Abstract
The purpose of the study was to investigate the effect of inhaling 1600 μg of salbutamol (SAL) on 30 m sprint before and after the Yo-Yo Intermittent Recovery test. In a randomised cross over single blind study 13 male non-asthmatic, football players volunteered (mean ± SD; age 18.1 ± 0.9 years; weight 69.5 ± 8.3 kg; height 1.78 ± 0.07 m). Participants completed two visits and were randomly assigned to either (SAL) or (PLA) treatment and performed a set of three sprints of 30 m before and after the Yo-Yo Intermittent Recovery Test (Yo-Yo IRT). Best sprint and mean sprint were analysed in addition to the distance covered during the Yo-Yo IRT; rating of perceived exertion and heart rate were collected at the end of each level completed. Repeated measures ANOVA were performed to investigate changes in performance between groups. Following the inhalation of supra-therapeutic salbutamol dose (1600 μg) neither 30 m sprint time (PLA 4.43 ± 0.14 s; SAL 4.44 ± 0.15 s, p = 0.76) nor distance covered in the Yo-Yo IRT test reported significant variation between PLA conditions (1660 ± 217 m) and SAL (1610 ± 229 m, p = 0.16). Moreover, lactate values, heart rate and RPE did not differ significantly between groups. The inhalation of 1600 μg salbutamol does not enhance 30 m sprint performance in non-fatigued and fatigue conditions. Our findings suggest when football players acutely inhale double the permitted dose of salbutamol, as indicated in the World Anti-Doping Agency List of Prohibited Substances and Methods, they will not experience improvements in sprint or endurance performance.
Key points.
Investigate the potential ergogenic action of inhaled 1600 μg salbutamol on 30 m sprint performance.
Investigate the potential ergogenic action of inhaled 1600 μg salbutamol on 30 m sprint performance after fatigue protocol.
Investigate the potential ergogenic action of inhaled 1600 μg salbutamol before and after a Yo-Yo intermittent recovery test.
Investigate the effects of inhaled short-acting beta-2 agonist administration on both aerobic and anaerobic exercise replicating intermittent sports performance.
Key words: Football, salbutamol, sprint, asthma, doping
Introduction
Athletes with asthma related conditions are permitted to use inhaled salbutamol in accordance with current WADA regulations (WADA, 2019). Although a therapeutic dose of inhaled salbutamol ranges from 200-400 μg, football players are permitted to inhale up to 800 μg in any 12 hours period and 1600 μg in a 24 hours period. Limited data is available to explain whether football players may gain an ergogenic advantage from doses of inhaled salbutamol up to 1600 μg.
Recent research by Dickinson et al. (2015) suggests that doses of up to 1600 μg of inhaled salbutamol do not improve repeated sprint performance following a 52 minute football specific treadmill running protocol. However, others have observed improvements in sprint cycling after the inhalation of salbutamol 180 μg (Signorile et al., 1992) and 15 mg Terbutaline (Kalsen et al., 2016). The latter authors suggested the 8% improvement in power output over a 10 s sprint cycling performance was associated with a greater fatigue resistance in type II fibers due to increased rates of glycogenolysis and glycolysis. Furthermore, when well trained swimmers inhale a combination of salbutamol (1600 μg), formoterol (36 μg) and salmeterol (200 μg), their 100 m sprint performance is enhanced by 1 s (Kalsen et al., 2013). Recently, Hostrup et al. (2016) demonstrated that initial Wingate sprint performance was enhanced after 8 mg oral salbutamol administration but subsequent sprints were not enhanced in the same way.
Acute use of short acting β2-agonists may result in ergogenic action leading to improvements in peak power and one-off sprint performance. However, limited data suggest repeated sprint performance, with and without pre fatigue, is not enhanced. The purpose of this study is to investigate the potential ergogenic action of inhaled 1600 μg salbutamol on 30 m sprint performance in football players before and after the Yo-Yo intermittent recovery test (Yo-Yo IRT).
Methods
Participants
Thirteen male football players volunteered and provided written and verbal informed consent. All players took part in amateur football league, competing once a week and trained specifically for football at least three times a week. All participants were free from respiratory disease including asthma related conditions, cardiopulmonary disease, metabolic disease and musculoskeletal injury (Table 1).
Table 1.
Descriptive features of participants (n = 13) expressed as mean and standard deviation (Mean ± SD)
| Parameters | |
|---|---|
| Age (years) | 18.1 ± 0.9 |
| Height (m) | 1.78 ± 0.07 |
| Weight (kg) | 69.5 ± 8.3 |
| BMI (kg·m-2) | 22 ± 1.7 |
| VO2Max (mL/min/kg) | 51.2 ± 3.4 |
| HRMax (bpm) | 198 ± 4.0 |
| Training (h/week | 8 ± 1.4 |
| RPEMax (u.a.) | 9 ± 1.0 |
BMI, body mass index; VO2Max, maximal oxygen uptake
The participants completed all assessments at the same time of the day (within 1 h), separated by a minimum of 48 hours. They were instructed to sleep for at least 7 hours, drink at least 35 mL/kg/day of water, refrain from consumption of alcohol for 24 hours prior to the visit and avoid vigorous exercise on the day before each of the following visits.
Ethical approval (no. 521415) for this study was provided by the University of Kent, Faculty of Science, Local Research Ethics Committee.
Experimental protocol
Participants visited the respective testing facilities on three separate occasions, the first of which was a familiarization session. The final two visits were completed in a single blind random order and separated by two days. On each of these separate occasions players were required to complete three maximal 30 m sprints followed by the Yo-Yo IRT and followed by a further three maximal 30 m sprints, performed 5 minutes after the cessation of the Yo-Yo IRT (Figure 1).
Figure 1.
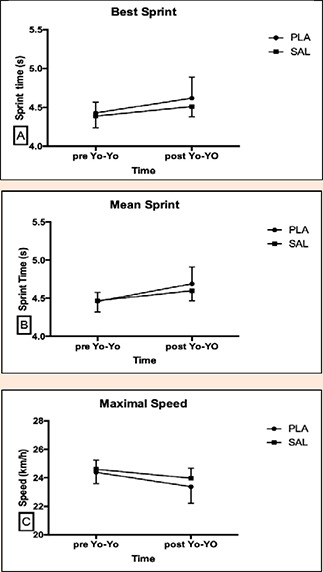
Best Sprint (A), Mean Sprint (B), Maximal speed (C) at pre and post Yo-Yo IRT. Data are presented by Mean and SD.
Fifteen minutes prior to the initiation of the first three 30 m sprints players inhaled one of the following treatments, via a pocket chamber:
Treatment 1: 16 inhalations of placebo containing water vapour (PLA).
Treatment 2: 16 inhalations of 100μg salbutamol (SAL; Ventolin Evohaler- GlaxoSmithKline, UK).
The order of PLA and SAL was randomized and counterbalanced by an order generated by online software (randomization.com).
Motivation and Mood questionnaires
Before each visit participants completed questionnaires to measure motivation to train and mood via the Brunel Mood Scale and Matthews questionnaire (Matthews et al., 2001; Terry et al., 2003). The Brunel Mood Scale (BRUMS) was used to quantify current mood at baseline, before the 30 m sprint (pre-sprint) and post-sprint. The questionnaire included 24 items (e.g. “angry, uncertain, miserable, tired, nervous, and energetic”) divided into six subscales: anger, confusion, depression, fatigue, tension, and vigour. Each item was answered on a five point scale (0 = not at all, 1 = a little, 2 = moderately, 3 = quite a bit, 4 = extremely), and each subscales, with the respective relevant items, was calculated with a raw score in the range of 0–16. For this experiment, only scores for the fatigue and vigour subscales were considered as subjective ratings of fatigue.
Motivation. Motivation related to Sprint and Yo-Yo test was measured using the success motivation and intrinsic motivation scales (Matthews et al., 2001). Each scale consisted of 7 items scored from 0 to 4 points (0 = not at all, 1 = a little bit, 2 = somewhat, 3 = very much, 4 = extremely). Motivation questionnaire was administered immediately before the 30 m sprint test.
Sprint test
Before the assessments all participants completed the 11+ warm-up (Bizzini and Dvorak, 2015) concluding with standardised mobility and flexibility exercises. After the warm up football players completed three 30 m maximal sprints with 60 s passive recovery time between each sprint (Taskin, 2008). Five minutes following the end of the Yo-Yo IRT, participants completed a further three 30 m sprints with a 60 s active recovery between each one.
Consistent verbal encouragement was given to the players during each sprint. Players were encouraged to complete the sprints as fast as they could. In order to record the time to cover the distance, a system of light gates (Brower Timing, Fairlee, Vermont, USA) were utilised. Heart rate (HR) was recorded throughout the sprints using heart rate monitor and watch (Polar m400 HR, Polar Oy Finland); peak HR was recorded for each condition. The mean time of the three efforts and the best sprint time were recorded. At the end of each set of three sprints, a drop of blood (0.5 μL) was collected from the ear to obtain a capillary blood lactate sample (Lactate Pro, Arkray KDK, Japan).
Yo-Yo test (Yo-Yo IRT)
The Yo-Yo IRT test consisted of repeated 2 x 20 m shuttle runs at a progressively increasing speed controlled by audio bleeps (Krustrup et al., 2003). Between each running bout the players had a 10 s active rest period, in a space of 5 m where they are encouraged to walk or jog. Following the completion of each stage players were asked to provide a rating of perceived exertion on a scale between 6 and 20 (Borg, 1982). When the players failed on two occasions to complete the 2 x 20 m within the required time the test was concluded and the distance covered was recorded. Heart rate was recorded during the entire fitness trial.
Statistical analysis
All data are presented as mean ± SD unless otherwise stated. Assumptions of statistical tests for normal distribution and sphericity of data were checked as appropriate and Greenhouse-Geisser correction to the degrees of freedom was applied when violations to sphericity were found. Repeated measures ANOVAs were used to determine the effect of time (Sprint Pre vs Sprint Post) and condition (SAL vs. PLA) on peak power, mean power and highest speed reached during 30-m sprint. This same analysis was adopted to investigate vigor (pre-treatment vs. post-treatment), motivation (pre-treatment) and each physiological and perceptual parameter (Heart rate, Blood Lactate and RPE) at iso-time [end of warm-up (0 min) and each Yo-Yo IRT level completed].
Heart rate and RPE during the Yo-Yo IRT were analyzed from the start of the test (level 5) up to the end of level 16; beyond this point only the highest value was recorded and considered their maximum, as football players began to drop out of the test from level 16 onwards. Significance was set at p ≤ 0.05 (2-tailed) for all analyses and effect sizes for the repeated measures ANOVAs were calculated as partial eta squared (η²p), using the small = 0.02, medium = 0.13 and large = 0.26 interpretation for effect size. All data analysis was conducted using the statistical packages for social science (SPSS, version 21, IBM, U.S.).
Results
All thirteen-football players completed the Yo-Yo IRT test and the sprint assessment in both conditions, PLA and SAL. None of the participants reported unpleasant symptoms such as tachycardia, nausea, headache or sleep disturbance after inhaling 1600 μg salbutamol; none of the participants could correctly distinguish the difference between the PLA and SAL conditions.
30 m Sprint Pre and Post Yo-Yo IRT
30 m sprints conducted pre and post Yo-Yo IRT demonstrated no difference between PLA and SAL conditions for mean sprint time (p = 0.152, η²p = 0.163), best sprint time (p = 0.753 η²p = 0.008); highest speed reached (p = 0.859, η²p = 0.003; Figure 1).
Blood lactate following the pre Yo-Yo IRT 20 m sprints was not different between SAL and PLA (p = 0.450, η²p = 0.021). The post Yo-Yo IRT 30 m sprint lactate values were not different between SAL and PLA conditions (p = 0.882, η²p = 0.007; Figure 2).
Figure 2.
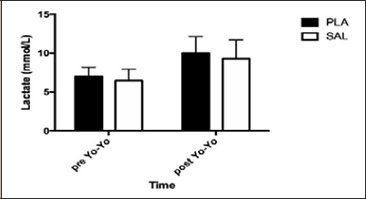
Lactate values collected after sprint pre Yo-Yo IRT and after sprint post Yo-Yo IRT. Data are presented by Mean and SD.
HR was not different between PLA and SAL (p = 0.123) during the sprint pre and post Yo-Yo IRT.
Yo-Yo Intermittent Recovery Test
The distance covered during the Yo-Yo IRT did not differ between PLA and SAL (p = 0.153, η²p = 0.224; Figure 3). No significant changes were observed between PLA and SAL during the Yo-Yo IRT for HR (p = 0.102, η²p = 0.095) or RPE (p = 0.195, η²p = 0.134). HR was not different between PLA and SAL at any level of the Yo-Yo IRT (Figure 4). RPE was not different between PLA and SAL at any level of the Yo-Yo IRT (Figure 4).
Figure 3.
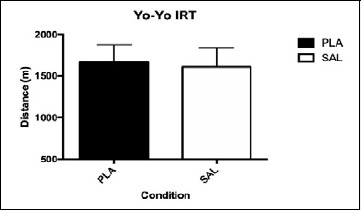
Distance covered by SAL and PLA during the Yo-Yo IRT. Data are presented by Mean and SD.
Figure 4.
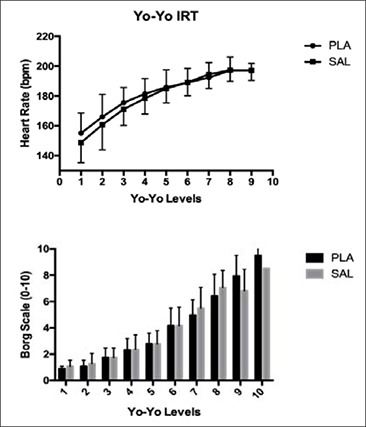
Heart rate and RPE response at the end of each Yo-Yo IRT level completed by the participants. Data are presented by Mean and SD. Level 10 in heart rate graph is missing because not all participants were able to complete it.
Mood and Motivation questionnaire
The mood questionnaire revealed a significant decrease in vigor over time in both conditions (p = 0.01) with no main effect of condition (p = 0.49, η²p =0.01) or condition × time interaction (p = 0.29, η²p =0.17; Table 2). No differences were found for intrinsic motivation (p = 0.62, η²p =0.09) and motivation to succeed the task (p = 0.78, η²p =0.02; Table 2)
Table 2.
Vigor at pre and post test and Motivation at pre test in SAL1600 and PLA conditions (Mean ± SD).
| SAL | PLA | |||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| BRUMS vigour (A.U.) | 5.4 ± .3 | 3.3 ± .5* | 5.7 ± .6 | 3.6 ± .4* |
| Motivation (A.U.) | 5.3 ± .5 | 5.1 ± .4 | ||
A.U. = arbitrary units; SAL = salbutamol; PLA = placebo.
* p < 0.01;
Discussion
Our study suggests that non-asthmatic football players who inhale 1600 μg of salbutamol do not experience improvements in 30 m sprint performance pre or post the Yo-Yo IRT test. Furthermore, Yo-Yo IRT performance, HR, blood lactate or RPE are not altered as a result of prior inhalation of 1600 μg of salbutamol.
Our results are similar to previous findings from our research group (Dickinson et al., 2015), where we did not observe any significant change in repeated sprints following a running protocol that simulated one half of a football match. The absence of ergogenic action on 30 m sprint performance found in this study could be a summation of factors including the type of drug and the dosage used in our studies compared to other studies. While acute doses of inhaled salbutamol up to 1600 μg does not seem to enhance 30 m sprint, 15 mg of inhaled terbutaline does increases 30 s cycling sprint peak and mean power by 3% in recreational athletes (Hostrup et al., 2014). High doses of inhaled terbutaline (15 mg) have also been shown to improve 10 s cycling sprint performance in moderately trained cyclists, with mean (8.3% ± 1.1%) and peak (7.8% ± 2.5%) power greater with terbutaline than with placebo (Kalsen et al., 2016). The improvements seen in other studies with regard to sprint and power performance could be due to greater potentiation of adrenergic receptors at very high dosages (Baker, 2010) and by the ability to induce a response at a given receptor induced by the drug administered. In regard to this, inhaled terbutaline may lead to same systemic concentration of doses administered orally with less side effects and similar plasma and urine half-life (Elers et al., 2012b).
Blood lactate is an indirect marker of muscle glycolysis; in our study we did not find a difference in blood lactate between PLA and SAL conditions. It has previously been reported that an 800μg dose of inhaled salbutamol can lead to detectable systemic concentrations of 10.95 ng/mL in healthy subjects (Elers et al. 2012a; 2012b). In our study we were not able to directly measure the concentration of salbutamol in the blood or in urine. We can therefore not assume that a dose of 1600 μg inhaled salbutamol, 15 minutes prior to the initial 30 m sprints, was a sufficient to result in significant systemic circulation. Future studies may benefit from indirectly monitoring systemic circulation of salbutamol by analysing ATP and phosphocreatine (PCr) to better understand the action of short acting β2-agonists on skeletal muscle cell beta-2 receptors. Previously, short acting β2-agonists has been shown to improve rates of glycolysis and glycogenolysis in the sarcoplasmic reticulum, improving 10 s sprint cycling performance (Kalsen et al., 2016). Low levels of circulating salbutamol may also explain why we had no reports of significant side effects from any of our participants.
Another way we could have estimated systemic circulation of salbutamol would be to measure urine concentration. In addition, performing this analyses would have also allowed us to investigate the potential for football players, who inhale double the permitted salbutamol, to be at risk of providing a urine sample during a doping test that exceeds the WADA 1000 ng·ml-1 threshold for an adverse analytical finding (WADA, 2019). Previous findings (Dickinson et al., 2014; 2015) suggest the acute inhalation of supra-maximal doses of salbutamol (up to 1600 μg) along-side acute loss of body mass of up to 5% can lead to individuals presenting with a urinary salbutamol concentration above 1200 ng·ml-1. Haase et al. (2016) confirmed that dehydration can significantly affect the concentration of salbutamol in urine samples. Based upon Dickinson et al, (2015) and Haase et al. (2016) results, WADA now has adjusted its methods to analyse the concentration of salbutamol with dehydration state of the athletes (WADA, 2019).
In our study, we did not observe any improvement in Yo-Yo IRT performance. This finding is similar to that of previous investigations that have demonstrated inhaled salbutamol does not improve endurance performance or VO2 peak (Elers et al., 2012). The available data from previous investigations has been analysed in a meta-analysis (Pluim et al., 2011), in which the authors concluded that endurance performance was not influenced by supra-therapeutic doses of inhaled salbutamol (up to 800 μg). Furthermore, a study by Elers et al. (2012) reported that inhaling acute supra-therapeutic doses (8mg) of salbutamol did not improve peak VO2 or oxygen kinetics.
The endurance component of football may be compromised if players inhale large doses of β2-agonists on a daily basis. When trained male participants inhaled terbutaline (4 mg) and completed high intensity interval training, over a four week period, they found that exercise performance and oxidative capacity improvements were attenuated when compared against a placebo group (Hostrup et al., 2018). Therefore, footballers frequently using high doses of inhaled β2-agonists may find the expected enhancements in endurance performance from their high intensity interval training are blunted.
In our study, we advised participants how to use the inhaler and observed them using the inhaler but we did not train them. Future studies may also benefit from thoroughly training inhaler technique with their participants to improve the delivery of salbutamol into the lower airways.
Conclusion
This study demonstrates inhalation of 1600 μg salbutamol does not improve 30 m sprint performance in trained amateur football players. Furthermore, our findings suggest that acute inhalation of 1600 μg salbutamol will not lead to improvements in Yo-Yo IRT performance in football players. Although 1600 μg of inhaled salbutamol is double the current permitted dose (800 μg) by WADA regulations (WADA, 2019), our study suggests footballers using inhaled salbutamol for asthma therapy within the doses permitted WADA regulations will not experience an ergogenic action in either their sprint or endurance performance.
Acknowledgements
The authors thank the staff and participants of Arconatese Football Club (Milano, IT) for their participation in this project. The authors also thank Mr Matteo Ferrari for his help with the technical support of the equipment and for his help with data collection. The experiments comply with the current laws of the country in which they were performed. The authors have no conflict of interest to declare.
Biographies

Michele MERLINI
Employment
Phd Student, School of Sport and Exercise Sciences, University of Kent, Chatham, UK
Degree
MSc
Research interests
Cognitive and doping aspects applied to the performance in various sports.
E-mail: michele.merlini1986@gmail.com

Marco BEATO
Employment
Senior Lecturer, Course Leader Strength & Conditioning, Research Lead Sport Science & Coaching, Department of Science and Technology, University of Suffolk, Ipswich, UK
Degree
PhD
Research interests
Sport science, strength and conditioning training, applied physiology, and performance analysis.
E-mail: M.Beato@uos.ac.uk

Samuele MARCORA
Employment
Professor and Director of Research, School of Sport and Exercise Sciences, University of Kent, Chatham, UK
Degree
PhD
Research interests
To find new ways to improve performance of endurance athletes, and reduce physical and mental fatigue in a variety of populations.
E-mail: S.M.Marcora@kent.ac.uk

John DICKINSON
Employment
Head of the Exercise Respiratory Clinic Reader, Director of Impact and Engagement, School of Sport and Exercise Sciences, University of Kent, Chatham, UK
Degree
PhD
Research interests
Respiratory problems in athletes.
E-mail: J.W.Dickinson@kent.ac.uk
References
- Baker JG. (2010) The selectivity of beta-adrenoceptor agonists at human beta1-, beta2- and beta3-adrenoceptors. British Journal of Pharmacology 160,1048-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizzini M., Dvorak J. (2015) FIFA 11+: An Effective Programme to Prevent Football Injuries in Various Player Groups Worldwide-a Narrative Review. British Journal of Sports Medicine 49, 577–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg G.A. (1982) Psychophysical Bases of Perceived Exertion. Medicine and Science in Sports and Exercise 14, 377–381. [PubMed] [Google Scholar]
- Dickinson J., Hu J., Chester N., Loosemore M., Whyte G. (2015) The Impact of Inhaled Salbutamol on Repeated Sprint Ability in Pre- Fatigued Soccer Players. Journal of Sports Medicine Doping Studies 5, 112-119. [Google Scholar]
- Dickinson J., Hu J., Chester N., Loosemore M., Whyte G. (2014) Impact of ethnicity, gender, and dehydration on the urinary excretion of inhaled salbutamol with respect to doping control. Clinical Journal of Sports Medicine 24, 482-489. [DOI] [PubMed] [Google Scholar]
- Elers J., Morkeberg J., Jansen T., Belhage B., Backer V. (2012) High-dose inhaled salbutamol has no acute effects on aerobic capacity or oxygen uptake kinetics in healthy trained men. Scandinavian Journal of Medicine and Science in Sports 22, 232-239. [DOI] [PubMed] [Google Scholar]
- Elers J., Pedersen L., Henninge J., Hemmersbach P., Dalhoff K., Backer V., (2012a) The pharmacokinetic profile of inhaled and oral salbutamol in elite athletes with asthma and nonasthmatic subjects. Clinical Journal of Sports Medicine 22, 140-145. [DOI] [PubMed] [Google Scholar]
- Elers J., Hostrup M., Pedersen L., Henninge J., Hemmersbach P., Dalhoff K., Backer V. (2012b) Urine and serum concentrations of inhaled and oral terbutaline. International Journal of Sports Medicine 33, 1026-1033. [DOI] [PubMed] [Google Scholar]
- Haase CB., Backer V., Kalsen A., Rzeppa S., Hemmersbach P., Hostrup M. (2016) The influence of exercise and dehydration on the urine concentrations of salbutamol after inhaled administration of 1600 μg salbutamol as a single dose in relation to doping analysis. Drug Test Anal. 8, 613-620. [DOI] [PubMed] [Google Scholar]
- Kalsen A., Hostrup M., Bangsbo J., Backer V. (2013). Combined Inhalation of beta2-agonists Improves Swim Ergometer Sprint Performance but Not High-intensity Swim Performance. Scandinavian Journal of Medicine and Science in Sports 24, 814–22. [DOI] [PubMed] [Google Scholar]
- Kalsen A., Hostrup M., Söderlund K., Karlsson S., Backer V., Bangsbo J. (2016) Inhaled Beta2-Agonist Increases Power Output and Glycolysis during Sprinting in Men. Medicine and Science in Sports and Exercise 48, 39–48. [DOI] [PubMed] [Google Scholar]
- Hostrup Morten, Anders Kalsen, Jens Bangsbo, Peter Hemmersbach, Sebastian Karlsson, Vibeke Backer. (2014). “High-Dose Inhaled Terbutaline Increases Muscle Strength and Enhances Maximal Sprint Performance in Trained Men.” European Journal of Applied Physiology 114, 2499–2508. [DOI] [PubMed] [Google Scholar]
- Hostrup M., Onslev J., Jacobson G., Wilson R., Bangsbo J. (2018) Chronic β2 -adrenoceptor agonist treatment alters muscle proteome and functional adaptations induced by high intensity training in young men. Journal Physiology 15,231-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostrup M., Kalsen A., Auchenberg M., Bangsbo J., Backer V. (2016) Effects of acute and 2-week administration of oral salbutamol on exercise performance and muscle strength in athletes. Scandinavian Journal of Medicine and Science in Sports 26, 8-16. [DOI] [PubMed] [Google Scholar]
- Krustrup P., Magni M., Amstrup T., Rysgaard T., Johansen J., Steensberg A., Pedersen P., Bangsbo J. (2003) The Yo-Yo Intermittent Recovery Test: Physiological Response, Reliability, and Validity. Medicine and Science in Sports and Exercise 35, 697–705. [DOI] [PubMed] [Google Scholar]
- Matthews G., Campbell S., Falconer S. (2001) Assessment of Motivational States in Performance Environments. Proceedings of the Human Factors and Ergonomics Society Annual Meeting 45, 906–910. [Google Scholar]
- Pluim B., Babette M., Olivier de Hon J., Bart Staal, Limpens J., Kuipers A., Shelley E. Overbeek A., Zwinderman Scholten R. (2011) β2-Agonists and Physical Performance. Sports Medicine 41, 39–57. [DOI] [PubMed] [Google Scholar]
- Signorile JF., Kaplan T., Applegate B., Perry AC. (1992) Effects of Acute Inhalation of the Bronchodilator, Albuterol, on Power Output. Medicine and Science in Sports and Exercise 24, 638–642. [PubMed] [Google Scholar]
- Taskin H. (2008) Evaluating Sprinting Ability, Density of Acceleration, and Speed Dribbling Ability of Professional Soccer Players with Respect to Their Positions. Journal of Strength and Conditioning Research 22, 1481–1486. [DOI] [PubMed] [Google Scholar]
- Terry P. C, Lane A.C., Fogarty JC. (2003) Construct Validity of the Profile of Mood States — Adolescents for Use with Adults. Psychology of Sport and Exercise 4, 125–139. [Google Scholar]
- W.A.D.A. (2019) Prohibited List. World Anti-Doping Agency. Available from URL: https://www.wadaama.org/sites/default/files/prohibited_list_2019_en.pdf. Accessed January 1, 2019.


