Abstract
Purpose
To report a case of orbital cellular epithelioid hemangioma (EH) in which FOSB and CAMTA1 immunostains were used to detect a cytogenetic rearrangement as an adjunctive tool in diagnosis.
Methods
Case report.
Results
A patient with a history of prior ligation of a presumed orbital varix presented with recurrent proptosis. Imaging revealed a highly vascular right orbital mass. Microscopic examination revealed a circumscribed neoplasm composed of plump epithelioid endothelial cells with copious mildly eosinophilic cytoplasm and relatively uniform vesicular nuclei. To aid in diagnosis, immunostains for FOSB and CAMTA1 were performed to detect corresponding cytogenetic rearrangements. The presence of multifocal nuclear positivity for FOSB, indicating FOSB genetic rearrangement, and negativity for CAMTA1 were considered reassuring features against a diagnosis of a malignant epithelioid hemangioendothelioma (EHE), supporting a diagnosis of benign cellular EH.
Conclusions
This case report demonstrates that the use of immunohistochemical stains to detect cytogenetic rearrangements may aid in the distinction between benign EH and malignant EHE. It also reminds providers of the clinical and histopathologic features of this lesion, which occurs rarely in the orbit, and helps clarify the evolving nomenclature surrounding epithelioid hemangioma.
Keywords: Epithelioid hemangioma, Cytogenetics, Angiolymphoid hyperplasia with eosinophilia
Established Facts
Epithelioid hemangioma (EH) occurs only rarely in the orbit, where it can have a diverse presentation with nonspecific radiographic and clinical features, making histopathology essential to diagnosis.
It is important to be able to distinguish benign epithelioid hemangioma from malignant vascular tumors such as epithelioid hemangioendothelioma (EHE) and high grade epithelioid angiosarcoma, but similar pathologic features can make definitive diagnosis challenging.
Novel Insights
To our knowledge, this is the first published case of orbital epithelioid cellular hemangioma in which FOSB and CAMTA1 immunostains were used to detect cytogenetic rearrangements that supplemented histopathology in diagnosis.
The presence of multifocal nuclear positivity for FOSB, indicating FOSB genetic rearrangement, and negativity for CAMTA1 were reassuring features against a diagnosis of a malignant epithelioid hemangioendothelioma (EHE), supporting a diagnosis of benign cellular epithelioid hemangioma (EH).
This paper also provides an overview of the complicated nomenclature surrounding epithelioid hemangioma, highlighting the distinction between this entity and Kimura's disease and the more recent delineation of 3 distinct subtypes of epithelioid hemangioma: typical, cellular, and angiolymphoid hyperplasiawith eosinophilia (ALHE).
Introduction
Epithelioid hemangioma (EH) is a rare, benign, vascular neoplasm that typically presents as subcutaneous nodules in the head and neck region but may also be found in other locations including bone, heart, spleen, colon, penis, and rarely the orbit, among others [1]. This lesion has previously been given a number of different designations, most commonly angiolymphoid hyperplasia with eosinophilia (ALHE) or histiocytoid hemangioma. On histopathology, EH features characteristic vascular channels lined by prominent endothelial cells with an epithelioid appearance. More recently, it was appreciated that the morphology of EH spans a diverse spectrum, with various appearances including intravascular growth, heavy inflammatory infiltrate, and a cellular/solid proliferation [2]. The diverse histopathologic appearance of this lesion makes diagnosis challenging, as it may be confused with a variety of other entities including Kimura disease (KD), malignant epithelioid hemangioendothelioma (EHE), or high grade epithelioid angiosarcoma. Furthermore, EH occurs only rarely in the orbit, where it can have a diverse presentation with nonspecific radiographic and clinical features, making histopathology essential to diagnosis and underscoring the importance of distinguishing this benign entity from malignant vascular tumors.
Here, we present a case of orbital cellular EH in which immunostains to detect cytogenetic rearrangements were used as an adjunct to histopathology in diagnosis. We review the clinical and histopathologic features of EH, as well as the evolving nomenclature surrounding this entity, including the recent distinction between typical, cellular, and ALHE variants of EH.
Case Report
A 58-year-old female presented with recurrent proptosis of the right orbit. Four years earlier, she had been worked up for a similar complaint at an outside institution. The lesion was thought to be consistent with orbital varix, with partial thrombosis causing dilation of the right superior ophthalmic vein and secondary prolapse of orbital fat. The patient underwent a right sided anterior orbitotomy to debulk the prolapsed superior orbital tissue, which was confirmed on pathology to be adipose tissue. Intraoperatively, a large, dilated vein was noted in the superonasal orbit. The vessel was ligated with two 4-0 silk sutures in an effort to decrease venous flow and prevent further swelling.
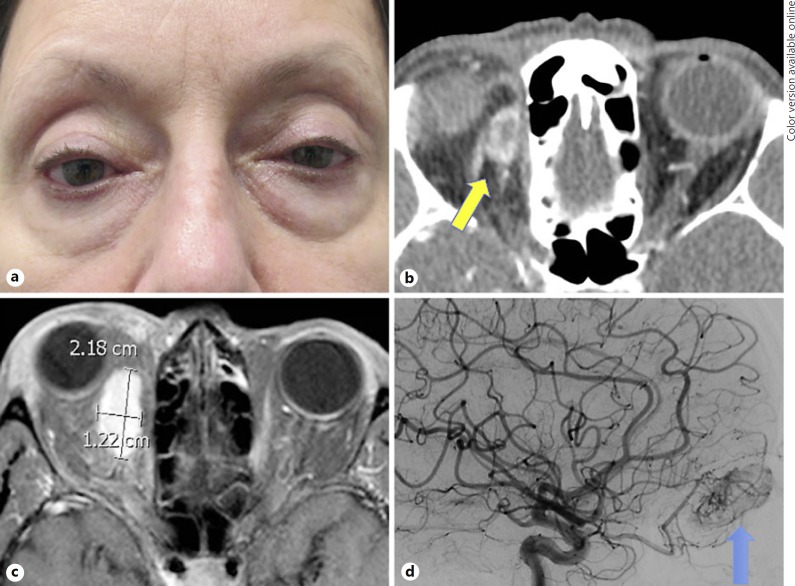
Following the procedure, the patient's orbital proptosis had improved for a period of several months before recurring, with gradually increasing orbital swelling and proptosis of the right eye over the subsequent 2-year period. At the time of subsequent presentation 29 months after the original orbitotomy, she was noted to have 5 mm of right-sided proptosis, with Hertel measurements of 22 and 17 and 3 mm of right hypoglobus (Fig. 1a). Visual acuity was 20/20 in both eyes. There was no afferent pupillary defect. She had 1 mm of right-sided mechanical ptosis, and moderate resistance to retropulsion of the right globe was noted. Confrontational visual fields and extraocular movements were full, and she was orthophoric in primary gaze. Slit lamp exam was significant only for moderate chemosis of the temporal conjunctiva in the right eye and nuclear sclerosis in both eyes. The patient acknowledged diplopia with upgaze. She denied any increase in swelling or discomfort with her head in a dependent position. Orbital CT revealed an ovoid, peripherally enhancing mass at the superomedial aspect of the right orbit (Fig. 1b). Subsequent orbital MRI/MRA confirmed a homogeneous, intensely enhancing mass within the right superomedial orbit, which measured 2.2 × 1.4 × 1.3 cm (Fig. 1c). The lesion did not change in size with Valsalva maneuver. Angiography of the lesion demonstrated a hypervascular mass arising from the branches of the ophthalmic artery (Fig. 1d), which could not be safely embolized via a transarterial approach without risk to the vision in the right eye. In order to decrease blood flow prior to excision of the lesion, interventional radiology performed direct puncture of the lesion under CT guidance, embolizing the anterior and central aspects of the tumor. The patient underwent excision of the lesion the following day. Intraoperatively, a soft tissue, intraconal mass was noted in the superonasal region of the orbit. The lesion was removed in its entirety.
Fig. 1.
a Clinical photograph demonstrating right-sided proptosis and hypoglobus. b Orbital CT with contrast revealing an ovoid, peripherally enhancing mass at the superomedial aspect of the right orbit (yellow arrow). c Orbital MRI with contrast demonstrating a homogeneous, intensely enhancing mass within the right superomedial orbit measuring 2.2 × 1.4 × 1.3 cm. d Arterial angiography demonstrating a hypervascular mass arising from the branches of the ophthalmic artery (blue arrow).
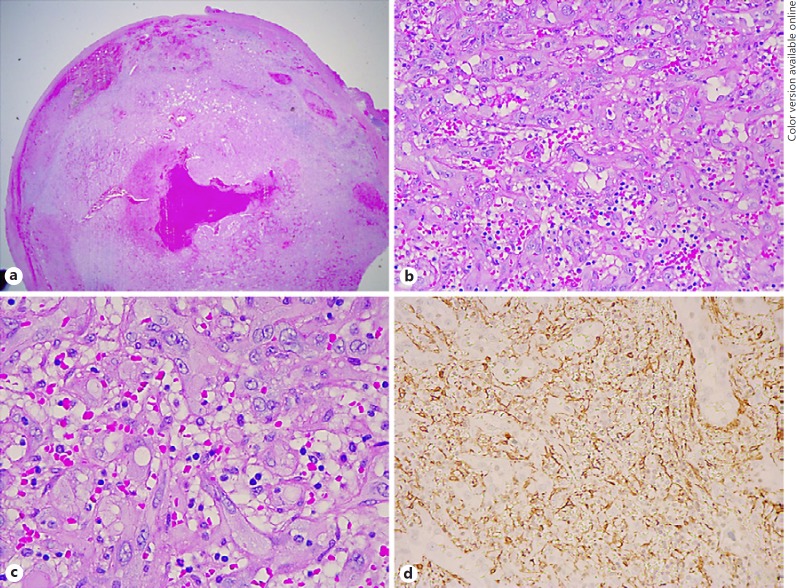
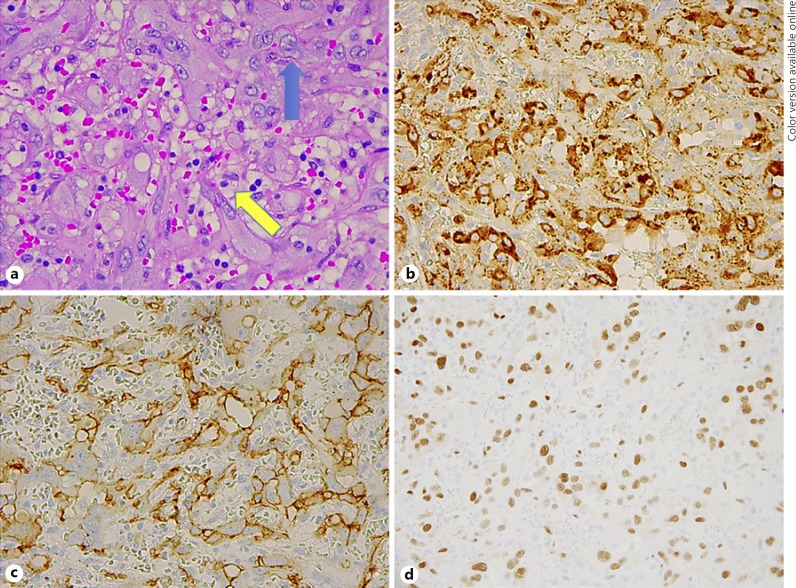
On gross description, the specimen was noted to be rubbery, red and purple in color, and measured 24 × 10 × 10 mm. Microscopic evaluation revealed a circumscribed but unencapsulated neoplasm composed of plump epithelioid endothelial cells with copious mildly eosinophilic cytoplasm, relatively uniform vesicular nuclei, and no evidence of cytologic atypia (Fig. 2a, b). In many areas, there was a well-developed vasoformative architecture. In the seemingly more solid areas, staining for SMA helped to highlight the presence of well-formed, closely packed vessels (Fig. 2c). The cells lining the vascular channels stained positively with Factor VIII and CD34, and negatively with S100 (Fig. 3a–c). There was multifocal nuclear positivity for FOSB (Fig. 3d), confirming the presence of an FOSB cytogenetic rearrangement, and negativity for CAMTA1. These cytogenetic results were considered to be reassuring features against malignant EHE, and a diagnosis of benign cellular EH was made.
Fig. 2.
a Low magnification image of the resected specimen, noted to be a circumscribed but unencapsulated neoplasm. b, c Higher magnification images reveal a neoplasm composed of plump epithelioid endothelial cells with copious palely eosinophilic cytoplasm and relatively uniform vesicular nuclei. In many areas, one can appreciate a well-developed vasoformative architecture. d In the seemingly more solid areas, staining for SMA helps to highlight the presence of well-formed, closely packed vessels. Hematoxylin and eosin: ×1.25 (a), ×20 (b), ×40 (c). d Smooth muscle actin: ×20.
Fig. 3.
a In addition to the plump epithelioid endothelial cells (blue arrow), pericytes with round, dark nuclei and clear cytoplasm can be seen lining the vascular channels in close proximity to the endothelial cells (yellow arrow). b, c The cells lining the vascular channels stained positively with Factor VIII and CD34. d Multifocal nuclear positivity for FOSB was present, indicating an FOSB gene rearrangement, while an immunostain for CAMTA1 was negative (not pictured). a Hematoxylin and eosin: ×40. b Factor VIII: X40. c CD34: ×40. d FOSB: ×40.
Discussion
The entity known as ALHE was first described by Wells and Whimster [3]. Rosai et al. [4] later proposed the term “histiocytoid hemangioma,” given the proliferation of distinct cells identified as “histiocytoid endothelial cells” in these lesions. The alternative term “EH” was later coined by Weiss and Enzinger [5]. This entity has also been referred to as an “intravenous atypical vascular proliferation” and an “inflammatory angiomatous nodule” [6, 7]. It was initially believed that subcutaneous ALHE was identical or closely related to an entity called KD, with ALHE thought to represent an earlier stage in the disease process and KD a later stage. Googe et al. [8] described ALHE and KD as two distinctive entities, with ALHE thought to be a primary neoplastic disorder of the vascular endothelium with a secondary inflammatory response and KD an allergic or autoimmune process. In subsequent years, KD has been recognized as differing from ALHE in other ways, including having a male predominance, significant lymphadenopathy, a higher incidence of peripheral blood eosinophilia, and the lack of a distinctive endothelial cell as a marker [9]. More recently, the entity of ALHE/EH itself has been further divided into typical, cellular and ALHE variants identified by Huang et al. [2]. Table 1 provides an overview of selected vascular lesions included in the differential diagnosis of EH of the orbit, highlighting clinical, histologic, immunohistochemical, and cytogenetic features that may help clinicians distinguish between these lesions. Based on histopathologic and cytogenetic analysis, our patient's lesion was deemed most consistent with the cellular variant of EH.
Table 1.
Clinical and histologic features of vascular lesions included in the differential diagnosis of epithelioid hemangioma
| Vascular lesion | Key clinical features | Key histologic features | Cytogenetics | Immunohistochemistry |
|---|---|---|---|---|
| Capillary hemangioma | Usually observed within the first few weeks of life; Rarely seen in adult patients; typically solitary red or purplish raised nodule on eyelid, of variable size; course is rapid growth followed by slow regression | Proliferation of capillary vessels and perivascular pericytes; often has considerable mitotic activity in proliferative phase; progressively replaced by connective tissue | Nonspecific or none reported | Positive for factor VIII, CD31; Juvenile hemangioma stains positive for GLUT-1 |
| Cavernous hemangioma | Well-circumscribed, slow-growing; presents as proptosis in adults; usually located in the retrobulbar region | Large, blood-filled, endothelial-lined vascular spaces. The walls may have adventitial fibrosis | Nonspecific or none reported | Positive for factor VIII, CD31 |
| EH: includes typical, cellular and ALHE variants | Rare vascular tumor; nodules, or erythematous subcutaneous papules, usually in the head/neck region of young females but can also involve organs and other soft tissue locations | Well circumscribed lesion composed of vessels lined by plump endothelial cells that protrude into the lumen in a“tombstone fashion”; vascular component features capillaries, arterioles, and venules; inflammation is usually lymphoplasmacytic with numerous eosinophils; Frequently demonstrates atypia and intracytoplasmic vacuoles | See below | Positive for factor VIII, CD31 |
| Typical EH | Usually located in bone and soft tissue sites | Epithelioid cells have eosinophilic cytoplasm, scattered vacuoles, vesicular nuclei, and prominent nucleoli; often a background inflammatory infiltrate which includes eosinophils | FOS gene rearrangements are common | |
| Cellular EH | Usually located in bone and soft tissue sites | Increased cellularity, solid growth pattern, moderate mitotic activity, and a mild inflammatory background | FOS gene rearrangements are common | |
| ALHE variant of EH | Usually located in the head/neck and extremities; often seen in the setting of prior trauma, with this history and the histopathology suggesting a reactive process, unlike the neoplastic appearance of other EH variants | Vascular“blow out” pattern associated with damaged medium-sized vessels and extensive inflammation, often with prominent lymphoid follicles | FOS gene rearrangements are uncommon | |
| KD | Subcutaneous nodules in the head and neck, most often in young, Asian males; probable allergic or autoimmune response; frequently associated with lymphadenopathy and peripheral blood eosinophilia | Proliferation of capillaries without endothelial cell atypia; occurs with lymphoid follicles, a marked eosinophilic infiltrate, and dense fibrosis | Nonspecific or none reported | Positive for factor VIII, CD31 |
| EHE | Rare, malignant vascular tumor, often with a relatively indolent course; most commonly occurs in the liver, lung, or veins of the extremities, but may be found at other sites especially in bones and skin; occurs over a broad age-range, with a slight female predilection | Strands or cords of epithelioid cells with abundant glassy eosinophilic cytoplasm and prominent cytoplasmic vacuolation, embedded in a chrondromyxoid or hyalinized stroma; Most EHE lack well-formed vascular channels, and display more significant cytologic atypia than EH, but less than EA | WWTR1-CAMTA1 fusion, or less commonly YAP1-TFE3 fusion | Positive for factor VIII, CD31; variable staining for SMA, CK |
| AVM | Non-neoplastic vascular lesion characterized by AV shunts; angiography demonstrates high flow angioma with feeder vessels; may see pulsating exophthalmos with a bruit, hemorrhage, or thrombosis | Large numbers of veins and arteries of different size; areas resembling a cavernous or capillary hemangioma are frequent, as are thromboses and calcification; can be combined with lymphatic vessels | Nonspecific or none reported | Positive for factor VIII, CD31; elastin stain may help distinguish between arteries and veins |
| KS | Low-grade vascular malignancy associated with HHV8 infection; clinical forms include: classical (elderly), endemic (African), iatrogenic (immunosuppresion), and AIDs-related; can present as a patch, plaque or nodule, which is classically solid, red-violet, slightly elevated, and involves eyelid skin or margin and extends to conjunctiva; Often multifocal | Proliferation of spindle-shaped cells, slit-like vascular channels, fibroblasts, inflammatory cells, and extravasated RBCs; minimal to no pleomorphism; HHV8 is a specific marker for KS | DNA fragments of HHV8 have been found in 90% of confirmed KS cases | Positive for factor VIII, CD31, and HHV8 |
| EA | Rare, highly malignant neoplasm of endothelial cell origin; most common in elderly white males; my begin as ill-defined red plaque that resembles a bruise/sty; borders may extend beyond margin of visible lesion; can metastasize, usually with hematogenous spread | Sheets of large, mild to moderately pleomorphic epithelioid cells, with abundant eosinophilic cytoplasm, vesicular nuclei, and prominent nucleoli; often with infiltrative growth and irregular anastomosing vascular channels; prominent cytologic atypia and frequent mitoses are common | varied cytogenetic abnormalities | positive for factor VIII, CD31, Vimentin, Fli-1; variable staining for CK, CD34, and EMA (in cutaneous lesions) |
EH, epithelioid hemangioma; KD, Kimura's Disease; EHE, epithelioid hemangioendothelioma; AVM, arteriovenous malformation; KS, Kaposi Sarcoma; EA, epithelioid angiosarcoma;
Clinically, EH is a rare condition that is most often characterized by single or multiple papules or nodules involving the dermis and subcutaneous tissue. It is more commonly seen in females than males. It shows a predilection for the head and neck region but more recently has been recognized to have a ubiquitous distribution with cases involving numerous organs and soft tissue locations. The true incidence of orbital EH is difficult to gauge because of the overlapping features with KD, with the earlier literature describing the two as analogous entities. A recent review of the literature reveals at least 44 cases of EH/ALHE involving the orbit/adnexal structures, with such involvement still thought to be relatively rare [9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19]. Within the ocular region, ALHE may involve a variety of structures, including the lacrimal glands, intra and extraconal spaces, conjunctiva, and eyelids [10, 11, 12, 13, 14, 15, 16, 17, 18]. While most often unilateral, cases of bilateral orbital involvement have been reported [19]. The clinical presentation is highly variable, with presenting features including eyelid swelling, proptosis, and chronic dacryoadenitis, and imaging results including lacrimal gland enlargement, orbital masses, or vascular malformations similar to arteriovenous malformations.
Histopathologically, EH is characterized by atypical vascular proliferation with a variable chronic inflammatory infiltrate, which may include lymphocytes, eosinophils, and lymphoid follicles [11]. The characteristic pathologic finding is that of plump, vacuolated endothelial cells with an epithelioid appearance lining vascular channels. On immunohistochemistry, the endothelial cells show reactivity for endothelial markers CD34, CD31, and Factor VIII [20].
Despite its benign classification, EH can occasionally show increased cellularity, cytologic atypia, and regional aggressive growth. The presence of cytologic features, which may overlap with malignant lesions, and the potentially aggressive clinical characteristics of EH make it a diagnostic challenge [21]. EH may be confused at one end of the spectrum (ALHE) with inflammatory conditions such as KD, and at the other end of the spectrum (cellular EH) with malignant EHE and high-grade epithelioid angiosarcoma [2]. In recent years, genetic abnormalities have been described that may aid in distinguishing between several of these entities, which may otherwise be difficult to distinguish on pathology alone. For example, recurrent chromosomal translocations have been described in malignant EHE, specifically t(1; 3) resulting in WWTR1-CAMTA1 fusion [21, 22], and YAP1-TFE3 fusion has been noted in the less common variant of EHE with vasoformative features [23]. These genetic abnormalities were not identified in benign EH, suggesting that cytogenetic analysis may be a useful adjunctive test to exclude a malignant epithelioid vascular tumor in difficult cases [23]. Antonescu et al. [21] also analyzed 2 intra-osseous EH cases with worrisome histologic features and discovered a ZFP36-FOSB fusion in 1 case and a WWTR1-FOSB chimeric transcript in the other. They subsequently screened 44 EH cases from different locations, with 7 additional EH revealing FOSB gene rearrangements [21]. In a follow-up study, Huang et al. [2] sought to investigate the prevalence of FOS rearrangement in a cohort of EH cases, which lacked FOSB rearrangement, finding FOS positivity in 17/58 cases tested (29%). The FOS gene encodes a transcription factor that forms part of an activating complex, which binds to elements of the promoter and enhancer regions of target genes, thereby regulating a variety of physiologic and tumorigenic processes, including cell proliferation and angiogenesis [2]. Currently, the presence of multifocal nuclear positivity for an FOSB or FOS genetic rearrangement are considered to be reassuring features arguing for a diagnosis of benign EH and against a diagnosis of a malignant EHE [2, 21].
In their study of 58 EH cases including cutaneous, soft tissue, and intra-osseous lesions, Huang et al. [2] noted that the majority of cases were unencapsulated with a hemorrhagic background and hemosiderin-laden histiocytes. Most of the tumors had a lobulated growth pattern, while 3 were more infiltrative and 2 were entirely confined within vascular lumen. The authors further divided these cases into typical (43%), cellular (36%), and ALHE (21%) variants based on histology. As its designation would suggest, the cellular variant was noted to have increased cellularity and a solid growth pattern. Nuclear atypia and necrosis were seen in a subset of all variants, but increased mitotic activity occurred mostly in the cellular variant.
Prominent inflammation with a marked eosinophilic infiltrate was seen across all subtypes, including 5 typical, 2 cellular, and 9 ALHE variants respectively [2]. Interestingly, all 12 ALHE cases in the study by Huang et al. [2] lacked FOS gene abnormalities, suggesting different pathogenesis for this type of EH as compared to the tumorigenesis of typical and cellular EH, in which the dysregulation of the FOS family of transcription factors is believed to play a key role. The unique histopathology of the ALHE variant, characterized by capillary proliferation around a large vessel, vascular damage, and extensive inflammation, is similarly distinct from the neoplastic appearance of other EH variants, and may also suggest a reactive process [2].
The histopathology in our case was suggestive of a benign EH, with distinction between this and malignant EHE further supported by immunohistochemical stains which showed multifocal nuclear positivity for FOSB, indicating FOSB genetic rearrangement, and negativity for CAMTA1. To our knowledge, this is the first published case of orbital epithelioid cellular hemangioma in which immunohistochemical stains were used to detect cytogenetic rearrangements that aided in diagnosis. This case demonstrates that such testing can be a valuable tool for distinguishing between benign and malignant endothelial neoplasms, particularly EH and EHE. It is also a reminder of the clinical and histopathologic characteristics of the benign entity of EH, which only rarely occurs in the orbit but must be included in the differential of vascular orbital lesions. Given the presumed persistent nature of this patient's lesion over many years, this case demonstrates the need for ongoing monitoring following ligation of presumed orbital varices, particularly those with atypical features, as these may prove to be more complex vascular lesions.
Statement of Ethics
The subject has given informed consent for this case report. The study was performed in accordance with the Declaration of Helsinki.
Disclosure Statement
The authors declare that they have no conflicts of interest or financial interests to disclose.
Author Contributions
All named authors satisfy the ICMJE Criteria for Authorship.
Acknowledgment
We would like to acknowledge Christopher Fletcher, MD, at Brigham and Women's Hospital for his expert consultation on this case. We are also grateful for the following support: Unrestricted Grant for Research to Prevent Blindness, New York, NY; Casey Eye Institute NIH Core Grant (P30 EY010572).
References
- 1.Fletcher CD, Bridge JA, Hogendoorn PC, Mertens F. WHO Classification of Tumours of Soft Tissue and Bone. 4th ed. Volume 5. Lyon, France: IARC Press; 2013. [Google Scholar]
- 2.Huang SC, Zhang L, Sung YS, Chen CL, Krausz T, Dickson BC, et al. Frequent FOS gene rearrangements in epithelioid hemangioma: a molecular study of 58 cases with morphologic reappraisal. Am J Surg Pathol. 2015 Oct;39((10)):1313–21. doi: 10.1097/PAS.0000000000000469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wells GC, Whimster IW. Subcutaneous angiolymphoid hyperplasia with eosinophilia. Br J Dermatol. 1969 Jan;81((1)):1–14. doi: 10.1111/j.1365-2133.1969.tb15914.x. [DOI] [PubMed] [Google Scholar]
- 4.Rosai J, Gold J, Landy R. The histiocytoid hemangiomas. A unifying concept embracing several previously described entities of skin, soft tissue, large vessels, bone, and heart. Hum Pathol. 1979 Nov;10((6)):707–30. doi: 10.1016/s0046-8177(79)80114-8. [DOI] [PubMed] [Google Scholar]
- 5.Weiss SW, Enzinger FM. Epithelioid hemangioendothelioma: a vascular tumor often mistaken for a carcinoma. Cancer. 1982 Sep;50((5)):970–81. doi: 10.1002/1097-0142(19820901)50:5<970::aid-cncr2820500527>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 6.Rosai J, Akerman LR. Intravenous atypical vascular proliferation. A cutaneous lesion simulating a malignant blood vessel tumor. Arch Dermatol. 1974 May;109((5)):714–7. doi: 10.1001/archderm.109.5.714. [DOI] [PubMed] [Google Scholar]
- 7.Jones EW, Bleehen SS. Inflammatory angiomatous nodules with abnormal blood vessels occurring about the ears and scalp (pseudo or atypical pyogenic granuloma) Br J Dermatol. 1969 Nov;81((11)):804–16. doi: 10.1111/j.1365-2133.1969.tb15948.x. [DOI] [PubMed] [Google Scholar]
- 8.Googe PB, Harris NL, Mihm MC., Jr Kimura's disease and angiolymphoid hyperplasia with eosinophilia: two distinct histopathological entities. J Cutan Pathol. 1987 Oct;14((5)):263–71. doi: 10.1111/j.1600-0560.1987.tb00498.x. [DOI] [PubMed] [Google Scholar]
- 9.Buggage RR, Spraul CW, Wojno TH, Grossniklaus HE. Kimura disease of the orbit and ocular adnexa. Surv Ophthalmol. 1999 Jul-Aug;44((1)):79–91. doi: 10.1016/s0039-6257(99)00064-8. [DOI] [PubMed] [Google Scholar]
- 10.Azari AA, Kanavi MR, Lucarelli M, Lee V, Lundin AM, Potter HD, et al. Angiolymphoid hyperplasia with eosinophilia of the orbit and ocular adnexa: report of 5 cases. JAMA Ophthalmol. 2014 May;132((5)):633–6. doi: 10.1001/jamaophthalmol.2013.8243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mukherjee B, Kadaskar J, Priyadarshini O, Krishnakumar S, Biswas J. Angiolymphoid hyperplasia with eosinophilia of the orbit and adnexa. Ocul Oncol Pathol. 2015 Sep;2((1)):40–7. doi: 10.1159/000433545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hidayat AA, Cameron JD, Font RL, Zimmerman LE. Angiolymphoid hyperplasia with eosinophilia (Kimura's disease) of the orbit and ocular adnexa. Am J Ophthalmol. 1983 Aug;96((2)):176–89. doi: 10.1016/s0002-9394(14)77785-2. [DOI] [PubMed] [Google Scholar]
- 13.Sánchez-Acosta A, Moreno-Arredondo D, Rubio-Solornio RI, Rodríguez-Martínez HA, Rodríguez-Reyes AA. Angiolymphoid hyperplasia with eosinophilia of the lacrimal gland: a case report. Orbit. 2008;27((3)):195–8. doi: 10.1080/01676830701804099. [DOI] [PubMed] [Google Scholar]
- 14.Cook HT, Stafford ND. Angiolymphoid hyperplasia with eosinophilia involving the lacrimal gland: case report. Br J Ophthalmol. 1988 Sep;72((9)):710–2. doi: 10.1136/bjo.72.9.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shields CL, Shields JL, Glass RN. Bilateral orbit involvement with angiolymphoid hyperplasia with eosinophilia. Orbit. 1995;9:89–95. [Google Scholar]
- 16.Bostad L, Pettersen W. Angiolymphoid hyperplasia with eosinophilia involving the orbita. A case report. Acta Ophthalmol (Copenh) 1982 Jun;60((3)):419–26. doi: 10.1111/j.1755-3768.1982.tb03033.x. [DOI] [PubMed] [Google Scholar]
- 17.Eisenberg E, Lowlicht R. Angiolymphoid hyperplasia with eosinophils: a clinical-pathological conference. J Oral Pathol. 1985 Mar;14((3)):216–23. doi: 10.1111/j.1600-0714.1985.tb00484.x. [DOI] [PubMed] [Google Scholar]
- 18.Kennedy SM, Pitts JF, Lee WR, Gibbons DC. Bilateral Kimura's disease of the eyelids. Br J Ophthalmol. 1992 Dec;76((12)):755–7. doi: 10.1136/bjo.76.12.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alder B, Proia A, Liss J. Distinct, bilateral epithelioid hemangioma of the orbit. Orbit. 2013 Feb;32((1)):51–3. doi: 10.3109/01676830.2012.739674. [DOI] [PubMed] [Google Scholar]
- 20.Urabe A, Tsuneyoshi M, Enjoji M. Epithelioid hemangioma versus Kimura's disease. A comparative clinicopathologic study. Am J Surg Pathol. 1987 Oct;11((10)):758–66. doi: 10.1097/00000478-198710000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Antonescu CR, Chen HW, Zhang L, Sung YS, Panicek D, Agaram NP, et al. ZFP36-FOSB fusion defines a subset of epithelioid hemangioma with atypical features. Genes Chromosomes Cancer. 2014 Nov;53((11)):951–9. doi: 10.1002/gcc.22206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanas MR, Sboner A, Oliveira AM, Erickson-Johnson MR, Hespelt J, Hanwright PJ, et al. Identification of a disease-defining gene fusion in epithelioid hemangioendothelioma. Sci Transl Med. 2011 Aug;3((98)):98ra82. doi: 10.1126/scitranslmed.3002409. [DOI] [PubMed] [Google Scholar]
- 23.Antonescu CR, Le Loarer F, Mosquera JM, Sboner A, Zhang L, Chen CL, et al. Novel YAP1-TFE3 fusion defines a distinct subset of epithelioid hemangioendothelioma. Genes Chromosomes Cancer. 2013 Aug;52((8)):775–84. doi: 10.1002/gcc.22073. [DOI] [PMC free article] [PubMed] [Google Scholar]