Abstract
Background
Intestinal failure (IF) in the adult is the result of a wide spectrum of disease. Acute mesenteric ischemia, postoperative short bowel due to a complicative course, and Crohn's disease are major causes of IF. Reconstructive surgery in the context of IF comprises a spectrum of procedures including stoma takedown, reversal of laparostomies, and closure of enteric fistulas.
Methods
This article is based on a PubMed-based literature search and personal experience in adult patients with IF.
Results
This review summarizes therapeutic options of reconstructive surgery in adult patients focusing on the main reasons of IF such as mesenteric ischemia, complicative previous surgery, and Crohn's disease. Indications and contraindications are discussed as well as the optimal time point of reconstructive surgery.
Conclusion
This overview summarizes surgical aspects in a special cohort of patients with a rare disease entity necessitating an interdisciplinary approach.
Keywords: Surgery, Intestinal failure, Short bowel, Crohn's disease, Mesenteric ischemia
Introduction
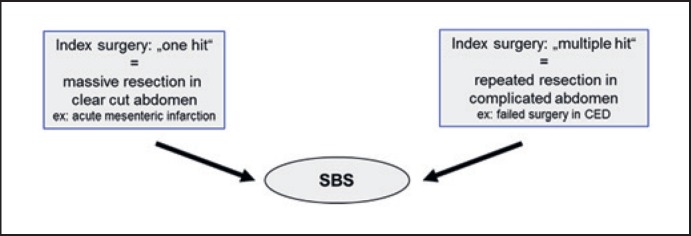
Intestinal failure (IF) is the inability of the bowel to provide the body with sufficient nutrients, fluid, and supplements. In 2015 the ESPEN group redefined IF as “the reduction of the gut function below the minimum necessary for the absorption of macronutrients and/or water and electrolytes, such as intravenous supplementation is required to maintain health and or growth” [1]. This group elaborated a functional classification of IF allowing the stratification of patients according to a time- and prognostic-based pattern. Causes of IF are diverse and can be categorized as shown in a simplified manner in Table 1. Reconstructive surgery in the context of IF is not a defined surgical procedure. In most cases it includes stoma reversal or closure of bowel fistulas in the presence or absence of a laparostomy. When planning reconstructive surgery in IF patients, it is essential to keep the primary cause which led to this situation in mind. It is helpful to stratify the case according to the index surgery as “one hit” versus “multiple hit” category (Fig. 1). IF with short bowel due to intestinal ischemia is most often a “one hit” abdomen, meaning that the procedure resulting in short bowel is extensive resection in a single surgical step. Short bowel due to Crohn's disease is in most cases the result of repeated and complicated procedures in the same patient. This aspect is relevant for planning reconstructive surgery in view of the expected fragility of the abdomen, difficulty in getting access to the abdominal cavity, and length of the operation.
Table 1.
Causes of intestinal failure
| Medical | Ischemic | Infiltrative | Obstructive | Functional |
|---|---|---|---|---|
| Trauma Surgical complication | arterial venous | desmoid carcinoid amyloidosis tumor | adhesions internal hernia volvulus | pseudo-obstruction Crohn's disease colitis radiation enteritis |
Fig. 1.
Role of the index surgery.
The focus of this review lies in personal experience with reconstructive surgery in adult IF patients, omitting surgical techniques of bowel lengthening procedures or intestinal transit modifying operations which have been summarized elsewhere [2].
Indication for Reconstructive Surgery
The indication for reconstructive surgery should be clearly defined (Table 2). The most relevant indication for reconstructive surgery is gain in bowel length. This can be reached either via pure lengthening procedures such as serial transverse enteroplasty (STEP) or the Bianchi procedure [3, 4, 5], which are mainly performed in pediatric patients optimizing rather volume-to-surface ratio than pure lengthening or via recruitment of bowel segments that are excluded from chyme passage as a result of previous surgery or a complication thereof. In this latter case a stoma is present and the wish of the patient to take down the stoma is one reason for reconstructive surgery. A well-designed stoma can, however, result in better quality of life than short bowel with colorectum in continuity but multiple uncontrolled bowel movements per day and night. A high enterostomy can harbor many problems such a skin irritation due to aggressive small bowel output. All efforts to “control” local problems such as high-dose intravenous proton pump inhibitors, medical treatment to lower stoma output (loperamide, opioids, or octreotide) [6], and professional stoma care should be undertaken to minimize local problems [7].
Table 2.
Indications for reconstructive surgery in intestinal failure patients
| Indication | Intention |
|---|---|
| Stoma takedown | |
| Gain of bowel length | Better intestinal absorption of macro- and micronutrients |
| Better intestinal absorption of fluid and electrolytes | |
| Catheter infection | Stoma may be associated with increased risk for catheter infection |
| Worsening liver function | Latent septic source from |
| – blind loop | |
| – colonic reservoir | |
| Patient wish | Difficult to care for stoma |
| Skin irritation/ulceration | |
| Quality of life improvement | |
| Laparostomy reversal | Catheter infection |
| Difficult to care for laparostomy | |
| Health care costs | |
| Patient wish | |
A clear indication for reconstructive surgery is the presence of a laparostomy with one or multiple enteric fistulas. This “abdominal catastrophe” is often the result of previous failed surgery for simple indications such as hernia repair or Crohn's disease. Previous publications estimated postoperative short bowel in up to 30% as the reason for IF and need for total parenteral nutrition (TPN) [8, 9, 10]. Once an entero-atmospheric fistula has developed spontaneous resolution is extremely rare [11]. Enteric fistulas are difficult to treat and almost impossible to treat in an ambulatory setting [12, 13]. Nevertheless, stabilization of these fistulas in the same manner as mentioned above for difficult-to-manage enterostomies is crucial and needs time. Patients' patience is often stressed, and sometimes psychological help is needed until the reconstructive surgical procedure can be performed.
A rare indication for reconstructive surgery is lowering or preventing catheter-related blood stream infection (CRBSI). The presence of a stoma or a laparostomy necessitating regular changes in wound dressing might harbor an increased risk of CRBSI. When venous access is already limited and pathophysiology suggests infection due to stoma, stoma takedown may be indicated since the presence of stoma is a risk factor for CRBSI [14].
Furthermore, a secondary deterioration of liver function known as IF-associated liver disease (IFALD), measured as elevated liver enzymes, can result from intrabdominal pathology such as blind loops or dilated bowel segments with bacterial overgrowth [15]. These indications are rare, and the success of reconstructive surgery cannot be guaranteed.
Contraindication for Reconstructive Surgery
When the risks of anesthesia and surgery outweigh the anticipated benefits, reconstructive surgery should better be denied. Detailed knowledge about the anatomy, the expected outcome, and the risk of surgery are mandatory to decide whether the operation is reasonable. In view of quality of life, the shorter the small bowel and the older the patient, the stricter the indication for reconstructive surgery should be. However, anastomosing very short small bowel segments to a segment of colon has been shown to improve quality of life by reducing the need for TPN [16]. A special situation is IF due to intestinal ischemia with subtotal loss of the small and large intestine including the right and transverse colon. In this case, reconstructive surgery with jejuno-colostomy results in frequent bowel passages. The compliance and the age of the patient in this case determines the subjective outcome.
High risk of anastomotic failure is a major contraindication for reconstructive surgery, although in most cases only a single anastomosis is necessary. So far, there are no data indicating that the risk of anastomotic dehiscence is significantly higher in IF patients on TPN provided that malnutrition and hypalbuminemia have successfully been (re)compensated. This is in contrast to a recent study in patients with left colectomy for colon cancer, which showed preoperative TPN as a major risk factor for anastomotic dehiscence [17]. Risk factors for anastomotic dehiscence are otherwise the same as in the “normal” surgical population [18]. Renal insufficiency with dialysis and generalized atherosclerosis are major risk factors [19, 20]. A contrast-enhanced CT scan best rules out compromised perfusion [21]. Risk calculators for anastomotic failure have been established mainly for colorectal surgery [22, 23]. An online module for calculating perioperative risks by the American College of Surgeons can be used (https://riskcalculator.facs.org/RiskCalculator/Outcome.jsp).
Reconstructive Surgery for:
Short Bowel
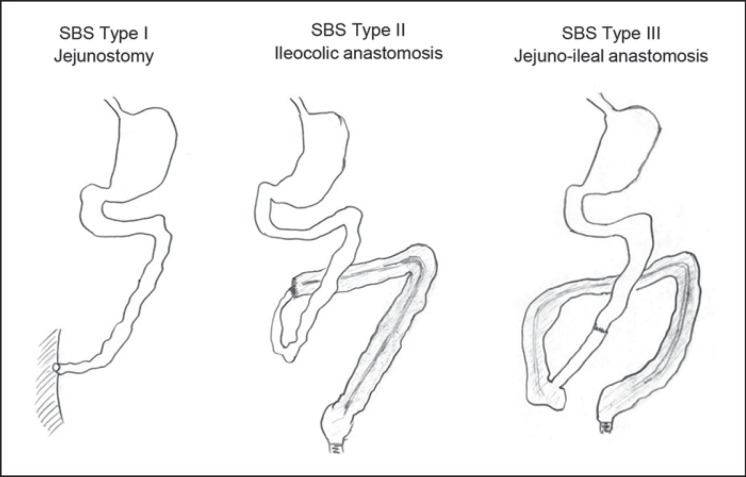
Short bowel syndrome is classified as Type I, II, or III depending on which anatomical situation is present (Fig. 2). Defining the underlying anatomic type of short bowel helps since it predicts outcome [9, 24]. When type I short bowel syndrome can be converted to Type II or III, prognosis both in terms of survival as well as for oral autonomy improves. Pathophysiology of such conversion lies not only in gaining bowel length but enhancing adaptation via distinct pathways such as endocrine signaling (GLP-2, aldosterone), hyperphagia, and probably the microbiome [25, 26, 27]. The clue is to know preoperatively how much bowel can be recuperated in view of length, quality, and anatomical origin. It is standard to measure bowel length at the end of the procedure when operating on patients with Crohn's disease, especially in redo procedures. However, this is not the standard of care yet in “abdominal catastrophes” as mentioned above. When dealing with such patients operating reports are often hard to get. Therefore, the remaining bowel length is often unknown. Imaging studies such as MRI, CT scan, or small bowel follow-through can be helpful in estimating bowel length [21]. The shorter the bowel the easier it is! Clinically judging bowel length by asking the patient when an orally taken meal passes through can also be helpful, especially in patients with enteric fistulas. When the colon length has to be determined a colon contrast enema shows it very precisely. It further has the advantage of ruling out colonic stenosis which could later impair bowel passage. Pathology reports help in evaluating the length of resected bowel but naturally make no statements on the remaining bowel length. Endoscopy plays no role in evaluating the remaining bowel length.
Fig. 2.
Types of short bowel syndrome.
Mesenteric Ischemia – Arterial and Venous – and Dissection
Arterial mesenteric ischemia is still one of the main reasons for massive bowel loss. In larger publications, mesenteric ischemia accounted for 10–40% of short bowel syndrome [8, 9, 10]. The clinical outcome of mesenteric ischemia was poor over many years and has improved only little over decades [28, 29, 30, 31]. However, due to the widespread availability of angio-CT, increased awareness, and the establishment of “intestinal stroke units” [32], the outcome of mesenteric ischemia has improved recently. Reestablishing arterial perfusion via embolectomy of the arterial thrombus or stenting of mesenteric vessels alone or in combination with bowel resection is crucial and strictly time dependent [33]. However, it is still a matter of debate whether bowel continuity should be restored early or late after revascularization [34]. Older guidelines [35] do not specify whether bowel resection should be followed by early or late anastomosis. The more recent European guideline from the Society of Vascular Surgery [36] and the World Society of Emergency Surgery [37] suggests that bowel continuity should be restored early during second- or third-look laparotomy. The reason for either leaving both ends blind or performing a stoma was the increased risk of anastomotic dehiscence when a primary anastomosis was performed directly after bowel revascularization and resection. Although experimental data suggest that acute ischemia impairs healing [38, 39] and can be improved by postponing anastomosis [40], there is no data on how often anastomotic dehiscence occurs after early reconstruction of bowel continuity following mesenteric ischemia in humans [41]. In my personal experience, anastomotic dehiscence can be reduced significantly (from 32% to 8%) if the bowel anastomosis is postponed from the first to the second or third laparotomy. Restoring bowel continuity can be easily postponed up to 10 days after the initial hit if necessary. An unstable clinical status, especially an ongoing septic process, forbids bowel reconstruction at any time point. Establishing an “intestinal stroke center” for patients with arterial mesenteric ischemia significantly lowers mortality and long-term IF. Therefore, improving interdisciplinary treatment for patients with arterial mesenteric ischemia is probably one of the best preventions [24, 42] for later reconstructive procedures.
Crohn's Disease
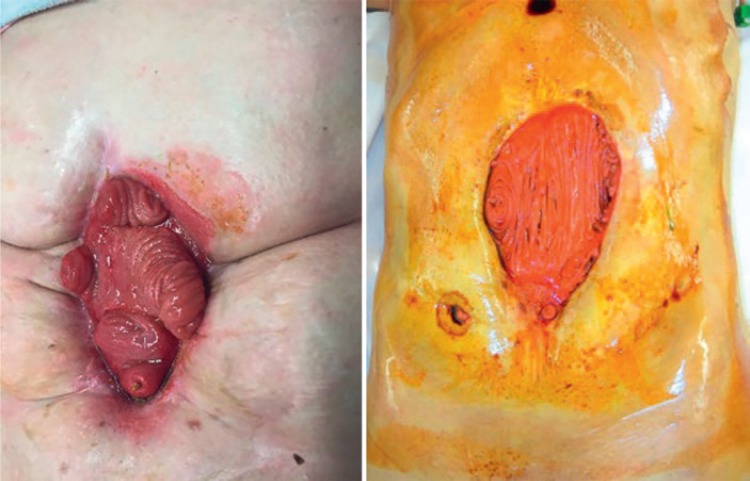
IF in patients with Crohn's disease is mainly due to complicated surgery, and seldom due to repeated noncomplicated surgical procedures [43, 44]. When IF in Crohn's patients is due to short bowel, a reconstructive surgical procedure is not an option and bowel transplantation can be considered. However, if IF in this patient population is due to an abdominal catastrophe and the expected gain of bowel length is sufficient, reconstructive surgery can be considered. In these cases, patients often present with an open abdomen or multiple fistulas (Fig. 3). The following aspects should be addressed preoperatively: (1) what kind of medical treatment for Crohn's disease is the patient taking and does it have to be tapered or intensified preoperatively? and (2) is there bowel with “active” Crohn's disease left which may become reactivated after bowel continuity and stool passage have been restored? Of note, preoperative endoscopy is often not helpful and can be misleading in judging Crohn's activity in excluded bowel segments [45]. In my personal experience, the decision about resecting Crohn's-affected bowel segment at the time of reconstructive surgery depends on the medical therapeutic options left. Otherwise, simultaneous resection is a good choice.
Fig. 3.
Laparostomies with small bowel fistulas.
Certain mutations in the NOD2 (nucleotide-binding oligomerization domain 2) gene are associated with Crohn's disease [46]. Patients with NOD2 deficiency exhibit worse outcomes compared to NOD2 wild type in Crohn's disease and in short bowel syndrome even in the absence of Crohn's [47, 48, 49, 50]. Whether the NOD2 status of a Crohn patient should be determined preoperatively and whether it has an influence on the choice of reconstructive surgery is unclear. Currently, it is not yet routine to determine NOD2 status as a prognostic marker. In a NOD2 knock-out mouse model, mice with a resectional short bowel syndrome have a worse outcome with regard to adaptation compared to wild-type mice [Berlin et al., in press].
Time Point of Reconstruction
There is no randomized study evaluating the best time point of reconstructive surgery after the last surgical intervention in patients with IF or short bowel. Most data are extrapolated from reversal of Hartman procedures done for perforated sigmoid diverticulitis and these data are controversial [51, 52]. Apart from the fact that many patients never undergo reversal and although early takedown of ileostomies as well as Hartmann reversal have been judged safe procedures [53, 54], most surgeons choose 6 months after the last procedure as the best time point. Resolution of the postinflammatory state and of adhesions are arguments in favor of waiting for this comparatively long period of time. In a French multicenter prospective trial in young patients with ileocecal resections due to Crohn's disease who received an end ileostomy, stoma reversal was safely done after 2–5 months [55]. A recent systematic review of timing of surgery in IF patients showed that a longer time interval between the last surgery resulted in fewer complications and in a lower recurrence rate of enteral fistulas [11].
The following aspects argue in favor of an early time point of reconstructive surgery: the index surgery was a single surgical procedure with no programmed lavage and no peritonitis, the patient is well reconstituted with a closed wound, and finally, the patient is well nourished. Patients with comorbidities often need more time to recover after the last surgery. Ideally, they should have recuperated to their preoperative functional status. However, a high enteric fistula can impair full recovery. The term of “prehabilitation” has come up and could be a method to improve outcome in this very special patient group [56].
When no information about the patient's history including intrabdominal anatomy with anastomosis, bowel length, and number of previous laparotomies is available, then it is safe to postpone reconstructive surgery for at least 6 months after the last procedure [21]. In my own experience, reconstructive surgery on 28 patients was safely performed at a median of 10 months after the last procedure. The more complex and complicated previous surgery was, the longer the time interval to reconstructive surgery should be. Waiting up to 12 months is safe. A close interdisciplinary discussion with the gastroenterologist about the ideal time point of surgery for a patient on TPN is mandatory.
Abdominal Approach and Abdominal Wall Closure
Abdominal wall access can be challenging in this patient cohort. Getting into the abdomen can take much time and is a matter of patience. A laparoscopic approach should not be undertaken in this situation and is contraindicated in most cases due to the risk of bowel perforation. Usually, the old scar from median laparotomy is excised and the upper abdomen is used as the first approach. When a skin graft was done on an open abdomen, the “pinch test” helps to evaluate whether the laparostomy is “ready to get into.” When the skin graft can be pinched and lifted from the underlying bowel, it can easily be dissected from the bowel.
When performing reconstructive surgery in IF patients, there is at least one anastomosis performed, resulting in contamination of the abdominal cavity. Nonresorbable mesh grafts for closure should not be used in the same setting. In our opinion bio-meshes play no role. It is preferable to close the abdominal wall by single interrupted or less often by continuous suture even when the tissue sutured is rather a “low quality” scar than fascia, since closure with autologous tissue prevents fistula formation. There are few data as to whether these patients need further hernia surgery [11] and how safely this can be done. Restricting abdominal pressure to lift no more than 5 kg for at least 6 weeks seems reasonable postoperatively.
Conclusion
Reconstructive surgery in adult patients with IF includes stoma takedown, closure of entero-atmospheric fistulas, and reanastomosing bowel segments with the goal to gain bowel length, improve short bowel syndrome, and ameliorate patient status in view of parenteral nutrition. The indication for such operations is rare. Although the surgical technique to perform these operations is not difficult, it is one of the most important steps in the multidisciplinary team work, leading, hopefully, to recovery from IF. Therefore, patients who need these complex reconstructive procedures should be referred to specialized centers.
Disclosure Statement
The author declares no conflict of interest.
Funding Sources
No funding was received for this study.
References
- 1.Pironi L, Arends J, Baxter J, Bozzetti F, Peláez RB, Cuerda C, et al. Home Artificial Nutrition & Chronic Intestinal Failure Acute Intestinal Failure Special Interest Groups of ESPEN ESPEN endorsed recommendations. Definition and classification of intestinal failure in adults. Clin Nutr. 2015 Apr;34((2)):171–80. doi: 10.1016/j.clnu.2014.08.017. [DOI] [PubMed] [Google Scholar]
- 2.Iyer KR. Surgical management of short bowel syndrome. JPEN J Parenter Enteral Nutr. 2014 May;38((1 Suppl)):53S–9S. doi: 10.1177/0148607114529446. [DOI] [PubMed] [Google Scholar]
- 3.Kim HB, Fauza D, Garza J, Oh JT, Nurko S, Jaksic T. Serial transverse enteroplasty (STEP): a novel bowel lengthening procedure. J Pediatr Surg. 2003 Mar;38((3)):425–9. doi: 10.1053/jpsu.2003.50073. [DOI] [PubMed] [Google Scholar]
- 4.Alberti D, Boroni G, Giannotti G, Parolini F, Armellini A, Morabito A, et al. “Spiral intestinal lenghtening and tailoring (SILT)” for a child with severely short bowel. Pediatr Surg Int. 2014 Nov;30((11)):1169–72. doi: 10.1007/s00383-014-3583-x. [DOI] [PubMed] [Google Scholar]
- 5.Bianchi A. Intestinal loop lengthening—a technique for increasing small intestinal length. J Pediatr Surg. 1980 Apr;15((2)):145–51. doi: 10.1016/s0022-3468(80)80005-4. [DOI] [PubMed] [Google Scholar]
- 6.Keller J, Panter H, Layer P. Management of the short bowel syndrome after extensive small bowel resection. Best Pract Res Clin Gastroenterol. 2004 Oct;18((5)):977–92. doi: 10.1016/j.bpg.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Pironi L, Corcos O, Forbes A, Holst M, Joly F, Jonkers C, et al. ESPEN Acute and Chronic Intestinal Failure Special Interest Groups Intestinal failure in adults: recommendations from the ESPEN expert groups. Clin Nutr. 2018 Dec;37((6 6 Pt A)):1798–809. doi: 10.1016/j.clnu.2018.07.036. [DOI] [PubMed] [Google Scholar]
- 8.Bruzoni M, Sudan DL, Cusick RA, Thompson JS. Comparison of short bowel syndrome acquired early in life and during adolescence. Transplantation. 2008 Jul;86((1)):63–6. doi: 10.1097/TP.0b013e3181734995. [DOI] [PubMed] [Google Scholar]
- 9.Messing B, Crenn P, Beau P, Boutron-Ruault MC, Rambaud JC, Matuchansky C. Long-term survival and parenteral nutrition dependence in adult patients with the short bowel syndrome. Gastroenterology. 1999 Nov;117((5)):1043–50. doi: 10.1016/s0016-5085(99)70388-4. [DOI] [PubMed] [Google Scholar]
- 10.Thompson JS, DiBaise JK, Iyer KR, Yeats M, Sudan DL. Postoperative short bowel syndrome. J Am Coll Surg. 2005 Jul;201((1)):85–9. doi: 10.1016/j.jamcollsurg.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 11.de Vries FE, Atema JJ, van Ruler O, Vaizey CJ, Serlie MJ, Boermeester MA. A Systematic Review and Meta-analysis of Timing and Outcome of Intestinal Failure Surgery in Patients with Enteric Fistula. World J Surg. 2018 Mar;42((3)):695–706. doi: 10.1007/s00268-017-4224-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evenson RA, Fischer JE. Behandlung enteraler Fisteln beim offenen Abdomen. Chirurg. 2006 Jul;77((7)):594–601. doi: 10.1007/s00104-006-1207-2. [DOI] [PubMed] [Google Scholar]
- 13.Schecter WP, Hirshberg A, Chang DS, Harris HW, Napolitano LM, Wexner SD, et al. Enteric fistulas: principles of management. J Am Coll Surg. 2009 Oct;209((4)):484–91. doi: 10.1016/j.jamcollsurg.2009.05.025. [DOI] [PubMed] [Google Scholar]
- 14.Santarpia L, Buonomo A, Pagano MC, Alfonsi L, Foggia M, Mottola M, et al. Central venous catheter related bloodstream infections in adult patients on home parenteral nutrition: Prevalence, predictive factors, therapeutic outcome. Clin Nutr. 2016 Dec;35((6)):1394–8. doi: 10.1016/j.clnu.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 15.Rezaie A, Pimentel M, Rao SS. How to Test and Treat Small Intestinal Bacterial Overgrowth: an Evidence-Based Approach. Curr Gastroenterol Rep. 2016 Feb;18((2)):8. doi: 10.1007/s11894-015-0482-9. [DOI] [PubMed] [Google Scholar]
- 16.Smith KH, Saunders JA, Nugent KP, Jackson AA, Stroud MA. Reduced parenteral nutrition requirements following anastomosis of a short residual colonic segment to a short jejunum. JPEN J Parenter Enteral Nutr. 2011 Nov;35((6)):732–5. doi: 10.1177/0148607111406504. [DOI] [PubMed] [Google Scholar]
- 17.Pellino G, Frasson M, García‐Granero A, Granero‐Castro P, Rodríguez JL, Flor‐Lorente B, et al. Predictors of complications and mortality following left colectomy with primary stapled anastomosis for cancer: results of a multicentric study with 1111 patients. Colorectal Disease. 2018;20((11)):986–995. doi: 10.1111/codi.14309. Available from: URL: https://onlinelibrary.wiley.com/doi/full/10.1111/codi.14309. [DOI] [PubMed] [Google Scholar]
- 18.Kingham TP, Pachter HL. Colonic anastomotic leak: risk factors, diagnosis, and treatment. J Am Coll Surg. 2009 Feb;208((2)):269–78. doi: 10.1016/j.jamcollsurg.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 19.Henne-Bruns D, Kramer K. Inzidenz, Risikofaktoren und Prävention der intestinalen Nahtinsuffizienz. Zentralbl Chir. 2013 Jun;138((3)):301–6. doi: 10.1055/s-0031-1271387. [DOI] [PubMed] [Google Scholar]
- 20.Thompson SK, Chang EY, Jobe BA. Clinical review: healing in gastrointestinal anastomoses, part I. Microsurgery. 2006;26((3)):131–6. doi: 10.1002/micr.20197. [DOI] [PubMed] [Google Scholar]
- 21.Vaizey CJ, Maeda Y, Barbosa E, Bozzetti F, Calvo J, Irtun Ø, et al. ESCP Intestinal Failure Group European Society of Coloproctology consensus on the surgical management of intestinal failure in adults. Colorectal Dis. 2016 Jun;18((6)):535–48. doi: 10.1111/codi.13321. [DOI] [PubMed] [Google Scholar]
- 22.Sammour T, Lewis M, Thomas ML, Lawrence MJ, Hunter A, Moore JW. A simple web-based risk calculator is superior to the surgeon's estimate of anastomotic leak after colon cancer resection. Tech Coloproctol. 2017;21((1)):35–41. doi: 10.1007/s10151-016-1567-7. Available from: URL: https://doi.org/ [DOI] [PubMed] [Google Scholar]
- 23.Rojas-Machado SA, Romero-Simó M, Arroyo A, Rojas-Machado A, López J, Calpena R. Prediction of anastomotic leak in colorectal cancer surgery based on a new prognostic index PROCOLE (prognostic colorectal leakage) developed from the meta-analysis of observational studies of risk factors. Int J Colorectal Dis. 2016 Feb;31((2)):197–210. doi: 10.1007/s00384-015-2422-4. [cited 2019 May 30] [DOI] [PubMed] [Google Scholar]
- 24.Carbonnel F, Cosnes J, Chevret S, Beaugerie L, Ngo Y, Malafosse M, et al. The role of anatomic factors in nutritional autonomy after extensive small bowel resection. J Parenteral and enteral. Nutrition. 1996;1996((20)):275–80. doi: 10.1177/0148607196020004275. [DOI] [PubMed] [Google Scholar]
- 25.Jeppesen PB. Modern treatment of short bowel syndrome. Curr Opin Clin Nutr Metab Care. 2013 Sep;16((5)):582–7. doi: 10.1097/MCO.0b013e328363bce4. [cited 2019 May 19] [DOI] [PubMed] [Google Scholar]
- 26.Rubin DC, Levin MS. Mechanisms of intestinal adaptation. Best Pract Res Clin Gastroenterol. 2016 Apr;30((2)):237–48. doi: 10.1016/j.bpg.2016.03.007. [cited 2019 May 19] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Billiauws L, Corcos O, Joly F. What's new in short bowel syndrome? Curr Opin Clin Nutr Metab Care. 2018 Jul;21((4)):313–8. doi: 10.1097/MCO.0000000000000473. [cited 2019 May 19] [DOI] [PubMed] [Google Scholar]
- 28.Eckstein HH. Die akute mesenteriale Ischämie. Resektion oder Rekonstruktion? Chirurg. 2003 May;74((5)):419–31. doi: 10.1007/s00104-003-0630-x. [DOI] [PubMed] [Google Scholar]
- 29.Brandt LJ, Boley SJ, American Gastrointestinal Association AGA technical review on intestinal ischemia. Gastroenterology. 2000 May;118((5)):954–68. doi: 10.1016/s0016-5085(00)70183-1. [DOI] [PubMed] [Google Scholar]
- 30.Kassahun WT, Schulz T, Richter O, Hauss J. Unchanged high mortality rates from acute occlusive intestinal ischemia: six year review. Langenbecks Arch Surg. 2008 Mar;393((2)):163–71. doi: 10.1007/s00423-007-0263-5. [DOI] [PubMed] [Google Scholar]
- 31.Gupta PK, Natarajan B, Gupta H, Fang X, Fitzgibbons RJ., Jr Morbidity and mortality after bowel resection for acute mesenteric ischemia. Surgery. 2011 Oct;150((4)):779–87. doi: 10.1016/j.surg.2011.07.079. [DOI] [PubMed] [Google Scholar]
- 32.Corcos O, Castier Y, Sibert A, Gaujoux S, Ronot M, Joly F, et al. Effects of a multimodal management strategy for acute mesenteric ischemia on survival and intestinal failure. Clin Gastroenterol Hepatol. 2013 Feb;11((2)):158–65.e2. doi: 10.1016/j.cgh.2012.10.027. [DOI] [PubMed] [Google Scholar]
- 33.Luther B, Mamopoulos A, Lehmann C, Klar E. The Ongoing Challenge of Acute Mesenteric Ischemia. Visc Med. 2018 Jul;34((3)):217–23. doi: 10.1159/000490318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nguyen BT, Blatchford GJ, Thompson JS, Bragg LE. Should intestinal continuity be restored after massive intestinal resection? Am J Surg. 1989 Dec;158((6)):577–9. doi: 10.1016/0002-9610(89)90197-9. [DOI] [PubMed] [Google Scholar]
- 35.American Gastroenterological Association Medical Position Statement: guidelines on intestinal ischemia. Gastroenterology. 2000 May;118((5)):951–3. doi: 10.1016/s0016-5085(00)70182-x. [DOI] [PubMed] [Google Scholar]
- 36.Björck M, Koelemay M, Acosta S, Bastos Goncalves F, Kölbel T, Kolkman JJ, et al. Esvs Guidelines Committee Editor's Choice - Management of the Diseases of Mesenteric Arteries and Veins: Clinical Practice Guidelines of the European Society of Vascular Surgery (ESVS) Eur J Vasc Endovasc Surg. 2017 Apr;53((4)):460–510. doi: 10.1016/j.ejvs.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 37.Bala M, Kashuk J, Moore EE, Kluger Y, Biffl W, Gomes CA, et al. Acute mesenteric ischemia: guidelines of the World Society of Emergency Surgery. World J Emerg Surg. 2017 Aug;12((1)):38. doi: 10.1186/s13017-017-0150-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuzu MA, Köksoy C, Kale IT, Tanik A, Terzi C, Elhan AH. Reperfusion injury delays healing of intestinal anastomosis in a rat. Am J Surg. 1998 Oct;176((4)):348–51. doi: 10.1016/s0002-9610(98)00198-6. [DOI] [PubMed] [Google Scholar]
- 39.Posma LA, Bleichrodt RP, Lomme RM, de Man BM, van Goor H, Hendriks T. Early anastomotic repair in the rat intestine is affected by transient preoperative mesenteric ischemia. J Gastrointest Surg. 2009 Jun;13((6)):1099–106. doi: 10.1007/s11605-009-0827-5. [DOI] [PubMed] [Google Scholar]
- 40.Posma LA, Bleichrodt RP, van Goor H, Hendriks T. A prolonged interval between deep intestinal ischemia and anastomotic construction does not impair wound strength in the rat. Int J Colorectal Dis. 2007 Dec;22((12)):1485–91. doi: 10.1007/s00384-007-0333-8. [DOI] [PubMed] [Google Scholar]
- 41.Park WM, Gloviczki P, Cherry KJ, Jr, Hallett JW, Jr, Bower TC, Panneton JM, et al. Contemporary management of acute mesenteric ischemia: factors associated with survival. J Vasc Surg. 2002 Mar;35((3)):445–52. doi: 10.1067/mva.2002.120373. [DOI] [PubMed] [Google Scholar]
- 42.Nuzzo A, Ronot M, Maggiori L, Corcos O. Rather than Surgical Technique, Dedicated Stroke Centers Improve Bowel and Life Outcomes in Acute Mesenteric Ischemia. J Clin Gastroenterol. 2017 doi: 10.1097/MCG.0000000000000970. [DOI] [PubMed] [Google Scholar]
- 43.Post S, Herfarth C, Böhm E, Timmermanns G, Schumacher H, Schürmann G, et al. The impact of disease pattern, surgical management, and individual surgeons on the risk for relaparotomy for recurrent Crohn's disease. Ann Surg. 1996 Mar;223((3)):253–60. doi: 10.1097/00000658-199603000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Agwunobi AO, Carlson GL, Anderson ID, Irving MH, Scott NA. Mechanisms of intestinal failure in Crohn's disease. Dis Colon Rectum. 2001 Dec;44((12)):1834–7. doi: 10.1007/BF02234463. [DOI] [PubMed] [Google Scholar]
- 45.Yantiss RK, Odze RD. Pitfalls in the interpretation of nonneoplastic mucosal biopsies in inflammatory bowel disease. Am J Gastroenterol. 2007 Apr;102((4)):890–904. doi: 10.1111/j.1572-0241.2007.01105.x. [DOI] [PubMed] [Google Scholar]
- 46.Strober W, Asano N, Fuss I, Kitani A, Watanabe T. Cellular and molecular mechanisms underlying NOD2 risk-associated polymorphisms in Crohn's disease. Immunol Rev. 2014 Jul;260((1)):249–60. doi: 10.1111/imr.12193. [cited 2019 Jun 8] [DOI] [PubMed] [Google Scholar]
- 47.Nasir BF, Griffiths L, Nasir A, Roberts R, Barclay M, Gearry R, et al. Perianal disease combined with NOD2 genotype predicts need for IBD-related surgery in Crohn's disease patients from a population-based cohort. J Clin Gastroenterol. 2013 Mar;47((3)):242–5. doi: 10.1097/MCG.0b013e318258314d. [DOI] [PubMed] [Google Scholar]
- 48.Tyler AD, Milgrom R, Stempak JM, Xu W, Brumell JH, Muise AM, et al. The NOD2insC polymorphism is associated with worse outcome following ileal pouch-anal anastomosis for ulcerative colitis. Gut. 2013 Oct;62((10)):1433–9. doi: 10.1136/gutjnl-2011-301957. [DOI] [PubMed] [Google Scholar]
- 49.Sehgal R, Berg A, Polinski JI, Hegarty JP, Lin Z, McKenna KJ, et al. Genetic risk profiling and gene signature modeling to predict risk of complications after IPAA. Dis Colon Rectum. 2012 Mar;55((3)):239–48. doi: 10.1097/DCR.0b013e31823e2d18. [DOI] [PubMed] [Google Scholar]
- 50.Schäffler H, Schneider N, Hsieh CJ, Reiner J, Nadalin S, Witte M, et al. NOD2 mutations are associated with the development of intestinal failure in the absence of Crohn's disease. Clin Nutr. 2013 Dec;32((6)):1029–35. doi: 10.1016/j.clnu.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 51.Horesh N, Lessing Y, Rudnicki Y, Kent I, Kammar H, Ben-Yaacov A, et al. Considerations for Hartmann's reversal and Hartmann's reversal outcomes-a multicenter study. Int J Colorectal Dis. 2017 Nov;32((11)):1577–82. doi: 10.1007/s00384-017-2897-2. [DOI] [PubMed] [Google Scholar]
- 52.Fleming FJ, Gillen P. Reversal of Hartmann's procedure following acute diverticulitis: is timing everything? Int J Colorectal Dis. 2009 Oct;24((10)):1219–25. doi: 10.1007/s00384-009-0747-6. [DOI] [PubMed] [Google Scholar]
- 53.Lasithiotakis K, Aghahoseini A, Alexander D. Is Early Reversal of Defunctioning Ileostomy a Shorter, Easier and Less Expensive Operation? World J Surg. 2016 Jul;40((7)):1737–40. doi: 10.1007/s00268-016-3448-7. [DOI] [PubMed] [Google Scholar]
- 54.Resio BJ, Jean R, Chiu AS, Pei KY. Association of Timing of Colostomy Reversal With Outcomes Following Hartmann Procedure for Diverticulitis. JAMA Surg. 2018 doi: 10.1001/jamasurg.2018.4359. [cited 2019 May 12] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fumery M, Seksik P, Auzolle C, Munoz-Bongrand N, Gornet JM, Boschetti G, et al. REMIND study group investigators Postoperative Complications after Ileocecal Resection in Crohn's Disease: A Prospective Study From the REMIND Group. Am J Gastroenterol. 2017 Feb;112((2)):337–45. doi: 10.1038/ajg.2016.541. [DOI] [PubMed] [Google Scholar]
- 56.Möller T, Becker T, Egberts JH. Präkonditionierung von Lunge und Kreislauf vor viszeral- oder thoraxchirurgischen Eingriffen. Chirurg. 2019;90((7)):529–36. doi: 10.1007/s00104-019-0943-z. [DOI] [PubMed] [Google Scholar]