Abstract
This study aimed to investigate the expression of PGC-1α/FNDC5/irisin induced by attenuation of high-fat diet (HFD)-induced bone accrual and determine whether swimming exercise could improve attenuating bone accrual through this mechanism. Eight-week-old Sprague–Dawley rats were divided into two groups for the first 8 weeks: CD, control diet (n = 10); and HFD, high-fat diet (n = 20). HFD-fed rats were again divided into two groups for further 8 weeks treatment: HFD (n = 10) and HFD with swimming exercise (HEx, n = 10). During this time, the CD group continuously fed the normal diet. Throughout the 16 weeks study period, the rats were weighed once every week. Samples were collected for analysis after last 8 weeks of treatment in the 16 weeks. Morphological and structural changes of the femur and tibial bone were observed using micro-CT, and Osteocalcin, CTX-1 and irisin levels in the blood were measured by enzyme-linked immunosorbent assay. The expression of IL-1, β-catenin, FNDC5 and PGC-1α, in the femur were evaluated by immunohistochemistry. Eight weeks of HFD increased body weight and epididymal fat mass and decreased bone mineral density (BMD). Subsequent 8 weeks of swimming exercise improved obesity, BMD, bone microstructure, and bone metabolic factors in the HEx group. The irisin levels in the blood and the expressions of FNDC5 and PGC-1α in the bone were significantly lower in the HFD group than in the CD group, but elevated in the HEx group than in the HFD group. Swimming exercise is effective in improving obesity-worsened bone health and increases blood irisin and bone PGC-1α and FNDC5 levels.
Key points.
Increased epididymal fat mass by high-fat diet attenuates bone accrual in growing rats.
Swimming exercise effectively improves the bone microstructure, which attenuate bone accrual by high-fat diet.
Swimming exercise improves bone accrual attenuation by reducing high-fat diet-induced inflammation and increasing expression of FNDC5 and PGC-1.
Key words: Obesity, swimming, bone metabolism, bone mineral density, bone microstructure
Introduction
Fat tissues are regarded as endocrine organs that play important roles in energy balance regulation and homeostasis. Fat tissue can be either white adipose tissue (WAT) or brown adipose tissue (BAT) (Kajimura and Saito, 2014). BAT is involved in body temperature regulation and energy consumption by generating heat without shivering, while WAT stores energy and is spread throughout the body (Yamauchi et al., 2003). However, excessive fat accumulation due to obesity elevates proinflammatory cytokines, such as tumor necrosis factor-α (TNF-α), interleukin (IL)-1, and IL-6 (Cao, 2011). They stimulate osteoclast differentiation and activity by regulating receptor activator of NF-κB (RANK), RANK ligand (RANKL), and osteoprotegerin pathways (Khosla, 2001), while restricting osteoblastogenesis. This has an adverse effect on bone health, causing reduced bone mineral density (BMD) and microstructural changes (Rosen and Bouxsein, 2006). Physical exercise is the preferred treatment for prevention of obesity-related poor bone health (Cosman et al., 2014), and exerts an anabolic effect on bone either directly through mechanical signals generated by muscle contraction or indirectly via endocrine regulation (Colaianni and Grano, 2015; DiGirolamo et al., 2013). Thus, bone metabolism represents a close interaction between muscle and bone (Robling and Turner, 2009). Weight-bearing exercises such as walking, running, and weight training have been used to improve bone metabolism, and non-weight bearing exercises such as swimming and cycling have been perceived as less effective (Abrahin et al., 2016). However, recent studies have reported that non-weight bearing exercises, such as swimming, are also effective in improving bone metabolism (Hart et al., 2001; Ju et al., 2015; Lu et al., 2016; Oh et al., 2016). In particular, Falcai et al. (2015) reported that 3 weeks of swimming improved BMD and microstructure as effectively as jumping exercises in rats with reduced bone mass by hindlimb suspension. These results suggest that muscle contraction alone, without weight loading, is sufficient to maintain and improve bone health. Mechanisms underlying improvement of bone metabolism by muscle contraction are regulated by gene expression or various hormones released in the bone or skeletal muscle tissues (Nordström et al., 2005). The newly identified irisin, an exercise-induced myokine, is cleaved from fibronectin type III domain containing protein 5 (FNDC5) and released into the serum during exercise (Boström et al., 2012).
Recent research reported that irisin inhibits osteoclast activity while increasing osteoblast differentiation in bone cells lines (Zhang et al., 2017). Systemic administration of irisin regulates bone anabolism through direct mechanism via β-catenin and indirect mechanism via browning of the WAT (Motyl et al., 2013; Rahman et al., 2013). Also, irisin is effective in reducing body fat and preventing and treating obesity by inducing energy expenditure (Calton et al., 2016). Thus, exercise-induced irisin may reduce osteoclast differentiation by decreased proinflammatory cytokines associated with fat accumulation, and increase anabolic factors such as β-catenin, which induces osteoblast differentiation. However, it has not been investigated whether this effect occurs in vivo as well as in vitro using cell lines.
This study was conducted to investigate the expression of PGC-1α/FNDC5/irisin following attenuation of bone accrual by high-fat diet and investigated whether swimming exercise could improve attenuating bone accrual through this mechanism.
Methods
Animal and diets
This study used 8-week-old male Sprague–Dawley rats (n = 30) obtained from the Damul Science Inc. (Daejeon, South Korea). The rats were raised in a plastic cage, with two rats in the same group per cage, at the Study Animal Center at J University. The temperature and humidity of the laboratory were set to 23–25°C and 70%–80%, respectively, with a 12-h light–dark cycle (08:00 – 20:00). This study was approved by the Institutional Animal Care and Use Committee of our university.
The study animals were acclimatized for 1 week and then randomly divided into the control diet (CD, n = 10) group receiving feed containing 3.5% fat and the high-fat diet (HFD) group (n = 20) receiving feed containing 45% fat. The rats were fed their corresponding feed for 8 weeks via free feeding (Table 1).
Table 1.
Composition of experimental diets.
| Control diet (g) | High-fat diet (g) | |
|---|---|---|
| Casein | 200.0 | 245.0 |
| L-Cystine | 3.5 | 3.5 |
| Corn starch | 397.5 | 85.0 |
| Maltodextrin | 35.0 | 115.0 |
| Sucrose | 100.0 | 200.0 |
| Lard | 20.0 | 195.0 |
| Soybean oil | 70.0 | 30.0 |
| Cellulose | 50.0 | 58.0 |
| Mineral mix | 30.5 | 43.0 |
| Calcium phosphate | 3.5 | 3.4 |
| Vitamin mix | 19.0 | 19.0 |
| Choline bitartrate | 2.5 | 3.0 |
| Red food color | — | 0.1 |
| Protein | 20.5 %kcal | 19.0 %kcal |
| Carbohydrate | 76.0 %kcal | 36.2 %kcal |
| Fat | 3.5 %kcal | 44.8 %kcal |
| Total | 100 %kcal | 100 %kcal |
The HFD group was further divided into the HFD control (HFD, n = 10) and high-fat diet with swimming exercise (HEx, n = 10) groups. Rats continued to receive HFD during the 8 weeks of swimming exercise. The CD group did not undergo any additional treatment and was used as the control group for the HFD group.
Swimming exercise
The swimming protocol consisted of 8 weeks of low-intensity swimming exercise, which was based on partial revisions to the methods by Terada and Tabata (2004) and Kim et al. (2017). The exercise groups were adapted to a round water tank (70 cm × 70 cm) containing water at 28 ± 2°C for 3 days (10–45 min/day). The rats then began swimming at 10 AM, 45 min/day for the first two weeks and 60 min/day for the last six weeks.
Sample collection
After 8 weeks of swimming exercise, the study animals were given an intra-abdominal injection (1 mL/kg body weight) of a 2:1:2 mixture of Zoletil (Virbac Laboratories), Rompun (Bayer Korea), and normal saline. Blood samples were collected from the abdominal aorta to analyze osteocalcin, CTX-1 and irisin concentration. The blood samples were left standing at room temperature for 30 min and centrifuged to isolate the serum. Epididymal fat was extracted to measure body fat mass. The sample was stored at –80°C until use. In addition, to observe morphological changes of the bone, the right tibial and femoral bones were isolated and stored in a 10% formalin tube until measurement. The left femoral bone was used to analyze expression of bone metabolic factors.
Measurement of bone morphological and structural in bone by micro computed tomography (CT)
BMD and microstructure of the tibia and femur were analyzed via micro-CT (SkyScan 1076, Bruker, Kontich, Belgium). An X-ray of 60 kA and 167 μA was irradiated, and the specimen was filtered through a 0.5 mm aluminum filter to produce a micro-CT image. The micro-CT image was then reconstructed to a gray-scale level using NRecon version 1.3 (SkyScan), and the reconstructed two-dimensional image was converted to a three-dimensional model using the CTAn and CTVox (SkyScan) software. In the CTAn software, 150 slides at 0.5 mm distance from the growth plate in the tibia and femur were set as the region of interest (ROI). Percent bone volume/total volume (BV/TV), trabecular thickness (Tb.Th), trabecular number (Tb.N), and trabecular separation (Tb.Sp) were measured within the ROI. In the CTVox (Skyscan) software, the 2D cross section and 3D structure of the trabecular bone were converted into images.
Hematoxylin and eosin (H&E) and immunohistochemistry (IHC) staining
To analyze morphological changes and expression of bone metabolic factors, IL-1, β-catenin, PGC-1α, and FNDC5 in the tibia and femur, bone tissues fixed in 10% formalin were demineralized in 10% formic acid for 7 days. The tissues were dehydrated with ethanol, cleared with xylene, and infiltrated and embedded in paraffin. The processed tissues were cut into about 5 μm slices with a microtome and were placed on a glass slide for H&E and IHC staining. IHC staining was performed using VECTASTAIN Universal Elite ABC Kit (Vector Laboratories, Burlingame, CA, USA) to detect expression of IL-1, β-catenin, PGC-1α, and FNDC5. The stained slides were observed using a microscope (Carl Zeiss, Oberkochen, Germany).
Enzyme-linked immunosorbent assay (ELISA) analyses
Blood osteocalcin, CTX-1, and irisin levels were measured using ELISA kits (MyBioSource Inc., San Diego, CA, USA). Then, 25 μL of the standard solution and serum from each sample were added to wells coated with antibody. After adding 100 μL of working reagent conjugate buffer, the solution was incubated for 2 hours at 37°C. The contents of each well were removed, and the wells were washed three times with a wash buffer. Then, 100 μL of the TMB reagent was added to each well and incubated for 30 min at 37°C. After adding 50 μL of stop solution, ELISA reader (Model 550 Microplate reader, Bio-Rad Inc., Hercules, CA, USA) was used to measure at 450 nm absorbance within 10 min, and the measurement was entered in the standard curve for calculation. The intra- and inter-assay coefficient of variation (CV) were <8% and 10% for CTX-1 and osteocalcin, and ≤8% and ≤12% for irisin, respectively.
Statistical analysis
The data are presented as mean ± standard error of the mean (SE). The increases in body weight and food intake were analyzed by two-way ANOVA with repeated measures. Independent t-tests were conducted for comparison of epididymal fat weight and BMD after 8 weeks of HFD. One-way ANOVA for the comparison of BMD and microstructure of bone, number of IL-1, β-catenin, PGC-1α, and FNDC5 in bone and blood parameters was used to compare the differences among groups after 8 weeks treatment in the 16 weeks. A Bonferroni post hoc test was conducted to determine the significance. The significance level was set at p<0.05. The statistical analyses were performed with SPSS 18.0 (SPSS Inc., Chicago, IL, USA).
Results
HFD increases body weight but does not change food intake
Changes in body weight during the 16 weeks experimental period (Diet and Exercise period each 8 weeks) are shown in Figure 1. The body weight of HFD group increased significantly after 4 weeks of diet period compared to CD group (p < 0.05). This body weight gain was slowed down by the swimming exercise, which started from the 8th week of HFD, and significantly decreased after 3 weeks swimming exercise than HFD group (p < 0.05).
Figure 1.
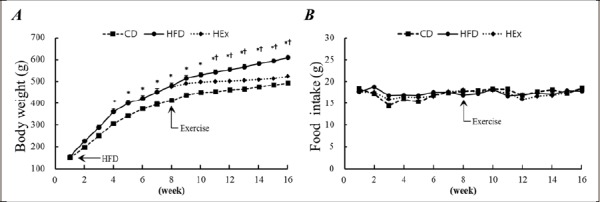
Body weight (A) was increased by high-fat diet, but food intake (B) does not different. Different letters indicates significant differences (p < 0.05) between groups at same time.
HFD increases epididymal fat mass and reduces BMD
As shown in Figure 2, epididymal fat mass of the HFD group significantly increased after 8 weeks of HFD than that in the CD group (p < 0.05). The femur and tibia BMD of the HFD group were significantly decrease after 8 weeks of HFD than that in the CD group (p < 0.05). These results suggest that HFD attenuates bone accrual in growing rats. We, therefore, evaluated the effect of 8 weeks of swimming exercise on the attenuated bone accrual by HFD. These results are shown in Figure 3 and 4 and Table 2.
Figure 2.
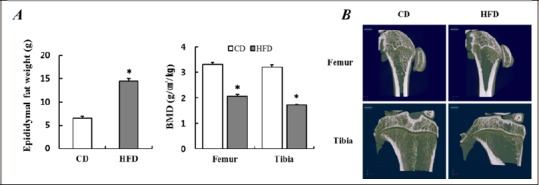
Increased epididymal fat mass and decreased BMD according to 8 weeks high-fat diet. (A) Comparison of epididymal fat mass and BMD after 8 weeks high-fat diet. (B) Representative micro-computed tomography image in femur and tibia bone after 8 weeks of high-fat diet. Data are presented as mean ± SE. * p < 0.05 vs. CD by t-test. CD, control diet; HFD, high-fat diet; BMD, bone mineral density.
Figure 3.
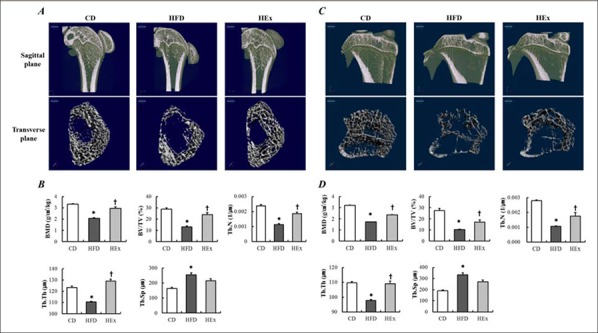
During the 16 weeks experimental period, the last 8 weeks swimming exercise improves the deterioration of trabecular bone by high-fat diet. Representative micro-computed tomography image and microstructure analysis of trabecular bone in the femur (A and B) and tibia (C and D) after 8 weeks of swimming exercise. Data are presented as mean ± SE. * p < 0.05 vs. CD, † p < 0.05 vs. HFD by one-way ANOVA. CD, control diet; HFD, high-fat diet; HEx, high-fat diet and swimming exercise. BMD, bone mineral density; BV/TV, bone volume/total volume; Tb.N, trabecular number; Tb.Th, trabecular thickness; Tb.Sp, trabecular separation.
Figure 4.
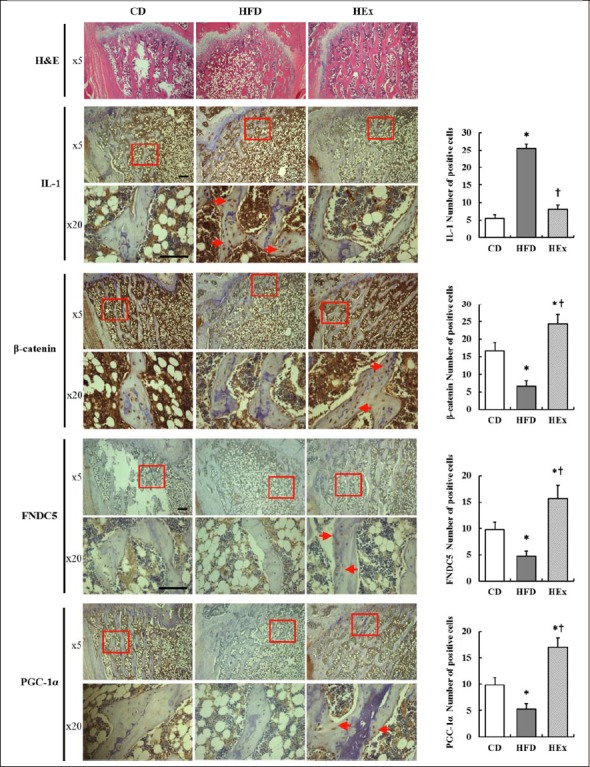
The expression of IL-1, β-catenine, FNDC5 and PGC-1α in the femur at the end of 16 weeks of experiment. Representative hematoxylin and eosin (H&E) staining of longitudinal section of femoral bone in each group. The magnification of each specimen was ×5 and ×20. Scale bars represent 50μm. Expression of IL-1 and β-catenine, FNDC5 and PGC-1α manifested by the brown staining of the DAB dye, was visualized in the nuclei (short red arrows) of femur by IHC. At least five slides from each specimen were quantified. CD, control diet; HFD, high-fat diet; HEx, high-fat diet and swimming exercise; IL-1, interleukin 1; FNDC5, Fibronectin type III domain-containing protein 5; PGC-1α, Peroxisome proliferator-activated receptor gamma coactivator 1-alpha. * p < 0.05 vs. ND, † p < 0.05 vs. HEx by one-way ANOVA.
Table 2.
Osteocalcin, CTX-1 and irisin level in serum at the end of experiment.
| Osteocalcin (pg/ml) | CTX-1 (ng/ml) | Irisin (ng/ml) | |
|---|---|---|---|
| CD | 0.141 ± 0.008 | 0.119 ± 0.008 | 2.129 ± 0.089 |
| HFD | 0.097 ± 0.007* | 0.179 ± 0.01* | 1.377 ± 0.113* |
| HEx | 0.124 ± 0.011† | 0.143 ± 0.018† | 1.922 ± 0.213† |
Data are presented as mean ± SE.
* p < 0.05 vs. ND group
† p < 0.05 vs. HFD group by one way ANOVA. CD, control diet; HFD, high-fat diet; HEx, high-fat diet and swimming exercise; CTX-1, C-telopeptide of type I collagen.
Swimming alleviates BMD and HFD worsened bone microstructure
Morphological and structural changes in the femur and tibia were investigated after 8 weeks of HFD and consecutive 8 weeks of swimming exercise. Bone morphological changes of the femur and tibia are respectively illustrated changes of the femur and tibia are respectively illustrated in sagittal and transverse plane images in Figure 3. Analysis results of femur and tibia bone were the same, and the detailed explanation is as follows: The HFD group showed a marked difference in the trabecular bone from that of the CD and HEx groups (Figure 3A and C). Regarding the structural changes of the femur (Figure 3B) and tibia (Figure 3D), BMD, BV/TV, Tb.N, and Tb.Th were significantly reduced in the HFD group than in the CD group (p < 0.05), but significantly increased in the HEx group compared to that in the HFD group (p < 0.05). This finding suggests that 8 weeks of swimming exercise effectively improves the femoral structure, which attenuate bone accrual by HFD (Figure 3B and D).
Swimming improves the expression of IL-1, β-catenin, FNDC5, and PGC-1α in the femoral bone worsened by HFD
Histological changes of the femur were examined using IHC after the last 8 weeks treatments in the 16 weeks period (Figure 4). The IL-1 level in the femoral bone was increased in the HFD group than in the CD but swimming exercise reduced HFD-induced IL-1 (p < 0.05). β-catenin, FNDC5, and PGC-1α levels were decrease in the HFD group than in the CD group (p < 0.05) but increased in HEx group compared to the CD and HFD group (p < 0.05). These results indicate that swimming exercise improves bone accrual attenuation by reducing HFD-induced inflammation and increasing expression of FNDC5 and PGC-1.
Swimming improves osteocalcin, CTX-1, and irisin level in the serum worsened by HFD
As shown in Table 2, concentrations of serum osteocalcin and irisin were significantly decrease in the HFD group than in the CD group but swimming exercise reversed this decrease (p < 0.05). Conversely, serum CTX-1 concentration was increased in the HFD group than in the CD group, but swimming exercise suppressed this increase (p < 0.05).
Discussion
To identify effective methods to improve bone health in rats with HFD induced attenuation of bone accrual, we administered a swimming exercise protocol. We first measured body weight, epididymal fat mass and BMD in femur and tibia after 8 weeks of HFD to determine that HFD attenuate bone accrual. Expression of inflammatory cytokines and induction of obesity from high fat diets adversely affect BMD as well as bone microstructure and bone remodeling (Anandacoomarasamy et al., 2009; Bartelt et al., 2010; Hsu et al., 2006; Park et al., 2006; Patsch et al., 2011). As shown in Figure 1 and 2, after 8 weeks of HFD, BMD decreased with increasing body weight and epididymal fat mass. We purchased 8 weeks old SD rats and sacrificed after 17 weeks later including 1 week of acclimation. Sengupta et al. (2005) SD rats reach peak bone mass at 3 month of age. Thus, the results of this study indicate that HFD attenuate bone accrual. HFD or HFD-induced obesity.
Exercise is highly effective not only for preventing and treating obesity, but also for improving obesity-induced poor bone health (Li et al., 2017). Exercise appears to affect bones directly through mechanical loading and indirectly through endocrine regulation during exercise (Cosman et al., 2014). The focus has been on the direct pathway thus far, utilizing a resistance exercise like weight training. However, resistance exercise for attenuated bone accrual is not often recommended because it increases the risk of injury to the musculoskeletal system caused by inappropriate training techniques, excessive loading, poorly designed equipment, ready access to the equipment (Faigenbaum et al., 2009). Therefore, if there is a relatively low risk of injuries and an effective exercise than a resistance exercise, this is a good intervention for attenuated bone accrual. Swimming is a safer exercise, where buoyancy lowers joint burden and risks of fall or fracture. Swimming exercise has been found to improve BMD and bone microstructure in menopause models in the studies by Ju et al. (2015) and Oh et al. (2016) aged rats in Hart et al. (2001) study, and in rats with HFD-induced obesity in Lu et al. (2016) study. In this context, the present study administered 8 weeks of swimming exercise in rats with HFD-induced obesity. We found that body weight and epididymal fat mass significantly decreased in the HEx groups (results not shown) compared to that in the HFD group. Analysis of the bone density and microstructure of the femur and tibia using micro-CT revealed that bone density, BV/TV, Tb.N, and Tb.Th significantly increased in the HEx group compared to that in the HFD group (Figure 3), while serum osteocalcin and CTX-1 (Table 2) and IL-1 and β-catenin (Figure 4) levels in the bone tissue were improved. These results are difficult to compare with the results of external mechanical loading like resistance exercise, however, we demonstrated that muscle contraction without weight-bearing can improve the attenuation of bone accrual associated with HFD. This beneficial effect on attenuated bone accrual may be associated with the regulation of various bone metabolites.
Hormones, cytokines, and myokines that are released in various body parts during exercise are involved in the physiological adaptation to exercise (Huh et al., 2012). In particular, irisin secreted from the skeletal muscle during physical activity is known to be effective in decreasing body fat by inducing an increase in energy consumption of WAT (Boström et al., 2012). In addition, recent studies have shown that irisin is associated with bone metabolism (Liu et al., 2013; Lu et al., 2016; Qiao et al., 2016). Irisin is involved in osteogenesis and prevention of fractures (Zhang et al., 2017). In an in vitro study by Qiao et al. (2016) treating MC3T3 cells (osteoblasts) with irisin stimulated osteoblast proliferation and increased the expression of osteoblastic transcription regulators and osteoblast differentiation markers. Furthermore, irisin treatment effectively inhibited RANKL-induced osteoclast differentiation and led to positive changes in BMD and microstructure (Zhang et al., 2017). The results of these previous studies suggest that irisin plays an important role in bone metabolism as well as body fat reduction. Our finding showed the PGC-1α and FNDC5 in the femur and blood irisin level were increase in the HEx group than in the HFD group. In the studies by Bashar et al. (2018), Yang et al. (2016) and Lu et al. (2016) 8-week swimming exercises have been shown to increase blood irisin concentrations, but no studies have examined the role of irisin in BMD and bone metabolism. In particular, the expressions of FNDC5 and PGC-1α in the femur were significantly increased in the HEx group than in the HFD group in this study. In addition, compared with the HFD group, the HEx group showed decreased levels of bone resorption factors such as CTX-1 and IL-1 and increased levels of bone formation factors such as osteocalcin and β-catenin in the blood and femoral bone (Table 2 and Figure 4). In this study, swimming exercise increased PGC-1α/FNDC5/irisin levels and had a positive effect on bone metabolism through direct pathways such as osteocalcin, β-catenin, and CTX-1 and indirect pathways such as IL-1 and body fat reduction. Resistance exercise such as weight training have been mainly used because of the focus on mechanical loading for maintenance and improvement of bone health. However, this study confirmed the effect of swimming exercise on body fat reduction and bone health improvement. In particular, we observed an increase in serum irisin concentration, an increase in PGC-1α/FNDC5 expression in the bone tissue, and a positive change in bone metabolism markers in the blood and bone tissue. Further studies will be needed to clarify the relationship between these mechanisms and bone metabolism. This study is meaningful in that swimming exercise can be an alternative to improving bone health in obese, aging, and patients with arthritis who have weight-bearing exercise restriction.
Conclusion
HFD negatively affects bone accrual in BMD, bone microstructure, and bone metabolism, of growing rats. Swimming exercise was found to decrease body weight, body fat, and pro-inflammatory cytokines that were elevated due to obesity, promote bone metabolism, and improve BMD and microstructure of the femur and tibia. In addition, swimming increased serum irisin levels and expression of PGC-1/FNDC5 in the bone. These results indicate that swimming can improve bone accrual, when it is attenuated by a high fat diet. Particularly, this study verified that swimming, which has been perceived negatively so far, improves bone health and that it is a safe and effective exercise program that decrease body fat and improves bone health for individuals who are obese and who cannot perform more vigorous or weight-bearing exercises.
Acknowledgements
Yun-Seok Kang and Jae-Cheol Kim contributed equally to this work. This work was supported by the Ministry of Education of the Republic of Korea and the National Research Foundation of Korea (NRF-2016S1A5B5A07920443). There is no conflict of interest. The present study complies with the current laws of the country in which it was performed.
Biographies
Yunseok KANG
Employment
Department of Sports Science, College of Natural Science, Chonbuk National University, Jeonju, South Korea
Degree
PhD
Research interests
Bone metabolism, Osteoporosis, Osteoarthritis, Exercise biochemistry
E-mail: kangys53@jbnu.ac.kr
Jaecheol KIM
Employment
Prof., Department of Sports Science, College of Natural Science, Chonbuk National University, Jeonju, South Korea
Degree
PhD
Research interests
Exercise biochemistry, exercise physiology, exercise nutrition
E-mail: kjc@jbnu.ac.kr
Jeongseok KIM
Employment
Department of Sports Science, College of Natural Science, Chonbuk National University, Jeonju, South Korea
Degree
PhD
Research interests
Muscle metabolism (mitochondrial biogenesis), Aging
E-mail: kjs2002dol@hanmail.net
Sanghyun KIM
Employment
Prof., Department of Sports Science, College of Natural Science, Chonbuk National University, Jeonju, South Korea
Degree
PhD
Research interests
Muscle metabolism (mitochondrial biogenesis), aging, exercise mimetics, muscle-bone crosstalk
E-mail: sh5275@jbnu.ac.kr
References
- Abrahin O., Rodrigues R.P., Marçal A.C., Alves E.A.C., Figueiredo R.C., Sousa E.C.d. (2016) Swimming and cycling do not cause positive effects on bone mineral density: a systematic review. Revista Brasileira de Reumatologia 56, 345-351. [DOI] [PubMed] [Google Scholar]
- Anandacoomarasamy A., Fransen M., March L. (2009) Obesity and the musculoskeletal system. Current Opinion in Rheumatology 21, 71-77. [DOI] [PubMed] [Google Scholar]
- Bartelt A., Beil F.T., Schinke T., Roeser K., Ruether W., Heeren J., Niemeier A. (2010) Apolipoprotein E-dependent inverse regulation of vertebral bone and adipose tissue mass in C57Bl/6 mice: modulation by diet-induced obesity. Bone 47, 736-745. [DOI] [PubMed] [Google Scholar]
- Bashar S.M., El-sherbeiny S.M.S., Boraie M.Z. (2018) Correlation between the blood level of irisin and the severity of acute myocardial infarction in exercise-trained rats. Journal of Basic and Clinical Physiology and Pharmacology 30, 59-71. [DOI] [PubMed] [Google Scholar]
- Boström P., Wu J., Jedrychowski M.P., Korde A., Ye L., Lo J.C., Rasbach K.A., Boström E.A., Choi J.H., Long J.Z. (2012) A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 481, 463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calton E.K., Soares M.J., James A.P., Woodman R.J. (2016) The potential role of irisin in the thermoregulatory responses to mild cold exposure in adults. American Journal of Human Biology 28, 699-704. [DOI] [PubMed] [Google Scholar]
- Cao J.J. (2011) Effects of obesity on bone metabolism. Journal Orthopaedic Surgery and Research 6, 30, June 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colaianni G., Grano M. (2015) Role of Irisin on the bone–muscle functional unit. BoneKEy Reports 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosman F., De Beur S., LeBoff M., Lewiecki E., Tanner B., Randall S., Lindsay R. (2014) Clinician’s guide to prevention and treatment of osteoporosis. Osteoporosis International 25, 2359-2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiGirolamo D.J., Kiel D.P., Esser K.A. (2013) Bone and skeletal muscle: neighbors with close ties. Journal of Bone and Mineral Research 28, 1509-1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faigenbaum A.D., Kraemer W.J., Blimkie C.J., Jeffreys I., Micheli L.J., Nitka M., Rowland T.W. (2009) Youth resistance training: updated position statement paper from the national strength and conditioning association. The Journal of Strength & Conditioning Research 23, S60-S79. [DOI] [PubMed] [Google Scholar]
- Falcai M., Zamarioli A., Okubo R., Paula F., Volpon J. (2015) The osteogenic effects of swimming, jumping, and vibration on the protection of bone quality from disuse bone loss. Scandinavian Journal of Medicine & Science in Sports 25, 390-397. [DOI] [PubMed] [Google Scholar]
- Farr J.N., Drake M.T., Amin S., Melton L.J., McCready L.K., Khosla S. (2014) In vivo assessment of bone quality in postmenopausal women with type 2 diabetes. Journal of Bone and Mineral Research 29, 787-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart K., Shaw J., Vajda E., Hegsted M., Miller S. (2001) Swim-trained rats have greater bone mass, density, strength, and dynamics. Journal of Applied Physiology 91, 1663-1668. [DOI] [PubMed] [Google Scholar]
- Hsu Y.-H., Venners S.A., Terwedow H.A., Feng Y., Niu T., Li Z., Laird N., Brain J.D., Cummings S.R., Bouxsein M.L. (2006) Relation of body composition, fat mass, and serum lipids to osteoporotic fractures and bone mineral density in Chinese men and women. The American Journal of Clinical Nutrition 83, 146-154. [DOI] [PubMed] [Google Scholar]
- Huh J.Y., Panagiotou G., Mougios V., Brinkoetter M., Vamvini M.T., Schneider B.E., Mantzoros C.S. (2012) FNDC5 and irisin in humans: I. Predictors of circulating concentrations in serum and plasma and II. mRNA expression and circulating concentrations in response to weight loss and exercise. Metabolism-Clinical and Experimental 61, 1725-1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju Y.-I., Sone T., Ohnaru K., Tanaka K., Fukunaga M. (2015) Effect of swimming exercise on three-dimensional trabecular bone microarchitecture in ovariectomized rats. Journal of Applied Physiology 119, 990-997. [DOI] [PubMed] [Google Scholar]
- Kajimura S., Saito M. (2014) A new era in brown adipose tissue biology: molecular control of brown fat development and energy homeostasis. Annual Review Physiology 76, 225-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosla S. (2001) Minireview: The OPG/RANKL/RANK System. Endocrinology 142, 5050-5055. [DOI] [PubMed] [Google Scholar]
- Kim J.C., Park G.D., Kim S.H. (2017) Inhibition of oxidative stress by antioxidant supplementation does not limit muscle mitochondrial biogenesis or endurance capacity in rats. Journal of Nutritional Science and Vitaminology 63, 277-283. [DOI] [PubMed] [Google Scholar]
- Li W., Xu P., Wang C., Ha X., Gu Y., Wang Y., Zhang J., Xie J. (2017) The effects of fat-induced obesity on bone metabolism in rats. Obesity Research & Clinical Practice 11, 454-463. [DOI] [PubMed] [Google Scholar]
- Lirani-Galvão A.P.R., Lazaretti-Castro M. (2010) Physical approach for prevention and treatment of osteoporosis. Arquivos Brasileiros de Endocrinologia & Metabologia 54, 171-178. [DOI] [PubMed] [Google Scholar]
- Liu J.-J., Wong M.D., Toy W.C., Tan C.S., Liu S., Ng X.W., Tavintharan S., Sum C.F., Lim S.C. (2013) Lower circulating irisin is associated with type 2 diabetes mellitus. Journal of Diabetes and its Complications 27, 365-369. [DOI] [PubMed] [Google Scholar]
- Lu Y., Li H., Shen S.-W., Shen Z.-H., Xu M., Yang C.-J., Li F., Feng Y.-B., Yun J.-T., Wang L. (2016) Swimming exercise increases serum irisin level and reduces body fat mass in high-fat-diet fed Wistar rats. Lipids in Health and Disease 15, 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magni P., Dozio E., Galliera E., Ruscica M., Corsi M. (2010) Molecular aspects of adipokine-bone interactions. Current Molecular Medicine 10, 522-532. [DOI] [PubMed] [Google Scholar]
- Messier S.P., Gutekunst D.J., Davis C., DeVita P. (2005) Weight loss reduces knee-joint loads in overweight and obese older adults with knee osteoarthritis. Arthritis & Rheumatism 52, 2026-2032. [DOI] [PubMed] [Google Scholar]
- Motyl K.J., Bishop K.A., DeMambro V.E., Bornstein S.A., Le P., Kawai M., Lotinun S., Horowitz M.C., Baron R., Bouxsein M.L. (2013) Altered thermogenesis and impaired bone remodeling in Misty mice. Journal of Bone and Mineral Research 28, 1885-1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordström A., Karlsson C., Nyquist F., Olsson T., Nordström P., Karlsson M. (2005) Bone loss and fracture risk after reduced physical activity. Journal of Bone and Mineral Research 20, 202-207. [DOI] [PubMed] [Google Scholar]
- Oh T., Tanaka S., Naka T., Igawa S. (2016) Effects of high-intensity swimming training on the bones of ovariectomized rats. Journal of Exercise Nutrition & Biochemistry 20, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J., Yoon S., Lee H., Jo H., Lee S., Kim Y., Kim Y., Shin Y. (2006) Burden of disease attributable to obesity and overweight in Korea. International Journal of Obesity 30, 1661. [DOI] [PubMed] [Google Scholar]
- Patsch J.M., Kiefer F.W., Varga P., Pail P., Rauner M., Stupphann D., Resch H., Moser D., Zysset P.K., Stulnig T.M. (2011) Increased bone resorption and impaired bone microarchitecture in short-term and extended high-fat diet–induced obesity. Metabolism-Clinical and Experimental 60, 243-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao X., Nie Y., Ma Y., Chen Y., Cheng R., Yin W., Hu Y., Xu W., Xu L. (2016) Irisin promotes osteoblast proliferation and differentiation via activating the MAP kinase signaling pathways. Scientific Reports 6, 18732, January 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman S., Lu Y., Czernik P.J., Rosen C.J., Enerback S., Lecka-Czernik B. (2013) Inducible brown adipose tissue, or beige fat, is anabolic for the skeleton. Endocrinology 154, 2687-2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robling A.G., Turner C.H. (2009) Mechanical signaling for bone modeling and remodeling. Critical Reviews in Eukaryotic Gene Expression 19, 319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen C.J., Bouxsein M.L. (2006) Mechanisms of Disease: is osteoporosis the obesity of bone? Nature Clinical Practice Rheumatology 2, 35, 01/01/online. [DOI] [PubMed] [Google Scholar]
- Sekiya I., Tang T., Hayashi M., Morito T., Ju Y.J., Mochizuki T., Muneta T. (2009) Periodic knee injections of BMP-7 delay cartilage degeneration induced by excessive running in rats. Journal of Orthopaedic Research 27, 1088-1092. [DOI] [PubMed] [Google Scholar]
- Sengupta S., Arshad M., Sharma S., Dubey M., Singh M. (2005) Attainment of peak bone mass and bone turnover rate in relation to estrous cycle, pregnancy and lactation in colony-bred Sprague–Dawley rats: suitability for studies on pathophysiology of bone and therapeutic measures for its management. The Journal of Steroid Biochemistry and Molecular Biology 94, 421-429. [DOI] [PubMed] [Google Scholar]
- Terada S., Tabata I. (2004) Effects of acute bouts of running and swimming exercise on PGC-1α protein expression in rat epitrochlearis and soleus muscle. American Journal of Physiology-Endocrinology and Metabolism 286, E208-E216. [DOI] [PubMed] [Google Scholar]
- Yamauchi T., Kamon J., Ito Y., Tsuchida A., Yokomizo T., Kita S., Sugiyama T., Miyagishi M., Hara K., Tsunoda M. (2003) Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 423, 762. [DOI] [PubMed] [Google Scholar]
- Yang X., Yuan H., Li J., Fan J., Jia S., Kou X., Chen N. (2016) Swimming intervention mitigates HFD-induced obesity of rats through PGC-1α-irisin pathway. European Review for Medical and Pharmacological Sciences 20, 2123-2130. [PubMed] [Google Scholar]
- Zhang J., Valverde P., Zhu X., Murray D., Wu Y., Yu L., Jiang H., Dard M.M., Huang J., Xu Z., Tu Q., Chen J. (2017) Exercise-induced irisin in bone and systemic irisin administration reveal new regulatory mechanisms of bone metabolism. Bone Research 5, 16056. [DOI] [PMC free article] [PubMed] [Google Scholar]


