Abstract
Nivolumab plus ipilimumab combined therapy is one of the promising drugs that enhance the anti- immune response in patients with advanced melanoma. Therefore, to increase its response rate is of great interest to dermatologists. Recent reports suggested that, since CD8+ T cells after the administration of ICIs increase the RANKL expression to induce an immunosuppressive tumor microenvironment in melanoma, denosumab might enhance the anti-tumor effects of immune checkpoint inhibitors, such as nivolumab and ipilimumab. In this report, we present a case of multiple metastatic melanoma with nivolumab, ipilimumab plus denosumab combined therapy.
Keywords: Nivolumab plus ipilimumab combined therapy, Denosumab, RANKL, Advanced melanoma, Bone metastasis
Introduction
Nivolumab plus ipilimumab combined therapy is one of the promising drugs that enhance the anti- immune response in patients with advanced melanoma [1], and is recommended by the NCCN guidelines for cutaneous melanoma as a first-line therapy [2] in spites of its high toxicity. Although BRAF inhibitor combo is also recommended by the NCCN guidelines as a first-line for BRAF mutated advanced melanoma [2], its efficacy is limited in advanced melanoma with multiple organ metastases [3].
Denosumab, a fully human monoclonal antibody to RANKL, has been permitted for the treatment of metastatic bone tumors [4], and is reported to enhance the anti-tumor effects of immune checkpoint inhibitors [5]. In this report, we present a case of multiple metastatic melanoma with nivolumab, ipilimumab plus denosumab combined therapy.
Case Report
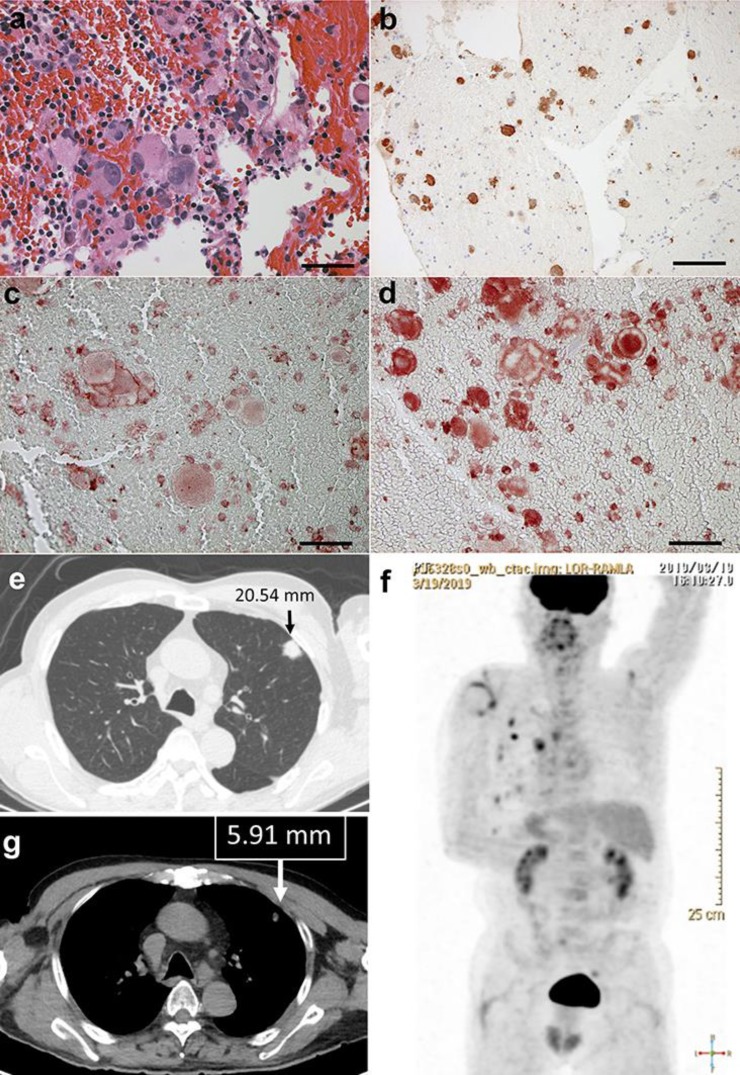
A 70-year-old Japanese man visited our outpatient clinic with a 3-month history of multiple lung metastasis of melanoma. He had undergone resection for malignant melanoma on the back 15 years before, and had been followed up in our clinics for 10 years without any recurrence of the tumor. Five years after our follow-up period, an abnormal shadow of the lung was pointed out in a regular health check-up. Then, he consulted a respiratory physician. Computed tomography (CT) showed multiple lung and pleural nodules, and hilar and mediastinal lymph node swelling. Biopsy from the mediastinal lymph node showed markedly atypical melanocytes (Fig. 1a) that were positive for S-100 (Fig. 1b), Melan A, HMB45, RANK (Fig. 1c) and RANKL (Fig. 1d). From the above findings, the diagnosis was multiple metastatic melanoma in the lung. At his initial visit, we performed positron emission tomography (PET-CT), which revealed cervical (C3) and rib metastasis in addition to lung (Fig. 1e), pleural and lymph node metastases (Fig. 1f). The serum lactate dehydrogenase (LDH) level was in the upper limit of the normal range (222 U/L). The THxID kit revealed that the metastatic lymph node tumor possessed the BRAFV600E mutation. In addition, the serum level of CXCL5 was 811.8 ng/mL. Since the patient had metastases in 4 organs (>3 organs), suggesting that dabrafenib plus trametinib combined therapy might not be effective 3, nivolumab (80 mg/body/every three weeks) was given in combination with ipilimumab (3 mg/kg/every three weeks) for 4 cycles. In addition, since this patient showed metastatic melanoma of the bone, we administered denosumab 120 mg every month. The increased levels of soluble (s)CD163 at 6 weeks was 20.54 (ng/mL). We administered prednisolone (10∼30 mg/day) for the treatment of uveitis (G2) and skin rash (G2) one month after the initial administration. Follow-up PET-CT two months after the combination therapy suggested significant regression of the lung nodules (Fig. 1g), pleural nodules, lymph nodes and bone metastases. Three months after the final administration of this combined therapy, these metastatic nodules remained regressed, and there were no immune-related adverse events except for uveitis (G2) and skin rash (G2).
Fig. 1.
Biopsy from mediastinal lymph node: markedly atypical melanocytes (a), which were positive for S-100 (b), RANK (c) and RANKL (d). Multiple lung metastases (e), pleural, lymph node metastases, and bone metastases on PET-CT at initial visit (f). After treatment, the multiple lung metastases had decreased (g).
Discussion
Recently, pre-clinical reports suggested that the receptor of activated nuclear factor kappa B ligand (RANKL) blockade enhances the therapeutic effects of immune chokepoint inhibitors [5, 6, 7]. For example, Ahern et al. reported that the administration of anti-RANKL antibodies (Abs) enhanced the anti-tumor effects of anti-PD1 Abs plus anti-CTLA4 Abs by the suppression of RANKL+ PD1highCD8 T cells in a B16F10 mouse melanoma model [6]. In another report, anti-RANKL Abs enhanced the therapeutic effects of ipilimumab in the terminal stage of metastatic melanoma patients [7]. In a mouse model, co-administration of anti-RANKL Abs with anti-CTLA4 Abs enhanced CD8 mediated anti-tumor immune response in B16F10 melanoma [8]. These reports suggested that anti-RANKL Abs, denosumab, might enhance the anti-melanoma effects of ipilimumab with or without nivolumab.
RANK and its ligand RANKL are critically involved in metastasis formation in breast, prostate or renal cancer [4]. In addition, Kupas et al. reported that the RANK expression on melanoma cells is increased in parallel with tumor progression [9]. Moreover, not only tumor cells, but also tumor-associated macrophages (TAMs) and Langerhans cells express RANK [10, 11], and RANK/ RANKL signaling increases the production of immunosuppressive chemokines such as CCL17 [12]. These reports suggested that the blockade of RANK/ RANKL signaling in advanced melanoma might be useful to improve the tumor microenvironment in advanced melanoma.
In this report, we present a case of multiple metastatic melanoma treated with nivolumab, ipilimumab plus denosumab combined therapy. Although this case presented high baseline levels of CXCL5, suggesting a response to anti-PD1 Abs monotherapy [13], we selected nivolumab plus ipilimumab combined therapy with denosumab because of other prognostic factors at baseline (metastases in 4 organs). As we predicted by our biomarker systems, the increased levels of soluble (s)CD163 at 6 weeks suggested an effective response to anti-PD1 Abs based therapy [14]. Indeed, PET-CT at 3 months after the initial treatment revealed a partial response to this combined therapy. Since a previous clinical study suggested that the response rate of dabrafenib plus trametinib combined therapy in patients with advanced melanoma with multiple organ metastases is limited [3], we selected nivolumab plus ipilimumab combined therapy in the present case. Since we present only a single case, further cases and phase II clinical trial are needed to evaluate the efficacy of this combined therapy.
Statement of Ethics
The patient gave written informed consent. The protocol for this human study was approved by the ethics committee of Tohoku University Graduate School of Medicine, Sendai, Japan (Permit No: 2017–1-064). All methods were performed in accordance with the relevant guidelines and regulations.
Disclosure Statement
The authors have no conflicting interests to declare.
Funding Sources
This study was supported in part by the Japan Agency for Medical Research and Development (19cm0106434h0002).
Author Contributions
Fujimura T designed the research study. Yoshida S, Fujimura T, Kambayashi Y, Amagai R and Hashimoto A treated the patients and acquired the clinical data and samples. Fujimura T gathered and analyzed the ELISA data. Fujimura T wrote the manuscript. Fujimura T and Aiba S supervised the study.
References
- 1.Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, et al. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med. 2017 Oct;377((14)):1345–56. doi: 10.1056/NEJMoa1709684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.NCCN Clinical Practice Guidelines in Oncology (NCCN GuidelinesⓇ) Melanoma Version 2.2019-March 12, 2019 https://www.nccn.org/professionals/physician_gls/pdf/cutaneous_melanoma.pdf In. 2019.
- 3.Long GV, Flaherty KT, Stroyakovskiy D, Gogas H, Levchenko E, de Braud F, et al. Dabrafenib plus trametinib versus dabrafenib monotherapy in patients with metastatic BRAF V600E/K-mutant melanoma: long-term survival and safety analysis of a phase 3 study. Ann Oncol. 2017 Jul;28((7)):1631–9. doi: 10.1093/annonc/mdx176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Azim H, Azim HA., Jr Targeting RANKL in breast cancer: bone metastasis and beyond. Expert Rev Anticancer Ther. 2013 Feb;13((2)):195–201. doi: 10.1586/era.12.177. [DOI] [PubMed] [Google Scholar]
- 5.Angela Y, Haferkamp S, Weishaupt C, Ugurel S, Becker JC, Oberndörfer F, et al. Combination of denosumab and immune checkpoint inhibition: experience in 29 patients with metastatic melanoma and bone metastases. Cancer Immunol Immunother. 2019 Jul;68((7)):1187–94. doi: 10.1007/s00262-019-02353-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahern E, Harjunpää H, O'Donnell JS, Allen S, Dougall WC, Teng MW, et al. RANKL blockade improves efficacy of PD1-PD-L1 blockade or dual PD1-PD-L1 and CTLA4 blockade in mouse models of cancer. OncoImmunology. 2018 Feb;7((6)):e1431088. doi: 10.1080/2162402X.2018.1431088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smyth MJ, Yagita H, McArthur GA. Combination Anti-CTLA-4 and Anti-RANKL in Metastatic Melanoma. J Clin Oncol. 2016 Apr;34((12)):e104–6. doi: 10.1200/JCO.2013.51.3572. [DOI] [PubMed] [Google Scholar]
- 8.Ahern E, Harjunpää H, Barkauskas D, Allen S, Takeda K, Yagita H, et al. Co-administration of RANKL and CTLA4 Antibodies Enhances Lymphocyte-Mediated Antitumor Immunity in Mice. Clin Cancer Res. 2017 Oct;23((19)):5789–801. doi: 10.1158/1078-0432.CCR-17-0606. [DOI] [PubMed] [Google Scholar]
- 9.Kupas V, Weishaupt C, Siepmann D, Kaserer ML, Eickelmann M, Metze D, et al. RANK is expressed in metastatic melanoma and highly upregulated on melanoma-initiating cells. J Invest Dermatol. 2011 Apr;131((4)):944–55. doi: 10.1038/jid.2010.377. [DOI] [PubMed] [Google Scholar]
- 10.Kambayashi Y, Fujimura T, Furudate S, Asano M, Kakizaki A, Aiba S. The Possible Interaction between Receptor Activator of Nuclear Factor Kappa-B Ligand Expressed by Extramammary Paget Cells and its Ligand on Dermal Macrophages. J Invest Dermatol. 2015 Oct;135((10)):2547–50. doi: 10.1038/jid.2015.199. [DOI] [PubMed] [Google Scholar]
- 11.Fujimura T, Kambayashi Y, Furudate S, Kakizaki A, Hidaka T, Aiba S. Possible mechanisms of the crosstalk between Langerhans cells and regulatory T cells in extramammary Paget disease by receptor activator of nuclear factor kappa B (RANK) ligand/RANK pathways. Br J Dermatol. 2017 Feb;176((2)):387–94. doi: 10.1111/bjd.14864. [DOI] [PubMed] [Google Scholar]
- 12.Fujimura T, Kambayashi Y, Furudate S, Asano M, Kakizaki A, Aiba S. Receptor Activator of NF-κB Ligand Promotes the Production of CCL17 from RANK+ M2 Macrophages. J Invest Dermatol. 2015 Nov;135((11)):2884–7. doi: 10.1038/jid.2015.209. [DOI] [PubMed] [Google Scholar]
- 13.Fujimura T, Sato Y, Tanita K, Lyu C, Kambayashi Y, Amagai R, et al. Association of baseline serum levels of CXCL5 with the efficacy of nivolumab in advanced melanoma. Front Med (Lausanne) 2019 Apr;6:86. doi: 10.3389/fmed.2019.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujimura T, Sato Y, Tanita K, Kambayashi Y, Otsuka A, Fujisawa Y, et al. Serum level of soluble CD163 may be a predictive marker of the effectiveness of nivolumab in patients with advanced cutaneous melanoma. Front Oncol. 2018 Nov;8:530. doi: 10.3389/fonc.2018.00530. [DOI] [PMC free article] [PubMed] [Google Scholar]