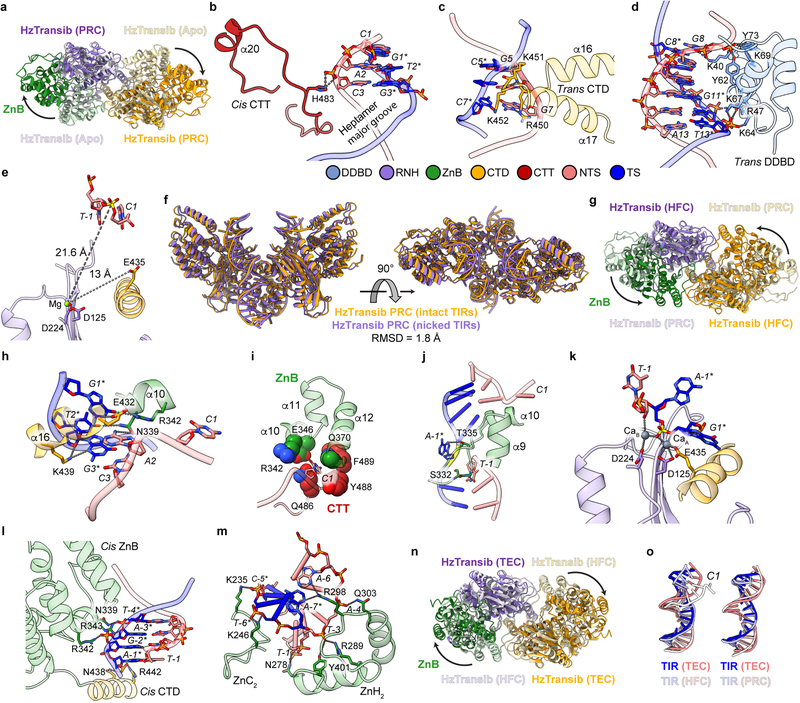
Extended Data Fig. 6. TIR recognition in HzTransib PRC, HFC and TEC.
a, Superimposition of HzTransib dimer in PRC (dark colors) and apo (pale colors) structures by their DDBD illustrates the large conformational changes of ZnB domains (green in one subunit). b–e, TIR recognition in HzTransib PRC. b, Interactions between HzTransib CTT and the heptamer. Hydrogen bonds are shown as gray dotted lines. Labels for nucleotide residues are italic. c, Interactions between HzTransib and last three base pairs of heptamer. d, Interactions between HzTransib and transposon end DNA downstream of heptamer. e, Active site of HzTransib PRC structure. Distances between Mg2+ ion and scissile phosphate or E435 are indicated. f, The front and top views of two HzTransib PRC structures (incubated with either intact or nicked TIRs at 4°C) superimposed by their DDBD domains. The HzTransib nicked PRC complex is referred to as a PRC because of its strong structural resemblance to the intact DNA PRC. Depending on reaction conditions (temperature and divalent cation; see Methods), the nicked TIR substrate can be incorporated into either a nicked PRC or the HFC. g, Superimposition of HzTransib dimer in HFC and PRC structures by their DDBD shows the inward movements of ZnB domains and dimer closure. h–k, TIR recognition in HzTransib HFC. h, Interactions between HzTransib and the first three base pairs of heptamer. i, The first nucleotide of the heptamer (C1) is flipped out and buried in a pocket. j, Interactions between HzTransib α9-α10 loop and TIR at heptamer-flanking DNA junction. k, Active site of HzTransib HFC structure. l, Interactions between HzTransib and TIR flanking DNA in PRC. m, Interactions between HzTransib ZnB domain and TIR flanking DNA in HFC. n, Superimposition of HzTransib dimer in TEC and HFC structures by their DDBD shows the outward movements of ZnB domains. o, Comparison of transposon end DNA in TEC to that in HFC or in PRC. Mg2+ and Ca2+ ions are green and slate gray, respectively; other structure elements are colored as in Fig. 2b. Scissile phosphate in each structure is highlighted in yellow.