atack JR. Selective labelling of diazepam-insensitive GABAA receptors in vivo using [3H]Ro 15-4513. Br J Pharmacol 2005;146:817–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Brooks-Kayal AR, Russek SJ. Regulation of GABAA receptor gene expression and Epilepsy In: th, Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, eds. Jasper’s Basic Mechanisms of the Epilepsies. Bethesda (MD): National Center for Biotechnology Information (US) [PubMed] [Google Scholar]
- 201.Grabenstatter HL, Cogswell M, Cruz Del Angel Y, et al. Effect of spontaneous seizures on GABAA receptor alpha4 subunit expression in an animal model of temporal lobe epilepsy. Epilepsia 2014;55:1826–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Grabenstatter HL, Del Angel YC, Carlsen J, et al. The effect of STAT3 inhibition on status epilepticus and subsequent spontaneous seizures in the pilocarpine model of acquired epilepsy. Neurobiol Dis 2014;62:73–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Grabenstatter HL, Russek SJ, Brooks-Kayal AR. Molecular pathways controlling inhibitory receptor expression. Epilepsia 2012;53 S9:71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Roberts DS, Raol YH, Bandyopadhyay S, et al. Egr3 stimulation of GABRA4 promoter activity as a mechanism for seizure-induced up-regulation of GABA(A) receptor alpha4 subunit expression. PNAS 2005;102:11894–11899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Lorenz D, Deuschl G. Update on pathogenesis and treatment of essential tremor. Curr Opin Neurol 2007;20:447–452. [DOI] [PubMed] [Google Scholar]
- 206.Lee CH, MacKinnon R. Structures of the human HCN1 hyperpolarization-activated channel. Cell 2017;168:111–120.e111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Biel M, Wahl-Schott C, Michalakis S, Zong X. Hyperpolarization-activated cation channels: from genes to function. Physiol Rev 2009;89:847–885. [DOI] [PubMed] [Google Scholar]
- 208.Notomi T, Shigemoto R. Immunohistochemical localization of Ih channel subunits, HCN1-4, in the rat brain. J Comp Neurol 2004;471:241–276. [DOI] [PubMed] [Google Scholar]
- 209.Zolles G, Wenzel D, Bildl W, et al. Association with the auxiliary subunit PEX5R/Trip8b controls responsiveness of HCN channels to cAMP and adrenergic stimulation. Neuron 2009;62:814–825. [DOI] [PubMed] [Google Scholar]
- 210.Heuermann RJ, Jaramillo TC, Ying SW, et al. Reduction of thalamic and cortical Ih by deletion of TRIP8b produces a mouse model of human absence epilepsy. Neurobiol Dis 2016;85:81–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Lyman KA, Han Y, Chetkovich DM. Animal models suggest the TRIP8b-HCN interaction is a therapeutic target for major depressive disorder. Expert Opin Ther Targets 2017;21:235–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Han Y, Heuermann RJ, Lyman KA, Fisher D, Ismail QA, Chetkovich DM. HCN-channel dendritic targeting requires bipartite interaction with TRIP8b and regulates antidepressant-like behavioral effects. Mol Psychiatry 2017;22:458–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Lyman KA, Han Y, Heuermann RJ, et al. Allostery between two binding sites in the ion channel subunit TRIP8b confers binding specificity to HCN channels. J Biol Chem 2017;292:17718–17730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Shaikh AG, Miura K, Optican LM, Ramat S, Tripp RM, Zee DS. Hypothetical membrane mechanisms in essential tremor. J Transl Med 2008;6:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.DiFrancesco D, Borer JS. The funny current: cellular basis for the control of heart rate. Drugs 2007;67 Suppl 2:15–24. [DOI] [PubMed] [Google Scholar]
- 216.Wahl-Schott C, Biel M. HCN channels: structure, cellular regulation and physiological function. Cell Mol Life Sci 2009;66:470–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Morgan JC, Sethi KD. Drug-induced tremors. Lancet Neurol 2005;4:866–876. [DOI] [PubMed] [Google Scholar]
- 218.Young RR, Growdon JH, Shahani BT. Beta-adrenergic mechanisms in action tremor. N Engl J Med 1975;293:950–953. [DOI] [PubMed] [Google Scholar]
- 219.Leigh PN, Jefferson D, Twomey A, Marsden CD. Beta-adrenoreceptor mechanisms in essential tremor; a double-blind placebo controlled trial of metoprolol, sotalol and atenolol. J Neurol Neurosurg Psychiatry 1983;46:710–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Drayer DE. Lipophilicity, hydrophilicity, and the central nervous system side effects of beta blockers. Pharmacotherapy 1987;7:87–91. [DOI] [PubMed] [Google Scholar]
- 221.Ondo W Essential tremor: what we can learn from current pharmacotherapy. Tremor Other Hyperkinet Mov 2016;6:356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Rainbow TC, Parsons B, Wolfe BB. Quantitative autoradiography of beta 1- and beta 2-adrenergic receptors in rat brain. PNAS 1984;81:1585–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Daly JW, Padgett W, Creveling CR, Cantacuzene D, Kirk KL. Cyclic AMP-generating systems: regional differences in activation by adrenergic receptors in rat brain. J Neurosci 1981;1:49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Long MA, Deans MR, Paul DL, Connors BW. Rhythmicity without synchrony in the electrically uncoupled inferior olive. J Neurosci 2002;22:10898–10905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Bal T, McCormick DA. Synchronized oscillations in the inferior olive are controlled by the hyperpolarization-activated cation current I(h). J Neurophysiol 1997;77:3145–3156. [DOI] [PubMed] [Google Scholar]
- 226.Luthi A, McCormick DA. H-current: properties of a neuronal and network pacemaker. Neuron 1998;21:9–12. [DOI] [PubMed] [Google Scholar]
- 227.Matsumoto-Makidono Y, Nakayama H, Yamasaki M, et al. Ionic basis for membrane potential resonance in neurons of the inferior olive. Cell Rep 2016;16:994–1004. [DOI] [PubMed] [Google Scholar]
- 228.Biggio G, Costa E, Guidotti A. Pharmacologically induced changes in the 3’:5’-cyclic guanosine monophosphate content of rat cerebellar cortex: difference between apomorphine, haloperidol and harmaline. J Pharmacol Exp Ther 1977;200:207–215. [PubMed] [Google Scholar]
- 229.Flora ED, Perera CL, Cameron AL, Maddern GJ. Deep brain stimulation for essential tremor: a systematic review. Mov Disord 2010;25:1550–1559. [DOI] [PubMed] [Google Scholar]
- 230.Lewis AS, Chetkovich DM. HCN channels in behavior and neurological disease: too hyper or not active enough? Mol Cell Neurosci 2011;46:357–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Ohno Y, Shimizu S, Tatara A, et al. Hcn1 is a tremorgenic genetic component in a rat model of essential tremor. PLoS One 2015;10:e0123529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Nishitani A, Tanaka M, Shimizu S, et al. Involvement of aspartoacylase in tremor expression in rats. Exp Anim 2016;65:293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Nava C, Dalle C, Rastetter A, et al. De novo mutations in HCN1 cause early infantile epileptic encephalopathy. Nat Genet 2014;46:640–645. [DOI] [PubMed] [Google Scholar]
- 234.Hopfner F, Deuschl G. Is essential tremor a single entity? Eur J Neurol 2018;25:71–82. [DOI] [PubMed] [Google Scholar]
- 235.Clark LN, Louis ED. Essential tremor. Handb Clin Neurol 2018;147:229–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Clark LN, Louis ED. Challenges in essential tremor genetics. Rev Neurol 2015;171:466–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Odgerel Z, Hernandez N, Park J, Ottman R, Louis ED, Clark LN. Whole genome sequencing and rare variant analysis in essential tremor families. bioRxiv The Preprint Server for Biology 2018;doi: 10.1101/248443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Bergareche A, Bednarz M, Sanchez E, et al. SCN4A pore mutation pathogenetically contributes to autosomal dominant essential tremor and may increase susceptibility to epilepsy. Hum Mol Genet 2015;24:7111–7120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Leng XR, Qi XH, Zhou YT, Wang YP. Gain-of-function mutation p.Arg225Cys in SCN11A causes familial episodic pain and contributes to essential tremor. J Hum Genet 2017;62:641–646. [DOI] [PubMed] [Google Scholar]
- 240.Liu X, Hernandez N, Kisselev S, et al. Identification of candidate genes for familial early-onset essential tremor. Eur J Hum Genet 2016;24:1009–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Trimmer JS. Subcellular localization of K+ channels in mammalian brain neurons: remarkable precision in the midst of extraordinary complexity. Neuron 2015;85:238–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Salinas M, Duprat F, Heurteaux C, Hugnot JP, Lazdunski M. New modulatory alpha subunits for mammalian Shab K+ channels. J Biol Chem 1997;272:24371–24379. [DOI] [PubMed] [Google Scholar]
- 243.Smith P, Arias R, Sonti S, et al. A Drosophila model of essential tremor. Sci Rep 2018; 8: 7664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Llinas RR. The intrinsic electrophysiological properties of mammalian neurons: insights into central nervous system function. Science 1988;242:1654–1664. [DOI] [PubMed] [Google Scholar]
- 245.Perez-Reyes E Molecular physiology of low-voltage-activated t-type calcium channels. Physiol Rev 2003;83:117–161. [DOI] [PubMed] [Google Scholar]
- 246.Sarnthein J, Jeanmonod D. High thalamocortical theta coherence in patients with Parkinson’s disease. J Neurosci 2007;27:124–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Sinton CM, Krosser BI, Walton KD, Llinas RR. The effectiveness of different isomers of octanol as blockers of harmaline-induced tremor. Pflugers Archiv 1989;414:31–36. [DOI] [PubMed] [Google Scholar]
- 248.Coutelier M, Blesneac I, Monteil A, et al. A recurrent mutation in CACNA1G alters Cav3.1 T-type calcium-channel conduction and causes autosomal-dominant cerebellar ataxia. Am J Hum Genet 2015;97:726–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Cain SM, Snutch TP. Voltage-gated calcium channels and disease. Biofactors 2011;37:197–205. [DOI] [PubMed] [Google Scholar]
- 250.Eckle VS, Shcheglovitov A, Vitko I, et al. Mechanisms by which a CACNA1H mutation in epilepsy patients increases seizure susceptibility. J Physiol 2014;592:795–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Huc S, Monteil A, Bidaud I, Barbara G, Chemin J, Lory P. Regulation of T-type calcium channels: signalling pathways and functional implications. Biochim Biophys Acta 2009;1793:947–952. [DOI] [PubMed] [Google Scholar]
- 252.Kumar P, Kumar D, Jha SK, Jha NK, Ambasta RK. Ion channels in neurological disorders. Adv Protein Chem Struct Biol 2016;103:97–136. [DOI] [PubMed] [Google Scholar]
- 253.Zhang Y, Zhang XF, Fleming MR, et al. Kv3.3 Channels bind Hax-1 and Arp2/3 to assemble a stable local actin network that regulates channel gating. Cell 2016;165:434–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Bergareche A, Bednarz M, Sanchez E, et al. SCN4A pore mutation pathogenetically contributes to autosomal dominant essential tremor and may increase susceptibility to epilepsy. Hum Mol Genet 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Bilen J, Bonini NM. Drosophila as a model for human neurodegenerative disease. Annu Rev Genet 2005;39:153–171. [DOI] [PubMed] [Google Scholar]
- 256.Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 1993;118:401–415. [DOI] [PubMed] [Google Scholar]
- 257.McGurk L, Berson A, Bonini NM. Drosophila as an In Vivo Model for human neurodegenerative disease. Genetics 2015;201:377–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Merner ND, Girard SL, Catoire H, et al. Exome sequencing identifies FUS mutations as a cause of essential tremor. Am J Hum Genet 2012;91:313–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Willcox BJ, Donlon TA, He Q, et al. FOXO3A genotype is strongly associated with human longevity. PNAS 2008;105:13987–13992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Reiter LT, Potocki L, Chien S, Gribskov M, Bier E. A systematic analysis of human disease-associated gene sequences in Drosophila melanogaster. Genome Res 2001;11:1114–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Holmes G The cerebellum of man. Brain 1939;62:1939. [Google Scholar]
- 262.Walker AE, Botterell EH. The syndrome of the superior cerebellar peduncle in the monkey. Brain 1937;60:329–353. [Google Scholar]
- 263.Thach WT, Goodkin HP, Keating JG. The cerebellum and the adaptive coordination of movement. Annu Rev Neurosci 1992;15:403–442. [DOI] [PubMed] [Google Scholar]
- 264.Goldberger ME, Growdon JH. Pattern of recovery following cerebellar deep nuclear lesions in monkeys. Exp Neurol 1973;39:307–322. [DOI] [PubMed] [Google Scholar]
- 265.Vilis T, Hore J. Central neural mechanisms contributing to cerebellar tremor produced by limb perturbations. J Neurophysiol 1980;43:279–291. [DOI] [PubMed] [Google Scholar]
- 266.Vilis T, Hore J. Effects of changes in mechanical state of limb on cerebellar intention tremor. J Neurophysiol 1977;40:1214–1224. [DOI] [PubMed] [Google Scholar]
- 267.Gemba H, Sasaki K, Yoneda Y, Hashimoto S, Mizuno N. Tremor in the monkey with a cerebellar lesion. Exp Neurol 1980;69:173–182. [DOI] [PubMed] [Google Scholar]
- 268.Monzee J, Drew T, Smith AM. Effects of muscimol inactivation of the cerebellar nuclei on precision grip. J Neurophysiol 2004;91:1240–1249. [DOI] [PubMed] [Google Scholar]
- 269.Elble RJ, Schieber MH, Thach WT Jr. Activity of muscle spindles, motor cortex and cerebellar nuclei during action tremor. Brain Res 1984;323:330–334. [DOI] [PubMed] [Google Scholar]
- 270.Matsushita M, Iwahori N. Structural organization of the interpositus and the dentate nuclei. Brain Res 1971;35:17–36. [DOI] [PubMed] [Google Scholar]
- 271.Harvey RJ, Porter R, Rawson JA. Discharges of intracerebellar nuclear cells in monkeys. J Physiol 1979;297:559–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Flament D, Vilis T, Hore J. Dependence of cerebellar tremor on proprioceptive but not visual feedback. Exp Neurol 1984;84:314–325. [DOI] [PubMed] [Google Scholar]
- 273.Elble RJ, Deuschl G. Tremor In: Brown WF, Bolton CF, Aminoff M, eds. Neuromuscular function and disease: basic, clinical and electrodiagnostic aspects. Philadelphia: W. B. Saunders Co., 2002: 1759–1779. [Google Scholar]
- 274.Hore J, Flament D. Changes in motor cortex neural discharge associated with the development of cerebellar limb ataxia. J Neurophysiol 1988;60:1285–1302. [DOI] [PubMed] [Google Scholar]
- 275.Gorassini M, Prochazka A, Taylor JL. Cerebellar ataxia and muscle spindle sensitivity. J Neurophysiol 1993;70:1853–1862. [DOI] [PubMed] [Google Scholar]
- 276.Zackowski KM, Bastian AJ, Hakimian S, et al. Thalamic stimulation reduces essential tremor but not the delayed antagonist muscle timing. Neurology 2002;58:402–410. [DOI] [PubMed] [Google Scholar]
- 277.Groppa S, Herzog J, Falk D, Riedel C, Deuschl G, Volkmann J. Physiological and anatomical decomposition of subthalamic neurostimulation effects in essential tremor. Brain 2014;137:109–121. [DOI] [PubMed] [Google Scholar]
- 278.Li Volsi G, Pacitti C, Perciavalle V, Sapienza S, Urbano A. Interpositus nucleus influences on pyramidal tract neurons in the cat. Neuroscience 1982;7:1929–1936. [DOI] [PubMed] [Google Scholar]
- 279.Carpenter MB, Hanna GR. Effects of thalamic lesions upon cerebellar dyskinesia in the rhesus monkey. J Comp Neurol 1962;119:127–147. [DOI] [PubMed] [Google Scholar]
- 280.Asanuma C, Thach WT, Jones EG. Distribution of cerebellar terminations and their relation to other afferent terminations in the ventral lateral thalamic region of the monkey. Brain Res 1983;286:237–265. [DOI] [PubMed] [Google Scholar]
- 281.Stepniewska I, Sakai ST, Qi HX, Kaas JH. Somatosensory input to the ventrolateral thalamic region in the macaque monkey: potential substrate for parkinsonian tremor. J Comp Neurol 2003;455:378–395. [DOI] [PubMed] [Google Scholar]
- 282.Elble RJ. Tremor disorders. Curr Opin Neurol 2013;26:413–419. [DOI] [PubMed] [Google Scholar]
- 283.Voogd J What we do not know about cerebellar systems neuroscience. Front Syst Neurosci 2014;8:227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Schieber MH, Thach WT Jr. Trained slow tracking. II. Bidirectional discharge patterns of cerebellar nuclear, motor cortex, and spindle afferent neurons. J Neurophysiol 1985;54:1228–1270. [DOI] [PubMed] [Google Scholar]
- 285.Thach WT, Schieber MH, Mink J, Kane S, Horne M. Cerebellar relation to muscle spindles in hand tracking. Prog Brain Res 1986;64:217–224. [DOI] [PubMed] [Google Scholar]
- 286.Ohye C, Shibazaki T, Hirai T, et al. A special role of the parvocellular red nucleus in lesion-induced spontaneous tremor in monkeys. Behav Brain Res 1988;28:241–243. [DOI] [PubMed] [Google Scholar]
- 287.Nathan PW, Smith MC. The rubrospinal and central tegmental tracts in man. Brain 1982;105:223–269. [DOI] [PubMed] [Google Scholar]
- 288.Carpenter MB. A study of the red nucleus in the rhesus monkey; anatomic degenerations and physiologic effects resulting from localized lesions of the red nucleus. J Comp Neurol 1956;105:195–249. [DOI] [PubMed] [Google Scholar]
- 289.Carpenter MB, Correll JW. Spinal pathways mediating cerebellar dyskinesia in rhesus monkey. J Neurophysiol 1961;24:534–551. [DOI] [PubMed] [Google Scholar]
- 290.Hakimian S, Norris SA, Greger B, Keating JG, Anderson CH, Thach WT. Time and frequency characteristics of Purkinje cell complex spikes in the awake monkey performing a nonperiodic task. J Neurophysiol 2008;100:1032–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Keating JG, Thach WT. Nonclock behavior of inferior olive neurons: interspike interval of Purkinje cell complex spike discharge in the awake behaving monkey is random. J Neurophysiol 1995;73:1329–1340. [DOI] [PubMed] [Google Scholar]
- 292.Keating JG, Thach WT. No clock signal in the discharge of neurons in the deep cerebellar nuclei. J Neurophysiol 1997;77:2232–2234. [DOI] [PubMed] [Google Scholar]
- 293.Musacchio T, Purrer V, Papagianni A, et al. Non-motor symptoms of essential tremor are independent of tremor severity and have an impact on quality of life. Tremor Other Hyperkinet Mov 2016;6:361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Frison G, Favretto D, Zancanaro F, Fazzin G, Ferrara SD. A case of beta-carboline alkaloid intoxication following ingestion of Peganum harmala seed extract. Forensic Sci Int 2008;179:e37–43. [DOI] [PubMed] [Google Scholar]
- 295.Haubenberger D, Hallett M. Essential Tremor. N Engl J Med 2018;378:1802–1810. [DOI] [PubMed] [Google Scholar]
- 296.Fekete R, Jankovic J. Revisiting the relationship between essential tremor and Parkinson’s disease. Mov Disord 2011;26:391–398. [DOI] [PubMed] [Google Scholar]