Abstract
Purpose:
To identify biological factors that may yield a therapeutic advantage of proton therapy versus photon therapy. Specifically, the role of non-homologous end-joining (NHEJ) and homologous recombination (HR) in the survival of cells in response to clinical photon and proton beams.
Methods and Materials:
We irradiated HT1080, M059K (DNA-PKcs+/+), HCC1937 human cancer cell linesand their isogenic counterparts HT1080-shDNA-PKcs, HT1080-shRAD51IND, M059J (DNA-PKcs−/−) and HCC1937-BRCA1 (BRCA1 complemented) to assess cell clonogenic survival and γ-H2AX radiation-induced foci (RIF). Cells were irradiated with either clinically relevant photons or one of three proton linear energy transfer (LET) values.
Results:
Our results indicate that NHEJ deficiency is more important in dictating cell survival than proton LET. Cells with disrupted HR through BRCA1 mutation showed increased radiosensitivity only for high-LET protons whereas RAD51 depletion showed increased radiosensitivity for both photons and protons. DNA double strand breaks (DSBs), assessed by γ-H2AX-RIF, showed greater numbers after 24 h in cells exposed to higher LET protons. We also observed that NHEJ-deficient cells were unable to repair the vast majority of DSBs after 24 h.
Conclusions:
BRCA1 mutation significantly sensitizes cells to protons but not photons. Loss of NHEJ renders cells hypersensitive to radiation, whereas the relative importance of HR increases with LET across several cell lines. This may be attributable to the more clustered damage induced by higher LET protons which are harder to repair through NHEJ. This highlights the importance of tumor biology in dictating treatment modality, as well as suggesting BRCA1 as a potential biomarker for proton therapy response. Our data also supports the use of pharmacologic inhibitors of DNA repair to enhance the sensitivity to different radiation types but also raises issues for normal tissue toxicity.
Keywords: Proton therapy, radiation, DNA repair, homologous recombination, non-homologous end joining
INTRODUCTION
Proton beams are more likely to generate clustered DNA lesions, which include single (SSBs) and double (DSBs) strand breaks, and base damage, all clustered within a region of 10 to 20 base pairs, whereas photons produce relatively fewer clustered DNA lesions (1). Several studies have characterized the increase in clustered DSB lesions as a function of linear energy transfer (LET) (2–7). Failure to repair these lesions in a timely fashion leads to greater probability of misrepair and consequently cell death (8). The outcome may be determined by a cell’s tolerance of DNA damage, which is thought to be dictated by the ability of a cell to repair its DNA and thresholds for death and survival signals within each cell line and even at the individual cell level (8).
Cells have numerous mechanisms to repair different types of lesions in nuclear DNA, which in turn promote cell survival (8). The two canonical pathways of DSB DNA repair are non-homologous end-joining (NHEJ) and homologous recombination (HR). NHEJ operates in all phases of the cell cycle (9) and involves bridging of two DNA ends followed by the creation of blunt ends and subsequent ligation. NHEJ is dependent upon the DNA protein kinase catalytic subunit (DNA-PKcs) (10). In HR, DNA is copied from a homologous DNA strand, requiring the cell to be in the S or G2 phase of the cell cycle (11), a process that operates with slower temporal kinetics than NHEJ (11). Proteins such as BRCA1 and RAD51 are involved in HR repair (12).
In the context of clustered DSB lesions, HR resects much of the clustered DSB lesion and instigates repair based on homologous sequences from the homologous template, whereas NHEJ is inhibited owing to lack of binding substrates, or competing repair processes operating on different lesions. Several studies have shown that in non-human cell lines NHEJ is the main pathway to repair radiation-induced damage independently of LET (13–16). Fontana et al. (17) found that NHEJ deficiency in human cell lines through defects in DNA-PKcs makes cells extremely sensitive to photons and protons. They also showed that HR deficiency through RAD51 silencing or treatment with SAHA makes cells more sensitive to protons than photons and they suggested that HR defects can more specifically sensitize cells to protons. However, the Fontana et al. study was limited to a single proton-LET located in the middle of a spread out Bragg peak (SOBP). Also they pointed out that no HR inhibitors that directly and specifically target HR are currently available. Differently than the previous work, we demonstrate that BRCA1 mutation, a clinically relevant gene that causes HR deficiency, preferentially sensitizes cells to high-LET protons but not to photons. We also show that NHEJ is by far the most important pathway to repair radiation-induced damage – independently of proton-LET, but the role of HR relative to NHEJ increases as a function of proton-LET.
METHODS AND MATERIALS
We used the human cancer cell lines M059J, M059K, HCC1937, and HT1080. M059K and M059J glioblastoma cells are an isogenic pair isolated from the same patient, with M059J lacking DNA-PKcs activity (18,19). HCC1937 is a triple-negative breast cancer cell line with a mutation in the BRCA1 gene (20). We also used isogenic HT1080 in which RAD51 or PRKDC were silenced (HT1080-shRAD51IND and HT1080-shDNA-PKcs, respectively) (21) as well as an HCC1937 isogenic cell line in which BRCA1 has been restored (HCC1937-BRCA1) (20). All cell lines were authenticated and confirmed to be negative for mycoplasma contamination at the XXXX facility. Further details on cell culture are available in Supplemental Method 1.
For clinical relevance, we used clinical photon (6-MV x-ray at 10 cm depth) and proton (fluence-weighted LET values of 1.1, 2.5, and 7.3 keV/μm) beams throughout this work (Supplemental Method 2–4).
Clonogenic cell survival assays were conducted for all cell lines. At least three independent biological replicates were performed. Each replicate was done at least in triplicate (Supplemental Method 5). γ-H2AX radiation induced foci (RIF) (marker for DSBs) were assessed for the M059K and M059J cell lines (Supplemental Method 6). Clonogenic survival data was fit using the linear-quadratic model. To quantify cell survival, we used the dose at which the survival fraction falls to 10% (D10%). To characterize the role of HR versus NHEJ as a function of LET, we defined the sensitization enhancement ratio (SER) as the ratio between the D10% for DNA-repair proficient cells and D10% for DNA-repair deficient cells for a given radiation condition. This factor quantifies the sensitization of a given treatment and/or DNA repair deficiency relative to the DNA repair proficient exposed to photons.
Statistical analysis was performed using GraphPad Prism (GraphPad Software, Inc., San Diego, CA). Quantities of interest, including parameters of the cell survival curve (D10% and SER) and foci number, were compared using a two-tailed Student’s t-test. A statistically significant difference was defined at P<0.05. All error bars and quoted uncertainties are represented as one standard deviation of the mean.
RESULTS
HR deficiency sensitizes cells to high-LET protons
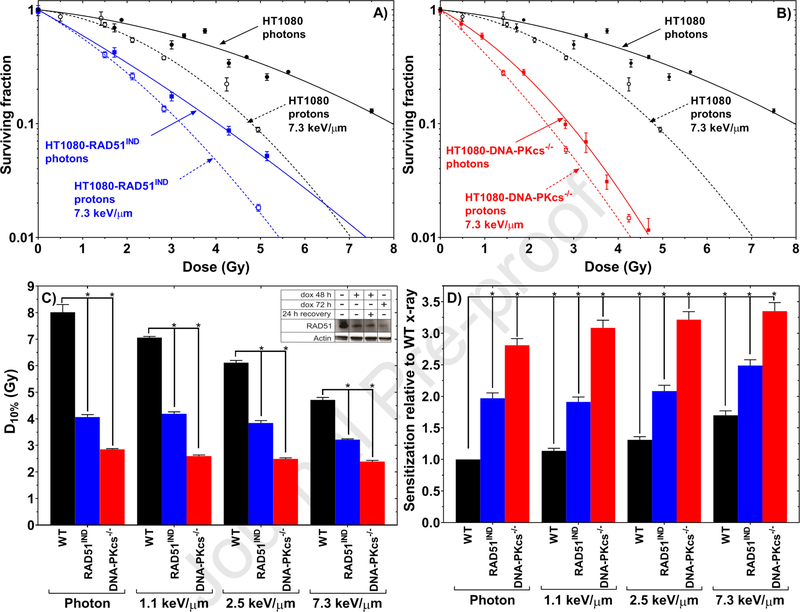
HT1080 cells were found to be more sensitive to protons than photons; silencing RAD51 increased the sensitivity to both radiation types, with protons producing higher sensitization (Fig. 1A). Similar effects were observed for silencing DNA-PKcs, although those cells were generally more radiosensitive (Fig. 1B). HR- deficient HT1080-shRAD51IND cells were 1.98 ± 0.08 (P<0.001) times more sensitive to photons compared with their HR-proficient counterpart (Fig. 1C–D). HT1080-shRAD51IND cells were 2.49 ± 0.09 (P < 0.001) times more sensitive to high-LET protons (7.3 keV/μm) compared with their HR-proficient counterpart exposed to photons. When we examined response of HR-proficient cells to high-LET protons (7.3 keV/μm), sensitivity was increased by only 1.71±0.07 (P<0.001) fold over response to photons. In addition to D10%, other aspects of the survival curve such as D50% and the surviving fraction at 2 Gy showed similar trends (Supplementary Table 2–3).
Fig. 1.
(A) Clonogenic cell survival curves of isogenic pairs of HT1080 cells that are either proficient in HR or deficient in HR (HT1080-shRAD51IND) exposed to photons or 7.3 keV/μm protons after doxycycline treatment. (B) Clonogenic cell survival curves of isogenic pairs of HT1080 cells that are either NHEJ-proficient (HT1080) or -deficient (HT1080-shDNA-PKcs) exposed to photons or to high-LET protons after doxycycline treatment. (C) D10% from the clonogenic survival curves of HT1080, HT1080-shRAD51IND, and HT1080-shDNA-PKcs cells after exposure to photons or protons at three LET values. Inset, western blots to confirm depletion of RAD51 expression after doxycycline treatment (Supplemental Method 7). (D) Sensitization relative to wild-type (WT) (DNA repair-proficient) cells exposed to photons. Note that for comparison and visualization the HT1080 photon data is repeated in A and B.
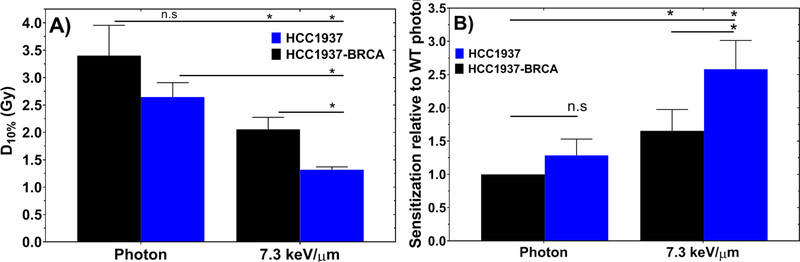
We further evaluated the role of defects in HR in cell lines irradiated with low-LET photons and high-LET protons using the HCC1937 and HCC1937-BRCA1 isogenic pair. HR-deficient HCC1937 cells and HR-proficient HCC1937-BRCA1 cells showed no significant difference (1.29±0.24, P>0.2 – n.s.) in response to photons but the HR-deficient HCC1937 cells were 1.55 ± 0.17 (P<0.05) times more sensitive to high-LET protons (7.3 keV/μm) than were HCC1937-BRCA cell lines also exposed to high-LET protons (Fig. 2).
Fig. 2.
(A) D10% from the clonogenic survival curves of HCC1937 and HCC1937-BRCA cells after exposure to photons or protons. (B) Sensitization relative to wild-type (WT) (DNA repair-proficient) cells exposed to photons.
NHEJ is the primary pathway for repairing low- and high-LET radiation-induced DNA damage
NHEJ-deficient HT1080-shDNA-PKcs cells were 2.81±0.11 (P<0.001) times more sensitive to photons than were their NHEJ-proficient counterpart (Fig. 1D). Further, high-LET protons (7.3 keV/μm) sensitized the NHEJ-deficient HT1080-shDNA-PKcs cells by 3.35±0.14 (P<0.001) times compared with its NHEJ-proficient HT1080 counterpart exposed to photons (Fig. 1D). Notably, NHEJ-deficient HT1080-shDNA-PKcs cells were 2.81±0.10 times more sensitive to photons than its NHEJ-proficient HT1080 counterpart also exposed to photons. Thus, the sensitivity of HT1080-shDNA-PKcs cells varied by only 1.19±0.07 between photons and high-LET protons (7.3 keV/μm) (Fig. 1D).
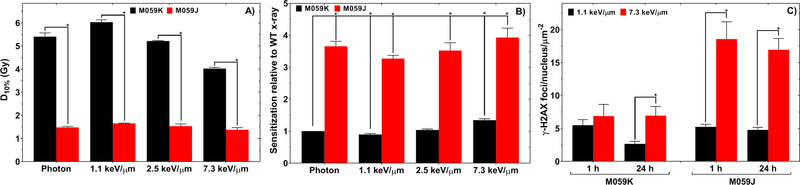
We further confirmed that NHEJ is the main repair pathway for promoting cell survival in response to both low- and high-LET radiation by using M059K and M059J cells. The response of NHEJ-deficient M059J cells to photons (D10% = 1.48±0.05 Gy) was no different (P>0.05) than the response to protons (D10% = 1.37±0.09 Gy) (Fig. 3A). In the M059K cell line we found a similar response as was found in the HT1080-shDNA-PKcs cell line, with inability to perform NHEJ being associated with a 3.9±0.3 (P<0.001) fold change in response to protons compared with the NHEJ-proficient M059K counterpart exposed to photons (Fig. 3B).
Fig. 3.
(A) D10% from the clonogenic survival curves of M059K and M059J cell lines. Cells were exposed to photons or one of three proton-LET values. (B) Sensitization relative to the photon exposed M059K cell. (C) γ-H2AX RIF at 1 h and 24 h after irradiation with 1.1 keV/μm or 7.3-keV/μm protons in M059K and M059J cell lines.
We found that at 7.3 keV/μm, M059J cells had 2.4±0.5 (P<0.05) times more persistent radiation-induced γ-H2AX foci at 24 h relative to M059K cells (Fig. 3C and Supplementary Fig. 2), indicating that M059J cells cannot effectively repair radiation-induced DSBs. At 1 h and 24 h after irradiation, there were 3.5±0.6 (P<0.001) and 3.5±0.5 (P<0.001) more foci per M059J nucleus per particle for 7.3 keV/μm compared with 1.1 keV/μm protons, respectively. Our foci data also showed that on average, the γ-H2AX foci surface area for cell lines exposed to 7.3 keV/μm were larger than those for the 1.1-keV/μm protons (Supplementary Fig. 2).
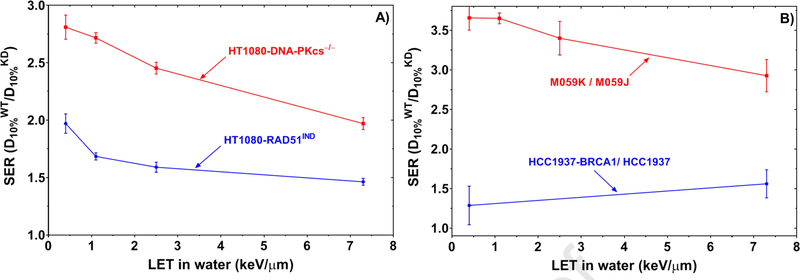
The role of HR increases with LET
For NHEJ-deficient cells, the SER decreased as a function of LET (Fig. 4A), indicating that the contribution of NHEJ relative to other repair pathways decreases with LET. The SER for HR-deficient cells reached a plateau for the LET range from 2.5 keV/μm to the highest LET we investigated in this work (7.3 keV/μm), suggesting that the role of HR relative to NHEJ increases with LET. DNA-repair-deficient cells showed a larger effect for low-LET photons than for high-LET protons except for the HCC1937 cell line, in which the SER increased for high-LET radiation (Fig. 4B). This finding is in line with previous reports that high-LET radiation produces DNA lesions that are much harder to repair than those produced by low-LET radiation (13,16).
Fig. 4.
(A) SER values for HT1080-shRAD51IND (HR-deficient) and HT1080-shDNA-PKcs (NHEJ-deficient) cells relative to their wild-type HT1080 cells (DNA repair-proficient). (B) SER values for M059J cells (NHEJ-deficient) relative to M059K cells (NHEJ-proficient) and HCC1937 cells (HR-deficient) relative to HCC1937-BRCA1 cells (HR-proficient).
DISCUSSION
Differently than other studies that used non-human cell lines (13–16), our study focused on understanding the relationship between clinically relevant proton LET and DNA repair defects in human cancer cell lines. We demonstrated for the first time that BRCA1 mutation affects the response to protons but not to photons. This has direct clinical implications in that our data suggests that patients with germline BRCA1 mutations may respond better to protons than photons. On the other hand, RAD51 depletion affects the response to both photons and protons, which confirms previous results (17). We also showed that DNA-PKcs is of critical importance for survival following radiation exposure irrespective of LET (17). Our findings strongly support that biological factors such as DNA repair defects may be as important, or even more important, than physical factors such as LET in dictating response to radiation in human cancer cell lines.
When DSB repair pathways were disrupted, radiosensitivity increased regardless of radiation quality. This effect was greatest for NHEJ deficiency, but in the setting of increased LET, the relative importance of HR increased. Other studies have described an increase in DSB clusters after high-LET protons relative to low- LET photons and that these lesions may require HR to perform satisfactory repair (13,17,22,23). Here we show that 7.3 keV/μm protons are 3.5±0.6 (average of 1 h and 24 h measurements) times more efficient in creating DSB RIF than were 1.3 keV/μm protons (Fig. 3C). To the best of our knowledge, this is the first time that RIF efficiency has been quantified in clinical proton beams. Our approach used detailed Monte Carlo simulations of the experimental conditions combined with accurate dosimetry to determine the fluence of protons at the cell’s irradiation geometry (Supplemental Method 3–4).
To assess how the repair of radiation-induced DNA damage, in particular DSB repair, is affected by the DNA repair pathway status, we investigated the relevance of HR versus NHEJ. Cells that are deficient in NHEJ are sensitive to both photons and protons, which supports the theory that NHEJ has a major role in the repair of low- and high-LET radiation in human cancer cell lines. Indeed, our findings indicate that NHEJ is by far the most important pathway for repairing radiation-induced DSBs, independent of proton-LET. This agrees with other studies in non-human cell lines that targeted the same pathways (15,16). This was also observed in response to a variety of heavier ions including carbon and neon at much higher LET values compared to the LET values from this study (15,16,24,25). Over the range of LET values investigated here, NHEJ remained the most important pathway, but its relative role decreased as LET increased (Fig. 4A and B). HT1080-shDNA-PKcs and M059J cells were only marginally sensitized (1.19±0.07 and 1.08±0.09, respectively) after high-LET protons (7.3 keV/μm) compared to photons, which indicates that NHEJ deficiency is more important than proton LET in dictating cell survival (Fig. 1D and Fig. 3B). Higher-LET radiation can produce short DSB fragments that may prevent the Ku protein from efficiently binding to DSB sites (24). Ku binding is an essential step in canonical NHEJ and recruiting DNA-PKcs. However, survival in DNA-PKcs-deficient cells differs little after photon versus proton exposure, regardless of LET. This suggests that NHEJ repairs most lethal lesions. When this pathway is disrupted, it seems that alternatives to canonical NHEJ such as microhomology-mediated end joining or HR cannot accommodate the loss of NHEJ and thus small numbers of lesions seem to be enough to establish cell death, on which the LET has little impact.
Our findings further showed that the role of HR increases as a function of LET. This was likely a consequence of more clustered lesions induced by higher LET radiation. In fact others have demonstrated that HR deficiency through XRCC3 mutation resulted in an increased amount of persistent DSB foci after protons versus photons (13). HR deficiency in our HT1080 system renders cells more radiosensitive, with greater radiosensitization from high-LET relative to low-LET. Our data suggests that this is dependent on the point at which the pathway is inhibited because BRCA1 mutation rendered significant radiosensitization to high-LET protons only. Combining HR deficiency with high-LET radiation suggests a larger differential response relative to low-LET radiation, although the magnitude of the radiosensitization is lower than NHEJ deficiency. Notably, for germline mutations that cause HR or NHEJ deficiency or systemic pharmacologic inhibition of these pathways, care should be taken with respect to normal tissue toxicity (26), because this greater radiosensitization is predicted to occur across all tissues.
We investigated two aspects of HR deficiency, before and after DNA end resection (BRCA1 and RAD51, respectively). Important proteins that dictate the pathway chosen include BRCA1, which functions before DNA end resection (27). Mutant BRCA1 was previously reported to generate protein products that are still able to perform some wild type functions but lack tumor suppressor activity (28). Our results indicate that HR deficiency via BRCA1 mutation causes cells to be more sensitive to protons compared with the isogenic cell line in which BRCA1 is restored, but minimal differences were noted for photons. We believe that high-LET protons create a population of clustered DSB lesions that require HR, clusters that are not present in low-LET protons or photons. Mechanistically, it is possible that mutant BRCA1 fails to aid in the de-phosphorylation of 53BP1 that has accumulated at DNA DSBs (27). As a result, the cell cannot make the switch to HR owing to the continual presence of 53BP1 and inhibition of end resection, resulting in cell death (27). Previous studies by others have shown increased sensitivity of HCC1937 versus HCC1937-BRCA1 to137Cs γ-irradiation (29,30). Our results show no significant difference in response to 6 MV photons, which are similar in LET to 137Cs γ-irradiation. We believe this discrepancy could be due to differences in culture conditions and colony counting, including the time allowed for colony formation, and how colonies were defined.
Partial depletion of RAD51, on the other hand, showed a radiosensitization effect for both photons and protons, with the effect enhanced for protons. RAD51 operates after resection around the double-strand lesion and is therefore important after a cell initiates HR repair (31). The selection of HR in response to simple DSB lesions may reflect the presence of available substrates and the cell-cycle stage (32). However, if a cell commits to HR with extensive end resection and is subsequently unable to complete the process, this may lead to an unrepaired lesion, failed replication, and cell death (6,33).
In summary, we showed that BRCA1 mutation renders radiosensitivity to protons but not photons in the HCC1937 cell line. We also showed that in multiple distinct human cell lines, DNA repair status is an important driver of radiosensitivity – more so than LET. These results agree with the results from Fontana et al. (17) and published data using non-human cell lines across a variety of particle beams and LET values (13–16). Deficiency in NHEJ in particular makes cells more radiosensitive to both low- and high-LET radiation. On the other hand, the relative role of HR versus NHEJ increases as a function of LET, but NHEJ remains the main DSB repair pathway. These effects may be mimicked by using pharmacologic inhibitors of critical DNA repair proteins, a result with potential impact for translational research, particularly in ongoing clinical trials (e.g., NCT02516813). These results also highlight potential issues with the current RBE associated with proton beams, currently set at 1.1. Our data highlight that RBE may be less than 1.1 at LET values associated with SOBP regions. However, at the end of the proton range RBE likely exceeds 1.1 as shown by our data (Supplementary Table 5). These high RBE values require special attention when proton beams are aimed at critical structures. Our results corroborate that RBE has a strong dependence on DNA repair capacity and type of cancer cell line but also demonstrate that it depends on specific DNA repair proteins. Our results also highlight that combination of radiation with DNA repair deficiency either from pharmacological inhibition or germline mutations may increase normal tissue toxicity. It is then essential to use the state of art radiotherapy delivery techniques to spare normal tissue. We encourage additional translational research evaluating the combination of DNA repair inhibitors in conjunction with LET modulation. Clinical translation will require further studies in animal models, particularly immune-competent animals, to understand potential micro-environmental effects in combination with systemic immune responses, which have now been intimately linked to DNA damage (21,34,35).
Supplementary Material
ACKNOWLEDGMENTS
This work was supported in part by funds from: the Cancer Prevention and Research Institute of Texas grant RP170040 (G.O.S.); the Division of Radiation Oncology, MD Anderson (R.M., D.R.G. and G.O.S.); the University Cancer Foundation via the Sister Institution Network Fund at the University of Texas MD Anderson Cancer Center (G.O.S.); the Cancer Center Support (Core) Grant CA016672 to MD Anderson. The authors thank Dr. Shane R. Stecklein from The University of Kansas Cancer Center for providing the HCC1937-BRCA1 cell line. Dr. Narayan Sahoo at the MD Anderson Proton Therapy Center for proton beam-time scheduling at the MD Anderson Proton Center, and Christine F. Wogan of the Division of Radiation Oncology at MD Anderson for editing the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
COMPETING INTERESTS
G.O.S. has research funds from Elekta AB and S.F.S. has research funds from Varian Medical Systems, Inc.
REFERENCES
- 1.Mavragani IV, Nikitaki Z, Souli MP, et al. Complex DNA damage: A route to radiation-induced genomic instability and carcinogenesis. Cancers (Basel) 2017;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hada M, Georgakilas AG Formation of clustered DNA damage after high-let irradiation: A review. J Radiat Res 2008;49:203–10. [DOI] [PubMed] [Google Scholar]
- 3.Carter RJ, Nickson CM, Thompson JM, et al. Complex DNA damage induced by high linear energy transfer alpha-particles and protons triggers a specific cellular DNA damage response. Int J Radiat Oncol Biol Phys 2018;100:776–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lorat Y, Timm S, Jakob B, et al. Clustered double-strand breaks in heterochromatin perturb DNA repair after high linear energy transfer irradiation. Radiother Oncol 2016;121:154–161. [DOI] [PubMed] [Google Scholar]
- 5.Friedland W, Schmitt E, Kundrat P, et al. Comprehensive track-structure based evaluation of DNA damage by light ions from radiotherapy-relevant energies down to stopping. Sci Rep 2017;7:45161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asaithamby A, Hu B, Chen DJ Unrepaired clustered DNA lesions induce chromosome breakage in human cells. Proceedings of the National Academy of Sciences of the United States of America 2011;108:8293–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okayasu R, Okada M, Okabe A, et al. Repair of DNA damage induced by accelerated heavy ions in mammalian cells proficient and deficient in the non-homologous end-joining pathway. Radiation research 2006;165:59–67. [DOI] [PubMed] [Google Scholar]
- 8.Roos WP, Thomas AD, Kaina B. DNA damage and the balance between survival and death in cancer biology. Nat Rev Cancer 2016;16:20–33. [DOI] [PubMed] [Google Scholar]
- 9.Löbrich M, Jeggo P A process of resection-dependent nonhomologous end joining involving the goddess artemis. Trends in biochemical sciences 2017;42:690–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang HHY, Pannunzio NR, Adachi N, et al. Non-homologous DNA end joining and alternative pathways to double-strand break repair. Nat Rev Mol Cell Biol 2017;18:495–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jeggo PA, Geuting V, Lobrich M The role of homologous recombination in radiation-induced double-strand break repair. Radiother Oncol 2011;101:7–12. [DOI] [PubMed] [Google Scholar]
- 12.Shibata A Regulation of repair pathway choice at two-ended DNA double-strand breaks. Mutation research 2017;803–805:51–55. [DOI] [PubMed] [Google Scholar]
- 13.Grosse N, Fontana AO, Hug EB, et al. Deficiency in homologous recombination renders mammalian cells more sensitive to proton versus photon irradiation. Int J Radiat Oncol Biol Phys 2014;88:175–81. [DOI] [PubMed] [Google Scholar]
- 14.Genet SC, Maeda J, Fujisawa H, et al. Comparison of cellular lethality in DNA repair-proficient or -deficient cell lines resulting from exposure to 70 mev/n protons or 290 mev/n carbon ions. Oncology reports 2012;28:1591–6. [DOI] [PubMed] [Google Scholar]
- 15.Maeda J, Fujii Y, Fujisawa H, et al. Hyperthermia-induced radiosensitization in cho wild-type, nhej repair mutant and hr repair mutant following proton and carbon-ion exposure. Oncology letters 2015;10:2828–2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gerelchuluun A, Manabe E, Ishikawa T, et al. The major DNA repair pathway after both proton and carbon- ion radiation is nhej, but the hr pathway is more relevant in carbon ions. Radiat Res 2015;183:345–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fontana AO, Augsburger MA, Grosse N, et al. Differential DNA repair pathway choice in cancer cells after proton- and photon-irradiation. Radiother Oncol 2015;116:374–80. [DOI] [PubMed] [Google Scholar]
- 18.Allalunis-Turner MJ, Barron GM, Day RS 3rd,, et al. Isolation of two cell lines from a human malignant glioma specimen differing in sensitivity to radiation and chemotherapeutic drugs. Radiat Res 1993;134:349–54. [PubMed] [Google Scholar]
- 19.Lees-Miller SP, Godbout R, Chan DW, et al. Absence of p350 subunit of DNA-activated protein kinase from a radiosensitive human cell line. Science 1995;267:1183–5. [DOI] [PubMed] [Google Scholar]
- 20.Stecklein SR, Kumaraswamy E, Behbod F, et al. Brca1 and hsp90 cooperate in homologous and non-homologous DNA double-strand-break repair and g2/m checkpoint activation. Proceedings of the National Academy of Sciences of the United States of America 2012;109:13650–13655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhattacharya S, Srinivasan K, Abdisalaam S, et al. Rad51 interconnects between DNA replication, DNA repair and immunity. Nucleic Acids Res 2017;45:4590–4605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hojo H, Dohmae T, Hotta K, et al. Difference in the relative biological effectiveness and DNA damage repair processes in response to proton beam therapy according to the positions of the spread out bragg peak. Radiat Oncol 2017;12:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Q, Ghosh P, Magpayo N, et al. Lung cancer cell line screen links fanconi anemia/brca pathway defects to increased relative biological effectiveness of proton radiation. International Journal of Radiation Oncology*Biology*Physics 2015;91:1081–1089. [DOI] [PubMed] [Google Scholar]
- 24.Wang H, Wang X, Zhang P, et al. The ku-dependent non-homologous end-joining but not other repair pathway is inhibited by high linear energy transfer ionizing radiation. DNA Repair 2008;7:725–733. [DOI] [PubMed] [Google Scholar]
- 25.Takahashi A, Kubo M, Ma H, et al. Nonhomologous end-joining repair plays a more important role than homologous recombination repair in defining radiosensitivity after exposure to high-let radiation. Radiat Res 2014;182:338–344. [DOI] [PubMed] [Google Scholar]
- 26.Brown JM Beware of clinical trials of DNA repair inhibitors. International Journal of Radiation Oncology Biology Physics 2019;103:1182–1183. [DOI] [PubMed] [Google Scholar]
- 27.Isono M, Niimi A, Oike T, et al. Brca1 directs the repair pathway to homologous recombination by promoting 53bp1 dephosphorylation. Cell Rep 2017;18:520–532. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y, Krais JJ, Bernhardy AJ, et al. Ring domain–deficient brca1 promotes parp inhibitor and platinum resistance. The Journal of Clinical Investigation 2016;126:3145–3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DelloRusso C, Welcsh PL, Wang W, et al. Functional characterization of a novel brca1-null ovarian cancer cell line in response to ionizing radiation. Molecular Cancer Research 2007;5:35. [DOI] [PubMed] [Google Scholar]
- 30.Scully R, Ganesan S, Vlasakova K, et al. Genetic analysis of brca1 function in a defined tumor cell line. Molecular Cell 1999;4:1093–1099. [DOI] [PubMed] [Google Scholar]
- 31.Li X, Heyer WD Homologous recombination in DNA repair and DNA damage tolerance. Cell Res 2008;18:99–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hustedt N, Durocher D The control of DNA repair by the cell cycle. Nature Cell Biology 2016;19:1. [DOI] [PubMed] [Google Scholar]
- 33.Biehs R, Steinlage M, Barton O, et al. DNA double-strand break resection occurs during non-homologous end joining in g1 but is distinct from resection during homologous recombination. Mol Cell 2017;65:671–684.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mackenzie KJ, Carroll P, Martin CA, et al. Cgas surveillance of micronuclei links genome instability to innate immunity. Nature 2017;548:461–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harding SM, Benci JL, Irianto J, et al. Mitotic progression following DNA damage enables pattern recognition within micronuclei. Nature 2017;548:466–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.