Abstract
We have previously reported on the activity of different extracts from Astronium sp. against Candida albicans, with the hydroethanolic extract prepared from leaves of A. urundeuva, an arboreal species widely distributed in arid environments of South America and often used in folk medicine, displaying the highest in vitro activity. Here we have further evaluated the antifungal activity of this extract against strains of C. albicans and C. glabrata, the two most common etiological agents of candidiasis. The extract was tested alone and loaded into a nanostructured lipid system (10% oil phase, 10% surfactant and 80% aqueous phase, 0.5% Poloxamer 407®). In vitro susceptibility assays demonstrated the antifungal activity of the free extract and the microemulsion against both Candida species, with increased activity against C. glabrata, including collection strains and clinical isolates displaying different levels of resistance against the most common clinically used antifungal drugs. Checkerboard results showed synergism when the free extract was combined with amphotericin B against C. albicans. Serial passage experiments confirmed development of resistance to fluconazole but not to the free extract upon prolonged exposure. Although preformed biofilms were intrinsically resistant to treatment with the extract, it was able to inhibit biofilm formation by C. albicans at concentrations comparable to those inhibiting planktonic growth. Cytotoxicity assays in different cell lines as well as an alternative model using Artemia salina L. confirmed a good safety profile of the both free and loaded extracts, and an in vivo assay demonstrated the efficacy of the free and loaded extracts when used topically in a rat model of vaginal candidiasis. Overall, these results reveal the promise of the A. urundeuva leaves extract to be further investigated and developed as an antifungal.
Keywords: Astronium urundeuva, Candida sp. biofilm, checkerboard, resistance, vulvovaginal candidiasis
Introduction
There are approximately 200 species in the genus Candida, several of which can inhabit several environments in the body such as the oral cavity, oropharynx, bronchial secretions, skin folds, feces, urine and vagina (Achkar and Fries, 2010; Khan et al., 2018; Qadir and Asif, 2019). Some species are limited to certain body regions, but others, including C. albicans, the most common cause of candidiasis associated with approximately 50% of cases, are widely distributed. Warmer and more humid places, such as the oral and the vaginal cavity, are considered most favorable for the development of the infection. For example, vulvovaginal candidiasis (VVC) is considered the most common infection affecting around 75% of women at least once in their lifetime, which could also present frequent episodes of recurrence (Santos Ramos et al., 2015). Among the non-albicans Candida species, C. glabrata is considered the second most frequent causative agent of candidiasis (Gabaldón and Carreté, 2016; Whaley et al., 2017). Some infections are also caused by more than one Candida species, especially oral candidiasis, which may involve both C. albicans and C. glabrata (Nakamura-Vasconcelos et al., 2017). Very often these infections, including mucosal and invasive candidiasis, are associated with biofilm formation, with biofilms serving as a protective environment against external insults (Uppuluri et al., 2009).
The current antifungal arsenal is mostly limited to amphotericin B, azoles and echinocandins (Pierce et al., 2013). The high incidence of the different forms of candidiasis in an increasing number of compromised patients and the development of resistance against these conventional antifungal agents point to the urgent need to discover and develop novel therapeutics against infections caused by Candida spp. (Ngo et al., 2016; Perlin et al., 2017). Natural products have historically represented the main starting point of compounds for therapeutic use and a majority of antibiotics have been traditionally obtained from natural sources. Several plants have shown important in vitro and in vivo antimicrobial activities, justifying even more the intense search for traditional medicine focused on antimicrobial characterization of plants. The biological activity of medicinal plants from different regions of the world has been studied by several groups of researchers based on tradition. A number of studies have reported the presence of various substances, including extracts and vegetable oils which are effective to control the growth of a wide variety of micro-organisms including fungi (Swamy et al., 2016; Rajkowska et al., 2017).
Astronium (Anacardiaceae) is a genus containing several medicinal species, including Astronium urundeuva (Allemão) Engl., commonly known as aroeira-preta or aroeira-do-sertão. This is an arboreal species widely distributed in South America, especially in the arid environment of Cerrado, pollinated by wind and containing a small flower. The size of this specie varies according to region of occurrence, but usually features 30 m high, with inflorescence between July and September and ripening of the fruit from September to October, consisting of a single seed (Machado and Oliveira, 2014; Leite et al., 2017). It is considered one of the most popular traditional medicinal plants in Northeastern Brazil as treatment to various diseases, including gastritis, gastric ulcers, cervicitis, vaginitis, hemorrhoids and as anti-inflammatory and natural healing (Calou et al., 2014; Machado and Oliveira, 2014; Resende et al., 2015). Previous studies reported the major components of Astronium sp. extracts, including chalcones, flavonoids precursors, essential oils, hydrolizable and condensed tannins that are considered to be the main responsible for the therapeutic activities. The presence of volatile oils justifies the typical aroma found in A. urundeuva leaves (Costa et al., 2014; Machado et al., 2016; Leite et al., 2017). We have previously reported on the in vitro antifungal activity of different extracts from a number of Astronium sp., with the hydroethanolic plant extract prepared from leaves of A. urundeuva displaying the most potent activity against Candida albicans (Bonifácio et al., 2015). Moreover this extract, both free and loaded into a nanostructured lipid system, displayed activity in an in vivo model of vulvovaginal candidiasis using Wistar rats (Bonifácio et al., 2015).
Here we have further evaluated the antifungal activity of this extract against different strains of C. albicans and C. glabrata, the two most common etiological agents of candidiasis. In vitro and in vivo results indicate the anticandidal activity and properties of both the free extract and an improved microemulsion formulation, and confirm the promise of the A. urundeuva leaves extract to be further developed as an antifungal for the treatment of candidiasis.
Materials and Methods
Preparation of and Hydroethanolic Extract of A. urundeuva Leaves
A. urundeuva leaves were collected in Bálsamo, São Paulo, Brazil, in November 2007 and authenticated in Campinas by Prof. Dr. Jorge Tamashiro (Institute of Biosciences, State University of Campinas – UNICAMP). A voucher specimen of this plant was deposited at the Herbarium of the Institute – UNICAMP and identified as SANO N° 1446. The hydroethanolic extract of A. urundeuva was obtained by exhaustive percolation method as previously described (Simões et al., 2017), and then, a rotary evaporator was used under reduced pressure (50°C). After being transferred to a tared glass, the extract was maintained in the fume hood until it was completely dried and was freeze-dried when necessary. For use in susceptibility experiments the free extract was prepared by solubilizing the crude extract in DMSO and miliQ water (at a ratio of 1:9) at a final concentration of 2000 μg/mL.
Preparation of a Loaded Extract
The loaded extract was prepared by loading the crude extract into a nanosystem composed of cholesterol (10%) as an oil phase, phosphate buffer saline (PBS, pH 7.4) as an aqueous phase (80%), and a surfactant mixture of soybean phosphatidylcholine (SPC)/polyoxyethylene (20) cetyl ether (Brij®58) (10%) as previously described (Bonifácio et al., 2015), with 0.5% of Poloxamer 407® added in order to promote better bioadhesion characteristics (for in vivo testing). The formulation was obtained by sonication in a rod sonicator (Q700 – Qsonica® 700 W) with an ice bath. Briefly, the mixture was sonicated for 10 min with an interval of 30 s every 2 min. After preparing the microemulsion, the extract was added to the formulation and then the sonication process was performed once again but this time for only 3 min in continuous mode.
In vitro Susceptibility Testing to Examine the Antifungal Activity Against Collection of Strains and Clinical Isolates of C. albicans and C. glabrata
We evaluated the activity of the free and loaded extracts against C. albicans ATCC 10231 and SC5314 strains, and against C. glabrata ATCC 2001 strain. In addition the free extract was also tested against some C. albicans strains that have mechanisms of resistance that have been previously characterized in the Lopez-Ribot laboratory (Perea et al., 2001; Li et al., 2004; Vila et al., 2015). We also used several C. albicans clinical isolates with previously characterized molecular mechanisms of azole resistance, TW2, TW3 and TW17, which represent a series of matched susceptible and resistant isolates from the same patient with oropharyngeal candidiasis (White, 1997). In addition, we used a set of genetically engineered C. albicans “gain of function” strains in key transcriptional regulators of azole resistance including TAC1, MRR1 and UPC2 (a kind gift from David Rogers) (Schubert et al., 2011; Flowers et al., 2012). In the case of C. glabrata, we also used a series of clinical isolates with different levels of resistance against azoles or echinocandins, which were provided by the Fungus Testing Laboratory (FTL) at The University of Texas Health Science Center at San Antonio. Antifungal susceptibility testing was performed using a broth microdilution technique following the methodology described in document M27-A3 published by the Clinical and Laboratory Standards Institute (CLSI) (Clinical and Laboratory Standards Institute, 2008) with minor modifications, with determination of MIC90 as the concentration that inhibits 90% of the fungal yeast growth when compared to the growth control. MIC values were initially evaluated by visual analysis and confirmed by spectrophotometry at 490 nm in a microtiter plate reader.
Determination of Minimum Fungicidal Concentration (MFC)
In order to verify that the extract was able to kill the yeast cells (fungicidal effect) the plates were also evaluated for MFC. Briefly, aliquots from each well from susceptibility testing assays were transferred to plates containing Sabouraud (SDA), which were then incubated at 37°C for 48 h. Results were evaluated by analyzing the presence or absence of growth in the SDA (Bagiu et al., 2012).
Activity of the Free Extract in Combination With Clinically Used Antifungals
We determined the in vitro activity of the free extract in combination with fluconazole (Pfizer Inc., New York, NY, United States), amphotericin B (Sigma Chemical Co., St. Louis, MO, United States) and caspofungin (Sigma Chemical Co., St. Louis, MO, United States) (as representatives of each class of antifungal drugs, azoles, polyenes and echinocandins) using a checkerboard titration method. A round-bottom 96-well microplate (Corning Inc., Corning, NY, United States) was filled with RPMI medium supplemented with L-glutamine (Cellgro; Corning, United States) and buffered with 165 mM morpholinepropanesulfonic acid (MOPS) (Thermo Fisher Scientific Inc., United States) at pH 7.0 (50 μL) from column 2–10. Drug A (established antifungal) (100 μL) was added in column 1 and serial 2-fold dilutions (50 μL) was performed from column 1–9. In another microplate, 50 μL of RPMI were dispensed from row B to H, and drug B (the free extract) (100 μL) was added to wells in row A (column 1–10) and subsequently diluted 2-fold from row A to G. The different dilutions of drug B were then transferred to the microplate containing dilutions of drug A. In this scheme, row H (columns 1–9) and column 10 (rows A-H) contain drugs A and B alone, respectively. The yeast inoculum (100 μL in RMPI medium) was added from columns 1 to 11 (rows A–H) at a final concentration of 1 × 103 cells/mL, and then microplates were incubated at 37°C for 48 h. Columns 11 and 12 represent growth and media controls. MIC values were evaluated by visual analysis and confirmed by spectrophotometry at 490 nm in a microtiter plate reader (Benchmark Microplate reader; Bio-Rad, CA, United States).
The results were analyzed using the Fractionary Inhibitory Concentration (FIC) indices, a non-parametric model based on the Loewe additivity theory. The FIC indices were defined as the following equation: FICI = (FICA + FICB), where: FICA = (MICAcombination/MICAalone) and FICB = (MICBcombination/MICBalone). MICAalone and MICBalone are the Minimum Inhibitory Concentrations (MIC) of drugs A and B alone and MICAcombination and MICBcombination are the concentrations of drugs A and B at the isoeffective combinations, respectively. Off-scale MICs were considered as the lowest or the highest concentrations tested in the microdilution. The FICI was interpreted as ‘synergy’ (FICI ≤ 0.5), ‘indifference’ (FICI > 0.5–4.0) and ‘antagonism’ (FICI > 4.0) (Hindler, 2010).
Serial Passage Experiments to Evaluate the Potential for the Development of Resistance
Once we had established the activity of our samples, we performed a series of serial passage experiments to evaluate the potential to induce resistance upon repeated exposure to the free extract, similar to previous experiments looking at development of resistance (Pierce et al., 2015; Romo et al., 2018). In these experiments, experimental C. albicans or C. glabrata populations were exposed to sub inhibitory concentrations of the free extract (at 1/2 the MIC50) or fluconazole. Cultures were placed in orbital shakers and every day, 10 μL from each culture was serially transferred into 1 mL of fresh medium containing the sample. These daily transfers were performed for 8 weeks and after the first week, the strains were exposed to the MIC50 and this was continued uninterrupted until the end of the experiment (in the case of fluconazole, as increased resistance was detected, the concentrations were doubled every week). After daily transfers, population samples were archived in 1 mL of 40% (vol/vol) glycerol at −80°C. Cells from populations recovered at different time points were then used for the determination of MICs against the free extract and fluconazole using the same 96-well microtiter plate methodology (Clinical and Laboratory Standards Institute, 2008).
Activity of the Free Extract Against Candida Biofilms
The activity of the free extract against C. albicans and C. glabrata biofilms under two different treatment modalities (inhibition of biofilm formation and activity against preformed biofilms) was evaluated using the 96-well microtiter plate of Candida biofilm formation and susceptibility testing (Pierce et al., 2008, 2010). Briefly, for inhibition of biofilm formation the media RPMI 1640 (50 μL) was added to each well in a flat-bottom 96-well microplate (Corning Inc., NY, United States), the free extract (50 μL) was added in column 1 and serially diluted until column 10 at concentrations ranging from 500 to 0.97 μg/mL. Then 50 μL of the yeast suspension at a concentration of 2 × 106 cells/mL were added to well of columns 1–11, and microtiter plates were incubated for 24 h at 37°C. To determine the activity against preformed biofilms, the yeast inoculum (100 μL of a suspension of 1 × 106 cells/mL in RPMI medium) was added to each well of a flat-bottom 96-well microplate and was incubated at 37°C for 24 h in order to allow for biofilm formation. After this period, the liquid was carefully aspirated not to touch the preformed biofilm, which was then washed with PBS (100 μL) two times in order to remove planktonic and non-adherent cells. In another microplate, the dilutions of the free extract were prepared from 1000 to 1.95 μg/mL, and transferred to the microtiter plate containing the preformed biofilms, which was incubated for an additional 24 h at 37°C. After the incubation periods, the liquid in each well of the microplates from both assays was carefully aspirated and the biofilm was washed with PBS (100 μL) twice in order to remove planktonic and non-adherent cells. The post-processing to measure the metabolic activity after the antifungal treatment was evaluated by XTT (Sigma) reduction assay as previously described by our group (Pierce et al., 2008, 2010). Finally, plates were read by spectrophotometry at 490 nm in a microtiter plate reader.
In vitro Cytotoxicity Assays Using Cell Lines
VERO (ATCC® CCL-81TM), MRC-5 (ATCC® CCL-171TM) and HaCaT cell lines were used to determine cytotoxicity of the free and loaded extracts by determining IC50 values (the highest concentration of the sample that allows the viability of at least 50% of the cells) according to Pavan et al. (2010). Cells were incubated at 37°C with 5% of CO2 in plates with a surface area of 12.50 cm2 in 10 mL of DMEM (Vitrocell®, Campinas, SP, Brazil) supplemented with 10% of fetal bovine serum, gentamicin sulfate (50 mg/L) and amphotericin B (2 mg/L). This technique entails collecting the cells using a solution of trypsin/EDTA (Vitrocell®), centrifuging the cells at 2,000 rpm for 5 min, counting the number of cells in a Neubauer chamber, determining viability using a 0.4% of Trypan blue solution – 0.25% (Sigma-Aldrich®, St. Louis, MO, United States), which is excluded from viable cells, and adjusting the cell concentration to 2.5 × 105 cells/mL in DMEM. Next, 200 μL of the suspension were deposited into each well of a 96-well microplate (KASVI®, Curitiba, PR, Brazil) and samples were then incubated at 37°C in an atmosphere of 5% of CO2 for 24 h to facilitate the attachment of the cells to the well (O’Brien et al., 2000). Dilutions of the tested samples were prepared to obtain concentrations ranging from 1,000 to 3.90 μg/mL. The dilutions were added to the cells after the medium and the non-adherent cells were removed. Cells were then incubated for an additional 24 h. The cytotoxicity of the samples was determined by adding 30 μL of resazurin and reading after an incubation period of approximately 6 h. The reading was performed using a microplate Spectrafluor Plus (TECAN®, Männedorf, Switzerland) reader using excitation and emission filters with wavelengths at 530 and 590 nm, respectively. This reading is based on the resazurin reduction to the highly fluorescent resorufin through cell viability. Non-viable cells fast lose their metabolic capacity in reducing resazurin and are not able to produce a fluorescent signal. Untreated cells (negative control or viable cells) and cells treated with doxorubicin (Sigma-Aldrich, St. Louis, MO, United States) at 100 nmol (positive control or dead cells), DMSO (5%) and microemulsion (without the active ingredient) were also used in each test as controls. The assays were performed three different times.
The selectivity index (SI) of the extract alone or loaded into the microemulsion was obtained by calculating the ratio of IC50 value divided by the MIC value for the fungal strain. A higher SI value indicates that the analyzed extract is more active against the yeast and less cytotoxic to the host, with an SI greater than 10 considered to be a promising value for the samples (Tuberculosis Drug Screening Program, 2001).
Toxicity Assays Using an Alternative in vivo Model of Artemia salina L.
The toxicity of the samples was also determined using an Artemia salina L. model. The amount of 25 mg of brine shrimp eggs (Natal, RN, Brazil) were incubated in a beaker (2000 mL) containing artificial salt water (temperature ranging from 20 to 30°C). The artificial salt water consisted of 23 g NaCl, 11 g of MgCl2 6H2O, 4 g of Na2SO4, 1.3 g of CaCl2 H2O, and 0.7 g of KCl dissolved in 1000 mL of distilled water. The pH was adjusted to 9.0 using 5 N NaOH to prevent Artemia larvae (nauplii) death due to the low pH during the incubation process. After 1 day, Saccharomyces cerevisiae was added to the beaker (0.6 g per liter of salt water) to cultivate the nauplii, which were collected for the experiment after 2 days of egg incubation. For the experiment, 120 μL of a suspension containing 10–15 nauplii was added to each well of a 96-well microtiter plate. In another 96-well microplate, samples were serially diluted resulting in concentrations ranging from 500 to 0.97 μg/mL. After 24 h of incubation, microplates were examined under a Lupa binocular microscope (×3.0), and the number of live nauplii in each was counted in order to determine the lethal dose (LD50) (Parra et al., 2001). Untreated nauplii (negative control or viable nauplii) and nauplii treated with potassium dichromate at concentrations ranging from 250 to 1.95 μg/mL (positive control or dead nauplii) were also tested as controls. According to Food and Drug Administration (FDA), LD50 can be defined as the sample concentration considered to be lethal to 50% of the organisms after being exposed to the sample to be tested. LD50 is normally expressed as a time-dependent variation, i.e., 24 h LD50. In all toxicity assays, DMSO 2% (Sigma-Aldrich®) and the microemulsion without the extract were used as controls. Assays were performed in three independent experiments.
Activity of the Free and Loaded Extracts in an in vivo Model of Vulvovaginal Candidiasis
The anti-C. albicans activity of the free and loaded extract was also tested using an in vivo model of vulvovaginal candidiasis This experiment was previously approved by the Ethics Committee on the Use of Animals in Research – CEUA of the School of Pharmaceutical Sciences – São Paulo State University (protocol number 28/2015).
Female Wistar rats (n = 6 per group; 150–200 g body weight, 8 weeks age) were immunosuppressed with cyclophosphamide (Sigma®, 20 mg/Kg, 0.3 mL – one dose) and pseudo-estrus state was induced with estradiol via subcutaneous administration (Sigma®, 0.2 mg/mL once a day until the rats reached an appropriate state). Vulvovaginal candidiasis infection was induced using the C. albicans strain SC5314. This strain was originally a clinical isolate obtained from a patient with disseminated candidiasis, and is also the strain used in the C. albicans genome sequencing project; as such it has been widely used in virulence studies (Gillum et al., 1984; Swindell et al., 2009; Zago et al., 2015). Moreover, it has been documented that this strain is highly filamentous and capable of forming robust biofilms that display high level of resistance to clinically used antifungals (Pierce et al., 2008). These attributes are ideal for the establishment of vaginal infection in the animal model used, since it is well established in the literature that vaginal candidiasis has a biofilm etiology (Harriott et al., 2010). After the infection with C. albicans SC5314 at a concentration of 5.0 × 107 cells/mL (intravaginal inoculation – 100 μL) the free and loaded extract (2× MIC) were topically administered to two different groups of animals twice a day (100 μL) during 8 days of treatment. Appropriate positive (infected and treated with antifungal cream consisted of tetracycline −25 mg/g + amphotericin B – 12.5 mg/g) and negative (infected and untreated) controls were also used (see Figure 2 for description of the different control and experimental groups). The efficacy of the treatment was examined by evaluating the fungal load during the course of the infection via determination of colony forming units (CFU) in vaginal lavage fluids (lavage performed with 100 μL of PBS). After discontinuing the treatment, determination of CFUs in vaginal lavages was performed during 7 consecutive days in order to verify if the disease could eventually reemerge. Statistical treatment was performed and significant differences (p < 0.05) were analyzed according to two-way ANOVA with post-test Tukey.
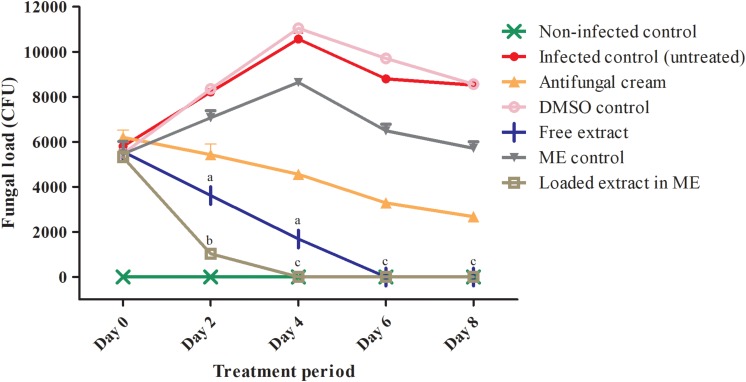
FIGURE 2.
Fungal loads detected in vaginal lavages from animals in different experimental and control groups on days 0, 2, 4, 6, and 8 of treatment. ME, microemulsion. Different letters in superscripts in columns indicate statistically significant differences (P < 0.05) according to two-way ANOVA with post-test Tukey. aComparison between the free extract and the DMSO control. bComparison between the loaded extract and the microemulsion control. cComparison between the loaded and the free extract. ∗Undetectable fungal burden for the animals treated with the free extract (days 6 and 8) and the loaded extract (days 4, 6, and 8).
Results and Discussion
In vitro Activity of the Extract Alone or Incorporated Into the Nanostructured System Against C. albicans and C. glabrata Collection Strains
C. albicans and C. glabrata represent the two most common causative agents of candidiasis, affecting an expanding population of at-risk patients. In particular, the number of C. glabrata infections has increased in the last few decades, which is generally attributed to the decreased susceptibility of this microorganism to azole antifungals (Whaley et al., 2017; Cavalheiro et al., 2018). Clearly, there is an interest in the development of novel therapeutic approaches for candidiasis that may overcome the problem of resistance (Srinivasan et al., 2014), which has led to an increased interest and reevaluation of the properties of natural products used in folk medicine in different parts of the world (Li and Lou, 2018). Our previous experiments indicated that the hydroethanolic extract prepared from leaves of A. urundeuva displayed the highest levels of activity against a C. albicans collection strain (ATCC 18804) among different types of extracts prepared from different plants from this same Astronium species (Bonifácio et al., 2015). Furthermore, the antifungal activity was retained when the extract was incorporated into a nanostructured lipid system (Bonifácio et al., 2015). Other groups have also reported on the antifungal activity of A. urundeuva bark and leaves against different species of Candida (Oliveira et al., 2017). Thus, in an initial set of experiments we used CLSI techniques to assess the antifungal activity of both the free extract and the extract incorporated in an improved microemulsion formulation against two other collection strains of C. albicans, and also expanded these studies to a C. glabrata collection strain (Table 1). As seen in the Table, and confirming our previous results, in vitro susceptibility testing results demonstrated similar levels of activity for the free and loaded extracts against both strains of C. albicans. Interestingly, an even more potent antifungal activity was detected against the C. glabrata collection strain used in these initial experiments.
TABLE 1.
Inhibitory effect of free and loaded extract on C. albicans and C. glabrata planktonic cells.
| Strain | C. albicans | C. glabrata | ||||
| Sample | ATCC 10231 | SC5314 | ATCC 2001 | |||
| MIC90 | MFC | MIC90 | MFC | MIC90 | MFC | |
| Free | 15.62 | 125 | 31.25 | 125 | 0.24 | 125 |
| Loaded into ME | 15.62 | 15.62 | 31.25 | 31.25 | 0.24 | 0.24 |
Values are in μg/mL. ME, microemulsion; MIC, minimum inhibitory concentration; MFC, minimum fungicidal concentration.
We also determined the MFCs for the free and the loaded extracts against the same collection strains. As seen in Table 1, a much higher concentration of the free extract was required to exert a fungicidal effect against the Candida strains. However, MFC values for the loaded extract against both species of Candida were the same as their corresponding MICs, indicating that a fungicidal effect was exerted at low concentrations.
Activity of the Free Extract Against C. albicans and C. glabrata Clinical Isolates Exhibiting Different Levels of Resistance Against Conventional Antifungals
As mentioned before, the emergence of resistance has become a significant clinical problem that often limits our ability to successfully treat Candida infections, also highlighting the need for the discovery and development of novel antifungal agents, particularly those active against species and strains that are resistant to conventional antifungals (Ngo et al., 2016; Perlin et al., 2017). In C. albicans, azole resistance typically develops upon prolonged therapy, whereas C. glabrata commonly shows decreased susceptibility to azole derivatives, with echinocandin resistance also being detected with increasing frequency in last decade (Ngo et al., 2016; Perlin et al., 2017).
In the case of C. albicans we examined the activity of our free extract against a number of clinical isolates recovered form HIV-infected patients with oropharyngeal candidiasis, including several isolates that are resistant to treatment with conventional antifungals and for which molecular mechanisms of resistance have been previously described (White, 1997; Perea et al., 2001). Additionally, we conducted susceptibility testing assays of the free extract against several laboratory generated strains overexpressing key transcriptional activators of drug resistance efflux pumps and the ergosterol pathway (Schubert et al., 2011; Flowers et al., 2012); including a gain-of-function strain in UPC2, leading to overexpression of ERG11 (the gene encoding lanosterol demethylase, the target enzyme of azole antifungals), as well as gain-of-function strains in TAC1 and MRR1, leading to overexpression of CDR and MDR genes, respectively, which encode efflux pumps involved in azole resistance (Schubert et al., 2011; Flowers et al., 2012). Table 2 contains information on each of these strains as well as the MIC values obtained for the free extract. As seen in Table 2, although most of the MIC values obtained for the different strains were mostly comparable to those obtained for collection strains. However, we detected elevated MIC values, particularly for strains MRR1R34A, 3731 and 2440, which could potentially indicate that the active component in the extract may be a substrate for the Mdr1 major facilitator also involved in azole resistance. Overall these results seem to confirm the antifungal activity of the free extract against a majority of C. albicans strains with decreased susceptibility against conventional antifungals, including both clinical isolates and laboratory-generated strains that show resistance against azoles.
TABLE 2.
Results (MIC values) of in vitro susceptibility testing of the free extract against C. albicans clinical isolates displaying different mechanisms of azole resistance and against laboratory generated strains with activating mutantions in key genes involved in azole resistance.
| Strain | Description | Overexpressed genes | Extract | Fluconazole |
| 4639 | F449S, T229A (Erg11p substitutions) | MDR1, CDR1 | 7.81 | >64a |
| 4617 | F449S, T229A (Erg11p substitutions) | N/A | 3.9 | 64a |
| 6482 | D116E, K128T, Y132H, D278N, G464S, P230L (Erg11p substitutions) | N/A | 125 | >64b |
| 2440 | V437I (Erg11p substitution) | MDR1, ERG11 | 250 | 64a |
| 3731 | F126L, K143R (Erg11p substitutions) | MDR1 | 500 | >64a |
| 412 | K128T (Erg11p substitution) | N/A | 62.5 | 0.5a |
| TW2 | Clinical isolate (drug resistance observed) | MDR1 | 62.5 | 2c |
| TW3 | Clinical isolate (drug resistance observed) | MDR1 | 62.5 | 8c |
| TW17 | Clinical isolate (multi drug resistance observed) Point mutation: R467K | CDR1, MDR1, ERG11 | 125 | 128c |
| TAC1R34A | Strain with homozygous activating mutation in TAC1 | TAC1, CDR1 and CDR2 | 125 | – |
| MRR1R34A | Strain with homozygous activating mutation in MRR1 | MRR1 and MDR1 | >500 | 3.13d |
| UPC2R14A | Strain with homozygous activating mutation in UPC2 | UPC2 and ERG11 | 15.62 | >256e |
Results for fluconazole are from previous publications using the same standardized methodologies and are provided for comparative purposes. Values are in μg/mL. Fluconazole MIC values from previous reports: aPerea et al., 2001; bLi et al., 2004; cWhite et al., 1997; dSchubert et al., 2011; eFlowers et al., 2012.
We then evaluated the activity of the free extract against a series of C. glabrata clinical isolates from the FTL collection that are resistant to azoles or echinocandins. Confirming our results using the C. glabrata collection strain (Table 3), the free extract showed very potent activity against all C. glabrata isolates tested, which was completely unaffected by their resistance patterns to clinically used antifungals. Thus, it would seem that the free extract may represent a promising alternative particularly for the treatment of C. glabrata infections, including those that may fail conventional therapy.
TABLE 3.
The inhibitory effect of the free extract and standard antifungals fluconazole and echinocandin against resistant C. glabrata clinical isolates.
| Cell line | Free extract | Fluconazole | Caspofungin | |||
| Sample | MIC50 | MIC90 | MIC50 | MIC90 | MIC50 | MIC90 |
| CgSM1 (azole-S) | 0.97 | 1.95 | 4–8 | 8 | – | – |
| CgSM3 (azole-R) | 0.97 | 1.95 | 62.5–125 | 125 | – | – |
| DI19-56 (azole-R) | <0.97 | 1.95–3.90 | >128 | >128 | – | – |
| DI19-57 (azole-R) | <0.97 | 1.95–3.90 | 64 | 128 | – | – |
| DI19-58 (azole-R) | <0.97 | 1.95–3.90 | >128 | >128 | – | – |
| DI19-59 (azole-R) | <0.97 | 1.95–3.90 | >128 | >128 | – | – |
| DI19-60 (azole-R) | <0.97 | 1.95–3.90 | 128 | >128 | – | – |
| DI19-61 (echino-R) | 0.97–1.95 | 3.90–7.81 | – | – | 4 | >4 |
| DI19-62 (echino-R) | 0.97–1.95 | 3.90–7.81 | – | – | > 4 | >4 |
| DI19-63 (echino-R) | 0.97–1.95 | 1.95 | – | – | > 4 | >4 |
| DI19-64 (echino-R) | 0.48–0.97 | 0.97 | – | – | 2–4 | 4 |
| DI19-65 (echino-R) | 0.48–0.97 | 1.95 | – | – | 2–4 | 4 |
Values are in μg/mL. azole-S, azole-susceptible strain; azole-R, azole-resistant strain; echino-R, echinocandin-resistant strain.
In vitro Activity of the Free Extract in Combination With Conventional Antifungal Agents
The antifungal activity detected for the free extract makes it a potential candidate for the development of combinatorial therapies together with currently used antifungal agents. In some cases, plant extracts with biological properties, i.e., antifungal, require such high concentrations to be effective that it could be toxic to healthy cells and cause many side effects. The same situation is also applicable to antifungals already used in clinical therapy, i.e., amphotericin B, that is considered nephrotoxic. Therefore, if these plant extracts could be used combined with antifungals, both at lower concentrations, it could lead to increased activity and reduce their negative side effects. To examine the activity of these combinations, we performed checkerboard assays in which different concentrations of the free extract were used in combination with different dilutions of fluconazole, caspofungin or amphotericin B, both against C. albicans and C. glabrata. As seen in Supplementary Table S1, after calculation of the respective FICIs, a majority of combinations showed indifference, with the notable exemption of the combinations between the free extract and amphotericin B against C. albicans, which resulted in synergism. Overall, these results indicate that the free extract could potentially be used in combination with currently used antifungal agents.
Serial Passage Experiments to Evaluate the Potential for the Induction of Resistance
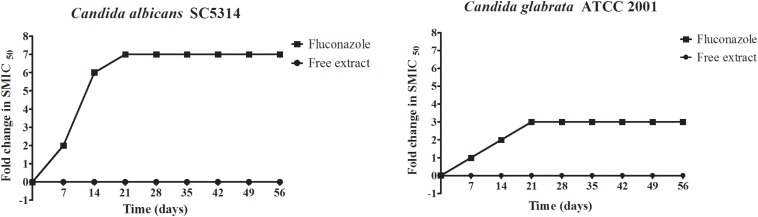
Candida has the ability to develop resistance upon prolonged and repeated exposure to fluconazole, which greatly complicates therapy (Morschhäuser, 2016; Berkow and Lockhart, 2017). Thus, we were interested in examining if this same conditions could lead to the development of resistance against the free extract, for which we performed serial passage experiments as previously described by our group (Pierce et al., 2015; Romo et al., 2018), both with the free extract and with fluconazole. The initial concentrations used for these experiments were half the MIC values during the first week, which was then increased to the MIC from weeks 2 to 8, at which time the experiment was ended. In the case of fluconazole, as decreased susceptibility was detected after successive passages, the concentrations were doubled each week. As shown in Figure 1, results indicated that C. albicans and C. glabrata did not develop resistance when exposed to the free extract, as the MIC values were identical at the beginning and end of serial passage experiments. This was in stark contrast to fluconazole which, as expected, led to the induction of resistance in both Candida species, with MIC values at the end of experiments being greater than 128 μg/mL.
FIGURE 1.
Serial passage of C. albicans SC5314 and C. glabrata ATCC 2001 in the presence of the free extract or fluconazole to assess the potential for the development of resistance.
Activity of the Free Extract Against Biofilms of C. albicans and C. glabrata
Candida species have the ability to form biofilms on different inert and biological surfaces, and it is well established that biofilm formation leads to high levels of resistance against most antifungal agents (Uppuluri et al., 2009; Rodrigues et al., 2017). Thus, we were interested in examining the activity of the free extract against C. albicans and C. glabrata biofilms. In a first set of experiments we evaluated the activity of the free extract against preformed biofilms of both Candida species, whereas a second set of experiments examined the ability of the free extract to inhibit biofilm formation. As seen in Table 4, preformed biofilms of C. albicans and C. glabrata were intrinsically resistant to the free extract. However, interestingly the extract was able to inhibit C. albicans biofilm formation at concentrations only slightly higher than those inhibiting growth of planktonic cells. The same was not true for C. glabrata, as the concentrations of free extract capable of inhibiting biofilm formation were much higher than those effective against planktonic growth. Interestingly, Trentin and co-workers (Trentin et al., 2013) studied stem-bark extracts from different plants, including A. urundeuva, and reported on the activity of this extract against Pseudomonas aeruginosa biofilms.
TABLE 4.
Inhibitory effect of the free extract on preformed biofilms and their ability to inhibit biofilm formation of Candida albicans and Candida glabrata.
| Strain | SMIC50 | |
| Inhibition | Preformed | |
| C. albicans SC5314 | 62.5 | 1000 |
| C. glabrata ATCC 2001 | 250 | 1000 |
Values are in μg/mL. SMIC, sessile minimum inhibitory concentration.
Toxicity Assays
Very often effective antimicrobial concentrations of natural products are relatively high, and their use may be limited due to toxicity (Van Vuuren and Holl, 2017; Wright, 2017). Our previous report pointed to the low cytotoxicity of the free and loaded extracts when tested in an in vitro system (Bonifácio et al., 2015). Here, we have expanded upon our initial observations by examining the cytotoxicity of the free extract in three different cells lines, and also using an alternative in vivo model in Artemia salina L. These experiments were performed to confirm lack of toxicity as a prelude to in vivo experiments (see below).
Table 5 shows the IC50 cytotoxicity values for the free and loaded extracts when tested in VERO, MRC-5 and HaCaT cell lines. When analyzing the cytotoxicity results of VERO cells line (an epithelial cell from green monkeys), we could observe that the extract loaded into the microemulsion presented a higher IC50 (greater than 1000 μg/mL) when compared to the free extract (536.1 μg/mL), and these values were 229 and 123 times greater, respectively, than IC50 value observed for doxorubicin (4.36 μg/mL for this cell line), a reference drug used in these assays. Regarding the MRC-5 cell line (a normal cell derived from human lungs widely used for phenotypic screening of drugs), both the free extract and the microemulsion had similar IC50 values (360 and 380 μg/mL, respectively), which also compared favorably to doxorubicin (IC50 of 1.90 μg/mL). Lower IC50 values were detected when the HaCaT (human keratinocytes) cell line was used, indicating a higher toxicity for this cell line compared to the others, although the IC50 values for both the free and loaded extracts (158 and 110 μg/mL, respectively) were still significantly higher than IC50 values for doxorubicin (6.79 μg/mL). When these IC50 values were compared to the corresponding MIC values for both C. albicans and C. glabrata (see Table 1), the resulting selectivity indices pointed toward a good safety profile associated with these extracts, with the calculated SI values generally higher than 10 in most instances, except for slightly lower values for the tests performed using HaCaT cells.
TABLE 5.
Biological assessments for the toxicity of the free and loaded extract of A. urundeuva leaves in different cell lines as assessed by IC50 values of the free and loaded extract in comparison to doxorubicin (used as control).
| Cell line | IC50 VERO | IC50 MRC-5 | IC50 HaCaT |
| Sample | |||
| Free extract | 536.1 | 360 | 158 |
| Loaded extract | >1000 | 380 | 110 |
| Doxorubicin | 4.36 | 1.90 | 6.79 |
Values are in μg/mL.
The toxicity of the free and loaded extracts were further evaluated in vivo using a novel alternative model of Artemia salina L., which is gaining favor for the evaluation of toxicity properties of a number of natural products (Krishnaraju et al., 2005; Amaral and Silva, 2008; Silva Filho et al., 2009; Nguta et al., 2011; Arcanjo et al., 2012; Mirzaei and Mirzaei, 2013; Lalisan et al., 2014; Rajabi et al., 2015). Our results demonstrated that nauplii viability was unaffected even when exposed to the highest concentration of both the free and loaded extracts tested (500 μg/mL), thereby demonstrating their lack of toxicity in this model. These results are also in agreement with those previously reported by Amaral and Silva (2008), using the same Artemia salina L. model to study the toxicity of different plant extracts, including A. urundeuva.
In vivo Efficacy of the Free and Loaded Extracts in a Rat Model of Vulvovaginal Candidiasis
We have previously reported on the activity of the free and loaded extracts in a rat model of vulvovaginal candidiasis using a different C. albicans strain (ATCC 18804) (Bonifácio et al., 2015). Also, the formulation of the microemulsion system used in this current report was slightly modified by the addition of 0.5% of Poloxamer 407® in order to increase its in vivo bioadhesive properties. Thus, we were interested in determining the efficacy of this improved formulation in the same model of vulvovaginal candidiasis, this time also using a different C. albicans strain (SC5314), which is the most common strain used in virulence studies (Gillum et al., 1984; Swindell et al., 2009; Zago et al., 2015).
As seen in Figure 2, on day 2, presence of CFUs in the lavage fluids recovered from rats in all infected groups confirmed the establishment of vaginal infection, and a trend toward decreased fungal burden was already observed in animals treated topically with either the free or the loaded extract. By day 4 no fungal burden was detected in lavage samples from animals treated with the extract loaded in the improved microemulsion formulation, and the same was true by day 6 in the case of animals treated with the free extract, which compared favorably to the control group of animals treated with a vaginal cream containing amphotericin B which still had significant levels of CFUs. We could also confirm that the control groups treated with vehicle only did not reduce infection, thereby proving that the antifungal activity was truly exhibited by the components from the extract. Interestingly, after the treatment period ended, we could detect a re-infection in the group treated with free extract and the positive control, but not in the case of animals treated with the extract loaded into the microemulsion system (not shown). Thus, together with our previous report (Bonifácio et al., 2015), these results provide further evidence of the in vivo antifungal activity of the extract in this model when used topically. Altogether results from this present study demonstrate an improved efficacy of the new microemulsion formulation as compared to our previous report (Bonifácio et al., 2015), with much faster clearance of infection despite the fact that the C. albicans strain used in this study forms robust biofilms that exhibit intrinsic resistance to common antifungals, as demonstrated also by the lack of efficacy of our positive control (antifungal cream).
Overall this study furthers our understanding on the antifungal activity of the plant (leaves) extract of A. urundeuva, both when used as a free extract and loaded into a microemulsion system. Our results demonstrate that compared to its activity against C. albicans, it displays an even more potent activity against C. glabrata, and organism that frequently shows decreased susceptibility to conventional antifungals, and preliminary studies indicate that it also displays activity against other Candida species, including C. krusei and C. parapsilosis, as well as against Cryptococcus neoformans (not shown). Moreover, the extract is active against a majority of clinical isolates of both C. albicans and C. glabrata that show resistance against clinically used antifungal agents, and is able to inhibit biofilm formation by both Candida species. The extract may be used in combination with conventional antifungals, to improve the efficacy of treatment that even against the clinical isolates considered resistant to most conventional drugs commonly used in treatments. In addition, results from serial passage experiments indicate that the extract is not likely to induce resistance in either C. albicans or C. glabrata, in stark contrast with results obtained for fluconazole. We have also expanded on the studies on the toxicity properties of the extract, including lack of toxicity in vivo in an alternative model of Artemia salina L. Our in vivo experiments in the rat model of Candida vaginitis demonstrate the efficacy of treatment with the free and loaded extract. Altogether, our results confirm the antifungal properties of the A. urundeuva leaves extract both in vitro and in vivo, and its promise to be further developed as an alternative treatment for the treatment of candidiasis and potentially other fungal infections.
Data Availability Statement
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
Ethics Statement
The animal study was reviewed and approved by the Ethics Committee on the Use of Animals in Research – CEUA of the School of Pharmaceutical Sciences – São Paulo State University (protocol number 28/2015).
Author Contributions
BB performed all the in vitro and in vivo assays and drafted the manuscript. TV contributed to the in vitro susceptibility assays. IM and MS contributed to the in vivo assay. PS and MC contributed to the microemulsion development. IS, ÉO, and FP contributed to the toxicity assay. LS and WV contributed to the extract obtention process. JL-R contributed to all the in vitro susceptibility assays, assisted in drafting, and reviewing the manuscript. TB conceived the project, supervised all the assays, assisted in drafting, and reviewing the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank Dr. David Rogers from the University of Tennessee Health Science Center for the gain-of-function strains, Dr. Nathan Wiederhold from the University of Texas Health Science Center San Antonio Fungal Testing Lab for the Candida glabrata clinical isolates and Semanti Sakar and Gina Wall for help with combinatorial studies.
Footnotes
Funding. This work was supported by grant #2009/52237-9, grant #2013/25432-0, grant #2013/25121-5, and grant #2015/19011-8 São Paulo Research Foundation (FAPESP), School of Pharmaceutical Sciences/UNESP, São Paulo, Brazil. This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001. Work in the JL-R laboratory is supported by grants numbered R01DE023510 and R01AI119554 from the National Institute of Dental and Craniofacial Research and the National Institute of Allergy and Infectious Diseases, respectively, with additional support provided by the Margaret Batts Tobin Foundation, San Antonio, TX, United States.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2019.02642/full#supplementary-material
References
- Achkar J. M., Fries B. C. (2010). Candida infections of the genitourinary tract. Clin. Microbiol. Rev. 23 253–273. 10.1128/CMR.00076-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral E. A., Silva R. M. G. (2008). Avaliação da toxidade aguda de angico (Anadenanthera falcata), pau-santo (Kilmeyera coreacea), aroeira (Myracrodruon urundeuva) e cipó-de-São-João (Pyrostegia venusta), por meio do bioensaio com Artemia salina. Perquirc̄re 5 1–16. [Google Scholar]
- Arcanjo D. D., Albuquerque A. C., Melo-Neto B., Santana L. C., Medeiros M. G., Citó A. (2012). Bioactivity evaluation against Artemia salina leach of medicinal plants used in brazilian northeastern folk medicine. Braz. J. Biol. 72 505–509. 10.1590/s1519-69842012000300013 [DOI] [PubMed] [Google Scholar]
- Bagiu R. V., Vlaicu B., Butnariu M. (2012). Chemical composition and in vitro antifungal activity screening of the Allium ursinum L. (Liliaceae). Int. Mol. Sci. 13 1426–1436. 10.3390/ijms13021426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkow E. L., Lockhart S. R. (2017). Fluconazole resistance in Candida species: a current perspective. Infect. Drug Resist. 10 237–245. 10.2147/IDR.S118892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifácio B. V., Ramos M. A. S., Silva P. B., Negri K. M. S., Lopes E. O., Souza L. P., et al. (2015). Nanostructured lipid system as a strategy to improve the anti-Candida albicans activity of Astronium sp. Int. J. Nanomedicine. 10 5081–5092. 10.2147/IJN.S79684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calou I., Bandeira M. A., Aguiar-Galvão W., Cerqueira G., Siqueira R., Neves K. R., et al. (2014). Neuroprotective properties of a standardized extract from Myracrodruon urundeuva Fr. All. (aroeira-do-sertão), as evaluated by a Parkinson’s disease model in rats. Parkinsons Dis. 2014 519615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalheiro M., Costa C., Silva-Dias A., Miranda I. M., Wang C., Pais P., et al. (2018). A transcriptomics approach to unveiling the mechanisms of in vitro evolution towards fluconazole resistance of a Candida glabrata clinical isolate. Antimicrob. Agents Chemother. 63:e995-18. 10.1128/AAC.00995-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinical and Laboratory Standards Institute (2008). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; M27-A3. Wayne, PA: Clinical and Laboratory Standards Institute. [Google Scholar]
- Costa O. B., Del Menezzi C. H. S., Benedito L. E. C., Resck I. S., Vieira R. F., Bizzo R. H. (2014). Essential oil constituents and yields from leaves of Blepharocalyx salicifolius (Kunt) O. Berg and Myracrodruon urundeuva (Allemão) collected during daytime. Int. J. For. Res. 2014 1–6. 10.1155/2014/982576 [DOI] [Google Scholar]
- Flowers S. A., Barker K. S., Berkow E. L., Toner G., Chadwick S. G., Gygax S. E., et al. (2012). Gain-of-function mutations in UPC2 are a frequent cause of ERG11 upregulation in azole-resistant clinical isolates of Candida albicans. Eukaryot. Cell. 11 1289–1299. 10.1128/EC.00215-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabaldón T., Carreté L. (2016). The birth of a deadly yeast: tracing the evolutionary emergence of virulence traits in Candida glabrata. FEMS Yeast Res. 16:fov110. 10.1093/femsyr/fov110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillum A. M., Tsay E. Y., Kirsch D. R. (1984). Isolation of the Candida albicans gene for orotidine-5’-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Mol. Gen. Genet. 198 179–182. 10.1007/bf00328721 [DOI] [PubMed] [Google Scholar]
- Harriott M. M., Lilly E. A., Rodriguez T. E., Fidel P. L., Noverr M. C. (2010). Candida albicans forms biofilms on the vaginal mucosa. Microbiology 156 3635–3644. 10.1099/mic.0.039354-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindler J. (2010). Clinical Microbiology Procedures Handbook. Washington: ASM Press. [Google Scholar]
- Khan M., Ahmed J., Gul A., Ikram A., Lalani F. K. (2018). Antifungal susceptibility testing of vulvovaginal Candida species among women attending antenatal clinic in tertiary care hospitals of Peshawar. Infect. Drug Resist. 11 447–456. 10.2147/IDR.S153116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnaraju A. V., Rao T. V. N., Sundararaju D., Vanisree M., Tsay S. H., Subbaraju G. V. (2005). Assessment of bioactivity of Indian medicinal plants using Brine shrimp (Artemia salina) lethality assay†. IJASE 3 125–134. [Google Scholar]
- Lalisan J., Nuñeza O. M., Uy M. M. (2014). Brine shrimp (Artemia salina) bioassay of the medicinal plant Pseudelephantopus spicatus from Iligan City, Philippines. Int. Res. J. Biological Sci. 3 47–50. [Google Scholar]
- Leite R. S., Souza V. G., Oliveira A. H., Chaves Júnior J. V., Salvador I. S., Andrade F. H. V., et al. (2017). Standardization and stability evaluation of dry extracts of Myracrodruon urundeuva allemão obtained by spray drier. Int. J. Pharm. Pharm. Sci. 9 154–159. [Google Scholar]
- Li G., Lou H. X. (2018). Strategies to diversify natural products for drug discovery. Med. Res. Rev. 38 1255–1294. 10.1002/med.21474 [DOI] [PubMed] [Google Scholar]
- Li X., Brown N., Chau A. S., López-Ribot J. L., Ruesga M. T., Quindos G., et al. (2004). Changes in susceptibility to posaconazole in clinical isolates of Candida albicans. J. Antimicrob. Chemother. 53 74–80. 10.1093/jac/dkh027 [DOI] [PubMed] [Google Scholar]
- Machado A. C., Oliveira R. C. (2014). Medicamentos fitoterápicos na odontologia: evidências e perspectivas sobre o uso da aroeira-do-sertão (Myracrodruon urundeuva Allemão). Rev. Bras. Plantas Med. 16 283–289. 10.1590/s1516-05722014000200018 [DOI] [Google Scholar]
- Machado A. C., Souza L. P., Saldanha L. L., Pieroni L. G., Matos A. A., Oliveira F. A., et al. (2016). “Aroeira” (Myracrodruon urundeuva) methanol extract: the relationship between chemical compounds and cellular effects. Pharm. Biol. 54 2737–2741. 10.1080/13880209.2016.1182555 [DOI] [PubMed] [Google Scholar]
- Mirzaei M., Mirzaei A. (2013). Comparison of the Artemia salina and Artemia uramiana bioassays for toxicity of 4 Iranian medicinal plants. Int. Res. J. Biological Sci. 2 49–54. [Google Scholar]
- Morschhäuser J. (2016). The development of fluconazole resistance in Candida albicans - an example of microevolution of a fungal pathogen. J. Microbiol. 54 192–201. 10.1007/s12275-016-5628-4 [DOI] [PubMed] [Google Scholar]
- Nakamura-Vasconcelos S. S., Fiorini A., Zanni P. D., Bonfim-Mendonça P. S., Godoy J. R., Almeida-Apolonio A. A., et al. (2017). Emergence of Candida glabrata in vulvovaginal candidiasis should be attributed to selective pressure or virulence ability? Arch. Gynecol. Obstet. 296 519–526. 10.1007/s00404-017-4465-y [DOI] [PubMed] [Google Scholar]
- Ngo H. X., Garneau-Tsodikova S., Green K. D. (2016). A complex game of hide and seek: the search for new antifungals. Med. Chem. Comm. 7 1285–1306. 10.1039/c6md00222f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguta J. M., Mbaria J. M., Gakuya D. W., Gathumbi P. K., Kabasa J. D., Kiama S. G. (2011). Biological screening of Kenyan medicinal plants using Artemia salina L. (Artemiidae). Pharmacologyonline 2 458–478. [Google Scholar]
- O’Brien J., Wilson I., Orton T., Pognan F. (2000). Investigation of the alamar blue (Resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur. J. Biochem. 267 5421–5426. 10.1046/j.1432-1327.2000.01606.x [DOI] [PubMed] [Google Scholar]
- Oliveira F. A., Rorato V. C., Almeida-Apolonio A. A., Rodrigues A. B., Barros A. L., Sangalli A., et al. (2017). In vitro antifungal activity of Myracrodruon urundeuva Allemão against human vaginal Candida species. An. Acad. Bras. Cienc. 89 2423–2432. 10.1590/0001-3765201720170254 [DOI] [PubMed] [Google Scholar]
- Parra A. L., Yhebra R. S., Sardiñas I. G., Buela L. I. (2001). Comparative study of the assay of Artemia salina L. and the estimate of the medium lethal dose (LD50 value) in mice, to determine oral acute toxicity of plant extracts. Phytomedicine 8 395–400. 10.1078/0944-7113-00044 [DOI] [PubMed] [Google Scholar]
- Pavan F. R., da S., Maia P. I., Leite S. R., Deflon V. M., Batista A. A., et al. (2010). Thiosemicarbazones, semicarbazones, dithiocarbazates and hydrazide/hydrazones: anti - Mycobacterium tuberculosis activity and cytotoxicity. Eur. J. Med. Chem. 45 1898–1905. 10.1016/j.ejmech.2010.01.028 [DOI] [PubMed] [Google Scholar]
- Perea S., López-Ribot J. L., Kirkpatrick W. R., McAtee R. K., Santillan R. A., Martinez M., et al. (2001). Prevalence of molecular mechanisms of resistance to azole antifungal agents in Candida albicans strains displaying high-level fluconazole resistance isolated from human immunodeficiency virus-infected patients. Antimicrob. Agents Chemother. 45 2676–2684. 10.1128/aac.45.10.2676-2684.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlin D. S., Rautemaa-Richardson R., Alastruey-Izquierdo A. (2017). The global problem of antifungal resistance: prevalence, mechanisms, and management. Lancet Infect. Dis. 17 e383–e392. 10.1016/S1473-3099(17)30316-X [DOI] [PubMed] [Google Scholar]
- Pierce C. G., Chaturvedi A. K., Lazzell A. L., Powell A. T., Saville S. P., Mchardy S. F., et al. (2015). A novel small molecule inhibitor of Candida albicans biofilm formation, filamentation and virulence with low potential for the development of resistance. NPJ Biofilms Microbiomes. 1:15012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce C. G., Srinivasan A., Uppuluri P., Ramasubramanian A. K., López-Ribot J. L. (2013). Antifungal therapy with an emphasis on biofilms. Curr. Opin. Pharmacol. 13 726–730. 10.1016/j.coph.2013.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce C. G., Uppuluri P., Tristan A. R., Wormley F. L., Mowat E., Ramage G., et al. (2008). A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nat. Protoc. 3 1494–1500. 10.1038/nport.2008.141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce C. G., Uppuluri P., Tummala S., Lopez-Ribot J. L. (2010). A 96 well microtiter plate-based method for monitoring formation and antifungal susceptibility testing of Candida albicans biofilms. J. Vis. Exp. 44:2287. 10.3791/2287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qadir M. I., Asif H. (2019). An overview to candidiasis - a Candida infection. Int. J. Adv. Res. Micro. Immunol. 2 1–3. [Google Scholar]
- Rajabi S., Ramazani A., Hamidi M., Naji T. (2015). Artemia salina as a model organism in toxicity assessment of nanoparticles. Daru 23:20. 10.1186/s40199-015-0105-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska K., Kunicka-Styczyńska A., Maroszyńska M. (2017). Selected essential oils as antifungal agents against antibiotic-resistant Candida spp.: in vitro study on clinical and food-borne isolates. Microb. Drug Resist. 23 18–24. 10.1089/mdr.2016.0001 [DOI] [PubMed] [Google Scholar]
- Resende F. A., Campos D. L., da Silva V. C., de Grandis R. A., Souza L. P., Leonardo Junior C. S., et al. (2015). Mutagenicity and chemopreventive activities of Astronium species assessed by Ames test. Regul. Toxicol. Pharmacol. 72 506–513. 10.1016/j.yrtph.2015.05.014 [DOI] [PubMed] [Google Scholar]
- Rodrigues C. F., Rodrigues M. E., Silva S., Henriques M. (2017). Candida glabrata biofilms: how far have we come? J. Fungi. 3:11. 10.3390/jof3010011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romo J. A., Pierce C. G., Esqueda M., Hung C. Y., Saville S. P., Lopez-Ribot J. L. (2018). In vitro characterization of a biaryl amide anti-virulence compound targeting Candida albicans filamentation and biofilm formation. Front. Cell. Infect. Microbiol. 8:227. 10.3389/fcimb.2018.00227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos Ramos M. A., Calixto G., de Toledo L. G., Bonifácio B. V., dos Santos L. C., de Almeida M. T., et al. (2015). Liquid crystal precursor mucoadhesive system as a strategy to improve the prophylactic action of Syngonanthus nitens (Bong.) Ruhland against infection by Candida krusei. Int. J. Nanomed. 10 7455–7466. 10.2147/IJN.S92638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert S., Barker K. S., Znaidi S., Schneider S., Dierolf F., Dunkel N., et al. (2011). Regulation of efflux pump expression and drug resistance by the transcription factors Mrr1, Upc2, and Cap1 in Candida albicans. Antimicrob. Agents Chemother. 55 2212–2223. 10.1128/AAC.01343-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva Filho C. R. M., Souza A. G., Conceição M. M., Silva T. G., Silva T. M. S., Ribeir A. P. L. (2009). Avaliação da bioatividade dos extratos de cúrcuma (Curcuma longa L., Zingiberaceae) em Artemia salina e Biomphalaria glabrata. Rev. Bras. Farmacogn. 19 919–923. [Google Scholar]
- Simões C. M. O., Schenkel E. P., de Mello J. C. P., Mentz L. A., Petrovick P. R. (2017). Farmacognosia: Do Produto Natural Ao Medicamento. Porto Alegre: Artmed. [Google Scholar]
- Srinivasan A., Lopez-Ribot J. L., Ramasubramanian A. K. (2014). Overcoming antifungal resistance. Drug Discov. Today Technol. 11 65–71. 10.1016/j.ddtec.2014.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swamy M. K., Akhtar M. S., Sinniah U. R. (2016). Antimicrobial properties of plant essential oils against human pathogens and their mode of action: an updated review. Evid. Based Complement. Alternat. Med. 2016 1–21. 10.1155/2016/3012462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swindell K., Lattif A. A., Chandra J., Mukherjee P. K., Ghannoum M. A. (2009). Parenteral lipid emulsion induces germination of Candida albicans and increases biofilm formation on medical catheter surfaces. J. Infect. Dis. 200 473–480. 10.1086/600106 [DOI] [PubMed] [Google Scholar]
- Trentin D. S., Silva D. B., Amaral M. W., Zimmer K. R., Silva M. V., Lopes N. P., et al. (2013). Tannins possessing bacteriostatic effect impair Pseudomonas aeruginosa adhesion and biofilm formation. PLoS One 8:e66257. 10.1371/journal.pone.0066257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuberculosis Drug Screening Program (2001). Search for new drugs for treatment of tuberculosis. Antimicrob. Agents Chemother. 45 1943–1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uppuluri P., Pierce C. G., López-Ribot J. L. (2009). Candida albicans biofilm formation and its clinical consequences. Future Microbiol. 4 1235–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Vuuren S., Holl D. (2017). Antimicrobial natural product research: a review from a South African perspective for the years 2009-2016. J. Ethnopharmacol. 208 236–252. 10.1016/j.jep.2017.07.011 [DOI] [PubMed] [Google Scholar]
- Vila T. V., Chaturvedi A. K., Rozental S., Lopez-Ribot J. L. (2015). In vitro activity of miltefosine against Candida albicans under planktonic and biofilm growth conditions and in vivo efficacy in a murine model of oral candidiasis. Antimicrob. Agents Chemother. 59 7611–7620. 10.1128/aac.01890-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whaley S. G., Berkow E. L., Rybak J. M., Nishimoto A. T., Barker K. S., David Rogers P. (2017). Azole antifungal resistance in Candida albicans and emerging non-albicans Candida species. Front. Microbiol. 7:2173. 10.3389/fmicb.2016.02173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White T. C. (1997). Increased mRNA levels of ERG16, CDR, and MDR1 correlate with increases in azole resistance in Candida albicans isolates from a patient infected with human immunodeficiency virus. Antimicrob. Agents Chemother. 41 1482–1487. 10.1128/aac.41.7.1482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White T. C., Pfaller M. A., Rinaldi M. G., Smith J., Redding S. W. (1997). Stable azole drug resistance associated with a substrain of Candida albicans from an HIV-infected patient. Oral Dis. 3(Suppl. 1), S102–S109. [DOI] [PubMed] [Google Scholar]
- Wright G. D. (2017). Opportunities for natural products in 21st century antibiotic discovery. Nat. Prod. Rep. 34 694–701. 10.1039/c7np00019g [DOI] [PubMed] [Google Scholar]
- Zago C. E., Silva S., Sanitá P. V., Barbugli P. A., Dias C. M. I., Lordello V. B., et al. (2015). Dynamics of biofilm formation and the interaction between Candida albicans and methicillin-susceptible (MSSA) and -resistant Staphylococcus aureus (MRSA). PLoS One 10:e0123206. 10.1371/journal.pone.0123206 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.