Abstract
Background
International dietary recommendations include guidance on healthy eating and weight management for people who have survived cancer; however dietary interventions are not provided routinely for people living beyond cancer.
Objectives
To assess the effects of dietary interventions for adult cancer survivors on morbidity and mortality, changes in dietary behaviour, body composition, health‐related quality of life, and clinical measurements.
Search methods
We ran searches on 18 September 2019 and searched the Cochrane Central Register of Controlled trials (CENTRAL), in the Cochrane Library; MEDLINE via Ovid; Embase via Ovid; the Allied and Complementary Medicine Database (AMED); the Cumulative Index to Nursing and Allied Health Literature (CINAHL); and the Database of Abstracts of Reviews of Effects (DARE). We searched other resources including reference lists of retrieved articles, other reviews on the topic, the International Trials Registry for ongoing trials, metaRegister, Physicians Data Query, and appropriate websites for ongoing trials. We searched conference abstracts and WorldCat for dissertations.
Selection criteria
We included randomised controlled trials (RCTs) that recruited people following a cancer diagnosis. The intervention was any dietary advice provided by any method including group sessions, telephone instruction, written materials, or a web‐based approach. We included comparisons that could be usual care or written information, and outcomes measured included overall survival, morbidities, secondary malignancies, dietary changes, anthropometry, quality of life (QoL), and biochemistry.
Data collection and analysis
We used standard Cochrane methodological procedures. Two people independently assessed titles and full‐text articles, extracted data, and assessed risk of bias. For analysis, we used a random‐effects statistical model for all meta‐analyses, and the GRADE approach to rate the certainty of evidence, considering limitations, indirectness, inconsistencies, imprecision, and bias.
Main results
We included 25 RCTs involving 7259 participants including 977 (13.5%) men and 6282 (86.5%) women. Mean age reported ranged from 52.6 to 71 years, and range of age of included participants was 23 to 85 years. The trials reported 27 comparisons and included participants who had survived breast cancer (17 trials), colorectal cancer (2 trials), gynaecological cancer (1 trial), and cancer at mixed sites (5 trials).
For overall survival, dietary intervention and control groups showed little or no difference in risk of mortality (hazard ratio (HR) 0.98, 95% confidence interval (CI) 0.77 to 1.23; 1 study; 3107 participants; low‐certainty evidence). For secondary malignancies, dietary interventions versus control trials reported little or no difference (risk ratio (RR) 0.99, 95% CI 0.84 to 1.15; 1 study; 3107 participants; low‐certainty evidence). Co‐morbidities were not measured in any included trials.
Subsequent outcomes reported after 12 months found that dietary interventions versus control probably make little or no difference in energy intake at 12 months (mean difference (MD) ‐59.13 kcal, 95% CI ‐159.05 to 37.79; 5 studies; 3283 participants; moderate‐certainty evidence). Dietary interventions versus control probably led to slight increases in fruit and vegetable servings (MD 0.41 servings, 95% CI 0.10 to 0.71; 5 studies; 834 participants; moderate‐certainty evidence); mixed results for fibre intake overall (MD 5.12 g, 95% CI 0.66 to 10.9; 2 studies; 3127 participants; very low‐certainty evidence); and likely improvement in Diet Quality Index (MD 3.46, 95% CI 1.54 to 5.38; 747 participants; moderate‐certainty evidence).
For anthropometry, dietary intervention versus control probably led to a slightly decreased body mass index (BMI) (MD ‐0.79 kg/m², 95% CI ‐1.50 to ‐0.07; 4 studies; 777 participants; moderate‐certainty evidence). Dietary interventions versus control probably had little or no effect on waist‐to‐hip ratio (MD ‐0.01, 95% CI ‐0.04 to 0.02; 2 studies; 106 participants; low‐certainty evidence).
For QoL, there were mixed results; several different quality assessment tools were used and evidence was of low to very low‐certainty. No adverse events were reported in any of the included studies.
Authors' conclusions
Evidence demonstrated little effects of dietary interventions on overall mortality and secondary cancers. For comorbidities, no evidence was identified. For nutritional outcomes, there was probably little or no effect on energy intake, although probably a slight increase in fruit and vegetable intake and Diet Quality Index. Results were mixed for fibre. For anthropometry, there was probably a slight decrease in body mass index (BMI) but probably little or no effect on waist‐to‐hip ratio. For QoL, results were highly varied. Additional high‐quality research is needed to examine the effects of dietary interventions for different cancer sites, and to evaluate important outcomes including comorbidities and body composition. Evidence on new technologies used to deliver dietary interventions was limited.
Plain language summary
Dietary intake in people living beyond cancer
Background Diet has been linked to cancer, and dietary guidelines are available for cancer prevention. People after cancer have been found to have higher rates of other conditions including cardiovascular disease, diabetes, and other cancers. It is therefore sensible for people after cancer to look at changing their diet. It was important to undertake this review to assess the evidence on dietary advice for people who have survived cancer.
Aim of the review This review evaluates evidence on dietary interventions for people after cancer.
Quality of evidence The quality of evidence is generally low to very low. Most studies did not evaluate dietary interventions for key review outcomes, particularly mortality and morbidity. However, a few study outcomes with moderate‐certainty evidence focused on dietary intake and physical measurements. Included studies compared dietary interventions versus control or usual care. We pooled data from similar randomised controlled trials (RCTs) to provide a summary estimate of the effects of an intervention, and we judged how confident (certain) we were of these findings by using an established method (GRADE).
Main findings We identified 25 RCTs involving 27 different comparisons. For some outcomes, we found absence of evidence for dietary interventions. We found some evidence showing that dietary interventions probably did not modify energy intake; however, some evidence shows what is probably a slight increase in fruit and vegetable intake (moderate‐certainty evidence). Evidence on dietary fibre was mixed for different advice on weight reducing or healthy eating. Dietary interventions compared to control probably improved the Diet Quality Index (moderate‐certainty evidence). For physical measurements, we found a probable reduction in body mass index (BMI) with dietary interventions compared to controls (moderate‐certainty evidence) but little evidence showing any change in waist‐to‐hip ratio (low‐certainty evidence). For quality of life (QoL), results were mixed due to the wide variety of tools used. No adverse events were reported.
Conclusion Available evidence shows that dietary interventions can be helpful in modifying fruit and vegetable servings and diet quality; modification of fibre intake was variable, and some benefits were seen for anthropometric measurements, including BMI. Most of the evidence is based on women with breast cancer, so more research is needed for patients with other cancers. Gaps identified in the evidence involved the use of new technologies, comorbidities, and body composition data.
Summary of findings
Summary of findings for the main comparison. Dietary intervention compared to control for people living beyond cancer.
| Dietary intervention compared to control for people living beyond cancer | ||||||
| Patient or population: people living beyond cancer Setting: community Intervention: dietary intervention Comparison: control | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with control | Risk with dietary intervention | |||||
| Mortality Follow‐up: 7.3 years | Study population | HR 0.98 (0.77 to 1.23) | 3107 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | ||
| 106 per 1000 | 104 per 1000 (82 to 128) | |||||
| Secondary cancers Follow‐up: 7.3 years | Study population | RR 0.99 (0.84 to 1.15) | 3107 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | ||
| 168 per 1000 | 166 per 1000 (141 to 193) | |||||
| Fruit and vegetable intake assessed as servings Follow‐up: 12 months | Mean fruit and vegetable intake was 4.56 servings | MD 0.41 servings higher (0.1 higher to 0.71 higher) | ‐ | 834 (5 RCTs) | ⊕⊕⊕⊝ Moderatec | |
| Fibre intake assessed as g Follow‐up: 12 months | Mean fibre intake was 15.6 g | MD 5.12 g higher (0.66 lower to 10.9 higher) | ‐ | 3127 (2 RCTs) | ⊕⊝⊝⊝ Very lowb,d | |
| Diet Quality Index Follow‐up: 12 months | Mean Diet Quality Index was 64.7 | MD 3.46 higher (1.54 higher to 5.38 higher) | ‐ | 747 (3 RCTs) | ⊕⊕⊕⊝ Moderatee | |
| Body mass index Follow‐up: 12 months | Mean body mass index was 29.63 kg/m² | MD 0.79 Kg/m2 lower (1.5 lower to 0.7 lower) | ‐ | 777 (4 RCTs) | ⊕⊕⊕⊝ Moderatee | |
| Waist‐to‐hip circumference assessed as cm Follow‐up: 12 months | Mean waist‐to‐hip circumference was ‐0.46 cm | MD 0.01 cm lower (0.04 lower to 0.02 higher) | ‐ | 106 (2 RCTs) | ⊕⊕⊝⊝ Lowc,f | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; HR: hazard ratio; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence. High‐certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate‐certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low‐certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low‐certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aInability to rate consistency as only one study. bConfidence intervals are not narrow. cDowngraded one level due to indirectness. dDowngraded two levels for high level of inconsistency between studies. eDowngraded one level for risk of bias. fDowngraded one level due to small sample sizes.
Background
Description of the condition
It is estimated that globally 18.1 million new cases of cancer and 9.6 million cases of death were due to cancer in 2018 (Bray 2018). It is estimated that 15.5 million Americans with a diagnosis of cancer were alive in 2016, and this number is anticipated to reach 20.3 million by 2026 (Miller 2016). In the UK, the number of cancer survivors has been growing over the last 30 years (Elliott 2011), and survival has increased steadily from 61.2% to 72.3% for patients diagnosed from 2000 to 2015 (Broggio 2019). Furthermore, survival estimation for most cancer sites was above 75% after one year and 50% after five years, with the exception of lung and stomach cancer (Broggio 2019). The proportion of people who survive cancer may be attributed to an increase in the aging population and advancements in anti‐cancer therapies (chemotherapy and radiotherapy), which have improved the outcomes of treatment (Aziz 2003; Lancet 2004). Over 60% of those living beyond a cancer diagnosis are over 65 years of age (Ravasco 2003), approximately 60% are female, and most are diagnosed initially with breast, prostate, or colorectal malignancy (Maddams 2009). However, negative factors influencing cancer survival have been highlighted and include lower socioeconomic status combined with higher levels of coexisting conditions and unhealthy lifestyle choices (Louwman 2010). It is now recognised that as survival increases, associated long‐term health issues of cancer will emerge as a significant public health concern (Mosher 2009), and this is reflected in healthcare strategies (Department 2010; Lippman 2004).
Health promotion initiatives aimed at improving the well‐being of people who have survived cancer are now essential to decrease comorbidities and improve quality of life (QoL). Focus groups have reported that people who have survived cancer are often confused regarding future strategies to improve their health and well‐being (Armes 2009; Marbach 2011).
For the purpose of this review, cancer survivors are defined as people living beyond a diagnosis of cancer after all treatment interventions have been discontinued, when treatment interventions may include surgery, chemotherapy, radiotherapy, and active hormone therapy. This review does not include patients with cancer who are undergoing active or palliative treatment.
Description of the intervention
International recommendations on how to maintain a healthy lifestyle are currently available from the World Cancer Research Fund (WCRF) and the American Institute for Cancer Research (AICR) for prevention of cancer and for guidance for those who are living beyond a cancer diagnosis (WCRF/AICR 2018; Kushi 2012). Healthy lifestyle changes recommended by WCRF/AICR 2018 have been linked to longevity. From a large European study, those who followed a higher proportion of healthy lifestyle recommendations had 34% reduced risk of mortality compared to those who adhered to fewer recommendations (Vergnaud 2013). Low compliance with the WCRF/AICR recommendations was significantly associated with increased hazard ratios (HRs) of dying from cancer or circulatory and respiratory disease (Vergnaud 2013). Healthy lifestyle recommendations for those living beyond cancer include maintaining a healthy weight throughout life; adopting an active lifestyle; consuming a healthy diet with emphasis on plant foods; and limiting alcoholic beverages (WCRF/AICR 2018). Dietary interventions include any method that is aimed at altering an individual’s food or drink intake.
How the intervention might work
Lifestyle factors predispose people to development of chronic disease and cancer. These include overweight or obesity, lack of physical activity, and high saturated fat intake combined with low intake of fruits and vegetables (Daar 2007). A plethora of data have linked chronic diseases, including diabetes and cardiovascular and respiratory disease, to lifestyle factors, so it would seem reasonable that these comorbidities among people who have survived cancer could be reduced by modifying lifestyle factors (Kushi 2012). Those who live beyond cancer have an elevated incidence of recurrent disease and other cancers, so they would potentially benefit from modifying their behaviour to adhere to the recommendations for cancer prevention. Furthermore, patients have been found to have a higher level of motivation to change lifestyle behaviours after a cancer diagnosis than they had before the diagnosis (Demark‐Wahnefried 2005; Ganz 2005; Satia 2004). A survey of modifications in health‐related behaviours demonstrated that two‐thirds of people surviving breast, colorectal, and prostate cancer made positive health‐related changes to their diet and changed usage of supplements up to two years after their cancer diagnosis (Patterson 2003). Others have reported that patients are willing to change their behaviour after receiving a diagnosis of cancer and that they have already made changes (Demark‐Wahnefried 2000).
Why it is important to do this review
Those who have survived cancer have not only an increased risk of secondary malignancies but also a higher incidence of comorbidities compared to the general population (Nord 2005). An increased incidence of cardiovascular disease, diabetes, and osteoporosis has been reported among survivors of cancer (Demark‐Wahnefried 2009; Hawkes 2013; Janssen‐Heijnen 2009). Genotype and lifestyle are considered significant contributory factors that lead to increased morbidity and cancer recurrence in people who have survived cancer (Daar 2007; Demark‐Wahnefried 2009). Furthermore, survivors of cancer use healthcare services and receive social welfare benefits more frequently than others (Nord 2005). In addition, it has been shown that those who have survived cancer visit their general practitioners more frequently than their non‐cancer counterparts (Khan 2011). Research has demonstrated that the poorer health status identified among survivors of cancer detrimentally influences QoL (Baker 2003). Among older people who have survived cancer, improved diet and enhanced physical activity have been shown to be associated with better vitality and functioning (Hewitt 2003).
This systematic review is important to determine which dietary interventions are effective for those who have survived cancer. Available evidence supports exercise initiatives for cancer survivors, in relation to health‐related QoL (Mishra 2012). In promoting lifestyle behaviours, it is difficult to unravel the contributions of individual components to overall health and well‐being. However, it would be useful to determine the most appropriate dietary interventions that are effective in people who have survived cancer to inform clinical practitioners, and to assist in improving the long‐term health of people who have survived cancer. Evidence on dietary interventions for survivors of cancer is now developing, so it is timely to review the literature to summarise the research, to inform clinical practice and policy development, and to identify gaps in the literature for further research.
Objectives
To assess the effects of dietary interventions for adult cancer survivors on morbidity and mortality, changes in dietary behaviour, body composition, health‐related quality of life, and clinical measurements.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) and cluster RCTs published in peer‐reviewed journals that compared a dietary intervention versus a control consisting of no intervention or written information.
Types of participants
All adult cancer survivors, defined as those who have lived beyond a cancer diagnosis that occurred after the age of 18 years and have completed all active anti‐cancer interventions, such as surgery, radiotherapy, chemotherapy, or hormone therapy. People with pre‐cancerous lesions were not included. People who had survived recurrent cancers were included if they had completed all anti‐cancer therapies.
Types of interventions
All dietary interventions were provided for healthy eating and weight loss or weight maintenance. Specific nutritional interventions, including those based only on food, were included. Dietary interventions needed to include multiple nutrients, fat, carbohydrate, protein, vitamins, and minerals. Dietary interventions based on a single food group were excluded. Oral supplements, including those with single or multiple nutrients, were excluded. Probiotic supplements were excluded, along with all intravenous nutrient solutions containing single or multiple nutrient administrations. All enteral feedings were also excluded.
Types of outcome measures
Primary outcomes
Overall survival (e.g. time to death from any cause)
Incidence of secondary malignancy or other cancer
Incidence of all comorbidities
Secondary outcomes
Dietary intake measured by dietary analysis using food frequency questionnaires, dietary recall, or food diaries, or assessed by dietary assessment methods
Body weight or anthropometric measurements including hip‐to‐waist ratios, skin fold thickness, or functional capacity measurements
Patient outcomes, including quality of life (QoL) questionnaires (e.g. EuroQoL Group Quality of Life Questionnaire based on 5 dimensions (EQ‐5D) (Szende 2014), Short Form (SF)‐36 (Bowling 1999))
Biochemical measurements, which may include lipid profiles or serum glucose as a surrogate marker (blood glucose levels, serum cholesterol, serum triglyceride levels)
Number of healthy eating changes made to habitual eating patterns
We will present a 'Summary of findings' table to report the following outcomes.
Overall mortality.
Secondary cancers.
Fruit and vegetable intake.
Fibre intake.
Diet Quality Index.
Body mass index (BMI).
Waist‐to‐hip ratio.
Search methods for identification of studies
Electronic searches
We searched the following electronic databases.
Cochrane Central Register of Controlled Trials (CENTRAL; 2019, Issue 9), in the Cochrane Library (Appendix 1).
MEDLINE via Ovid (1946 to September week 1 2019) (Appendix 2).
Embase via Ovid (1980 to 2019 week 37) (Appendix 3).
Allied and Complementary Medicine Database (AMED) (Ovid) (1985 to 31 October 2018) (Appendix 4).
Cumulative Index to Nursing and Allied Health Literature (CINAHL) (EBSCO host) (1937 to 31 October 2018) (Appendix 5).
Database of Abstracts of Reviews of Effects (DARE) (1994 to March 2015) (Appendix 6).
We identified all relevant articles on PubMed; we used the 'related articles' feature to carry out further searches for newly published articles. Reports in all languages were sought and translations carried out when necessary.
Searching other resources
We reviewed the reference lists of all retrieved articles and other reviews on the topic. We also searched the World Health Organization (WHO) International Clinical Trials Registry Platform (www.who.int/ictrp/en).
We searched metaRegister (www.isrctn.com/), Physicians Data Query (www.cancer.gov/publications/pdq), www.clinicaltrials.gov, and www.cancer.gov/about‐cancer/treatment/clinical‐trials for ongoing trials. If through these searches we identified ongoing trials that had not been published, we approached the principal investigators to ask for relevant data. We searched conference proceedings and abstracts through ZETOC (zetoc.mimas.ac.uk) and WorldCat Dissertations (www.worldcat.org/).
We handsearched abstracts from meetings held by the American Institute for Cancer Research (www.aicr.org/).
We also contacted investigators of eligible unpublished studies identified from the abstracts of conference proceedings to ask for relevant unpublished data, and we searched trial registries for additional studies.
Data collection and analysis
Selection of studies
Three review authors (DG, AMS, JS) independently assessed titles and abstracts retrieved from the searches to determine study relevance and eligibility. We excluded all papers that failed to meet the eligibility criteria. Two review authors retrieved and independently reviewed full‐text articles for potentially relevant studies, to assess whether they met the inclusion criteria. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram and Characteristics of included studies and Characteristics of excluded studies tables. A third review author was called upon to resolve any conflicts that arose during study selection. Multiple reports of the same study were linked. We translated any non‐English articles before assessment, as required.
Data extraction and management
We devised a standardised data collection form to facilitate collection of data from the included studies; we have provided this data extraction form in Appendix 7. We piloted and modified the data extraction form as required. Two review authors (DJ, SB) independently extracted data and discussed any discrepancies with a third review author (CT). We recorded the following information for each trial.
Year of publication, country of origin, source of funding, number of participants.
Study population: age, gender, location of tumour, previous therapy, cancer staging or classification.
Other baseline characteristics, including proportion of overweight or obese survivors (defined by body mass index > 25 kg/m² or nutrition status assessment derived from a validated tool), alcohol intake, smoking status, current physical activity, and socioeconomic group.
We expressed measurement of treatment effect as follows. For dichotomous variables, we calculated risk ratios (RRs) and expressed them with 95% confidence intervals (CIs). For continuous data expressed as means with standard deviations (SDs), we used mean differences (MDs) to show effect size. For data presented as time‐to‐event, if they were dichotomous, we used a log rank approach to calculate hazard ratios (HRs).
Assessment of risk of bias in included studies
We assessed risk of bias in included studies using the Cochrane tool (Higgins 2011). This included assessment of the following.
-
Selection bias.
Random sequence generation.
Allocation concealment.
-
Performance bias.
Blinding of participants and personnel (patients and treatment providers), although this may not be possible due to the nature of some of the interventions.
-
Detection bias.
Blinding of outcome assessment.
-
Attrition bias.
-
Incomplete outcome data: we recorded the proportion of participants whose outcomes were not reported at the end of the study and categorised them as follows.
Low risk of bias, if less than 80% of patients were assessed and reasons for loss to follow‐up or inadequate responses were similar in both treatment arms.
High risk of bias, if more than 80% of patients were assessed or reasons for loss to follow‐up or inadequate responses differed between treatment arms.
Unclear risk of bias, if the number of patients assessed was not reported.
-
-
Reporting bias.
Selective reporting of outcomes.
Other possible sources of bias.
Two review authors independently applied the 'Risk of bias' tool and resolved differences by discussion or by appeal to a third review author. We summarised results in both a 'Risk of bias' graph and a 'Risk of bias' summary. We interpreted results of meta‐analyses in light of the findings with respect to risk of bias.
Measures of treatment effect
Overall survival
Incidence of secondary malignancy or other cancer
Incidence of comorbidities
Dietary changes measured by dietary analysis using food frequency questionnaires, dietary recall, or food diaries, or assessed by dietary assessment methods
Changes in body weight or anthropometric measurements including hip‐to‐waist ratios, skin fold thickness, or functional capacity measurements
Patient outcomes, including QoL questionnaires
Biochemical measurements, which may include lipid profiles or serum glucose as a surrogate marker
Number of healthy eating changes made to habitual eating patterns
Details of type of intervention, including nutritional education, change behaviour techniques employed, and delivery method of the intervention (written, telephone, face‐to‐face, or Internet‐based)
Unit of analysis issues
We included cluster randomised trials. In these trials, individuals were randomised as a block, from one centre or one clinic, so we dealt with this on a trial‐by‐trial basis, depending on the study design.
Dealing with missing data
An intention‐to‐treat analysis was planned and we contacted study authors for any missing 'Risk of bias' information or outcome data required, if appropriate. We reported on levels of loss to follow‐up/inadequate follow‐up and assessed these as a source of potential bias. We planned to investigate, through sensitivity analyses, the effects of any imputed data on pooled effect estimates; however, we did not impute any data in the analysis, so we did not do this.
Assessment of heterogeneity
We assessed the heterogeneity of any combined studies in the meta‐analysis using I². If I² was greater than 30%, we examined possible reasons for heterogeneity in relation to clinical setting, study participants, and similarity of clinical parameters in studies.
Assessment of reporting biases
We searched multiple sources including trial registries as detailed above. We considered whether trials were undertaken and reported according to their trial protocol. We found an insufficient number of included studies to assess publication bias using a funnel plot, as detailed in Section 10.4 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Data synthesis
We used Review Manager 2014 for data synthesis. We conducted meta‐analyses only if we found studies reporting similar comparisons for the same outcomes. We performed meta‐analyses using the Mantel‐Haenszel random‐effects method for synthesis of dichotomous data due to the anticipated level of heterogeneity in these studies.
For continuous variables, we used inverse variance in a random‐effects model for suitable data for a meta‐analysis. One study reported time‐to‐event data, and we used the HR with the inverse variance fixed‐effect model. If we established that heterogeneity between studies was significant (I² > 30%), we explored possible causes of heterogeneity. If a meta‐analysis could not be undertaken, we provided a descriptive review of the studies.
'Summary of findings' for assessing certainty of evidence
We will present the overall certainty of evidence for each outcome according to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach, which takes into account issues related not only to internal validity (risk of bias, inconsistency, imprecision, publication bias) but also to external validity (e.g. directness of results) (Langendam 2013; Schünemann 2011). We will create a 'Summary of findings' table based on the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and we will use GRADEPro GDT 2014. We will use the GRADE checklist and GRADE Working Group certainty of evidence definitions (Meader 2014). We will downgrade the evidence from 'high‐certainty' by one level for serious (or by two levels for very serious) concerns for each limitation.
High‐certainty: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate‐certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low‐certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
Very low‐certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Subgroup analysis and investigation of heterogeneity
When data allowed, we planned and undertook subgroup analysis on different cancer types and on different dietary intervention methods delivered for specific interventions. This included subgroup analysis of interventions for weight management or analysis that looked at interventions delivered by different methods.
Sensitivity analysis
We undertook sensitivity analysis to evaluate effects of bias on the results by investigating the impact of trials that had a high or unclear level of bias. We evaluated separately each of the items assessed to indicate bias.
Results
Description of studies
Results of the search
See Characteristics of included studies,Characteristics of excluded studies,Characteristics of ongoing studies, and Studies awaiting classification.
Searches were performed up to 26 October 2018; we identified a total of 11,092 articles from databases, registers, and other sources as pre‐specified in the Search methods for identification of studies. After we had removed 1772 duplicates and had excluded 9154 records by title and abstract screening, because studies did not meet the inclusion criteria, or because they were reports of ongoing studies, we assessed 166 full‐text articles for eligibility. After full‐text screening, we identified 78 reports from 25 studies that met the inclusion criteria (Figure 1).
1.
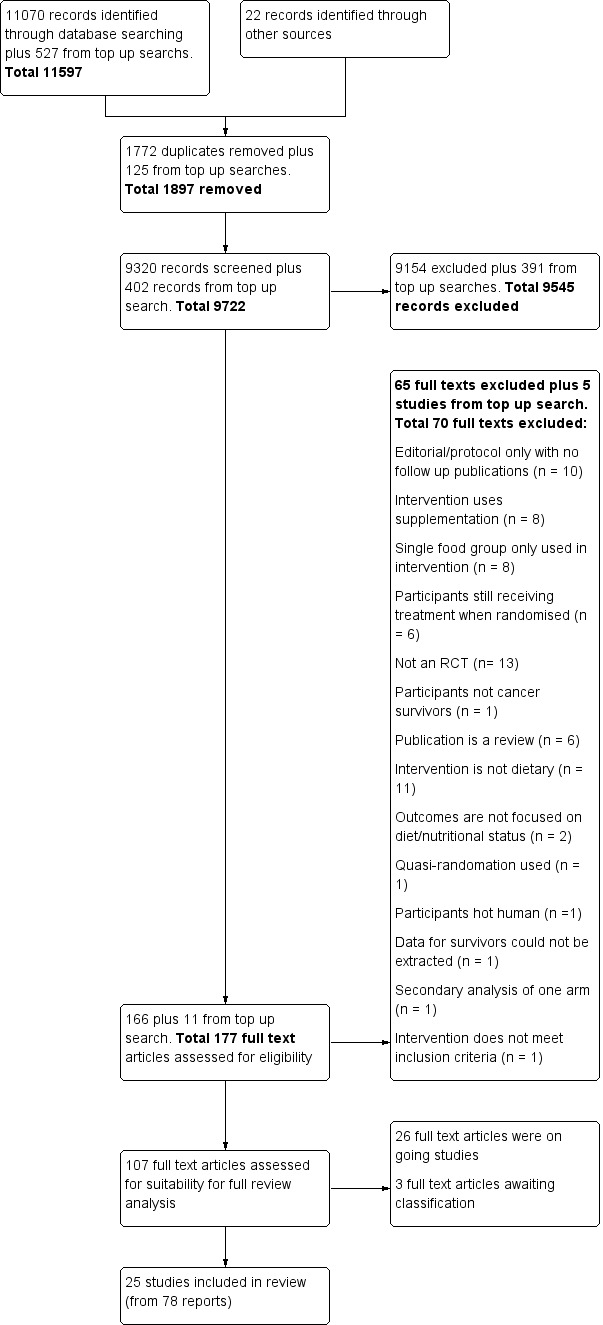
PRISMA flow diagram for study selection.
We performed a top‐up search from 1 November 2018 to 18 September 2019, and we searched the main databases (MEDLINE, Embase, and CENTRAL). We identified 527 studies, of which 125 were duplicates. After title and abstract screening, we excluded another 391 records. After full‐text screening of the remaining 11 studies, we excluded five additional studies (Goodwin 2019; Hagemann 2019; Koutoukidis 2019; Ligibel 2019; Park 2019), and we identified six studies for inclusion in the review. Of those studies, three are ongoing studies (Demark‐Wahnefried 2019; Groarke 2018; O'Connor 2018), and three are awaiting classification (Brown 2018; Parekh 2018; Zuniga 2019).
In total, through all the searches, we found 25 included studies, 26 ongoing studies, and three studies awaiting classification. After review of the results of studies awaiting classification, we concluded that their current inclusion would not change any conclusions of the review at this time (Figure 1).
We identified only one trial report for 10 studies (Bloom 2008; Bourke 2011; Kanera 2017; Kim 2011; Mefferd 2007; Park 2016; Sheppard 2016; Swisher 2015; Yun 2017; Zick 2017), and we found 15 studies with more than one trial report (Befort 2016; Demark‐Wahnefried 2006; Demark‐Wahnefried 2007; Demark‐Wahnefried 2014; Djuric 2002; Ghavami 2017; Greenlee 2013; Greenlee 2015; Gruenigen 2012; Harrigan 2016; Hawkes 2013; Morey 2009; Pierce 2007; Reeves 2017; Scott 2013). All additional reports are included under the main report in the reference list. The reports identified for each study are shown in Appendix 8.
Included studies
See Characteristics of included studies. All data for study outcomes were derived from published sources.
Study design
All included studies were RCTs that randomised participants to control or intervention arms. However, one RCT also incorporated pre‐ and post‐design, whereby women were randomised to delayed intervention as the control group (Bloom 2008). We included six reports of pilot studies (Bourke 2011; Djuric 2002; Greenlee 2013; Kim 2011; Reeves 2017; Zick 2017), along with one report of a feasibility study (Sheppard 2016).
Mortality and secondary cancers were recorded in one study (Pierce 2007). Dietary changes including total energy intake from dietary assessment and fruit and vegetable intake were reported. This outcome was reported as servings of fruits or vegetables separately per day, or as fruit and vegetable servings per day combined, or as the number of participants eating more than five portions of fruits or vegetables. Fibre intake was also reported along with the Diet Quality Index. Changes in anthropometric measures including body weight, body mass index, body composition, waist‐to‐hip ratio, waist circumference, and hip circumference, were reported. Quality of life was reported as Functional Assessment of Cancer Therapy ‐ General (FACT‐G), Functional Assessment of Cancer Therapy ‐ Breast (FACT‐B), Functional Assessment of Cancer Therapy ‐ Colorectal (FACT‐C), or Short Form (SF)‐36 with physical and mental health domains. Some studies also reported global health status using the European Organisation for Research and Treatment of Cancer (EORTC) questionnaire. One study reported biochemistry including total cholesterol and triglycerides (Mefferd 2007).
Participants
A total of 7259 participants were randomised in the 25 included studies, including 977 (13.5%) men and 6282 (86.5%) women. Most studies included women with breast cancer (Befort 2016; Bloom 2008; Demark‐Wahnefried 2014; Djuric 2002; Ghavami 2017; Greenlee 2013; Greenlee 2015; Harrigan 2016; Kim 2011; Mefferd 2007; Park 2016; Pierce 2007; Reeves 2017; Scott 2013; Sheppard 2016; Swisher 2015; Zick 2017). Two studies had discrepancies between total numbers of participants and genders reported (Kanera 2017; Yun 2017). Two studies included only participants with colorectal cancer (Bourke 2011; Hawkes 2013), one study included only women with uterine cancer (Gruenigen 2012), and three studies included a mixture of participants after survival of prostate, breast, or colorectal cancer (Demark‐Wahnefried 2006; Demark‐Wahnefried 2007; Morey 2009). Two studies reported mixed cancer sites (Kanera 2017; Yun 2017). Mean age reported ranged from 52.6 to 71 years, and range of age of included participants was 23 to 85 years.
Three studies recruited participants from ethnic minority groups. One study recruited black women (Sheppard 2016), and the other two recruited Hispanic and black women (Greenlee 2013; Greenlee 2015). Two studies targeted older adults (Demark‐Wahnefried 2006; Morey 2009). However, in total, mean participant age was reported as above 60 years in five studies (Demark‐Wahnefried 2006; Demark‐Wahnefried 2014; Hawkes 2013; Morey 2009; Zick 2017).
Eleven studies recruited participants based on nutritional status measurements in which body mass index (BMI) was greater than or equal to 25 kg/m² (Befort 2016; Demark‐Wahnefried 2014; Ghavami 2017; Greenlee 2013; Gruenigen 2012; Harrigan 2016; Mefferd 2007; Reeves 2017; Scott 2013; Sheppard 2016; Swisher 2015); one study incorporated lifestyle behaviours into the inclusion criteria (Hawkes 2013); and one study incorporated nutritional intake into the inclusion criteria (Kim 2011). One study included participants who consumed fewer than five portions of fruits and vegetables daily (Greenlee 2015).
Most interventions were provided in participants' own homes or in a community setting. In one instance, the intervention was provided at a university rehabilitation centre (Bourke 2011). In three instances, intervention was provided in a hospital or clinic environment (Mefferd 2007; Pierce 2007; Sheppard 2016), and one study provided the intervention at an exercise facility (Swisher 2015).
Interventions
The dietary interventions provided varied in relation to the person providing the intervention, the mode of provision (web, print, telephone, group, or face‐to‐face), and the frequency of contact with participants. For each study, these factors have been outlined in Table 2. Studies recorded dietary intake in different ways; some reported energy intake and nutrients, and others reported food groups or scales. In the results, we report dietary intake using energy intake, fruit servings per day, vegetable servings per day, fruit and vegetable servings per day in combination, fibre intake, and Diet Quality Index. The way dietary intake was recorded varied between studies, as did the methods of assessing dietary intake. Assessment methods included food diaries that were monitored by participants, three‐day diet recalls, diet history questionnaires, food frequency questionnaires, and three‐day diet diaries. All dietary assessment methods used are recorded in Table 2. Dietary intake was also reported over differing lengths of time; these are shown in Table 2. Due to lack of standardised methods of recording and assessing dietary intake, only a few studies could be incorporated into meta‐analyses, so we have given a narrative summary of results when appropriate.
1. Characteristics of dietary interventions, outcomes, and length of follow‐up.
| Author and date | Personnel providing dietary intervention | Description of intervention | Method of dietary assessment | Frequency of contact | Outcomes recorded | Follow‐up length | Nutritional status at recruitment | Behavioural change therapy | Control or comparison group |
| Befort 2016 | Registered dietitian or psychologist | Phase 1: 25‐week 60‐minute conference call sessions delivered to groups of 12 to 15 women. Phase 2: 26 biweekly conference calls | Not reported | Weekly | Anthropometry changes, participant costs, incremental cost‐effectiveness | 18 months | Not reported | Not reported | Provided 9 newsletters with the same content as intervention calls |
| Bloom 2008 | Trained healthcare professional |
Three 6‐hour group workshops | Fruit and vegetable and fat screener questionnaires | Monthly | QoL, knowledge, dietary changes |
6 months | Not reported | Not reported | Usual care |
| Bourke 2011 | Exercise physiologist | Nutrition advice pack and healthy eating seminars with supervised home‐based exercise sessions | 3‐Day food diary | Weekly | QoL, dietary and anthropometry changes | 12 weeks | Not reported | Not reported | Usual care |
| Demark‐Wahnefried 2006 | Dietitian/ counsellors |
Personalised workbook of diet and physical activity targets given on the basis of individual intake. Supported by telephone counselling | 3‐Day diet recall | 12 bimonthly 20‐ to 30‐minute sessions over 6 months | Anthropometry, physical function, diet quality | 6 months and 12 months | Nil | Transtheoretical model, social‐cognitive theory |
General written material |
| Demark‐Wahnefried 2007 | Mail‐based | Tailored mailed dietary material on increasing fruits and vegetables, reducing total fat, increasing exercise, providing lifestyle advice | Telephone interviews using diet history questionnaire | 7 newsletters at 6‐weekly intervals | Anthropometry, behaviour and dietary change, QoL |
1 year & 2 years |
Not reported | Transtheoretical model, social‐cognitive theory |
General written material |
| Demark‐Wahnefried 2014 | Not reported | Personalized workbook with goal‐setting. Advice on specific dietary intake and physical activity | 24‐Hour recall | Telephone interviews at 3 time points | Dietary intake, anthropometry, QoL |
12 months |
BMI ≥ 25 to 39.9 |
Social‐cognitive theory | Written material |
| Djuric 2002 | Dietitian | Individualised counselling via telephone contact provided |
3‐Day food diary | Counselling: weekly for first 3 months, biweekly for months 3 to 6, then monthly up to 30 months | Dietary intake, anthropometry | 12 months | Not reported | Social‐cognitive theory | Written materials |
| Djuric 2002 | Weight watchers groups | Weight watchers meetings only | 3‐Day food diary | Weekly | Anthropometry and dietary changes | 12 months | Not reported | Not reported | Written materials |
| Ghavami 2017 | Researcher but not specified | Individualised intervention promoting prescribed exercise (moderate exercise 3 to 5 days per week) and a balanced diet through stage‐matched telephone counselling and a workbook | Not reported | Weekly | QoL | 24 weeks | BMI > 25 | Not reported | Usual care |
| Greenlee 2013 | Staff trained under the CURVE programme | Group weight loss programme | Food frequency questionnaire 110‐item | 3‐ to 5‐day/week exercise 1‐hour nutrition course/week |
Anthropometry, metabolic and dietary changes | 12 months | BMI ≥ 25 | Not reported | Waiting list |
| Greenlee 2015 | Registered dietitian, nutritionist, chef | 9 group sessions (24 hours over 12 weeks) of nutrition intervention with culturally tailored curriculum | 24‐Hour dietary recall | Weekly/ monthly |
Anthropometry, dietary changes | 12 months | BMI 30.6 | Social‐cognitive theory, transtheoretical model | Booklets on healthy eating |
| Gruenigen 2012 | Dietitian | Individualised goal‐setting enabling self‐efficacy. Group sessions for nutrition and physical activity advice plus face‐to‐face counselling sessions |
24‐Hour recall | 10 weekly sessions followed by 6 biweekly sessions plus face‐to‐face counselling | Anthropometry and dietary changes | 12 months | BMI 25.0 to 39.9 vs > 40 in control | Social‐cognitive theory | Written material |
| Harrigan 2016 | Registered dietitian | In‐person counselling about nutrition, expertise, behaviour strategies | Food frequency questionnaire | Once per week (for month), then every second week (for months 2 and 3), then once per month (for months 4 to 6) | Anthropometry, dietary changes, biochemical changes, physical activity | 6 months | BMI 33.5 vs 34 in control | Social‐cognitive theory | |
| Harrigan 2016 | Registered dietitian | Telephone counselling about nutrition, expertise, behaviour strategies | Food frequency questionnaire | Once per week (for month), then every second week (for months 2 and 3), then once per month (for months 4 to 6) | Anthropometry, dietary changes, biochemical changes, physical activity | 6 months | BMI 31.8 | Social‐cognitive theory | |
| Hawkes 2013 | Health coach | Telephone health coaching focused on physical activity, weight management, dietary habits, smoking | Food frequency questionnaire | 11 phone sessions over 6 months | Anthropometry, dietary changes, QoL | 6 months | BMI ≥ 25 | Acceptance commitment therapy, mindfulness | Usual care |
| Kanera 2017 | Web‐based | Web‐based self‐management program with modules on diet, physical activity, depression, others | Dutch standard questionnaire on food consumption | 4 weeks after diet | Dietary changes, physical activity | 12 months | BMI 26.0 | Theory of planned behaviour, self‐regulated theory. integrated model of change | Usual care (waiting list) |
| Kim 2011 | Trained nurses | Workbook and individualised prescription for regular exercise and diet 30‐minute telephone calls |
3‐Day dietary recall, diet quality tool | Weekly | Dietary changes, Diet Quality Index, QoL, behavioural changes | 12 weeks | Not reported | Transtheoretical model | Usual care |
| Mefferd 2007 | Trained research assistant | Group exercise and diet modification sessions using behavioural treatment, weekly phone calls | Food diaries self‐monitored | Weekly sessions followed by once‐monthly sessions, then monthly sessions for 6 months | Anthropometry changes | 16 weeks | BMI ≥ 25 | Cognitive‐behavioural therapy | Waiting list |
| Morey 2009 | Health counsellor | PersonaIised tailored workbook and series of quarterly newsletters, along with a programme of telephone counselling and automated prompts | 24‐Hour dietary recall | Weekly and monthly telephone and counselling sessions over 12 months | Anthropometry changes, functionality | 12 months | BMI ≥ 25 and ≤ 40 | Transtheoretical model, social‐cognitive theory |
Usual care |
| Park 2016 | Mail‐based | Mail‐based lifestyle intervention | Paffenbarger activity questionnaire | Biweekly for 4 months | Dietary changes, feasibility, and adherence | 7 months | Not reported | Addressed behavioural skills but no specific therapy reported | Usual care |
| Pierce 2007 | Trained counsellors | Telephone counselling. Phase 1: build self‐efficacy to implement dietary targets. Phase 2: focus on self‐monitoring and barriers to adherence. Phase 3: focus on motivation 12 cooking classes were offered plus newsletters |
24‐Hour dietary recall | Average 18 counselling calls, 12 cooking classes, and 12 study newsletters. By 4 years, average 31 calls and 48 newsletters received | Overall survival, incidence of secondary cancer, comorbidities, anthropometry, dietary changes | 4 years | Not reported | Social‐cognitive theory | Writen materials |
| Reeves 2017 | Dietitian | Posted materials and telephone calls | 24‐Hour diet recall | Posted materials and up to 16 calls over 6 months | Anthropometry, dietary changes, QoL | 6 months | BMI ≥ 25 to 45 | Not reported | Usual care |
| Scott 2013 | Trained technician | Individual hypocaloric eating and supervised exercise sessions | 3‐Day diet diary | Weekly | Anthropometry, dietary changes, QoL | 24 weeks | BMI ≥ 25 | Not reported | Written material |
| Sheppard 2016 | Exercise physiologist, nutritionist, survivor coach | Group and individualised phone sessions | 4‐Day food diary | Every 2 weeks group session, phone calls on the weeks in between | Anthropometry changes, physiological function, QoL, biochemical changes | 12 weeks | BMI ≥ 25 and ≤ 40 | Motivational interviewing technique |
General health information |
| Swisher 2015 | Dietitian, exercise physiologist | Supervised and unsupervised exercise sessions, 2 individual dietary sessions | 3‐Day food diary | 3 times/week supervised 2 times unsupervised |
Anthropometry changes, physical function, QoL, biochemical changes | 12 weeks | BMI ≥ 25 | Not reported | Written materials |
| Yun 2017 | Trained health professional | 1‐hour health education session, 3‐hour leadership workshop, individual coaching by phone for 24 weeks | Validated questionnaire based on the Rules for National Cancer Prevention: dietary practice guideline | Weekly/monthly for different sessions | Physical activity, dietary changes, QoL, cancer survivors leadership | 12 months | BMI 22.05 | Transtheoretical model | Usual care |
| Zick 2017 | Registered dietitian | Individualised phone counselling | 24‐Hour dietary recall | Weekly for 4 weeks, then biweekly | Anthropometry, dietary changes, QoL, biochemical changes | 3 months | BMI 27.2 vs 29.2 | Brandura's social‐cognitive theory | General health curriculum sessions |
Kim 2011 assessed the Diet Quality Index using a scale on which a lower score indicated better diet quality (Patterson 1994). Two studies ‐ Demark‐Wahnefried 2006 and Demark‐Wahnefried 2014 ‐ used a Diet Quality Index score for which a higher score indicated better diet quality (Haines 1999).
Types of anthropometry measurements recorded were body weight, body mass index, weight loss, and hip‐to‐waist ratio. Study authors measured outcomes at different time points.
Quality of life was measured via FACT‐G, which has 27 questions, each of which is answered on a five‐point Likert scale ranging from zero (Not at all) to four (Very much). Questions are phrased such that higher numbers indicate a better health state. For over 20 cancer‐specific scales, such as FACT‐B for breast cancer and FACT‐C for colorectal cancer, higher scores are indicative of a better state of health.
The SF‐36 was also used to measure quality of life and consists of eight scaled scores, which are the weighted sums of the questions in each section. Each scale is directly transformed into a 0 to 100 scale on the assumption that each question carries equal weight. Lower score means greater disability. Higher score means less disability.
Comparators
The comparisons included in this review were usual care, written materials, or waiting list compared to dietary intervention. Two included studies had more than two arms meeting the inclusion criteria (Djuric 2002; Harrigan 2016). In these studies, the intervention arms were amalgamated when possible, using the guidance provided in Chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions for obtaining a joint mean, a standard deviation, and a comparison with control (Deeks 2011). When this was not possible, we provided a narrative account in the results.
Funding sources
Funding sources were the US National Institute of Health Research or the American Institute of Cancer Research in 13 studies (Befort 2016; Bloom 2008; Demark‐Wahnefried 2006; Demark‐Wahnefried 2007; Demark‐Wahnefried 2014; Djuric 2002; Greenlee 2015; Harrigan 2016; Mefferd 2007; Morey 2009; Pierce 2007; Sheppard 2016; Zick 2017). For two studies, funding was provided by the American Cancer Society (Gruenigen 2012; Swisher 2015). Three studies procured funding from personal foundations (Demark‐Wahnefried 2006; Greenlee 2013; Pierce 2007). For one study, funding was awarded from the National Institute for Health Research (NIHR) in the UK (Bourke 2011), and for another study from Germany, funding was provided by the National Cancer Institute (NCI) (Park 2016). Two studies received funding from the National Cancer Research Centre in South Korea (Kim 2011; Yun 2017), and two studies received funding from the Australian Government (Hawkes 2013; Reeves 2017). Commercial funding was stated only in Djuric 2002. Some studies received funding from more than one source (Demark‐Wahnefried 2006; Pierce 2007), and only one study did not state the funding source (Ghavami 2017).
Excluded studies
See Characteristics of excluded studies.
We excluded 70 articles after full‐text screening: 13 were not RCTs, 10 were editorials or protocols only with no follow‐up publications, eight were interventions using supplementation or enriched diet, six were reviews, six included participants who were still receiving treatment when randomised, one included participants who were not cancer survivors, 11 provided interventions that were not dietary, eight used single food groups only in the intervention, two had outcomes that were not focused on diet or nutritional status, one used quasi‐randomisation, one did not involve humans, one did not separate data for cancer survivors from data for carers, one conducted secondary analysis for only one arm, and the intervention for one study did not meet review inclusion criteria.
Risk of bias in included studies
We summarised risk of bias in the included studies in Figure 2 and displayed this information graphically in Figure 3.
2.
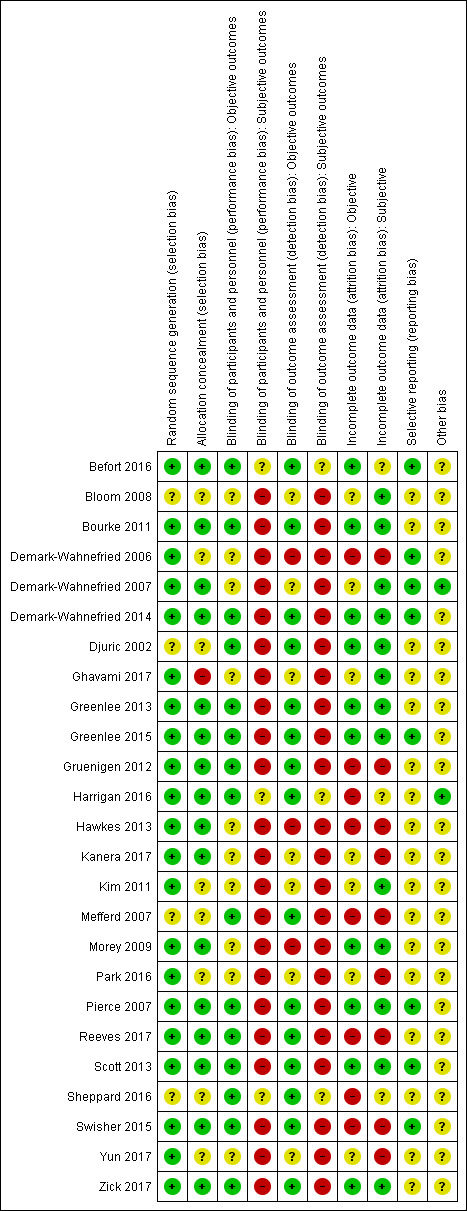
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
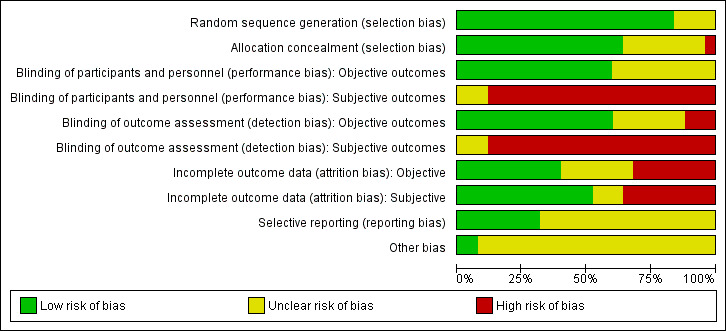
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
We assessed selection bias in the form of the risk of bias domains of random sequence generation and allocation concealment.
Random sequence generation
In relation to random sequence generation, we identified 21 studies with low risk of bias because randomisation by computer was used in seven studies (Bourke 2011; Gruenigen 2012; Hawkes 2013; Kanera 2017; Reeves 2017; Yun 2017; Zick 2017); block randomisation was used in seven studies (Demark‐Wahnefried 2006; Demark‐Wahnefried 2007; Greenlee 2013; Harrigan 2016; Morey 2009; Park 2016; Pierce 2007); an off‐site statistician was used in four studies (Befort 2016; Demark‐Wahnefried 2014; Greenlee 2015; Swisher 2015); a random numbers table was used in two studies (Ghavami 2017; Kim 2011); and an independent researcher carried out randomisation in one study (Scott 2013). Unclear risk was demonstrated in four studies, which lacked detail around the explanation of randomisation (Mefferd 2007; Sheppard 2016), and in one study, which provided no information in relation to randomisation (Bloom 2008; Djuric 2002).
Allocation concealment
We found unclear allocation concealment in eight studies, which provided little ‐ Mefferd 2007 and Yun 2017 ‐ or no detail ‐ Bloom 2008,Demark‐Wahnefried 2006,Djuric 2002,Kim 2011,Park 2016,Sheppard 2016. For 16 studies, risk was considered low as randomisation was undertaken independently (Befort 2016; Bourke 2011; Greenlee 2013; Gruenigen 2012; Hawkes 2013; Kanera 2017; Morey 2009; Pierce 2007; Reeves 2017; Scott 2013; Swisher 2015); the study staff involved with randomisation was blinded (Demark‐Wahnefried 2007; Harrigan 2016; Hawkes 2013); or sealed envelopes marked with a numerical code were used (Greenlee 2015; Zick 2017). One study was considered high risk as a random numbers table was used (Ghavami 2017).
Blinding
For evaluating performance bias, we assessed blinding of participants and personnel for both objective and subjective outcomes.
Blinding of participants and personnel
We identified unclear risk for objective outcomes in three studies in which no blinding took place due to the nature of the intervention (Demark‐Wahnefried 2006; Hawkes 2013; Morey 2009). For seven studies, we deemed the risk as unclear for objective outcomes because no information was provided (Bloom 2008); or no objective outcomes were reported (Demark‐Wahnefried 2007; Ghavami 2017; Kanera 2017; Kim 2011; Park 2016; Yun 2017). For the remaining 15 studies, we considered risk as low for objective outcomes, as it was clearly stated that blinding had been used or the outcome was not influenced by blinding (Befort 2016; Bourke 2011; Demark‐Wahnefried 2014; Djuric 2002; Greenlee 2013; Greenlee 2015; Gruenigen 2012; Harrigan 2016; Mefferd 2007; Pierce 2007; Reeves 2017; Scott 2013; Sheppard 2016; Swisher 2015; Zick 2017).
For subjective outcomes, risk of bias was high in 22 studies, which could not be blinded due to the nature of the study (Bloom 2008; Bourke 2011; Demark‐Wahnefried 2006; Demark‐Wahnefried 2007; Djuric 2002; Ghavami 2017; Greenlee 2013; Greenlee 2015; Gruenigen 2012; Hawkes 2013; Kanera 2017; Kim 2011; Mefferd 2007; Morey 2009; Park 2016; Pierce 2007; Reeves 2017; Scott 2013; Swisher 2015; Yun 2017; Zick 2017), or for which no further details were given (Demark‐Wahnefried 2014). Bias was unclear in three studies because subjective outcomes were not reported (Befort 2016; Harrigan 2016; Sheppard 2016). No study had low risk for subjective outcomes.
Blinding of outcome assessment
We identified high risk of bias for objective outcomes in three studies, which stated no blinding had been in place (Demark‐Wahnefried 2006; Hawkes 2013; Morey 2009). For seven studies, risk was unclear; for two of these studies, blinding of outcome assessment was not stated (Bloom 2008; Kim 2011); for one study, blinding was suggested but not enough detail was included (Demark‐Wahnefried 2007); and for four studies, objective measures were not reported (Ghavami 2017; Kanera 2017; Park 2016; Yun 2017). The remaining 15 studies were considered low risk as study authors clearly stated that blinding had been in place for the outcome assessment (Befort 2016; Bourke 2011; Demark‐Wahnefried 2014; Djuric 2002; Greenlee 2013; Greenlee 2015; Gruenigen 2012; Harrigan 2016; Mefferd 2007; Pierce 2007; Reeves 2017; Scott 2013; Sheppard 2016; Swisher 2015; Zick 2017).
Risk of bias for subjective outcomes was high in 22 studies because no blinding of outcomes assessment was reported and/or outcome measurement was patient self‐reported (Bloom 2008; Bourke 2011; Demark‐Wahnefried 2006; Demark‐Wahnefried 2007; Djuric 2002; Ghavami 2017; Greenlee 2013; Greenlee 2015; Gruenigen 2012; Hawkes 2013; Kanera 2017; Kim 2011; Mefferd 2007; Morey 2009; Park 2016; Pierce 2007; Reeves 2017; Scott 2013; Swisher 2015; Yun 2017; Zick 2017), or no further details were reported (Demark‐Wahnefried 2014). In three studies, risk was deemed to be unclear because no subjective outcomes were reported (Befort 2016; Harrigan 2016; Sheppard 2016). No study was considered at low risk for subjective outcomes.
Incomplete outcome data
Attrition bias was assessed in the form of incomplete outcome data for both objective and subjective outcomes.
Eight studies were considered to have high attrition bias for objective outcomes: uneven dropout between groups was reported in two studies ‐ in one study, dropout rates were 61% control and 16% intervention (Mefferd 2007), and in another study, dropout rates were 0% control and 18% intervention (Swisher 2015); and a large attrition rate was reported at between 21% and 49% in six studies (Demark‐Wahnefried 2006; Gruenigen 2012; Harrigan 2016; Hawkes 2013; Reeves 2017; Sheppard 2016). Ten studies were considered at low risk as the attrition rate was low or was similar between groups (Befort 2016; Bourke 2011; Demark‐Wahnefried 2014; Djuric 2002; Greenlee 2013; Greenlee 2015; Morey 2009; Pierce 2007; Scott 2013; Zick 2017).
Nine studies were considered to have high attrition bias for subjective outcomes due to unequal or high attrition rates (Demark‐Wahnefried 2006; Gruenigen 2012; Hawkes 2013; Kanera 2017; Mefferd 2007; Park 2016; Reeves 2017; Swisher 2015; Yun 2017). Unclear attrition bias was identified in three studies that did not report on subjective outcomes (Befort 2016; Harrigan 2016; Sheppard 2016). The remaining 13 studies were considered to be at low risk with low dropout rates, good adherence levels, and use of intention‐to‐treat analysis (Bloom 2008; Bourke 2011; Demark‐Wahnefried 2007; Demark‐Wahnefried 2014; Djuric 2002; Ghavami 2017; Greenlee 2013; Greenlee 2015; Kim 2011; Morey 2009; Pierce 2007; Scott 2013; Zick 2017).
Selective reporting
For evaluating reporting bias, we assessed selective reporting.
We found eight studies at low risk of bias as a protocol was available (Befort 2016; Demark‐Wahnefried 2006; Demark‐Wahnefried 2007; Demark‐Wahnefried 2014; Greenlee 2015; Pierce 2007; Scott 2013; Swisher 2015), and all primary and secondary outcomes of interest to the review were reported in the pre‐specified way. We judged most studies to have unclear reporting bias. For 14 studies, we found insufficient information to permit judgement as no protocol was available (Bloom 2008; Bourke 2011; Djuric 2002; Ghavami 2017; Greenlee 2013; Harrigan 2016; Kim 2011; Mefferd 2007; Morey 2009; Park 2016; Reeves 2017; Sheppard 2016; Yun 2017; Zick 2017). We found that two studies reported on primary outcome measures but did not report on all secondary outcome measures or at the time points specified in the protocol (Gruenigen 2012; Hawkes 2013), and one study did not report on all primary outcomes (Kanera 2017).
Other potential sources of bias
We judged two studies to have low risk of other bias (Demark‐Wahnefried 2007; Harrigan 2016), as reports provided sufficient detail for the review authors to have no other concerns regarding sources of bias. In the remaining 23 studies, information was insufficient to permit a judgement, so the risk of bias is unclear. Some studies used self‐reporting and thus may have been open to recall bias (Bourke 2011; Demark‐Wahnefried 2006; Hawkes 2013; Reeves 2017).
Effects of interventions
See: Table 1
Comparisons were dietary intervention versus control or usual care. Two studies reported more than one comparison (Djuric 2002; Harrigan 2016). See Table 1 for details about these studies regarding dietary interventions, follow‐up, and outcomes as well as certainty of evidence.
Primary outcomes
Overall survival
One study reported on mortality in participants with breast cancer (Pierce 2007). GRADE ratings for overall survival and for secondary malignancies or other cancers are shown in Table 1.
Rates of death in groups receiving dietary intervention compared to groups given control were similar (hazard ratio (HR) 0.98, 95% confidence interval (CI) 0.77 to 1.23; n = 3107; low‐certainty evidence; Analysis 1.1; heterogeneity was not applicable as only one study reported the number of deaths after 7.3 years' follow‐up among participants after breast cancer (Pierce 2007)). We downgraded the quality of evidence by one level for inability to assess consistency as there was only one study and one level, as the CIs were not narrow.
1.1. Analysis.
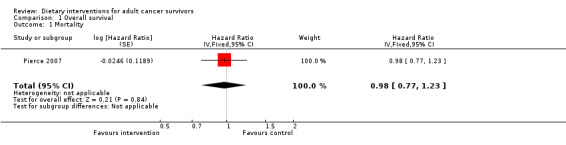
Comparison 1 Overall survival, Outcome 1 Mortality.
Incidence of secondary malignancy or other cancer
Dietary intervention compared to control may make little or no difference in secondary malignancies or other cancers (risk ratio (RR) 0.99, 95% CI 0.84 to 1.15; 3107 participants; low‐certainty evidence; Analysis 1.2; heterogeneity was not applicable as there was only one study) occurring within 7.3 years' follow‐up (Pierce 2007). We downgraded the quality of evidence by one level for inability to assess consistency as there was only one study and one level, as the CIs were not narrow.
1.2. Analysis.
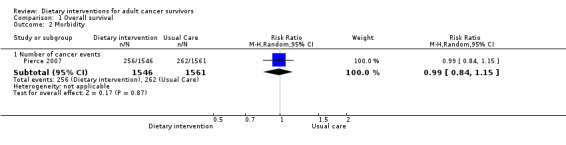
Comparison 1 Overall survival, Outcome 2 Morbidity.
Incidence of morbidities
This outcome was not reported in any of the included studies.
Secondary outcomes
Dietary changes
GRADE ratings for dietary changes are shown in Table 1.
Energy intake
A total of 11 studies reported total energy intake from dietary assessment (Bourke 2011; Demark‐Wahnefried 2014, Djuric 2002; Greenlee 2013; Greenlee 2015; Gruenigen 2012; Pierce 2007; Reeves 2017; Scott 2013; Sheppard 2016; Zick 2017).
We are uncertain if dietary intervention compared to control has any effect on energy intake at three months measured in kilocalories (mean difference (MD) ‐52.61 kcal, 95% CI ‐209.23 to 104.02; 115 participants; heterogeneity I² = 0%; very low‐certainty evidence) from three studies (Bourke 2011; Gruenigen 2012; Zick 2017). We downgraded the quality of evidence by one level due to risk of bias, one level for imprecision, and one level due to wide variation in effect estimates across studies.
Dietary intervention compared to control probably makes little or no difference in energy intake at six months measured in kilocalories (MD ‐47.67 kcal, 95% CI ‐142.33 to 46.99; 3236 participants; heterogeneity I² = 25%; moderate‐certainty evidence) from four studies (Demark‐Wahnefried 2014; Greenlee 2013; Gruenigen 2012; Pierce 2007). We downgraded the quality of evidence by one level due to wide variation in effect estimates across studies.
Dietary intervention compared to control probably makes little or no difference in energy intake at 12 months measured in kilocalories (MD ‐59.13 kcal, 95% CI ‐156.05 to 37.79; 3283 participants; heterogeneity I² = 42%; moderate‐certainty evidence) reported in five studies (Demark‐Wahnefried 2014; Greenlee 2013; Greenlee 2015; Gruenigen 2012; Pierce 2007). The data for energy intake are displayed in Analysis 2.1. We downgraded the quality of evidence by one level due to wide variation in effect estimates across studies.
2.1. Analysis.
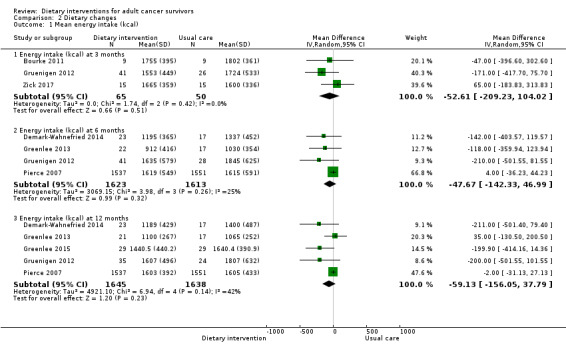
Comparison 2 Dietary changes, Outcome 1 Mean energy intake (kcal).
Subgroup analyses of studies on body mass index (BMI) greater than 25 kg/m² at three months ‐ Gruenigen 2012 and Zick 2017 ‐ and at six months ‐ Gruenigen 2012 and Greenlee 2013 ‐ showed very low‐certainty of evidence, and we are uncertain if dietary intervention has any effect on energy intake in this subgroup (Table 3). Greenlee 2015 presented energy intake as adjusted means at three months and at six months, and we are uncertain if dietary intervention has any effect on energy intake with very low‐certainty evidence (Table 3).
2. Dietary changes: adjusted and subgroup analyses of secondary outcomes.
| Outcome | Units or groups used for analysis | Time point (months) | Mean difference | 95% confidence interval | Participants (N) | Heterogeneity (%) | Certainty of evidence | Figure number |
| Energy intake EI (kcal) | Adjusted means | 3 | ‐637 | ‐ 819.79 to ‐454.21 | 67 | NA | Very low | Analysis 2.2 |
| Adjusted means | 6 | ‐548.1 | ‐ 753.22 to ‐342.98 | 61 | NA | Very low | Analysis 2.2 | |
| BMI > 25 kg/m² | 3 | ‐51.81 | ‐283.08 to 179.45 | 97 | 41 | Very low | Analysis 2.3 | |
| BMI > 25 kg/m² | 6 | ‐49 | ‐ 269.75 to 171.74 | 93 | 43 | Very low | Analysis 2.4 | |
| Fruit servings (day) | Uterine cancer | 6 | 0.2 | ‐ 0.57 to 0.97 | 69 | NA | Very low | Analysis 2.6 |
| Uterine cancer | 12 | 0.3 | ‐ 0.80 to 1.40 | 59 | NA | Very low | Analysis 2.7 | |
| Breast cancer | 6 | 0.8 | 0.58 to 1.02 | 3088 | NA | Moderate | Analysis 2.6 | |
| Breast cancer | 12 | 0.46 | ‐ 0.40 to 1.31 | 3146 | 74 | Moderate | Analysis 2.7 | |
| Hispanic population | 12 | ‐0.1 | ‐ 0.98 to 0.78 | 58 | NA | Very low | Analysis 2.8 | |
| Mixed population | 12 | 0.8 | 0.64 to 0.96 | 3088 | NA | Moderate | Analysis 2.3 | |
| Fruit and vegetable servings (day) | Adjusted means | 3 | 0.20 | ‐0.91 to 1.31 | 67 | NA | Very low | Analysis 2.13 |
| Adjusted means | 6 | 1.10 | ‐0.01 to 2.21 | 61 | NA | Very low | Analysis 2.13 | |
| Fibre intake (g) | Weight reduction advice | 6 | ‐0.20 | ‐3.52 to 3.12 | 39 | NA | Very low | Analysis 2.15 |
| Weight reduction advice | 12 | 2.10 | 0.24 to 3.96 | 39 | NA | Very low | Analysis 2.15 | |
| Healthy eating advice | 6 | 9.50 | 8.52 to 10.48 | 3088 | NA | Moderate | Analysis 2.16 | |
| Healthy eating advice | 12 | 8.00 | 7.30 to 8.70 | 3088 | NA | Moderate | Analysis 2.16 |
BMI: body mass index.
NA: not applicable.
We excluded one study from the meta‐analysis as it was considered to have unclear or high risk for all risk of bias domains (Djuric 2002). This study reported energy intake after 12 months and included three groups: the weight watchers group (2106 kcal, standard deviation (SD) 673, 13 participants), the individualised group (1833 kcal, SD 358, 9 participants), and the comprehensive intervention group (1899 kcal, SD 424, 8 participants) compared to the control group (2246 kcal, SD 660, 10 participants).
One study collected data from the intervention group and not from the control group, showing a reduction in energy intake when baseline was compared with measurements at 12 weeks of ‐207.3 kcal (SD 31.5) (Sheppard 2016). Two studies reported no difference in energy intake but did not provide any data (Reeves 2017; Scott 2013).
Fruit and vegetable intake
Fruit servings per day
Fifteen studies reported on fruit and vegetable intake (Bloom 2008; Demark‐Wahnefried 2006; Demark‐Wahnefried 2007; Greenlee 2013; Greenlee 2015; Gruenigen 2012; Harrigan 2016; Hawkes 2013; Kanera 2017; Morey 2009; Park 2016; Pierce 2007; Reeves 2017; Yun 2017; Zick 2017).
Data suitable for meta‐analysis on fruit servings per day were provided in three studies (Greenlee 2015; Gruenigen 2012; Pierce 2007).
It is uncertain if dietary intervention compared to control has any effect on fruit servings (MD 0.10 servings, 95% CI ‐0.82 to 1.02; 67 participants; heterogeneity not applicable; very low‐certainty evidence) in one study at three months (Gruenigen 2012). We downgraded the quality of evidence by one level due to risk of bias, by one level for imprecision, and by one level for inability to assess consistency as there was only one study.
Dietary intervention compared to control may slightly improve fruit servings (MD 0.62 servings, 95% CI 0.08 to 1.16; 3157 participants; I² = 54%; low‐certainty evidence) in two studies reporting fruit portions at six months (Gruenigen 2012; Pierce 2007). We downgraded the quality of evidence by one level due to risk of bias and by one level due to inconsistency between studies as there was only one study.
Dietary intervention compared to control may have little or no effect in improving fruit servings (MD 0.47 servings, 95% CI ‐0.13 to 1.07; 3205 participants; I² = 56%; low‐certainty evidence) in three studies at 12 months (Analysis 2.5; Figure 4; Greenlee 2015; Gruenigen 2012; Pierce 2007). We downgraded the quality of evidence by one level due to risk of bias and by one level due to inconsistency between studies.
2.5. Analysis.

Comparison 2 Dietary changes, Outcome 5 Mean fruit servings (per day).
4.
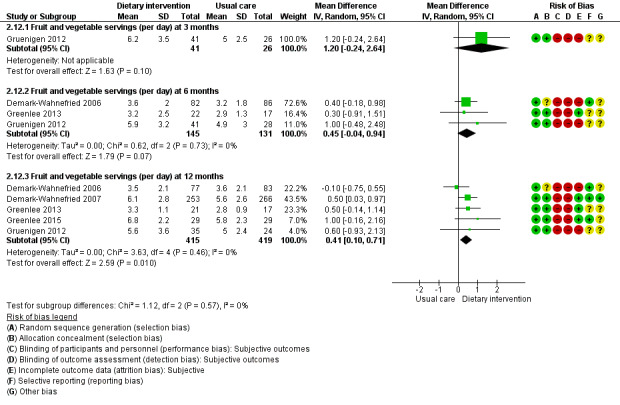
Forest plot of comparison: 2 Dietary changes, outcome: 2.12 Mean fruit and vegetable servings (per day).
Subgroup analysis on different cancer sites and on population differences was undertaken to explore the high heterogeneity observed in fruit servings.
Three studies were considered for cancer site differences.
It is uncertain if dietary intervention has any effect on fruit intake in uterine cancer survivors at six months and at 12 months with very low‐certainty evidence (Gruenigen 2012).
In breast cancer survivors, dietary intervention compared to control probably slightly improves fruit servings with moderate‐certainty evidence at six months, and evidence at 12 months shows that dietary intervention probably has little or no effect on fruit intake (Table 3; Greenlee 2015; Pierce 2007), with moderate‐certainty evidence.
High heterogeneity at 12 months was explored on the basis of population differences.
In a Hispanic population, we are uncertain whether dietary intervention has any effect on fruit servings with very low‐certainty evidence.
In a mixed population (85% white), dietary intervention compared to control probably slightly improves fruit servings with moderate‐certainty evidence (Table 3).
Vegetable servings per day
We are uncertain if dietary intervention compared to control has any effect on adjusted mean vegetable servings in Hispanic women with breast cancer at three months (MD 0.60, 95% CI ‐0.23 to 1.43; 67 participants; very low‐certainty evidence), at six months (MD 0.80 servings, 95% CI ‐0.03 to 1.63; 61 participants; very low‐certainty evidence), and at 12 months (MD 1.10 servings, 95% CI 0.35 to 1.85; 58 participants; very low‐certainty evidence; Analysis 2.9) in one study (Greenlee 2015). We downgraded the quality of evidence by one level due to risk of bias, by one level for imprecision, and by one level for inability to assess consistency as there was only one study.
We are uncertain if dietary intervention compared to control has any effect on vegetable servings in women with uterine cancer at three months (MD 1.20 servings, 95% CI 0.00 to 2.40; 67 participants; very low‐certainty evidence) and at six months and 12 months (six‐month MD 0.80 servings, 95% CI ‐0.37 to 1.97; 69 participants; very low‐certainty evidence; 12‐month MD 0.30 servings, 95% CI ‐0.85 to 1.45; 59 participants; heterogeneity not applicable; very low‐certainty evidence; Analysis 2.10) in one study (Gruenigen 2012). We downgraded the quality of evidence by one level due to risk of bias, by one level for imprecision, and by one level due to inability to assess consistency across studies as there was only one study.
We are uncertain if dietary intervention compared to control increases vegetable servings in women with breast cancer at three months (MD 3.70 servings, 95% CI 2.64 to 4.76; 30 participants; very low‐certainty evidence; Analysis 2.11) in one study (Zick 2017). We downgraded the quality of evidence by one level due to risk of bias, by one level due to imprecision, and by one level due to inability to assess consistency across studies as there was only one study.
Dietary intervention compared to control probably increases vegetable servings in women with breast cancer at six months (MD 4.50 servings, 95% CI 4.49 to 4.51; 3088 participants; moderate‐certainty evidence) and at 12 months (MD 3.90 servings, 95% CI 3.89 to 3.91; 3088 participants; moderate‐certainty evidence; Analysis 2.11) in one study (Pierce 2007). We downgraded the quality of evidence by one level due to inability to assess consistency across studies as there was only one study.
2.9. Analysis.
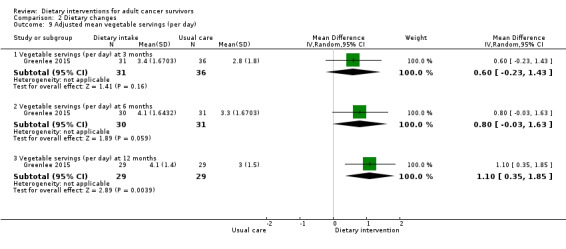
Comparison 2 Dietary changes, Outcome 9 Adjusted mean vegetable servings (per day).
2.10. Analysis.
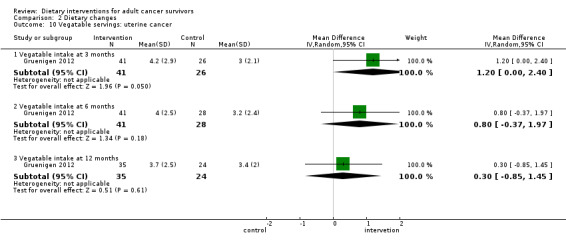
Comparison 2 Dietary changes, Outcome 10 Vegatable servings: uterine cancer.
2.11. Analysis.
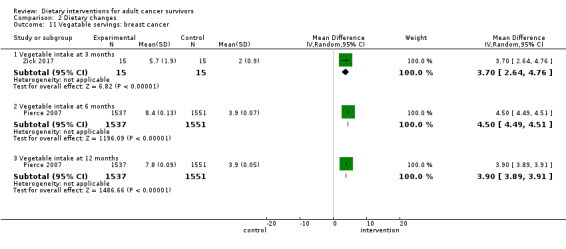
Comparison 2 Dietary changes, Outcome 11 Vegatable servings: breast cancer.
Fruit and vegetable servings per day
We are uncertain if dietary intervention compared to control has any effect on fruit and vegetable servings in women with uterine cancer at three months (MD 1.20 servings, 95% CI ‐0.24 to 2.64; 67 participants; very low‐certainty evidence; Analysis 2.12) in one study (Gruenigen 2012). We downgraded the quality of evidence by one level due to risk of bias and by one level due to inability to assess consistency across studies as there was only one study, and by one level for imprecision.
Dietary intervention compared to control may have little if any effect on fruit and vegetable servings in women with uterine cancer or breast cancer, and in a mixture of participants with breast or prostate cancer at six months (MD 0.45 servings, 95% CI ‐0.04 to 0.94; 276 participants; I² = 0%; low‐certainty evidence; Analysis 2.12) in three studies (Demark‐Wahnefried 2006; Greenlee 2013; Gruenigen 2012). We downgraded the quality of evidence by one level due to risk of bias and by one level due to indirectness.
Dietary intervention compared to control probably slightly increases fruit and vegetable servings in women with uterine cancer or breast cancer and in a mixture of participants at 12 months (MD 0.41 servings, 95% CI 0.10 to 0.71; 834 participants; I² = 0%; moderate‐certainty evidence; Analysis 2.12;Figure 4) in five studies (Demark‐Wahnefried 2006; Demark‐Wahnefried 2007; Greenlee 2013; Greenlee 2015; Gruenigen 2012). We downgraded the quality of evidence by one level due to indirectness.
2.12. Analysis.
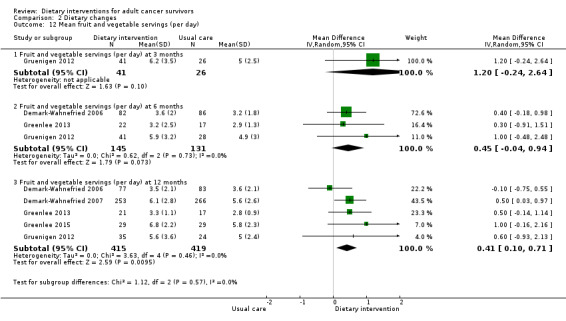
Comparison 2 Dietary changes, Outcome 12 Mean fruit and vegetable servings (per day).
Adjusted mean fruit and vegetable intake in women with breast cancer was reported in one study at three months and at six months, and we are uncertain if there is any effect of dietary intervention with very low‐certainty evidence (Table 3; Greenlee 2015).
One study reported adjusted between‐group differences in mean change for fruit servings (410 participants) at six months as 0.2 (95% CI ‐0.0 to 0.4), and at 12 months as 0.0 (95% CI ‐0.2 to 0.3) (Hawkes 2013); for vegetable servings (410 participants), the adjusted between‐group difference in mean change at six months was 0.4 (95% CI 0.2 to 0.7), and at 12 months was 0.2 (95% CI ‐0.1 to 0.5). Morey 2009 reported daily servings of fruits and vegetables in the manuscript as MD 1.11 (95% CI 0.76 to 1.47).
Seven studies did not provide data suitable for inclusion in the meta‐analysis, and one study did not report any data (Reeves 2017). One study did not report the number of servings but reported the percentage of participants who increased consumption of fruits and vegetables to more than five portions a day; 32% of participants ate more than five servings of fruits and vegetables in the control group compared to 31% (total 386) in the intervention group (Bloom 2008). One study reported fruit and vegetable portions in three arms of a trial as change from baseline at six months (in‐person intervention mean 1.2, SD 3.1, 33 vs telephone calls mean 1.1, SD 2.9, 34 vs control mean ‐0.3, SD 1.9, 33). For both intervention arms, results showed a difference when compared to the control arm (P = 0.017) (Harrigan 2016). One study reported fruit and vegetable intake at baseline, but subsequent data were presented in graphs at four months and at seven months (Park 2016). One study reported fruits and vegetables, stating there were differences between groups (P = 0.819 at three months and P = 0.413 at 12 months), and reported no other data (Yun 2017). One study reported vegetable consumption in grams per day at six months (intervention mean 146.6, SD 56.0, 184 participants vs control mean 124.9, SD 60.8, 219 participants) and at 12 months (intervention mean 95.3, SD 44.7, 166 participants vs control mean 81.4, SD 44.1, 210 participants) (Kanera 2017).
Fibre intake
We are uncertain if dietary intervention compared to control has any effect on fibre intake in participants with colon cancer at three months (MD 6.00 g, 95% CI 0.73 to 11.27; 18 participants; very low‐certainty evidence; Analysis 2.14) in one study (Bourke 2011). We downgraded the quality of evidence by one level due to risk of bias and by one level for inability to assess consistency as there was only one study, and by one level for imprecision. This comparison was also conducted at six months (MD 4.79 g, 95% CI ‐4.72 to 14.29; 3127 participants; I² = 97%; very low‐certainty evidence) and at 12 months (MD 5.12 g, 95% CI ‐0.66 to 10.90; 3127 participants; I² = 97%; very low‐certainty evidence; Analysis 2.14) in women with breast cancer in two studies (Greenlee 2013; Pierce 2007). We downgraded the quality of evidence by two levels due to inconsistency across studies and by one level as the CIs were not narrow.
2.14. Analysis.

Comparison 2 Dietary changes, Outcome 14 Fibre.
Because of the high level of inconsistency identified, we undertook subgroup analysis, as two studies had different aims for dietary intervention: one study gave weight‐reducing advice and enrolled participants with BMI ≥ 25 kg/m² (Greenlee 2013), and the other study aimed to encourage healthy eating for the intervention (Pierce 2007). We are uncertain from one study at six months and at 12 months whether dietary intervention for weight reduction has any effect on fibre intake with very low‐certainty evidence (Greenlee 2013). In another study of women with breast cancer, participants were encouraged to eat a healthy diet; at six months and at 12 months, dietary intervention compared to control probably increased fibre intake with moderate‐certainty evidence (Table 3; Pierce 2007).
Two studies looked at change scores (Harrigan 2016; Hawkes 2013). One of these studies reported fibre intake as grams per 1000 kcal (in‐person mean 5.6, SD 4.1, 33 participants, telephone mean 3.9, SD 5.3, 34 participants, control mean 1.3, SD 4.2, 33 participants) and performed between‐group comparisons with both intervention arms versus control (P < 0.017) (Harrigan 2016). In another study, change in fibre was expressed as grams per day adjusted for baseline values and reported at six months (intervention 0.3 g, standard error (SE) 0.6 vs control 1.0 g, SE 0.5; 410 participants) and at 12 months (intervention ‐0.2 g, SE 0.6 vs control 0.7 g, SE 0.6; 410 participants) (Hawkes 2013). One study reported differences between intervention and baseline at three months in grams per day (intervention 19.2, SD 12.2 vs control 13.4, SD 5.4; 22 participants); data were not available for the control arm at three months (Sheppard 2016).
Diet Quality Index
Four studies used the Diet Quality Index score (Demark‐Wahnefried 2006; Demark‐Wahnefried 2007; Demark‐Wahnefried 2014; Kim 2011).
We are uncertain if dietary intervention improves Diet Quality Index at three months (MD 0.90, 95% CI 0.29 to 1.52; 45 participants; very low‐certainty evidence; Analysis 2.18) in one study (Kim 2011). We downgraded the quality of evidence by one level due to imprecision, and by two levels due to risk of selection bias and lack of blinding.
Dietary intervention compared to control may improve Diet Quality Index at six months (MD 5.20, 95% CI 1.04 to 9.36; 182 participants; low‐certainty evidence; Analysis 2.17; Figure 5) in one study (Demark‐Wahnefried 2006). We downgraded the quality of evidence by one level due to no allocation concealment described and by one level due to inability to assess consistency as there was only one study.
2.18. Analysis.
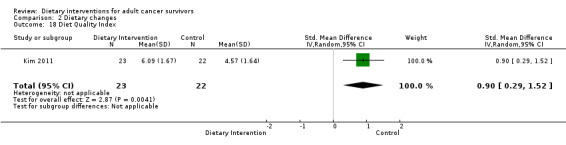
Comparison 2 Dietary changes, Outcome 18 Diet Quality Index.
2.17. Analysis.
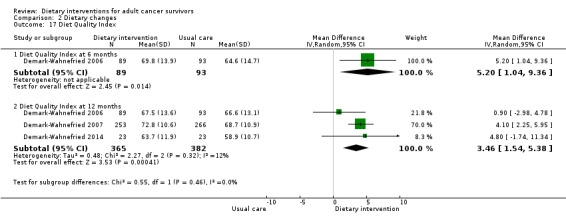
Comparison 2 Dietary changes, Outcome 17 Diet Quality Index.
5.
Forest plot of comparison: 2 Dietary changes, outcome: 2.18 Diet Quality Index .
Dietary intervention compared to control probably may improve Diet Quality Index at 12 months (MD 3.46, 95% CI 1.54 to 5.38; 747 participants; I² = 12%; moderate‐certainty evidence; Analysis 2.17; Figure 5) in three studies (Demark‐Wahnefried 2006; Demark‐Wahnefried 2007; Demark‐Wahnefried 2014). We downgraded the quality of evidence by one level due to risk of bias.
Changes in anthropometric measurements
Fifteen studies measured one or more outcomes for changes in anthropometric (physical) measures (Bourke 2011; Demark‐Wahnefried 2006; Demark‐Wahnefried 2007; Demark‐Wahnefried 2014; Djuric 2002; Greenlee 2013; Gruenigen 2012; Harrigan 2016; Hawkes 2013; Mefferd 2007; Morey 2009; Pierce 2007; Scott 2013; Sheppard 2016; Swisher 2015). GRADE ratings for changes in anthropometry are shown in Table 1.
Body weight
Dietary intervention compared to control may have little if any effect on body weight at three months (MD ‐3.52 kg, 95% CI ‐7.34 to 0.29; 247 participants; I² = 0%; low‐certainty evidence; Analysis 3.1) in six studies (Bourke 2011; Greenlee 2013; Gruenigen 2012; Mefferd 2007; Sheppard 2016; Swisher 2015). We downgraded the quality of evidence by one level due to risk of bias and by one level for imprecision.
Dietary intervention compared to control probably has little if any effect on body weight at six months (MD ‐2.84 kg, 95% CI ‐6.95 to 1.28; 190 participants; I² = 0%; moderate‐certainty evidence; Analysis 3.1) in three studies (Greenlee 2013; Gruenigen 2012; Scott 2013). We downgraded the quality of evidence by one level for imprecision.
Dietary intervention compared to control has little if any effect on body weight at 12 months (MD ‐0.80 kg, 95% CI ‐2.01 to 0.41; 3287 participants; I² = 0%; high‐certainty evidence; Analysis 3.1) in five studies that reported body weight (Demark‐Wahnefried 2014; Greenlee 2013; Greenlee 2015; Gruenigen 2012; Pierce 2007).
3.1. Analysis.

Comparison 3 Changes in anthropometry, Outcome 1 Mean weight (kg).
Adjusted weight was reported in Hispanic women with breast cancer at six months in one study (Greenlee 2015), and we are uncertain if dietary intervention compared to control has any effect on weight with very low‐certainty evidence (Table 4).
3. Anthropometric changes: adjusted and subgroup analyses of secondary outcomes.
| Outcome | Units or groups used for analysis | Time point (months) | Mean difference | 95% confidence interval | Participants (N) | Heterogeneity (%) | Certainty of evidence | Figure number |
| Body weight (kg) | Adjusted mean | 6 | ‐7 | ‐17.40 to 3.40 | 55 | NA | Very low | Analysis 3.2 |
| Weight change (kg) | Breast cancer | 6 | ‐3.6 | ‐5.56 to 1.64 | 90 | NA | Low | Analysis 3.3 |
| Breast, prostate, and colorectal cancer | 12 | 0.23 | ‐0.31 to 0.77 | 641 | NA | Low | Analysis 3.3 | |
| Weight loss (kg) | Breast cancer | 3 | 1 | ‐0.47 to 2.47 | 170 | NA | Moderate | Analysis 3.7 |
| Breast cancer | 6 | ‐1.6 | ‐3.06 to ‐0.14 | 170 | NA | Moderate | Analysis 3.7 | |
| BMI (kg/m²) | Mean difference | 6 | ‐0.6 | ‐1.15 to ‐0.05 | 410 | NA | Low | Analysis 3.6 |
| Mean difference | 12 | ‐0.44 | ‐0.64 to ‐0.25 | 1051 | 66 | Moderate | Analysis 3.6 | |
| Adjusted mean | 6 | ‐4.1 | ‐8.12 to ‐0.08 | 53 | NA | Very low | Analysis 3.4 | |
| Waist‐to‐hip ratio | Colon cancer | 3 | ‐0.11 | ‐0.17 to ‐0.05 | 18 | NA | Very low | Analysis 3.12 |
| Breast cancer | 3 | ‐0.01 | ‐0.02 to 0.01 | 163 | 0 | Low | Analysis 3.12 | |
| Waist circumference change (cm) | Breast cancer | 3 | ‐0.67 | ‐1.28 to ‐0.06 | 44 | NA | Very low | Analysis 3.14 |
| Breast cancer | 6 | ‐0.67 | ‐1.02 to ‐0.32 | 134 | 10 | Low | Analysis 3.14 | |
| Breast cancer | 12 | ‐0.15 | ‐0.74 to 0.44 | 44 | NA | Very low | Analysis 3.14 | |
| Adjusted mean | 6 | ‐3.9 | ‐11.1 to 3.31 | 50 | NA | Very low | Analysis 3.15 | |
| Hip circumference change (cm) | Breast cancer | 3 | 1.81 | 0.77 to 2.85 | 44 | NA | Very low | Analysis 3.13 |
| Breast cancer | 6 | ‐3.13 | ‐5.01 to ‐1.26 | 134 | 31 | Very low | Analysis 3.13 | |
| Breast cancer | 12 | 1.09 | ‐1.69 to 3.87 | 44 | NA | Very low | Analysis 4.7 |
NA: not applicable.
One study reported weight change at six months (Reeves 2017), showing that dietary intervention may slightly decrease body weight with low‐certainty evidence, and another study showed that dietary intervention may have little if any effect on body weight change at 12 months with low‐certainty evidence (Table 4; Morey 2009).
One study reported weight change in three arms after six months (in‐person ‐5.6, 95% CI ‐7.1 to ‐4.1 vs telephone ‐4.8, 95% CI ‐6.5 to ‐3.1 vs control ‐1.7, 95% CI ‐3.2 to ‐0.3) (Harrigan 2016). Djuric 2002 measured weight in kilograms, and figures in four arms without actual weights for each group and numbers for each arm at each time point were not documented clearly, so they were not included in the analysis.
One study reported weight loss at three months and at six months (Befort 2016). At three months, data showed there is probably little if any effect of dietary intervention on weight loss, but at six months there is probably a slight decrease in weight in the intervention group compared to the control group with moderate‐certainty evidence (Table 4).
Body mass index
Data expressed as means and standard deviations
Dietary intervention compared to control may slightly decrease BMI at three months (MD ‐1.80 kg/m², 95% CI ‐2.95 to ‐0.65; 247 participants; I² = 0%; low‐certainty evidence, Analysis 3.5; Figure 6) in six studies (Bourke 2011; Mefferd 2007; Scott 2013; Sheppard 2016; Swisher 2015; Zick 2017). We downgraded the quality of evidence by one level due to CI not being very narrow and due to risk of bias.
3.5. Analysis.
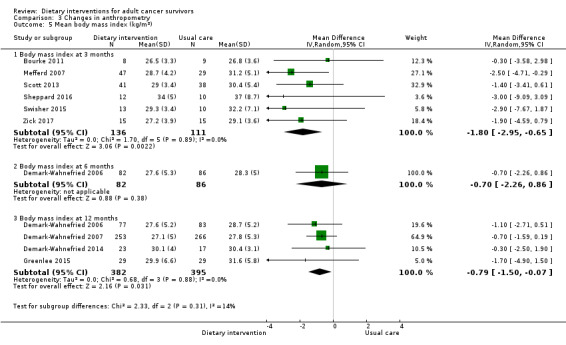
Comparison 3 Changes in anthropometry, Outcome 5 Mean body mass index (kg/m²).
6.
Forest plot of comparison: 3 Changes in anthropometry, outcome: 3.5 Mean body mass index (kg/m²).
We are uncertain if dietary intervention compared to control had any effect on BMI at six months (MD ‐0.70 kg/m², 95% CI ‐2.26 to 0.86; 168 participants; heterogeneity not applicable; very low‐certainty evidence; Analysis 3.5; Figure 6) in one study (Demark‐Wahnefried 2006). We downgraded the quality of evidence by two levels due to risk of bias and by one level due to inability to assess consistency as there was only one study.
Dietary intervention compared to control probably slightly decreased BMI at 12 months (MD ‐0.79 kg/m², 95% CI ‐1.50 to ‐0.07; 777 participants; I² = 0%; moderate‐certainty evidence; Analysis 3.5; Figure 6) in four studies (Demark‐Wahnefried 2006; Demark‐Wahnefried 2007; Demark‐Wahnefried 2014; Greenlee 2015). We downgraded the quality of evidence by one level due to risk of bias.
One study reported BMI as a mean difference at six months; data showed low‐certainty evidence and there may be a slight decrease in BMI in the intervention group compared to the control group (Hawkes 2013).
Two studies reported mean differences at 12 months, showing that dietary intervention probably slightly decreases BMI with moderate‐certainty evidence (Table 4; Hawkes 2013; Morey 2009). One study reported on adjusted mean BMI at six months, and we are uncertain if there is any effect of intervention on BMI with very low‐certainty evidence (Table 4; Greenlee 2015).
Body composition
Lean body tissue
We are uncertain whether dietary intervention has any effect on lean body mass at three months (MD ‐0.30, 95% CI ‐2.41 to 1.81; 76 participants; very low‐certainty evidence; Analysis 3.8) in one study (Mefferd 2007). We downgraded the quality of evidence by two levels due to risk of bias for sequence generation and blinding, by one level due to imprecision, and by one level due to inability to assess consistency as there was only one study.
3.8. Analysis.
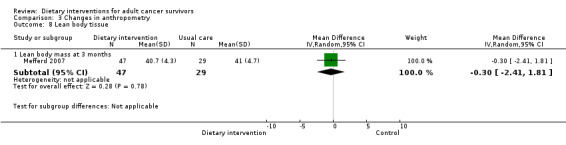
Comparison 3 Changes in anthropometry, Outcome 8 Lean body tissue.
Body fat
Dietary intervention compared to control may decrease percentage of body fat at three months (MD ‐4.97%, 95% CI ‐7.47 to ‐2.48; 99 participants; I² = 0%; low‐certainty evidence; Analysis 3.9) in two studies (Mefferd 2007; Swisher 2015). We downgraded the quality of evidence by one level due to risk of bias assessment and by one level due to imprecision.
We are uncertain if dietary intervention has any effect on percentage of body fat at six months (MD ‐0.01%, 95% CI ‐0.05 to 0.03; 39 participants; very low‐certainty evidence; Analysis 3.10) in one study (Greenlee 2013). We downgraded the quality of evidence by one level due to risk of bias assessment and by two levels due to small sample size and imprecision.
3.9. Analysis.
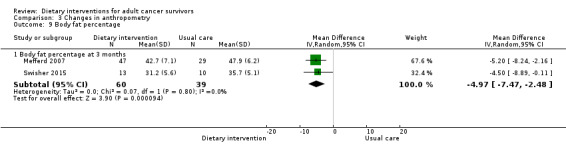
Comparison 3 Changes in anthropometry, Outcome 9 Body fat percentage.
3.10. Analysis.
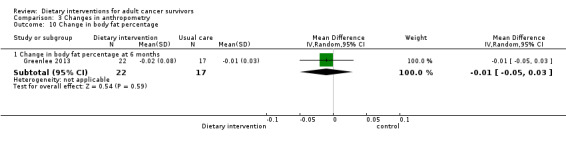
Comparison 3 Changes in anthropometry, Outcome 10 Change in body fat percentage.
One study reported percentage of body fat in 100 participants expressed as least squares means from a linear model, and the in‐person dietary intervention for six‐month change was ‐3.3 (95% CI ‐4.4 to ‐2.1; 33 participants) versus telephone intervention ‐2.4 (95% CI ‐3.7 to ‐1.2; 34 participants) versus control ‐1.7 (95% CI ‐2.8 to ‐0.5; 33 participants) (Harrigan 2016). One study reported on body fat, stating there was no difference between groups; data were not given (Scott 2013).
Waist‐to‐hip ratio
Dietary intervention compared to control may have little if any effect on waist‐to‐hip ratio at three months (MD ‐0.03, 95% CI ‐0.06 to 0.01; 181 participants; I² = 63%; low‐certainty evidence; Analysis 3.11) in five studies (Bourke 2011; Greenlee 2013; Mefferd 2007; Sheppard 2016; Swisher 2015). We downgraded the quality of evidence by one level due to risk of bias assessment and by one level due to inconsistency.
Dietary intervention compared to control may have little if any effect on waist‐to‐hip ratio at six months (MD ‐0.02, 95% CI ‐0.05 to 0.01; 118 participants; I² = 0%; low‐certainty evidence; Analysis 3.11) in two studies (Greenlee 2013; Scott 2013). We downgraded the quality of evidence by one level for imprecision due to small sample sizes and by one level for indirectness.
Dietary intervention compared to control may have little if any effect on waist‐to‐hip ratio at 12 months (MD ‐0.01, 95% CI ‐0.04 to 0.02; 106 participants; heterogeneity I² = 0%; low‐certainty evidence; Analysis 3.11) in two studies (Greenlee 2013; Greenlee 2015). We downgraded the quality of evidence by one level due to imprecision because of small sample size and by one level for indirectness because studies recruited only Hispanic women.
3.11. Analysis.
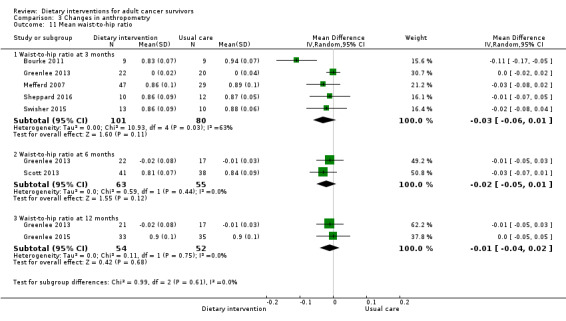
Comparison 3 Changes in anthropometry, Outcome 11 Mean waist‐to‐hip ratio.
We undertook subgroup analysis to explore heterogeneity in the waist‐to‐hip ratio in different cancer sites at three months. In one study on colon cancer survivors, we are uncertain whether dietary intervention had any effect on waist‐to‐hip ratio with very low‐certainty evidence (Bourke 2011). Studies on breast cancer survivors showed that dietary intervention may have little if any effect on waist‐to‐hip ratio with low‐certainty evidence (Table 4; Greenlee 2013; Mefferd 2007; Sheppard 2016; Swisher 2015). We downgraded the quality of evidence by one level due to risk of bias and by one level due to indirectness.
Waist circumference
Four studies reported waist circumference in means with SDs (Demark‐Wahnefried 2014; Greenlee 2015; Gruenigen 2012; Mefferd 2007).
We are uncertain whether dietary intervention decreases waist circumference at three months (MD ‐4.00 cm, 95% CI ‐12.53 to 4.53; 143 participants; I² = 66%; very low‐certainty evidence; Analysis 3.17) in two studies (Gruenigen 2012; Mefferd 2007). We downgraded the quality of evidence by two levels due to risk of bias assessment and by one level due to inconsistency.
Dietary intervention compared to control may have little if any effect on waist circumference at six months (MD ‐0.33 cm, 95% CI ‐4.79 to 4.14; 109 participants; I² = 0%; low‐certainty evidence; Analysis 3.17) in two studies (Demark‐Wahnefried 2014; Gruenigen 2012). We downgraded the quality of evidence by two levels due to wide CI.
Dietary intervention compared to control may have little if any effect on waist circumference at 12 months (MD ‐3.36 cm, 95% CI ‐7.55 to 0.82; 148 participants; I² = 17%; low‐certainty evidence; Analysis 3.17) in three studies (Demark‐Wahnefried 2014; Greenlee 2015; Gruenigen 2012). We downgraded the quality of evidence by two levels due to wide CI.
3.17. Analysis.

Comparison 3 Changes in anthropometry, Outcome 17 Mean waist circumference (cm).
Two studies reported changes in waist circumference (Greenlee 2013; Reeves 2017). One study reported on changes at three months, and we are uncertain if dietary intervention has any effect on waist circumference with very low‐certainty evidence (Greenlee 2013). Two studies reported on changes at six months, showing that dietary intervention may slightly decrease waist circumference with low‐certainty evidence (Greenlee 2013; Reeves 2017). One study reported on changes at 12 months, and we are uncertain if dietary intervention has any effect on waist circumference with very low‐certainty evidence (Table 4; Greenlee 2013).
One study reported on change in waist circumference in three arms after six months (in‐person ‐7.5, 95% CI ‐9.7 to ‐5.3 vs telephone ‐7.2, 95% CI ‐9.6 to ‐4.8 vs control ‐2.6, 95% CI ‐4.7 to ‐0.5) (Harrigan 2016).
One study reported an adjusted change in waist circumference at six months, and we are uncertain if dietary intervention had any effect on waist circumference with very low‐certainty evidence (Table 4; Greenlee 2015).
One study reported an adjusted mean difference in waist circumference (‐3.32 cm, 95% CI ‐1.53 to ‐5.11 cm; P < 0.001) (Scott 2013).
Hip circumference
Five studies reported on hip circumference (Greenlee 2013; Greenlee 2015; Harrigan 2016; Mefferd 2007; Reeves 2017).
Two studies reported data as means and SDs (Greenlee 2015; Mefferd 2007).
We are uncertain if dietary intervention compared to control has any effect on hip circumference (three months MD ‐5.40 cm, 95% CI ‐10.06 to ‐0.74; 76 participants; six months MD ‐3.20 cm, 95% CI ‐10.96 to 4.56; 50 participants; 12 months MD ‐4.00 cm, 95% CI ‐11.43 to 3.43; 49 participants; very low‐certainty evidence; Analysis 3.16). We downgraded the quality of evidence at each time point by one level due to risk of bias assessment, by two levels due to wide CI, and by one level due to inability to assess consistency due to one study.
3.16. Analysis.
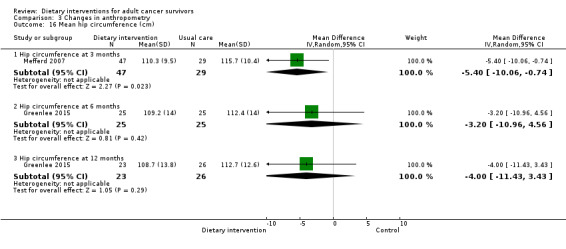
Comparison 3 Changes in anthropometry, Outcome 16 Mean hip circumference (cm).
Two studies reported hip circumference as change scores (Greenlee 2013; Reeves 2017). One study reported on changes at three months, and we are uncertain if dietary intervention has any effect on hip circumference change score with very low‐certainty evidence (Greenlee 2013). Two studies reported on changes at six months, and we are uncertain if there is any effect on hip circumference change score with very low‐certainty evidence (Greenlee 2013; Reeves 2017). One of these studies reported also on 12‐month change, and we are uncertain if dietary intervention has any effect on hip circumference change score (Table 4; Greenlee 2013).
One study reported change in hip circumference in three arms after six months (in‐person ‐6.9 cm, 95% CI ‐8.5 to ‐5.2 vs telephone ‐6.1 cm, 95% CI ‐8.0 to ‐4.3 vs control ‐3.1 cm, 95% CI ‐4.7 to ‐1.5) (Harrigan 2016).
Patient outcomes, including quality of life (QoL)
Eleven studies measured quality of life (Bourke 2011; Demark‐Wahnefried 2006; Demark‐Wahnefried 2007; Demark‐Wahnefried 2014; Hawkes 2013; Kim 2011; Mefferd 2007; Morey 2009; Reeves 2017; Scott 2013; Swisher 2015). GRADE ratings for QoL are shown in Table 5.
4. Dietary intervention compared to usual care for people living beyond cancer: quality of life.
| Dietary intervention compared to usual care for people living beyond cancer: quality of life | ||||||
| Patient or population: people living beyond cancer Setting: community Intervention: dietary intervention Comparison: usual care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with usual care | Risk with dietary intervention | |||||
| FACT‐G QoL Follow‐up: 12 months | Mean FACT‐G QoL was 93.15 | MD 0.52 lower (2.31 lower to 1.26 higher) | ‐ | 685 (2 RCTs) | ⊕⊝⊝⊝ Very lowa,b | |
| FACT‐B QoL Follow‐up: 6 months | Mean FACT‐B QoL was 114.1 | MD 4.9 higher (0.8 lower to 10.6 higher) | ‐ | 90 (1 RCT) | ⊕⊝⊝⊝ Very lowc,d,e | |
| SF‐36 physical domain/mean change Follow‐up: 12 months | Mean SF‐36 physical domain/mean change was 2.97 | MD 1.91 higher (0.45 higher to 3.37 higher) | ‐ | 1091 (3 RCTs) | ⊕⊕⊝⊝ Lowc | |
| SF‐36 mental domain/mean change Follow‐up: 12 months | Mean SF‐36 mental domain/mean change was 0.45 | MD 0.11 lower (3.29 lower to 3.08 higher) | ‐ | 1091 (3 RCTs) | ⊕⊝⊝⊝ Very lowa,f | |
| Global QoL Follow‐up: 12 months | Mean global QoL was 65.3 | MD 4.8 higher (0.79 lower to 10.39 higher) | ‐ | 174 (1 RCT) | ⊕⊝⊝⊝ Very lowc,d,e | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; FACT‐B: Functional Assessment of Cancer Therapy ‐ Breast; FACT‐G: Functional Assessment of Cancer Therapy ‐ General; MD: mean difference; QoL: quality of life; RCT: randomised controlled trial; SF‐36: Short Form 36. | ||||||
| GRADE Working Group grades of evidence. High‐certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate‐certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low‐certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low‐certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded two levels for risk of bias. bDowngraded one level as CI is not narrow. cDowngraded one level for risk of bias. dDowngraded one level due to lack of precision because of small sample size. eDowngraded one level due to inability to assess for consistency across studies as only one study. fDowngraded one level due to inconsistencies across studies.
FACT‐G QoL
We are uncertain if dietary intervention compared to control has any effect on FACT‐G score at six months (MD ‐2.50, 95% CI ‐5.80 to 0.80; 168 participants; very low‐certainty evidence; Analysis 4.1) in one study (Demark‐Wahnefried 2006). We downgraded the quality of evidence by two levels due to risk of bias assessment and by one level for inability to assess consistency across studies. This comparison was also done at 12 months (MD ‐0.52, 95% CI ‐2.31 to 1.26; 685 participants; I² = 0%; very low‐certainty evidence; Analysis 4.1) in two studies (Demark‐Wahnefried 2006; Demark‐Wahnefried 2007). We downgraded the quality of evidence by two levels due to risk of bias and by one level as CI was not very narrow.
4.1. Analysis.
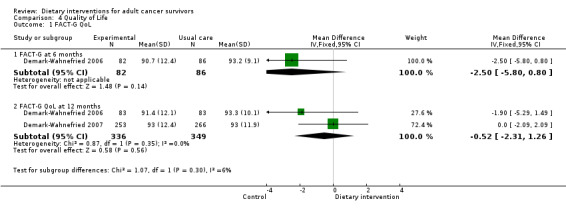
Comparison 4 Quality of Life, Outcome 1 FACT‐G QoL.
FACT‐B QoL
We are uncertain if dietary intervention compared to control has any effect on FACT‐B score at three months (MD 15.60, 95% CI ‐4.96 to 36.16; 23 participants; heterogeneity not applicable; very low‐certainty evidence) in one study (Swisher 2015). We downgraded the quality of evidence by one level due to risk of bias, by one level due to inability to assess consistency across studies as only one study, and by one level due to imprecision. This comparison was also performed at six months (MD 4.90, 95% CI ‐0.80 to 10.60; 90 participants; very low‐certainty evidence) in one study (Scott 2013). We downgraded the quality of evidence by one level due to risk of bias, by one level due to inability to assess consistency across studies as only one study, and by one level due to imprecision (Analysis 4.3).
4.3. Analysis.
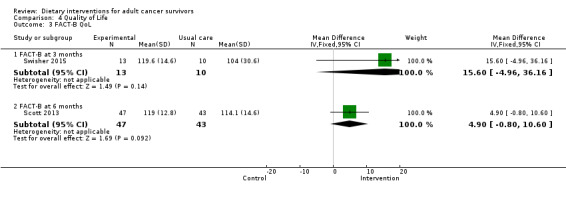
Comparison 4 Quality of Life, Outcome 3 FACT‐B QoL.
FACT‐C QoL
We are uncertain if dietary intervention compared to control affects FACT‐C score at three months (MD 14.00, 95% CI 2.87 to 25.13; 18 participants; very low‐certainty evidence; Analysis 4.2) in one study (Bourke 2011). We downgraded the quality of evidence by one level due to risk of bias, by one level due to inability to assess consistency across studies as there was only one study, and by one level due to imprecision.
4.2. Analysis.
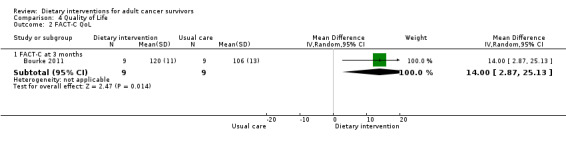
Comparison 4 Quality of Life, Outcome 2 FACT‐C QoL.
SF‐36 QoL mean change physical domain
We are uncertain if dietary intervention compared to control has any effect on SF‐36 physical score at six months (MD ‐0.15, 95% CI ‐1.59 to 1.28; 500 participants; I² = 0%; very low‐certainty evidence; Analysis 4.5) in two studies (Hawkes 2013; Reeves 2017). We downgraded the quality of evidence by one level as CIs were not narrow and by two levels for risk of bias assessment.
Dietary intervention compared to control may slightly improve SF‐36 physical score at 12 months (MD 1.91, 95% CI 0.45 to 3.37; 1091 participants; I² = 0%; low‐certainty evidence; Analysis 4.5) in three studies (Demark‐Wahnefried 2014; Hawkes 2013; Morey 2009). We downgraded the quality of evidence by two levels due to risk of bias assessment.
4.5. Analysis.
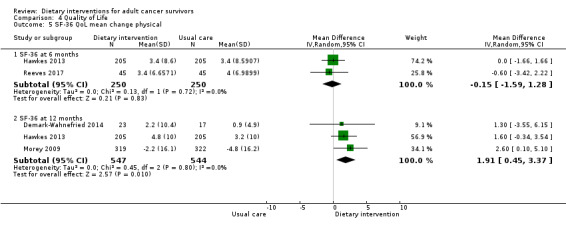
Comparison 4 Quality of Life, Outcome 5 SF‐36 QoL mean change physical.
One study reported on the SF‐36 QoL physical domain with means and SDs, and we are uncertain if dietary intervention has any effect on SF‐36 physical score with very low‐certainty evidence at six months and at 12 months (Table 6; Demark‐Wahnefried 2006).
5. Patient outcomes: adjusted and subgroup analyses of secondary outcomes.
| Outcome | Adjusted analysis/Factor considered | Time point (months) | Mean difference | 95% confidence Interval | Participants (N) | Heterogeneity (%) | Certainty of evidence | Figure number |
| SF‐36 QoL physical domain | Breast and prostate cancer | 6 | 2.2 | ‐4.62 to 9.02 | 168 | NA | Very low | Analysis 4.4 |
| Breast and prostate cancer | 12 | ‐0.20 | ‐7.42 to 7.02 | 160 | NA | Very low | Analysis 4.4 | |
| SF‐36 QoL mental health domain | Breast cancer | 12 | ‐4.3 | ‐9.96 to 1.09 | 40 | NA | Very low | Analysis 4.7 |
| Colorectal cancer | 12 | ‐0.7 | ‐2.64 to 1.24 | 410 | NA | Low | Analysis 4.7 | |
| Breast, prostate, and colorectal cancer | 12 | 7.04 | 5.25 to 8.83 | 641 | NA | Low | Analysis 4.7 |
NA: not applicable.
QoL: quality of life.
SF‐36: Short Form‐36.
SF‐36 QoL mental health domain mean change
Dietary intervention compared to control may have little if any effect on mean change in SF‐36 mental health score at six months (MD 0.70, 95% CI ‐0.96 to 2.36; 410 participants; low‐certainty evidence; Analysis 4.6) in one study (Hawkes 2013). We downgraded the quality of evidence by one level due to risk of bias assessment and by one level due to inability to assess consistency across studies as there was only one study.
We are uncertain if dietary intervention compared to control has any effect on mean change in SF‐36 mental health score at 12 months (MD ‐0.11, 95% CI ‐3.29 to 3.08; 1091 participants; I² = 79%; very low‐certainty evidence; Analysis 4.6) in three studies (Demark‐Wahnefried 2014; Hawkes 2013; Morey 2009). We downgraded the quality of evidence by two levels due to risk of bias assessment and by one level for inconsistency across studies.
4.6. Analysis.
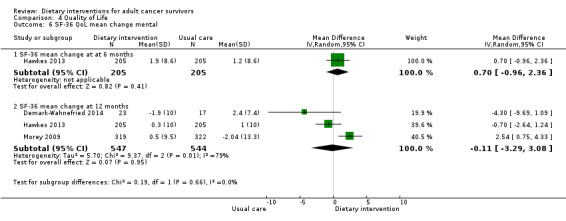
Comparison 4 Quality of Life, Outcome 6 SF‐36 QoL mean change mental.
We undertook subgroup analysis to investigate high heterogeneity at 12 months by investigating data for different cancer sites. In participants with breast cancer, we are uncertain if dietary intervention has any effect on mean change in SF‐36 mental health score with very low‐certainty evidence in one study (Demark‐Wahnefried 2014).
In colorectal cancer patients, dietary intervention may have little or no effect on SF‐36 mental health score with low‐certainty evidence in one study (Hawkes 2013).
In a mixed group of breast, prostate, and colorectal cancer survivors, dietary intervention may slightly improve mean change in SF‐36 mental health score with low‐certainty evidence in one study (Table 5; Morey 2009).
Cancer‐related fatigue
We are uncertain if dietary intervention compared to control has any effect on cancer‐related fatigue at six months (MD 1.40, 95% CI ‐0.26 to 3.06; 410 participants; very low‐certainty evidence) and at 12 months (MD 1.00, 95% CI ‐0.81 to 2.81; 410 participants; heterogeneity not applicable; very low‐certainty evidence; Analysis 4.8) in one study (Hawkes 2013). We downgraded the quality of evidence by two levels due to risk of bias assessment and by one level due to inability to assess consistency across studies as only one study is included.
4.8. Analysis.
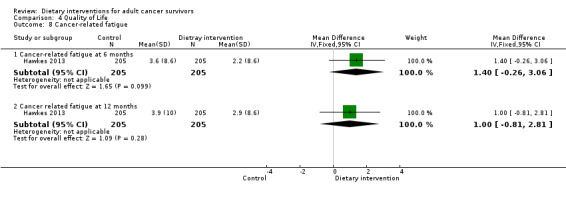
Comparison 4 Quality of Life, Outcome 8 Cancer‐related fatigue.
Global QoL
We are uncertain if dietary intervention compared to control has any effect on Global QoL at three months (MD 4.71, 95% CI 2.22 to 7.21; 220 participants; I² = 15%; very low‐certainty evidence; Analysis 4.9) in two studies (Kim 2011; Yun 2017). We downgraded the quality of evidence by two levels due to risk of bias and by one level due to wide confidence intervals. We also performed this comparison at six months in one study (MD 33.97, 95% CI 28.97 to 38.97; 80 participants; very low‐certainty evidence) (Ghavami 2017), and at 12 months in another study (MD 4.80, 95% CI ‐0.79 to 10.39; 174 participants; very low‐certainty evidence; Analysis 4.9) (Yun 2017). We downgraded the quality of evidence by two levels due to risk of bias assessment, imprecision, and inability to assess consistency due to only one study per outcome.
4.9. Analysis.
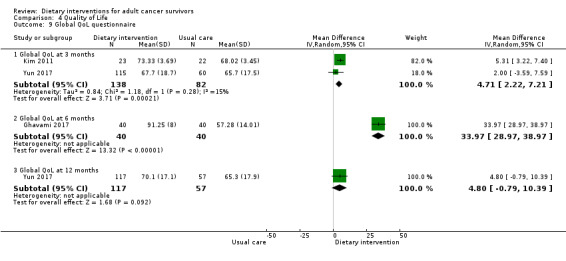
Comparison 4 Quality of Life, Outcome 9 Global QoL questionnaire.
Biochemical measures
GRADE assessment is shown in Table 7.
6. Dietary intervention compared to usual care for people living beyond cancer: biochemical outcomes.
| Dietary intervention compared to usual care for people living beyond cancer: biochemical outcomes | ||||||
| Patient or population: people living beyond cancer Setting: community Intervention: dietary intervention Comparison: usual care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with usual care | Risk with dietary intervention | |||||
| Total cholesterol assessed as mg/dL Follow‐up: 3 months | Mean total cholesterol was 209.5 | MD 18.2 lower (38.27 lower to 1.87 higher) | ‐ | 76 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,c | |
| Total cholesterol assessed as mg/dL Follow‐up: 6 months | Mean total cholesterol was 93.51 | MD 5.94 higher (2.45 higher to 9.43 higher) | ‐ | 83 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,c | |
| Triglycerides assessed as mg/dL Follow‐up: 3 months | Mean triglycerides was 175.4 | MD 40.6 lower (76.8 lower to 4.4 lower) | ‐ | 76 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,c | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RCT: randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence. High‐certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate‐certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low‐certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low‐certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level for risk of bias. bDowngraded one level due to lack of precision because of small sample size. cDowngraded one level due to inability to assess consistency across studies.
We are uncertain if dietary intervention has any effect on total cholesterol level at three months (MD ‐18.20 mg/dL, 95% CI ‐38.27 to 1.87; 76 participants; heterogeneity not applicable; very low‐certainty evidence) or at six months (MD 5.94 mg/dL, 95% CI 2.45 to 9.43; 83 participants, very low‐certainty evidence; Analysis 5.1), or on triglycerides at three months (MD ‐40.60 mg/dL, 95% CI ‐76.80 to ‐4.40; 76 participants; very low‐certainty evidence; Analysis 5.2) in one study (Mefferd 2007). We downgraded the quality of evidence due to risk of bias assessment, imprecision, and inability to assess consistency across studies.
5.1. Analysis.
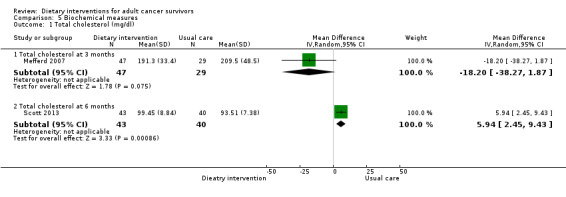
Comparison 5 Biochemical measures, Outcome 1 Total cholesterol (mg/dl).
5.2. Analysis.
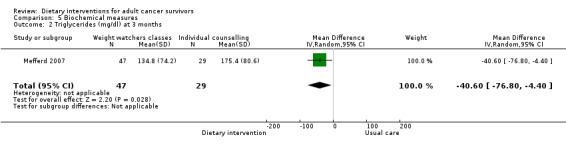
Comparison 5 Biochemical measures, Outcome 2 Triglycerides (mg/dl) at 3 months.
Adverse events
This outcome was not reported with dietary interventions.
Discussion
Summary of main results
This review includes 25 studies involving 7259 participants; we compared dietary intervention versus usual care or control and included no other comparisons. Most participants were women with breast cancer; we found a small amount of research on colorectal, prostate, and gynaecological cancer survivors. The evidence was very mixed overall for reported outcomes (mortality, dietary intake, anthropometry, quality of life, and biochemistry). See Table 1. The main findings are summarised below.
Effect of dietary intervention on mortality and morbidity
Low‐certainty evidence assessed after 7.3 years suggested little if any benefit of dietary intervention for survival or secondary malignancy. Other comorbidities were not recorded.
Effect of dietary intervention on dietary intake
Evidence assessed for dietary intervention after 12 months showed little or no effect on energy intake (moderate‐certainty evidence). When fruit and vegetable servings were combined, dietary intake was probably increased with dietary intervention (moderate‐certainty evidence), although for dietary fibre, the results were mixed. Evidence showed a probably positive effect of dietary intervention on Diet Quality Index (moderate‐certainty evidence).
Effect of dietary intervention on anthropometry
Evidence assessed after 12 months showed little or no effect of dietary intervention on body weight (high‐certainty evidence). Evidence showed a probably slight decrease in body mass index after 12 months of dietary intervention (moderate‐certainty evidence). Evidence suggested little or no effect of dietary intervention on waist‐to‐hip ratio after 12‐months (low‐certainty evidence). We found no evidence on measurements of body composition.
Effect of dietary intervention on quality of life after 12 months
We are uncertain about effects on Functional Assessment of Cancer Therapy (FACT), Short Form (SF)‐36, cancer‐related fatigue, and global quality of life measures after 12 months of dietary intervention based on the evidence found (very low‐certainty evidence).
Effect of dietary intervention on biochemistry
We are uncertain if dietary intervention has any effect on total cholesterol or triglycerides (very low‐certainty evidence).
Overall completeness and applicability of evidence
For this review, we searched only for studies including people living beyond cancer. We therefore excluded all studies that recruited participants at the start of their treatment programme or at any point in the care pathway when they were receiving treatment. We excluded from this review studies including people receiving hormone treatment who still had a malignant tumour.
Evidence of effectiveness for most outcomes evaluated is incomplete, particularly for mortality, morbidity, some nutrients, body composition measurements, and quality of life (QoL) measures related to patient‐reported outcomes. In addition, evidence was often incompletely reported with missing standard deviations of the mean and reported differences in weights and measurements that varied from grams to number of servings and number of people consuming recommended amounts of food or nutrient. When we encountered missing data, we contacted some study authors to request further data, and only one study author provided data; however we did not incorporate these data into the review. This made it difficult to perform meta‐analyses for these outcomes, and when we did perform meta‐analyses, we included multiple analyses to present results reported in different formats. In some instances, heterogeneity was high, so we performed subgroup analyses to explore these based on ‘a priori’ criteria including different cancer types and varying nutritional status.
When data were included but not in a form suitable for meta‐analysis, we tried to report these data in a narrative format when possible; however, we did not grade this evidence.
In terms of specific outcomes, we found evidence on the primary outcome mortality, which showed little if any difference between groups, although there may be limitations in the time frame the evidence was reported, as it may have not been sufficient for dietary interventions to influence overall survival. Morbidity as a surrogate marker was not reported by any of the studies identified for this review, apart from secondary malignancies. Dietary interventions were found to have little effect on energy levels, and several studies reported this with a moderate level of certainty after 12 months. For fruit and vegetable intake, dietary intervention probably had some positive effects. We found evidence showing that diet quality was probably improved with dietary intervention, although results for dietary fibre were mixed. We found limited evidence for some cancer sites and noted differences on subgroup analyses conducted to examine sites of cancer and nutritional status.
Researchers reported anthropometry using different measurements, some self‐reported. For body mass index, we found sufficient evidence to ascertain the effects of dietary interventions. Studies reporting body composition for fat and muscle as outcomes were lacking, so the evidence base is incomplete.
For QoL measurements, included studies reported a variety of measurements, which meant there was no compelling evidence by which to determine overall effect. We found no evidence on patient‐reported outcomes.
The evidence found was primarily based on women with breast cancer, so these results have limited generalisability to people with cancer at different sites.
Quality of the evidence
Most evidence is generally of low or very low‐certainty as assessed by GRADE (Higgins 2011), with most studies not evaluating effects of dietary interventions on key review outcomes, particularly the primary outcomes of mortality and morbidity (Table 8). Nonetheless, a few included studies did stand out, as they provided moderate‐certainty evidence on dietary intake and anthropometry (Table 9; Table 10). These studies included people with breast cancer and mixed cancer groups. Evidence was often undermined by unclear study methods or by study design limitations and small sample sizes, which led to downgrading of the evidence due to imprecision of estimates. Outcomes were often measured via self‐report tools and findings were poorly controlled in terms of definitions and judgements, particularly for some dietary assessment methods. Studies included were frequently downgraded for risk of bias due to absence of blinding for subjective outcomes. In addition, compliance with dietary interventions was seldom reported or assessed by objective tools. Adequate reporting of compliance with interventions is particularly problematic for dietary intervention studies. Furthermore, blinding of participants and professionals to dietary interventions is difficult when the intervention is based on food.
7. Dietary intervention compared to control for people living beyond cancer: mortality and morbidity.
| Dietary intervention compared to control for people living beyond cancer: mortality and morbidity | ||||||
| Patient or population: people living beyond cancer Setting: community Intervention: dietary intervention Comparison: control | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with control | Risk with dietary intervention | |||||
| Mortality Follow‐up: 7.3 years | Study population | HR 0.98 (0.77 to 1.23) | 3107 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | ||
| 106 per 1000 | 104 per 1000 (82 to 128) | |||||
| Secondary cancers Follow‐up: 7.3 years | Study population | RR 0.99 (0.84 to 1.15) | 3107 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | ||
| 168 per 1000 | 166 per 1000 (141 to 193) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; HR: hazard ratio; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence. High‐certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate‐certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low‐certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low‐certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aInability to rate consistency as only one study. bConfidence intervals are not narrow and no overlap is associated with effect estimates.
8. Dietary intervention compared to usual care for people living beyond cancer: dietary outcomes.
| Dietary intervention compared to usual care for people living beyond cancer: dietary outcomes | ||||||
| Patient or population: people living beyond cancer Setting: community Intervention: dietary intervention Comparison: usual care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with usual care | Risk with dietary intervention | |||||
| Energy intake/mean assessed as kcal Follow‐up: 12 months | Mean energy intake/mean was 1503 kcal | MD 59.13 kcal fewer (156.05 fewer to 37.79 more) | ‐ | 3283 (5 RCTs) | ⊕⊕⊕⊝ Moderatea | |
| Fruit and vegetable servings assessed as servings Follow‐up: 12 months | Mean fruit and vegetable servings was 4.56 servings | MD 0.41 servings higher (0.1 higher to 0.71 higher) | ‐ | 834 (5 RCTs) | ⊕⊕⊕⊝ Moderateb | |
| Fibre intake assessed as g Follow‐up: 12 months | Mean fibre intake was 15.6 g | MD 5.12 g higher (0.66 lower to 10.9 higher) | ‐ | 3127 (2 RCTs) | ⊕⊝⊝⊝ Very lowc,d | |
| Diet Quality Index Follow‐up: 12 months | Mean Diet Quality Index was 64.7 | MD 3.46 higher (1.54 higher to 5.38 higher) | ‐ | 747 (3 RCTs) | ⊕⊕⊕⊝ Moderatee | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RCT: randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence. High‐certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate‐certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low‐certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low‐certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level due to wide variation in effect estimates across studies. bDowngraded one level due to indirectness. cDowngraded two levels for high level of inconsistency between studies. dDowngraded one level as CI is not narrow. eDowngraded one level due to risk of bias.
9. Dietary intervention compared to usual care for people living beyond cancer: anthropometry outcomes.
| Dietary intervention compared to usual care for people living beyond cancer: anthropometry outcomes | ||||||
| Patient or population: people living beyond cancer Setting: community Intervention: dietary intervention Comparison: usual care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with usual care | Risk with dietary intervention | |||||
| Weight/mean assessed as kg Follow‐up: 12 | Mean weight/mean was 81.94 kg | MD 0.8 kg lower (2.01 lower to 0.41 higher) | ‐ | 3287 (5 RCTs) | ⊕⊕⊕⊕ High | |
| Body mass index/mean assessed as kg/m² follow up: 12 | Mean body mass index/mean was 29.63 kg/m² | MD 0.79 kg/m² lower (1.5 lower to 0.07 lower) | ‐ | 777 (4 RCTs) | ⊕⊕⊕⊝ Moderatea | |
| Waist‐to‐hip ratio assessed as cm Follow‐up: 12 months | Mean waist‐to‐hip ratio was 0.46 cm | MD 0.01 cm lower (0.04 lower to 0.02 higher) | ‐ | 106 (2 RCTs) | ⊕⊕⊝⊝ Lowb,c | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RCT: randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence. High‐certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate‐certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low‐certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low‐certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level due to risk of bias assessment. bDowngraded one level for indirectness as studies included only Hispanic women. cDowngraded one level due to imprecision because of small sample size.
Evidence rated as moderate‐certainty evidence was obtained primarily from women with breast cancer who were included in large studies conducted in the USA. Other evidence of low to moderate‐certainty was obtained from Australia among participants with colorectal cancer, and from the USA among participants with mixed cancer types.
A small number of studies reported on secondary outcomes of quality of life and biochemical measures; thus evidence on changes that may be attributed to dietary intervention is weak (Table 5; Table 7).
Potential biases in the review process
Evidence has been presented as outcomes, as the different dietary interventions provided were difficult to categorise into any meaningful comparisons and trial authors reported multiple variations in methods used to provide dietary intervention. Some interventions were provided by telephone, others face‐to‐face or by mail, and others were web‐based. Different healthcare professionals provided information to participants including dietitians, healthcare workers, and trained coaches. The dietary intervention in some instances incorporated behavioural change theory, and in other instances did not involve behavioural change theory. Some included studies used a variety of modes to provide dietary interventions during the same study.
Our definition of cancer survivorship may have led to potential biases in the approach to this review. Consequently, we may have favoured inclusion of studies on women with breast cancer and excluded studies on men with prostate cancer who have ongoing malignancies, which can be managed with biological agents and hormone therapies. The inclusion of a large proportion of studies on breast cancer has led to a bias in the review towards women. We are therefore uncertain if some of the evidence presented relates to men who have survived cancer. The definition of cancer survivors excluded studies that provided dietary interventions before cancer treatment, but those interventions could have been based on the same recommendations for survivorship.
Search methods
We did not handsearch all related journals and conference proceedings; we looked only at abstracts from the American Institute of Cancer Research that were available online. This may have led to our missing some abstracts. This decision was made at the protocol stage because to search all nutrition journals by hand would have been time‐consuming and would have been very resource‐intensive. We searched a number of databases and attempted to track down all reports of the same studies. However, due to the large number of reports identified and the volume of research reported in this area, some studies and reports may have been missed, even though efforts to follow the methods outlined in the protocol were extensive.
Study selection
We included only randomised controlled trials (RCTs), and we included only studies that reported a comparison between dietary intervention and usual care or written materials. We did not include studies that compared dietary interventions or different diets in people who had survived cancer. This limits our findings on comparison of dietary interventions to people who have survived cancer.
Data extraction and analysis
When studies reported outcomes for multiple time points, we tried to report the time points at three, six, and 12 months, and for mortality at the last time point reported. When data were missing and we could calculate from the reports, we did so, and we used Cochrane tools to assist in some calculations. However, if data were not available and were not retrieved by contacting authors, we reported findings in a narrative format. We used the random‐effects model for all meta‐analyses, irrespective of statistical heterogeneity, as at the protocol stage, we anticipated that clinical heterogeneity with respect to study populations and interventions would be high across all included studies. All subgroup analyses that were performed were pre‐specified in the protocol. Subgroup analyses were performed by cancer site and nutritional status. We did not grade the certainty of evidence for narrative results.
Agreements and disagreements with other studies or reviews
A number of other reviews have presented evidence on dietary interventions in people who have survived cancer (Demark‐Wahnefried, 2015; Goode 2015; Pierce 2009a; Reeves 2014; Roberts, 2017; Stacey, 2015). One review evaluated data from dietary intervention studies in people who had survived cancer based on mode of provision for the intervention, which could be telephone conversations, digital interaction, or face‐to‐face meetings (Goode 2015).
In this review, most study participants received information via the telephone, which concurs with the findings presented. The conclusions suggest that included evidence supports broad reach modalities, particularly the telephone, to provide lifestyle interventions (Goode 2015). The favourable opinions regarding broad reach approaches compared to face‐to‐face and group sessions are not directly supported by the data reported in our review, as we have presented data based on outcomes. It is difficult to classify dietary interventions, as a large proportion of trials are multi‐modal in approach, and interventions were delivered with different levels of follow‐up and by a range of personnel (Table 2). In addition, some of the dietary interventions that we identified had behavioural change strategies incorporated while others did not (Table 2).
Suggestions are made on how to gather more data on long‐term outcomes and for cancer types other than breast cancer in combination with health economic data (Goode 2015). A review of digital health provided contradictory conclusions challenging the concept that broad‐ranging dietary interventions are effective by suggesting that a review of digital health interventions revealed mixed results from a narrative synthesis of any effects on diet (Roberts, 2017). Added to this debate are the findings from a review of theory‐based nutritional behaviour change interventions for cancer survivors, which reported that including a behaviour change strategy led to improvement in at least one aspect of dietary intake, although no consensus was reached regarding mode of provision of the intervention from the evidence reviewed in these studies (Stacey, 2015).
Mortality and morbidity
One review discussed in length differences among studies including participants with breast cancer that have had a long enough time frame to evaluate overall survival and comorbidities (Pierce 2009a). We did not find any effect on our primary outcome measures, which included mortality and secondary malignancies, from one very large included study providing low‐certainty evidence (Pierce 2007). However, another large RCT, again including participants with breast cancer, demonstrated differences between groups favouring the intervention (Chlebowski, 2006). We excluded this trial from our review as participants were recruited while they were still potentially receiving active therapy within 365 days after surgery for breast cancer. This study showed that for participants who did not relapse, the hazard ratio (HR) for an event in the dietary intervention group compared to the control group was 0.76 (95% confidence interval (CI) 0.60 to 0.98), for recurrence‐free survival was 0.71 (95% CI 0.53 to 0.94), and for disease‐free survival was 0.81 (95% CI 0.65 to 099). However for overall survival, the HR was 0.98 (95% CI 0.65 to 1.21) (Chlebowski, 2006).
Differences in results from the two large studies in breast cancer cohorts could be due to the different samples recruited; one study recruited women between the ages of 48 and 79 years (Chlebowski, 2006), and the study included in this review recruited participants from 18 to 70 years of age (Pierce 2007). In Pierce 2009a, it is pointed out in the discussion that there was a difference between trials in time after diagnosis when participants were recruited, as well as in disease severity. Participants in Chlebowski, 2006 were likely to be younger and more highly educated, and were less likely to be obese.
It can be concluded that there may be overall benefit for survival when dietary interventions are provided to subgroups of people with breast cancer (Pierce 2009a). However, there is a paucity of evidence on these primary outcomes in other cancer cohorts.
Dietary intake
Another review concurred with our findings, highlighting beneficial effects on diet quality from dietary interventions in cancer survivorship (Demark‐Wahnefried, 2015).
Anthropometry
A narrative review of the evidence from intervention trials concurs with the findings of this review, demonstrating benefits of dietary intervention for weight loss and supporting our results in relation to body mass index for people who have survived cancer after 12 months of intervention (Demark‐Wahnefried, 2015). We reported on evidence from women with breast cancer including four studies that showed a slight reduction in body mass index (moderate‐certainty evidence). However, not all of these studies had dietary interventions that were aimed at weight reduction. Data from meta‐analysis on mean weight measured between groups in kilograms showed no differences among five studies after 12 months, although it has to be acknowledged that mean weight is not an ideal outcome to show differences between groups because it does not take into account changes as a proportion of overall body size. A further review of weight loss in women with breast cancer showed positive effects of dietary interventions on weight loss but also reported benefit in decreasing waist‐to‐hip ratios (Reeves 2014). We found mixed results for waist‐to‐hip ratios across cancer sites. Dietary interventions showed some benefit with variable effect sizes in women with breast cancer, but not in women with uterine cancer.
Quality of life
It was highlighted by another review that improvements in QoL resulted from dietary interventions (Demark‐Wahnefried, 2015). We found mixed results depending on the measurement tool and the QoL domain used. Benefits were seen in physical domains for a mixed group of cancer survivors, but not consistently in mental health domains, and mixed results on global QoL were noted over time.
Authors' conclusions
Implications for practice.
Moderate‐certainty evidence shows that dietary interventions can modify food and nutrient intakes and can positively affect some anthropometric measurements, particularly for women after breast cancer. Outcomes that showed evidence of some probable improvement were intake of fruits and vegetables and diet quality. Energy intake was not shown to be affected by dietary interventions. Body mass index was probably slightly reduced with dietary interventions. Lack of evidence from one included study suggests that dietary interventions improve overall mortality, and we found no evidence on the effects of dietary interventions on morbidities. Short‐term changes in dietary intake and in anthropometric measurements were not always shown to be sustained over a longer period.
In relation to quality of life (QoL), we are uncertain about study results.
Included studies described some positive benefits of dietary intervention for cancer survivors, although inconsistencies between included studies suggest that people may benefit differently from dietary interventions in terms of alterations in dietary intake and anthropometry, depending on type of cancer, nutritional status, and regular eating habits.
Different personnel administered the dietary interventions, but dietitians did so in most studies. It is important to note that training and appropriate understanding are required when dietary interventions are provided to people who have survived cancer. Some studies described in detail the behaviour change strategies used, and it is important to note that as modifications to dietary intake represent a behaviour change, it is essential to incorporate these behaviours into dietary interventions used in practice.
Implications for research.
Implications for future research include the following.
We identified 26 ongoing studies that are relevant to this review, including 11 studies on breast cancer, three on colorectal cancer, three on gynaecological cancer, and nine on mixed cancer types, indicating that this area is developing rapidly. Studies are now being undertaken to examine previously neglected cancer sites. In addition, long‐term follow‐up data on participants already randomised into cohorts are being reported, adding to the evidence base as studies mature.
Evidence in some areas is incomplete or lacking, and further research is required, including evaluation of dietary interventions for participants with cancer at sites other than the breast. These tumour sites could include colorectal or endometrial cancer, where a link has been established between occurrences and either dietary intake or obesity, making these important areas for future research. Moreover, a number of large ongoing studies recruiting participants with colorectal cancer will add substantially to the evidence.
In relation to measurement of outcomes, we found a lack of data on body composition when routinely available imaging was used to evaluate changes in body composition. Use of mobile or digital applications to deliver interventions was limited, and this may well be an approach that will facilitate delivery of dietary interventions in the future. Developing outcomes that make use of digital technologies and routine imaging in assessment of dietary interventions would add to future research. No studies in this review included comorbidities as an outcome measure, and this is a huge reason why modifying dietary intake studies that include comorbidities as an outcome would enhance findings in future research.
We identified some high‐quality studies, although not all trials followed CONSORT guidelines and could be improved in relation to quality and standards of reporting. There were only a few studies that attempted to blind assessors; this would be a feasible way to decrease risk of bias in future research.
This review is focused on survivors who were enrolled into studies after all treatments were completed, so it would be interesting to undertake further review of dietary interventions in patients who are living with cancer, particularly in light of the advancement of biological agents and hormone therapies used in prostate, breast, and gynaecological cancers. The optimal time to administer dietary interventions for healthy eating within cancer pathways for all cancer sites is a topic that remains controversial, as different studies included participants at different time points after their cancer treatment. Determining the optimal time to deliver dietary interventions is therefore worthy of further research.
Studies investigating dietary interventions and changes in anthropometrics need long‐term follow‐up to identify differences in clinical outcomes including mortality and morbidity. This is often difficult due to the resource implications of conducting studies with long‐term follow‐up. The use of surrogate markers as early indicators for tumour recurrence may offer an advantage in studies where cancer recurrence is an important outcome in the evaluation of dietary interventions.
Notes
Nil.
Acknowledgements
We thank Jo Morrison for clinical and editorial advice, Jo Platt for designing the search strategy, and Gail Quinn, Clare Jess, and Tracey Harrison for their contributions to the editorial process.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Gynaecological, Neuro‐oncology and Orphan Cancer Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS, or the Department of Health.
The authors and the Cochrane Gynaecological, Neuro‐oncology and Orphan Cancers Team are grateful to the following peer reviewers for their time and comments: Andy Bryant, Patrica Jupp, Karen Moody, and Clare Shaw.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Neoplasms] explode all trees #2 (cancer* or tumor* or tumour* or neoplas* or malignan* or carcinoma* or adenocarcinoma* or choriocarcinoma* or leukemia* or leukaemia* or metastat* or sarcoma* or teratoma*):ti,ab #3 #1 or #2 #4 MeSH descriptor: [Diet] explode all trees #5 MeSH descriptor: [Nutrition Assessment] explode all trees #6 MeSH descriptor: [Nutrition Therapy] explode all trees #7 MeSH descriptor: [Nutrition Disorders] explode all trees #8 MeSH descriptor: [Food Habits] explode all trees #9 MeSH descriptor: [Food Preferences] explode all trees #10 MeSH descriptor: [Food] explode all trees #11 (diet* or nutrition* or nutrient* or food* or feed* or eat* or drink*):ti,ab #12 (fat* or carbohydrate* or protein* or fruit* or vegetable* or fibre* or fiber* or fish* or meat* or poultry or dairy or salt* or sugar* or cereal* or nut* or seed* or alcohol* or caffeine):ti #13 (macrobiotic or ketogenic or vegetarian or (low adj (glycemic* or glycaemic*))):ti #14 #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 #15 MeSH descriptor: [Survivors] explode all trees #16 (survivor* or survival*):ti,ab #17 #15 or #16 #18 #3 and #14 and #17
Appendix 2. MEDLINE Ovid search strategy
1 exp neoplasms/ 2 (cancer* or tumor* or tumour* or neoplas* or malignan* or carcinoma* or adenocarcinoma* or choriocarcinoma* or leukemia* or leukaemia* or metastat* or sarcoma* or teratoma*).ti,ab. 3 1 or 2 4 exp diet/ 5 exp nutrition assessment/ 6 exp nutrition therapy/ 7 exp nutrition disorders/ 8 exp food habits/ 9 food preferences/ 10 exp food/ 11 (diet* or nutrition* or nutrient* or food* or feed* or eat* or drink*).ti,ab. 12 (fat* or carbohydrate* or protein* or fruit* or vegetable* or fibre* or fiber* or fish* or meat* or poultry or dairy or salt* or sugar* or cereal* or nut* or seed* or alcohol* or caffeine).ti. 13 (macrobiotic or ketogenic or vegetarian or (low adj (glycemic* or glycaemic*))).ti. 14 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 15 survivors/ 16 (survivor* or survival*).ti,ab. 17 15 or 16 18 3 and 14 and 17 19 randomized controlled trial.pt. 20 controlled clinical trial.pt. 21 randomized.ab. 22 placebo.ab. 23 clinical trials as topic.sh. 24 randomly.ab. 25 trial.ti. 26 19 or 20 or 21 or 22 or 23 or 24 or 25 27 18 and 26 28 exp animals/ not humans.sh. 29 27 not 28
key: [mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier]
Appendix 3. Embase search strategy
1 exp neoplasm/ 2 (cancer* or tumor* or tumour* or neoplas* or malignan* or carcinoma* or adenocarcinoma* or choriocarcinoma* or leukemia* or leukaemia* or metastat* or sarcoma* or teratoma*).ti,ab. 3 1 or 2 4 exp nutrition/ 5 exp nutritional disorder/ 6 (diet* or nutrition* or nutrient* or food* or feed* or eat* or drink*).ti,ab. 7 (fat* or carbohydrate* or protein* or fruit* or vegetable* or fibre* or fiber* or fish* or meat* or poultry or dairy or salt* or sugar* or cereal* or nut* or seed* or alcohol* or caffeine).ti. 8 (macrobiotic or ketogenic or vegetarian or (low adj (glycemic* or glycaemic*))).ti. 9 4 or 5 or 6 or 7 or 8 10 cancer survivor/ 11 (survivor* or survival*).ti,ab. 12 10 or 11 13 3 and 9 and 12 14 crossover procedure/ 15 double‐blind procedure/ 16 randomized controlled trial/ 17 single‐blind procedure/ 18 random*.mp. 19 factorial*.mp. 20 (crossover* or cross over* or cross‐over*).mp. 21 placebo*.mp. 22 (double* adj blind*).mp. 23 (singl* adj blind*).mp. 24 assign*.mp. 25 allocat*.mp. 26 volunteer*.mp. 27 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 28 13 and 27
key: mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword ti‐title ab=abstract
Appendix 4. AMED Ovid search strategy
1 exp neoplasms/ 2 (cancer* or tumor* or tumour* or neoplas* or malignan* or carcinoma* or adenocarcinoma* or choriocarcinoma* or leukemia* or leukaemia* or metastat* or sarcoma* or teratoma*).ti,ab. 3 1 or 2 4 Diet/ 5 exp nutrition assessment/ 6 exp Nutrition Therapy/ 7 exp Nutrition Disorders/ 8 Food Habits/ 9 Food Preferences/ 10 exp Food/ 11 (diet* or nutrition* or nutrient* or food* or feed* or eat* or drink*).ti,ab. 12 (fat* or carbohydrate* or protein* or fruit* or vegetable* or fibre* or fiber* or fish* or meat* or poultry or dairy or salt* or sugar* or cereal* or nut* or seed* or alcohol* or caffeine).ti,ab. 13 (macrobiotic or ketogenic or vegetarian or (low adj (glycemic* or glycaemic*))).ti,ab. 14 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 15 survivors/ 16 (survivors or survival*).ti,ab. 17 15 or 16 18 3 and 14 and 17 19 randomized controlled trial.pt. 20 controlled clinical trial.pt. 21 randomized.ab. 22 placebo.ab. 23 clinical trials as topic.sh. 24 randomly.ab. 25 trial.ti. 26 19 or 20 or 21 or 22 or 23 or 24 or 25 27 18 and 26 28 exp animals/ not humans.sh. 29 27 not 28
Appendix 5. CINAHL EBSCO search strategy
Advanced search option
Cancer and Survivorship and Diet and nutrition
All text search with Boolean/phase selected
Appendix 6. DARE search strategy
Advanced search option
Cancer and| Survivorship and Diet and nutrition
All fields searched MeSH Terms
Appendix 7. Data extraction sheet
| Form version/date |
| Version 2, 1 Feb 2016 |
| Review Title |
| Dietary Interventions in Adult Cancer Survivors |
| Date form completed |
| Name of review author completing this form |
| Study ID (Surname Year: as it will appear in RevMan) |
| Study title |
| Country of origin |
| Funding (including source, amount, if stated). |
| Notes(Unpublished – for own use) Eg. References to be followed up, source of information (especially if multiple reports of same trial, or unpublished data/personal communication included). |
Methods:
| Aim of intervention (As stated in the trial report/s. What was the problem that this intervention was designed to address?) |
| Aim of study (As stated in the trial report/s. What was the trial designed to assess?) |
| Study design |
| Methods of recruitment of participants (How were potential participants approached and invited to participate?) |
| Inclusion/exclusion criteria for participation in study |
| Informed consent obtained? (Yes/No/Unclear) |
| Ethical approval (Yes/No/Unclear) |
| Statistical methods and their appropriateness (if relevant) |
| Consumer involvement (eg. In design of study and/or intervention; in delivery of intervention; in evaluation of intervention; in interpretation of study findings) |
Participants:
| Description (eg. Patients/consumers; carers; parents of patients/consumers; health professionals; well people in the community) |
| Geographic location (eg. City/State/Country) |
| Setting (eg. Community, home, primary health centre, acute care hospital, extended care facility) |
| Number: (Eligible, excluded, refused to take part, randomised to intervention, randomised to control, excluded post randomisation, withdrawn, lost to follow‐up, died, included in analysis, included for each outcome) |
| Age: range, mean (standard deviation) |
| Gender |
| Ethnicity |
| Location of treated tumour |
| Therapy previously received for cancer |
| Cancer staging or classification |
| Baseline: proportion of overweight/obese survivors (defined by BMI > 25 kg/m²or nutritional assessment tool) |
| Baseline: alcohol intake (units/week) |
| Baseline: smoking status |
| Baseline: physical activity levels |
| Socioeconomic group |
| Other health problem/s (if relevant) |
Interventions:
| Details of intervention, including theoretical basis (with key references), aim, content, type (nutritional education, weight management), delivery method (written, telephone, face‐to‐face, Internet), setting, behaviour change techniques employed (motivational interviewing, goal setting). (Capture this information for each arm of the study, eg. Intervention A, Intervention B…) |
| Details of control/usual or routine care |
| Details of co‐interventions in all groups (co‐interventions may be separate from the intervention of interest for this review, or they may be other similar elements in a suite of interventions having a common purpose. Record all relevant information) |
| Delivery of intervention (eg. stages, timing, frequency, duration) (for each intervention included in the study, eg. Intervention A; Intervention B…) |
| Details of providers (Who delivers the intervention?; number of providers; training of providers in delivery of intervention) |
| Intervention quality (if relevant): (Record any information on the quality of the intervention ‐ assessed by study authors, by others, or by you ‐ such as the evidence base of the intervention, or the quality of staff training for intervention delivery) |
| Fidelity/integrity (Was the intervention delivered as intended? Record any assessment of this) |
Outcomes:
| Principal and secondary outcome measures (tick [P] all those included in this study and state if primary or secondary) |
| · Overall survival [ ] · Incidence of secondary malignancy or other cancer [ ] · Incidence of comorbidities [ ] · Dietary changes measured by diaries, FFQ, recall or assessment methodology [ ] · Changes in weight/anthropometry (incl hip:waist, skin folds, function) [ ] · Quality of life [ ] · Biochemical measure (lipid profiles, serum glucose) [ ] · No. of healthy eating changes made to habitual eating patterns [ ] · Other (please state): |
| Methods of assessing outcome measures (eg, phone survey, questionnaire, physical measurements (for each outcome)). State unit of measure or scale used (if scale, state direction) |
| Validity and reliability of outcome measures |
| Methods of follow‐up for non‐respondents |
| Timing of outcome assessment (including frequency, length of follow‐up (for each outcome)) |
| Adverse events (eg, complaints, levels of dissatisfaction, adverse incidents, side effects)) |
Assessment of risk of bias for RCTs, quasi‐RCTs, and CBAs (used to complete the ‘Risk of Bias’ tables in RevMan 5)
| Domain | Review authors’ judgement | Support for judgement | |
| Selection bias | Random sequence generation* |
High, Unclear, or Low risk? |
Describe the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups. (Adequate: random number table, computer generated. Inadequate: alternation, DOB, date of admission, hospital number) If you are including only RCTs in your review, papers marked ‘high risk’ should be excluded as they are not truly randomised. |
| Allocation concealment |
High, Unclear, or Low risk? |
Describe the method used to conceal the allocation sequence in sufficient detail to determine whether intervention allocations could have been foreseen in advance of, or during, enrolment. (Decision to accept or reject a participant must be made without knowledge of the treatment assigned) (Adequate: centralised randomisation or sequentially numbered opaque envelopes Inadequate: open list of random numbers, coin toss, alternation Unclear: just stating that a table, list or sealed envelopes were used) |
|
| Performance bias |
Blinding of participants and personnel Assessments should be made for each main outcome (or class of outcomes) |
High, Unclear, or Low risk? |
Describe all measures used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. Provide any information relating to whether the intended blinding was effective. |
| Detection bias |
Blinding of outcome assessment Assessments should be made for each main outcome (or class of outcomes). |
High, Unclear, or Low risk? |
Describe all measures used, if any, to blind outcome assessors from knowledge of which intervention a participant received. Provide any information relating to whether the intended blinding was effective. If the outcome is objective (eg. length of hospital stay) the rating should be ‘Low risk' |
| Attrition bias |
Incomplete outcome data Assessments should be made for each main outcome (or class of outcomes). |
High, Unclear, or Low risk? |
Describe the completeness of outcome data for each main outcome, including attrition and exclusions from the analysis. State whether attrition and exclusions were reported, the numbers in each intervention group (compared with total randomised participants), reasons for attrition/exclusions where reported, and any re‐inclusions in analyses performed by the review authors (ITT) |
| Reporting bias | Selective reporting |
High, Unclear, or Low risk? |
State how the possibility of selective outcome reporting was examined by the review authors, and what was found. |
| Other bias |
Other sources of bias See the Cochrane Handbook 8.15.1 for further examples of potential threats to validity. |
High, Unclear, or Low risk? |
State any important concerns about bias not addressed in the other domains in the tool. If particular questions/entries were pre‐specified in the review’s protocol, responses should be provided for each question/entry (early stopping, baseline imbalances, choice of design, funding source) |
Notes (These are published in the table Characteristics of Included Studies)
|
For example: · Contact with author (Yes (information obtained)/No) (SEE NOTE ON PAGE 1) · Power calculation? · Record if the study was translated from a language other than English. · Record if the study was a duplicate publication. |
Results
All data are numbers (of patients/units), not percentages
Dichotomous outcomes
| Outcome | Timing of outcome assessment (days/months) | Intervention group* | Control group | Notes | ||
| Observed (n) | Total (N) | Observed (n) | Total (N) | |||
*Note: add additional columns if there is more than one intervention group, eg. Intervention Group A, Intervention Group B…
Continuous outcomes
| Outcome | Timing of outcome assessment (days/months) | Intervention group | Control group | Notes | ||||
| *Mean / Mean change | Standard deviation | N | *Mean / Mean change | Standard deviation | N | |||
*delete as appropriate
Appendix 8. Reports for included studies
For the 'FRESH START' trial there were seven reports identified. The study design and primary study report for the "FRESHSTART" trial was reported in Demark‐Wahnefried 2007.
For the 'CANCHANGE' study there were eight reports identified, the primary study report used in this review was Hawkes 2013.
For the study on exercise and hypocaloric eating there were three trial reports, the primary report was Scott 2013.
For the 'Leading the Way in Exercise and Diet' (LEAD) study there were four reports, Demark‐Wahnefried 2006 was the primary report.
For the 'Daughters and Mothers Against Breast Cancer' (DAMES) study there was one other report identified Demark‐Wahnefried 2014 was the primary report.
For the 'Women's Healthy Eating and Living' (WHEL) study there were 17 trial reports identified. The primary report was Pierce 2007.
For the 'Survivors of Uterine Cancer Empowered by Exercise and Healthy Eating' (SUCCEED) study there were seven reports identified and the primary report was Gruenigen 2012.
For the 'Reach out to Enhance Wellness' (RENEW) study there were two reports identified. The primary report was Morey 2009.
For the 'Lifestyle Exercise and Nutrition' (LEAN) study there were three reports identified and the primary report was Harrigan 2016.
For the study by Greenlee 2013, there were five reports identified.
For the study by Greenlee 2015 there were two other report identified.
For the study by Reeves 2017 there was one other report identified.
For the study by Djuric 2002 there were two other reports identified.
For the study by Befort 2016 there was one other reported identified.
For the study by Ghavami 2017 there was one other reported identified.
Data and analyses
Comparison 1. Overall survival.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 1 | Hazard Ratio (Fixed, 95% CI) | 0.98 [0.77, 1.23] | |
| 2 Morbidity | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Number of cancer events | 1 | 3107 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.84, 1.15] |
Comparison 2. Dietary changes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean energy intake (kcal) | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Energy intake (kcal) at 3 months | 3 | 115 | Mean Difference (IV, Random, 95% CI) | ‐52.61 [‐209.23, 104.02] |
| 1.2 Energy intake (kcal) at 6 months | 4 | 3236 | Mean Difference (IV, Random, 95% CI) | ‐47.67 [‐142.33, 46.99] |
| 1.3 Energy intake (kcal) at 12 months | 5 | 3283 | Mean Difference (IV, Random, 95% CI) | ‐59.13 [‐156.05, 37.79] |
| 2 Adjusted mean energy intake (kcal) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Energy intake (kcal) at 3 months | 1 | 67 | Mean Difference (IV, Random, 95% CI) | ‐637.0 [‐819.79, ‐454.21] |
| 2.2 Energy intake (kcal) at 6 months | 1 | 61 | Mean Difference (IV, Random, 95% CI) | ‐548.1 [‐753.22, ‐342.98] |
| 3 Subgroup analysis energy intake (kcal) BMI > 25 kg/m² at 3 months | 2 | 97 | Mean Difference (IV, Random, 95% CI) | ‐51.81 [‐283.08, 179.45] |
| 4 Subgroup analysis energy intake (kcal) BMI > 25 kg/m² at 6 months | 2 | 93 | Mean Difference (IV, Random, 95% CI) | ‐49.00 [‐269.75, 171.74] |
| 5 Mean fruit servings (per day) | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Fruit servings (per day) at 3 months | 1 | 67 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.82, 1.02] |
| 5.2 Fruit servings (per day) at 6 months | 2 | 3157 | Mean Difference (IV, Random, 95% CI) | 0.62 [0.08, 1.16] |
| 5.3 Fruit servings (per day) at 12 months | 3 | 3205 | Mean Difference (IV, Random, 95% CI) | 0.47 [‐0.13, 1.07] |
| 6 Fruit servings for each cancer site at 6 months | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 Uterine cancer | 1 | 69 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.57, 0.97] |
| 6.2 Breast cancer | 1 | 3088 | Mean Difference (IV, Random, 95% CI) | 0.80 [0.58, 1.02] |
| 7 Fruit servings for each cancer site at 12 months | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 Uterine cancer | 1 | 59 | Mean Difference (IV, Random, 95% CI) | 0.30 [‐0.80, 1.40] |
| 7.2 Breast cancer | 2 | 3146 | Mean Difference (IV, Random, 95% CI) | 0.46 [‐0.40, 1.31] |
| 8 Fruit servings in different ethnic groups: breast cancer at 12 months | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.1 Hispanic population | 1 | 58 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.98, 0.78] |
| 8.2 Mixed (85% white) population | 1 | 3088 | Mean Difference (IV, Random, 95% CI) | 0.80 [0.64, 0.96] |
| 9 Adjusted mean vegetable servings (per day) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 9.1 Vegetable servings (per day) at 3 months | 1 | 67 | Mean Difference (IV, Random, 95% CI) | 0.60 [‐0.23, 1.43] |
| 9.2 Vegetable servings (per day) at 6 months | 1 | 61 | Mean Difference (IV, Random, 95% CI) | 0.80 [‐0.03, 1.63] |
| 9.3 Vegetable servings (per day) at 12 months | 1 | 58 | Mean Difference (IV, Random, 95% CI) | 1.10 [0.35, 1.85] |
| 10 Vegatable servings: uterine cancer | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 10.1 Vegatable intake at 3 months | 1 | 67 | Mean Difference (IV, Random, 95% CI) | 1.20 [0.00, 2.40] |
| 10.2 Vegatable intake at 6 months | 1 | 69 | Mean Difference (IV, Random, 95% CI) | 0.80 [‐0.37, 1.97] |
| 10.3 Vegatable intake at 12 months | 1 | 59 | Mean Difference (IV, Random, 95% CI) | 0.30 [‐0.85, 1.45] |
| 11 Vegatable servings: breast cancer | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 11.1 Vegetable intake at 3 months | 1 | 30 | Mean Difference (IV, Random, 95% CI) | 3.7 [2.64, 4.76] |
| 11.2 Vegetable intake at 6 months | 1 | 3088 | Mean Difference (IV, Random, 95% CI) | 4.5 [4.49, 4.51] |
| 11.3 Vegetable intake at 12 months | 1 | 3088 | Mean Difference (IV, Random, 95% CI) | 3.90 [3.89, 3.91] |
| 12 Mean fruit and vegetable servings (per day) | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 12.1 Fruit and vegetable servings (per day) at 3 months | 1 | 67 | Mean Difference (IV, Random, 95% CI) | 1.20 [‐0.24, 2.64] |
| 12.2 Fruit and vegetable servings (per day) at 6 months | 3 | 276 | Mean Difference (IV, Random, 95% CI) | 0.45 [‐0.04, 0.94] |
| 12.3 Fruit and vegetable servings (per day) at 12 months | 5 | 834 | Mean Difference (IV, Random, 95% CI) | 0.41 [0.10, 0.71] |
| 13 Adjusted mean fruit and vegetable servings (per day) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 13.1 Fruit and vegetable servings (per day) at 3 months | 1 | 67 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.91, 1.31] |
| 13.2 Fruit and vegetable servings (per day) at 6 months | 1 | 61 | Mean Difference (IV, Random, 95% CI) | 1.10 [‐0.01, 2.21] |
| 14 Fibre | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 14.1 Fibre intake at 3 months | 1 | 18 | Mean Difference (IV, Random, 95% CI) | 6.0 [0.73, 11.27] |
| 14.2 Fibre intake at 6 months | 2 | 3127 | Mean Difference (IV, Random, 95% CI) | 4.79 [‐4.72, 14.29] |
| 14.3 Fibre intake at 12 months | 2 | 3127 | Mean Difference (IV, Random, 95% CI) | 5.12 [‐0.66, 10.90] |
| 15 Fibre intake in participants on weight reduction | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 15.1 6 months | 1 | 39 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐3.52, 3.12] |
| 15.2 12 months | 1 | 39 | Mean Difference (IV, Random, 95% CI) | 2.10 [0.24, 3.96] |
| 16 Fibre intake in participants advised on health eating | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 16.1 6 months | 1 | 3088 | Mean Difference (IV, Random, 95% CI) | 9.5 [8.52, 10.48] |
| 16.2 12 months | 1 | 3088 | Mean Difference (IV, Random, 95% CI) | 8.0 [7.30, 8.70] |
| 17 Diet Quality Index | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 17.1 Diet Quality Index at 6 months | 1 | 182 | Mean Difference (IV, Random, 95% CI) | 5.20 [1.04, 9.36] |
| 17.2 Diet Quality Index at 12 months | 3 | 747 | Mean Difference (IV, Random, 95% CI) | 3.46 [1.54, 5.38] |
| 18 Diet Quality Index | 1 | 45 | Std. Mean Difference (IV, Random, 95% CI) | 0.90 [0.29, 1.52] |
2.2. Analysis.
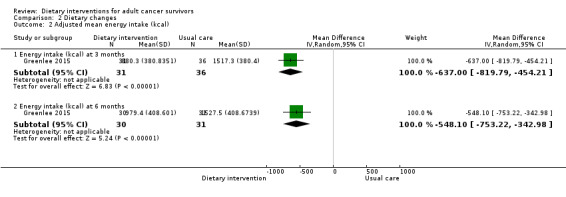
Comparison 2 Dietary changes, Outcome 2 Adjusted mean energy intake (kcal).
2.3. Analysis.
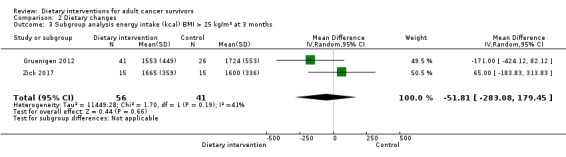
Comparison 2 Dietary changes, Outcome 3 Subgroup analysis energy intake (kcal) BMI > 25 kg/m² at 3 months.
2.4. Analysis.

Comparison 2 Dietary changes, Outcome 4 Subgroup analysis energy intake (kcal) BMI > 25 kg/m² at 6 months.
2.6. Analysis.
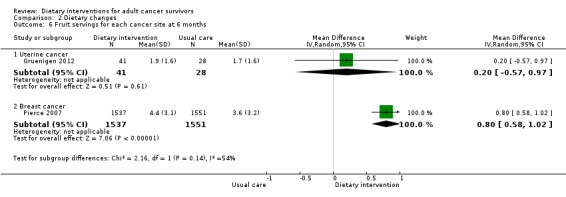
Comparison 2 Dietary changes, Outcome 6 Fruit servings for each cancer site at 6 months.
2.7. Analysis.
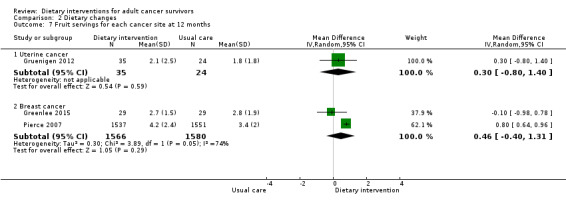
Comparison 2 Dietary changes, Outcome 7 Fruit servings for each cancer site at 12 months.
2.8. Analysis.
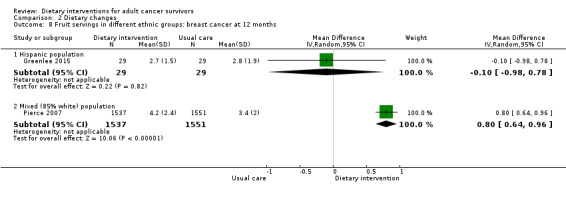
Comparison 2 Dietary changes, Outcome 8 Fruit servings in different ethnic groups: breast cancer at 12 months.
2.13. Analysis.

Comparison 2 Dietary changes, Outcome 13 Adjusted mean fruit and vegetable servings (per day).
2.15. Analysis.
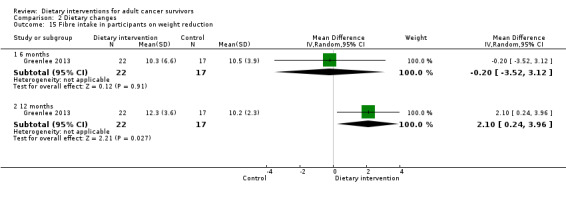
Comparison 2 Dietary changes, Outcome 15 Fibre intake in participants on weight reduction.
2.16. Analysis.
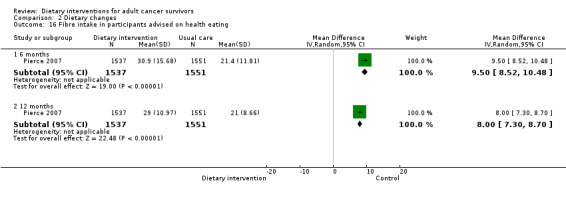
Comparison 2 Dietary changes, Outcome 16 Fibre intake in participants advised on health eating.
Comparison 3. Changes in anthropometry.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean weight (kg) | 10 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Weight at 3 months | 6 | 247 | Mean Difference (IV, Random, 95% CI) | ‐3.52 [‐7.34, 0.29] |
| 1.2 Weight at 6 months | 3 | 190 | Mean Difference (IV, Random, 95% CI) | ‐2.84 [‐6.95, 1.28] |
| 1.3 Weight at 12 months | 5 | 3287 | Mean Difference (IV, Random, 95% CI) | ‐0.80 [‐2.01, 0.41] |
| 2 Adjusted mean weight (kg) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Weight at 6 months | 1 | 55 | Mean Difference (IV, Random, 95% CI) | ‐7.0 [‐17.40, 3.40] |
| 3 Weight change | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Weight change at 6 months | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | ‐3.6 [‐5.56, ‐1.64] |
| 3.2 Weight change at 12 months | 1 | 641 | Mean Difference (IV, Fixed, 95% CI) | 0.23 [‐0.31, 0.77] |
| 4 Adjusted mean body mass index (kg/m²) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Body mass index at 6 months | 1 | 53 | Mean Difference (IV, Random, 95% CI) | ‐4.10 [‐8.12, ‐0.08] |
| 5 Mean body mass index (kg/m²) | 10 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Body mass index at 3 months | 6 | 247 | Mean Difference (IV, Random, 95% CI) | ‐1.80 [‐2.95, ‐0.65] |
| 5.2 Body mass index at 6 months | 1 | 168 | Mean Difference (IV, Random, 95% CI) | ‐0.70 [‐2.26, 0.86] |
| 5.3 Body mass index at 12 months | 4 | 777 | Mean Difference (IV, Random, 95% CI) | ‐0.79 [‐1.50, ‐0.07] |
| 6 Mean difference body mass index (kg/m²) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 Body mass index at 6 months | 1 | 410 | Mean Difference (IV, Fixed, 95% CI) | ‐0.6 [‐1.15, ‐0.05] |
| 6.2 Body mass index at 12 months | 2 | 1051 | Mean Difference (IV, Fixed, 95% CI) | ‐0.44 [‐0.64, ‐0.25] |
| 7 Weight loss | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 Weight loss at 3 months | 1 | 170 | Mean Difference (IV, Random, 95% CI) | 1.0 [‐0.47, 2.47] |
| 7.2 Weight loss at 6 months | 1 | 170 | Mean Difference (IV, Random, 95% CI) | ‐1.60 [‐3.06, ‐0.14] |
| 8 Lean body tissue | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.1 Lean body mass at 3 months | 1 | 76 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐2.41, 1.81] |
| 9 Body fat percentage | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 9.1 Body fat percentage at 3 months | 2 | 99 | Mean Difference (IV, Random, 95% CI) | ‐4.97 [‐7.47, ‐2.48] |
| 10 Change in body fat percentage | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 10.1 Change in body fat percentage at 6 months | 1 | 39 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.05, 0.03] |
| 11 Mean waist‐to‐hip ratio | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 11.1 Waist‐to‐hip ratio at 3 months | 5 | 181 | Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.06, 0.01] |
| 11.2 Waist‐to‐hip ratio at 6 months | 2 | 118 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.05, 0.01] |
| 11.3 Waist‐to‐hip ratio at 12 months | 2 | 106 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.04, 0.02] |
| 12 Waist‐to‐hip ratio for each cancer site at 3 months | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 12.1 Colon cancer | 1 | 18 | Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.17, ‐0.05] |
| 12.2 Breast cancer | 4 | 163 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.02, 0.01] |
| 13 Hip circumference change scores | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 13.1 Hip circumference change at 3 months | 1 | 44 | Mean Difference (IV, Random, 95% CI) | 1.81 [0.77, 2.85] |
| 13.2 Hip circumference change at 6 months | 2 | 134 | Mean Difference (IV, Random, 95% CI) | ‐3.13 [‐5.01, ‐1.26] |
| 13.3 Hip circumference change at 12 months | 1 | 44 | Mean Difference (IV, Random, 95% CI) | 1.09 [‐1.69, 3.87] |
| 14 Waist circumference change scores | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 14.1 Waist circumference change at 3 months | 1 | 44 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.67 [‐1.28, ‐0.06] |
| 14.2 Waist circumference change at 6 months | 2 | 134 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.67 [‐1.02, ‐0.32] |
| 14.3 Waist circumference change at 12 months | 1 | 44 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.74, 0.44] |
| 15 Adjusted mean waist circumference (cm) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 15.1 Waist circumference at 6 months | 1 | 50 | Mean Difference (IV, Random, 95% CI) | ‐3.90 [‐11.11, 3.31] |
| 16 Mean hip circumference (cm) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 16.1 Hip circumference at 3 months | 1 | 76 | Mean Difference (IV, Random, 95% CI) | ‐5.40 [‐10.06, ‐0.74] |
| 16.2 Hip circumference at 6 months | 1 | 50 | Mean Difference (IV, Random, 95% CI) | ‐3.20 [‐10.96, 4.56] |
| 16.3 Hip circumference at 12 months | 1 | 49 | Mean Difference (IV, Random, 95% CI) | ‐4.0 [‐11.43, 3.43] |
| 17 Mean waist circumference (cm) | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 17.1 Waist ratio at 3 months | 2 | 143 | Mean Difference (IV, Random, 95% CI) | ‐4.00 [‐12.53, 4.53] |
| 17.2 Waist ratio at 6 months | 2 | 109 | Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐4.79, 4.14] |
| 17.3 Waist ratio at 12 months | 3 | 148 | Mean Difference (IV, Random, 95% CI) | ‐3.36 [‐7.55, 0.82] |
3.2. Analysis.

Comparison 3 Changes in anthropometry, Outcome 2 Adjusted mean weight (kg).
3.3. Analysis.
Comparison 3 Changes in anthropometry, Outcome 3 Weight change.
3.4. Analysis.
Comparison 3 Changes in anthropometry, Outcome 4 Adjusted mean body mass index (kg/m²).
3.6. Analysis.
Comparison 3 Changes in anthropometry, Outcome 6 Mean difference body mass index (kg/m²).
3.7. Analysis.
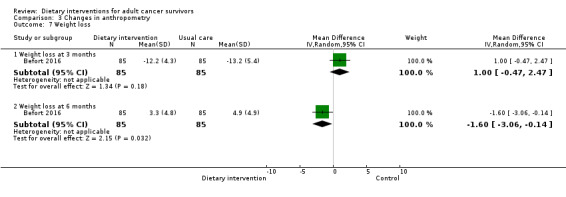
Comparison 3 Changes in anthropometry, Outcome 7 Weight loss.
3.12. Analysis.

Comparison 3 Changes in anthropometry, Outcome 12 Waist‐to‐hip ratio for each cancer site at 3 months.
3.13. Analysis.
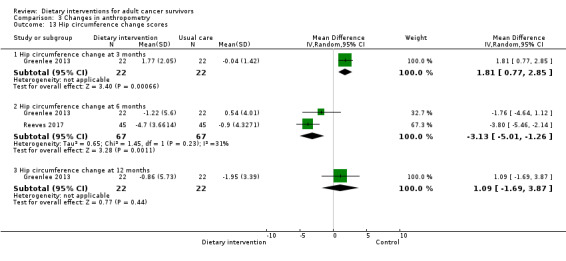
Comparison 3 Changes in anthropometry, Outcome 13 Hip circumference change scores.
3.14. Analysis.
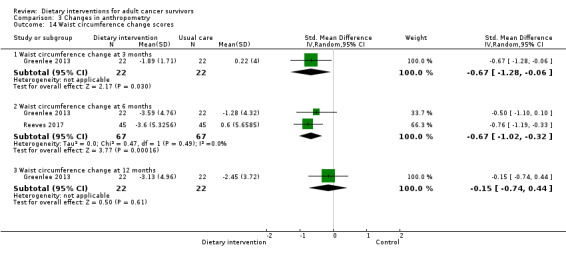
Comparison 3 Changes in anthropometry, Outcome 14 Waist circumference change scores.
3.15. Analysis.
Comparison 3 Changes in anthropometry, Outcome 15 Adjusted mean waist circumference (cm).
Comparison 4. Quality of Life.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FACT‐G QoL | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 FACT‐G at 6 months | 1 | 168 | Mean Difference (IV, Fixed, 95% CI) | ‐2.5 [‐5.80, 0.80] |
| 1.2 FACT‐G QoL at 12 months | 2 | 685 | Mean Difference (IV, Fixed, 95% CI) | ‐0.52 [‐2.31, 1.26] |
| 2 FACT‐C QoL | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 FACT‐C at 3 months | 1 | 18 | Mean Difference (IV, Random, 95% CI) | 14.0 [2.87, 25.13] |
| 3 FACT‐B QoL | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 FACT‐B at 3 months | 1 | 23 | Mean Difference (IV, Fixed, 95% CI) | 15.60 [‐4.96, 36.16] |
| 3.2 FACT‐B at 6 months | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | 4.90 [‐0.80, 10.60] |
| 4 SF‐36 QoL physical function score | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 SF‐36 QoL at 6 months | 1 | 168 | Mean Difference (IV, Random, 95% CI) | 2.20 [‐4.62, 9.02] |
| 4.2 SF‐36 QoL at 12 months | 1 | 160 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐7.42, 7.02] |
| 5 SF‐36 QoL mean change physical | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 SF‐36 at 6 months | 2 | 500 | Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐1.59, 1.28] |
| 5.2 SF‐36 at 12 months | 3 | 1091 | Mean Difference (IV, Random, 95% CI) | 1.91 [0.45, 3.37] |
| 6 SF‐36 QoL mean change mental | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 SF‐36 mean change at at 6 months | 1 | 410 | Mean Difference (IV, Random, 95% CI) | 0.7 [‐0.96, 2.36] |
| 6.2 SF‐36 mean change at 12 months | 3 | 1091 | Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐3.29, 3.08] |
| 7 SF‐36 QoL mean change mental for each cancer site at 12 months | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 Breast cancer | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐4.3 [‐9.69, 1.09] |
| 7.2 Colorectal cancer | 1 | 410 | Mean Difference (IV, Random, 95% CI) | ‐0.7 [‐2.64, 1.24] |
| 7.3 Breast, colorectal, and prostate cancer | 1 | 641 | Mean Difference (IV, Random, 95% CI) | 7.04 [5.25, 8.83] |
| 8 Cancer‐related fatigue | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 Cancer‐related fatigue at 6 months | 1 | 410 | Mean Difference (IV, Fixed, 95% CI) | 1.4 [‐0.26, 3.06] |
| 8.2 Cancer related fatigue at 12 months | 1 | 410 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐0.81, 2.81] |
| 9 Global QoL questionnaire | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 9.1 Global QoL at 3 months | 2 | 220 | Mean Difference (IV, Random, 95% CI) | 4.71 [2.22, 7.21] |
| 9.2 Global QoL at 6 months | 1 | 80 | Mean Difference (IV, Random, 95% CI) | 33.97 [28.97, 38.97] |
| 9.3 Global QoL at 12 months | 1 | 174 | Mean Difference (IV, Random, 95% CI) | 4.80 [‐0.79, 10.39] |
4.4. Analysis.
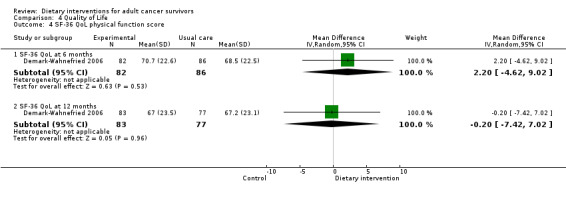
Comparison 4 Quality of Life, Outcome 4 SF‐36 QoL physical function score.
4.7. Analysis.
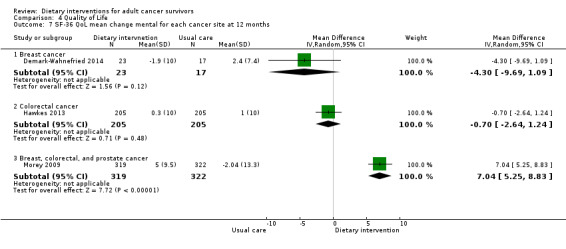
Comparison 4 Quality of Life, Outcome 7 SF‐36 QoL mean change mental for each cancer site at 12 months.
Comparison 5. Biochemical measures.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total cholesterol (mg/dl) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Total cholesterol at 3 months | 1 | 76 | Mean Difference (IV, Random, 95% CI) | ‐18.20 [‐38.27, 1.87] |
| 1.2 Total cholesterol at 6 months | 1 | 83 | Mean Difference (IV, Random, 95% CI) | 5.94 [2.45, 9.43] |
| 2 Triglycerides (mg/dl) at 3 months | 1 | 76 | Mean Difference (IV, Random, 95% CI) | ‐40.60 [‐76.80, ‐4.40] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Befort 2016.
| Methods |
Design: 2‐phase randomised controlled trial Country: USA Accrual dates: NR Trial Reg: NR |
|
| Participants |
Number randomised: 172 721 screened, 505 excluded; 216 attended study orientation visit, 6 excluded; 210 entered weight loss intervention, 38 excluded; 87 assigned to group phone counselling; 85 assigned to newsletter; 76 assessed at month 18 in the phone counselling group; 78 assessed at month 18 in the newsletter group; 85 included in the primary analysis in the phone counselling group; 83 included in primary analysis in the newsletter group Inclusion: post‐menopausal breast cancer survivors. Residing in rural areas. BMI 27 to 45 kg/m². Age 75 years or younger. Diagnosis of stage 0 to IIIc disease within the last 10 years. Completion of treatment at least 3 months before study entry. Clearance from oncologist given Exclusion: pending joint replacement; serious cardiac or pulmonary condition; insulin‐dependent diabetes; history of bariatric surgery; current substance abuse; major depression; binge eating disorder Gender: female Age, mean: 58 (SD 8.1) years Type of cancer: breast Therapy previously received for cancer: anti‐hormone 55.8%; surgery, lumpectomy 61.0%; surgery, mastectomy 48.8%; chemo 67.4%; radiation 72.1% Cancer stage: stage 0: 8.1%, stage I: 44.2%, stage II: 33.7%, stage III: 14.0% Ethnicity: Caucasian 99.4% Baseline physical activity: NR Education: high school 21.5%, some college 41.9%, bachelors degree 20.9%, masters/doctorate 15.7% |
|
| Interventions |
Comparison: lifestyle bi‐weekly group phone‐based counselling vs mailed newsletters Intervention: phase 1: 25‐week 60‐minute conference call sessions conducted by a registered dietitian or psychologist, delivered to groups of 12 to 15 women at a time; phase 2: 26 bi‐weekly conference call sessions with the same counsellor Control: phase 1: as above; phase 2: 9 newsletters |
|
| Outcomes |
Weight change from 6 to 18 months: weighed in light clothing in a fasting state using calibrated scales Program and participant costs and incremental cost‐effectiveness ratios: counsellor tracked time ‐ participant time spent in sessions, self‐monitoring, and reading materials Duration of follow‐up: 18 months |
|
| Notes | Funding: NIH/NCI R01CA155014 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Study statistician generated the randomisation |
| Allocation concealment (selection bias) | Low risk | Randomisation was blinded from both participants and research staff until the beginning of phase 2 |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Two‐phase study with diet and physical activity states blinded for phase 1 was undertaken, but as it was a diet and exercise intervention, it is difficult to see how this was applied logistically. However, outcome is not likely to be influenced by lack of complete blinding |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Unclear risk | No subjective outcomes |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Outcome is not likely to be influenced by lack of complete blinding |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | No subjective outcomes |
| Incomplete outcome data (attrition bias) Objective | Low risk | Attrition was 2% and attrition was similar in both groups |
| Incomplete outcome data (attrition bias) Subjective | Unclear risk | No subjective outcomes |
| Selective reporting (reporting bias) | Low risk | All primary outcomes reported |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Bloom 2008.
| Methods |
Design: randomised trial pre‐post design Country: USA Accrual dates: NR Trial Reg: NR |
|
| Participants |
Number randomised: 404 940 women were screened, and 194 were ineligible, mainly due to cancer recurrence or new cancer. From 746 who were eligible, 27 could not be scheduled for interview, 315 refused, and 260 were interested in participating but could not commit to the requirements of the study Inclusion: women who had been cancer‐free for 5 years after diagnosis of breast cancer; who were 50 years of age or younger at diagnosis and agreed to participate in a minimum of 2 monthly workshops and 2 interviews Exclusion: NR Age, years: 23 to 39: 13%; 40 to 44: 29%; 45 to 50: 58% Gender: female Type of cancer: breast Therapy previously received for cancer: chemotherapy only 21%, radiation only 21%, chemo and radio 34%, tamoxifen 39% Cancer stage: in situ 18%, local 54%, regional 26%, remote 1% Ethnicity: Euro‐American 76%, African American 5%, Latina 7%, Asian 10%, Other 2% Baseline physical activity: 77% of control group and 71% of intervention group exercised on 2 or more days per week for at least 30 minutes Education: 61% were college graduates; 80% were employed at least part time |
|
| Interventions |
Comparison: workshops vs delayed intervention Intervention: a series of three 6‐hour‐long workshops were conducted on Saturdays at 1‐month intervals. Through a variety of activities and presentations, each workshop addressed 4 cross‐cutting themes, including targeting unmet informational needs, promoting exercise and nutrition, improving communication skills, and providing and receiving emotional support. Workshops were delivered by trained health professionals, including a medical oncologist who specialised in breast cancer, a pharmacist, 2 attorneys, a gynaecologist, an exercise physiologist, a representative of the women’s healthy eating and living (WHEL) study, and a fitness instructor Control: women in the control group (delayed intervention) were invited to attend a 1‐day educational workshop following completion of the post‐test assessment |
|
| Outcomes |
Dietary changes: fruits and vegetables questionnaire and fat screeners questionnaire (Block, 1986 and 1990) Quality of life: changes in knowledge score and changes in communication No. of healthy eating changes made: survey on how many fruits and vegetables consumed each day, and how often non‐fat and low‐fat foods were consumed Duration of follow‐up: 6 months |
|
| Notes | Funding: NIH, NCI R01‐CA 078951 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk | No objective outcomes appropriate for this review |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | No blinding; outcome is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | No objective outcomes appropriate for this review |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | No blinding of outcome assessment; outcome measurement is likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) Objective | Unclear risk | No objective outcomes appropriate for this review |
| Incomplete outcome data (attrition bias) Subjective | Low risk | 4% loss to follow‐up, leaving a sample of 387/404. ITT was used |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Study authors refer to some women also being involved in a previous dietary study |
Bourke 2011.
| Methods |
Design: prospective, randomised, controlled pilot trial Country: UK Accrual dates: NR Trial Reg: NR |
|
| Participants |
Number randomised: 18 180 participants were identified through nurse‐led follow‐up clinics; 29 responded as interested, 11 were screened out due to ineligibility, 9 were randomised to intervention, 9 were randomised to control, 1 was lost to follow‐up Inclusion: histologically confirmed colon cancer, which had been resected 6 to 24 months previously Exclusion: existing participation in regular physical activity; Karnofsky rating < 80; unstable angina; uncontrolled hypertension; recent myocardial infarction or a pacemaker Age, years (SD): 52 to 80 (69) Intervention arm: 67.9 (5.7) Control arm: 70.3 (8.7) Gender: Intervention: 5 male, 4 female Control: 7 male, 2 female Ethnicity: NR Type of cancer: colon Therapy previously received for cancer: surgery: n = 17, chemotherapy: n = 6, palliative care: n = 1 Cancer stage: NR Baseline physical activity: chair sit‐to‐stand reps Intervention arm: 10 median (range 8 to 16) Control arm: 10 median (range 7 to 16) Education: NR |
|
| Interventions |
Comparison: supervised and home‐based exercise with dietary advice vs cancer follow‐up service Intervention: 12‐week lifestyle intervention made up of supervised and home‐based exercise sessions and dietary advice. Exercise sessions took place within a dedicated exercise suite at Sheffield Hallam University and were supervised by an experienced exercise physiologist. During the first 6 weeks, participants attended 2 group‐based supervised exercise sessions per week. During the final 6 weeks, participants attended the university facility once a week and were asked to perform 2 home‐based exercise sessions a week. Participants were also provided with a nutrition advice info pack on a fortnightly basis throughout the intervention and engaged in healthy eating seminars in a group format Control: holistic nurse‐led colorectal cancer follow‐up service |
|
| Outcomes |
Dietary changes: 3‐day diaries kept by the participant to self‐report food intake Changes in weight/anthropometry: weight, height, BMI, and hip‐to‐waist ratio measured by exercise physiologist Quality of life: FACT‐C used to assess disease‐specific QoL Duration of follow‐up: 12 weeks |
|
| Notes | Funding: eNIHR Cardiovascular Biomedical Research Unit | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomised by an independent researcher via code numbers using nQuery statistical software |
| Allocation concealment (selection bias) | Low risk | Randomisation was undertaken by a senior academic who was not directly involved in recruitment or assessment of patients. The randomisation sequence was not disclosed to the researcher responsible for day‐to‐day running of the trial until patients had completed baseline assessments |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | No blinding; outcome is not likely to be influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | No blinding; outcome is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Assessor of outcomes was blinded to group allocation |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | No blinding of outcome assessment; outcome measurement was patient self‐reported and therefore is likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) Objective | Low risk | Only 1 person dropped out due to stroke (6% attrition). ITT was used |
| Incomplete outcome data (attrition bias) Subjective | Low risk | Only 1 person dropped out due to stroke (6% attrition). ITT was used |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information |
Demark‐Wahnefried 2006.
| Methods |
Design: randomised controlled trial Country: USA Accrual dates: NR Trial Reg: NR |
|
| Participants |
Number randomised: 182 3290 identified, 2431 cases with complete physician and contact data, 2037 approved for contact, 688 consented to participate, 506 ineligible, 182 enrolled into study and completed telephone interview, 93 randomised to control arm, 89 randomised to intervention arm, 168 completed 6‐month follow‐up telephone interview, subset of 33 completed performance tests and anthropometrics, 160 completed 12‐month follow‐up phone call, subset of 31 consisted of 22 dropouts, included in ITT Inclusion criteria and exclusion criteria: patients were excluded if conditions precluded unsupervised exercise (uncontrolled congestive heart failure or angina, recent myocardial infarction, or breathing difficulties requiring oxygen use or hospitalisation; use of a mobility aid other than a cane; plans to have hip or knee replacement) or a high fruit and vegetable diet (kidney failure or chronic warfarin use); had progressive malignant disease or additional primary tumours; were unable to participate fully in telephone counselling or in mailed material interventions (severe hearing or speaking impairment, inability to speak/write English, or mental incompetence); reported fewer than 2 physical function deficits (unlikely to experience change in physical function); were already routinely exercising or adhering to a low‐fat, high‐fruit and vegetable diet Age: intervention: 71.5 mean (SD 4.4), control: 71.9 mean (SD 5.6) years Gender: Female: intervention: n = 51 (57.3%), control: n = 53 (57.0%) Male: intervention: n = 38 (42.7%), control: n = 40 (43%) Ethnicity: White: intervention: n = 73 (82.0%), control: n = 77 (82.8%) African American: intervention: n = 13 (14.6%), control: n = 14 (15.0%) Other/Unknown: intervention: n = 3 (3.4%), control: n = 2 (2.2%) Type of cancer: breast or prostate Therapy previously received for cancer: NR Cancer stage: NR Baseline physical activity: NR Education: NR |
|
| Interventions |
Comparison: diet and exercise counselling and tailored materials vs general health counselling Intervention: telephone counselling and tailored print materials aimed at increasing exercise and improving overall diet Control: general health counselling and materials |
|
| Outcomes |
Physical function: SF‐36 Diet: Diet Quality Index (DQI) from 3‐day dietary recalls Weight and height: self‐reported QoL: functional assessment of cancer therapy breast and prostate quality of life instrument Duration of follow‐up: 12‐months |
|
| Notes | Funding: supported by Grants No. AG11268 and CA106919 from the National Institutes of Health, the Mary Duke Biddle Foundation, Grant No. NR07795 (E.C.C.), and the Susan G. Komen Foundation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Eligible participants were block randomly assigned to the study |
| Allocation concealment (selection bias) | Unclear risk | Details on how this was done were not reported |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk | No blinding, but the intervention would have been difficult to blind |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | No blinding; outcome was patient self‐reported and therefore is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Objective outcomes | High risk | No blinding of outcome assessment; outcome measurement was patient self‐reported and therefore is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | No blinding of outcome assessment; outcome measurement was patient self‐reported and therefore is likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) Objective | High risk | 22% lost to follow‐up, leaving a sample of 160/182. ITT was used |
| Incomplete outcome data (attrition bias) Subjective | High risk | 22% lost to follow‐up, leaving a sample of 160/182. ITT was used |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported |
| Other bias | Unclear risk | Insufficient information for judgement |
Demark‐Wahnefried 2007.
| Methods |
Design: randomised, single‐blinded, parallel‐group, attention‐control active treatment‐controlled phase II clinical trial Country: USA Accrual dates: July 2002 to October 2005 Trial Reg: NR |
|
| Participants |
Number randomised: 543 Out of 2155, 543 were eligible (1612 excluded), 271 were randomised to the intervention arm, and 272 were randomised to the control arm At 1‐year follow‐up, 519 were included in the analysis: 18 in the intervention arm were lost to follow‐up, and 6 in the control arm were lost to follow‐up. 271 in the intervention arm and 272 in the control arm were included in 1‐year follow‐up ITT analysis At 2‐year follow‐up, 489 were included in the analysis: from 1‐year to 2‐year follow‐up, an additional 30 were lost to death, illness, no longer wanting to participate, and unable to be contacted Inclusion: early‐stage (in situ, localised, or regional) breast and prostate cancer, identified within 9 months of diagnosis Exclusion: conditions precluding unsupervised exercise (i.e. uncontrolled congestive heart failure or angina, recent myocardial infarction, or breathing difficulties requiring oxygen use or hospitalisation; walker or wheelchair use; plans to have hip or knee replacement); conditions precluding a high fruit and vegetable diet (kidney failure or chronic warfarin use); progressive cancer or additional primary tumour; non‐English speakers/writers Age: range 22 to 85, 57 mean (SD 10.8) years Gender: 44% male, 56% female from all who responded; 29% of respondents were ineligible Type of cancer: breast and prostate Therapy previously received for cancer: 44% radiation therapy including brachytherapy, 27% chemotherapy, 85% surgery Cancer stage: Breast: 8% stage 0, 29% stage I, 17% stage II, 3% stage III Prostate: 17% stage 0, 23% stage II, 3% unknown Ethnicity: 83% white; 13% black; 4% other Baseline physical activity: minutes of activity per week Intervention: 53.4 mean (SD 112.7) Control: 44.6 mean (SD 89.1) Education: 12% < high school graduate, 30% some college or associate experience, 58% college graduate/post graduate |
|
| Interventions |
Comparison: telephone counselling and tailored print materials on diet and exercise vs general health counselling Intervention: participants received sequentially tailored mailed materials, in 2 behavioural domains. These were consistent with the transtheoretical model, and health messages were customised to the participant’s stage of readiness to promote behaviour change Control: participants received standardised mailed materials on improving cancer survivors’ diet and exercise behaviours. The intervention was delivered over 10 months and involved personalised mailed printed materials promoting fruit and vegetable consumption followed by 7 newsletters at 6‐weekly intervals |
|
| Outcomes |
Dietary changes: telephone interviews using diet history questionnaire (Diet Quality Index score, total percentage of calories from fat, total percentage of calories from saturated fat, number of daily servings of fruits and vegetables) Changes in BMI: telephone interview using self‐reported data (kg/m²). Measured heights and weights performed on a 23% subsample using wall mounted stadiometer and calibrated platform scales (weight expressed as BMI; kg/m²) QoL: telephone interview using Functional Assessment of Cancer Therapy ‐ General (FACT‐G) score Biochemical measures: phlebotomy performed on a 23% subsample after a 4‐hour fast. Analysis of HDL cholesterol (mg/dL), C‐reactive protein, insulin, IL‐6, and alpha‐carotene No. of healthy eating changes made: telephone interview No. of lifestyle behaviour changes made: telephone interview Changes in level of physical activity: telephone interview using 7‐day physical activity recall (minutes per week) No. of adverse events: toll‐free number provided for participants to ring and record events at any time. Events categorised by a committee blinded to random assignment status as serious (life‐threatening, permanently debilitating, or requiring hospitalisation overnight) or non‐serious (all other events) Participants' opinion on helpfulness of the intervention: telephone interview using 5‐point Likert scale from completely to not at all helpful (no direction stated – assume 1 is completely helpful) Duration of follow‐up: 24 months |
|
| Notes | Funding: National Institutes of Health, American Institute of Cancer Research, Susan G. Komen Foundation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random assignment lists were generated by a project statistician using software of the Cancer and Leukaemia Group B |
| Allocation concealment (selection bias) | Low risk | Implemented in a blinded fashion at an office that was physically removed from the main study office |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk | No objective outcomes appropriate for this review |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | No blinding; outcome was patient self‐reported and therefore is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | No objective outcomes appropriate for this review |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | No blinding of outcome assessment; outcome measurement was patient self‐reported and therefore is likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) Objective | Unclear risk | No objective outcomes appropriate for this review |
| Incomplete outcome data (attrition bias) Subjective | Low risk | Attrition was low ‐ 4.4% at 1 year and 10% at 2 years. ITT was used in the final analysis, inputting no change in behaviour across time for dropouts |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | None |
Demark‐Wahnefried 2014.
| Methods |
Design: 2‐centred, single‐blinded, parallel‐group Country: USA Accrual dates: October 2007 to October 2009 Trial Reg: NCT00630591 |
|
| Participants | Overweight or obese post‐menopausal survivors of breast cancer and their overweight or obese adult daughters from USA Number randomised: 68 dyads 2517 eligible breast cancer survivors identified, 2306 gained permission to mail out a study invitation, 2244 living cases had a contactable/usable address, 139 responded and had eligible daughters – further invitation sent, 85 dyads met inclusion criteria and were still interested – sent full study consent, 70 dyads provided consent, 18 dyads in control arm, 25 dyads in individually tailored arm, 25 dyads in team tailored arm, 59 dyads completed 6‐month follow‐up, 63 dyads completed 12‐month follow‐up Inclusion and Exclusion: women diagnosed with AJCC stage 0 to III breast cancer who had completed primary treatment but were within 5 years of diagnosis with no evidence of progressive disease or second primary tumours, and who had a biological daughter and were aged > 21 years. Both mother and daughter had to meet the following criteria: BMI 25 to 39.9 kg/m². No pre‐existing medical conditions that would preclude adherence to the intervention. Ability to speak and write English. Community dwelling in the USA, Puerto Rico, or Guam. Not currently exercising for at least 150 minutes. Not enrolled in any other weight loss programmes Gender: female Age: mothers: 61.3 mean (SD 7.4) years Ethnicity: non‐Hispanic white: n = 100 (74%), Hispanic white: n = 10 (7%), African American: n = 24 (18%), Asian: n = 2 (1%) Type of cancer: breast Cancer stage: stage 0: n = 12 (18%), stage I: n = 29 (43%), stage II: n = 21 (31%), stage III: n = 3 (4%), missing: n = 3 (4%) Baseline physical activity: Mothers in: control arm = 31.9 min/week, individual arm = 39.8 min/week, team arm = 32.4 min/week Education: Mothers: less than high school: n = 1 (1.5%), high school graduate: n = 18 (26.9), some college: n = 25 (37.3%), college graduate: n = 23 (34.3%) |
|
| Interventions |
Comparison: tailored diet and exercise delivered individually vs tailored diet and exercise that emphasised the mother‐daughter bond vs standard diet and exercise materials Intervention: mother‐daughter dyads were randomly assigned to (1) a tailored diet and exercise intervention that was delivered in parallel and individually to mothers and daughters (intervention arm); (2) a tailored diet and exercise intervention that emphasised the mother‐daughter bond in a team‐based approach (TEAM arm); or (3) an attention control arm that received standardised diet and exercise materials (control arm) Control: received a copy of the National Cancer Institute brochure, “Facing Forward,” and the American Institute for Cancer Research publication, “Facts on Weight Management and Cancer,” which were included in a personalised binder |
|
| Outcomes |
Dietary changes: 2‐part telephone interview to gather dietary data using the interactive Nutrition Data System revised software (NCC food and nutrient database system v.2006, Minneapolis) Changes in weight/anthropometry: physical measures of height, weight, BMI, and waist circumference Quality of life: 2‐part telephone interview using the SF‐36 No. of healthy eating changes made: 2‐part telephone interview to gather data on total energy intake; energy from fats, sugars, and alcohol; fruit and vegetable, whole grain, dairy, meat, sodium, and sat fat intake to derive a Healthy Eating Index Score Duration of follow‐up: 12 months |
|
| Notes | Funding: National Institutes of Health | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Dyads were randomly assigned by an off‐site statistician to 1 of the 3 arms, within strata defined based on the race of the mother (white/non‐white) |
| Allocation concealment (selection bias) | Low risk | Dyads were randomly assigned by an off‐site statistician to 1 of the 3 arms, within strata defined based on the race of the mother (white/non‐white) |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Single‐blind trial; however, no further detail is given. Outcome is not likely to be influenced by lack of complete blinding |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Single‐blind trial; however, no further detail is given. Outcome was patient self‐reported and therefore is likely to be influenced by lack of complete blinding |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Single‐blind trial; however, no further detail is given. Outcome is not likely to be influenced by lack of complete blinding |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Single‐blind trial; however, no further detail is given. Outcome measurement was patient self‐reported and therefore is likely to be influenced by lack of complete blinding |
| Incomplete outcome data (attrition bias) Objective | Low risk | 10% were lost to follow‐up, with very similar dropout between arms |
| Incomplete outcome data (attrition bias) Subjective | Low risk | 10% were lost to follow‐up, with very similar dropout between arms |
| Selective reporting (reporting bias) | Low risk | All primary outcomes reported |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Djuric 2002.
| Methods |
Design: randomised pilot study to test an individualised approach towards weight loss in obese women who have had a diagnosis of breast cancer Country: USA Accrual dates: NR Trial Reg: NR |
|
| Participants |
Number randomised: 48 13 in control arm, 10 in weight watchers arm, 13 in individualised arm, 11 in comprehensive arm, 9 dropped out over the first 12 months Inclusion and Exclusion: ages 18 to 70 years. Stage I or II breast cancer that had been diagnosed within the past 4 years. Free of any recurrence as confirmed by a physician. Chemotherapy or radiation therapy had to be completed at least 3 months previously with the exception of tamoxifen Age: range 36 to 70 years Gender: female Type of cancer: breast Therapy previously received for cancer: chemotherapy: n = 30 (63%), on tamoxifen at recruitment: n = 30 (63%) Cancer stage: stage I or II Baseline physical activity: NR Ethnicity: white: n = 35 (73%), African American: n = 12 (25%) Education: college graduate: n = 30 (63%), employed at recruitment: n = 42 (88%) |
|
| Interventions |
Comparison: participants were randomly assigned to 1 of 4 groups: control, weight watchers (WW), individualised counselling, or a combination of WW and Individualised counselling Intervention: WW arm: women were encouraged to attend WW meetings but received no other dietary or exercise advice Individualised arm: contacts by the dietitian were scheduled weekly for the first 3 months, biweekly for months 3 to 6, and monthly thereafter. All individual contacts were made by telephone appointment. A monthly group meeting was also arranged, which women were encouraged to attend. In addition, all women received a monthly packet of written information on various weight loss topics The counselling approach used the theoretical framework of Bandura's social‐cognitive theory Comprehensive arm: participants received individualised counselling and were also asked to attend weekly WW meetings One dietitian provided the counselling sessions. The dietitian had over 10 years of experience in weight loss counselling in clinical settings. Meetings were organised via weight watchers groups, which are well known internationally and follow their own standards and training Control: participants received the National Cancer Institute's “Action Guide to Healthy Eating” and “Food Guide Pyramid” pamphlets, but they received no other dietary or exercise advice. They met with a dietitian at baseline and at 3, 6, and 12 months for outcome assessments. Controls were allowed to follow a weight reduction diet on their own if desired |
|
| Outcomes |
Dietary changes: 3‐day food records were completed at baseline and at 3, 6, and 12 months. Food records were kept on forms that enumerated foods eaten, time of day, and amount eaten. A registered dietitian taught the women how to keep food diaries and how to estimate portion sizes. The records were reviewed together with the dietitian and the participant at each of the 4 data collection points. Nutrient calculations were performed using the Minnesota Nutrition Data System Research software Changes in weight/anthropometry: at baseline and at 3, 6, 9, and 12 months, women were weighed in clothing but without shoes using a professional beam scale, and percentage of body fat was measured using tetra‐polar bioelectrical impedance. Height was measured at baseline only Biochemical measure: information taken from Jen 2004 – at baseline and at 3, 6, and 12 months, fasting blood samples were obtained. Blood samples were centrifuged, and plasma was stored at ‐70 degrees centigrade until analyses were conducted for glucose, triglycerides, cholesterol, insulin, and leptin Duration of follow‐up: 30 months |
|
| Notes | Funding: Grant RO3 CA89761 from NIH; the weight watchers group, Farmington Hills, Michigan; and the Ford Motor Company fund | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned. Insufficient information about the sequence generation process to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | No blinding; outcome is not likely to be influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | No blinding; outcome was patient self‐reported and therefore is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | No blinding; outcome is not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | No blinding of outcome assessment; outcome measurement was patient self‐reported and therefore is likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) Objective | Low risk | Attrition was 19% and was similar across groups |
| Incomplete outcome data (attrition bias) Subjective | Low risk | Attrition rate was low |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Ghavami 2017.
| Methods |
Design: randomised controlled trial Country: Iran Accrual dates: 2012 to 2015 Trial Reg: NR |
|
| Participants |
Number: 80 No other details about eligibility and recruitment Inclusion: women with BMI > 25 and classified as disease stage I to III; must have completed breast cancer primary treatment (surgery, chemotherapy, radiotherapy) at least 3 months ago, and not more than 18 months ago; on Nolvadex (tamoxifen) and other endocrine treatments but not hormone replacement therapy (HRT). Must be willing and able to attend supervised exercise sessions at least 3 times per week for a period of 24 weeks, with the intention of achieving an 80% minimum compliance target for attendance. Must be adults (18 years and above); must be able to read and write in Persian. Must receive a certificate from a cardiologist to participate in exercise sessions Exclusion: metastatic breast cancer; inoperable or active loco‐regional disease. Following alternative/complementary diets or taking high‐dose antioxidant supplement. Physical/psychiatric impairment that would seriously impair physical mobility. Severe nausea, anorexia, or other disease affecting health (e.g. arthritis, multiple sclerosis). Use of HRT or oral contraceptives within the past 4 months (HRT is not commonly prescribed in women who are recovering from breast cancer treatment). Engaged in exercise at the beginning of study (2 or more times per week for at least 30 minutes per session during the previous 3 months). Unable for other reasons to continue to participate in research Gender: female Age: mean 48.99 (SD 9.42) years Ethnicity: Asian Type of cancer: breast Therapy previously received for cancer: chemotherapy with tamoxifen or radiation therapy Cancer stage: stage I, II, or III Baseline physical activity: NR Education: primary school n = 19; secondary school n = 7; high school n = 40; greater than high school n = 14 |
|
| Interventions |
Comparison: lifestyle intervention vs usual care Intervention: 12‐week individualised intervention promoting prescribed exercise and a balanced diet through stage‐matched telephone counselling and a workbook. Moderate exercise 3 to 5 days per week and individual healthy eating advice and written information Control: usual care with no specific details |
|
| Outcomes |
Quality of life: EORCT QLQ‐C30 30‐item questionnaire Duration of follow‐up: 12 months |
|
| Notes | Funding: NR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used a random numbers table |
| Allocation concealment (selection bias) | High risk | Used a random numbers table |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk | No objective outcomes |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Participants and personnel not blinded |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | No objective outcomes |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | No blinding; outcome is patient self‐report |
| Incomplete outcome data (attrition bias) Objective | Unclear risk | No objective outcomes |
| Incomplete outcome data (attrition bias) Subjective | Low risk | No patients lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available; unclear if there was selective reporting |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Greenlee 2013.
| Methods |
Design: pilot randomised controlled trial Country: USA Accrual dates: NR Trial Reg: NR |
|
| Participants |
Number randomised: 42 112 agreed to be screened, 70 not enrolled, 22 randomised to intervention (1 dropout), 20 randomised to control (3 dropouts), 38 retained for full 12 months Inclusion: age 21 to 70 years; self‐identified as Hispanic or of African descent (African American or Caribbean). Diagnosis of stage 0 to IIIa breast cancer. Completed surgery, chemotherapy, and radiation therapy at least 6 months before. No evidence of recurrent or metastatic disease. BMI > 25 kg/m². Sedentary (defined as physically active to the point of sweating < 20 minutes per week). Not actively engaged in a weight loss programme. Non‐smoker. Haemoglobin A1c < 8%; blood pressure < 140/90. Low‐density lipoprotein cholesterol < 150 mg/dL. Both pre‐menopausal and post‐menopausal women were included in this pilot study to explore the feasibility aspect of the study in both populations Exclusion: smokers because investigators held it was more important to engage these women in a smoking cessation programme than a weight loss programme. Uncontrolled diabetes, hypertension, and hypercholesterolaemia as a precaution to avoid exacerbation of underlying cardiac and/or metabolic conditions in an intervention that was in the early testing phase Age: intervention: mean 52.6 (SD 8.0); control: mean 48.6 (SD 9.6) years Gender: female Type of cancer: breast Treatment previously received for cancer (%): Intervention: 19.1 mastectomy, 81.0 lumpectomy, 76.2 lymph node dissection, 86.4 radiation, 81.8 chemotherapy, 18.2 tamoxifen, 45.5 aromatase Control: 45.0 mastectomy, 65.0 lumpectomy, 60.0 lymph node dissection, 65.0 radiation, 75.0 chemotherapy, 50.0 tamoxifen, 40.0 aromatase Cancer stage (%): intervention: 50.0 stage I, 27.3 stage II, 13.6 stage III. Control: 35.0 stage I, 40.0 stage II, 15.0 stage III Ethnicity: Intervention: 22.7% African descent, 77.2% Hispanic descent Control: 20.0% African descent, 80.0% Hispanic descent Baseline physical activity: Occupational index: intervention: 2.6 mean (SD 0.5), control: 2.8 mean (SD 0.5) Active living Index: intervention: 2.5 mean (SD 0.7), control : 2.8 mean (SD 0.5) Sports and exercise index: intervention: 1.5 mean (SD 0.2), control: 1.5 mean (SD 0.3) Physical fitness VO2 max (mL/kg/min): intervention: 17.9 mean (SD 3.1), control: 19 mean (SD 4.0) Education (%): less than high school: intervention 36.4, control 25; high school or GED: intervention 18.2, control 55; some college: intervention 13.6, control 10; college or higher: intervention 31.8, control 10 |
|
| Interventions |
Comparison: 6‐month Curves weight loss programme vs wait‐list control Intervention: participants received 6 months of the Curves weight loss programme, followed by 6 months of observation, during which they could engage in any diet and physical activity of their choice Control: in the wait‐list control arm, participants were observed for 6 months, during which they were asked not to change their physical activity or diet, followed by 6 months of the Curves programme. To allow women randomised into the immediate intervention arm to initiate participation in the nutrition courses as part of a group, randomisation occurred at 5 different time points throughout the course of the study |
|
| Outcomes |
Anthropometric measures: weight and height were measured using a calibrated electronic scale (SR Instruments, Tonowanda, NY) and stadiometer (Genentech Accustat, San Francisco, CA). Waist and hip circumferences were measured by trained study staff using a Gulick II tape measure (Country Technology, Gays Mills, WI) DEXA and body composition: body composition was measured by DEXA using the Hologic QDR 4500 densitometer (Hologic, Waltham, MA). Baseline and 6‐month measurements used the same densitometer, software, and scan speed Serum metabolic marker analyses: serum samples were analysed in batches after all samples were collected Cholesterol (total, high‐density lipoprotein, indirect low‐density lipoprotein), triglycerides, glucose, and hsCRP: measured via an Integra 400 Plus automated chemistry analyser (Roche Diagnostics, Indianapolis, IN). Radioimmunoassays were used to measure insulin (Siemens, Deerfield, IL), total ghrelin (LINCO Research, St. Charles, MO), and adiponectin (Millipore, Billerica, MA) Diet: dietary intake was assessed using the Spanish version of the Block Questionnaire, with text in both Spanish and English on each page (28). The original 110‐item Block Questionnaire was developed using NHANES dietary recall data, which include representation of African Americans. The Spanish version of the Block Questionnaire includes additional food items typical of diets among Hispanics Duration of follow‐up: 12 months |
|
| Notes | Funding: this research was supported by Gateway for Cancer Research (H.G.), Women At Risk (H.G), the Susan G. Komen Foundation (D.L.H.), and Grant Number UL1 RR024156 from the National Center for Research Resources, a component of the National Institutes of Health (NIH) and NIH Roadmap for Medical Research | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised via randomly permuted blocks |
| Allocation concealment (selection bias) | Low risk | Randomised via randomly permuted blocks |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | No blinding of participants or staff, but intervention delivered by an outside agency |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | No blinding; outcome was patient self‐reported and therefore is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | No blinding of outcome assessment; outcome measurement is not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | No blinding of outcome assessment; outcome measurement was patient self‐reported and therefore is likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) Objective | Low risk | Attrition was 9% and dropout from each group was similar. ITT was used |
| Incomplete outcome data (attrition bias) Subjective | Low risk | Attrition was 9% and dropout from each group was similar. ITT was used |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Greenlee 2015.
| Methods |
Design: randomised controlled trial Country: USA Accrual dates: January 2011 to March 2012 Trial Reg: NCT01414062 |
|
| Participants |
Number randomised: 70 405 identified, 156 did not meet inclusion criteria, 142 refused, 37 unable to contact, 34 allocated to intervention, 36 allocated to control, 4 lost to intervention follow‐up, 5 lost to control follow‐up, 30 analysed in intervention at 6 months, 31 analysed in control at 6 months Inclusion: Spanish‐speaking women. History of stage 0 to III breast cancer. At least 3 months post treatment. No evidence of metastatic disease. Age 21 years or older. Hispanic descent and fluent Spanish. No uncontrolled diabetes mellitus. No uncontrolled comorbidities. Non‐smoker. Mean intake of < 5 servings of fruits and vegetables per day. Access to a functional phone or cell phone. Not currently active in a dietary change programme Gender: female Age: intervention: mean 55.1 (SD 9.1), control: mean 58.0 (SD 10.1) years Type of cancer: breast Therapy received for cancer: Intervention: mastectomy 44.1%, radiation 70.6%, chemo 44.1%, anti‐hormonal 73.5% Control: mastectomy 44.4%, radiation 55.6%, chemo 52.8%, anti‐hormonal 72.2% Cancer stage: Intervention: ductal carcinoma in situ 35.3%, I 32.4%, II 14.7%, III 11.8%, locally advanced 5.9% Control: ductal carcinoma in situ 22.2%, I 44.4%, II 25.0%, III 2.8%, locally advanced 2.8% Ethnicity: Intervention: black 20.6%, white 41.2%, Native American 5.9%, mixed 14.7% Control: black 30.6%, white 38.9%, Native American 0.0%, mixed 16.7% Baseline physical activity: 519 mean (SD 584) minutes per week Education: Intervention: less than high school 35.5%, high school 32.4%, some college 14.7%, college degree or higher 14.7% Control: less than high school 33.3%, high school 19.4%, some college 41.7%, college degree or higher 5.6% |
|
| Interventions |
Comparison: 9 sessions of culturally based education focusing on nutrition, cooking, and food shopping vs written dietary recommendations Intervention: 9‐session nutritional intervention programme over 12 weeks Control: 22‐page Spanish‐language healthy eating for breast cancer survivors |
|
| Outcomes |
Biochemical measures: fasting blood Body composition: anthropometric measures Dietary intake: three 24‐hour recalls, interviewer‐administered diet questionnaire, telephone call every month to assess diet Duration of follow‐up: 12 months |
|
| Notes | Funding: National Cancer Institute R21CA152903 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation sequence was generated by the study biostatistician |
| Allocation concealment (selection bias) | Low risk | Randomisation was sealed in envelopes marked with a numerical code |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | No blinding; outcome is not likely to be influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Participants and personnel not blinded |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | No blinding; outcome is not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | No blinding; outcome was patient self‐reported and therefore is likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) Objective | Low risk | Attrition was 13% and was similar from each group (4 from intervention and 5 from control) |
| Incomplete outcome data (attrition bias) Subjective | Low risk | Attrition was 13% and was similar from each group (4 from intervention and 5 from control) |
| Selective reporting (reporting bias) | Low risk | All primary outcomes reported |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Gruenigen 2012.
| Methods |
Design: 2‐group randomised trial Country: USA Accrual dates: NR Trial Reg: NR |
|
| Participants |
Number randomised: 75 398 letters sent to patients from the tumour registry, 114 patients responded to the letter (28.6%), 17 were unable to commit to the longitudinal study, and 22 were not interested/unknown reason, 75 (18.9%) were eligible and consented, 41 were assigned to the intervention and 34 to the control. Adherence to the intervention was 84.1%, with 100% assessed at 3 months, 100% at 6 months, and 85.4% at 12 months. In the control group, 76.5% were assessed at 3 months, 82.4% at 6 months, and 70.6% at 12 months. Attrition in the overall trail was 21.3% Inclusion: women with histologically confirmed stage I or II EC. Had undergone surgery consisting of total abdominal hysterectomy, bilateral salpingo‐oophorectomy, with no evidence of disease. Diagnosed in the previous 3 years. Body mass index ≥ 25 (overweight/obese). Performance status 0 to 2. Medical clearance from primary care physician. Approval to contact from treating gynaecological oncologist Exclusion: individuals unable to read the consent form. Severe depression, dementia, or cognitive deficit. Unavailable for longitudinal follow‐up assessments. Pre‐existing medical condition that was a barrier to unsupervised walking Gender: female Age: intervention: 57.0 mean (SD 8.6) years, control: 58.9 mean (SD 10.9) years Ethnicity: Intervention: 87.8% Caucasian, 9.8% African American, 2.4% Other Control: 94.1% Caucasian, 2.9% African American, 2.9% Other Type of cancer: uterine cancer Therapy previously received for cancer: 39.0% in intervention group and 35.3% in control group had received prior radiation therapy Cancer stage: I and II Education: Intervention: 14.6% < high school, 9.8% 12th grade, 36.6% some college, 39.0% college graduate or higher Control: 11.8% < high school, 23.5% 12th grade, 23.5% some college, 41.2% college graduate or higher |
|
| Interventions |
Comparison: lifestyle intervention (SUCCEED) group that received nutrition, exercise, and behavioural modification counselling vs usual care (UC) group Intervention: 16 group sessions were conducted (10 weekly followed by 6 biweekly) in the SUCCEED group. Physician face‐to‐face counselling occurred at 3, 6, and 12 months. Additional support was provided from the dietitian in the form of newsletters, emails, and telephone calls Control: patients randomised to the usual care group received an information brochure ("Healthy Eating and Physical Activity Across Your Lifespan," "Better Health and You") |
|
| Outcomes |
Changes in weight/anthropometry: weight change at 12 months measured via scales Dietary changes: energy and fruit and vegetable intake measured by 2 (1 weekday, 1 weekend day) 24‐hour recalls Anthropometrics: measured by a registered dietitian with 24‐hour recalls carried out by trained interviewers Duration of follow‐up: 12 months |
|
| Notes | Funding: American Cancer Society | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was stratified using block sizes of 6 or 8 |
| Allocation concealment (selection bias) | Low risk | Randomisation was stratified using block sizes of 6 or 8 |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | No blinding; outcome is not likely to be influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | No blinding; outcome was patient self‐reported and therefore is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | No blinding; outcome is not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | No blinding of outcome assessment; outcome measurement is likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) Objective | High risk | Attrition was high at 21% and was uneven across groups ‐ 35/41 85% intervention and 24/34 71% control |
| Incomplete outcome data (attrition bias) Subjective | High risk | Attrition was high at 21% and was uneven across groups ‐ 35/41 85% intervention and 24/34 71% control |
| Selective reporting (reporting bias) | Unclear risk | All primary outcomes reported, but not all secondary outcomes |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Harrigan 2016.
| Methods |
Design: randomised controlled trial: 3 arms Country: USA Accrual dates: June 2011 to December 2012 Trial Reg: NR |
|
| Participants |
Number randomised: 100 825 screened via telephone, 429 ineligible, 296 not interested, 33 in‐person counselling, 34 telephone counselling, 33 usual care Inclusion: breast cancer survivors with BMI > 25 kg/m², diagnosed in the 5 years before enrolment with stage 0 to 3 breast cancer, had completed chemotherapy and/or radiation therapy at least 3 months before enrolment. Physically able to exercise, agree to be randomly assigned, give informed consent to participate in all study activities. Had to be accessible by phone and English literate Exclusion: women who were pregnant or intending to become pregnant in the next year, recent stroke or myocardial infarction, severe uncontrolled mental illness Gender: all female Age: 59.0 mean (SD 7.5) years Type of cancer: breast Adjuvant treatment after surgery: none: n = 15 (15%), radiation only: n = 36 (36%), chemotherapy only: n = 22 (22%), radiation and chemotherapy: n = 54 (54%) Cancer stage: 0: n = 15 (15%), 1: n = 51 (51%), 2: n = 24 (24%), 3: n = 7 (7%), unknown: 3 (3%) Ethnicity: non‐Hispanic white: n = 91 (91%) Baseline physical activity: moderate to vigorous‐intensity physical activity (minutes/week): 99 mean (SD 127) Education: high school degree: n = 8 (8%), some college: n = 26 (26%), college degree: n = 29 (29%), graduate degree: n = 37 (37%) |
|
| Interventions |
Comparison: in‐person counselling vs telephone weight loss counselling vs usual care Intervention: weight loss intervention adapted from the diabetes prevention programme. Both in‐person and telephone counselling groups received the same lifestyle intervention Control: usual care group received the American Institute for Cancer Research nutrition and physical activity brochures and were referred to the Yale Cancer Centre Survivorship clinic. ITT was used |
|
| Outcomes |
Diet: 120‐item food frequency questionnaire Height: stadiometer (nearest 0.1 cm) Weight: measured while wearing light indoor clothing, without shoes (nearest 0.1 kg) Waist: measurement taken at smallest waist circumference (nearest 0.1 cm) Body fat: dual‐energy x‐ray absorptiometry scans (DEXA) performed to assess body fat with a Hologic 4500 scanner Serum biomarkers: fasting (> 12 hours) blood draw was performed. Serum samples were stored at ‐80 degrees C until assayed Duration of follow‐up: 6 months |
|
| Notes | Funding: American Institute of Cancer Research | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Permuted‐block randomisation with random block size performed by study biostatistician |
| Allocation concealment (selection bias) | Low risk | Participants randomly assigned by blinded study staff using unmarked envelopes |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | No blinding; outcome is not likely to be influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Unclear risk | Subjective outcomes not reported |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Study staff blinded to randomisation and lab technicians blinded to treatment assignment |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Subjective outcomes not reported |
| Incomplete outcome data (attrition bias) Objective | High risk | Attrition was 49% and was uneven across groups |
| Incomplete outcome data (attrition bias) Subjective | Unclear risk | Subjective outcomes not reported |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Low risk | No other concerns |
Hawkes 2013.
| Methods |
Design: 2‐armed prospective randomised controlled trial Country: Australia Accrual dates: October 2008 to June 2009 Trial Reg: ACTRN 2608000399392 |
|
| Participants |
Number randomised: 410 1141 colorectal cancer survivors assessed for eligibility, 57 excluded, 124 no response from doctor, 78 doctor refusal, 205 allocated to intervention, 171 completed 6‐month follow‐up, 34 lost to follow‐up, 159 completed 12‐month follow‐up, 12 lost to follow‐up, 205 allocated to usual care, 176 completed 6‐month follow‐up, 29 lost to follow‐up, 163 completed 12‐month follow‐up, 13 lost to follow‐up Inclusion and Exclusion: persons age ≥ 18 years residing in Queensland. Histologically confirmed diagnosis of primary CRC (i.e. C18‐C20, C218) within the previous 12 months. Queensland Cancer Registry notification from 1 October 2008, to 30 June 2009. Ability to understand and provide written informed consent in English. No metastatic disease (confirmed during screening interview). No medical condition limiting adherence to an unsupervised PA programme (as confirmed by their referring physician). A telephone. ≥ 1 poor health behaviour consistent with Australian recommendations (i.e. exercise < 150 minutes per week, < 2 servings of fruit or < 5 servings of vegetables per day, or overweight (BMI > 25 kg/m²)) Gender: Intervention: male 106, female 99 Control: male 115, female 90 Age: Intervention: mean 64.9 (SD 10.8) years Control: mean 67.8 (SD 9.2) years Type of cancer: colorectal Therapy previously received for cancer: Intervention: surgery 95.6%, chemo 41.5%, other therapy 11.7% Control: surgery 96.6%, chemo 39.5%, other therapy 14.2% Cancer stage: Intervention: A 17.6%, B 31.7%, C 22.0%, D 28.8% Control: A 19.0%, B 25.9%, C 23.4%, D 31.7% Baseline physical activity: NR Education: completed high school: intervention: 89.8%, control: 91.7% |
|
| Interventions |
Comparison: 6‐Month telephone‐delivered health coaching (HC) intervention commencing within 12 months of CRC diagnosis vs usual care (UC) Intervention: 11 telephone‐delivered HC sessions, a participant handbook, regular motivational postcard prompts, a pedometer, and the quarterly study newsletter sent to UC participants. The intervention was based on Acceptance Commitment Therapy (ACT). ACT interventions have been successfully used to enhance HRQoL and to promote positive health behaviours for various health conditions including chronic pain, diabetes, epilepsy, smoking, and obesity or weight management Control: UC participants received 4 freely available educational brochures produced by Cancer Council Australia on understanding CRC and cutting cancer risk, diet, and physical activity. Participants also received a quarterly study newsletter to enhance participant retention and were contacted for all follow‐up assessments |
|
| Outcomes |
HRQoL: Short Form–36 Cancer‐related fatigue: 13‐item Functional Assessment of Chronic Illness Therapy Fatigue Scale Weight management: BMI (kg/m²) using self‐reported height and weight measurements Dietary intake: Cancer Council Victoria Food Frequency Questionnaire (Dietary intake was quantified for total and saturated fats (percent of kJ intake), fibre (grams per day), fruits and vegetables (servings per day) (Cancer Council Victoria Food Frequency Questionnaire shows acceptability reliability and validity compared with 7‐day weighted food records and has been successfully used via telephone among cancer survivors) Duration of follow‐up: 12 months |
|
| Notes | Funding: supported by the Australian Government through Cancer Australia | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned to HC (n = 205) or UC (n = 205) at a ratio of 1 to 1 via a computer‐generated random number sequence |
| Allocation concealment (selection bias) | Low risk | Allocation sequence was generated by Can‐Change computer application developers and was concealed from project investigators and from the project manager who assigned participants to groups |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk | Participants were not blinded but the intervention could not really be blinded |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Participants were not blinded; outcome was patient self‐reported and therefore is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Objective outcomes | High risk | Participants were not blinded; outcome measurement was patient self‐reported and therefore is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Participants were not blinded; outcome measurement was patient self‐reported and therefore is likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) Objective | High risk | At 12 months, retention was 77.6% for the intervention group and 79.5% for the control group (P = 0.47). Similar dropout between groups. ITT was used |
| Incomplete outcome data (attrition bias) Subjective | High risk | At 12 months, retention was 77.6% for the intervention group and 79.5% for the control group (P = 0.47). Similar dropout between groups. ITT was used |
| Selective reporting (reporting bias) | Unclear risk | All primary outcomes reported, but not all secondary outcomes |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Kanera 2017.
| Methods |
Design: randomised controlled trial Country: Netherlands Accrual dates: November 2013 to June 2014 Trial Reg: Dutch Trial Register NTR3375 |
|
| Participants |
Number randomised: 518 1303 eligible, 785 no participation (decline before consent 100, not meeting inclusion criteria 5, computer literacy 10, unknown 670), 231 allocation to control group (no consent 7, not meeting inclusion criteria 2, baseline incomplete 13), 221 follow‐up for 6 months (lost 10), 212 follow‐up for 12 months (lost 19), 231 allocation to intervention group (no consent 6, not meeting inclusion criteria 15, baseline incomplete 13), 188 follow‐up for 6 months (lost 43), 169 follow‐up for 12 months (lost 62) Inclusion: 18 years of age or older. Previously diagnosed with cancer. Successful completion of the main treatment period. Up to 1 year ago. Receive aftercare (e.g. preventive hormonal therapy) or medical check‐ups. No cancer during last medical check‐up. All types of cancer. No groups were excluded (on condition that the main treatment period was successfully completed). Ability to speak and read the Dutch language. Access to the web and minimal Internet experience (monthly access) Exclusion: serious medical, psychiatric, or cognitive disease that would interfere with participation Gender: female: intervention: n = 183 (79.2 %), control: n = 186 (80.5%) Age: Intervention: mean 55.6 (SD 11.5) years Control: mean 56.2 (SD 11.3) years Ethnicity: NR Type of cancer: various (breast cancer: intervention = 70%, control = 71%) Therapy previously received for cancer: Surgery, chemotherapy, radiation: intervention: n = 86 (37.2%), control: n = 108 (46.8%) Surgery, chemotherapy: intervention: n = 61 (26.4%), control = 48 (20.8%) Surgery, radiation: intervention: n = 46 (19.9%), control: n = 30 (13.0%) Other: intervention: n = 38 (16.5%), control: n = 45 (19.5%) Cancer stage: NR Baseline physical activity: Weekly days > 30 minutes PA; intervention 4.9 mean (SD 1.9); control 4.6 mean (SD 2.0) Light PA minutes p/w: intervention 1521.5 mean (SD 897.9); control 1430.2 mean (SD 897.7) Moderate PA minutes p/w: intervention 595.9 mean (SD 620.5); control 526.5 mean (SD 546.5) Vigorous PA minutes p/w: intervention 231.0 mean (SD 323.9); control 238.0 mean (SD 426.0) Education: Low: intervention n = 76 (32.9%); control n = 97 (42.0%) Medium: intervention n = 76 (32.9%); control n = 70 (30.3%) High: intervention n = 79 (34.2%); control n = 64 (27.7%) |
|
| Interventions |
Comparison: online intervention questionnaire vs wait‐list control Intervention: cancer survivors in the experimental group who enter the online portal receive general information on dealing with distress, obtaining social support, self‐managing disease, and optimising healthy lifestyles. They are free to fill out a questionnaire on personal needs, which will lead them to tailored advice about the mentioned topics. Also information about possible other helpful interventions and social workers is provided. Cancer survivors in the experimental group are free to enter the online portal as often as they want to Wait‐list control: provided with access to online intervention after the last measurement |
|
| Outcomes |
Physical activity: Self‐reported Questionnaire to Assess Health Enhancing Physical Activity – SQUASH Vegetable consumption: Dutch Standard questionnaire on Food Consumption Duration of follow‐up: 12 months |
|
| Notes | Funding: KWF Kankerbestrijding, Netherlands Laboratory for Lifelong Learning (NELLL) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised allocation (ratio of 1:1) was automatically performed by means of a digital randomiser after centralised registration of participants |
| Allocation concealment (selection bias) | Low risk | Randomised allocation (ratio of 1:1) was automatically performed by means of a digital randomiser after centralized registration of participants |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk | No objective outcome measures |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Participants and personnel not blinded. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | No objective outcome measures |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Outcome patient self‐reported |
| Incomplete outcome data (attrition bias) Objective | Unclear risk | No objective outcome measures |
| Incomplete outcome data (attrition bias) Subjective | High risk | High attrition rate from intervention group (62 compared to 19 in control group). ITT used |
| Selective reporting (reporting bias) | Unclear risk | Primary outcomes: physical activity and fruit and vegetable consumption reported Primary outcome of well‐being not reported |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Kim 2011.
| Methods |
Design: randomised controlled trial Country: South Korea Accrual dates: NR Trial Reg: NR |
|
| Participants |
Number randomised: 45 951 participants identified from centre; 188 agreed to participate; 143 were excluded due to not meeting inclusion criteria, failing to complete screening tests, refusing to participate, or still receiving treatment; 23 randomised to intervention; 22 randomised to control; 5 lost to follow‐up in intervention arm; 4 lost to follow‐up in control arm Inclusion: women aged 20 years or older with stage 0 to III breast cancer. Primary treatment completed. Unmet behaviour goals or a poor diet as measured by the Diet Quality Index Exclusion: progressive disease. Additional primary tumours. Currently being treated for cancer. Condition that precludes unsupervised exercise or could interfere with fruit and vegetable diet. Any of the following contraindications for exercise: serum platelets lower than 100,000/mm³. Serum haemoglobin lower than 10 g/dL, body temp 37.8 or higher. White blood cell count 11,000/mm³ or higher Gender: female Age: Intervention: 44.6 mean (SD 9.9) years Control: 47.1 mean (SD 7.3) years Type of cancer: breast Therapy previously received for cancer (n): mastectomy 6, breast‐conserving surgery 39, radiation therapy 35, chemotherapy 37, hormone therapy 36 Cancer stage (n): stage 0: 5, stage I: 19, stage II: 15, stage III: 6 Ethnicity: NR Baseline physical activity: NR Education(n): high school or less: 29, completed university: 16 |
|
| Interventions |
Comparison: simultaneous stage‐matched exercise and diet intervention vs control Intervention: stage‐matched telephone counselling complemented with a workbook, individualised prescription for regular exercise, balanced diet programme based on guidelines for survivors (Doyle 2006) and guidelines of the Korean Nutrition Society. Prescription of exercise and diet was delivered weekly by 2 specially trained nurses during 30‐minute telephone counselling session Control: details not stated |
|
| Outcomes |
Dietary changes: assessed by 3‐day dietary recall and the Diet Quality Index tool for the Korean population Quality of life: European Organisation for Research and Treatment of Cancer quality of life questionnaire was used to measure functional status and global QoL Behaviour change towards consumption of a healthy diet: participants were assessed on their readiness to change based on TTM stages of change Duration of follow‐up: 12 weeks |
|
| Notes | Funding: National Cancer Centre grant | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table, although there is the possibility of selection bias due to the low level of enrolment |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk | No objective outcomes appropriate for this review |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | No blinding; outcome was patient self‐reported and therefore is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | No objective outcomes appropriate for this review |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | No blinding of outcome assessment; outcome measurement was patient self‐reported and therefore was likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) Objective | Unclear risk | No objective outcomes appropriate for this review |
| Incomplete outcome data (attrition bias) Subjective | Low risk | Attrition was 20%,but was similar in both groups, with 5 dropouts in the intervention arm and 4 in the control arm. Analyses were performed on an ITT basis |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Mefferd 2007.
| Methods |
Design: randomised controlled trial Country: USA Accrual dates: 2002 (4‐month period) Trial Reg: NR |
|
| Participants |
Number randomised: 85 736 sent a letter, 296 responded, 211 were excluded (most did not meet the overweight criteria, and other common reasons included not interested, did not have invasive breast cancer, and were in another nutrition study), 56 randomised to Intervention, 49 randomised to control, 9 dropout from intervention, 76 included in analysis (29 control and 47 intervention) Inclusion: breast cancer survivors who met the following inclusion criteria: 18 years of age and older; diagnosed with stage I to IIIA breast cancer within the previous 14 years; completed initial treatments (i.e. surgery, adjuvant chemotherapy, radiation therapy); initial BMI > 25.0 kg/m² and a minimum of 15 kg over ideal weight as defined by Metropolitan Life Insurance tables (1983); willingness and ability to attend group meetings and to maintain contact with investigators for 12 months; ability to provide dietary and exercise data by telephone at prescribed intervals Exclusion: inability to participate in physical activity because of severe disability (e.g. severe arthritic condition). Vulnerable individuals, such as pregnant women, were also excluded Gender: female Age: mean 56.3 (SD 8.2) years; range 34 to 72 years Ethnicity: non‐Hispanic white n = 71 (93%), Hispanic n = 2 (3%), African American n = 1 (1%), Pacific Islander n = 1 (1%), Other n = 1 (1%) Type of cancer: breast Therapy previously received for cancer: NR Cancer stage: Ia: n = 35 (46%), 1b: n = 3 (4%), Iia: n = 26 (34%), IIb: n = 10 (13%), IIIa: n = 2 (3%), tamoxifen use: n = 37 (54%) Baseline physical activity: moderate + vigorous physical activity at baseline (hours/week) Control: mean 4.0 (SD 3.4) Intervention: mean 3.2 (SD 2.0) Education: High school n = 6 (8%), some college n = 21 (28%), college graduate n = 40 (53%), postgraduate n = 9 (12%) |
|
| Interventions |
Comparison: weekly 6‐week intervention with cognitive‐behavioural therapy for obesity vs wait‐list control Intervention: once weekly, 16‐week intervention or wait‐list control The intervention incorporated elements of CBT for obesity, addressing a reduction in energy intake, as well as exercise, with the goal of an average of 1 hour a day of moderate to vigorous activity. Body weight, total and regional body fat (by dual energy X‐ray absorptiometry), waist and hip circumference, and blood lipids were assessed at baseline and after 16 weeks of intervention Control: wait‐list group |
|
| Outcomes |
Physical measures for body composition: anthropometry, scan, blood lipids Duration of follow‐up: 16 weeks |
|
| Notes | Funding: NIH grants CA90413 and CA101489 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised |
| Allocation concealment (selection bias) | Unclear risk | Randomised |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | No blinding; outcome is not likely to be influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | No blinding; outcome was patient self‐reported and therefore is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | No blinding; outcome is not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | No blinding of outcome assessment; outcome measurement is not likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) Objective | High risk | Attrition was 11%, but dropout rates between groups ‐ 61% control and 16% intervention ‐ were unequal. ITT was not used |
| Incomplete outcome data (attrition bias) Subjective | High risk | Attrition was 11%, but dropout rates between groups ‐ 61% control and 16% intervention ‐ were unequal. ITT was not used |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Morey 2009.
| Methods |
Design: randomised controlled trial Country: USA Accrual dates: July 2017 to May 2007 Trial Reg: NCT00303875 |
|
| Participants |
Number randomised: 641 67,161 individuals were identified, 47,146 were excluded (duplicates, deceased, missing contact details), Study letters of invitation were mailed to 20,015 individuals, 2156 expressed interest and were sent an eligibility questionnaire, 1208 responses were received, 567 individuals were ineligible Intervention (n = 319) or delayed intervention control group (n = 322) Inclusion: breast, prostate, and colorectal cancer. Diagnosed at least 5 years ago. Aged 65 years or older. No evidence of progressive disease or second cancer Exclusion: physician denied permission to contact patient. Institutionalised individuals. BMI < 25 or > 40. Severe hearing or speaking impairment. Non‐English speaking or writing. Contraindications to unsupervised exercise (angina, myocardial infarction < 6 months, congestive heart failure, chronic obstructive pulmonary disease, plan to have a hip or knee replacement, walker or wheelchair use, recent stroke with hemiparesis). Already performing more than 150 minutes of moderate to vigorous exercise per week Age: Intervention: mean 73.0 (SD 5.0) years Control: mean 73.1 (SD 5.1) years Gender: Male: intervention: n = 147 (46.1%) Control: n = 145 (45.0%) Ethnicity: white: intervention: n = 284 (89.0%), control: n = 285 (88.5%) Type of cancer: Breast: intervention: n = 143 (44.8%), control: n = 146 (45.3%) Prostate: intervention: n = 131 (41.1%), control: n = 130 (40.4%) Colorectal: intervention: n = 45 (14.1%), control: n = 46 (14.3%) Therapy previously received for cancer: none stated Cancer staging: NR Baseline physical activity: NR Education: some college education: intervention: n = 201 (63.0%), control: n = 94 (60.2%) |
|
| Interventions |
Comparison: 12‐month diet and exercise intervention delivered via telephone counselling and tailored mailed materials vs delayed intervention control Intervention: personally tailored workbook and series of quarterly newsletters, along with a programme of telephone counselling and automated prompts (i.e. 15 sessions and 8 prompts over the 12‐month period) Control: delayed intervention, wait‐list control |
|
| Outcomes |
Dietary intake: data were averaged from 2 unannounced 24‐hour recalls, using the interactive Nutrition Data System for Research software, version 2006 (Nutrition Coordinating Center, Minneapolis, Minnesota) Self‐reported height and weight: collected for estimation of BMI and weight loss Duration of follow‐up: 12 months |
|
| Notes | Funding: supported by National Institutes of Health grants CA106919 and P30AG028716, and grant E3386R from Veterans Affairs Research and Development | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation method by race, cancer type, and sex with even distribution into an intervention or delayed intervention control group |
| Allocation concealment (selection bias) | Low risk | Randomisation carried out by a statistician with no participant contact |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk | No blinding; intervention would have been difficult to blind |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | No blinding; outcome was patient self‐reported and therefore is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Objective outcomes | High risk | No blinding of outcome assessment; outcome measurement was patient self‐reported and therefore is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | No blinding of outcome assessment; outcome measurement was patient self‐reported and therefore is likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) Objective | Low risk | Attrition even across groups. 88 dropouts with reasons (14% lost to follow‐up), but all participants included in the final analysis |
| Incomplete outcome data (attrition bias) Subjective | Low risk | Attrition even across groups. 88 dropouts with reasons (14% lost to follow‐up), but all participants included in the final analysis |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Park 2016.
| Methods |
Design: randomised controlled trial Country: Berlin Accrual dates: September 2011 to October 2013 Trial Reg: NCT01819324 |
|
| Participants |
Number randomised: 173 Invitation letter 1397, ClinicalTrials.gov 16, main hospital 866, returned to sender 124, received 177 (excluded 4, not eligible 3, did not complete required materials 1), TTMI 57, follow‐up for 4 months 40 (lost 17), follow‐up for 7 months 43 (3 came back), SLM 58, follow‐up for 4 months 43 (lost 15), follow‐up for 7 months 41 (lost 2), control 58, follow‐up for 4 months 50 (lost 8), follow‐up for 7 months 47 (lost 3) Inclusion: first diagnosed with breast cancer in the past 1.5 years. Stage 0 to 2 breast cancer. No prior adjuvant treatment for another cancer. Can read and write English. Not participating in other health behaviour research right now Exclusion: apparently serious mental disturbance, male breast cancer survivors Gender: female Age: TTMI mean 55.73 (SD 10.91); SLM mean 57.74 (SD 10.7); UC mean 55.72 (SD 10.92) years Ethnicity: White: TTMI n = 54 (94.7%); SLM n = 56 (96.6%); UC n = 53 (93.0%) African American: TTMI n = 1 (1.8%); SLM n = 1 (1.7%); UC n = 2 (3.5%) Hispanics: TTMI n = 1 (1.8%); SLM n = 0; UC n = 1 (1.8%) Other: TTMI n = 1 (1.8%); SLM n = 1 (1.7%); UC n = 1 (1.8%) Type of cancer: breast Therapy previously received for cancer: Current cancer treatment No: TTMI n = 35 (64.8%); SLM n = 38 (70.4%); UC n = 33 (58.9%) Yes: TTMI n = 19 (35.2%); SLM n = 16 (29.6%); UC n = 23 (41.1%) Cancer stage: NR Baseline physical activity: exercise time (minutes): NR Education: Less than high school: TTMI n = 0; SLM n = 1 (1.7%); UC n = 0 High school/GED 6: TTMI n = 7 (10.5%); SLM (12.1%); UC n = 8 (14.0%) Some college: TTMI n = 11 (19.3%); SLM n = 9 (15.5%); UC n = 8 (14.0%) 2‐year college degree (Associate's): TTMI n = 7 (12.3%); SLM n = 4 (6.9%); UC n = 5 (8.8%) 4‐year college degree (BA/BS): TTMI n = 17 (29.8%); SLM n = 23 (39.7%); UC n = 20 (35.1%) Graduate degree (MA, PhD): TTMI n = 16 (28.1%); SLM n = 13 (22.4%); UC n = 12 (21.0%) Professional degree (MD, JD): TTMI n = 0; SLM n = 1 (1.7%); UC n = 4 (7.0%) |
|
| Interventions |
Comparison: mail‐based teachable moment materials vs mail‐based standard lifestyle management vs usual care Experimental: targeting the teachable moment: receiving targeting the teachable moment intervention materials (focused on health behaviours and issues specific to breast cancer survivors) every other week for 4 months Active comparator: standardised lifestyle management: receiving standardised lifestyle management materials (focused mostly on health behaviours) every other week for 4 months No intervention: usual care: receiving SLM materials at the end of 7 months |
|
| Outcomes |
Primary outcome measures: Changes in eating habits measured by the National Cancer Institute Quick Food Scan Diet habits (amount of fat and fruit/vegetable uptake) Changes in physical activity measured by the Paffenbarger physical activity questionnaire Frequency and amount of time for physical exercise Secondary outcome measures: Changes in coping strategies measured by the Brief COPE Coping strategies (e.g. problem‐focused coping, emotional approach coping) Changes in self‐efficacy measured by the general self‐efficacy questionnaire General sense of perceived self‐efficacy Changes in social support measured by the Interpersonal Support Evaluation List (ISEL) Social support Changes in life meaning measured by the Meaning in Life Questionnaire (MLQ) Presence of meaning and search for meaning Duration of follow‐up: 7 months |
|
| Notes | Funding: National Cancer Institute | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants completed baseline questionnaires (T1) and then were randomly assigned (using a blocked design to ensure equal numbers of participants in each group) to 1 of 3 groups |
| Allocation concealment (selection bias) | Unclear risk | Participants completed baseline questionnaires (T1) and then were randomly assigned (using a blocked design to ensure equal numbers of participants in each group) to 1 of 3 groups |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk | No objective outcome measures |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Participants and personnel not blinded |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | No objective outcome measures |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Patient self‐report |
| Incomplete outcome data (attrition bias) Objective | Unclear risk | No objective outcome measures |
| Incomplete outcome data (attrition bias) Subjective | High risk | Dropout from intervention groups: targeting the teachable moment 25% and standard lifestyle management 30% vs 19% control |
| Selective reporting (reporting bias) | Unclear risk | Protocol could not be located; insufficient information to make a judgement |
| Other bias | Unclear risk | Insufficient information to make a judgement |
Pierce 2007.
| Methods |
Design: multi‐institutional randomised controlled trial Country: USA Accrual dates: 1995 to 2000 (month not specified) Trial Reg: NCT00003787 |
|
| Participants |
Number randomised: 3107 7572 breast cancer patients screened; 4463 excluded due to not meeting inclusion criteria, declining to participate, or unable to commit to study; 1546 randomised to intervention; 1561 randomised to control. In the intervention group, 16 were lost to follow‐up and 2 withdrew. In the control group, 8 were lost to follow‐up and 19 withdrew. 1537 included in intervention group primary analysis, 1551 included in control group primary analysis Inclusion: diagnosis of a primary operable invasive breast carcinoma categorised by American Joint Committee on Cancer (edition IV) criteria. Stage I (≥ 1 cm), stage II, or stage IIIA within the past 4 years. Age at diagnosis between 18 and 70 years. Treatment with axillary dissection and total mastectomy or lumpectomy followed by primary breast radiation. No current or planned chemotherapy. No evidence of recurrent disease or new breast cancer since completion of initial local treatment. No other cancer in the past 10 years Exclusion: objective evidence of recurrent disease; current enrolment in another dietary clinical trial. Diagnosis with a comorbidity requiring a specific diet or using a medication that contraindicated a high‐fibre diet. Pregnancy. Receiving oestrogen replacement therapy, including vaginal oestrogen creams. Cirrhosis. Other primary or recurrent invasive cancer within the last 10 years (other than non‐melanoma skin cancer or carcinoma of the cervix in situ). Unable to commit to the intervention schedule. Life‐threatening disease or medical condition, other than breast cancer, that would interfere with participation in an 8‐year diet study. Any previous diagnosis of invasive breast carcinoma Age: Intervention: mean 53.3 (SD 8.9) years Control: mean 53.0 (SD 9.0) years Gender: female Type of cancer: breast Therapy previously received for cancer: Intervention: mastectomy 52.8%, breast‐sparing surgery 47.2%, radiation 61%, adjuvant chemotherapy 71.2%, ever anti‐oestrogen use 19.5% Control: mastectomy 51.6%, breast‐sparing surgery 48.4%, radiation 62%, adjuvant chemotherapy 68.6%, ever anti‐oestrogen use 65.3% Cancer stage: Intervention: I 15.6%, II 40.3%, III 35.9%, unspecified 8.3% Control: I 15.8%, II 40%, III 35.9%, unspecified 8.3% Ethnicity: Intervention: white 85%, African American 4%, Hispanic 5.7%, Asian American 3%, mixed/other 2.3% Control: white 85.6%, African American 3.7%, Hispanic 5%, Asian American 3.2%, mixed/other 2.5% Baseline physical activity: NR Education: NR |
|
| Interventions |
Comparison: intensive counselling to adopt a dietary pattern high in fruits, vegetables, and fibre and low in fat vs advice to follow the 5‐a‐day diet Intervention: telephone counselling, supplemented with 12 cooking classes in the first year and monthly newsletters throughout the study Control: women randomised to the comparison group were provided with print materials (from the US Department of Agriculture and the National Cancer Institute) describing a diet with a recommended daily intake of 5 servings of vegetables and fruits, more than 20 g of fibre, and less than 30% total energy intake from fat |
|
| Outcomes |
Overall survival: National Death Index was searched using social security number, name, and date of birth Incidence of secondary malignancy/comorbidities: during telephone interview, clinical staff queried participants regarding the occurrence of any new or existing medical diagnoses. Any report of breast cancer event or death triggered a confirmation interview and collection of medical records and/or death certificate Dietary changes: assessed by sets of 4 prescheduled 24‐hour dietary recalls conducted by telephone on random days over a 3‐week period, stratified for weekend vs weekday. Recalls were scheduled at baseline and at 1 year, 4 years, and 6 years and on 50% random samples at 6, 24, and 36 months Changes in weight and blood analysis: clinic visits conducted at baseline, 1 year, 2 or 3 years (randomly determined), 4 years, and 6 years included measured weight and venepuncture. Separated blood samples were stored in cryovials in −80°C freezers for later analysis Duration of follow‐up: 6 years |
|
| Notes | Funding: study was initiated with support from the Walton Family Foundation and was continued with funding from National Cancer Institute grant CA 69375. Some data were collected from general clinical research centres (National Institutes of Health grants M01‐RR00079 and M01‐RR00827) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned to intervention or comparison group via a random permuted‐block design stratified by tumour stage, age, and clinical site |
| Allocation concealment (selection bias) | Low risk | Allocation of participants was conducted by the clinical site coordinator running the study’s randomisation computer programme, which automatically stamped the assigned study group in the database |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | No blinding; outcome is not likely to be influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | No blinding; outcome is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | No blinding; outcome is not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | No blinding of outcome assessment; outcome measurement is likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) Objective | Low risk | Minimal dropout with 1% lost to follow‐up overall and similar numbers dropping out of the intervention and control groups. ITT was used |
| Incomplete outcome data (attrition bias) Subjective | Low risk | Minimal dropout with 1% lost to follow‐up overall and similar numbers dropping out of the intervention and control groups. ITT was used |
| Selective reporting (reporting bias) | Low risk | All primary outcomes reported |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Reeves 2017.
| Methods |
Design: 2‐armed randomised controlled trial Country: Australia Accrual dates: July 2009 to June 2010 Trial reg: NR Consent: written informed consent was obtained |
|
| Participants |
Number randomised: 90 927 identified, 743 obtained oncologist consent and were sent a letter, 248 consented to contact, 45 randomised to the intervention, 45 randomised to usual care, 5 lost to follow‐up in the intervention arm, 11 lost to follow‐up in the usual care arm, 45 analysed in each group Inclusion: women survivors after a diagnosis of stage I to III breast cancer. Age 18 to 75 years. Diagnosis of breast cancer approximately 9 to 18 months before. Completed primary cancer treatment. BMI 25 to 40 kg/m². Resided within 50 km of the state capital Brisbane. English speaking Exclusion: diagnosis of ductal carcinoma in situ or distant metastatic disease. Previous diagnosis of invasive breast cancer. Any other cancer in the past 5 years. Contraindications to participation in an unsupervised exercise or weight loss programme. Self‐reported mental health condition that would interfere with study participation. Using or planning to use weight loss medications. Previously had or planning to have bariatric surgery. Not contactable for the duration of the study Gender: female Age: mean 55.3 (SD 8.7) years Ethnicity: Caucasian 96.7% Type of cancer: breast Therapy received for cancer: surgery only 3.3%; surgery and chemo 14.4%; surgery and radiotherapy 28.9%; surgery, chemo, and radiotherapy 53.3%; trastuzumab 13.3%; endocrine treatment 73.3% Cancer stage: stage I 48.9%, stage II 33.3%, stage III 17.8% Baseline physical activity: NR Education: high school or less 32.2%, trade/technical 30.0%, university or higher 37.8% |
|
| Interventions |
Comparison: telephone diet and physical activity intervention vs usual care Intervention: weight loss intervention of diet and physical activity. Intervention included posted materials and up to 16 calls from a dietitian over a 6‐month period Control: mailed brief feedback after assessments; after 6‐month assessment, provided with the intervention workbook and a diary |
|
| Outcomes |
Changes in weight/anthropometry: objectively measured during the in‐person assessment Dietary changes: self‐reported dietary intake by two 24‐hour dietary recall telephone interviews assessed at baseline and at 6 months. Dietary intake included vegetables, energy, energy density, carbohydrates, total fat, and saturated fat Healthy eating changes: dietary strategies recorded included portion control, dietary self‐monitoring, and reducing fat intake Quality of life: assessed by SF‐36; fatigue was measured on the 13‐item FACIT; fatigue and body image were assessed by body image and relationship scale Duration of follow‐up: data collected at baseline and at 6 months |
|
| Notes | Funding: early career researcher grant from the University of Queensland, project grant funding from the National Health and Medical Research Council and Queensland Health Core Infrastructure Funding | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation sequence and group allocation were generated by a staff member not involved with the study using a computer‐generated random number sequence, with block sizes of 6 |
| Allocation concealment (selection bias) | Low risk | Randomisation sequence and group allocation were generated by a staff member not involved with the study using a computer‐generated random number sequence, with block sizes of 6 |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | No blinding; outcome is not likely to be influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Participants and personnel not blinded |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Suggested outcome assessors blinded to patient groups, but not enough detail provided. However, outcome is not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | No blinding; outcome was patient self‐reported and therefore is likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) Objective | High risk | Greater attrition in control group than in intervention group (75.6% vs 88.9%); missing data imputed |
| Incomplete outcome data (attrition bias) Subjective | High risk | Greater attrition in control group than in intervention group (75.6% vs 88.9%); missing data imputed |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Scott 2013.
| Methods |
Design: randomised controlled trial Country: UK Accrual dates: NR Trial Reg: ISRCTN08045231 |
|
| Participants |
Number randomised: 90 523 sent recruitment letter, 371 did not respond to letter, 86 enquiries from other sources, 238 total number of enquiries, 60 excluded, 64 refused to participate, 14 could not contact, 10 declined after familiarisation, 47 allocated to intervention group, 43 allocated to control, 6 lost to follow‐up intervention, 5 lost to follow‐up control, 41 completed 6‐month intervention assessments, 38 completed 6‐month control assessments Inclusion: overweight women with BMI 25 kg/m². Completed surgery, chemotherapy, and radiotherapy for early‐stage breast cancer (stage I to III) 3 to 18 months previously. Receiving adjuvant endocrine treatments and yet to complete a 1‐year course of adjuvant trastuzumab. Subject to acceptable cardiac function determined by a multi‐gated acquisition (MUGA) scan and consultant approval Exclusion: concomitant HRT or oral contraceptives; metastatic or active loco‐regional disease. Physical or psychiatric impairment limiting physical mobility. Severe nausea, anorexia, or other condition precluding participation in exercise. Following alternative/complementary diets. Taking high‐dose antioxidant supplements. Engaged in regular exercise Gender: female Age: Intervention: 55.6 mean (SD 10.2) years Control: 55.9 mean (SD 8.9) years Type of cancer: breast Therapy previously received for cancer: Mastectomy: intervention: n = 28 (60%), control: n = 9 (21%) Breast‐conserving surgery: intervention: n = 19, control: (40%) n = 34 (79%) Chemotherapy: intervention: n = 27 (57%), control: n = 23 (54%) Radiotherapy: intervention: n = 40 (85%), control: n = 35 (81%) Tamoxifen: intervention: n = 23 (49%), control: n = 22 (51%) Aromatase inhibitor: intervention: n = 14 (30%), control: n = 11 (26%) Trastuzumab: intervention: n = 4 (9%), control: n = 6 (14%) Lymphedema: intervention: n = 10 (21%), control: n = 15 (35%) Cancer stage: all early stage, I to III Ethnicity: white: intervention: n = 46 (98%), control: n = 42 (98%) Baseline physical activity: Resting heart rate, beats/min at baseline: intervention: 78 mean (SD 12), Control: 74 mean (SD 11) Education (SES): Secondary and A levels: intervention: n = 18 (38%), control: n = 12 (28%) Degree: intervention: n = 8 (17%), control: n = 8 (19%) Vocational qualifications: intervention n = 6 (13%), control: n = 2 (5%) |
|
| Interventions |
Comparison: exercise and hypocaloric healthy eating programme vs control Intervention: 24‐week lifestyle intervention with 3 weekly supervised exercise sessions and an individually tailored hypocaloric healthy eating programme. Each participant also received one‐to‐one individualised dietary advice and written information (‘‘Weight Loss on a Plate,’’ Scottish Dietetic Association). Additional weekly small‐group nutrition education seminars included topics such as dietary fat intake, hydration, achieving a healthy balanced diet, and alcohol consumption Control: a healthy eating booklet, "Eat Well" (Food Standards Agency, UK), which also included brief advice on keeping active |
|
| Outcomes |
Weight: measured by a standard technique BMI: measured by a standard technique Waist circumference: measured by a standard technique Waist‐ to‐hip ratio: measured by a standard technique % body fat: measured using bioelectrical impedance QoL: measured using the Functional Assessment of Cancer Therapy ‐ General (FACT‐G), including the breast subscale (FACT‐B) Dietary intake: measured using 3‐day diet diaries, which were analysed for total energy and macronutrient intake (NetWisp 3: Tinuviel Software Systems, Cheshire, UK) Duration of follow‐up: 6 months |
|
| Notes | Funding: American Institute for Cancer Research (Grant number 05A008‐REV) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomly allocated (1:1 ratio) to 1 of 2 groups: (1) lifestyle intervention group, or (2) control group. Randomisation was performed by an independent researcher at the Clinical Trials Research Unit, University of Leeds |
| Allocation concealment (selection bias) | Low risk | Randomisation sequence was not disclosed until patients had completed their baseline assessments |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | No blinding; outcome is not likely to be influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | No blinding; outcome is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | No blinding; outcome is not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | No blinding of outcome assessment; outcome measurement is likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) Objective | Low risk | Attrition 12%. Missing outcome data balanced in numbers across invention and control groups. ITT with missing data inputted |
| Incomplete outcome data (attrition bias) Subjective | Low risk | Attrition 12%. Missing outcome data balanced in numbers across invention and control groups. ITT with missing data inputted |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Unclear risk | Not reported |
Sheppard 2016.
| Methods |
Design: 2‐arm randomised controlled trial Country: USA Accrual dates: December 2010 to January 2012 Trial Reg: NR |
|
| Participants |
Number randomised: 31 106 screened, 37 ineligible, 69 eligible, 50 consent, 19 declined participation, 19 not randomised, 15 intervention, 16 control, 12 completed, 1 withdrew, 3 lost to follow‐up, 10 completed, 5 withdrew Inclusion: self‐identified as African American. Overweight or obese (BMI ≥ 25 kg/m² and ≤ 40 kg/m². Sedentary (exercises less than 60 min/week for previous 6 months). Early‐stage/localised breast cancer 6 months. 5 years post active treatment. Ability to read and speak English. Ability to provide informed consent Exclusion: history of other cancers (except basal or squamous cell carcinoma). Recurrence of breast cancer. Current enrolment in another physical activity or dietary clinical trial or commercial programmes like Weight Watchers. Inability to commit to the intervention schedule. Telephone inaccessibility. Pre‐existing conditions that preclude adherence to an unsupervised exercise programme. Failure to provide medical clearance. Morbidly obese (BMI ≥ 40 kg/m²) Gender: female Age: mean 54.7 (SD 9.8) years Type of cancer: breast Therapy previously received for cancer: NR Cancer staging: early stage/localised Ethnicity: African American Baseline physical activity: Control (minutes/week (mean ± SD)): vigorous 37.5 ± 71.4, moderate 53.6 ± 60.9, walking 100.0 ± 87.6, total 205.0 ± 196.9 Metabolic equivalents (MET) (minutes/week (mean ± SD)): vigorous 300 ± 570.9, moderate 214.5 ± 243.5, walking 313.5 ± 283.9, total 688.5 ± 794.2 Intervention: (minutes/week (mean ± SD)): vigorous 37.5 ± 84.5, moderate 62.5 ± 129.8, walking 140.7 ± 229.0, total 291.7 ± 387.0 Metabolic equivalents (MET) (minutes/week (mean ± SD)): vigorous 300 ± 675.8, moderate 250.0 ± 519.2, walking 464.4 ± 755.7, total 291.7 ± 387.0 Education: NR |
|
| Interventions |
Comparison: Stepping STONE intervention (physical activity and diet intervention) vs control (general health info for cancer survivors) Intervention: 12‐week Stepping STONE intervention based on theory of planned behaviour and social‐cognitive theory. Participants met once every 2 weeks for 90‐minute group session – 30 minutes physical activity and 60 minutes educational session. On weeks not meeting, individual telephone coaching sessions with a trained survivor coach for 15 minutes Control: general health information for cancer survivors – facing forward life after cancer treatment. Offered intervention at completion of the study |
|
| Outcomes |
Weight, waist, and hip circumference: physical measurement Food intake: participants recorded food intake for 4 days Duration of follow‐up: 12 weeks |
|
| Notes | Funding: National Cancer Institute R21CA149996, Biostatistics and Bioinformatics Shared Resource and Tissue Culture Shared Resource NCI grant P30CA51008 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | No blinding; outcome is not likely to be influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Unclear risk | Not reported. No subjective outcomes appropriate for this review |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | No blinding; outcome is not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Not reported. No subjective outcomes appropriate for this review |
| Incomplete outcome data (attrition bias) Objective | High risk | Overall attrition rate is high (29%) and is likely to have clinical relevance |
| Incomplete outcome data (attrition bias) Subjective | Unclear risk | Not reported. No subjective outcomes appropriate for this review |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Swisher 2015.
| Methods |
Design: randomised controlled clinical trial, with a 2:1 randomisation scheme Country: USA Accrual dates: NR Trial Reg: NCT01498536 |
|
| Participants |
Number randomised: 23 (13 intervention, 10 control). 66 survivors invited to participate, 28 enrolled Inclusion: stage I, II, or III invasive breast cancer. > 3 months after completion of active treatment. Body mass index (BMI) > 25 kg/m². Confirmed ER/PR/HER2 neu‐negative status (per pathology report); younger than 80 years of age Exclusion: significant cardiac disease, renal failure; significant symptomatic lymphoedema. Physical or psychological comorbidities that would prohibit exercise testing or participation. Diagnosis of diabetes mellitus. Active smoking status Gender: female Age: Intervention: mean 53.8, range: 43 to 65 years Control: mean 53.6, range: 36 to 71 years Ethnicity: NR Type of cancer: breast Therapy previously received for cancer: Surgery: intervention (13): lumpectomy 13, mastectomy 5, radiation (Y/N) 13/5, chemotherapy (Y/N) 17/1 Control (10): lumpectomy 6, mastectomy 4, radiation (Y/N) 7/1, chemotherapy (Y/N) 7/1 Cancer stage: I, II, or III (no further detail provided). Baseline physical activity: exercise time (minutes): intervention: 12.4, control: 13.8 Education: NR |
|
| Interventions |
Comparison: moderate exercise and diet counselling vs usual care Intervention: get fit for the fight programme. Programme consisted of supervised, moderate‐intensity aerobic exercise 3 times per week at the exercise facility and 2 unsupervised sessions per week at home. Dietary counselling consisted of 2 individual sessions with the study dietitian ‐ a specialist in nutrition for cancer patients. Participants completed a 3‐day diet record during baseline testing before meeting individually with the dietitian Control: written materials about healthy eating for cancer survivors and suggestions on ways to achieve regular physical activity. Not instructed to avoid diet change or exercise |
|
| Outcomes |
Weight loss (body mass, BMI, % fat): BMI was calculated from height and weight measurements without shoes and in light clothing Waist and hip circumferences: measured via a spring‐loaded tape measure Body fat percentage: calculated from skin fold measurement at 7 body sites according to American College of Sports Medicine standards Quality of life: Function After Cancer Therapy ‐ Breast (FACT‐B) Duration of follow‐up: 12 weeks |
|
| Notes | Funding: institutional research grant from the American Cancer Society | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was determined a priori by the study statistician, and group assignments were placed in opaque envelopes |
| Allocation concealment (selection bias) | Low risk | Randomisation was determined a priori by the study statistician, and group assignments were placed in opaque envelopes |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | No blinding; outcome is not likely to be influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | No blinding; outcome is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | No blinding; outcome is not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | No blinding of outcome assessment; outcome measurement is likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) Objective | High risk | Uneven dropout between groups ‐ 0% control and 18% intervention. ITT was used with some data but not all |
| Incomplete outcome data (attrition bias) Subjective | High risk | Uneven dropout between groups ‐ 0% control and 18% intervention. ITT was used with some data but not all |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Yun 2017.
| Methods |
Design: randomised controlled trial Country: South Korea Accrual dates: April 2012 to August 2013 Trial Reg: NCT01527409 |
|
| Participants |
Number randomised: 248 546 eligible patients, 298 excluded; Leach Program group: 115 (69.3%) participants completed the 12‐month course at 3 months, and 117 (70.5%) at 6 to 12 months; UC group, 60 (73.2%) participants completed the course at 3 months and 57 (71.3%) at 12 months Inclusion: ≥ 20 years old. Platelet count ≥ 100,000/mm³. Serum haemoglobin ≥ 10 g/dL. Not already met 2 or more behavioural goals aimed for in the study. Energy expenditure achieved by at least moderate exercise for at least 150 minutes/week. Intake of ≥ 5 servings of fruits and vegetables per day. Total score > 72 points in the Post Traumatic Growth Inventory Exclusion: currently receiving cancer treatment. Progressive malignant disease or recurrent, metastasised, or additional primary cancer. Condition that might compromise adherence to an unsupervised exercise programme (e.g. uncontrolled congestive heart failure or angina, recent myocardial infarction, breathing difficulties requiring oxygen use or hospitalisation, inability to walk without a walker or wheelchair, planning to receive hip or knee replacement surgery). Condition that could interfere with ingestion of a diet high in vegetables and fruits (e.g. kidney failure, need for chronic warfarin). Serious psychological disorder (e.g. bipolar disease, schizophrenia, eating disorder). Infection (body temperature ≥ 37.2°C or WBC ≥11,000 mm³). Visual or motor dysfunction. Pregnant Gender: male 42 (20.39), female 164 (79.61) Age: mean 50.68 (SD 9.43) years Type of cancer: stomach (n = 51), lung (n = 5), breast (n = 123), colorectal (n = 11), gynaecological (n = 9), other (n = 1) Therapy previously received for cancer: Missing ‐ 10, surgery ‐ 195 (99.49), radiotherapy‐ 100 (51.02), chemotherapy ‐ 119 (60.71), hormonal therapy ‐ 51 (43.59) Cancer stage: stage 0 ‐ 5 (2.51), stage 1 ‐ 100 (52.67), stage 2 ‐ 66 (33.17), stage 3 ‐ 20 (10.05), stage 4 ‐ 2 (1.01), other (5/6) ‐ 6 (3.02) Ethnicity: Korean Education: high school graduate or less 105 (51.47), college graduate 99 (48.53) |
|
| Interventions |
Comparison: LEACH (physical activity, diet, and distress) programme vs usual care Intervention: 1‐hour health education workshop (physical activity, dietary habits, and distress management) and 3‐hour leadership workshop (Seven Habits of Highly Effective People With Cancer). Next, the Intervention group was also offered individual coaching by telephone for a 24‐week period Control: encouraged to continue their usual care and given a health education booklet on physical activity, dietary habits, and distress management |
|
| Outcomes |
Physical activity: measured in METs (kcal/kg/week) Diet: a validated questionnaire on fruit and vegetable intake Post‐traumatic growth: measured using the 21‐item Post‐Traumatic Growth Inventory Duration of follow‐up: outcomes were measured at 0, 3, 6, and 12 months, but data from the 6‐month period were not included in the analysis due to lack of participants |
|
| Notes | Funding: national cancer centre, national R&D programme for cancer control, ministry of health and welfare, Republic of Korea | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | With the aid of a computerised random number generator, we randomly assigned eligible participants |
| Allocation concealment (selection bias) | Unclear risk | With the aid of a computerised random number generator, we randomly assigned eligible participants |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk | No objective outcome measures |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Participants and personnel not blinded |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | No objective outcome measures |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Self‐report quality of life |
| Incomplete outcome data (attrition bias) Objective | Unclear risk | No objective outcome measures |
| Incomplete outcome data (attrition bias) Subjective | High risk | All participants accounted for, but 30% dropout in control and intervention groups |
| Selective reporting (reporting bias) | Unclear risk | Protocol could not be located; insufficient information to make a judgement |
| Other bias | Unclear risk | Insufficient information to permit a judgement |
Zick 2017.
| Methods |
Design: pilot randomised clinical trial Country: USA Accrual dates: January 2014 to April 2015 Trial Reg: NCT01902745 |
|
| Participants | Breast cancer survivors, completed treatment with reported persistent fatigue Number randomised: 30 50 assessed for eligibility, 20 excluded, 15 allocated to fatigue reduction diet (FRD), 15 allocated to general health curriculum (GHC), 30 completed treatment and were included in the analysis Inclusion: 18 years of age or older. BMI between 18.5 and 35 kg/m². Diagnosis of local regional breast cancer (stage 0 to IIIa). Completed all cancer treatments except for hormonal therapy and herceptin. At least 1 year previously, reported persistent fatigue starting on or after cancer diagnosis. Score > 4 on the brief fatigue inventory. Low fruit and vegetable intake (fewer than 5.5 servings per day) Exclusion: diagnosis pf untreated anaemia. Hypothyroid or hyperthyroid and supplemented with omega 3 fatty acids. On a medically prescribed diet. Pregnant. Wanting to become pregnant or lactating. Planning to start or stop any chronic supplements or medication within 6 weeks before or throughout the study Gender: female Age: FRD: mean 64.4 (SD 10.0) years GHC: mean 60.4 (SD 9.35) years Type of cancer: breast Cancer stage: FRD: stage 0: 7%, stage 1: 27%, stage 2: 47%, stage 3: 13%, unknown: 7% GHC: stage 0: 33%, stage 1: 20%, stage 2: 27%, stage 3: 13%, unknown: 0% Therapy received for cancer: FRD: surgery 100%, chemo 73%, radiation 86%, hormone 12% GHC: surgery 100%, chemo 53%, radiation 73%, hormone 67% Ethnicity: FRD: 93% white GHC: 93% white Baseline physical activity: NR Education: NR |
|
| Interventions |
Comparison: fatigue reduction diet vs general health curriculum Intervention: individualised counselling using the theoretical framework of Bandura's social‐cognitive theory, delivered via 6 brief 15‐minute telephone counselling calls by a registered dietitian and based on dietary intake Control: individualised counselling using the theoretical framework of Bandura's social‐cognitive theory, delivered via 6 brief 15‐minute telephone counselling calls by a study staff member and based on general health (with no dietary info) |
|
| Outcomes |
Severity and impact of fatigue: 9‐item Brief Fatigue Inventory Sleep quality: 19‐item PSQI Dietary intakes: 7‐day food diaries and 24‐hour recalls Adherence to dietary goal: daily food checklists and serum fatty acids Duration of follow‐up: 3 months |
|
| Notes | Funding: supported by grants from the James Stuart and Barbara Padnos Research Funds for Cancer Research and the NIH CTSA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation code was computer‐generated in blocks of size 6 by the study biostatistician. Study personnel who had no contact with participants or study data placed the randomisation assignments in sequentially numbered opaque envelopes |
| Allocation concealment (selection bias) | Low risk | Upon randomisation, the next number in the sequence was chosen, and the envelope was opened, indicating treatment assignment |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | No blinding; outcome is not likely to be influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Participants and personnel not blinded |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | No blinding; outcome is not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Patient self‐report |
| Incomplete outcome data (attrition bias) Objective | Low risk | Low attrition (1 participant dropout from the intervention group). ITT was used |
| Incomplete outcome data (attrition bias) Subjective | Low risk | Low attrition (1 participant dropout from the intervention group). ITT was used |
| Selective reporting (reporting bias) | Unclear risk | Protocol could not be located; insufficient information to permit a judgement |
| Other bias | Unclear risk | Insufficient information to make a judgement |
ACT: Acceptance Commitment Therapy.
BMI: body mass index.
CBT: cognitive‐behavioural therapy.
CRC: colorectal cancer.
DEXA: dual‐energy X‐ray absorptiometry.
DQI: Diet Quality Index.
EC: endometrial cancer.
EORCT QLQ‐C30: 30‐item questionnaire of the European Organisation for Research and Treatment.
FACIT: Functional Assessment of Chronic Illness Therapy.
FACT‐B: Functional Assessment of Cancer Therapy ‐ Breast.
FACT‐C: Functional Assessment of Cancer Therapy ‐ Colorectal.
FACT‐G: Functional Assessment of Cancer Therapy ‐ General.
FRD: fatigue reduction diet.
GHC: general health curriculum
HDL: high‐density lipoprotein.
HRQoL: health‐related quality of life.
HRT: hormone replacement therapy.
IL‐6: interleukin‐6.
ITT: intention‐to‐treat.
MET: metabolic equivalent.
NHANES: National Health and Nutrition Examination Survey.
NR: not reported.
PA: physical activity.
PSQI: Pittsburgh Sleep Quality Index.
QoL: quality of life.
R&D: research and development.
SD: standard deviation.
SES: supplementary education services.
SF‐36: Short Form‐36.
SLM: standard lifestyle method.
TTMI: Targeting the Teachable Moment Intervention.
UC: usual care.
WHEL: Women's Healthy Eating and Living study.
WW: Weight Watchers.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abrams 2014 | Editorial article |
| Anderson 2014 | Participants are not cancer survivors |
| Arends 2014 | Single food group only (fruit and vegetable) used in the intervention: this is not sufficient to represent healthy eating |
| Arnold 2015 | Intervention uses supplementation |
| Boddie 2010 | Intervention uses supplementation |
| Bourke 2014 | Not an RCT |
| Brewer 2015 | Not an RCT |
| Campbell 2009 | Single food group only (fruit and vegetable) used in the intervention: this is not sufficient to represent healthy eating |
| Capozzi 2012 | Protocol article with no follow‐up publications |
| Chlebowski 2008 | As in paper by Reddy 2005 (WINS trial): participants were not survivors but were early‐stage breast cancer patients. In addition, some participants were receiving chemotherapy at recruitment |
| Chlebowski 2013 | Review article |
| Chlebowski 2015 | (WINS Trial) Participants were not survivors but were early‐stage breast cancer patients. In addition, some participants were receiving chemotherapy at recruitment |
| De Waele 2015 | Intervention took place at time of treatment |
| del Rocio Berglund 2012 | (FASEB abstract 626.6) Intervention uses supplementation |
| del Rocio Berglund 2012a | (FASEB abstract 1024.2) Intervention uses supplementation |
| Delvin 2014 | Participants were not human |
| Demark‐Wahnefried 2008 | Intervention took place before and during participants' chemotherapy treatment |
| Dennis Parker 2014 | Not an RCT |
| Djuric 2010 | Participants were still receiving chemotherapy when randomised to the intervention |
| Emond 2010 | Intervention was not dietary |
| Ferdowsian 2007 | Review article |
| Flynn 2010 | Intervention uses olive oil ‐ enriched or low‐fat diet |
| Frensham 2014 | Intervention was not dietary, and trial was quasi‐randomised |
| Fukui 2014 | Intervention was acupuncture ‐ not dietary |
| Garrett 2013 | Not an RCT |
| George 2015 | Not an RCT |
| Giallauria 2014 | Intervention was not dietary |
| Goodwin 2019 | On active treatment |
| Hagemann 2019 | Study design |
| Haggerty 2014 | Intervention was focused on comparison of 2 technologies |
| Hershman | Intervention was not dietary |
| Ho 2013 | Protocol article with no follow‐up publications |
| Hung 2013 | Participants received stem cell transplantation |
| James 2011 | Protocol article with no follow‐up publications |
| James 2015 | Data for cancer survivors could not be extracted |
| Ko 2010 | Uses the same data from the NC SRIDES study (Campbell 2009), which was excluded because the intervention used only a single food group, which is not representative of healthy eating |
| Koner 2012 | Not an RCT |
| Koutoukidis 2019 | Study design |
| Kwiatkowski 2017 | Dietray intervention was not clearly specified |
| Lee 2014 | Intervention was not dietary |
| Lee 2018 | 2 × 2 factorial randomised controlled trial, where results are collapsed so the comparison of interest (dietary intervention vs control) is not presented in the manuscript |
| Li 2008 | Intervention uses supplementation |
| Ligibel 2019 | Review |
| Lynch 2014 | Outcomes were focused on sedentary behaviour ‐ not on nutritional status/dietary intake |
| McCarroll 2014 | Intervention uses supplementation |
| McDonald 2014 | Protocol article with no follow‐up publications |
| Moriya 2014 | Intervention uses immune‐enhancing diet |
| Nelson 2008 | Review article |
| O'Neill 2010 | Recruited participants were receiving androgen deprivation therapy for treatment of prostate cancer |
| Park 2019 | Design |
| Pasanisi 2009 | Not sure if trial is randomised. Not enough details on participants, methods, or outcomes |
| Paxton 2012 | Intervention uses supplementation |
| Pellegrini 2014 | Review article |
| Rack 2010 | Review article and protocol with no follow‐up publications. |
| Reddy 2005 | (WINS Trial) Participants were not survivors but were early‐stage breast cancer patients. In addition, some participants were receiving chemotherapy at recruitment |
| Rock 2015 | Intervention was not dietary |
| Sedlacek 2011 | Not an RCT |
| Song 2015 | Not an RCT |
| Stacey 2017 | Results reported only data for the intervention group at 12 months ‐ not for the control group |
| Stricker 2013 | Participants were quasi‐randomised |
| Thiebaut 2006 | Editorial article |
| Thomas 2009 | Not an RCT; conducted for development of a tool only |
| Thompson 2012 | Not an RCT |
| Thomson 2014 | Not an RCT |
| Tyagi 2005 | Intervention was not dietary |
| Urowitz 2012 | Not an RCT |
| Van Der Werf 2015 | Protocol only |
| Villasenor 2014 | Outcomes focused on inflammation ‐ not on nutritional status/dietary intake |
| Vona‐Davis 2015 | Primarily intervention was exercise‐based. Dietary intervention included only advice on reduction of calorie intake |
| Xing 2014 | Review article |
RCT: randomised controlled trial.
Characteristics of studies awaiting assessment [ordered by study ID]
Brown 2018.
| Methods |
Design: phase II randomised trial Country: USA Accrual dates: NR Trial Reg: NCT01978899 |
| Participants |
Number randomised: 60 Inclusion: body mass index (BMI) > 25.0 kg/m²; completion of all surgery, chemotherapy, and/or radiation at least 1 month before study enrolment (concurrent treatment with adjuvant hormonal or biologic therapies was acceptable); ECOG performance status of 0 (fully active without restriction) or 1 (restricted in physically strenuous activity, but ambulatory); and ability to walk 2 city blocks and to speak and read English Exclusion: other serious medical conditions such as unstable cardiovascular disease or digestive disorders that would preclude participation in a physical activity and dietary intervention Gender: female n = 58, male n = 3 Age: 52 ± 9 years Type of cancer: Breast: intervention 24 (80%), control 22 (73%) Gynecological: intervention 0 (0%), control 4 (13%) Hematological: intervention 2 (7%), control 2 (7%) Genitourinary: intervention 1 (3%), control 1 (3%) Gastrointestinal: intervention 1 (3%), control 1 (3%) Sarcoma: intervention 2 (7%), control 0 (0%) Therapy previously received for cancer: Chemotherapy: intervention 19 (63%), control 21 (70%) Radiation: intervention 22 (73%), control 18 (60%) Hormone: intervention 20 (67%), control 22 (73%) Cancer stage: NR Ethnicity: White:intervention 26 (87%), control 26 (87%) Black: intervention 2 (7%), control 1 (3%) Other: intervention 2 (7%), control 3 (10%) Education: High school or less: intervention 1 (3%), control 4 (13%) Some college: intervention 5 (17%), control 5 (17%) College degree or more: intervention 24 (80%), control 21 (70%) |
| Interventions |
Comparison: weight loss intervention vs wait‐list control Intervention: weight loss intervention participated in a 15‐week, in‐person, group‐based programme that was led by a health coach with a background in nutrition and an exercise physiologist. Behavioural content of the programme described herein was modelled after the Lifestyle Intervention in Adjuvant Treatment of Early Breast Cancer (LISA) study Control: wait‐list control |
| Outcomes | Primary aim of the study was to evaluate the efficacy of the intervention in lowering body mass. Secondary aims of the study were to evaluate effects of the intervention on body composition, physical fitness, and concentrations of serum biomarkers linked to cancer risk and prognosis |
| Notes | Funding: a grant from the Friends of Dana‐Farber Cancer Institute |
Parekh 2018.
| Methods |
Design: pilot randomised controlled trial Country: USA Accrual dates: NR Trial Reg: NR |
| Participants |
Number randomised: 59 Inclusion: 18+ years of age; diagnosed with breast cancer; speak, read, and understand English fluently; completed prescribed treatment (surgery, chemotherapy, and/or radiation therapy) within the previous 6 months at the Perlmutter Cancer Center. Willing to attend all scheduled data collection visits, to complete questionnaires, and to attend educational sessions Exclusion: pregnant women and women with a poor prognosis (survival < 6 months per oncologist) Gender: female Age: mean 57.7 years Type of cancer: breast Therapy previously received for cancer: NR Cancer stage: NR Ethnicity: Asian: intervention 1 (3.6), control 2 (6.5) Black or African American: intervention 8 (28.6), control 5 (16.1) White: intervention 19 (67.9), control 21 (67.7) American Indian/Alaskan Native: intervention 0, control 2 (6.5) Other race: intervention 0, control 1 (3.2) Education: Did not complete high school: intervention 0, control 0 High school: intervention 2 (7.1), control 0 Some college: intervention 7 (25.0), control 9 (29.0) Bachelor’s degree: intervention 7 (25.0), control 11 (35.5) Master’s degree: intervention 11 (39.3), control 11 (35.5) PhD: intervention 1 (3.6), control 0 |
| Interventions |
Comparison: 6 nutritional education sessions vs brochure information Intervention: nutrition literacy training curriculum education on breast cancer and nutrition in the context of the disease, over a period of 3 months (6 sessions; 12 hours in total) Control: nutrition information brochures developed by the American Institute for Cancer Research for cancer survivors |
| Outcomes | Nutrition literacy: assessment instrument for breast cancer patients (NLit‐BCa) was administered at baseline and at completion of the study Fruit and vegetable intake: estimated using a validated block screener that ranks individuals with regard to consumption Height and body weight: measured by standard procedures using a stadiometer; weight was measured by standard procedures using a digital weighing scale Health literacy: Newest Vital Sign (NVS) tool was developed by Pfizer Inc. and is a validated method to assess health literacy |
| Notes | Funding: New York University’s Perlmutter Cancer Center (PCC) for internal funding |
Zuniga 2019.
| Methods |
Design: 2‐arm randomised controlled trial Accrual dates: NR Trial Reg: NR |
| Participants |
Number randomised: 153 Inclusion: overweight and obese (BMI ≥ 25 kg/m²), early‐stage (0 to III), English‐speaking breast cancer survivors who had completed their treatment 2 or more months before study enrolment Exclusion: NR Gender: female Age: intervention: mean (SD) 55.3 ± 10.3 years; control: mean (SD) 58.4 ± 8.2 years Type of cancer: breast Therapy previously received for cancer: Surgery: intervention 57 (95.0), control 60 (92.3) Chemotherapy: intervention 37 (61.7), control 45 (69.2) Radiation: intervention 38 (63.3), control 39 (60.0) Hormonal therapy: intervention 16 (26.7), control 26 (40.0) Antibody therapy: intervention 6 (10.0), control 7 (10.8) Reconstruction: intervention 23 (38.3), control 25 (38.5) Cancer stage: Stage 0: intervention 5 (8.3), control 7 (10.8) Stage 1: intervention 18 (30.0), control 17 (26.2) Stage 2: intervention 20 (33.3), control 18 (27.7) Stage 3: intervention 8 (13.3), control 13 (20.0) Don’t know: intervention 9 (15.0), control 10 (15.4) Ethnicity: Anglo: intervention 25 (41.7), control 28 (43.1) Latino: intervention 31 (51.7), control 33 (50.8) Other: intervention 4 (6.7), control 4 (6.2) Education: High school graduate or less: intervention 9 (15.0), control 7 (10.8) Some college/Assoc degree: intervention 24 (40.0), control 17 (26.2) College graduate or higher: intervention 27 (45.0), control 41 (63.1) |
| Interventions |
Comparison: individualised anti‐inflammatory dietary vs standard care Intervention: individualised anti‐inflammatory dietary prescriptions and behaviour change cues through 6 monthly workshops (culinary demonstrations, recipes, and meal planning), reinforced by evidence‐ and theory‐based patient navigation, motivational interviewing, and tailored newsletters personalised to individual readiness for change Control: minimal nutritional information at baseline, monthly American Institute for Cancer Research informational brochures, and 2 telephone calls before assessment appointments |
| Outcomes | Anthropometric data: Questionnaires for Barriers to Care, social support, depression scale (CES‐D), coping self‐esteem, family health history, health Behaviour change, Cancer Worry Scale, FACT‐G, FACT‐B (Breast Cancer Functional Assessment of Cancer Therapy Scale), self‐efficacy, IPAQ short (last 7 days), PSS‐14 (Perceived Stress Scale), Brief Family Life Questionnaire, Apter Motivational Style Profile, stages of change 3‐Day food diary Biomarkers |
| Notes | Funding: supported by Susan G. Komen (SAB08‐0005); Redes en Accion: The National Latino Cancer Research Network (U54CA153511); the Institute for Health Promotion Research at UT Health San Antonio; and the UT Health San Antonio Mays Cancer Center through the NCI Cancer Center Support Grant (P30 CA054174) |
BMI: body mass index.
CES‐D: Center for Epidemiologic Studies Depression Scale.
ECOG: Eastern Cooperative Oncology Group.
FACT‐B: Functional Assessment of Cancer Therapy ‐ Breast.
FACT‐G: Functional Assessment of Cancer Therapy ‐ General.
IPAQ: International Physical Activity Questionnaire.
NR: not reported.
PSS‐14: Perceived Stress Scale.
Characteristics of ongoing studies [ordered by study ID]
Alberts 2008.
| Trial name or title | Diet and physical activity change or usual care in improving survival in patients with previously treated stage II, III, or IV ovarian, fallopian tube, or primary peritoneal cancer |
| Methods | Randomised phase III trial |
| Participants | Patients with previously treated stage II, III, or IV ovarian, fallopian tube, or primary peritoneal cancer. After treatment, participants will be randomised to either healthy lifestyle counselling or usual care |
| Interventions |
Group 1: (lifestyle intervention) Participants receive a dietary intervention designed to promote increased levels of plasma carotenoids, to control weight, and to ensure adequacy of micronutrient intake. Participants also undergo a physical activity intervention comprising a moderately low aerobic regimen, face‐to‐face counselling on how to read food labels to estimate grams of fat per serving, and telephone counselling by a lifestyle intervention counsellor once a week for 4 weeks, then twice a month for 6 months, monthly for the subsequent 6 months, and then once every other month for 11 months. Participants complete daily fat gram and step diaries at least 3 times per week GROUP 2: (comparison lifestyle) Participants receive a study notebook containing general study‐related information. Participants are not asked to record diet or physical activity but are provided a single sample diary in their study notebook. Participants receive telephone contact on a sliding scale similar to the intervention group, but at less frequent intervals (22 vs 33 calls over the course of the intervention) After completion of the study, participants are followed every 3 months for 2 years, every 6 months for 3 years, and then annually thereafter |
| Outcomes | Biomarkers (e.g. total carotenoid), survival, compliance, QoL, bowel function, sleep duration/quality, anthropometry, dietary intake, telomere length Biomarkers to be measured at baseline and at 6, 12, and 24 months. After completion of the study, participants are followed every 3 months for 2 years, every 6 months for 3 years, and then annually thereafter |
| Starting date | July 2008 |
| Contact information | David Alberts |
| Notes |
Anderson 2017.
| Trial name or title | The women’s wellness after cancer program: a multi‐site, single‐blinded, randomised controlled trial protocol |
| Methods | Single‐blinded, multi‐centre, randomised controlled trial |
| Participants | Women treated for blood, breast, and gynaecological cancer within 24 months of completion of chemotherapy (primary or adjuvant) and/or radiotherapy |
| Interventions | Intervention: comprises an evidence‐based interactive iBook and journal, web interface, and virtual health consultations by an experienced cancer nurse trained in delivery of the WWACP. The 12‐week intervention focuses on evidence‐based health education and health promotion after a cancer diagnosis, incorporating promotion of physical activity, good diet, smoking cessation, and reduction of alcohol intake, plus strategies for sleep and stress management. The programme is based on Bandura’s social‐cognitive theoretical framework Control: usual care |
| Outcomes | The primary outcome is health‐related quality of life, as measured by the Functional Assessment of Cancer Therapy ‐ General (FACT‐G). Secondary outcomes are menopausal symptoms as assessed by the Greene Climacteric Scale; physical activity elicited with the Physical Activity Questionnaire Short Form (IPAQ‐SF); sleep measured by the Pittsburgh Sleep Quality Index; habitual dietary intake monitored with the Food Frequency Questionnaire (FFQ); alcohol intake and tobacco use measured by the Australian Health Survey and anthropometric measures including height, weight, and waist‐to‐hip ratio. All participants were assessed with these measures at baseline (at the start of the intervention), at 12 weeks (at completion of the intervention), and at 24 months (to determine the level of sustained behaviour change) |
| Starting date | 21/10/2014 |
| Contact information | Prof Debra Anderson School of Nursing and Midwifery Griffith University / Menzies Health Institute Building G16, Clinical Sciences Gold Coast Campus Parklands Drive Southport Queensland 4222 debra.anderson@griffith.edu.au |
| Notes |
Asprey 2009.
| Trial name or title | Promotion of healthy lifestyle and risk modification for cancer survivors and their partners/caregivers (ENRICH: exercise and nutrition routine improving cancer health) |
| Methods | A wait‐list randomised controlled trial |
| Participants | Patients who have been diagnosed with cancer of any type and have completed all active treatment. Participants are either cancer survivors or carers |
| Interventions | The ENRICH intervention provides education and information for participants to set up their own home‐based walking programme with pedometer, their own resistance training programme with Gymstick (exercise stick with elastic tubing), and healthy diet info provided via 6 face‐to‐face, 2‐hour sessions, over an 8‐week period The wait‐list control group will receive the intervention after the programme has been evaluated |
| Outcomes | Physical activity, self‐reported step counts, fruit and vegetable intake, BMI, waist circumference, social support levels, QoL, mediators of physical activity changes Outcome measures at baseline and at 8 weeks (endpoint), 20 weeks, and 12 months |
| Starting date | December 2009 |
| Contact information | Ms Garielle Asprey 153 Dowling Street, Woolloomooloo NSW 2011., Australia +61 2 93341772 gabriellea@nswcc.org.au |
| Notes |
Blomhoff 2012.
| Trial name or title | Effect of the new Norwegian food‐based dietary guidelines on chronic diseases in colorectal cancer |
| Methods | Parallel randomised controlled trial |
| Participants | Patients diagnosed with colorectal cancer stage I to III, who have finished cancer treatment and are 50 to 75 years of age. Participants are randomised to an intensive dietary intervention, based on the new Norwegian food‐based dietary guidelines; in addition, focus is placed on foods that have been shown to inhibit inflammation or oxidative stress |
| Interventions | The intervention will aid participants in undertaking a diet based on the new Norwegian food‐based dietary guidelines. This will include access to clinical nutritionists, free food, food discounts, cooking courses, cookbook/recipes, study website, and organised physical activity The control group will receive counselling on physical activity only |
| Outcomes | Biomarkers of comorbid conditions, oxidative stress and inflammation, compliance tested by biomarkers in blood, physical function, grip strength, physical activity level, dietary pattern Outcomes measured at baseline and at 6 months and 12 months |
| Starting date | March 2012 |
| Contact information | Rune Blomhoff University of Oslo |
| Notes |
Cirauqui 2014.
| Trial name or title | Prevention of breast cancer recurrence through weight control, diet, and physical activity intervention (PREDICOP) |
| Methods | Randomised controlled trial |
| Participants | Breast cancer patients with stage I, II, IIIA (or T1‐3, N0‐N2, M0) at diagnosis, randomised to intervention or control group |
| Interventions | Intervention group: lifestyle intervention combining weight control, diet, and physical activity Control group: minimal diet intervention and minimal physical activity intervention |
| Outcomes | Time frame: 5 years from recruitment day Primary outcome measures: time to local and distant recurrence Secondary outcome measures: overall survival, disease‐free survival, quality of life (time frame: baseline, 1 year, and 3 years) Other outcome measures: changes in biomarkers (time frame: baseline and 1 year ) |
| Starting date | January 2014 |
| Contact information | Contact: Antonio Agudo, MD, PhD; +34 932607401; a.agudo@iconcologia.net Contact: Noemie Travier, MSc; +34 932607401; ntravier@iconcologia.net |
| Notes |
Clinton 2014.
| Trial name or title | Harvesting health programme in improving diet and physical activity level in cancer survivors |
| Methods | Pilot clinical trial with single group assignment |
| Participants | Cancer survivors who have completed cancer treatment within the previous 12 months. Participants must have a computer, Internet access, and an active email account |
| Interventions | Intervention arm: supportive care (Harvesting Health Programme). Participants undergo a series of 10 educational and training sessions over 1 hour every 2 weeks, comprising education on current research, evidence‐based health guidelines, application techniques, reference materials specific to extended‐stage cancer survivors, and recommendations and personal health goals for survivorship |
| Outcomes | Behaviour change, biomarker levels assessed by values for the health and wellness index, diet, physical activity, QoL Outcomes measured from baseline up to 12 months |
| Starting date | October 2014 |
| Contact information | Steven Clinton Ohio State University, Comprehensive Cancer Centre |
| Notes |
Coups 2009.
| Trial name or title | Internet‐based weight loss program for colorectal cancer survivors. |
| Methods | Randomised phase I wait‐list trial |
| Participants | Patients who have survived colorectal cancer (stage I to III) and completed treatment up to 10 years ago. Participants must have no current evidence of cancer and must have access to the Internet at home or at work |
| Interventions | Arm I (12‐week Internet‐based weight loss intervention): patients attend an in‐person 60‐minute session with a health educator. The health educator will provide basic weight loss advice according to established guidelines and advice on diet modification to reduce calorie intake. The health educator will also recommend gradual increase in physical activity and will help patients set a realistic target weight to achieve at the end of the 12‐week intervention period. The health educator will introduce the participant to the intervention website and will assist in setting up access. Participants are given a 1‐page written summary on how to use the website. Participants are advised to log in to the website twice a week during the 12‐week intervention period Arm II (wait‐list control): patients are instructed to continue their usual dietary and physical activity routines during a 12‐week period. After the waiting period, patients receive the Internet‐based weight loss intervention for 12 weeks as in arm I |
| Outcomes | Patients in both arms complete surveys at baseline and at 12 months to assess sociodemographics, disease and treatment characteristics, prior Internet experience, depressive symptoms, weight, dieting and weight loss experiences, weight loss expectations, physical activity, and dietary outcome expectancies Patients in arm II complete an additional follow‐up survey at 24 weeks |
| Starting date | December 2009 |
| Contact information | Elliot Coups Rutgers, The State University of New Jersey |
| Notes |
Demark‐Wahnefried 2019.
| Trial name or title | |
| Methods |
Design: single‐blinded randomised clinical trial Country: USA Accrual dates: NR Trial Reg: NCT04000880 |
| Participants | Age 50 years or older; resident of Alabama, Mississippi, North Carolina, or Tennessee in the United States; diagnosed with multiple myeloma or localised kidney or ovarian cancer; or (localised (includes in situ) through regional) breast, colorectum, endometrium, or prostate cancer |
| Interventions | Participants will participate in 1 of 3 arms
|
| Outcomes | Primary outcomes: Change in dietary quality and intake (patient‐reported outcome) Change in body weight Change in physical activity and sleep Physical activity and sleep will be measured objectively via blank screen accelerometers, which are small devices (1 × 2 inches) that will be worn at the waist during waking hours and switched to a wrist band during sleep for a 7‐day period at each assessment point Secondary outcomes: Change in waist circumference Waist circumference Change in muscle mass To assess muscle mass, 3 days before home assessment, participants will take a capsule containing deuterium‐labelled creatine (creatine is a substance commonly found in protein‐containing foods, and deuterium is a naturally occurring element), administration of which is proven as safe with several studies conducted in humans across the lifespan from pre‐term infants to elders Change in physical performance Participants will complete the Senior Fitness Battery during in‐person assessments, which includes chair stands, 3‐metre walk, sit‐to‐stand, reaching (stretching exercises of the arms and legs), and the 2‐minute step test Change in physical activity (patient‐reported outcome) Physical activity will be measured via validated questionnaires (e.g. the Godin Leisure Time Exercise Questionnaire) Change in quality of life (patient‐reported outcome) Participants will complete questionnaires (PROMIS Cancer‐Related Item Bank and Short Form‐12) to self‐report quality of life Change in healthcare utilisation (patient‐reported outcome) Participants will complete a healthcare utilisation survey to capture physician and emergency room visits and hospitalisations |
| Starting date | June 2019 |
| Contact information | Wendy Demark‐Wahnefried, PhD, University of Alabama at Birmingham; tel. 833‐535‐7934; AMPLIFY@UABMC.EDU |
| Notes | Protocol |
Dittus 2012.
| Trial name or title | An Internet‐based weight loss and exercise intervention for breast cancer survivors (iWEB) |
| Methods | Single‐arm intervention of a 24‐week weight loss and exercise intervention |
| Participants | Survivors of breast cancer who have completed all treatment 2 to 12 months before study initiation. BMI between 27 and 50 kg/m² with access to a computer and the Internet |
| Interventions | Patients will participate in weekly online chats about behavioural and diet modifications led by a qualified facilitator. Participants will also engage in increasing amounts of aerobic activity throughout the course of the intervention. The intervention will last 6 months, and diet and body measures will be taken at baseline and at the end |
| Outcomes | Diet measures to work out total calorie and fat intake, anthropometrics (weight, BMI, body fat %), active energy expenditure, inflammatory biomarkers Outcomes measured at baseline and at 6 months (intervention endpoint) |
| Starting date | July 2012 |
| Contact information | Kim L Dittus, University of Vermont |
| Notes |
Ferrante 2016.
| Trial name or title | Virtual weight loss programme in maintaining weight in African American breast cancer survivors |
| Methods | Randomised controlled trial |
| Participants | Breast cancer survivors with previous invasive carcinoma at stage IA to IIIC, randomised to control or intervention group |
| Interventions | Experimental: group I (SparkPeople programme). Patients receive one 30‐minute session with the research assistant for training on how to use the SparkPeople website, and may request additional training if needed. Patients are instructed to self‐monitor their diet at least weekly using SparkPeople and physical activity levels daily using the Fitbit monitoring device, which integrates with the SparkPeople programme. Patients receive weekly motivational reminders to log into the website for 3 months via email, text, or phone, based on patient preference (active phase). Patients then enter the maintenance phase for an additional 3 months without reminders Active comparator: group II (wait‐list). Patients receive the weight loss handout and a Fitbit health monitoring device and proceed with their usual life. After 6 months, patients receive the SparkPeople treatment as in Group I |
| Outcomes | Time frame: up to 12 months Primary outcome measures: changes in weight, recruitment, retention rate Secondary outcome measures: changes in caloric intake, in cardio‐metabolic risk factors, in cardiopulmonary fitness, in physical activity, in quality of life for social‐cognitive theory variables Patient feedback on programme, as measured by semi‐structured interview |
| Starting date | May 2015 |
| Contact information | Principal Investigator: Jeanne Ferrante, 732‐743‐3222; Rutgers Cancer Institute of New Jersey, New Brunswick, New Jersey, United States 08903 |
| Notes |
Frensham 2013.
| Trial name or title | STRIDE (Steps Toward Improving Diet and Exercise): an online lifestyle intervention for cancer survivors living in South Australia |
| Methods | Parallel randomised controlled trial |
| Participants | Women and men with any type of cancer (except skin cancer) treated with curative intent. Not undergoing any active treatment at point of recruitment |
| Interventions | Intervention participants will take part in a 12‐week lifestyle programme, during which they will wear a pedometer and will use an online resource. The main focus of the online resource is a step log, where participants enter their daily step counts, perceptions of exertion, and feelings daily. Based on this information, they will be emailed weekly step count goals. The website will also include a virtual notice board, where community service organisers can advertise events and activities related to physical activity and healthy eating. Info on healthy eating will be provided based on the cancer council Australian nutrition guidelines, which supports the recommendations provided in the Australian Guide to Healthy Eating |
| Outcomes | Adherence measured by logins to the website and follow‐up telephone calls every 2 weeks, anthropometry, physical and psychological health data, physical activity, QoL, dietary habits Outcomes measured at baseline and at endpoint (12 weeks) |
| Starting date | April 2013 |
| Contact information | Miss Lauren Frensham, University of South Australia, GPO Box 2417, Adelaide SA 5001, Australia +61 08 8302 2680 lauren.frensham@mymail.unisa.edu.au |
| Notes |
Greenlee 2011.
| Trial name or title | Exercise, diet, and counselling in improving weight loss in overweight female breast or colorectal cancer survivors |
| Methods | 12‐month phase II single group assignment trial |
| Participants | Women with a previous diagnosis of stage I, II, or III invasive breast cancer or colorectal cancer with no evidence of the disease at the time of registration. Participants must have a BMI of 25 kg/m² or above and must be willing to attend the Curves fitness centre at least 3 times per week for 12 months |
| Interventions | Participants are instructed to practice 30 to 45 minutes of medium‐hard exercise 5 to 7 days a week at a Curves fitness centre or outside Curves for 12 months. Participants receive written materials on physical activity guidelines and the Curves fitness and weight management plan and are instructed to follow a higher carb diet plan, which promotes a 1500‐kcal/d diet that is high in fruits and vegetables consisting of 30% protein, 45% carbohydrates, and 25% fat. In addition, participants receive the dietary guidelines for cancer survivors, which recommend eating 5 or more servings of fruit/vegetables per day or a diet that is high in whole grains, low in sat fat, low in sugary foods, and low in alcohol. Participants receive 14 behavioural counselling sessions by telephone with the goal of increasing intervention adherence and participant retention. Each session lasts 40 minutes and occurs weekly on weeks 1 to 5 and then every 6 weeks by month 6. Participants also receive monthly email newsletters with health tips and motivational messages |
| Outcomes | Dietary intake, QoL, anthropometric measures, body fay %, biomarkers Outcomes measured at baseline and at 6 months and 12 months. Further participant follow‐up at 24 and 36 months |
| Starting date | October 2011 |
| Contact information | Heather Greenlee, Herbert Irving Comprehensive Cancer Centre, Southwest Oncology Group |
| Notes |
Groarke 2018.
| Trial name or title | |
| Methods |
Design: pilot randomised controlled trial retrospective Country: Ireland Accrual dates: NR Trial Reg: ISRCTN18676721 |
| Participants | Overweight/obese cancer survivors |
| Interventions | The study is employing a 2 groups (experimental and control) x 3 time points (baseline, 3 months, 6 months) mixed analysis of variance design to investigate the impact of a personalised solution (wear a Fitbit activity monitor, using a personalised dietary or physical activity intervention that will employ an educational component along with a shared decision‐making and goal‐setting model) vs standard care on primary and secondary health outcomes |
| Outcomes | Primary outcomes: Average daily step count, measured via Fitbit device continuously for 6 months BMI and weight Secondary outcomes: Sleep quality, measured via Fitbit device continuously for 6 months The following measures are recorded Physical fitness, measured using 6‐minute walk test ‐ resting HR, BP, SpO2, recovery HR, BP, SpO2 Dietary behaviour, measured using Food Frequency Questionnaire General health status (MOS SF‐36; Ware et al, 2000), fatigue (Mendoza, Wang, Cleeland, et al., 1999), self‐efficacy (Schwarzer & Jerusalem, 2010), exercise self‐efficacy (Bandura, 2006), exercise‐related social support (Sallis et al, 1987) |
| Starting date | August 2017 |
| Contact information | Dr. Jenny Groarke, National University of Ireland, Galway |
| Notes | Protocol |
Heinrich 2015.
| Trial name or title | ASCOT: lifestyle study for cancer survivors |
| Methods | Randomised controlled trial, single randomisation only |
| Participants | Patients who received a diagnosis of breast, colorectal, or prostate cancer in 2012/2013, and who express an interest in taking part in a trial of a lifestyle programme from 7 NHS Trusts across London and Essex |
| Interventions | Intervention group: the ‘Healthy Habits for Life’ intervention consists of a self‐guided printed booklet designed to help cancer survivors make healthy lifestyle behaviours habitual. Participants receive a telephone call from a researcher who will talk participants through the printed booklet, to check understanding, and to answer any questions and encourage engagement with the material Usual care group: did not receive any specific advice |
| Outcomes | Time frame: 0, 3, and 6 months Primary outcome measures: composite health behaviour risk index Secondary outcome measures: alcohol; dietary intake, physical activity, quality of life, sleep, smoking status |
| Starting date | February 2015 |
| Contact information | Dr Rebecca J Beeken; r.beeken@ucl.ac.uk |
| Notes |
Ho Wai‐chu 2012.
| Trial name or title | Diet and physical activity intervention in CRC survivors |
| Methods | A 12‐month phase II feasibility trial |
| Participants | Both males and females with histologically proven colorectal adenocarcinoma and within 1 year of completion of main cancer treatment |
| Interventions | Participants will be randomised in a 2 x 2 factorial design for 2 targeted behaviours prescribed over 12 months: Dietary intervention to meet the target of (1) < 5 servings of red/processed meat weekly; < 2 servings would be processed meat 2.2 servings of refined grains daily Physical activity intervention with the following targets: (1) general health target ‐ 30 minutes of moderate to vigorous physical activity (MVPA) 5 days per week (i.e. 10 MET‐hours/week); (b) cancer outcome target ‐ 60 minutes of MVPA 5 days per week (i.e. 18 to 20 MET‐hours/week) Meeting both dietary and physical activity target interventions No Intervention: usual care ‐ following general lifestyle advice in accordance with the recommendations of the Department of Health in Hong Kong available in the public domain Primary outcome measure is whether target levels of PA and dietary intake could be met at the end of the intervention |
| Outcomes | Outcomes were measured at 6, 12, 18, and 24 months: Physical activity, dietary changes, rates of compliance, determinants of compliance, facilitators and barriers to intervention, measurement of theoretical constructs underlying physical activity, dietary interventions Outcomes measured post intervention only at 12 and 24 months: Body composition, physical fitness, quality of life |
| Starting date | October 2012 |
| Contact information | Dr. Ho Wai‐cho Judy, The University of Hong Kong. |
| Notes |
Irwin 2014.
| Trial name or title | Lifestyle, exercise, and nutrition study 2 (LEAN 2) |
| Methods | A parallel randomised controlled trial |
| Participants | Those with a diagnosis of breast cancer ‐ American Joint Committee on Cancer (AJCC) stages 0 to IIIC, with BMI > 25 kg/m² Completed surgery, chemotherapy, and radiation at least 2 months ago and physically able to exercise |
| Interventions | Intervention arm: intervention will be based on the Diabetes Prevention Program weight loss programme, which uses a combination of reduced caloric intake, increased physical activity, and behaviour therapy. Content of the weight loss programme will be similar for the in‐person and telephone interventions, but the approach will vary (i.e. in‐person vs telephone counselling). Participants will be taught diet, exercise, and behaviour change strategies via the telephone (weekly calls for month 1, every other week for months 2 and 3, and monthly for months 4 to 6). All lessons and diet and physical activity logs will be mailed to participants at the beginning of the programme. Participants will record their daily diet and exercise in the logs Control arm: usual care/wait‐list ‐ at 6 months, participants in the wait‐list group may choose to participate in the 11 sessions either in‐person or via telephone or a combination of the two modes of delivery. They will also be offered the opportunity to return to Yale at 12 months (immediately after the end of the 6‐month counselling sessions) to have weight and DEXA measured |
| Outcomes | BMI, weight, height, body fat percentage, hormones, physical activity, dietary intake All outcomes are measured at baseline and at 6 months |
| Starting date | April 2014 |
| Contact information | Dr Melinda L Irwin, Yale University, USA 203 785 6392 Melinda.irwin@yale.edu |
| Notes |
Jernigan 2015.
| Trial name or title | Weight loss referral for healthier survivorship in obese stage I and II endometrial cancer survivors or atypical hyperplasia |
| Methods | Pilot clinical trial with single group assignment |
| Participants | Women with a history of stage I or II endometrial cancer or a diagnosis of complex atypical hyperplasia and a BMI of at least 30 kg/m² |
| Interventions | Patients are referred to a weight loss specialist for assistance with weight loss, and medical chart reviews are performed at baseline and every 3 months for 24 months |
| Outcomes | Accrual with intervention defined as number of patients who agree to participate, compliance with intervention, weight loss, compliance with lifestyle changes, incidence of obesity, progression‐free survival, overall survival, recurrence rate, level of functioning, quality of life, and symptoms Outcomes are collected at baseline, at 12 months, and at 24 months. Patients are also contacted at 90 days to determine whether they have initiated any weight loss interventions |
| Starting date | January 2015 |
| Contact information | Amelia Jernigan, Case Comprehensive Cancer Centre |
| Notes |
Lawler 2014.
| Trial name or title | Get healthy after breast cancer ‐ examining the feasibility and acceptability of referring breast cancer survivors to the NSW ‘Get Healthy Service’ – a telephone‐delivered programme targeting physical activity, healthy diet, and weight loss |
| Methods | A single‐group feasibility study |
| Participants | Females, aged 18 to 75 years, with a first diagnosis of stage I to III breast cancer (unilateral or bilateral). To have completed primary treatment with curative intent (i.e. initial surgery, chemotherapy, radiation therapy) within the past 12 months (endocrine or targeted therapies may be ongoing). Scheduled to return to the breast cancer clinic where they were recruited into the study for a follow‐up appointment within approximately 6 months of starting the study |
| Interventions | Participants will receive 6 months of telephone counselling from the 'NSW Get Healthy Information and Coaching Service' (GHS). The GHS is a free telephone counselling programme designed to help participants increase their physical activity, improve their diet, and achieve and maintain a healthy weight. Participants receive up to 10 coaching calls, with an average duration of 13 minutes, over a period of 6 months (up to 6 calls during the first 12 weeks, and up to 4 calls in the remaining 14 weeks). GHS health coaches are all university qualified health professionals, such as psychologists, nurses, dietitians, exercise physiologists, sports scientists, social workers, and physiotherapists. They receive further training to ensure they meet the requirements of the GHS |
| Outcomes | GHS uptake and number of calls (from GHS records), women’s satisfaction with the GHS, weight, dietary intake, physical activity, quality of life, fatigue, depression, body image, menopausal symptoms Outcomes measured at baseline and at 6 months (endpoint) |
| Starting date | January 2014 |
| Contact information | Dr Sheleigh Lawler, The University of Queensland, School of Population Health, Cancer Prevention Research Centre, Level 4 Public Health Building, Herston Road, HERSTON, QLD, 4006, Australia +61 7 3365 5544 s.lawler@sph.uq.edu.au |
| Notes |
Lechner 2012.
| Trial name or title | Lifestyle change, self‐management, and problem‐solving in daily life in cancer survivors; an online portal for change and support |
| Methods | Parallel randomised controlled trial |
| Participants | Patients who were previously diagnosed with cancer and successfully completed the main treatment period, up to 1 year ago |
| Interventions | Cancer survivors in the experimental group who enter the online portal to receive general information on dealing with distress, obtaining social support, self‐managing disease, and optimising healthy lifestyles. They are free to fill out a questionnaire on personal needs, which will lead them to tailored advice about the mentioned topics. Also information about possible other helpful interventions and social workers is provided. Cancer survivors in the experimental group are free to enter the online portal as often as they want during 1 year The wait‐list control group will be provided with access to the online intervention after the last measurement |
| Outcomes | Psychosocial distress, anxiety and depression, quality of life, physical activity, smoking behaviour, alcohol consumption, dietary behaviour, habit strength, self‐management, empowerment, perceived social support, perceived peer support All outcomes are measured at baseline and at 3, 6, and 12 months |
| Starting date | March 2012 |
| Contact information | Prof. L. Lechner, University Maastricht (UM), Open University the Netherlands |
| Notes |
Ligibel 2013.
| Trial name or title | Healthy living after cancer: weight management pilot study |
| Methods | A 16‐week crossover randomised controlled trial |
| Participants | History of any malignancy and completed all adjuvant surgery, chemotherapy, and/or radiation at least 1 month before study enrolment (patients receiving ongoing hormonal or biologic therapy are eligible to participate). BMI > 25 kg/m² and ECOG performance status of 0 or 1. Physically able to exercise and physician consent to start a weight loss programme |
| Interventions | Immediate weight loss programme group: patients will also be provided with exercise and dietary goals to implement at home. The programme consists of 16 sessions focused on reducing calories and increasing exercise. Sample goals of the programme include reducing weight by 1 or 2 pounds per week and increasing exercise to at least 150 minutes of moderate exercise (such as walking) per week. Weight loss sessions will take place once per week for 16 weeks at Dana‐Farber. The participant will meet with a dietitian and an exercise specialist weekly during the sessions to set diet and exercise goals for the week. Each session will consist of discussion of a diet and/or exercise topic for 30 minutes and 30 minutes of group exercise. The participant will also be given a pedometer to help keep track of his/her exercise, a cookbook with low‐calorie recipes, and a journal to keep track of their exercise and the food eaten each day. The participant will bring the journal to each weight loss session to review with study staff Delayed Weight Loss Programme Group ‐ will take part in the weight loss intervention after the 16‐week control period |
| Outcomes | Weight, anthropometrics, QoL, physical activity, body image Assessment of outcomes will occur at baseline (pre‐randomisation), at the end of the 16‐week intervention or control period, and at 32 weeks |
| Starting date | October 2013 |
| Contact information | Dr Jennifer Ligibel, Dana‐Farber Cancer Institute jligibel@partners.org |
| Notes |
Mathews 2012.
| Trial name or title | My lifestyle intervention of food and exercise (MyLIFE) |
| Methods | Parallel randomised controlled trial |
| Participants | Female patients, age 21 to 65 with a history of stage 1, 2, or 3 breast cancer. To have completed primary treatments (chemotherapy, radiation, and/or surgical treatment) for breast cancer (with or without maintenance therapy) within the last 3 months to 5 years of providing consent. Be willing/able to attend groups and assessments in Gainesville or Jacksonville. BMI of 27 to 45 kg/m² and weight‐stable (i.e. not lost/gained ≥ 10 lbs in the preceding 6 months, or since the end of primary treatment) |
| Interventions | Tailored lifestyle intervention (TLI) ‐ participants randomised to the TLI condition will receive a 3‐month weight management programme tailored to the specific needs of women in remission from breast cancer Commercial Weight Loss Programme (CWLP) ‐ participants randomised to the CWLP condition will receive a 3‐month commercial weight loss programme (i.e. Weight Watchers) at no cost |
| Outcomes | Body weight, inflammatory/metabolic disease markers associated with breast cancer recurrence, HDL cholesterol, blood glucose control, caloric intake, body composition, waist circumference, sagittal abdominal diameter, physical activity, health‐related quality of life, self‐efficacy to abstain from eating, frequency of participants' utilisation of specific weight management strategies, systolic and diastolic blood pressure Outcomes measured at baseline, at 3 months, and at 9 months |
| Starting date | June 2012 |
| Contact information | Dr Anne Mathews 352 392 1991 anne.mathews@ufl.edu |
| Notes |
O'Connor 2018.
| Trial name or title | |
| Methods |
Design: a randomised controlled trial Country: USA Accrual dates: NR Trial Reg: NCT03751449 |
| Participants | Men or women with a history of breast cancer who have completed treatment ≥ 6 months ago (not including hormonal therapy, Zometa, or other non‐chemotherapy/radiation cancer treatment at the discretion of the principal investigator) |
| Interventions | This trial studies how well exercise and nutritional education work in improving physical function and quality of life in older breast cancer survivors GROUP I (ACTIVE TREATMENT): participants complete a home‐based aerobic and resistance exercise programme and receive nutrition education for 12 weeks GROUP II (WAITLIST): participants are placed on a wait‐list for 12 weeks and then complete a home‐based aerobic and resistance exercise programme and receive nutrition education for 12 weeks |
| Outcomes | Primary outcomes: Activity levels for all participants will be collected using the Fitbit applications and a weekly activity log Quality of life as assessed by the Self‐Geriatric Assessment Measure (GA‐Self‐Assessment) Secondary outcomes: Diet quality as assessed by the ASA24 website Sleep as assessed by the Pittsburgh Sleep Quality Index (PSQI) The PSQI measures the quality and pattern of sleep in adults. There are 19 items. Each item is weighted on a 0 to 3 interval scale Anxiety and the effect of a home‐based aerobic and resistance exercise and nutrition education intervention questionnaire 19‐item questionnaire that measures the stages of the self‐determination continuum with respect to motivation to exercise Centers for Epidemiologic Studies Depression Scale (CESD‐R) |
| Starting date | November 2018 |
| Contact information | Tracey O'Connor, Roswell Park Cancer Institute; 716‐845‐7785; tracey.oconnor@roswellpark.org |
| Notes | Protocol |
Park 2013.
| Trial name or title | A lifestyle intervention for breast cancer survivors |
| Methods | A 4‐month parallel randomised controlled trial |
| Participants | Women diagnosed with stage 0 to 2 breast cancer in the past 1.5 years. Must have received no prior adjuvant treatment for another cancer. Can read and write English and not participating in other health behaviour research upon recruitment |
| Interventions | Participants will be assigned randomly to 1 of 3 groups: (1) newly developed mail‐based intervention (Targeting the Teachable Moment Intervention; TTMI), (2) standard lifestyle intervention (SLM), and (3) usual care. Both TTMI and SLM focus on health behaviours; however TTMI additionally addresses psychosocial issues specific to breast cancer survivors All participants will complete questionnaires at the time they start the study, at the end of 4 months, and 3 months later for a follow‐up If participants are assigned to any of the intervention groups, they will receive materials every other week for 4 months. If participants are assigned to the usual care group, they will receive the same materials as the standard lifestyle intervention et the end of the 7 months |
| Outcomes | Eating habits, physical activity, coping strategies, self‐efficacy, social support, life meaning Outcome measures at baseline, 4 months, and 7 months |
| Starting date | April 2011 |
| Contact information | Dr Crystal L. Park, University of Connecticut, USA |
| Notes |
Pasanisi 2012.
| Trial name or title | A randomized controlled trial of diet, physical activity, and breast cancer recurrences ‐ the DIANA‐5 study |
| Methods | A multi‐centre randomised controlled trial |
| Participants | 2000 breast cancer patients at high risk of recurrence are being randomised to 2 groups. Compliance is being monitored through weight and hormonal‐metabolic change. The main analysis will be done by intention‐to‐treat |
| Interventions | Of the 2000 patients, 1000 received the WCRF Decalogue for dietary prevention of cancer, and 1000 received an active support (kitchen courses, physical activity classes, and common meals) |
| Outcomes | Body weight and triglycerides |
| Starting date | 2012 |
| Contact information | P. Pasanisi. Istituto Nazionale Tumori, Department of Predictive and Preventive Medicine, Milan, Italy |
| Notes |
Quintiliani 2015.
| Trial name or title | Weight management among breast cancer survivors |
| Methods | Single‐group intervention trial |
| Participants | Women who had a breast cancer diagnosis (self‐reported) 2 or more years ago. Last cancer treatment (including surgery, radiation, or chemotherapy (self‐reported)) was 6 or more months ago. Current ownership of an iOS or an Android‐based platform smart phone and home Wi‐Fi. Ability to speak and read in English. Overweight or obese (body mass index ≥ 25) |
| Interventions | Experimental: mHealth Platform, the primary interventions employed in the study are Wi‐Fi enabled tracking devices, text message communications, and behavioral counselling The mHealth intervention for cancer survivors devised by the investigators consists of several components: (1) a commercially available smart phone app that captures patients' behavioural data (steps, sleep, weight) using devices (a FitBit and a FitBit scale), (2) text messages to participants to collect additional data (foods eaten, eating habits), and (3) phone sessions with a non‐professionally trained health counsellor about diet and physical activity behaviours Investigators propose to test feasibility and preliminary outcomes on weight, behaviours, psychological factors, and participant engagement in the intervention of our mHealth counselling intervention among 20 breast cancer survivors |
| Outcomes | Body weight, steps per day, diet Intake, sleep, engagement with the intervention from baseline, frequency of diet intake tracking |
| Starting date | February 2015 |
| Contact information | Dr Lisa Quintiliani 617 638 2777 lisa.quintiliani@bmc.org |
| Notes |
Resnick 2014.
| Trial name or title | Protein‐sparing modified fast intervention for weight loss in obese endometrial cancer survivors |
| Methods | A pilot single‐group study |
| Participants | Patients previously diagnosed with endometrial cancer and successfully treated through surgery. Body mass index (BMI) > 30 kg/m² and > 8 weeks removed from surgery to treat the endometrial cancer |
| Interventions | This pilot clinical trial studies protein‐sparing modified fast (PSMF) intervention for weight loss in obese endometrial cancer survivors. The PSMF is a diet that is very low in carbohydrates and calories and is designed to induce fast, safe weight loss. The diet consists of only lean meats (beef, pork, poultry, and seafood) in amounts adequate to meet protein requirements based on the individual's body weight Experimental: supportive care (PSMF). Participants will take part in a Protein‐Sparing Modified Fast (PSMF) intervention for weight loss. Participants will undergo a dietary intervention high in protein for 6 weeks, or until they have lost 15% of their body weight. This intervention will be followed by weight maintenance, during which participants will reintroduce non‐starchy vegetables to their diet. At this time, participants will also receive informational material and dietary education, which teaches participants how to read nutrition labels and calculate carbohydrate loads in foods. Participants are given the Obesity and Weight‐Loss Quality of Life Questionnaire to survey the impact of the intervention |
| Outcomes | Weight loss, cholesterol and triglycerides, markers of inflammation (C‐reactive protein, interleukin 6), glucose, number of dropouts, percentage of positive urinary ketone tests as a marker of dietary adherence, adverse events, quality of life After completion of the study, participants are followed up at 2 weeks and at 4 weeks, and then at 2, 3, 4, 5, and 6 months |
| Starting date | May 2014 |
| Contact information | Kimberly Resnick, Case Comprehensive Cancer Center, USA 216 844 3954 kimberly.resnick@uhhospitals.org |
| Notes |
AJCC: American Joint Committee on Cancer.
BMI: body mass index.
BP: blood pressure.
CESD‐R: Center for Epidemiologic Studies Depression Scale Revised.
CWLP: commercial weight loss programme.
ECOG: Eastern Cooperative Oncology Group.
FACT‐G: Functional Assessment of Cancer Therapy ‐ General.
FFQ: Food Frequency Questionnaire.
HDL: high‐density lipoprotein.
HR: heart rate.
IPAQ‐SF: International Physical Activity Questionnaire Short Form.
MET: metabolic equivalent.
MOS SF‐36: Medical Outcomes Study Short Form Health Survey.
MVPA: moderate to vigorous physical activity.
PA: physical activity.
PSMF: protein‐sparing modified fast.
PSQI: Pittsburgh Sleep Quality Index.
QoL: quality of life.
SLM: standard lifestyle method.
SpO2: peripheral capillary oxygen saturation.
TLI: tailored lifestyle intervention.
TTMI: Targeting the Teachable Moment intervention.
Differences between protocol and review
For all outcomes, we reported differences between intervention and control groups for all variables ‐ not changes from baseline to different time points. We have amended the method to reflect this. We reported outcomes for the last time point measured as there was a large amount of inconsistency in the evidence base between frequency of measurements at different time points and in the number of time points reported in studies included in the review. Outcomes for dietary intake reported included total energy, dietary fibre, and fruits and vegetables. We did not report data on fat, protein, or carbohydrate, or on micronutrients, as nutrients were often reported in different ways. We did not report nutrients expressed as a percentage of energy. This excluded other nutrients including protein, carbohydrate, and micronutrients reported.
Studies that compared multiple interventions to control: we did not compare the different interventions with one other, only each intervention versus control (weight watchers trial; Djuric 2002). We excluded studies with no control group that provided usual care or made written information available to the general public.
Contributions of authors
AMS, JS, and DG undertook all title and abstract checking along with data extraction. SB, JS, and DG undertook the analysis and the narrative review. SB wrote the discussion and the abstract. MP advised on statistics. All review authors have inputted on how the data were analysed. The methodological aspects of the systematic review protocol have been commented on by CT, and SL and MP have reviewed the plan for data analysis. AMS and SB undertook all risk of bias assessments.
The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Gynaecological Cancer Group. The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the NIHR, the National Health Service, or the Department of Health.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Macmillan Cancer Care, UK.
Dr Burden was supported by a Post Doctoral Fellowship
Declarations of interest
Sorrel Burden: none known. Debra J Jones: none known. Jana Sremanakova: I would like to declare the following existing/potential conflict of interest: salary paid to institution by Marie Curie. Anne Marie Sowerbutts: none known. Simon Lal: none known. Mark Pilling: none known. Chris Todd: none known.
New
References
References to studies included in this review
Befort 2016 {published data only}
- Befort CA, Klemp JR, Fabian C, Perri MG, Sullivan DK, Schmitz KH, et al. Protocol and recruitment results from a randomized controlled trial comparing group phone‐based versus newsletter interventions for weight loss maintenance among rural breast cancer survivors. Contemporary Clinical Trials 2014;37(2):261‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Befort CA, Klemp JR, Sullivan DK, Shireman T, Diaz FJ, et al. Weight loss maintenance strategies among rural breast cancer survivors: the rural women connecting for better health trial. Obesity (Silver Spring, Md) 2016;24(10):2070‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bloom 2008 {published data only}
- Bloom JR, Stewart SL, D'Onofrio CN, Luce J, Banks PJ. Addressing the needs of young breast cancer survivors at the 5 year milestone: can a short‐term, low intensity intervention produce change?. Journal of Cancer Survivorship 2008;2(3):190‐204. [DOI] [PubMed] [Google Scholar]
Bourke 2011 {published data only}
- Bourke L, Thompson G, Gibson DJ, Daley A, Crank H, Adam I, et al. Pragmatic lifestyle intervention in patients recovering from colon cancer: a randomized controlled pilot study. Archives of Physical Medicine and Rehabilitation 2011;92(5):749‐55. [DOI] [PubMed] [Google Scholar]
Demark‐Wahnefried 2006 {published data only}
- Demark‐Wahnefried W, Clipp EC, Morey MC, Pieper CF, Sloane R, Snyder DC, et al. Lifestyle intervention development study to improve physical function in older adults with cancer: outcomes from project. Journal of Clinical Oncology 2006;24(21):3465‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demark‐Wahnefried W, Morey MC, Clipp EC, Pieper CF, Snyder DC, Sloane R, et al. Leading the way in exercise and diet (Project LEAD): intervening to improve function among older breast and prostate cancer survivors. Controlled Clinical Trials 2003;24(2):206‐23. [DOI] [PubMed] [Google Scholar]
- Demark‐Wahnefried W, Morey MC, Clipp EC, Pieper CF, Snyder DC, Sloane R, et al. Leading the way in exercise and diet (Project LEAD): intervening to improve function among older breast and prostate cancer survivors. Controlled Clinical Trials 2003;24(2):206‐23. [DOI] [PubMed] [Google Scholar]
- Demark‐Wahnefried W, Morey MC, Clipp EC, Snyder DC, Sloane R, Pieper CF, et al. Results of project LEAD (Leading the Way in Exercise and Diet) ‐ a trial testing an intervention of telephone‐counselling and mailed materials in improving physical functioning among older breast and prostate cancer survivors. Journal of Clinical Oncology 2005;23(16):763. [Google Scholar]
Demark‐Wahnefried 2007 {published data only}
- Christy SM, Mosher CE, Sloane R, Snyder DC, Lobach DF, Demark‐Wahnefried W. Long‐term dietary outcomes of the FRESH START intervention for breast and prostate cancer survivors. Journal of the American Dietetic Association 2011;111(12):1844‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demark‐Wahnefried W, Clipp E, Lipkus I, Lobach D, Peterson B, Snyder D, et al. Results of FRESH START: a randomized controlled trial to improve diet and exercise behaviours in breast and prostate cancer survivors. Journal of Clinical Oncology 2006;24(18):Suppl 20. [Google Scholar]
- Demark‐Wahnefried W, Clipp EC, Lipkus IM, Lobach D, Snyder DC, Sloane R, et al. Main outcomes of the FRESH START trial: a sequentially tailored, diet and exercise mailed print intervention among breast and prostate cancer survivors. Journal of Clinical Oncology 2007;25(19):2709‐18. [DOI] [PubMed] [Google Scholar]
- Demark‐Wahnefried W, Clipp EC, McBride C, Lobach DF, Lipkus I, Peterson B, et al. Design of FRESH START: a randomized trial of exercise and diet among cancer survivors. Medicine and Science in Sports and Exercise 2003;35(3):415‐24. [DOI] [PubMed] [Google Scholar]
- Mosher CE, Lipkus I, Sloane R, Snyder DC, Lobach DF, Demark‐Wahnefried W. Long‐term outcomes of the FRESH START trial: exploring the role of self‐efficacy in cancer survivors' maintenance of dietary practices and physical activity. Psycho‐Oncology 2013;22(4):876‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottenbacher AJ, Day RS, Taylor WC, Sharma SV, Sloane R, Snyder DC, et al. Long‐term physical activity outcomes of home‐based lifestyle interventions among breast and prostate cancer survivors. Supportive Care in Cancer 2012;20:2483‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder DC, Sloane R, Lobach D, Lipkus IM, Peterson B, Kraus W, et al. Differences in baseline characteristics and outcomes at 1‐ and 2‐year follow‐up of cancer survivors accrued via self‐referral versus cancer registry in the FRESH START Diet and exercise trial. Cancer Epidemiological Biomarkers and Prevention 2008;17:1288‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Demark‐Wahnefried 2014 {published data only}
- Demark‐Wahnefried W, Jones LW, Snyder DC, Sloane RJ, Kimmick GG, Hughes DC, et al. Daughters and mothers against breast cancer (DAMES): main outcomes of a randomized controlled trial of weight loss in overweight mothers with breast cancer and their overweight daughters. Cancer 2014;120(16):2522‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tometich DB, Mosher CE, Winger JG, Badr HJ, Snyder DC, Sloane RJ, et al. Effects of diet and exercise on weight‐related outcomes for breast cancer survivors and their adult daughters: an analysis of the DAMES trial. Supportive Care in Cancer 2017;25(8):2559‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Djuric 2002 {published data only}
- Darga LL, Magnan M, Mood D, Hryniuk WM, DiLaura NM, Djuric Z. Quality of life as a predictor of weight loss in obese, early‐stage breast cancer survivors. Oncology Nursing Forum 2007;34(1):86‐92. [DOI] [PubMed] [Google Scholar]
- Djuric Z, DiLaura NM, Jenkins I, Darga L, Jen CK, Mood D, et al. Combining weight‐loss counseling with the weight watchers plan for obese breast cancer survivors. Obesity Research 2002;10(7):657‐65. [DOI] [PubMed] [Google Scholar]
- Jen KL, Djuric Z, DiLaura NM, Buison A, Redd JN, Maranci V, et al. Improvement of metabolism among obese breast cancer survivors in differing weight loss regimens. Obesity Research & Clinical Practice 2004;12:306‐12. [DOI] [PubMed] [Google Scholar]
Ghavami 2017 {published data only}
- Ghavami H, Akyolcu N. 22nd Annual Conference of the International Society for Quality of Life Research. Quality of Life Research 2015;24 Suppl 1:1‐191. [DOI] [PubMed] [Google Scholar]
- Ghavami HAN, Akyolcu N. Effects of a lifestyle interventions program on quality of life in breast cancer survivors. Uluslararasi Hematoloji‐Onkoloji Dergisi 2017;2:91‐9. [Google Scholar]
Greenlee 2013 {published data only}
- Delgado‐Cruzata L, Zhang W, Tsai WY, Valdovinos C, Wang Q, Crew K, et al. Effects of lifestyle modification on global DNA methylation in minority breast cancer survivors. Cancer Research 2012;72(8):Suppl. [4482] [Google Scholar]
- Greenlee H, Awad D, Crew KD, Kalinsky K, Maurer M, Brafman L, et al. Influence of clinic‐based survivorship intervention on dietary changes and lifestyle recommendations among Hispanic and non‐Hispanic women following adjuvant therapy for breast cancer. Cancer Research 2013;73(24):Suppl. [P3‐08‐12] [DOI] [PubMed] [Google Scholar]
- Greenlee H, Crew K, Ferguson K, McKinley P, Rundle A, Ogedegbe G, et al. Facilitators and barriers to recruitment for an exercise and dietary intervention study in minority breast cancer survivors. Cancer Research 2009;69(2):Suppl. [5077] [Google Scholar]
- Greenlee H, Crew K, McKinley P, Rundle A, Tsai W, Mata J, et al. Effects of a combined physical activity and dietary change intervention on weight loss in minority breast cancer survivors. Cancer Research 2009;69(24):Suppl. [1038] [Google Scholar]
- Greenlee HA, Crew KD, Mata JM, McKinley PS, Rundle AG, Zhang W, et al. A pilot randomized controlled trial of a commercial diet and exercise weight loss program in minority breast cancer survivors. Obesity (Silver Spring, Md) 2013;21(1):65‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Greenlee 2015 {published data only}
- Aycinena AC, Jennings KA, Gaffney AO, et al. Cocinar Para Su Salud! Development of a Culturally Based Nutrition Education Curriculum for Hispanic Breast Cancer Survivors Using a Theory‐Driven Procedural Model. Diet & Nutrition Interventions 2017; Vol. 44, issue 1:13‐21. [DOI] [PubMed]
- Greenlee H, Gaffney AO, Aycinena AC, Koch P, Contento I, Karmally W, et al. Cocinar Para Su Salud: randomized controlled trial of a culturally based dietary intervention among Hispanic breast cancer survivors. Academy of Nutrition and Dietetics 2015;115(5):709‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenlee H, Gaffney AO, Aycinena AC, Koch P, Contento I, et al. Long‐term diet and biomarker changes after a short‐term intervention among Hispanic breast cancer survivors: the Cocinar para su salud randomized controlled trial. Cancer Epidemiology, Biomarkers & Prevention 2016;25(11):1491‐502. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gruenigen 2012 {published data only}
- Gruenigen VV, Frasure H, Kavanagh MB, Janata J, Waggoner S. Survivors of uterine cancer empowered by exercise and healthy diet (SUCCEED): a randomized trial. Gynecologic Oncology 2012;125(3):699‐704. [DOI] [PubMed] [Google Scholar]
- Kavanagh MB, Gruenigen VE, Courneya KS, Gibbons HE, Waggoner SE, Lerner E. Effects of a lifestyle intervention on nutrient intake in overweight/obese endometrial cancer survivors. European‐e Journal of Clinical Nutrition and Metabolism 2009;4(3):143‐7. [Google Scholar]
- McCarroll ML, Armbruster S, Frasure HE, Gothard MD, Gil KM, Kavanagh MB, et al. Self‐efficacy, quality of life, and weight loss in overweight/obese endometrial cancer survivors (SUCCEED): a randomized controlled trial. Gynecological Oncology 2014;132:397‐402. [DOI] [PubMed] [Google Scholar]
- McCarroll ML, Frasure HE, Gil KM, Kavanagh MB, Waggoner S, gruenigen VE. Quality of life in a randomized lifestyle intervention program in overweight/obese endometrial cancer survivors. 4th Biennial Meeting of the International Gynecologic Cancer Society, 2012; Oct 13‐16; Vancouver (BC) Canada. 2012. [E118]
- Nock NL, Frasure H, Gruenigen VV, Dimitropoulos A. Using neuroimaging to demonstrate changes in food behaviour in obese endometrial cancer survivors enrolled in a lifestyle intervention. 29th Annual Scientific Meeting of the Obesity Society; 2011 Jan 1‐5; Orlando, (FL) United States. 2011. [S172]
- Gruenigen VE, Courneya KS, Gibbons HE, Kavanagh MB, Waggoner SE, Lerner E. Feasibility and effectiveness of a lifestyle intervention program in obese endometrial cancer patients: a randomized trial. Gynecologic Oncology 2008;109(1):19‐26. [DOI] [PubMed] [Google Scholar]
- Gruenigen VE, Gibbons HE, Kavanagh MB, Janata JW, Lerner E, Courneya KS. A randomized trial of a lifestyle intervention in obese endometrial cancer survivors: quality of life outcomes and mediators of behavior change. Health Quality of Life Outcomes 2009;7:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Harrigan 2016 {published data only}
- Harrigan M, Cartmel B, Loftfield E, Sanft T, Chagpar AB, Zhou Y, et al. Randomized trial comparing telephone versus in‐person weight loss counseling on body composition and circulating biomarkers in women treated for breast cancer: The lifestyle, exercise and nutrition (LEAN) study. Journal of Clinical Oncology 2016;34:669‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftfield E, Harrigan M, Li F, Cartmel B, Zhou Y, Playdon M, et al. Effect of weight loss intervention on inflammatory and metabolic markers in breast cancer survivors: the lifestyle, exercise, and nutrition (LEAN) study. Journal of Clinical Oncology 2014;32(5):Suppl. [1505] [Google Scholar]
- Santf TB, Harrigan M, Cartmel B, Playdon M, Zhou Y, Loftfield E, et al. Effect of weight history on ability to lose weight after a 6‐month randomized controlled weight loss trial on overweight breast cancer survivors: the lifestyle, exercise, and nutrition (LEAN) study. Journal of Clinical Oncology 2014;34(3):Suppl 174. [20591] [Google Scholar]
Hawkes 2013 {published data only}
- Hawkes A, Pakenham K, Courneya K, Peter B, Chambers S. 'Canchange': a trial of a telephone‐delivered lifestyle intervention for colorectal cancer (CRC) survivors. 37th Annual Scientific Meeting of the Clinical Oncological Society of Australia; 2010; Melbourne (VIC) Australia. 2010.
- Hawkes A, Pakenham K, Courneya KS, Patrao T. A randomised controlled trial of the effects of a telephone‐delivered program on health behaviours and quality of life for colorectal cancer survivors ('canchange'). 38th Annual Scientific Meeting of the Clinical Oncological Society of Australia; 2011; Perth (WA) Australia. 2011.
- Hawkes AL, Chambers SK, Pakenham KI, Patrao TA, Baade PD, Lynch BM, et al. Effects of a telephone‐delivered multiple health behavior change intervention (CanChange) on health and behavioral outcomes in survivors of colorectal cancer: a randomized controlled trial. Journal of Clinical Oncology 2013;31(18):2313‐21. [DOI] [PubMed] [Google Scholar]
- Hawkes AL, Gollschewski S, Lynch BM, Chambers S. A telephone‐delivered lifestyle intervention for colorectal cancer survivors 'CanChange': a pilot study. Psycho‐Oncology 2009;18(4):449‐55. [DOI] [PubMed] [Google Scholar]
- Hawkes AL, Gollschewski S, Lynch BM, Chambers SK. Developing and pilot testing a telephone‐delivered lifestyle intervention for colorectal cancer survivors ‐ 'Canchange'. 36th Annual Scientific Meeting of the Clinical Oncological Society of Australia; 2009; Gold Coast (QLD) Australia. 2009.
- Hawkes AL, Lynch BM, Owen N, Aitken JF. Lifestyle factors associated concurrently and prospectively with co‐morbid cardiovascular disease in a population‐based cohort of colorectal cancer survivors. European Journal of Cancer 2011;47(2):267‐76. [DOI] [PubMed] [Google Scholar]
- Hawkes AL, Pakenham K, Courneya KS, Patrao TA. A randomized trial of the effects of a multiple health behaviour intervention for colorectal cancer survivors on quality of life and psychosocial outcomes ('CanChange'). 39th Annual Scientific Meeting and IPOS 14th World Congress of Psycho‐Oncology; Brisbane (QLD) Australia. 2012.
- Hawkes AL, Pakenham KI, Courneya KS, Gollschewski S, Baade P, Gordon LG, et al. A randomized controlled trial of a tele‐based lifestyle intervention for colorectal cancer survivors (CanChange): study protocol. BMC Cancer 2009;9:286. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kanera 2017 {published data only}
- Kanera IM, Willems RA, Bolman CAW, Mesters I, Verboon P, Lechner L. Long term effects of a web based cancer aftercare intervention on moderate physical activity and vegetable consumption among early cancer survivors: a randomized controlled trial. Journal of Behavioural Nutrition and Physical Activity 2017;14(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2011 {published data only}
- Kim SH, Shin MS, Lee HS, Lee ES, Ro JS, Kang HS, et al. Randomized pilot test of a simultaneous stage‐matched exercise and diet intervention for breast cancer survivors. Oncology Nursing Forum 2011;38(2):97‐106. [DOI] [PubMed] [Google Scholar]
Mefferd 2007 {published data only}
- Mefferd K, Nichols JF, Pakiz B, Rock CL. A cognitive behavioral therapy intervention to promote weight loss improves body composition and blood lipid profiles among overweight breast cancer survivors. Breast Cancer Research and Treatment 2007;104(2):145‐52. [DOI] [PubMed] [Google Scholar]
Morey 2009 {published data only}
- Demark‐Wahnefried W, Morey MC, Sloane R, Snyder DC, Miller PE, Hartman TJ, et al. Reach out to enhance wellness home‐based diet‐exercise intervention promotes reproducible and sustainable long‐term improvements in health behaviors, body weight, and physical functioning in older, overweight/obese cancer survivors. Journal of Clinical Oncology 2012;30(19):2354‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey MC, Snyder DC, Sloane R, Cohen HJ, Peterson B, Hartman TJ, et al. Effects of home‐based diet and exercise on functional outcomes among older, overweight long‐term cancer survivors: RENEW: a randomized controlled trial. Journal of the American Medical Association 2009;301(18):1883‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Park 2016 {published data only}
- Park CL, Cho D, Salner AL, Dornelas E. A randomized controlled trial of two mail‐based lifestyle interventions for breast cancer survivors. Supportive Care in Cancer 2016;24:3037‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pierce 2007 {published data only}
- Gold EB, Pierce JP, Natarajan L, Stefanick ML, Laughlin GA, Caan BJ, et al. Dietary pattern influences breast cancer prognosis in women without hot flashes: the women's healthy eating and living trial. Journal of Clinical Oncology 2009;27:352‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Bardwell WA, Natarajan L, Flatt SW, Rock CL, Newman VA, et al. Correlates of physical activity level in breast cancer survivors participating in the women's healthy eating and living (WHEL) study. Breast Cancer Research and Treatment 2007;101:225‐32. [DOI] [PubMed] [Google Scholar]
- Hyder JA, Thomson CA, Natarajan L, Madlensky L, Pu M, Emond J, et al. Adopting a plant‐based diet minimally increased food costs in WHEL study. American Journal of Health Behaviour 2009;33:530‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones VE, Hollenbach K, Rock C, Faerber S, Haan M, Gold E, et al. The women's health eating and living (WHEL) study: a nutritional intervention study in breast cancer survivors. Breast Cancer Research and Treatment 2000;64(1):49. [Google Scholar]
- Pierce JP, Faerber S, Wright FA, Newman V, Flatt SW, Kealey S, et al. Feasibility of a randomized trial of a high‐vegetable diet to prevent breast cancer recurrence. Nutrition and Cancer 1997;28:282‐8. [DOI] [PubMed] [Google Scholar]
- Pierce JP, Faerber S, Wright FA, Rock CL, Newman V, Flatt SW, et al. A randomized trial of the effect of a plant‐based dietary pattern on additional breast cancer events and survival: the women's healthy eating and living (WHEL) study. Controlled Clinical Trials 2002;23(6):728‐56. [DOI] [PubMed] [Google Scholar]
- Pierce JP, Natarajan L, Caan BJ, Flatt SW, Kealey S, Gold EB, et al. Dietary change and reduced breast cancer events among women without hot flashes after treatment of early‐stage breast cancer: subgroup analysis of the women's healthy eating and living study. American Journal of Clinial Nutrition 2009;89:1565‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce JP, Natarajan L, Caan BJ, Parker BA, Greenberg ER, Flatt SW, et al. Influence of a diet very high in vegetables, fruit, and fiber and low in fat on prognosis following treatment for breast cancer: the women's healthy eating and living (WHEL) randomized trial. Journal of the American Medical Association 2007;298(3):289‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce JP, Natarajan L, Sun S, Al‐Delaimy W, Flatt SW, Kealey S, et al. Increases in plasma carotenoid concentrations in response to a major dietary change in the women's healthy eating and living study. Cancer Epidemiological Biomarkers & Prevention 2006;15:1886‐92. [DOI] [PubMed] [Google Scholar]
- Pierce JP, Newman VA, Flatt SW, Faerber S, Rock CL, Natarajan L, et al. Telephone counseling intervention increases intakes of micronutrient‐ and phytochemical‐rich vegetables, fruit and fiber in breast cancer survivors. Journal of Nutrition 2004;134:452‐8. [DOI] [PubMed] [Google Scholar]
- Rock CL, Flatt SW, Thomson CA, Stefanick ML, Newman VA, Jones L, et al. Plasma triacylglycerol and HDL cholesterol concentrations confirm self‐reported changes in carbohydrate and fat intakes in women in a diet intervention trial. Journal of Nutrition 2004;134(2):342‐7. [DOI] [PubMed] [Google Scholar]
- Rock CL, Flatt SW, Thomson CA, Stefanick ML, Newman VA, Jones LA, et al. Effects of a high‐fiber, low‐fat diet intervention on serum concentrations of reproductive steroid hormones in women with a history of breast cancer. Journal of Clinical Oncology 2004;22:2379‐87. [DOI] [PubMed] [Google Scholar]
- Saquib N, Natarajan L, Rock CL, Flatt SW, Madlensky L, Kealey S, et al. The impact of a long‐term reduction in dietary energy density on body weight within a randomized diet trial. Nutrition Cancer 2008;60(1):31‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saquib N, Rock CL, Natarajan L, Flatt SW, Newman VA, Thomson CA, et al. Does a healthy diet help weight management among overweight and obese people?. Health Education & Behaviour 2009;36(3):518‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson CA, Rock CL, Caan BJ, Flatt SW, Al‐Delaimy WA, Newman VA, et al. Increase in cruciferous vegetable intake in women previously treated for breast cancer participating in a dietary intervention trial. Nutrition and Cancer 2007;57:11‐9. [DOI] [PubMed] [Google Scholar]
- Thomson CA, Rock CL, Giuliano AR, Newton TR, Cui H, Reid PM, et al. Longitudinal changes in body weight and body composition among women previously treated for breast cancer consuming a high‐vegetable, fruit and fibre, low‐fat diet. European Journal of Nutrition 2005;44(1):18‐25. [DOI] [PubMed] [Google Scholar]
- Thomson CA, Rock CL, Thompson PA, Caan BJ, Cussler E, Flatt SW, et al. Vegetable intake is associated with reduced breast cancer recurrence in tamoxifen users: a secondary analysis from the women's healthy eating and living study. Breast Cancer Research & Treatment 2011;125:519‐27. [DOI] [PubMed] [Google Scholar]
Reeves 2017 {published data only}
- Reeves M, Winkler E, Mccarthy N, Lawler S, Terranova C, Hayes S, et al. Dietary outcomes following a six‐month weight loss intervention for breast cancer survivors: living well after breast cancer [The living well after breast cancer pilot trial: a weight loss intervention for women following treatment for breast cancer]. Asia‐Pacific Journal of Clinical Oncology. 2017; Vol. 13:125‐36. [DOI: 10.1111/ajco.12332] [DOI] [PubMed]
- Terranova C, Lawler S, Winkler E, Eakin E, Reeves M. Dietary outcomes following a six‐month weight loss intervention for breast cancer survivors: living well after breast cancer. World Cancer Congress. Melbourne, Australia, 2014; Vol. 10:2014:255.
Scott 2013 {published data only}
- Saxton JM, Daley A, Woodroofe N, Coleman R, Powers H, Mutrie N, et al. Study protocol to investigate the effect of a lifestyle intervention on body weight, psychological health status and risk factors associated with disease recurrence in women recovering from breast cancer treatment. BMC Cancer 2006;6:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton JM, Scott EJ, Daley AJ, Woodroofe M, Mutrie N, Crank H, et al. Effects of an exercise and hypocaloric healthy eating intervention on indices of psychological health status, hypothalamic‐pituitary‐adrenal axis regulation and immune function after early‐stage breast cancer: a randomized controlled trial. Breast Cancer Research 2014;16(2):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott E, Daley AJ, Doll H, Woodroofe N, Coleman RE, Mutrie N, et al. Effects of an exercise and hypocaloric healthy eating program on biomarkers associated with long‐term prognosis after early‐stage breast cancer: a randomized controlled trial. Cancer Causes & Control 2013;24(1):181‐91. [DOI] [PubMed] [Google Scholar]
Sheppard 2016 {published data only}
- Sheppard VB, Hicks J, Makambi K, Hurtado‐de‐Mendoza A, Demark‐Wahnefried W, Adams‐Campbell L. The feasibility and acceptability of a diet and exercise trial in overweight and obese black breast cancer survivors: the Stepping STONE study. Contemporary Clinical Trials 2016;46:106‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Swisher 2015 {published data only}
- Swisher AK, Abraham J, Bonner D, Gilleland D, Hobbs G, Kurian S, et al. Exercise and dietary advice intervention for survivors of triple‐negative breast cancer: effects on body fat, physical function, quality of life, and adipokine profile. Supportive Care in Cancer 2015;23(10):2995‐3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yun 2017 {published data only}
- Yun YH, Young AL, Myung KS, Jin AN, Byung HK, Sohee L, et al. A randomized controlled trial of physical activity, dietary habit, and distress management with the Leadership and Coaching for Health (LEACH) program for disease‐free cancer survivors. BioMed Central 2017;17(1):298. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zick 2017 {published data only}
- Zick SM, Colacino J, Cornellier M, Khabir T, Surnow K, Djuric Z. Fatigue reduction diet in breast cancer survivors: a pilot randomized clinical trial. Breast Cancer Research and Treatment 2017;161:299‐310. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Abrams 2014 {published data only}
- Abrams DI. Milking the evidence: diet does matter. Journal of Clinical Oncology 2014;32(22):2290‐2. [DOI] [PubMed] [Google Scholar]
Anderson 2014 {published data only}
- Anderson A, Craigie A, Caswell S, Treweek S, Stead M, Macleod M, et al. The impact of bodyweight and physical activity intervention (BeWEL) initiated through a national colorectal cancer screening programme: randomized controlled trial. British Medical Journal 2014;348:1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
Arends 2014 {published data only}
- Arends J, Theobald S, Schmid J, Bartsch HH. Nutritional training increases long‐term fruit and vegetable consumption in women with early breast cancer ‐ a randomized controlled trial. Metabolism and Nutrition in Oncology 2014;2(1):1‐7. [Google Scholar]
Arnold 2015 {published data only}
- Arnold K, Andridge R, Logan A, Yee L, Lustberg M, Orchard T. Healthy eating index scores are associated with inflammatory markers in breast cancer survivors. Federation of American Societies for Experimental Biology 2015;29(1):Suppl. [Google Scholar]
Boddie 2010 {published data only}
- Boddie AM, Berglund MS, Hernandez M, Paxton RJ, Hajek RA, Valero‐Hernandez MA, et al. Preliminary results from the ovarian nutrition education (ONE) study. Federation of American Societies for Experimental Biology 2010;24(1):Suppl. [Google Scholar]
Bourke 2014 {published data only}
- Bourke L, Gilbert S, Hooper R, Steed LA, Joshi M, Catto JW, et al. Lifestyle changes for improving disease‐specific quality of life in sedentary men on long‐term androgen‐deprivation therapy for advanced prostate cancer: a randomized controlled trial. European Urology 2014;66(3):51‐2. [DOI] [PubMed] [Google Scholar]
Brewer 2015 {published data only}
- Brewer KC, Peterson CE, Beckmeyer‐Borowko A, Otoo MA, Hoskins K, Davis F, et al. Access to high quality food and survival from ovarian cancer: an analysis of Cook County, Illinois. Gynecologic Oncology 2015;137(Suppl 1):103‐4. [Google Scholar]
Campbell 2009 {published data only}
- Campbell MK, Carr C, Devellis B, Switzer B, Biddle A, Amamoo MA, et al. A randomized trial of tailoring and motivational interviewing to promote fruit and vegetable consumption for cancer prevention and control. Annals of Behavioral Medicine 2009;38(2):71‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Capozzi 2012 {published data only}
- Capozzi LC, Lau H, Reimer RA, McNeely M, Giese‐Davis J, Culos‐Reed SN. Exercise and nutrition for head and neck cancer patients: a patient oriented, clinic‐supported randomized controlled trial. BMC Cancer 2012;12:446. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chlebowski 2008 {published data only}
- Chlebowski RT, Blackburn GL, Hoy MK, Thomson CA, Giuliano, AE, McAndrew P, et al. Survival analyses from the Women's Intervention Nutrition Study (WINS) evaluating dietary fat reduction and breast cancer outcome. Journal of Clinical Oncology 2008;26(15 Suppl):12. [Abstract nr 522] [Google Scholar]
Chlebowski 2013 {published data only}
- Chlebowski RT. Nutrition and physical activity influence on breast cancer incidence and recurrence. 13th International Conference of the Primary Therapy of Early Breast Cancer; 2013 Mar13‐16; St.Gallen, Switzerland. 2013:5.
Chlebowski 2015 {published data only}
- Chlebowski RT, Blackburn GL. Final survival analysis from the randomized women's intervention nutrition study (WINS) evaluating dietary intervention as adjuvant breast cancer therapy. Cancer Research 2015;75(9):Suppl. [Google Scholar]
del Rocio Berglund 2012 {published data only}
- Rocio Berglund M, Paxton RJ, Garcia‐Prieto C, Hernandez M, Hajek RA, Handy BC, et al. A randomized parallel‐group dietary feasibility study for stage II‐IV ovarian cancer survivors. Federation of American Societies for Experimental Biology 2012;26(1):Suppl. [Google Scholar]
del Rocio Berglund 2012a {published data only}
- Rocio Berglund M, Paxton RJ, Garcia‐Prieto C, Hernandez M, Hajek RA, Handy BC, et al. Evaluation of a telephone‐delivered dietary behavior intervention for ovarian cancer survivors. Federation of American Societies for Experimental Biology 2012;26(1):Suppl. [Google Scholar]
Delvin 2014 {published data only}
- Delvin E, Souberbielle JC, Viard JP, Salle B. Role of vitamin D in acquired immune and autoimmune diseases. Critical Reviews in Clinical Labroratory Sciences 2014;51(4):232‐47. [DOI] [PubMed] [Google Scholar]
Demark‐Wahnefried 2008 {published data only}
- Demark‐Wahnefried W, Case LD, Blackwell K, Marcom PK, Kraus W, Aziz N, et al. Results of a diet/exercise feasibility trial to prevent adverse body composition change in breast cancer patients on adjuvant chemotherapy. Clinical Breast Cancer 2008;8(1):70‐9. [DOI] [PubMed] [Google Scholar]
Dennis Parker 2014 {published data only}
- Dennis Parker EA, Sheppard VB, Adams‐Campbell L. Compliance with national nutrition recommendations among breast cancer survivors in "stepping stone". Integrative Cancer Therapies 2014;13(2):114‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
De Waele 2015 {published data only}
- Waele E, Mattens S, Honore P, Spapen H, Greve J, Pen JJ. Nutrition therapy in cachectic cancer patients. The Tight Caloric Control (TiCaCo) pilot trial. Appetite 2015;91:298‐301. [DOI] [PubMed] [Google Scholar]
Djuric 2010 {published data only}
- Djuric Z, Ellsworth J, Rapai M, Weldon A, Kim J, Sen A. A diet and exercise intervention in women being treated for breast cancer. American Association for Cancer Research 2010;70(8):Suppl. [Google Scholar]
Emond 2010 {published data only}
- Emond JA, Patterson RE, Natarajan L, Laughlin GA, Gold EB, Pierce JP. The protective effect of a dietary intervention on additional breast cancer events is limited to those with higher baseline testosterone. Cancer Research 2010;70(24):Suppl. [Google Scholar]
Ferdowsian 2007 {published data only}
- Ferdowsian HR, Barnard ND. The role of diet in breast and prostate cancer survival. Ethnicity & Disease 2007;17(2 Suppl 2):18‐22. [PubMed] [Google Scholar]
Flynn 2010 {published data only}
- Flynn MM, Reinert SE. Comparing an olive oil‐enriched diet to a standard lower‐fat diet for weight loss in breast cancer survivors: a pilot study. Journal of Women's Health 2010;19(6):1155‐61. [DOI] [PubMed] [Google Scholar]
Frensham 2014 {published data only}
- Frensham LJ, Zarnowiecki DM, Parfitt G, Stanley RM, Dollman J. Steps toward improving diet and exercise for cancer survivors (STRIDE): a quasi‐randomized controlled trial protocol. BMC Cancer 2014;14:428. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fukui 2014 {published data only}
- Fukui JA, Rothwell A, Danesh H, Adelson KB, Morris GJ, Irie H, et al. Comparison of weight loss among early‐stage breast cancer patients post chemotherapy: nutrition education in combination with weight loss acupuncture versus nutrition education alone. Journal of Clinical Oncology 2014;32:5. [Google Scholar]
Garrett 2013 {published data only}
- Garrett CR, Bekaii‐Saab TS, Ryan T, Fisher GA, Clive S, Kavan P, et al. Randomized phase 2 study of pegylated SN‐38 (EZN‐2208) or irinotecan plus cetuximab in patients with advanced colorectal cancer. John Wiley and Sons Inc. 2013;119(24):4223‐30. [DOI] [PubMed] [Google Scholar]
George 2015 {published data only}
- George SM, Ballard R, Shikany JM, Crane TE, Neuhouser ML. A prospective analysis of diet quality and endometrial cancer among 84,415 postmenopausal women in the Women's Health Initiative. Annals of Epidemiology 2015;25(10):788‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Giallauria 2014 {published data only}
- Giallauria F, Gentile M, Chiodini P, Berrino F, Mattiello A, Maresca L, et al. Exercise training reduces high mobility group box‐1 protein levels in women with breast cancer: findings from the DIANA‐5 study. Monaldi Archives for Chest Disease 2014;82(2):61‐7. [DOI] [PubMed] [Google Scholar]
Goodwin 2019 {published data only}
- Goodwin PJ, Segal R, Vallis M, Ligibel JA, Pond GR, Robidoux A, et al. Lifestyle intervention study (LISA) in early breast cancer (BC): an RCT of the effects of a telephone‐based weight loss intervention (with educational materials) vs educational materials alone on disease‐free survival (DFS). Cancer Research. 2019; Vol. 79, issue 4:Suppl 1.
Hagemann 2019 {published data only}
- Hagemann AR, Leon A, Liu J, Kuroki LM, Thaker PH, McCourt CK, et al. Behavioral weight loss interventions are insufficient to address the obesity crisis in endometrial cancer survivors: results of a randomized, controlled trial. Gynecologic Oncology 2019;154:Suppl 1. [Google Scholar]
Haggerty 2014 {published data only}
- Haggerty AF, Allison K, Sarwer DB, Spitzer J, Raggio G, Chu C. The use of technology‐based weight loss intervention for endometrial cancer survivors with obesity. 45th Annual Meeting on Women's Cancer of the Society of Gynecologic Oncology; 2014 Mar 22‐25;Tampa, FL United States. 2014:50‐1.
Hershman {published data only}
- Hershman DL, Greenlee H, Awad D, Kalinsky K, Maurer M, Kranwinkel G, et al. Randomized, single blind trial comparing limited and intensive survivorship interventions following adjuvant therapy in a multiethnic cohort of breast cancer survivors. Cancer Research 2012;72(24):Suppl. [Google Scholar]
Ho 2013 {published data only}
- Ho JW, Lee AM, Macfarlane DJ, Fong DY, Leung S, Cerin E, et al. Study protocol for "Moving Bright, Eating Smart" ‐ a phase 2 clinical trial on the acceptability and feasibility of a diet and physical activity intervention to prevent recurrence in colorectal cancer survivors. BMC Public Health 2013;13:487. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hung 2013 {published data only}
- Hung Y, Bauer J, Horsley P, Coll J, Bashford J, Isenring E. Telephone delivered nutrition and exercise counselling for cancer survivors following autologous stem cell transplantation ‐ a pilot, randomised controlled trial. International MASCC/ISOO Symposium: Supportive Care in Cancer; 2013 Jun 27‐29; Berlin, Germany. 2013:165‐6.
James 2011 {published data only}
- James EL, Stacey F, Chapman K, Lubans DR, Asprey G, Sundquist K, et al. Exercise and nutrition routine improving cancer health (ENRICH): the protocol for a randomized efficacy trial of a nutrition and physical activity program for adult cancer survivors and carers. BMC Public Health 2011;11:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
James 2015 {published data only}
- James EL, Stacey FG, Chapman K, Boyes AW, Burrows T, Girgis A, et al. Impact of a nutrition and physical activity intervention (ENRICH) (Exercise and Nutrition Routine Improving Cancer Health) on health behaviors of cancer survivors and carers: a pragmatic randomized controlled trial. BMC Cancer 2015;15:710. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ko 2010 {published data only}
- Ko LK, Campbell MK, Lewis MA, Earp J, Devellis B. Mediators of fruit and vegetable consumption among colorectal cancer survivors. Journal of Cancer Survivorship 2010;4(2):149‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Koner 2012 {published data only}
- Koner S, Basak J, Chakraborty A, Bhattacharya Majumder D, Dasgupta S, et al. Community therapy by early breast cancer detection and its prevention by lifestyle modification. IMPAKT Breast Cancer Conference; 2012 May 3‐5; Brussels Belgium. 2012:49.
Koutoukidis 2019 {published data only}
- Koutoukidis DA, Beeken RJ, Manchanda R, Burnell M, Ziauddeen N, Michalopoulou M, et al. Diet, physical activity, and health‐related outcomes of endometrial cancer survivors in a behavioral lifestyle program: the Diet and Exercise in Uterine Cancer Survivors (DEUS) parallel randomized controlled pilot trial. International Journal of Gynecological Cancer 2019;29(3):531‐40. [DOI] [PubMed] [Google Scholar]
Kwiatkowski 2017 {published data only}
- Kwiatkowski F, Mouret‐Reynier M‐A, Duclos M, et al. Long‐term improvement of breast cancer survivors' quality of life by a 2‐week group physical and educational intervention: 5‐year update of the 'PACThe' trial. British Journal of Cnacer 2017; Vol. 116, issue 11:1389–93. [DOI] [PMC free article] [PubMed]
Lee 2014 {published data only}
- Lee MK, Yun YH, Park HA, Lee ES, Jung KH, Noh DY. A Web‐based self‐management exercise and diet intervention for breast cancer survivors: pilot randomized controlled trial. International Journal of Nursing Studies 2014;51(12):1557‐67. [DOI] [PubMed] [Google Scholar]
Lee 2018 {published data only}
- Lee CF, Ho JWC, Fong DYT, Macfarlane DJ, Cerin E, Lee AM, et al. Dietary and physical activity interventions for colorectal cancer survivors: a randomized controlled trial. Science Report 2018;8(1):5731. [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2008 {published data only}
- Li Z, Aronson WJ, Arteaga JR, Hong K, Thames G, Henning SM, et al. Feasibility of a low‐fat/high‐fiber diet intervention with soy supplementation in prostate cancer patients after prostatectomy. European Journal of Clinical Nutrition 2008;62(4):526‐36. [DOI] [PubMed] [Google Scholar]
Ligibel 2019 {published data only}
- Ligibel JA, Basen‐Engquist K, Bea JW. Weight management and physical activity for breast cancer prevention and control. American Society of Clinical Oncology Educational Book 2019;39:e22‐e33. [DOI] [PubMed] [Google Scholar]
Lynch 2014 {published data only}
- Lynch BM, Courneya KS, Sethi P, Patrao TA, Hawkes AL. A randomized controlled trial of a multiple health behavior change intervention delivered to colorectal cancer survivors: effects on sedentary behavior. Cancer 2014;120(17):2665‐72. [DOI] [PubMed] [Google Scholar]
McCarroll 2014 {published data only}
- McCarroll ML, Armbruster S, Frasure HE, Gothard MD, Gil KM, Kavanagh M, et al. Self‐efficacy, quality of life, and weight loss in overweight/obese endometrial cancer survivors (SUCCEED): a randomized controlled trial. Gynecological Oncology 2014;132(2):397‐402. [DOI] [PubMed] [Google Scholar]
McDonald 2014 {published data only}
- McDonald C, Bauer J, Capra S, Coll J. The muscle mass, omega‐3, diet, exercise and lifestyle (MODEL) study ‐ a randomised controlled trial for women who have completed breast cancer treatment. BMC Cancer 2014;14:264. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moriya 2014 {published data only}
- Moriya T, Fukatsu K, Okamoto K, Shinto E, Ueno H, Hase K, et al. Effects of preoperative use of an immune‐enhancing diet on postoperative complications and long‐term outcome: a randomized clinical trial in colorectal cancer surgery in Japanese patients. 36th European Society for Clinical Nutrition and Metabolism, ESPEN Congress; 2014 Sep 6‐9; Geneva Switzerland. 2014:247.
Nelson 2008 {published data only}
- Nelson N. Dietary intervention trial reports no effect on survival after breast cancer. Journal of the National Cancer Institute 2008;100(6):386‐7. [DOI] [PubMed] [Google Scholar]
O'Neill 2010 {published data only}
- O'Neill RF, Haseen F, Murray LJ, O'Sullivan JM, Cantwell MM. A randomised controlled trial to evaluate the efficacy of a 6 month dietary and physical activity intervention for prostate cancer patients receiving androgen deprivation therapy. Trials 2010;9(3):431‐40. [DOI] [PubMed] [Google Scholar]
Park 2019 {published data only}
- Park SH, Knobf MT, Kerstetter J, Jeon S. Adherence to American Cancer Society guidelines on nutrition and physical activity in female cancer survivors: results from a randomized controlled trial (Yale fitness intervention trial). Cancer Nursing 2019;42(3):242‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pasanisi 2009 {published data only}
- Pasanisi P, Villarini A, Bruno E, Raimondi M, Gargano G, Berrino F. Benefits of a healthy diet for cancer patients. Joint ECCO 15‐34th ESMO Multidisciplinary Congress; 2009 Sep 20‐24; Berlin Germany. 2009:45.
Paxton 2012 {published data only}
- Paxton RJ, Garcia‐Prieto C, Rocio Berglund M, Hernandez M, Hajek RA, Handy BC, et al. A randomized parallel‐group dietary feasibility study for stage II‐IV ovarian cancer survivors. Gynecological Oncology 2012;124(3):410‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pellegrini 2014 {published data only}
- Pellegrini M. Diet and lifestyle in the prevention of cancer recurrence. European Biotechnology Congress; 2014 May 15‐18;Lecce Italy. 2014:16‐17.
Rack 2010 {published data only}
- Rack B, Andergassen U, Neugebauer J, Salmen J, Hepp P, Sommer H, et al. The German SUCCESS C study ‐ the first European lifestyle study on breast cancer. Breast Care (Basel, Switzerland) 2010;5(6):395‐400. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reddy 2005 {published data only}
- Reddy GK, Tripathy D. Dietary fat reduction improves relapse‐free survival in postmenopausal women previously treated for early‐stage breast cancer: results from a phase III women's intervention nutrition study. Clinical Breast Cancer 2005;6(2):112‐4. [Google Scholar]
Rock 2015 {published data only}
- Rock CL, Flatt SW, Byers TE, Colditz GA, Demark‐Wahnefried W, Ganz PA, et al. Results of the exercise and nutrition to enhance recovery and good health for you (ENERGY) trial: a behavioral weight loss intervention in overweight or obese breast cancer survivors. Journal of Clinical Oncology 2015;33(28):3169‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sedlacek 2011 {published data only}
- Sedlacek SM, Playdon MC, Wolfe P, McGinley JN, Wisthoff MR, Daeninck EA, et al. Effect of a low fat versus a low carbohydrate weight loss dietary intervention on biomarkers of long term survival in breast cancer patients (CHOICE): study protocol. BMC Cancer 2011;11:287. [DOI] [PMC free article] [PubMed] [Google Scholar]
Song 2015 {published data only}
- Song S, Hwang E, Moon HG, Noh DY, Lee JE. Dietary patterns and health‐related quality of life among Korean breast cancer survivors. Federation of American Societies for Experimental Biology 2015;29(1):Suppl. [Google Scholar]
Stacey 2017 {published data only}
- Stacey FG, Lubans DR, Chapman K, Bisquera A, James EL. Maintenance of lifestyle changes at 12‐month follow‐up in a nutrition and physical activity trial for cancer survivors. American Journal of Health Behaviour 2017;41(6):784‐95. [DOI] [PubMed] [Google Scholar]
Stricker 2013 {published data only}
- Stricker CT, Palmer SC, Panzer SL, Syrjala KL, Baker KS, McCabe MS, et al. Breast cancer survivors' outcomes and satisfaction following delivery of a survivorship care plan: results of a multicenter trial. Cancer Research 2013;73(24):Suppl. [Google Scholar]
Thiebaut 2006 {published data only}
- Thiebaut AC, Schatzkin A, Ballard‐Barbash R, Kipnis V. Dietary fat and breast cancer: contributions from a survival trial. Journal of the National Cancer Institute 2006;98(24):1753‐55. [DOI] [PubMed] [Google Scholar]
Thomas 2009 {published data only}
- Thomas R, Williams MMA, Prasannan C. Development of a post adjuvant exit lifestyle toolbox. Joint ECCO 15 ‐ 34th ESMO Multidisciplinary Congress; 2009; Sep 20‐24; Berlin Germany. 2009:210.
Thompson 2012 {published data only}
- Thompson HJ, Sedlacek SM, Paul D, Wolfe P, McGinley JN, Playdon MC, et al. Effect of dietary patterns differing in carbohydrate and fat content on blood lipid and glucose profiles based on weight‐loss success of breast‐cancer survivors. Breast Cancer Research 2012;14(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thomson 2014 {published data only}
- Thomson CA, Crane TE, Wertheim BC, Neuhouser ML, Li W, et al. Diet quality and survival after ovarian cancer: results from the Women's Health Initiative. Journal of the National Cancer Institute 2014;106(11):314. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tyagi 2005 {published data only}
- Tyagi P. Updated data from the Iressa survival in lung cancer trial. Clinical Lung Cancer 2005;6(6):340‐2. [DOI] [PubMed] [Google Scholar]
Urowitz 2012 {published data only}
- Urowitz S, Chiu W, Cockburn M, Dunlop B, Fierini D, Himel D, et al. Building recipes and understanding nutrition for cancer‐survivor health (BRUNCH). Journal of Nutrition, Education & Behaviour 2012;44(4):384‐6. [DOI] [PubMed] [Google Scholar]
Van Der Werf 2015 {published data only}
- Werf A, Blauwhoff‐Buskermolen S, Langius JA, Berkhof J, Verheul HM, van der Schueren MA. The effect of individualized nutritional counseling on muscle mass and treatment outcome in patients with metastatic colorectal cancer undergoing chemotherapy: a randomized controlled trial protocol. BMC Cancer 2015;15:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
Villasenor 2014 {published data only}
- Villasenor A, Flatt SW, Marinac C, Natarajan L, Pierce JP, Patterson RE. Postdiagnosis C‐reactive protein and breast cancer survivorship: findings from the WHEL study. Cancer Epidemiology, Biomarkers and Prevention 2014;23(1):189‐99. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vona‐Davis 2015 {published data only}
- Vona‐Davis L, Abraham J, Bonner D, Gilleland D, Hobbs G, Kurian S, et al. Effect of a 12‐week supervised physical activity and healthy eating program on body weight, functional capacity and serum biomarkers in survivors of triple‐negative breast cancer: a randomized, controlled trial. Cancer Research 2015;75(9):Suppl. [Google Scholar]
Xing 2014 {published data only}
- Xing MY, Xu SZ, Shen P. Effect of low‐fat diet on breast cancer survival: a meta‐analysis. Asian Pacific Journal of Cancer Prevention 2014;15(3):1141‐4. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Brown 2018 {published data only}
- Brown JC, Yung RL, Gobbie‐Hurder A, Shockro L, O'Connor K, Campbell N, et al. Randomized trial of a clinic‐based weight loss intervention in cancer survivors. Journal of Cancer Survivorship 2018;12(2):186‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Parekh 2018 {published data only}
- Parekh N, Jiang J, Buchan M, Meyers M, Gibbs H, Krebs P. Nutrition literacy among cancer survivors: feasibility results from the Healthy Eating and Living Against Breast Cancer (HEAL‐BCa) study: a pilot randomized controlled trial. Journal of Cancer Education 2018;33(6):1239‐49. [DOI] [PubMed] [Google Scholar]
Zuniga 2019 {published data only}
- Zuniga KE, Parma DL, Muñoz E, Spaniol M, Wargovich M, Ramirez AG. Dietary intervention among breast cancer survivors increased adherence to a Mediterranean‐style, anti‐inflammatory dietary pattern: the Rx for Better Breast Health Randomized Controlled Trial. Breast Cancer Research and Treatment 2019;173(1):145‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
Alberts 2008 {published data only}
- Diet and physical activity change or usual care in improving progression‐free survival in patients with previously treated stage II, III, or IV ovarian, fallopian tube, or primary peritoneal cancer. www.clinicaltrials.gov/ct2/show/NCT00719303. First received 21 July 2008.
Anderson 2017 {published data only}
- The women’s wellness after cancer program: a multisite, single‐blinded, randomised controlled trial protocol. BMC Cancer 2017; Vol. 17:98. [DOI] [PMC free article] [PubMed]
Asprey 2009 {published data only}
- Promotion of healthy lifestyle and risk modification for cancer survivors and their partners/caregivers (ENRICH: Exercise and Nutrition Routine Improving Cancer Health). https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=320954. First received 18 December 2009.
Blomhoff 2012 {published data only}
- Effect of the new Norwegian food based dietary guidelines on chronic diseases in colorectal cancer survivors (NFS). https://clinicaltrials.gov/ct2/show/NCT01570010. First received 4 April 2012.
Cirauqui 2014 {published data only}
- Prevention of breast cancer recurrence through weight control, diet, and physical activity intervention (PREDICOP). https://clinicaltrials.gov/ct2/show/NCT02035631. First received 14 January 2014.
Clinton 2014 {published data only}
- Harvesting health program in improving diet and physical activity level in cancer survivors. https://clinicaltrials.gov/ct2/show/NCT02268188. First received 20 October 2014.
Coups 2009 {published data only}
- Internet‐based weight‐loss program for colorectal cancer survivors. https://clinicaltrials.gov/ct2/show/NCT01032590. First received 15 December 2009.
Demark‐Wahnefried 2019 {published data only}
- Adapting multiple behavior interventions that effectively improve cancer survivor health cancer survivor health (AMPLIFY). https://clinicaltrials.gov/ct2/show/NCT04000880. First received 27 June 2019.
Dittus 2012 {published data only}
- An internet‐based weight loss and exercise intervention for breast cancer survivors (iWEB). https://clinicaltrials.gov/ct2/show/NCT01728506. First received 19 November 2012.
Ferrante 2016 {published data only}
- eHealth weight loss program in African American breast cancer survivors. https://clinicaltrials.gov/ct2/show/NCT02699983. First received 7 March 2016.
Frensham 2013 {published data only}
- STRIDE (Steps TowaRd Improving Diet and Exercise): an online lifestyle intervention for cancer survivors living in South Australia. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=363608. First received 29 April 2013.
Greenlee 2011 {published data only}
- S1008: exercise, diet, & counseling in improving weight loss in overweight female breast or colorectal cancer survivors. https://clinicaltrials.gov/ct2/show/NCT01453452. First received 17 October 2011.
Groarke 2018 {published data only}
- A pilot trial to investigate the impact of a personalised self‐management lifestyle programme using mobile technology on the health and well‐being of cancer survivors. http://www.isrctn.com/ISRCTN18676721. First received 2 May 2018.
Heinrich 2015 {published data only}
- Advancing survivorship after cancer: outcomes trial. http://www.isrctn.com/ISRCTN17421871. First received 29 July 2015.
Ho Wai‐chu 2012 {published data only}
- Diet and physical activity intervention in CRC survivors. https://clinicaltrials.gov/ct2/show/NCT01708824. First received 17 October 2012.
Irwin 2014 {published data only}
- Lifestyle, exercise and nutrition study 2 (LEAN 2). https://clinicaltrials.gov/ct2/show/NCT02110641. First received 10 April 2014.
Jernigan 2015 {published data only}
- Weight loss referral for healthier survivorship in obese stage I‐II endometrial cancer survivors or atypical hyperplasia. https://clinicaltrials.gov/ct2/show/NCT02342730. First received 21 January 2015.
Lawler 2014 {published data only}
- Get Healthy after Breast Cancer ‐ examining the feasibility and acceptability of referring breast cancer survivors to the NSW ‘Get Healthy Service’ – a telephone‐delivered program targeting physical activity, healthy diet and weight loss. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=365601. First received 22 January 2014. [DOI] [PubMed]
Lechner 2012 {published data only}
- Lifestyle change, self‐management, and problem solving in daily life in cancer survivors; an online portal for change and support. http://apps.who.int/trialsearch/Trial2.aspx?TrialID=NTR3375. First received 29 March 2012.
Ligibel 2013 {published data only}
- Healthy living after cancer: weight management pilot study. https://clinicaltrials.gov/ct2/show/NCT01978899. First received 8 November 2013.
Mathews 2012 {published data only}
- My lifestyle intervention of food and exercise (MyLIFE). https://clinicaltrials.gov/ct2/show/NCT01630499. First received 28 June 2012.
O'Connor 2018 {published data only}
- Exercise and nutrition education in improving physical function and quality of life in older breast cancer survivors. https://clinicaltrials.gov/ct2/show/NCT03751449. First received 22 November 2018.
Park 2013 {published data only}
- A lifestyle intervention for breast cancer survivors (TTMI). https://clinicaltrials.gov/ct2/show/NCT01819324. First received 27 March 2013.
Pasanisi 2012 {published data only}
- 1177 A randomized controlled trial of diet, physical activity and breast cancer recurrences – the DIANA‐5 study. https://www.ejcancer.com/article/S0959‐8049(12)71772‐0/abstract. July 2012V; Vol. 48, issue Suppl 5:S283–S284.
Quintiliani 2015 {published data only}
- Weight management among breast cancer survivors. https://clinicaltrials.gov/ct2/show/NCT02387671. First received 13 March 2015.
Resnick 2014 {published data only}
- Protein‐sparing modified fast intervention for weight loss in obese endometrial cancer survivors. https://clinicaltrials.gov/ct2/show/NCT02135562. First received 12 May 2014.
Additional references
Armes 2009
- Armes J, Crowe M, Colbourne L, Morgan H, Murrells T, Oakley C, et al. Patients' supportive care needs beyond the end of cancer treatment: a prospective, longitudinal survey. Journal of Clinical Oncology 2009;27(36):6172‐9. [DOI] [PubMed] [Google Scholar]
Aziz 2003
- Aziz NM, Rowland JH. Trends and advances in cancer survivorship research: challenge and opportunity. Seminars in Radiation Oncology 2003;13(3):248‐66. [DOI] [PubMed] [Google Scholar]
Baker 2003
- Baker F, Haffer SC, Denniston M. Health‐related quality of life of cancer and noncancer patients in Medicare managed care. Cancer 2003;97(3):674‐81. [DOI] [PubMed] [Google Scholar]
Bowling 1999
- Bowling A, Bond M, Jenkinson C, Lamping DL. Short Form 36 (SF‐36) Health Survey questionnaire: which normative data should be used? Comparisons between the norms provided by the Omnibus Survey in Britain, the Health Survey for England and the Oxford Healthy Life Survey. Journal of Public Health 1999;21(3):255–70. [DOI] [PubMed] [Google Scholar]
Bray 2018
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians 2018;68(6):394‐424. [DOI] [PubMed] [Google Scholar]
Broggio 2019
- Broggio J, King A. Geographic patterns of cancer survival in England Adults diagnosed 2011 to 2015 and followed up to 2016. www.ons.gov.uk/releases/geographicpatternsofcancersurvivalinenglandadultsdiagnosed2011to2015andfollowedupto2016. Accessed 26 June 2019.
Chlebowski, 2006
Daar 2007
- Daar AS, Singer PA, Persad DL, Pramming SK, Matthews DR, Beaglehole R, et al. Grand challenges in chronic non‐communicable diseases. Nature 2007;7169:494‐6. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1 (updated March 2011). London, UK: The Cochrane Collaboration.
Demark‐Wahnefried 2000
- Demark‐Wahnefried W, Peterson B, McBride C, Lipkus I, Clipp E. Current health behaviours and readiness to pursue life‐style changes among men and women diagnosed with early stage prostate and breast carcinomas. Cancer 2000;88(3):674‐84. [PubMed] [Google Scholar]
Demark‐Wahnefried 2005
- Demark‐Wahnefried W, Aziz NM, Rowland JH, Pinto BM. Riding the crest of the teachable moment: promoting long‐term health after the diagnosis of cancer. Journal of Clinical Oncology 2005;23(24):5814‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Demark‐Wahnefried 2009
- Demark‐Wahnefried W, Jones LW. Promoting a healthy lifestyle among cancer survivors. Hematology‐Oncology Clinics of North America 2009;22(2):319‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Demark‐Wahnefried, 2015
Department 2010
- Department of Health Macmillan Cancer Support & NHS Improvements. National Cancer Survivorship Initiative: Vision. www.dh.gov.uk/publications (accessed 2010).
Elliott 2011
- Elliott J, Fallows A, Staetsky L, Smith PWF, Foster CL, Maher EJ, et al. The health and well‐being of cancer survivors in the UK: findings from a population‐based survey. British Journal of Cancer 2011;105(Suppl 1):S11‐S20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ganz 2005
- Ganz PA. A teachable moment for oncologists: cancer survivors, 10 million strong and growing!. Journal of Clinical Oncology 2005;23(24):5458‐60. [DOI] [PubMed] [Google Scholar]
Goode 2015
GRADEPro GDT 2014 [Computer program]
- GRADE Working Group, McMaster University, Hamilton (ON). GRADEpro Guideline Development Tool (GDT)[www.gradepro.org]. Version [2015]. GRADE Working Group, McMaster University, Hamilton (ON), 2014.
Haines 1999
- Haines PS, Siega‐Riz AM, Popkin BM. The Diet Quality Index revised: a measurement instrument for populations. Journal of the American Dietetic Association 1999;99(6):697‐704. [DOI] [PubMed] [Google Scholar]
Hewitt 2003
- Hewitt M, Rowland JH, Yancik R. Cancer survivors in the United States: age, health, and disability. Journals of Gerontology 2003;58(1):82‐91. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). London, UK: The Cochrane Collaboration, 2011.
Janssen‐Heijnen 2009
- Janssen‐Heijnen MLG, Szerencsi K, Schans SAM, Maas Huub AAM, Widdershoven JW, Coebergh JWW. Cancer patients with cardiovascular disease have survival rates comparable to cancer patients within the age‐cohort of 10 years older without cardiovascular morbidity. Critical Reviews in Oncology/Hematology 2009;76(3):196‐207. [DOI] [PubMed] [Google Scholar]
Khan 2011
- Khan NF, Ward AM, Watson E, Rose PW. Consulting and prescribing behaviour for anxiety and depression in long‐term survivors of cancer in the UK. European Journal of Cancer 2011;46(18):3339‐44. [DOI] [PubMed] [Google Scholar]
Kushi 2012
- Kushi LH, Doyle C, McCullough M, Rock CR, Demark‐Wahnefried W, Bandera EV, et al. American Cancer Society Guidelines on Nutrition and Physical Activity for Cancer Prevention. Reducing the risk of cancer with healthy food choices and physical activity. CA: A Cancer Journal for Clinicians 2012;62(1):30‐67. [DOI] [PubMed] [Google Scholar]
Lancet 2004
- The Lancet. Cancer survivors: living longer, and now, better. Lancet 2004;364(9452):2153‐4. [DOI] [PubMed] [Google Scholar]
Langendam 2013
- Langendam MW, Akl EA, Dahm P, Glasziou P, Guyatt G, Schunemann HJ. Assessing and presenting summaries of evidence in Cochrane Reviews. Systematic Reviews 2013;23(2):81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lippman 2004
- Lippman SM, Levin B, Brenner DE, Gordon GB, Aldige CR, Kramer BS, et al. Cancer prevention and the American Society of Clinical Oncology. Journal of Clincial Oncology 2004;22(19):3848‐51. [DOI] [PubMed] [Google Scholar]
Louwman 2010
- Louwman WJ, Aarts MJ, Houterman S, Lenthe FJ, Coebergh JWW, Janssen‐Heijnen MLG. A 50% higher prevalence of life‐shortening chronic conditions among cancer patients with low socioeconomic status. British Journal of Cancer 2010;103(11):1742‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Maddams 2009
- Maddams J, Brewster D, Gavin A, Steward J, Elliott J, Utley M, et al. Cancer prevalence in the United Kingdom: estimates for 2008. British Journal of Cancer 2009;101(3):541‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marbach 2011
- Marbach TJ, Griffie J. Patient preferences concerning treatment plans, survivorship care plans, education, and support services. Oncology Nursing Forum 2011;38(3):335‐42. [DOI] [PubMed] [Google Scholar]
Meader 2014
- Meader N, King K, Llewellyn A, Norman G, Brown J, Rodgers M, et al. A checklist designed to aid consistency and reproducibility of GRADE assessments: development and pilot validation. Systematic Reviews 2014;3:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Miller 2016
- Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, et al. Cancer treatment and survivorship statistics. Cancer Journal for Clinicians 2016;66(4):271‐89. [DOI] [PubMed] [Google Scholar]
Mishra 2012
- Mishra SI, Scherer RW, Geigle PM, Berlanstein DR, Topaloglu O, Gotay CC, et al. Exercise interventions on health‐related quality of life for cancer survivors. Cochrane Database of Systematic Reviews 2012, Issue 8. [DOI: 10.1002/14651858.CD007566] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mosher 2009
- Mosher CE, Sloane R, Morey MC, Snyder DC, Cohen HJ, Miller PE, et al. Associations between lifestyle factors and quality of life among older long‐term breast, prostate, and colorectal cancer survivors. Cancer 2009;115(17):4001‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nord 2005
- Nord C, Mykletun A, Thorsen L, Bjøro T, Fosså SD. Self‐reported health and use of health care services in long‐term cancer survivors. International Journal of Cancer 2005;114(2):307‐16. [DOI] [PubMed] [Google Scholar]
Patterson 1994
- Patterson RE, Haines PS, Popkin BM. Diet quality index: capturing a multidimensional behavior. Journal of the American Dietetic Association 1994;94(1):57‐64. [DOI] [PubMed] [Google Scholar]
Patterson 2003
- Patterson RE, Neuhouser ML, Hedderson MM, Schwartz SM, Standish LJ, Bowen DJ. Changes in diet, physical activity, and supplement use among adults diagnosed with cancer. Journal of the American Dietetic Association 2003;103(3):323‐8. [DOI] [PubMed] [Google Scholar]
Pierce 2009a
- Pierce JP. Diet and breast cancer prognosis: making sense of the Women's Healthy Eating and Living and Women's Intervention Nutrition Study trials. Current Opinion in Obstetrics & Gynecology 2009;21(1):86‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ravasco 2003
- Ravasco P, Monteiro‐Grillo I, Vidal PM, Camilo ME. Nutritional deterioration in cancer: the role of disease and diet. Clinical Oncology 2003;15(8):443‐50. [DOI] [PubMed] [Google Scholar]
Reeves 2014
- Reeves MM, Terranova CO, Eakin EG, Demark‐Wahnefried W. Weight loss intervention trials in women with breast cancer: a systematic review. Obesity Reviews 2014;15(9):749‐68. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roberts, 2017
- Roberts AL, Fisher A, Smith L, Heinrich M, Potts HWW. Digital health behaviour change interventions targeting physical activity and diet in cancer survivors: a systematic review and meta‐analysis. Journal of Cancer Survivorship 2017; Vol. 11, issue 6:704‐19. [DOI] [PMC free article] [PubMed]
Satia 2004
- Satia JA, Campbell MK, Galanko JA, James A, Carr C, Sandler RS. Longitudinal changes in lifestyle behaviors and health status in colon cancer survivors. Cancer Epidemiology Biomarkers & Prevention 2004;13(6):1022‐31. [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and ‘Summary of findings' tables. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.training.cochrane.org/handbook.
Stacey, 2015
- Stacey FG, James EL, Chapman K, Courneya KS, Lubans DR. A systematic review and meta‐analysis of social cognitive theory‐based physical activity and/or nutrition behavior change interventions for cancer survivors. Journal of Cancer Survivorship 2015; Vol. 11, issue 9:305‐38. [DOI] [PMC free article] [PubMed]
Szende 2014
- SzendE A, Janssen B, Cabases J. Self‐reported Population Health: An International Perspective Based on EQ‐5D. https://link.springer.com/book/10.1007%2F978‐94‐007‐7596‐1: Springer Open, 2014. [PubMed] [Google Scholar]
Vergnaud 2013
- Vergnaud AC, Romaguera D, Peeters PH, Gils CH, Chan DS, Romieu I, et al. Adherence to the World Cancer Research Fund/American Institute for Cancer Research guidelines and risk of death in Europe: results from the European prospective investigation into nutrition and cancer cohort study. American Journal of Clinical Nutrition 2013;97(5):1107‐20. [DOI] [PubMed] [Google Scholar]
WCRF/AICR 2018
- World Cancer Research Fund/American Institute for Cancer Research. Continuous update project. www.wcrf.org/dietandcancer/resources. Accessed 25 June 2019.
References to other published versions of this review
Burden 2014
- Burden S, Gibson DJ, Todd C, Gratton EK, Pilling M, Lal S. Dietary interventions for adult cancer survivors. Cochrane Database of Systematic Reviews 2014, Issue 9. [DOI: 10.1002/14651858.CD011287] [DOI] [PMC free article] [PubMed] [Google Scholar]


