Abstract
Background
Radioactive iodine (RAI)-refractory differentiated thyroid cancer (DTC) is a rare form of DTC which poses a therapeutic challenge due to the scarcity of effective treatment options. In recent years several tyrosine kinase inhibitors targeting specific molecular pathways involved in its pathogenesis have been investigated, such as sorafenib, lenvatinib, and sunitinib. These appear to be associated with improved progression-free survival (PFS).
Objectives
We aim to describe our experience with sorafenib and sunitinib in the treatment of RAI-refractory metastatic DTC and to evaluate and compare their efficacy and adverse effect profiles.
Method
A total of 28 patients with RAI-refractory metastatic DTC were included - 26 had first-line treatment with sorafenib (8 subsequently switched to sunitinib, most due to disease progression) and 2 with sunitinib. We evaluated PFS and best radiological response achieved with each agent as primary endpoints. The secondary objective was to describe adverse effects and safety profile.
Results
Mean PFS was 10.8 months with sorafenib and 6 months with sunitinib as a second-line treatment. Best overall response was partial remission (PR) with either agent – PR rate of 30.7% with sorafenib and 37.5% with second-line sunitinib. All treatment courses had registered adverse effects and 13.9% justified definitive treatment cessation.
Conclusions
Sorafenib and sunitinib appear to be effective treatment options in delaying disease progression of patients with RAI-refractory metastatic DTC, with an acceptable safety profile. Interestingly, sunitinib appears to show some efficacy even in patients who experience disease progression on sorafenib.
Keywords: Sorafenib, Sunitinib, Thyroid cancer, Tyrosine kinase inhibitors
Introduction
Differentiated thyroid cancer (DTC) is the most common form of thyroid carcinoma [1]. Most cases of DTC can be effectively treated with surgery followed by levothyroxine therapy and radioactive iodine (RAI) in selected patients [2]. Although rare, in some cases the disease is refractory to RAI. Such patients have a poor prognosis as there is a lack of effective treatment options [3, 4, 5].
To date, various alterations in molecular pathways have been identified as playing a part in the pathogenesis of thyroid cancer and these represent potential treatment targets [6]. As such, several multitargeted tyrosine kinase inhibitors (TKIs) have been investigated for the treatment of RAI-refractory thyroid cancer with the objective of inhibiting the MAPK pathway and angiogenesis [3].
Sorafenib, a TKI which inhibits VEGFR 1, 2, and 3, RET (including RET/PTC), RAF (including BRAFV600E), and platelet-derived growth factor receptor beta (PDGFRβ), has already been evaluated in a phase 3 study (the DECISION trial) with reports of increased progression-free survival (PFS) [7]. In November 2013 sorafenib was approved by the Food and Drug Administration (FDA) for the treatment of advanced and progressive RAI-refractory DTC. In 2014 it received approval from the European Medicines Agency (EMA) for the same indication.
Lenvatinib, another multikinase inhibitor, has also been evaluated in a phase 3 study (the SELECT trial) with improvements in PFS and was already approved by the FDA and EMA in 2015.
Sunitinib is another drug targeting VEGFRs and PDGFRs with only three published phase 2 trials [8, 9, 10]. These described median PFS between 8.0 and 13.1 months with sunitinib and response rates (variably defined) between 22 and 31%.
In 2010, Cabanillas et al. [11] published real-world data on their experience with off-label sorafenib and sunitinib use in patients with widely metastatic progressive DTC. Both drugs appeared to be effective with most patients achieving stable disease or partial remission (PR). Since then, and given the availability of these drugs, our centre decided to adopt a similar therapeutic approach. Here we present our centre's results and experience with the use of sorafenib and sunitinib in patients with metastatic DTC from 2009 to 2016.
Materials and Methods
Patients
We reviewed the clinical files of all patients with metastatic DTC which were treated with either sorafenib or sunitinib in our institution from 2009 to 2016. We considered the following inclusion criteria: adult patients (≥18 years of age) with the diagnosis of metastatic DTC (as confirmed by histological analysis of thyroidectomy specimens or by cytological findings in the case of patients who did not undergo surgery) who had disease progression in the past 12 months before starting sorafenib or sunitinib and who had at least 2 months of therapy. Patients were considered as having RAI-refractory disease if they had at least one lesion without RAI uptake on whole-body scintigraphy or had documented progression within a year after RAI treatment or had persistent disease after the administration of a cumulative activity of more than 22 GBq (600 mCi) of RAI [3, 12].
Study Design
Most included patients (26 out of 28 patients) were initially started on sorafenib and were maintained on the drug as long as there was no documented disease progression or important side effects limiting its use. After stopping sorafenib, sunitinib was proposed when considered clinically appropriate – when it was considered the patient would have potential benefits from the drug which surpassed its potential negative impact on his/her quality of life – and was indeed started on a group of 8 patients.
Most patients started sorafenib with 400 mg orally twice a day, and sunitinib was commenced on a regimen of 50 mg orally once daily for 4 weeks followed by 2 weeks off. Every patient was evaluated regularly by an endocrinologist with documentation of adverse effects on each visit – at most every 4 weeks.
Our objective was to determine PFS (considered as the time until radiologically confirmed progression or death from oncologic disease) and best radiological response achieved with sorafenib and sunitinib treatment as first- and second-line TKI therapies. The secondary objective was to describe safety issues associated with these therapeutic agents and their side effect profile.
Radiographic and Biochemical Assessment
Patients had a radiological evaluation every 3 months after starting treatment and disease progression was classified at that time according to RECIST criteria 1.1. [13, 14]. CT and neck ultrasound were the imaging techniques most frequently used throughout the follow-up to define disease status. Measurements of TSH-suppressed thyroglobulin (Tg) levels were carried out also at 3-month intervals.
Statistical Analyses
Quantitative data is described as mean (± standard deviation) and qualitative data as percentage. Kaplan-Meier curves were used to describe PFS. Statistical analysis was done using IBM SPSS Statistics version 25.
Results
Patient Characteristics
From January 2009 to December 2016, 35 patients were treated with sorafenib or sunitinib due to metastatic DTC or poorly differentiated thyroid carcinoma (PDTC) (Table 1). Of these, a total of 8 patients were excluded from the analysis for the following reasons: 6 because they did not complete more than 2 months of therapy (5 died as a consequence of disease progression and 1 had severe mucositis which lead to treatment discontinuation) and 2 because there was a substantial lack of data in the patient's file.
Table 1.
Baseline patient characteristics
| Gender | |
| Male | 13 (46.4) |
| Female | 15 (53.6) |
| Age, years | 57.1±11.5 |
| At diagnosis | |
| At first TKI therapy initiation | 62.5±11.2 |
| Histology | |
| Not submitted to surgery | 3 (10.7) |
| Papillary | 14 (50.0) |
| Follicular | 6 (21.4) |
| Poorly differentiated | 5 (17. 8) |
| Metastasis location | |
| Lungs | 25 (89.3) |
| Lymph node | 19 (67.9) |
| Bone | 14 (50. 0) |
| Liver | 2 (7.1) |
| Pleura | 1 (3.6) |
| Other | 2 (7.1) |
| Serum Tg at baseline, ng/mL | 29,914.1±72,637.5 |
| Prior treatments | |
| Radioactive iodine1 | 25 (89.3) |
| Radiotherapy | 17 (60. 7) |
| Chemotherapy | 0 (0) |
| Tyrosine kinase inhibitor | |
| Sorafenib | 18 (64.3) |
| Sunitinib | 2 (7.1) |
| Sorafenibfollowed bysunitinib | 8 (28.6) |
Values aren (%) or mean ± standard deviation, as appropriate.
Mean cumulative activity = 363.8 mCi.
Twenty-eight patients were included in this series, with a mean age at first-line TKI therapy initiation of 62.5 ± 11.2 years. Three patients did not undergo thyroid surgery before starting TKI therapy as they either had unresectable tumour or the surgical risk was considered too high; however, the cytological result was consistent with DTC in these cases. Of the 25 patients who had thyroidectomy performed, 14 had a diagnosis of PTC (4 classical variant, 6 follicular variant, 1 mixed classical and follicular variant, 1 insular variant, 1 tall-cell variant, and 1 mixed classical and trabecular variant), 5 had a PDTC, and the other 6 patients had follicular thyroid cancer (3 of them Hürthle cell carcinoma). Mean baseline Tg was 29,914.1 ± 72,637.5 ng/mL and only 1 patient had positive anti-Tg antibodies. All except for the 3 patients who did not undergo surgery had prior RAI therapy – all of them considered RAI-refractory and 8 had some RAI avid metastases on the last performed scintigraphy. Seventeen patients received external beam radiation due to local disease persistence (9 patients) and bone (9 patients) or brain metastasis (1 patient); these lesions were not considered for the RECIST assessments.
A total of 36 treatment courses were evaluated - 26 with sorafenib and 10 with sunitinib. Mean treatment duration was 14.8 ± 12.7 months (range 2–52 months) with sorafenib and 10.3 ± 5.8 months (range 2–22 months) with sunitinib.
Response Evaluation: First-Line Treatments
Twenty-eight patients were evaluated - 2 had first-line therapy with sunitinib and 26 with sorafenib.
With sorafenib, the best overall response was PR - 8 patients (30.7%) who experienced 9–36 months until disease progression (mean of 20.0 months). Ten patients (38.4%) achieved stable disease while 8 (30.7%) had progressive disease despite TKI therapy. Fourteen patients (53.8%) had a durable response – considered as patients who had more than 6 months of non-progressive disease. Mean treatment duration was 14.7 ± 13.1 months (range 2–52 months).
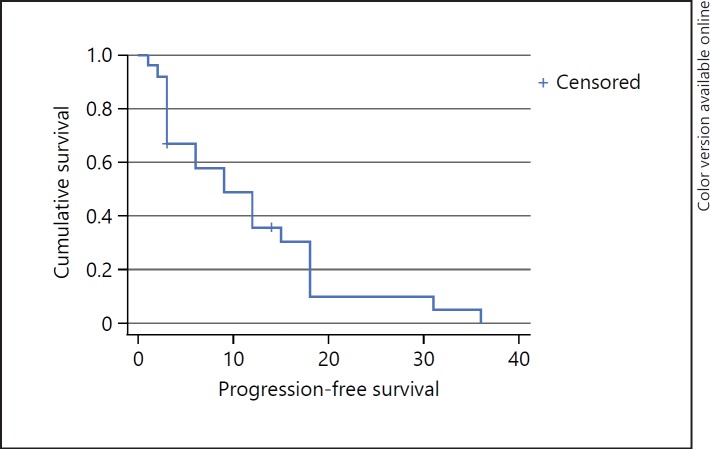
Mean PFS was 10.8 ± 9.2 months. Two patients did not progress on sorafenib but discontinued therapy due to drug adverse effects. Plotted PFS is displayed in a Kaplan-Meier curve in Figure 1.
Fig. 1.
Survival function. Kaplan-Meier analysis of progression-free survival (PFS) of sorafenib-treated patients. Mean PFS was 10.8 ± 9.2 months.
There was a Tg decrease during treatment in 15 patients (57.7%) – most (11 patients) achieved their minimum Tg concentration at 3 months of therapy. By the time of drug discontinuation, 9 patients (34.6%) had serum Tg concentration lower than at baseline.
There were a total of 5 deaths while on sorafenib therapy - 4 related to oncological disease progression and 1 with gastrointestinal bleeding attributed to sorafenib. Mean time between treatment initiation and death was 25.9 ± 17.4 months.
Two patients were considered as taking sunitinib as first-line TKI therapy. One was due to the development of acute pancreatitis very shortly after sorafenib was started while the other started with sunitinib in 2011, when sorafenib was yet to receive official therapeutic approval by the EMA and published results were similar between these two agents, given drug availability at the time. Of these 2 patients, one had PR for 12 months while the other had stable disease for 18 months before disease progression. Both patients died after therapy was discontinued – one 36 months after sunitinib was started and the other after 27 months.
Response Evaluation: Second-Line Treatments
Eight patients (27.6% of those included in our series) initiated sunitinib as a second-line TKI therapy after sorafenib - 7 patients due to disease progression while on sorafenib and 1 patient due to severe side effects.
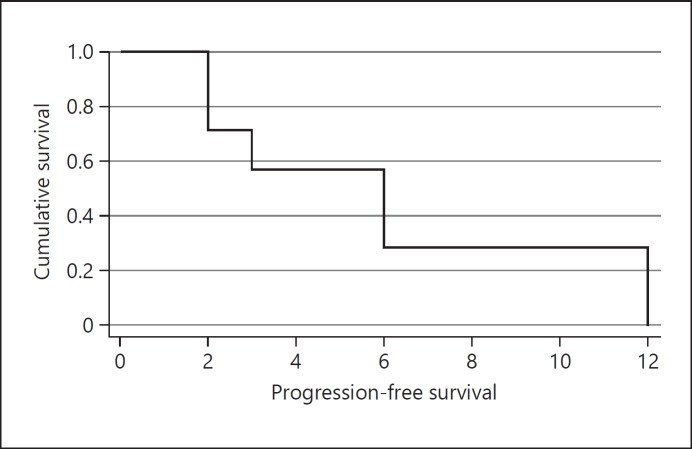
The best observed response was PR, which was observed in 3 patients (37.5% – all decreased lymph node target lesions) - 2 of them had disease progression 12 months before while the other had no progression by the time therapy was discontinued (due to hepatic failure). The other 5 patients experienced disease progression between 2 and 6 months after starting sunitinib. Mean PFS was 6.1 ± 4.3 months. Plotted data is displayed in a Kaplan-Meier curve in Figure 2.
Fig. 2.
Survival function. Kaplan-Meier analysis of PFS progression-free survival (PFS) of second-line sunitinib-treated patients. Median PFS was 6.1 ± 4.3 months.
There was a serum Tg decrease in 4 patients. However, the last measured Tg was increased, as compared with baseline value, in every patient. One patient died due to acute hepatic failure, attributed to sunitinib. One patient was still alive by the time of data retrieval - 2 years after stopping sunitinib. Mean time between treatment initiation and death was 13.6 ± 8.2 months.
Adverse Effects
Adverse effects were reported in all treatment courses [15] (Table 2). Diarrhoea and fatigue were the most common adverse effects, followed by anorexia and hypertension (particularly in the sorafenib-treated patients). Mucocutaneous effects were also very common – reported 39 times (34 with sorafenib and 5 with sunitinib). Thirteen patients (50.0%) treated with sorafenib and 8 (80%) with sunitinib required reduction of drug daily doses due to toxicity. Temporary drug withdrawal was also needed in 6 patients (23.1%) who received sorafenib and 8 (80%) who received sunitinib. There was one death attributed to sorafenib – a patient who developed severe gastrointestinal haemorrhage 2 months after starting the drug. One patient developed acute hepatic failure after 10 months of sunitinib, leading to patient death. Four patients experienced several mucocutaneous adverse effects with sorafenib which justified treatment discontinuation.
Table 2.
Sorafenib and sunitinib adverse effects
| Sorafenib |
Sunitinib |
Total |
||||
|---|---|---|---|---|---|---|
| grades≥3 | all grades | grades≥3 | all grades | n | % | |
| Diarrhoea | 3 | 12 | 1 | 4 | 16 | 44.4 |
| Fatigue | 2 | 10 | 1 | 5 | 15 | 41.7 |
| Anorexia | 3 | 11 | 1 | 4 | 15 | 41.7 |
| Hypertension | 1 | 9 | 1 | 6 | 15 | 41.7 |
| Hand-footsyndrome | 2 | 12 | 1 | 2 | 14 | 38.9 |
| Alopecia | 0 | 10 | 0 | 0 | 10 | 27.8 |
| Vomiting | 2 | 7 | 0 | 3 | 10 | 27.8 |
| Skin rash | 0 | 7 | 1 | 2 | 9 | 25.0 |
| Mucositis | 1 | 5 | 0 | 1 | 6 | 16.7 |
| Haemorrhage | 1 | 0 | 0 | 3 | 3 | 8.3 |
| Thrombocytopenia | 0 | 0 | 0 | 3 | 3 | 8.3 |
| Arthralgia | 1 | 1 | 0 | 1 | 2 | 5.6 |
| Acute hepatic failure | 0 | 0 | 1 | 1 | 1 | 2.8 |
| Hyponatremia | 0 | 0 | 0 | 1 | 1 | 2.8 |
Grading according to Common Terminology Criteria for Adverse Events (CTCAE) v5.0[15].
Discussion/Conclusion
Successful treatment of patients with thyroid cancer of follicular origin can usually be achieved by means of surgery followed by levothyroxine therapy and RAI. However, in cases refractory to RAI alternatives treatment modalities are needed. To date, several TKIs have demonstrated effectiveness in clinical trials and in a few real-world retrospective studies, leading to their increasing use in clinical practice in recent years.
Regarding sorafenib, the DECISION trial was a landmark phase 3 study where patients with RAI-refractory locally advanced or metastatic DTC treated with sorafenib showed (as compared with placebo) improved PFS (10.8 months) and response rates (12.2% of patients) [7]. Since then, various studies outside clinical trials have reported mean PFS of 7.2–19 months [11, 16, 17]. In our series, patients treated with sorafenib as the first-line TKI had a mean PFS of 10.8 months, which is very similar to results published in the DECISION trial and in the retrospective study of the TUTHYREF network [7, 16]. The PR rate was 30.7% in our series, somewhat higher than that was described in the previously mentioned studies (12.2–15%) and this could perhaps be explained by different sample sizes and compositions and by the fact that we did not include in our analyses patients who died before completing 2 months of therapy. The mean duration of response was 20 months – a number much higher than the 10.2 months of the DECISION trial although the number of patients who had a PR in our series was very small and the range was very extensive (9–36 months). Durable responses were seen in 53.8% of patients, similar to that reported by Cabanillas et al. [11] (66%), although that study included 2 patients treated with sunitinib.
Sunitinib use in DTC is still off-label and largely based on results from phase 2 trials [8, 9, 10]. In our series we present patients treated with sunitinib either after withdrawal of sorafenib (due to disease progression or important adverse effects limiting its use) in 8 patients or upfront in 2. Considering only patients who had sunitinib as a second-line TKI, the PR rate was 37.5% and mean PFS was 6 months while other studies describe PR rates of 8–28% and mean PFS of 6.5–8 months. It is difficult to make a direct comparison of our findings with other studies due to various reasons such as small sample sizes and different sample compositions since several series included, for instance, patients with anaplastic and medullary thyroid cancer and addressed patients with first- and second-line treatment as a whole [8, 9, 10, 16]. Still, our study seems to add to the interesting hypothesis that patients who have disease progression on sorafenib can still have a worthwhile clinical response with sunitinib and, as such, should still be considered for this drug. Randomized clinical trials directly addressing this issue and comparing different TKIs are greatly needed.
Adverse effects are common with the use of TKIs and can sometimes force dose reductions and drug withdrawal [18]. In our series, 13.9% of treatment courses had to be definitively suspended due to adverse effects and 58.3% needed dose reductions. All of our patients reported at least one drug adverse effect in every treatment course provided with either sorafenib or sunitinib. This is in line with results of large trials such as DECISION and THYSU as well as other retrospective studies [7, 10, 17]. The most commonly reported adverse effects were mucocutaneous changes with either drug, which is in line with what others have reported with sorafenib but not sunitinib [7, 10]. One patient died as a direct consequence of acute hepatic failure while on sunitinib, with no other evident cause besides drug toxicity. This has previously been described in a few other cases although mildly elevated liver enzymes are the most common form of reported hepatoxicity [19, 20]. There was one death of a patient on sorafenib which was attributed to gastrointestinal haemorrhage as a drug adverse effect – this is somewhat surprising as haemorrhagic events described in this setting are usually low-grade events [21].
Our study does have certain limitations such as its retrospective and observational nature, the small sample size, and the very small number of patients who were treated with sunitinib as the first-line TKI. Also of note, our inclusion criterion of a minimum of 2 months of therapy differentiates our study from others. The fact that we decided to include 3 patients who did not undergo surgery and were not submitted to RAI treatment could have influenced treatment responses – none of them had a PR to sorafenib.
In summary, in our population of DTC patients with progressive advanced disease we observed somewhat positive results with sorafenib and sunitinib regarding treatment response rate (with a reasonable duration of response) and PFS – in line with previous clinical trials and retrospective studies. Patients who experienced disease progression on sorafenib also appeared to have some benefit of subsequent treatment with sunitinib. Adverse effects were very frequent although they only limited drug use in a minority of patients. In conclusion, we consider sorafenib and sunitinib as valid treatment options for delaying the progression of disease in patients with advanced progressive DTC.
Statement of Ethics
The authors have no ethical conflicts to disclose. This study was conducted in accordance with the principles of the Declaration of Helsinki. All patients gave their informed consent and the protocol was approved by the institute's committee on clinical research. No animal experiments were performed.
Disclosure Statement
The authors declare they have no conflict of interest.
Funding Sources
The authors declare there were no funding sources for the execution of the research and paper.
Author Contributions
Francisco Sousa Santos: data collection and analyses, data interpretation, and writing of the final paper. Rita Joana Santos: data collection, data interpretation, writing of manuscript draft, and revision of the final paper. Valeriano Leite: study conception/design and manuscript revision.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018 Jan;68((1)):7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016 Jan;26((1)):1–133. doi: 10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schlumberger M, Sherman SI. Approach to the patient with advanced differentiated thyroid cancer. Eur J Endocrinol. 2012 Jan;166((1)):5–11. doi: 10.1530/EJE-11-0631. [DOI] [PubMed] [Google Scholar]
- 4.Haugen BR, Sherman SI. Evolving approaches to patients with advanced differentiated thyroid cancer. Endocr Rev. 2013 Jun;34((3)):439–55. doi: 10.1210/er.2012-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carneiro RM, Carneiro BA, Agulnik M, Kopp PA, Giles FJ. Targeted therapies in advanced differentiated thyroid cancer. Cancer Treat Rev. 2015 Sep;41((8)):690–8. doi: 10.1016/j.ctrv.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 6.Riesco-Eizaguirre G, Santisteban P. ENDOCRINE TUMOURS: Advances in the molecular pathogenesis of thyroid cancer: lessons from the cancer genome. Eur J Endocrinol. 2016 Nov;175((5)):R203–17. doi: 10.1530/EJE-16-0202. [DOI] [PubMed] [Google Scholar]
- 7.Brose MS, Nutting CM, Jarzab B, Elisei R, Siena S, Bastholt L, et al. DECISION investigators Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet. 2014 Jul;384((9940)):319–28. doi: 10.1016/S0140-6736(14)60421-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bikas A, Kundra P, Desale S, Mete M, O'Keefe K, Clark BG, et al. Phase 2 clinical trial of sunitinib as adjunctive treatment in patients with advanced differentiated thyroid cancer. Eur J Endocrinol. 2016 Mar;174((3)):373–80. doi: 10.1530/EJE-15-0930. [DOI] [PubMed] [Google Scholar]
- 9.Carr LL, Mankoff DA, Goulart BH, Eaton KD, Capell PT, Kell EM, et al. Phase II study of daily sunitinib in FDG-PET-positive, iodine-refractory differentiated thyroid cancer and metastatic medullary carcinoma of the thyroid with functional imaging correlation. Clin Cancer Res. 2010 Nov;16((21)):5260–8. doi: 10.1158/1078-0432.CCR-10-0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ravaud A, de la Fouchardière C, Caron P, Doussau A, Do Cao C, Asselineau J, et al. A multicenter phase II study of sunitinib in patients with locally advanced or metastatic differentiated, anaplastic or medullary thyroid carcinomas: mature data from the THYSU study. Eur J Cancer. 2017 May;76:110–7. doi: 10.1016/j.ejca.2017.01.029. [DOI] [PubMed] [Google Scholar]
- 11.Cabanillas ME, Waguespack SG, Bronstein Y, Williams MD, Feng L, Hernandez M, et al. Treatment with tyrosine kinase inhibitors for patients with differentiated thyroid cancer: the M. D. Anderson experience. J Clin Endocrinol Metab. 2010 Jun;95((6)):2588–95. doi: 10.1210/jc.2009-1923. [DOI] [PubMed] [Google Scholar]
- 12.Schmidt A, Iglesias L, Klain M, Pitoia F, Schlumberger MJ. Radioactive iodine-refractory differentiated thyroid cancer: an uncommon but challenging situation. Arch Endocrinol Metab. 2017 Jan-Feb;61((1)):81–9. doi: 10.1590/2359-3997000000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009 Jan;45((2)):228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz LH, Litière S, de Vries E, Ford R, Gwyther S, Mandrekar S, et al. RECIST 1.1-Update and clarification: from the RECIST committee. Eur J Cancer. 2016 Jul;62:132–7. doi: 10.1016/j.ejca.2016.03.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Cancer Insituite Common Terminology Criteria for Adverse Events version 5.0 2017
- 16.Massicotte MH, Brassard M, Claude-Desroches M, Borget I, Bonichon F, Giraudet AL, et al. Tyrosine kinase inhibitor treatments in patients with metastatic thyroid carcinomas: a retrospective study of the TUTHYREF network. Eur J Endocrinol. 2014 Mar;170((4)):575–82. doi: 10.1530/EJE-13-0825. [DOI] [PubMed] [Google Scholar]
- 17.Molina-Vega M, García-Alemán J, Sebastián-Ochoa A, Mancha-Doblas I, Trigo-Pérez JM, Tinahones-Madueño F. Tyrosine kinase inhibitors in iodine-refractory differentiated thyroid cancer: experience in clinical practice. Endocrine. 2018 Feb;59((2)):395–401. doi: 10.1007/s12020-017-1499-7. [DOI] [PubMed] [Google Scholar]
- 18.Resteghini C, Cavalieri S, Galbiati D, Granata R, Alfieri S, Bergamini C, et al. Management of tyrosine kinase inhibitors (TKI) side effects in differentiated and medullary thyroid cancer patients. Best Pract Res Clin Endocrinol Metab. 2017 Jun;31((3)):349–61. doi: 10.1016/j.beem.2017.04.012. [DOI] [PubMed] [Google Scholar]
- 19.Guillen SS, Meijer M, de Jongh FE. Lethal acute liver failure in a patient treated with sunitinib. BMJ Case Rep. 2016 Mar;2016:2015–7. doi: 10.1136/bcr-2015-213624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mueller EW, Rockey ML, Rashkin MC, Pharm D, Rashkin MC. Sunitinib-related fulminant hepatic failure: case report and review of the literature. Pharmacotherapy. 2008 Aug;28((8)):1066–70. doi: 10.1592/phco.28.8.1066. [DOI] [PubMed] [Google Scholar]
- 21.Dai C, Zhou F, ShaoJiang-Hua, Wu L-Q, Yu X, Yin X-B. Bleeding risk in cancer patients treated with sorafenib: A meta-analysis of randomized controlled trials. J Cancer Res Ther. 2018;14((12)):948–56. doi: 10.4103/0973-1482.188430. [DOI] [PubMed] [Google Scholar]