Abstract
This study aimed to assess the release of cardiac damage biomarkers jointly with cardiac autonomic modulation after a mountain ultramarathon. Such knowledge and the possible relationship of these markers with race time is of primary interest to establish possible recommendations upon athletes’ recovery and return to training following these competitions. Forty six athletes enrolled in the Penyagolosa Trails CSP115 race (118 km and a total positive elevation of 5439 m) took part in the study. N-terminal pro-brain natriuretic peptide (NT-proBNP) and high-sensitive cardiac troponin T (hs-TNT) concentrations as well as linear and nonlinear heart rate variability (HRV) were evaluated before and after the race. NT-proBNP and hs-TNT significantly increased post-race; fifty percent of the finishers surpassed the Upper Reference Limit (URL) for hs-TNT while 87% exceeded the URL for NT-proBNP. Overall and vagally-mediated HRV were diminished and cardiac autonomic modulation became less complex and more predictable following the race. More pronounced vagal modulation decreases were associated with higher levels of postexertional NT-proBNP. Moreover, rise in hs-TNT and NT-proBNP was greater among faster runners, while pre-race overall and vagally-mediated HRV were correlated with finishing time. Participation in a 118-km ultratrail induces an acute release of cardiac damage biomarkers and a large alteration of cardiac autonomic modulation. Furthermore, faster runners were those who exhibited a greater rise in those cardiac damage biomarkers. In light of these findings, an appropriate recovery period after ultraendurance races appears prudent and particularly important among better performing athletes. At the same time, HRV analysis is shown as a promising tool to assess athletes’ readiness to perform at their maximum level in an ultraendurance race.
Key points.
Faster runners were those who exhibited a greater rise in cardiac damage biomarkers (hs-TNT and NT-proBNP).
An appropriate recovery period after ultraendurance races appears prudent and particularly important among better performing athletes.
HRV analysis is shown as a promising tool to assess athletes’ readiness to perform at their maximum level in an ultraendurance race.
Key words: Heart rate variability, heart rate complexity, cardiac damage, ultratrail, performance, recovery
Introduction
The beneficial effects of moderate and regular exercise on overall health are irrefutable (Warburton et al., 2006); however, the acute and chronic consequences of prolonged and exhaustive endurance exercise remain unclear, especially on the heart (Abela and Sammut, 2017; Sharma et al., 2015). Indeed, numerous studies have documented elevations in biomarkers consistent with cardiac damage (i.e., cardiac troponins [cTn]) in apparently healthy individuals following marathon and ultra-marathon races. A meta-analysis which included 1120 endurance athletes from 26 studies employing second and third-generation cTn assays, reported an incidence rate (i.e. cTn concentration above the Upper Reference Limit, defined as the 99th percentile of a healthy population, URL) of 47% (Shave et al., 2007). Moreover, several studies have observed concomitant transient abnormalities in both ventricular (Passaglia et al., 2013; Vitiello et al., 2013) and atrial function (Wilhelm et al., 2014). This fact, together with the drastic rise in the popularity of ultra-distance mountain races over the last few years (Hoffman et al., 2010), has heightened concern among runners, scientists and clinicians alike. Hence, considering this scenario, it seems relevant to undertake more studies assessing the cardiac impact of ultraendurance races.
Meanwhile, changes in cardiac autonomic activity have been postulated to reflect exercise-induced changes in cardiac performance and haemodynamics (Stanley et al., 2013). However, research about the impact of ultraendurance exercise on the autonomic control of the heart rate (HR) is still limited (Foulds et al., 2014; Gratze et al., 2005; Hautala et al., 2001; Scott et al., 2009), and only Scott et al. (2009) assessed HR variability (HRV) jointly with cardiac damage biomarkers release (i.e., N-terminal pro-brain natriuretic peptide, NT-proBNP; cardiac troponin T, cTnT). Interestingly, in this latter study, although no significant changes were noted in HRV (with the exception of mean HR) following the 160-km Western States Endurance Run (WSER), authors found a significant positive correlation between delta changes in high frequency modulation of HRV measured in normalized units (HFnu) and NT-proBNP.
Furthermore, none of the previous studies assessing the impact of ultraendurance exercise upon cardiac autonomic modulation have employed nonlinear indices. Several authors have argued that the evaluation of nonlinear oscillations in the HR signal (i.e. complexity and fractal properties) offers an additional qualitative insight into cardiac autonomic modulation, characterized by less dependency on HR and lower inter-individual and intra-individual variation (Nicolini et al., 2012). Indeed, changes in complexity and fractal properties of HR dynamics have been previously reported in the absence of changes in HRV measures, suggesting that nonlinear methodologies not only compliment the analysis of HRV but it may also be more sensitive than the latter (Millar et al., 2009a).
Similarly to the inclusion of chaos theory and fractal mathematics in the assessment of HR dynamics, in the last years the laboratory technical improvements have led to the current availability of high-sensitivity (hs) assays to assess cardiac troponins. These assays are characterized by lower limits of detection and improved reliability compared to second and third-generation cTn assays (Giannitsis et al., 2009). However, given to their recent introduction in the field, only a small number of studies have measured hs-TNT following strenuous exercise (Vilela et al., 2014). NT-proBNP and hs-TNT are integral to the diagnosis of acute coronary syndromes (ACS) and congestive heart failure (CHF); however, the significance of elevations in these biomarkers in athletes in the absence of ACS or CHF remains uncertain (Donnellan and Phelan, 2018). Therefore, the assessment of NT-proBNP and hs-TNT jointly with HR dynamics following an ultraendurance mountain race could contribute to a better understanding of cardiac consequences of such extreme endurance events and thus enable establishing possible recommendations upon athletes’ recovery and return to training following these competitions. Our study hypothesis was that participants would sustain a significant release of cardiac-specific biomarkers jointly with a cardiac autonomic impairment. We also hypothesized that pre-race HR dynamics would be associated with performance.
Methods
Participants
Forty six male recreational ultra-endurance athletes were recruited to participate in the study. Selected athletes were required to have previously completed at least one ultra-marathon (>60 km). This research was developed at the Penyagolosa Trails CSP115 race in 2014 (May 17th - 18th), which hosted the 1st edition of the Spanish Ultra-Endurance Championship. The track consisted of 118 km, starting at an altitude of 40 m and finishing at 1280 m above the sea level, with a total positive and negative elevation of 5439 and 4227 m respectively. All subjects were fully informed of the procedure and gave their written consent to participate. They were also allowed to withdraw from the study at will. A questionnaire was used to collect demographic information as well as training and competition history. The main characteristics of the sample are presented in Table 1. The investigation was conducted according to the Declaration of Helsinki and approval for the project was obtained from the research Ethics Committee of the University Jaume I of Castellon.
Table 1.
Final sample (n=46) main characteristics (mean±SD).
| Age (years) | 42 ± 7.49 |
| Weight (kg) | 74.06 ± 7.73 |
| Height (m) | 1.74 ± 0.05 |
| BMI (kg/m2) | 24.56 ± 1.94 |
| Years since 1st Ultra-marathon (>60-km) | 4.63 ± 3.97 |
| Ultramarathons (>60-km) races before event | % |
| 1 | 37.2 |
| 2 | 16.3 |
| 3 | 9.3 |
| 4 | 14 |
| 5 | 4.7 |
| > 5 | 18.6 |
| Average weekly sessions | 4.37 ± 1.45 |
| Average weekly training volume (hours) | % |
| < 12 | 48.8 |
| 12-15 | 37.2 |
| 16-20 | 9.3 |
| > 20 | 4.7 |
| Average weekly training volume (km) | % |
| < 50 | 16.3 |
| 50-75 | 46.5 |
| 75-100 | 23.3 |
| 100-150 | 9.3 |
| > 150 | 4.7 |
BMI, Body Mass Index
Assessment of cardiac autonomic modulation
HR beat-to-beat data was acquired during 10 min of supine rest in the afternoon the day before the race and following race completion, in both cases before blood collection. Participants were informed to avoid caffeine and exercise in the 12 h before pre-race testing and a large meal in the previous 4 h. Following the race, the initiation of HR recording occurred within 5 min from crossing the finish line. In that time span, participants were allowed neither to drink nor to eat. Measurements were performed using a Polar RS800 HR monitor together with a Polar Wearlink Wind electrode transmitter (Polar Electro, Kempele, Finland), after application of conductive gel as recommended by the manufacturer. This instrument has been previously validated for the accurate measurement of RR intervals in young and middle-aged men (Wallen et al., 2012). Respiratory rate was not controlled to not interfere in athletes’ recovery, although they were asked to avoid irregular respiration. Normal respiratory rate does not result in significantly different heart rate-derived indices compared with controlled breathing (Bloomfield et al., 2001).
RR intervals were transferred to Polar Pro Trainer 5 software (Polar Electro, Kempele, Finland) and afterwards analyzed using Kubios HRV Analysis Software 2.0 (The Biomedical Signal and Medical Imaging Analysis Group, University of Kuopio, Finland). The whole analysis process was carried out by the same researcher to ensure consistency (Martinez-Navarro et al., 2018). Artifacts were identified and corrected according to manufacturer’s recommendations (Tarvainen et al., 2008), and only those recordings with <1% of artefacts were considered. The following indices were obtained from the last 5-min segment of both recordings: mean HR; the standard deviation of normal RR intervals (SDNN) as a measure of overall variability and the root-mean-square difference of successive normal RR intervals (RMSSD) as a measure of vagal modulation; the short-term scaling exponent (4 to 11 beats, α1) from Detrended Fluctuation Analysis to estimate sympathovagal balance and fractal correlation properties; and Sample Entropy (SampEn) to provide an indication of the complexity of the time-series under these circumstances (Nicolini et al., 2012; Tulppo et al., 2005). Time domain indices were chosen instead of spectral indices because of its greater intraindividual reproducibility (Al Haddad et al., 2011).
Assessment of cardiac damage biomarkers
Blood samples were collected from an antecubital vein by venipuncture at baseline (in the afternoon the day before the race) and after completion of the ultra-endurance competition (following the HR recording) using BD Vacutainer PST II tubes; then they were centrifuged at 3500 rpm for ten minutes and kept at 4°C during transport to NISA Rey Don Jaime Hospital for analysis of the biochemical variables. Hs-TNT was measured quantitatively with the new high-sensitive enzyme immunoassay based on electrochemiluminescence technology (ECLIA), using a Cobas e411 analyzer (Roche Diagnostics, Penzberg, Germany). Detailed descriptions of this assay have been previously published (Giannitsis et al., 2010). The Limit of the Blank (LoB) of this assay is 3 ng/L and the URL, defined as the 99th percentile of a healthy population, 14 ng/L. The approximate hs-TnT equivalent to the upper limit of 30 ng/L for the 4th generation cTnT assay is 50 ng/L (Giannitsis et al., 2010). NT-proBNP was assessed with an Elecsys proBNP ECLIA using a Cobas 6000 e601 analyzer (Roche Diagnostics, Basel, Switzerland). The URL for NT-proBNP was considered to be 125 ng/l (McMurray et al., 2012).
Statistical analysis
Statistical analyses were carried out using the Statistical Package for the Social Sciences software (IBM SPSS Statistics for Windows, version 22.0, IBM Corp., Armonk, NY). After testing for normal distribution (Kolmogorov-Smirnov test, with Lilliefor’s correction), SDNN, RMSDD and NT-proBNP were logarithmically transformed to allow parametric comparisons. Notwithstanding, data are provided as raw values (i.e. not logarithmically transformed) to facilitate the interpretation of the results. HR dynamics indices and lnNT-proBNP were compared before and after the race using Student’s t-tests. Possible relationships between post-race cardiac damage biomarkers and HR dynamics indices with performance, demographic and training data were assessed using Pearson correlation analyses. The same statistical procedure was applied to analyze possible associations between and pre-race and post-race HR dynamics indices and cardiac damage biomarkers. Post-race values for cardiac damage biomarkers and HR dynamics indices for each subject were related to the individual baseline level to define the delta scores (Δ):
| Δ(fold increase) = (post-race value – pre-race value)/pre-race value |
Due to the nature of hs-TNT data, pre-post changes were assessed on a case-by-case basis and values below the LoB (<3 ng/l) were set to 3 ng/l to calculate delta percentages. The meaningfulness of the outcomes was estimated through the effect size (ES, means divided by the standard deviation): an ES<0.5 was considered small; between 0.5-0.8, moderate; and greater than 0.8, large (Thomas et al., 2005). Likewise, correlations >0.5 were considered large, 0.3-0.5, moderate and <0.3, small. The significance level was set at p < 0.05 and data are presented as means and standard deviations (±SD).
Results
Thirty athletes successfully completed the race with an average finish time of 21 h 59 min ± 4 h 1 min. The finishers/starters ratio for the subjects of the present study (i.e. 65.2%) was similar to the ratio when all male race participants were considered (64.7%), whereas the average finish time was somewhat faster when all male race participants were considered (21 h 21 min ± 4 h 26 min). All levels of performance were represented in our sample as shown by their rank ranging from 30th to 288th place (of 294 male finishers). Unfortunately, 2 participants were excluded from HR dynamics analyses due to an excessive number of artifacts (>1%) in their HR recordings.
Cardiac damage biomarkers
The concentration of cardiac damage biomarkers measured in post-race samples was remarkably increased as compared with values obtained on baseline specimens. At baseline, concentrations of serum hs-TNT were negative (i.e., below the LoB) in 23 subjects and none participant displayed a value above the URL. After the race, 27 athletes (90%) showed measurable values of hs-TNT; 15 of those runners (50%) displayed values exceeding the URL but only 1 participant surpassed the threshold for suspicion of myocardial injury. The post-race range of concentrations varied from <3 (3 participants) to 78.1 ng/L. Change in hs-TNT was largely and inversely associated with finishing time (r= -0.51; p < 0.01; see Figure 1) and a moderate inverse relationship was also found between changes in hs-TNT and SampEn (r = -0.38; p < 0.05). The exclusion of the only participant which exceeded the 50 ng/L threshold increased the magnitude of the associations between Δ hs-TNT and both finishing time (r = -0.67; p < 0.01) and Δ SampEn (r = -0.49; p < 0.01).
Figure 1.
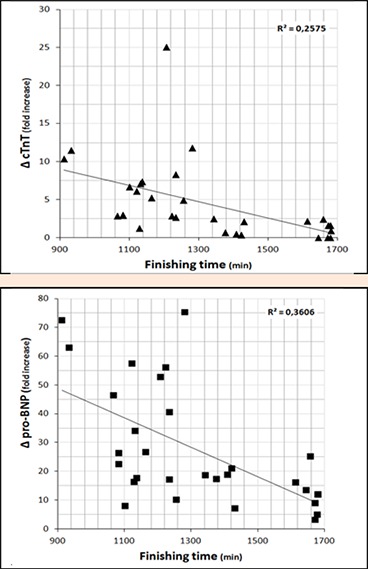
Relationship between finishing time (x-axis) and cardiac biomarkers release (y-axis). Linear regression (R2) is included.
At baseline, mean NT-proBNP was 20.90 ± 15.84 ng/L. After the CSP115 race, NT-proBNP levels were significantly and largely higher (443 ± 280.61 ng/L, p < 0.01, ES = 2.16), with a post-race range of NT-proBNP concentrations from 37.3 to 1109 ng/L. Pre-race, none athlete had NT-proBNP concentrations which exceeded the URL for healthy subjects, while 26 participants (86.7%) had post-race levels above this clinical threshold. Change in NT-proBNP was largely and inversely correlated with finishing time (r = -0.60; p < 0.01; see Figure 1). A moderate inverse relationship was also found between changes in NT-proBNP and lnRMSSD (r = -0.41; p < 0.05). On the contrary, baseline NT-proBNP was unrelated to Δ NT-proBNP or performance, and change in cardiac damage biomarkers (i.e., Δ hs-TNT nor Δ NT-proBNP) was neither correlated to age or training-related variables (i.e. years since 1st ultra-marathon and average training weekly sessions); although average training weekly sessions showed a tendency towards significance with Δ NT-proBNP (r = 0.32; p = 0.09). Besides, Δ NT-proBNP and Δ hs-TNT were positive and largely correlated (r = 0.65; p < 0.01).
Cardiac autonomic modulation
HR and both linear and nonlinear HR dynamics indices significantly changed from pre-race to post-race. HR was significantly and largely increased (58.81 ± 7.89 vs 76.31 ± 9.63 bpm, p < 0.01, ES=2.02) whereas overall HRV (i.e. lnSDNN) and vagally-mediated HRV (i.e. lnRMSSD) were significantly and largely decreased post-race (44.51 ± 19.11 vs 25.83 ± 14.68 ms, p < 0.01, ES = -1.20; 43.58 ± 23.92 vs 19.92 ± 14.16 ms, p < 0.01, ES = -1.39). There was also a significant and large increase of α1 (1.12 ± 0.25 vs 1.43 ± 0.24, p < 0.01, ES = 1.32) concomitantly with a significant and large reduction of SampEn (1.71 ± 0.27 vs 1.48 ± 0.28, p < 0.01, ES = -0.85). Pre-race lnSDNN and lnRMSSD were moderately and inversely correlated with finishing time (r = -0.46 and r= -0.40; p < 0.05; see Figure 2). Besides, a moderate relationship was found between finishing time and change in HR and SampEn (r = -0.43 and r = 0.44; p < 0.05). Finally, all linear and nonlinear HR dynamics indices at baseline were inversely correlated with their respective pre-post change (r value range = -0.82 to -0.39; p < 0.05).
Figure 2.
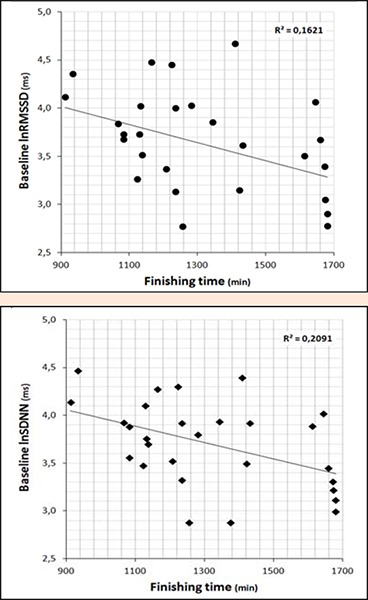
Relationship between finishing time (x-axis) and baseline HRV (y-axis). Linear regression (R2) is included.
Discussion
The results of the present study demonstrate that ultra-distance mountain races, such as Penyagolosa Trails CSP115, induce a great increase in cardiac damage biomarkers together with a large alteration of cardiac autonomic modulation.
Cardiac damage biomarkers
A large significant increase was recorded in NT-proBNP (27.32 ± 20.77-fold rise; 20.90 ± 15.84 vs. 443 ± 280.61 ng/L) and more than 86% of the participants displayed post-race levels of NT-proBNP above the URL. Previous studies involving ultraendurance events found similar results to ours (Ohba et al., 2001; Scott et al., 2009; Stelzer et al., 2015); whereas following shorter races (i.e., road half-marathon and marathon) there has been described a smaller release of NT-proBNP (Shave et al., 2007). Altogether, our results endorse previous suggestion that rise in NT-proBNP as a consequence of prolonged strenuous exercise seems to be related to the duration of the race (Scharhag et al., 2005; Serrano-Ostariz et al., 2011; Shave et al., 2007).
Fifty percent of the participants in our study surpassed the URL for hs-TNT following the race. As noted in the introduction, only a handful of studies have assessed high-sensitivity cardiac troponins following strenuous exercise. During the 216-km Badwater Ultra-marathon 4 out of 10 runners displayed hs-TNT concentrations above the URL (Giannitsis et al., 2009); whereas the prevalence of post-road marathon values exceeding the URL ranged between 86 and 94% (Vilela et al., 2014). Our data thus reinforce previous assumption that the proportion of troponin-positive participants in ultraendurance events is lower than that reported in shorter distances (i.e. road marathons), probably due to the lower exercise intensities in these competitions (Shave et al., 2007). Actually, albeit measured with the less accurate 2nd and 3rd generation cTn assays, following the 161-km WSER only 20% of the athletes exceeded the URL (Scott et al., 2009) and barely two runners did it (16.7%) after completing a 24-h ultra-marathon (Passaglia et al., 2013).
Furthermore, the observation that cTn is significantly risen following common recreational physical activities such as a 1-h kettlebell or a spinning class (Duttaroy et al., 2012; Savukoski et al., 2015) suggest that troponin elevations are common following short but vigorous exercise and not necessarily a product of a prolonged and exhaustive effort. Indeed, although some abnormalities in ventricular and atrial function have been observed in subjects acutely after endurance exercise (Passaglia et al., 2013; Vitiello et al., 2013; Wilhelm et al., 2014), both follow-up studies (Scharhag et al., 2006) and cardiac magnetic resonance imaging examinations (O’Hanlon et al., 2010) indicate that there is no evidence that these acute effects may translate into an irreversible cardiac damage. Besides, the fact that normalization of cTn concentrations has been demonstrated to occur within 24h after exercise reinforces the physiological, rather than pathological, meaning of exercise-derived cTn release (Shave et al., 2010).
Nevertheless, the association found between finishing time and change in both NT-proBNP and hs-TNT implies that faster runners released greater amounts of cardiac damage biomarkers during the race. The same relationship between finishing time and cardiac troponin I has been reported following the 166-km Ultra Trail du Mont Blanc (Vitiello et al., 2013) and the 161-km Leadville (Khodaee et al., 2015). Besides, exercise intensity has been demonstrated to be an important factor to trigger the release of cardiac troponins (Serrano-Ostariz et al., 2011; Shave et al., 2007). Therefore, it may be reasonable to think that faster runners sustain a greater cardiac damage during an ultraendurance event. However, exercise-induced NT-proBNP production has been suggested to have cytoprotective and growth-regulating effects (Giannitsis et al., 2009; Scharhag et al., 2005); similarly, the release of cTn during exercise has been attributed in the last years to physiologic adaptive and reversible damage rather than pathological irreversible damage (Donnellan and Phelan, 2018; Giannitsis et al., 2009; Shave et al., 2010); hence, we speculate that greater release of cardiac damage biomarkers in faster runners is reflective of the capacity to stress their cardiovascular system to a greater extent during exercise. Moreover, very recently Legaz-Arrese et al. (2015) have reported that a 14-week endurance training intervention resulted in better performance and higher values of hs-TNT following a 60-min running time trial. These authors suggested that post-intervention higher values of hs-TNT could reflect a physiological heart adaptation to exercise training stimulus, similar to what happens with heart size, and thus should not be interpreted as a risk or hazardous consequence of achieving a greater training status.
The large and positive relationship found between Δ NT-proBNP and Δ hs-TNT adds more controversy to an already debated topic. Following a timeline, Ohba et al. (2001) formerly showed a significant correlation between NT-proBNP and cTnT after running a 100-km ultra-marathon and suggested that this relationship implied that exercise-induced release of cTnT was mainly attributable to myocardial cell necrosis. On the contrary, most of the subsequent reports employing more specific cTnT assays (i.e., 3rd generation) did not find such a relationship (Scharhag et al., 2005). However, other studies assessing hs-TNT have shown both correlated (Giannitsis et al., 2009) and uncorrelated outcomes (Scherr et al., 2011). Therefore, it remains an open question whether there exists a relationship or not between the exercise-induced release of those two biomarkers. In either case, that association would not involve evidence of myocardial cell necrosis as formerly suggested by Ohba et al. (2001), but rather an interrelated physiologic response to cardiac stress imposed by strenuous exercise, accordingly with the hypothesis stated in the preceding paragraph.
Cardiac autonomic modulation
As far as we are concerned, this is the first study to assess nonlinear HR dynamics following an ultraendurance event. Our results showed that cardiac autonomic modulation became less complex and more predictable following the race (i.e., lower SampEn and higher α1). Previous investigations assessing the immediate recovery from short-lasting high-intensity workouts have achieved similar results (Blasco-Lafarga et al., 2013; Goulopoulou et al., 2009; Millar et al., 2009b), although in the study from Blasco-Lafarga et al. (2013) SampEn showed no change following a supramaximal Judo test. Interestingly, the magnitude of the change in SampEn was the smallest among linear and nonlinear indices in our research; thus it may be argued that complexity properties of HR behaviour are more resilient (i.e., compared to vagal and sympathetic drive indices) to exercise stress. In either case, the extreme duration of an ultradistance mountain race (21 h 59 min ± 4 h 4 min) appears to induce a similar immediate alteration in HR dynamics as a short-lasting but high-intensity workout. Further studies should delve into the recovery timeline of nonlinear HR dynamics following an ultraendurance event.
At the same time, our significant and large reduction in both overall and vagally mediated HRV coincides with previous studies involving mountain marathon races (Bernardi et al., 1997; Murrell et al., 2007) and ultraendurance events (Foulds et al., 2014; Gratze et al., 2005; Hautala et al., 2001). Only one study has reported non-statistical changes in HRV following an ultraendurance event (160-km WSER; Scott et al., 2009). Moreover, our increase in α1, which is considered also a sympathovagal balance index (Tulppo et al., 2005), is consistent with post-race sympathetic predominance observed in previous studies using spectral domain indices (Bernardi et al., 1997; Foulds et al., 2014; Gratze et al., 2005; Murrell et al., 2007).
In addition, cardiac damage and reduction in vagal modulation seems to be interrelated, so that those athletes who showed the greatest post-race reductions in parasympathetic modulation (i.e., lnRMSSD) also displayed the greatest increases in NT-proBNP. A similar pattern (a larger HRV reduction in runners with higher post-race hs-TNT) has been described following a 30-km cross-country running race (Aagaard et al., 2014). However, it opposes to the results of Scott et al. (2009), who found a positive association between changes in HFnu and NT-proBNP following a 160-km ultratrail. The reason for the discrepancy with this latter study may lie in the lower intraindividual reproducibility of spectral domain indices and lower reliability of relative indices (i.e., expressed in normalized units) in situations of low total spectral power (i.e., post-exercise) (Al Haddad et al., 2011).
Our outcomes also showed a significant relationship between pre-race cardiac autonomic modulation, post-race HRV delta scores and performance; namely, those athletes with higher baseline overall and vagally-mediated HRV showed larger changes in those variables, and moreover, achieved a greater performance in the race. A previous study failed to find any relationship between baseline HRV (i.e. HFnu and LFnu indices) and performance in an Ironman (Gratze et al., 2005). Nevertheless, our results are in accordance with previous studies (Blasco-Lafarga et al., 2013; Buchheit and Gindre, 2006; Cataldo et al., 2018) and the “autonomic resource hypothesis” proposed by Hynynen et al. (2008), which suggests that a better baseline profile of cardiac autonomic modulation allows further usage of autonomic resources during self-paced exercise and contribute to a better performance. Further studies should elucidate whether a better baseline profile of cardiac autonomic modulation is also related to a faster intermediate and long-term recovery (i.e., from 1h after the race onwards) following an ultraendurance event.
Conclusion
Participation in a 118-km ultratrail induces an acute release of cardiac damage biomarkers together with a large alteration of both linear and nonlinear indices of cardiac autonomic modulation. Furthermore, the magnitude of cardiac damage biomarkers increase and cardiac autonomic modulation disturbance appear to be interrelated and greater among faster runners. This leads us to suggest that faster runners are able to stress their cardiovascular system to a greater extent during self-paced prolonged endurance exercises. In light of these findings, an appropriate recovery period after ultraendurance races seems prudent and particularly important among better performing athletes.
Finally, our results show that those athletes with higher baseline overall and vagally-mediated HRV displayed larger pre-post changes in those variables and achieve faster finishing times in the race; therefore, HRV analysis is proposed as a coach’s tool to assess athletes’ readiness to perform at their maximum level in an ultraendurance race.
Limitations
We recognise that a single assessment immediately post-exercise might not reflect peak concentrations of cardiac damage biomarkers and does not allow any inference on the recovery timeline of cardiovascular alterations found (i.e. cardiac autonomic impairment and cardiac damage) (Legaz-Arrese et al., 2015; Stanley et al., 2013); however, obtaining further sampling (i.e. blood draws and HR recordings) was extremely difficult and even unethical due to the location of the finish line (Penyagolosa Trails CSP115 is a non-circular race and start and finish line are 80-km apart).
Acknowledgements
Current research could be carried out thanks to the collaboration of Vithas-Nisa Hospitals group and “Cátedra Endavant Villarreal CF de l’Esport”. Authors are also grateful to Penyagolosa Trails organization, athletes who participate in the study and volunteers. Experiments performed during this research comply with the current laws of Spain. The authors have no conflicts of interests to declare.
Biographies
Ignacio MARTÍNEZ-NAVARRO
Employment
Exercise Physiologist at Sports Health Unit of the Vithas-Nisa 9 Octubre Hospital and Professor of the Physical Education and Sports Department at the University of Valencia.
Degree
PhD
Research interests
Clinical aspects of exercise, sports physiology and performance, especially in long-distance runners.
E-mail: ignacio.martinez-navarro@uv.es
Juan Miguel SÁNCHEZ
Employment
Cardiologist at General University Hospital of Castellon and Sports Health Unit of the Vithas-Nisa 9 Octubre Hospital.
Degree
MD
Research interests
Cardiological aspects of exercise, especially in long-distance runners.
E-mail: jumisag1@hotmail.com
Eladio J. COLLADO-BOIRA
Employment
Professor and Vice dean of the Faculty of Health Sciences at the Jaume I University of Castellon.
Degree
PhD
Research interests
Clinical aspects of exercise, sports physiology and performance, especially in long-distance runners.
E-mail: colladoe@uji.es
Barbara HERNANDO
Employment
Postdoctoral researcher at Medicine Department of the Jaume I University of Castellon.
Degree
PhD
Research interests
The genetics of skin sensitivity to sunlight and skin cancer susceptibility. Analyzing specific biomarkers in ultradistance runners.
E-mail: hernandb@uji.es
Nayara PANIZO
Employment
Nephrologist at Clinical Universitary Hospital of Valencia.
Degree
MD, PhD
Research interests
Clinical aspects of exercise, especially in long-distance runners; clinical nephrology and hemodialysis.
E-mail: nayapanizo@gmail.com
Carlos HERNANDO
Employment
Head of Sports Service and Professor of the Education and Specific Didactics Department at the Jaume I University of Castellon.
Degree
PhD
Research interests
Clinical aspects of exercise, sports physiology and performance, especially in long-distance runners.
E-mail: hernando@uji.es
References
- Aagaard P., Sahlen A., Bergfeldt L., Braunschweig F. (2014) Heart rate and its variability in response to running-associations with troponin. Medicine Science Sports Exercise 46, 1624-1630, August. [DOI] [PubMed] [Google Scholar]
- Abela M., Sammut L. (2017) Cardiac troponin: more than meets the eye. Postgraduate Medical Journal 93, 762-765, December. [DOI] [PubMed] [Google Scholar]
- Al Haddad H., Laursen P.B., Chollet D., Ahmaidi S., Buchheit M. (2011) Reliability of resting and postexercise heart rate measures. International Journal Sports Medicine 32, 598-605, August. [DOI] [PubMed] [Google Scholar]
- Bernardi L., Passino C., Robergs R., Appenzeller O. (1997) Acute and persistent effects of a 46-kilometer wilderness trail run at altitude: cardiovascular autonomic modulation and baroreflexes. Cardiovascular Research 34, 273-80, May. [DOI] [PubMed] [Google Scholar]
- Blasco-Lafarga C., Martinez-Navarro I., Mateo-March M. (2013) Is baseline cardiac autonomic modulation related to performance and physiological responses following a supramaximal Judo test? PLoS One 8, e78584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomfield D.M., Magnano A., Bigger J.T., Jr., Rivadeneira H., Parides M., Steinman R.C. (2001) Comparison of spontaneous vs. metronome-guided breathing on assessment of vagal modulation using RR variability. American Journal Physiology-Heart Circulatory Physiology 280, H1145-1150. [DOI] [PubMed] [Google Scholar]
- Buchheit M., Gindre C. (2006) Cardiac parasympathetic regulation: respective associations with cardiorespiratory fitness and training load. American Journal Physiology-Heart Circulatory Physiology 291, H451-8, July. [DOI] [PubMed] [Google Scholar]
- Cataldo A., Bianco A., Paoli A., Cerasola D., Alagna S., Messina G., Zangla D., Traina M. (2018) Resting sympatho-vagal balance is related to 10 km running performance in master endurance athletes. European Journal of Translational Myology 28, 7051, Jan 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnellan E., Phelan D. (2018) Biomarkers of Cardiac Stress and Injury in Athletes: What Do They Mean? Current Heart Failure Reports 15, 116-122, April. [DOI] [PubMed] [Google Scholar]
- Duttaroy S., Thorell D., Karlsson L., Borjesson M. (2012) A single-bout of one-hour spinning exercise increases troponin T in healthy subjects. Scandinavian Cardiovascular Journal 46, 2-6. [DOI] [PubMed] [Google Scholar]
- Foulds H.J., Cote A.T., Phillips A.A., Charlesworth S.A., Bredin S.S., Burr J.F., Drury C.T., Ngai S., Fougere R.J., Ivey A.C., Warburton D.E. (2014) Characterisation of baroreflex sensitivity of recreational ultra-endurance athletes. European Journal Sport Science 14, 686-694. [DOI] [PubMed] [Google Scholar]
- Giannitsis E., Kurz K., Hallermayer K., Jarausch J., Jaffe A.S., Katus H.A. (2010) Analytical validation of a high-sensitivity cardiac troponin T assay. Clinical Chemistry 56, 254-261, February. [DOI] [PubMed] [Google Scholar]
- Giannitsis E., Roth H.J., Leithauser R.M., Scherhag J., Beneke R., Katus H.A. (2009) New highly sensitivity assay used to measure cardiac troponin T concentration changes during a continuous 216-km marathon. Clinical Chemistry 55, 590-592, March. [DOI] [PubMed] [Google Scholar]
- Goulopoulou S., Fernhall B., Kanaley J.A. (2009) Hemodynamic responses and linear and non-linear dynamics of cardiovascular autonomic regulation following supramaximal exercise. Europeam Journal Applied Physiology 105, 525-531, March. [DOI] [PubMed] [Google Scholar]
- Gratze G., Rudnicki R., Urban W., Mayer H., Schlogl A., Skrabal F. (2005) Hemodynamic and autonomic changes induced by Ironman: prediction of competition time by blood pressure variability. Journal Applied Physiology (1985) 99, 1728-1735, November. [DOI] [PubMed] [Google Scholar]
- Hautala A., Tulppo M.P., Makikallio T.H., Laukkanen R., Nissila S., Huikuri H.V. (2001) Changes in cardiac autonomic regulation after prolonged maximal exercise. Clinical Physiology 21, 238-245, March. [DOI] [PubMed] [Google Scholar]
- Hoffman M.D., Ong J.C., Wang G. (2010) Historical analysis of participation in 161 km ultramarathons in North America. International Journal History Sport 27, 1877-91. [DOI] [PubMed] [Google Scholar]
- Hynynen E., Uusitalo A., Konttinen N., Rusko H. (2008) Cardiac autonomic responses to standing up and cognitive task in overtrained athletes. International Journal Sports Medicine 29, 552-558, July. [DOI] [PubMed] [Google Scholar]
- Khodaee M., Spittler J., VanBaak K., Changstrom B.G., Hill J.C. (2015) Effects of Running an Ultramarathon on Cardiac, Hematologic, and Metabolic Biomarkers. International Journal Sports Medicine 36, 867-871, Nov. [DOI] [PubMed] [Google Scholar]
- Legaz-Arrese A., Lopez-Laval I., George K., Puente-Lanzarote J.J., Mayolas-Pi C., Serrano-Ostariz E., Revilla-Marti P., Moliner-Urdiales D., Reverter-Masia J. (2015) Impact of an endurance training program on exercise-induced cardiac biomarker release. American Journal Physiology-Heart Circulatory Physiology 308, H913-20, Apr 15. [DOI] [PubMed] [Google Scholar]
- Martinez-Navarro I., Chiva-Bartoll O., Hernando B., Collado E., Porcar V., Hernando C. (2018) Hydration Status, Executive Function, and Response to Orthostatism After a 118-km Mountain Race: Are They Interrelated? Journal Strength Conditioning Research 32, 441-449, February. [DOI] [PubMed] [Google Scholar]
- McMurray J.J., Adamopoulos S., Anker S.D., Auricchio A., Bohm M., Dickstein K., Falk V., Filippatos G., Fonseca C., Gomez-Sanchez M.A., Jaarsma T., Kober L., Lip G.Y., Maggioni A.P., Parkhomenko A., Pieske B.M., Popescu B.A., Ronnevik P.K., Rutten F.H., Schwitter J., Seferovic P., Stepinska J., Trindade P.T., Voors A.A., Zannad F., Zeiher A., Bax J.J., Baumgartner H., Ceconi C., Dean V., Deaton C., Fagard R., Funck-Brentano C., Hasdai D., Hoes A., Kirchhof P., Knuuti J., Kolh P., McDonagh T., Moulin C., Reiner Z., Sechtem U., Sirnes P.A., Tendera M., Torbicki A., Vahanian A., Windecker S., Bonet L.A., Avraamides P., Ben Lamin H.A., Brignole M., Coca A., Cowburn P., Dargie H., Elliott P., Flachskampf F.A., Guida G.F., Hardman S., Iung B., Merkely B., Mueller C., Nanas J.N., Nielsen O.W., Orn S., Parissis J.T., Ponikowski P. (2012) ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. European Journal Heart Failure 14, 803-869, August. [DOI] [PubMed] [Google Scholar]
- Millar P.J., Rakobowchuk M., Adams M.M., Hicks A.L., McCartney N., MacDonald M.J. (2009a) Effects of short-term training on heart rate dynamics in individuals with spinal cord injury. Autonomic Neuroscience 150, 116-121, Oct 5. [DOI] [PubMed] [Google Scholar]
- Millar P.J., Rakobowchuk M., McCartney N., MacDonald M.J. (2009b) Heart rate variability and nonlinear analysis of heart rate dynamics following single and multiple Wingate bouts. Applied Physiology Nutrition Metabolism 34, 875-883, Oct. [DOI] [PubMed] [Google Scholar]
- Murrell C., Wilson L., Cotter J.D., Lucas S., Ogoh S., George K., Ainslie P.N. (2007) Alterations in autonomic function and cerebral hemodynamics to orthostatic challenge following a mountain marathon. Journal Applied Physiology (1985) 103, 88-96, July. [DOI] [PubMed] [Google Scholar]
- Nicolini P., Ciulla M.M., De Asmundis C., Magrini F., Brugada P. (2012) The prognostic value of heart rate variability in the elderly, changing the perspective: from sympathovagal balance to chaos theory. Pacing Clinical Electrophysiology 35, 622-638, May. [DOI] [PubMed] [Google Scholar]
- O’Hanlon R., Wilson M., Wage R., Smith G., Alpendurada F.D., Wong J., Dahl A., Oxborough D., Godfrey R., Sharma S., Roughton M., George K., Pennell D.J., Whyte G., Prasad S.K. (2010) Troponin release following endurance exercise: is inflammation the cause? a cardiovascular magnetic resonance study. Journal Cardiovascular Magnetic Resonance 12, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohba H., Takada H., Musha H., Nagashima J., Mori N., Awaya T., Omiya K., Murayama M. (2001) Effects of prolonged strenuous exercise on plasma levels of atrial natriuretic peptide and brain natriuretic peptide in healthy men. American Heart Journal 141, 751-758, May. [DOI] [PubMed] [Google Scholar]
- Passaglia D.G., Emed L.G., Barberato S.H., Guerios S.T., Moser A.I., Silva M.M., Ishie E., Guarita-Souza L.C., Costantini C.R., Faria-Neto J.R. (2013) Acute effects of prolonged physical exercise: evaluation after a twenty-four-hour ultramarathon. Arquivos Brasileiros Cardiologia 100, 21-28, January. [DOI] [PubMed] [Google Scholar]
- Savukoski T., Mehtala L., Lindahl B., Venge P., Pettersson K. (2015) Elevation of cardiac troponins measured after recreational resistance training. Clinical Biochemistry 48, 803-6, August. [DOI] [PubMed] [Google Scholar]
- Scott J.M., Esch B.T., Shave R., Warburton D.E., Gaze D., George K. (2009) Cardiovascular consequences of completing a 160-km ultramarathon. Medicine Science Sports Exercise 41, 26-34, January. [DOI] [PubMed] [Google Scholar]
- Scharhag J., Herrmann M., Urhausen A., Haschke M., Herrmann W., Kindermann W. (2005) Independent elevations of N-terminal pro-brain natriuretic peptide and cardiac troponins in endurance athletes after prolonged strenuous exercise. American Heart Journal 150, 1128-1134, December. [DOI] [PubMed] [Google Scholar]
- Scharhag J., Urhausen A., Schneider G., Herrmann M., Schumacher K., Haschke M., Krieg A., Meyer T., Herrmann W., Kindermann W. (2006) Reproducibility and clinical significance of exercise-induced increases in cardiac troponins and N-terminal pro brain natriuretic peptide in endurance athletes. European Journal Cardiovascular Prevention Rehabilitation 13, 388-397, June. [DOI] [PubMed] [Google Scholar]
- Scherr J., Braun S., Schuster T., Hartmann C., Moehlenkamp S., Wolfarth B., Pressler A., Halle M. (2011) 72-h kinetics of high-sensitive troponin T and inflammatory markers after marathon. Medicine Science Sports Exercise 43, 1819-1827, Oct. [DOI] [PubMed] [Google Scholar]
- Serrano-Ostariz E., Terreros-Blanco J.L., Legaz-Arrese A., George K., Shave R., Bocos-Terraz P., Izquierdo-Alvarez S., Bancalero J.L., Echavarri J.M., Quilez J., Aragones M.T., Carranza-Garcia L.E. (2011) The impact of exercise duration and intensity on the release of cardiac biomarkers. Scandinavian Journal Medicine Science Sports 21, 244-249, Apr. [DOI] [PubMed] [Google Scholar]
- Sharma S., Merghani A., Mont L. (2015) Exercise and the heart: the good, the bad, and the ugly. European Heart Journal 36, 1445-53, Jun 14. [DOI] [PubMed] [Google Scholar]
- Shave R., Baggish A., George K., Wood M., Scharhag J., Whyte G., Gaze D., Thompson P.D. (2010) Exercise-induced cardiac troponin elevation: evidence, mechanisms, and implications. Journal American College Cardiology 56, 169-176, Jul 13. [DOI] [PubMed] [Google Scholar]
- Shave R., George K.P., Atkinson G., Hart E., Middleton N., Whyte G., Gaze D., Collinson P.O. (2007) Exercise-induced cardiac troponin T release: a meta-analysis. Medicine Science Sports Exercise 39, 2099-2106, December. [DOI] [PubMed] [Google Scholar]
- Stanley J., Peake J.M., Buchheit M. (2013) Cardiac parasympathetic reactivation following exercise: implications for training prescription. Sports Medicine 43, 1259-1277, Dec. [DOI] [PubMed] [Google Scholar]
- Stelzer I., Kropfl J.M., Fuchs R., Pekovits K., Mangge H., Raggam R.B., Gruber H.J., Pruller F., Hofmann P., Truschnig-Wilders M., Obermayer-Pietsch B., Haushofer A.C., Kessler H.H., Machler P. (2015) Ultra-endurance exercise induces stress and inflammation and affects circulating hematopoietic progenitor cell function. Scandinavian Journal Medicine Science Sports 25, e442-450, October. [DOI] [PubMed] [Google Scholar]
- Tarvainen M., Niskanen J., Lipponen J., Ranta-aho P., Karjalainen P. (2008). Kubios HRV—A Software for Advanced Heart Rate Variability Analysis. In: 4th European Conference of the International Federation for Medical and Biological Engineering, Heidelberg, S.B., Ed, Springer, Antwerp, Belgium, pp. 1022-1025. [Google Scholar]
- Thomas J., Nelson J., Silverman S. (2005) Research Methods in Physical Activity. Champaign: Human Kinetics. [Google Scholar]
- Tulppo M.P., Kiviniemi A.M., Hautala A.J., Kallio M., Seppanen T., Makikallio T.H., Huikuri H.V. (2005) Physiological background of the loss of fractal heart rate dynamics. Circulation 112, 314-319, Jul 19. [DOI] [PubMed] [Google Scholar]
- Vilela E.M., Bastos J.C., Rodrigues R.P., Nunes J.P. (2014) High-sensitivity troponin after running--a systematic review. Netherlands Journal Medicine 72, 5-9, January. [PubMed] [Google Scholar]
- Vitiello D., Rupp T., Bussiere J.L., Robach P., Polge A., Millet G.Y., Nottin S. (2013) Myocardial damages and left and right ventricular strains after an extreme mountain ultra-long duration exercise. International Journal Cardiology 165, 391-392, May 10. [DOI] [PubMed] [Google Scholar]
- Wallen M.B., Hasson D., Theorell T., Canlon B., Osika W. (2012) Possibilities and limitations of the Polar RS800 in measuring heart rate variability at rest. European Journal Applied Physiology 112, 1153-1165, March. [DOI] [PubMed] [Google Scholar]
- Warburton D.E., Nicol C.W., Bredin S.S. (2006) Health benefits of physical activity: the evidence. Canadian Medical Association Journal 174, 801-809, Mar 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm M., Zueger T., De Marchi S., Rimoldi S.F., Brugger N., Steiner R., Stettler C., Nuoffer J.M., Seiler C., Ith M. (2014) Inflammation and atrial remodeling after a mountain marathon. Scandinavian Journal Medicine Science Sports 24, 519-525, June. [DOI] [PubMed] [Google Scholar]


