Abstract
Objective
Non-infectious myelitis in SLE may be due to SLE myelitis, comorbid multiple sclerosis (MS), or neuromyelitis optica (NMO). We compared characteristics of these three conditions in SLE patients at a large academic institution.
Methods
We searched for neurologic diagnoses of SLE myelitis, NMO myelitis, and MS myelitis among 2,297 patients with at least four 1997 ACR revised criteria for SLE between 2000 and 2015. Each subject was reviewed by a neurologist to confirm the underlying neurologic diagnosis. Demographic, clinical, laboratory, and radiographic data were extracted and compared using Fisher’s exact test, analysis of variance, and Wilcoxon rank-sum test.
Results
Fifteen of the 2,297 subjects with SLE (0.7%) met criteria for a spinal cord syndrome: seven had SLE myelitis, three had AQP4 seropositive NMO, and five had MS. The median SLEDAI-2K score at time of neurologic syndrome presentation was higher in SLE myelitis subjects (8, IQR 7–16) compared to subjects with NMO (6, IQR 0–14) or MS (2, IQR 0–4), p=0.02. Subjects with SLE myelitis were also more likely to have elevated anti-dsDNA antibodies at presentation (86%) compared to subjects with NMO (33%) or MS (0%), p=0.03.
Conclusion
Myelitis occurs rarely among patients with SLE. Compared to subjects with SLE + NMO and subjects with SLE + MS, subjects with SLE myelitis had higher SLE disease activity at presentation.
Keywords: Systemic Lupus Erythematosus, Neuropsychiatric Lupus, Anti-DNA antibodies
INTRODUCTION
Systemic lupus erythematosus (SLE) is a chronic disease characterized by autoreactivity of the innate and adaptive immune systems, leading to autoantibody production and immune complex deposition within tissues.1 It is estimated to affect approximately 161,000 to 322,000 adults within the United States (US), and typically involves multiple organ systems.2 Neurologic manifestations of SLE include, among others, seizures, psychosis, acute confusional state, neuropathy, stroke, and myelitis.3 Myelitis, or inflammation of the spinal cord, occurs in 1–2% of patients with SLE and may present with motor, sensory, or autonomic deficits below the level of spinal inflammation, leading to significant morbidity.4 Several case series and small case-control studies have examined patients with SLE myelitis and have found that the clinical presentation, laboratory evaluation, and radiographic features of this disease are often heterogeneous.5–20 In addition, several other autoimmune conditions may affect the spinal cord. Among them, multiple sclerosis and anti-aquaporin-4 antibody (AQP4) mediated neuromyelitis optica (NMO) may be difficult to distinguish clinically from SLE myelitis.21,22 Differentiating between these three conditions is important because they require different treatment approaches.23, 24, 25 Thus, we sought to compare the demographic, clinical, laboratory, and radiographic characteristics of these three conditions within an SLE registry from a large academic hospital in Boston, Massachusetts (MA).
PATIENTS AND METHODS
Subjects were identified by searching the Brigham and Women’s Hospital Lupus Center Registry comprised of 2,297 patients with at least four 1997 American College of Rheumatology (ACR) revised criteria for SLE.26 All included subject records were reviewed by an attending rheumatologist to confirm the diagnosis of SLE. Neurologic diagnoses within this population were identified by text string searches within electronic medical records for the terms “myelitis”, “NMO”, “neuromyelitis optica”, and “multiple sclerosis” between January 1, 2000 and December 31, 2015. Each subject’s record was then reviewed by an attending neurologist (SB) to confirm the diagnosis of SLE myelitis, AQP4 seropositive NMO, or MS. Subjects with positive AQP4 antibodies were, by definition, classified as NMO (as all patients had myelitis and would thus meet the International Panel for NMO Diagnosis (IPND) diagnostic criteria).27 MS was classified based on the 2010 McDonald Criteria.28 Patients were excluded if they did not have clinical, laboratory, and imaging data at the time of spinal cord syndrome presentation.
Data were extracted regarding demographics (age at time of presentation, sex, race), clinical factors (years since onset of SLE symptoms, presence of concurrent SLE flare, sensory loss, weakness, bowel/bladder dysfunction, concurrent optic neuritis, treatment, follow-up course), laboratory features (cerebrospinal fluid (CSF) profile, inflammatory markers, complement levels, autoantibody profiles), and radiographic features (lesion number, pattern, contrast enhancement, and follow-up resolution). Bowel/bladder dysfunction included urinary urgency, urinary hesitancy, or fecal incontinence; constipation was not included. Inflammatory markers included erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP). Complement levels included C3, C4, and/or CH50. Specific autoantibodies included anti-double stranded DNA antibody (anti-dsDNA), lupus anticoagulant (LAC), anticardiolipin (aCL) IgM and IgG antibodies, and anti-beta-2 glycoprotein-I (anti-β2GPI) IgM and IgG antibodies. In addition, the SLE Disease Activity Index 2000 score (SLEDAI-2K)29 at the time of presentation was determined for each patient. Neurologic impairment at the time of presentation and at 1-year follow-up was measured using the American Spinal Injury Association Impairment Scale (AIS), with categories including complete motor and sensory loss (A), complete motor loss with preserved sensation (B), incomplete motor loss with muscle strength <3/5 (C), incomplete motor loss with muscle strength ≥3/5 (D), and normal function (E).30
Although the expanded disability status scale (EDSS) is commonly used to measure disability caused by MS or NMO, this study utilized the AIS because the EDSS also includes other aspects of neurologic dysfunction not related to the spinal cord. Characteristics of these three groups were compared using Fisher’s exact test for categorical variables and analysis of variance for continuous variables. Wilcoxon rank-sum test was used for SLEDAI-2K score, ESR, and CRP level as these values were not normally distributed. Data were analyzed using SAS 9.4 (Cary, North Carolina, US). This study was approved by the Institutional Review Board (IRB) of Brigham and Women’s Hospital (2016P002848). The IRB granted a waiver for the requirement of informed consent by study subjects given the retrospective nature of the study design. The primary data from this study can be obtained by contacting the first author listed above.
RESULTS
Fifteen of the 2,297 subjects with SLE (0.7%) met criteria for a spinal cord syndrome: seven had SLE myelitis, three had AQP4+ NMO, and five had MS. Demographic, clinical, laboratory, and magnetic resonance imaging (MRI) characteristics of the study population are displayed in Table 1. There were no significant demographic differences between these three groups, with mean age at presentation in the 5th decade for all three groups. The majority of subjects in all three groups were white females.
Table 1.
Characteristics of subjects with SLE myelitis, SLE + NMO, and SLE + MS at neurologic event presentation
| SLE myelitis (n=7) | SLE + NMO (n=3) | SLE + MS (n=5) | p-value | |
|---|---|---|---|---|
| Demographic Characteristics | ||||
| Age, mean ± SD years | 41 ± 10 | 46 ± 14 | 40 ± 13 | 0.80 |
| Sex | 0.67 | |||
| Female | 5 (71) | 3 (100) | 5 (100) | |
| Male | 2 (29) | 0 (0) | 0 (0) | |
| Racea | 1.00 | |||
| White | 6 (86) | 3 (100) | 5 (100) | |
| Black | 1 (14) | 0 (0) | 0 (0) | |
| Years since SLE onset, mean ± SD | 10 ± 11 | 22 ± 15 | 16 ± 3 | 0.23 |
| Clinical Characteristics | ||||
| SLE flare | 3 (43) | 0 (0) | 0 (0) | 0.25 |
| Median SLEDAI-2K score (IQR) | 8 (7–16) | 6 (0–14) | 2 (0–4) | 0.02 |
| Sensory loss | 6 (86) | 1 (33) | 3 (60) | 0.39 |
| Weakness | 4 (57) | 2 (67) | 1 (20) | 0.41 |
| Bowel or bladder symptoms | 3 (60) | 1 (33) | 1 (20) | 0.77 |
| Optic neuritis | 2 (29) | 2 (67) | 0 (0) | 0.13 |
| Laboratory Characteristics | ||||
| Elevated anti-dsDNA antibody | 6 (86) | 1 (33) | 0 (0) | 0.03 |
| Hypocomplementemia | 4 (57) | 1 (33) | 1 (33) | 1.00 |
| Antiphospholipid antibody positive | 5 (71) | 0 (0) | 1 (20) | 0.15 |
| Median ESR in mm/hr (IQR)b | 19 (12–45) | 22 (13–34) | 11 (5–20) | 0.24 |
| Median CRP in mg/L (IQR)b | 7 (2–13) | 5 (2–8) | 0.6 (0.6–2) | 0.07 |
| Oligoclonal bands in CSF | 2(67) | 0 (0) | 2 (100) | 0.23 |
| MRI Characteristics | ||||
| Single spinal lesion | 5 (71) | 1 (50) | 1 (25) | 0.39 |
| Contrast-enhancing lesion(s) | 4 (67) | 1 (50) | 1 (25) | 0.48 |
| Longitudinally extensive myelitisc | 2 (29) | 1 (50) | 1 (25) | 1.00 |
SLE=systemic lupus erythematosus; NMO=neuromyelitis optica; MS=multiple sclerosis; SD=standard deviation; SLEDAI-2K= SLE Disease Activity Index 2000; IQR=interquartile range; dsDNA=double-stranded deoxyribonucleic acid; ESR=erythrocyte sedimentation rate; mm/hr=millimeters per hour; CRP=C-reactive protein; mg/L=milligrams per liter; CSF=cerebrospinal fluid; MRI=magnetic resonance imaging.
All patients were non-Hispanic.
Reference range for ESR is 0–20 mm/hr. Reference range for CRP is 0–8.0 mg/L.
Longitudinal extension: involving ≥3 vertebral segments.
As far as clinical characteristics, the median SLEDAI-2K score at time of neurologic syndrome presentation was higher in SLE myelitis subjects (8, interquartile range (IQR) 7–16) compared to subjects with NMO (6, IQR 0–14) or MS (2, IQR 0–4), p=0.02. None of the subjects in the NMO or MS group were having a concurrent SLE flare at the time of neurologic syndrome presentation, whereas 43% of the SLE myelitis subjects were having a flare at presentation. For those with concurrent SLE flare, the other involved organ systems included the skin and joints. Optic neuritis was present in both the SLE myelitis group (n=2) and the NMO group (n=2). Importantly, all subjects presented with clinical evidence of myelitis including weakness, sensory loss, and/or bowel or bladder dysfunction. Diagnosis of spinal cord syndrome occurred within 21 days of symptom onset for 6 out of 7 subjects with SLE myelitis, 2 out of 3 subjects with NMO, and 3 out of 5 patients with MS.
As described by Birnbaum et al, we did categorize our 15 subjects as either white matter myelitis (characterized by spasticity and/or hyperreflexia) or gray matter myelitis (characterized by flaccidity and/or hyporeflexia).10 Of our 15 subjects, only 2 had evidence of gray matter myelitis (1 was in the SLE + NMO group and 1 was in the SLE + MS group). Unlike Birnbaum et al, we did not find that those with gray matter myelitis had irreversible paraplegia, CSF profile consistent with bacterial meningitis, or evidence of high SLE disease activity at presentation. Only one of the subjects with gray matter myelitis had urinary symptoms, and 100% had a monophasic course (as compared to 82% of those with white matter myelitis). There was no statistical difference between those with SLE myelitis, SLE + NMO, or SLE + MS in regard to white matter vs gray matter myelitis (p=0.6).
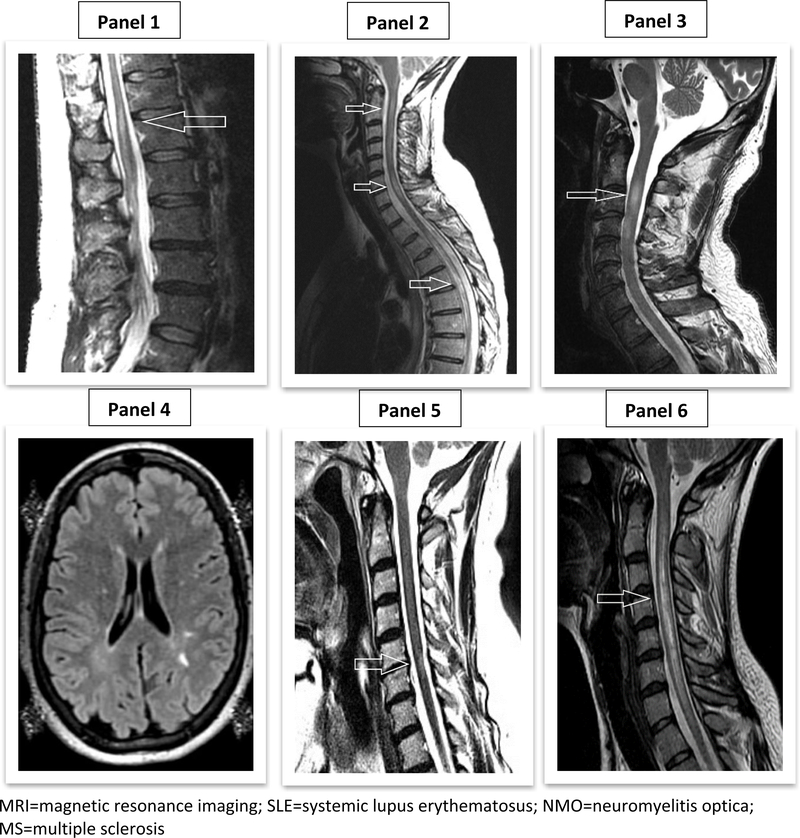
A greater proportion of subjects in the SLE myelitis group had elevated anti-dsDNA antibodies at presentation (86%) compared to subjects with NMO (33%) or MS (0%), p=0.03. There were otherwise no statistically significant differences in laboratory characteristics between these three groups, although there was increased antiphospholipid antibody positivity in the SLE myelitis group as compared to the NMO and MS groups (71% vs. 0% vs. 20%, p=0.15) and higher CRP in the SLE myelitis and NMO groups compared to the MS group (p=0.07). On cerebrospinal fluid (CSF) analysis, oligoclonal bands were seen in the SLE myelitis and MS groups, but not in the NMO group. The majority of subjects did not have inflammatory CSF features such as pleocytosis, elevated protein, or low glucose. Additionally, there was no difference in the presence of inflammatory CSF features between the 3 groups. Spinal MRI characteristics did not differ between the groups (Figure 1). The radiographic pattern of longitudinally extensive myelitis involving at least three vertebral segments was seen in all three groups. In all three groups, 100% of subjects who had a repeat MRI of the spine ≥6 months after spinal disease onset had persistent lesions.
Figure 1.
Examples of Spinal MRI Findings in SLE Myelitis (Panels 1-3), Brain and Spinal MRI Findings in SLE + MS (Panels 4-5), and Spinal MRI Findings in SLE + NMO (Panel 6)
All subjects in the SLE myelitis and NMO groups received steroids at presentation; 3/5 subjects in the MS group did. In the SLE myelitis group, two subjects received cyclophosphamide (29%) and one subject received plasma exchange (14%). In the NMO group, one subject received plasma exchange + mycophenolate mofetil (33%) and one subject received rituximab (33%). In the MS group, one subject received mycophenolate mofetil (20%) and one subject received azathioprine (20%). Outcomes did not differ significantly between these three groups (Table 2). Most subjects had a single episode of myelitis, remained on immunosuppression through last follow-up, and were able to ambulate independently at last follow-up. One year after spinal disease onset, all subjects were either in AIS category D (incomplete motor loss, ≥4/5 strength) or category E (normal function).
Table 2.
Comparison of outcomes among subjects with SLE myelitis, SLE + NMO, and SLE + MS
| SLE myelitis (n=7) | SLE + NMO (n=3) | SLE + MS (n=5) | p-value | |
|---|---|---|---|---|
| Mean follow-up time ± SD years | 7 ± 5 | 3 ± 3 | 6 ± 3 | 0.33 |
| Full response to acute treatment | 1 (14) | 0 (0) | 1 (20) | 1.00 |
| Recurrence of disease | 1 (14) | 1 (33) | 0 (0) | 0.67 |
| AIS Category E (normal) at 1 year | 3 (43) | 1 (50) | 3 (60) | 1.00 |
| Immunosuppression at last visit | 6 (86) | 3 (100) | 5 (100) | 1.00 |
| Independent ambulation at last visit | 6 (86) | 2 (67) | 4 (80) | 1.00 |
| MRI lesion(s) after ≥6 months | 6 (100) | 1 (100) | 4 (100) | N/A |
SLE=systemic lupus erythematosus; NMO=neuromyelitis optica; MS=multiple sclerosis; SD=standard deviation; AIS=American Spinal Injury Association Impairment Scale; MRI=magnetic resonance imaging; N/A=not applicable.
DISCUSSION
Myelitis is a rare complication of SLE which may lead to significant morbidity, and thus it is important to identify this condition and differentiate it from similarly presenting conditions which may occur in patients with SLE (such as NMO and MS) so that it may be promptly and appropriately treated. This study of 15 patients with SLE and spinal cord syndromes seen at a large academic hospital in Boston, MA sought to compare the demographics, clinical presentation, laboratory and radiographic features, and treatment outcomes of SLE myelitis, SLE + NMO, and SLE + MS from 2000–2015.
The functional deficits associated with these three conditions are particularly concerning given that they tend to affect young and middle-aged adults, with a mean age at onset in the 5th decade of life in our study. Other studies have similarly reported a mean age at SLE myelitis onset of 25–42 years10, 13–14, NMO median age at onset in the late 4th decade,31 and MS mean age at onset of 28–31 years.32 Interestingly, our small study population was more likely to be white (93%) than the general SLE population of the hospital which participated in the study (66% white). Although our study population was small, this suggests that white patients with SLE may be more likely to develop spinal cord syndromes or there may be disparities in the diagnosis which favor white patients. Other studies of SLE myelitis have reported study populations that are 36–46% white10, in comparison to the SLE adult population within the US, which is approximately 55% white.2 The majority of patients with NMO are non-white31, 33–35, in contrast to MS, which is most common amongst patients of northern European ancestry.32
Optic neuritis is a cardinal feature of NMO, but we also found that approximately 30% of subjects in the SLE myelitis group had optic neuritis, in line with the previously noted association between SLE myelitis and optic neuritis.13 We found that subjects in the SLE myelitis group had higher disease activity at presentation as measured by median SLEDAI-2K scores and proportion of subjects with elevated anti-dsDNA antibodies. This may provide clinicians with a diagnostic clue favoring SLE myelitis over SLE + NMO or SLE + MS in practice. The majority of our SLE myelitis group had antiphospholipid antibodies (71%), which has been noted in other studies.9, 13–14, 20 This is in contrast to the general SLE population in which 30–40% have antiphospholipid antibodies.36 The majority of subjects in the NMO and MS groups did not have antiphospholipid antibodies, but these differences did not reach statistical significance. It has been proposed that hypercoagulability associated with antiphospholipid antibodies may play a role in the pathophysiology of SLE myelitis by leading to spinal cord ischemia, however a previous study of 70 patients found no evidence that anticoagulation improves outcomes in these patients.37
A study by Birnbaum et al published in 2009 categorized patients with SLE myelitis into white matter myelitis (characterized by spasticity and hyperreflexia) and gray matter myelitis (characterized by flaccidity and hyporeflexia).10 They found that white matter myelitis was associated with NMO and presence of antiphospholipid antibodies, whereas gray matter myelitis was associated with irreversible paraplegia, monophasic course, higher SLEDAI scores, urinary retention, and CSF analysis resembling bacterial meningitis. However, when we classified our 15 subjects into white matter myelitis (N=13) and gray matter myelitis (N=2), we did not find any of these associations reported by Birnbaum et al. Longitudinally extensive myelitis (LEM) is classically associated with NMO (though short-segment lesions can occur) 38, 39, and we did see LEM in a greater proportion of our NMO group as compared to the MS and SLE myelitis groups (although not statistically significant). This pattern may also occur in SLE myelitis as noted in several studies.7–8, 11,14 Additionally, we found that radiographic improvement may temporally lag clinical improvement, as all subjects with follow-up imaging had persistent radiographic lesions at 6 months or later. Thus, it appears that following a patient’s exam and symptoms may be more likely to assist with prognostication than subsequent imaging.
The outcomes of subjects were relatively favorable; 87% of subjects were treated with steroids at myelitis onset and 87% had a single episode of myelitis. All subjects had either minor impairment in motor function (strength ≥4/5) or normal function at 1-year follow-up. Previous studies have suggested that the addition of cyclophosphamide to steroids in SLE myelitis is associated with improved outcomes.12,13, 25 In support of this previous finding, the only subject in our study who had a full response to acute treatment in the SLE myelitis group received both cyclophosphamide and steroids. Azathioprine is also a first-line agent for SLE myelitis, with plasma exchange used in refractory cases.25 In contrast, steroids in combination with rituximab or azathioprine has often been among first-line management options for NMO, with plasma exchange reserved for refractory cases.23 Lastly, MS flares are typically managed with steroids followed by plasma exchange in refractory cases.24 The long-term treatment approaches for these three conditions differ, highlighting the need for accurate identification in the clinical setting.
Strengths of our study include extensive follow-up time for our study population (mean 5.7 years, range 6 months-13 years) and comprehensive clinical, laboratory, and MRI data. Limitations include our small study population and retrospective study design. Furthermore, the neurologic outcomes of our study population were favorable at 1 year, which may have been due to milder disease at presentation and/or earlier diagnosis (the majority of our study population was diagnosed with a spinal cord syndrome within 21 days of symptom onset). The neurologic outcomes of subjects in our study was overall similar to the findings of a 2015 study by Saison et al12, which showed that after a median of 5.9 years of follow-up, only 3 of 18 patients with SLE myelitis required aid with ambulation. In summary, in these rare instances of myelitis among the SLE registry population, we found that compared to subjects with SLE + NMO and subjects with SLE + MS, subjects with SLE myelitis had significantly higher SLE disease activity at myelitis onset as indicated by SLEDAI-2K scores and elevated anti-dsDNA antibody levels. Further studies with larger patient populations are needed in order to determine how best to differentiate these 3 conditions in clinical practice.
Acknowledgments
FUNDING
This research was supported by NIH K24 AR 066109 (Dr. Costenbader) and NIH P30 AR072577 (VERITY).
Footnotes
DECLARATION OF CONFLICTING INTERESTS
The Author(s) declare(s) that there is no conflict of interest.
REFERENCES
- 1.Choi J, Kim ST, Craft J. The pathogenesis of systemic lupus erythematosus – an update. Curr Opin Immunol 2012; 24: 651–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Helmick CG, Felson DT, Lawrence RC, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States: Part I. Arthritis Rheum 2008; 58: 15–25. [DOI] [PubMed] [Google Scholar]
- 3.Petri M, Orbai AM, Alarcón GS, et al. Derivation and validation of Systemic Lupus International Collaborating Clinics Classification criteria for systemic lupus erythematosus. Arthritis Rheum 2012; 64: 2677–2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.West SG. Neuropsychiatric lupus. Rheum Dis Clin North Am 1994; 20: 129–158. [PubMed] [Google Scholar]
- 5.Schulz SW, Shenin M, Mehta A, Kebede A, Fluerant M, Derk CT. Initial presentation of acute transverse myelitis in systemic lupus erythematosus: demographics, diagnosis, management and comparison to idiopathic cases. Rheumatol Int 2012; 32: 2623–2627. [DOI] [PubMed] [Google Scholar]
- 6.Espinosa G, Mendizábal A, Mínguez S, et al. Transverse myelitis affecting more than 4 spinal segments associated with systemic lupus erythematosus: clinical, immunological, and radiological characteristics of 22 patients. Semin Arthritis Rheum 2010; 39: 246–256. [DOI] [PubMed] [Google Scholar]
- 7.Lu X, Gu Y, Wang Y, Chen S, Ye S. Prognostic factors of lupus myelopathy. Lupus 2008; 17: 323–328. [DOI] [PubMed] [Google Scholar]
- 8.Téllez-Zenteno JF, Remes-Troche JM, Negrete-Pulido RO, Dávila-Maldonado L. Longitudinal myelitis associated with systemic lupus erythematosus: clinical features and magnetic resonance imaging of six cases. Lupus 2001; 10: 851–856. [DOI] [PubMed] [Google Scholar]
- 9.D’Cruz DP, Mellor-Pita S, Joven B, et al. Transverse myelitis as the first manifestation of systemic lupus erythematosus or lupus-like disease: good functional outcome and relevance of antiphospholipid antibodies. J Rheumatol 2004; 31: 280–285. [PubMed] [Google Scholar]
- 10.Birnbaum J, Petri M, Thompson R, Izbudak I, Kerr D. Distinct subtypes of myelitis in systemic lupus erythematosus. Arthritis Rheum 2009; 60: 3378–3387. [DOI] [PubMed] [Google Scholar]
- 11.Li XY, Xiao P, Xiao HB, et al. Myelitis in systemic lupus erythematosus frequently manifests as longitudinal and sometimes occurs at low disease activity. Lupus 2014; 23: 1178–1186. [DOI] [PubMed] [Google Scholar]
- 12.Saison J, Costedoat-Chalumeau N, Maucort-Boulch D, et al. Systemic lupus erythematosusassociated acute transverse myelitis: manifestations, treatments, outcomes, and prognostic factors in 20 patients. Lupus 2015; 24: 74–81. [DOI] [PubMed] [Google Scholar]
- 13.Kovacs B, Lafferty TL, Brent LH, DeHoratius RJ. Transverse myelopathy in systemic lupus erythematosus: an analysis of 14 cases and review of the literature. Ann Rheum Dis 2000; 59: 120–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hryb JP, Chiganer E, Contentti EC, Di Pace JL, Lessa C, Perassolo MB. Myelitis in systemic lupus erythematosus: clinical features, immunological profile and magnetic resonance imaging of five cases. Spinal Cord Ser Cases 2016; 2: 16005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quintanilla-González L, Atisha-Fregoso Y, Llorente L, Fragoso-Loyo H. Myelitis in systemic lupus erythematosus: clinical characteristics and effect in accrual damage. A single-center experience. Lupus 2017; 26: 248–254. [DOI] [PubMed] [Google Scholar]
- 16.Mok CC, Lau CS, Chan EY, Wong RW. Acute transverse myelopathy in systemic lupus erythematosus: clinical presentation, treatment, and outcome. J Rheumatol 1998; 25: 467–473. [PubMed] [Google Scholar]
- 17.Harisdangkul V, Doorenbos D, Subramony SH. Lupus transverse myelopathy: better outcome with early recognition and aggressive high-dose intravenous corticosteroid pulse treatment. J Neurol 1995; 242: 326–331. [DOI] [PubMed] [Google Scholar]
- 18.Provenzale JM, Barboriak DP, Gaensler EH, Robertson RL, Mercer B. Lupus-related myelitis: serial MR findings. AJNR Am J Neuroradiol 1994; 15: 1911–1917. [PMC free article] [PubMed] [Google Scholar]
- 19.Salmaggi A, Lamperti E, Eoli M, et al. Spinal cord involvement and systemic lupus erythematosus: clinical and magnetic resonance findings in 5 patients. Clin Exp Rheumatol 1994; 12: 389–394. [PubMed] [Google Scholar]
- 20.Lavalle C, Pizarro S, Drenkard C, Sánchez-Guerrero J, Alarcón-Segovia D. Transverse myelitis: a manifestation of systemic lupus erythematosus strongly associated with antiphospholipid antibodies. J Rheumatol 1990; 17: 34–37. [PubMed] [Google Scholar]
- 21.Shahmohammadi S, Doosti R, Shahmohammadi A, et al. Autoimmune diseases associated with Neuromyelitis Optica Spectrum Disorders: A literature review. Mult Scler Relat Disord 2019; 27: 350–363. [DOI] [PubMed] [Google Scholar]
- 22.Jácome Sánchez EC, García Castillo MA, González VP, Guillén López F, Correa Díaz EP. Coexistence of systemic lupus erythematosus and multiple sclerosis. A case report and literature review. Mult Scler J Exp Transl Clin 2018; 4: 2055217318768330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trebst C, Jarius S, Berthele A, et al. Update on the diagnosis and treatment of neuromyelitis optica: recommendations of the Neuromyelitis Optica Study Group (NEMOS). J Neurol 2014; 261: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamout BI, Alroughani R. Multiple Sclerosis. Semin Neurol 2018; 38: 212–225. [DOI] [PubMed] [Google Scholar]
- 25.Chiganer EH, Hryb JP, Carnero Contentti E. Myelitis and Lupus: Clinical Manifestations, Diagnosis and Treatment. Review. Reumatol Clin 2017; 13: 344–348. [DOI] [PubMed] [Google Scholar]
- 26.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997; 40: 1725. [DOI] [PubMed] [Google Scholar]
- 27.Wingerchuk DM, Banwell B, Bennett JL, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology 2015; 85: 177–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 2018; 17: 162–173. [DOI] [PubMed] [Google Scholar]
- 29.Gladman DD, Ibanez D, Urowitz MB. Systemic lupus erythematosus disease activity index 2000. J Rheumatol 2002; 29: 288–291. [PubMed] [Google Scholar]
- 30.Maynard FM Jr, Bracken MB, Creasey G, et al. International standards for neurological and functional classification of spinal cord injury. American Spinal Injury Association. Spinal Cord 1997; 35: 266–274. [DOI] [PubMed] [Google Scholar]
- 31.Sellner J, Boggild M, Clanet M, et al. EFNS guidelines on diagnosis and management of neuromyelitis optica. Eur J Neurol 2010; 17: 1019–1032. [DOI] [PubMed] [Google Scholar]
- 32.Gooden DS. The epidemiology of multiple sclerosis: insights to disease pathogenesis. Handb Clin Neurol 2014; 122: 231–266. [DOI] [PubMed] [Google Scholar]
- 33.Bukhari W, Prain KM, Waters P, et al. Incidence and prevalence of NMOSD in Australia and New Zealand. J Neurol Neurosurg Psychiatry 2017; 88: 632–638. [DOI] [PubMed] [Google Scholar]
- 34.Flanagan EP, Cabre P, Weinshenker BG, et al. Epidemiology of aquaporin-4 autoimmunity and neuromyelitis optica spectrum. Ann Neurol 2016; 79: 775–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hor JY, Lim TT, Chia YK, et al. Prevalence of neuromyelitis optica spectrum disorder in the multiethnic Penang Island, Malaysia, and a review of worldwide prevalence. Mult Scler Relat Disord 2018; 19: 20–24. [DOI] [PubMed] [Google Scholar]
- 36.Petri M Epidemiology of the antiphospholipid antibody syndrome. J Autoimmun 2000; 15: 145–151. [DOI] [PubMed] [Google Scholar]
- 37.Katsiari CG, Giavri I, Mitsikostas DD, Yiannopoulou KG, Sfikakis PP. Acute transverse myelitis and antiphospholipid antibodies in lupus. No evidence for anticoagulation. Eur J Neurol 2011; 18: 556–563. [DOI] [PubMed] [Google Scholar]
- 38.Tobin WO, Weinshenker BG, Lucchinetti CF. Longitudinally extensive transverse myelitis. Curr Opin Neurol 2014; 27: 279–289. [DOI] [PubMed] [Google Scholar]
- 39.Flanagan EP, Weinshenker BG, Krecke KN, et al. Short myelitis lesions in aquaporin-4-IgG-positive neuromyelitis optica spectrum disorders. JAMA Neurol 2015; 72: 81–87. [DOI] [PMC free article] [PubMed] [Google Scholar]