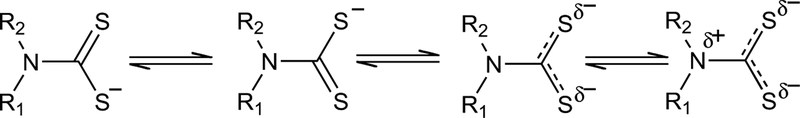Abstract
Background:
Considerable evidence demonstrates the importance of dithiocarbamates especially disulfiram as anticancer drugs. However there are no systematic reviews outlining how their metal-binding ability is related to their anticancer activity. This review aims to summarize chemical features and metal-binding activity of disulfiram and its metabolite DEDTC, and discuss different mechanisms of action of disulfiram and their contributions to the drug’s anticancer activity.
Methods
We undertook a disulfiram-related search on bibliographic databases of peer-reviewed research literature, including many historic papers and in vitro, in vivo, preclinical and clinical studies. The selected papers were carefully reviewed and summarized.
Results:
More than five hundreds of papers were obtained in the initial search and one hundred eighteen (118) papers were included in the review, most of which deal with chemical and biological aspects of Disulfiram and the relationship of its chemical and biological properties. Eighty one (81) papers outline biological aspects of dithiocarbamates, and fifty seven (57) papers report biological activity of Disulfiram as an inhibitor of proteasomes or inhibitor of aldehyde dehydrogenase enzymes, interaction with other anticancer drugs, or mechanism of action related to reactive oxygen species. Other papers reviewed focus on chemical aspects of dithiocarbamates.
Conclusion:
This review confirms the importance of chemical features of compounds such as Disulfiram to their biological activities, and supports repurposing DSF as a potential anticancer agent.
Keywords: Disulfiram, metal, dithiocarbamate, proteasome inhibitor, anticancer drug, new use of old drugs
1. INTRODUCTION
Cancer is a disease characterized by uncontrolled and abnormal cell growth. If this growth and dispersion are not curved, it can result in metastases and increase the risk of death associated with this disease. Tumor cells can undergo cell death by various routes, including a natural process known as apoptosis or programmed cell death. If the tumor doesn’t present metastatic features, initial treatment involves surgery or therapy with radiation of the primary lesion and nearby lymph nodes, in order to prevent its spread and growth. However, in many cases, tumor cells may remain after the initial treatment having potential to be re-established in a new tumor with more aggressive phenotype which is much more difficult to treat and again will have the risk of metastasis [1].
One major problem of chemotherapy and radiotherapy is their toxicity. Toxic side effects are usually associated with high doses, necessary for the induction of cell cancer death, which however, negatively contributes to the patient’s body and recovery. For this reason, it is important to discover specific anticancer drugs that can selectively induce cell death in tumor cells without effects in normal cells [2].
The typical studies related with new anticancer drugs must involve compounds that have selective potency to malignancies over normal cells via preclinical and then clinical tests. This strategy is time-consuming and expensive. A current approach being taken is a combination of several drugs that target and inhibit different metabolic pathways in cancer cells, with each drug being used at a low dose to reduce total toxicity.
In addition, repurposing an old drug for a new use, such as cancer therapy is a very attractive, exciting field. Antabuse or DSF serves as a great example of this approach. DSF has been repurposed as a potential anticancer drug due to its unique properties of known profile in human body, low-cost, little side effects and high selectivity against different cancers and synergistic activity with other drugs. Many of the biological properties of DSF seem to require its complexation with metals.
About 50 years of use is reported of DSF as an antialcoholism drug and now is being used in the treatment of cancer [3–10]. The pharmacology of DSF in patients and cell lines is well-known [11]. DSF is a promissory drug against cancer because of easy availability, cost effectiveness and less adverse effects than classical drugs cancerostatics. Another important characteristic is the ability of DSF to show tumor proteasome activities thud representing a new, promising approach to proteasome inhibition. In addition, DSF is also able to suppress different cancer-associated pathways including ROS, PIK, MAPK, NF-κB, ALDH, EGFR/Src/VEGF and others [12, 13].
2. CHEMICAL ASPECTS
2.1. General Structures of Dithiocarbamates and Disulfiram
2.1.1. Dithiocarbamates (DTC)
Dithiocarbamates (DTC) belong to a class of compounds known as 1,1-dithiolates, that include dithiophosphates, dithiopohosphinates, dithiocarbimates and other related compounds. DTC compounds comprise a large group of molecules having condensed structure R1-R2-NCSS-R3, which are chemically the semiamides of dithiocarbonic acid or dithioacids of low molecular weight [14]. The structures in Fig. (1) shows a distribution of four canonical forms of the dithiocarbamate ligands, all having an IR band in the regions 1480–1550 cm−1 corresponding to C-N bond and UV-Vis band in the regions 450 nm assignating to C-S2− bond, which appear and can be changed when they form a metal-containing complex [15]. These compounds can be easily synthetized from primary or secondary amines, and should have good solubility in water or organic solvents depending upon the nature of the cation [16].
Fig. (1).
Structures of resonance of dithiocarbamates.
One important characteristic of these compounds is their ability to be amphiphilic, lipophilic or hydrophilic depending to the R-substitutions. Normally, bifunctional or unifunctional compounds are present in these groups, which means that two different or equal chemical entities can be found in the same molecule resulting in different chemical and physical properties. For example, solubility of such a compound in both water and octanol/water mixture can be used to predict whether and how it could cross cellular membrane [17].
DTC salts are soluble in water and relatively insoluble in common organic compounds and are stable under basic and neutral conditions. However, in some cases, they can be decomposed to form isothiocyanate (basic conditions), amines, and CS2 (acid conditions). DTC can also undergo oxidation to form thiuram disulfide, through a pathway via a single electron detachment [18].
2.1.2. Disulfiram (DSF)
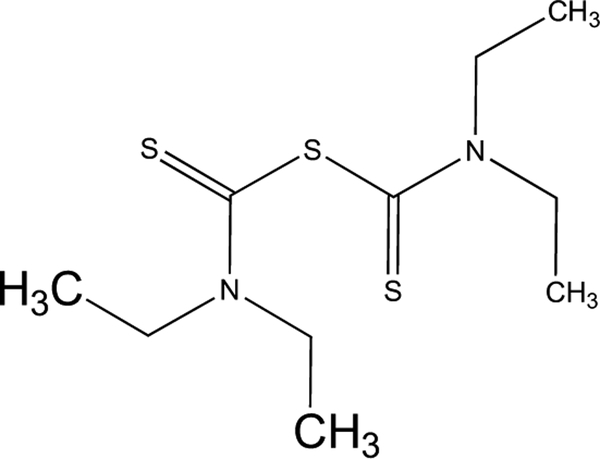
Disulfiram (DSF, Fig. 2) or tetraethylthiuram disulphide is a disulfide derivative of DEDTC. The chemical formula of DSF is C10H20N2S4 or ((C2H5)2NCS)2S2. DSF is more lipophilic than DEDTC, an important feature of DSF is that it can cross cellular membrane more easily than DEDTC. Therefore, DSF is a better drug than DEDTC for the potential treatment of human diseases.
Fig. (2).
Structure of Disulfiram.
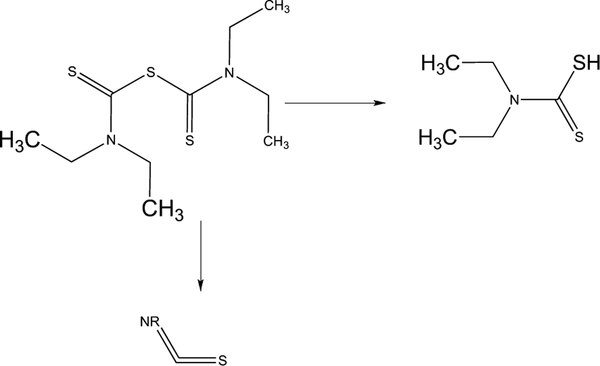
DSF can be decomposed under acidic conditions or upon reduction of its disulphide bridge to yield two diethyldithiocarbamate molecules which can then chelate copper and induce stimulation of Reactive Oxygen Species (ROS) by copper. Additionally, DSF could produce Reactive Nitrogen Species (RNS) (Fig. 3) [19].
Fig. (3).
Products of decomposition of Disulfiram.
The super reactivity of dithiocarbamates and disulfiram, is mainly related with two aspects. One aspect is their ability to chelate metals generally forming stable complex (e.g. copper, iron, zinc) and the second aspect is their high affinity for protein with thiol groups, which might be responsible for the range of adverse effects [20] and their roles as either a potent apoptosis inhibitor or inducer, depending on the circumstances [10, 21].
2.2. Interaction of DSF and DTC with Metals: Copper, Zinc, Cadmiun and Others
Dithiocarbamates are mono-anionic chelating ligands which form stable complexes with metal elements, including vanadium, chromium, molybdenum, manganese, iron, ruthenium, osmium, cobalt, nickel, platinum, copper, silver, gold, zinc, cadmium and mercury [17, 18, 22–26].
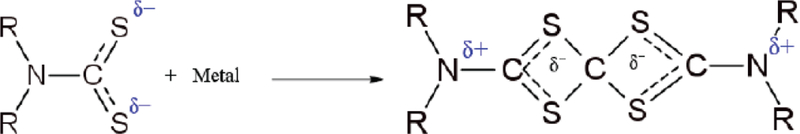
In these complexes, DTC stabilizes the metal ions with high or low oxidation states. The reaction of the formation of some complexes is related to the presence of the sulphur atoms and a change in the localization of a positive charge from the metal ion to the complex, which becomes more stable in resonance forms (Fig. 4).
Fig. (4).
Formation of complex DTC-Metal.
The complexes of DTCs with metals can be prepared by adding a basic metal solution (ammonium or alkali) to a solution of DTC. These complexes are sparingly soluble in water but more soluble in non-polar organic solvents such as chloroform, carbon tetrachloride and other. The ratio of 1: 2 (metal: dithiocarbamate) is mostly used, and the desired metal complex precipitates upon stirring the mixture for a few hours at room temperature, which can be purified by recrystallization [16, 17, 26, 27].
DTC-Metal complexes have a general formula of (RR’NCS2)2M where ‘M’ represents a divalent metal such as copper, cadmium, zinc, iron, manganese, etc. However, at high pH, the monoalkyldithiocarbamates form a further transient complex of the type (R1NCS2)M, which subsequently decomposes to the metal sulfide and isothiocyanate [5]. Complex of DTC with Pb(II), Zn(II), and Hg(II) are colorless in contrast to Cu(II), Ni(II), Co(II) and Fe(II)/(III) complexes which are intensely colored observed with the presence of UV-Vis band of wavelength between 400–700 nm characteristic S-Metal bond. The dithiocarbamate complexes are soluble in nonpolar solvents such as di-chloromethane and chloroform, but insoluble in polar solvents such as acetone, ethanol, methanol, dimethyl sulfoxide, acetonitrile and water [23, 28–31].
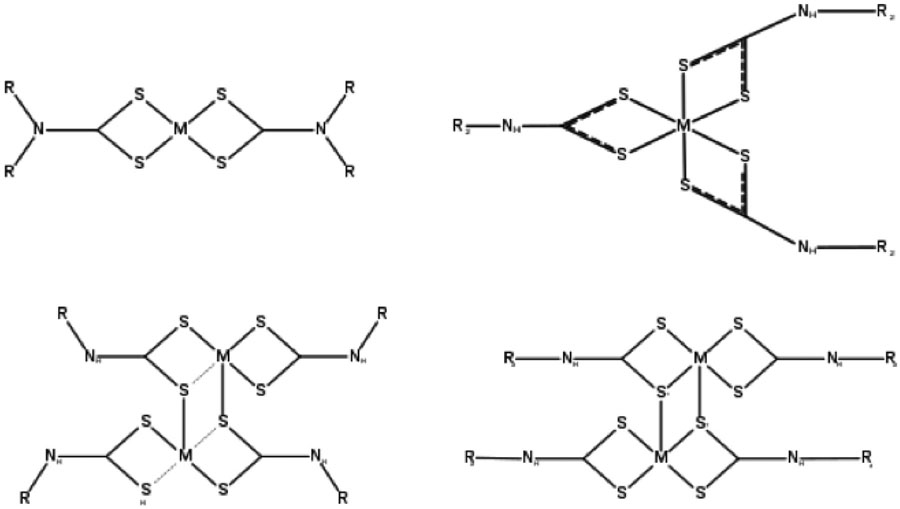
In a metal-dithiocarbamate complex, DTC generally binds to metals in a symmetrical chelation but in some cases, other coordination modes are present, such as the monodentate and bidentate modes being the most prevalent. Most of the DTC complexes have a planar shape as that of sterically non-demanding ligands and can be electronically tuned by substituents [23, 31]. These complexes can be stabilized as different metals with different oxidations states. This can be attributed to the existence of soft dithiocarbamate and hard thioureide resonance forms. The latter results from the delocalization of the nitrogen lone pair onto the sulfurs, and consequently their complexes tend to have a rich electrochemistry [6]. In these complexes, the alkoxy groups or R1–R2 substituents are not involved in the coordination to the metal centres [11, 33]. Different forms of binding between metal and DTC are shown in Fig. (5).
Fig. (5).
Different structures of metal-DTC complex.
In recent years, a number of studies have demonstrated that various DTCs induce metal uptake and metal-dependent apoptosis in a variety of cancer cell lines [3–13, 32]. In a chemical context, interest in dithiocarbamates compounds has increased due to their ability to coordinate different metals allowing determination of two or more metals in different samples (e.g. waste water or biological samples) simultaneously. For this reason, it is important to have knowledge of the coordinating behaviors of the dithiocarbamates complexes [33].
On the other hand, ternary complexes with nitric oxide have been synthetized, which could result in two compounds with different structures, depending on the number of nitric oxide complexes in the molecule. These complexes in-vitro do not inhibit proliferation in cancer cell lines which could be explained when UV-Vis spectra is studied. These compounds did not show a characteristic band at wavelenght 450 nm specific to S-Metal bond, suggesting that there is no direct interaction with metal, but interaction in this case is S-Metal-NO [28, 34, 35].
Because disulfiram is a thiuram disulphide of dithiocarbamate, products of its decomposition are similar to that of dithiocarbamate and have similar properties, such as, being able to form complexes with metals. The interaction of DSF with copper (II) chloride in solution evidences a rapid formation of the bis(N,N-diethyl dithiocarbamato) copper(II) complex in situ [19].
The DEDTC-Cu complex can be formed by the addition of copper (II) salts to DSF and a color change (from colorless to brown color) is observed most likely due to the formation of DEDTC-Cu(II) complex. Also, this same complex can be formed from a mixture of Cu(II) and DSF via spontaneous decomposition of DSF in the presence of water and copper(II) to form a complex with a theoretical 93% yield. It has been found that UV-Vis spectra of this complex have two different wavelengths both of which show similar spectra with a characteristic band in 450 nm suggesting an interaction between S from dithiocarbamate and copper [19]. The DSF-Metal complexes have similar chemical and physical properties to DEDTC-metal complexes, being soluble in nonpolar solvents but insoluble in polar solvents, and being able to form colorful complex with different metal (Table 1).
Table 1.
Spectrophotometric data from different DEDTC complexes used as anticancer agents.
| Complex | Color | Max Wavelength (nm) |
|---|---|---|
| DEDTC – Cu | Dark Brown | 270 - 290 - 450 |
| DEDTC – Zn | White | 260 – 280 |
| DEDTC – Cd | White | 260 – 285 |
| DEDTC – Co | Yellow | 245 - 270 - 480 - 640 |
| DEDTC – Ni | Yellow | 245 - 320 - 390 - 480 - 630 |
| DEDTC – Pb | White | 240 – 280 |
| DEDTC – Fe | Reddish Yellow | 260 - 280 - 480 - 640 |
2.3. Reactions with Reactives Oxygen Species
Reactive oxygen species (ROS) are a group of chemical molecules that can be easily converted to oxidizing agents or radicals [36], which can be grouped into two categories: free radical and non radical. Free radicals are species that exist independently with one or more unpaired electron like hydroxyl radical (·OH), anion hydroxyl (−OH) or anion radical superoxide (O2·−). Non radical species are molecules with a higher oxidant capacity such as hydrogen peroxide (H2O2), ozone (O3), or singlet oxygen (1O2) [37]. ROS are extremely trasient species due to their high chemical reactivity, and can cause damage on macromolecules as DNA, proteins, carbohydrates, and lipids in a cell [38]. However, antioxidants [reduced glutathione (GSH), catalase, and superoxide dismutase (SOD)] usually help to prevent damage from ROS in normal conditions [39].
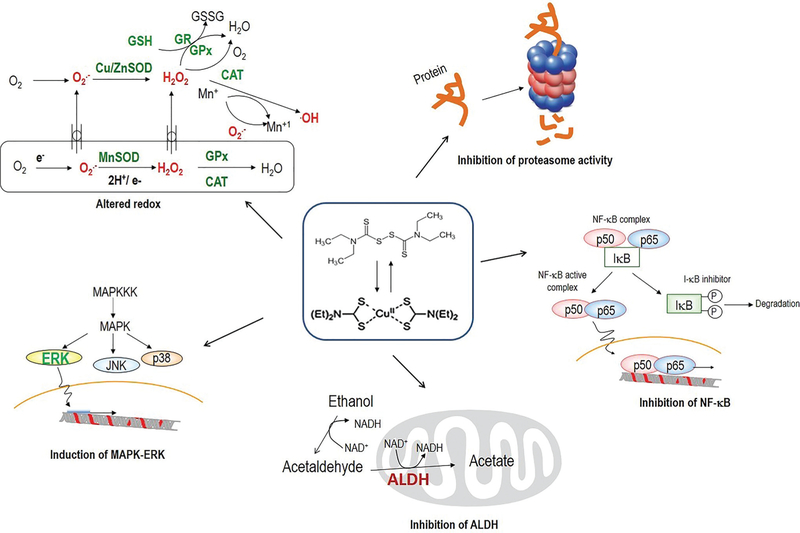
DTC has been reported to be able to alter ROS concentration and has antioxidant activity. The ROS-modulating ability of DTCs is associated with their metal-binding activity [17–19, 22–31] (Fig. 6).
Fig. (6).
Summary of mechanism of action of Disulfiram as an anticancer drug.
Metals, DSF-metal and DTC-metal complexes can produce reactive oxygen species using Fenton reaction and Haber Weiss Reactions. In these reactions, metals in the most lowest state of oxidation [for example, Fe (II), Cu (I), Ti (III), Cr (II), and Co (II)] can react with hydrogen peroxide (Fenton Reactions) and the products of this reaction, hydroxyl radical and the oxidized metal ion, can initiate another reaction (Haber-Weiss reaction), and then oxidized metallic ion is reduced by a radical anion superoxide. This important reaction regenerates the metal allowing it to engage with Fenton Reactions. In this manner, the metal is regenerated and consequently highly toxic hydroxyl species are produced [40–42].
Diethyldithiocarbamate also reacts with hydroperoxides, producing a compound whose absorption spectrum is similar to that of disulfiram, which is unable to generate a chain reaction with O2·−, unlike dithiothreitol. The diethyldithiocarbamate free radical may give an with O2, as for other thiols, but not propagate an oxidation chain. Similarly, the oxidation mechanism of diethyldithiocarbamate by hydrogen peroxide is different from that of glutathione [43, 44].
Diethyldithiocarbamate is especially active in scavenging the reactive oxygen species O2·− and H202. These oxidations yield mainly the hydrated form of disulfiram. In view of this, the reactivity of DEDTC toward hydrogen peroxide and superoxide radical was evaluated, showing that this compound is able to induce the formation of reactive oxygen species and, DSF works in similar condition in which fenton and Haber-Weiss Reactions occur [45]. DEDTC is a good free radical scavenger due their ability to trap free radicals, in some case forming complexes or new radicals [46].
2.4. Reactions with Thiols Groups
The free-radical chemistry of the dithiocarbamates is very complex, which includes production of not only ROS but also non-protein thiol compounds. Furthermore, some products of dithiocarbamates can react directly with molecules like GSH to generate more free radicals. Superoxide radical generation may be due to the interaction of some compounds with GSH and oxygen [47]. Once generated intracellularly, metal ions in their state of lower oxidation (Men+) can interact with ROS via the coupling between Fenton and Haber-Weiss reactions. Upon interaction with either superoxide (O2−), peroxyl (RO2), or hydroxyl (OH) radicals, DEDTC is oxidized to a thiyl radical, (Et)2NC(S)S, which can dimerize to form disulfiram [45, 46].
DEDTC was shown to possess a peroxidase-like activity, which utilizes exclusively glutathione as a substrate for the reduction of H2O2 and a limited number of organic hydroperoxides [48, 49].
The reaction of DSF is similar to that of H2O2 with DEDTC; generation of thiuram disulfide plays an important role in the copper-dependent GSH oxidation and apoptosis [17, 20, 21].
Besides, disulfiram in aqueous medium is converted to DEDTC by thiols as glutathione, being important in their activity in biological media, like glutathione peroxidase. However, this reaction can be reversed because the oxidations of DEDTC are complex and form several transients, producing the formation of reactive intermediates. [17, 20, 21].
3. BIOCHEMICAL TARGETS FOR ANTICANCER ACTIVITY
Many anticancer properties of Disulfiram have been demonstrated in different pre-clinical models of breast, prostate, myeloma, leukemia, lung cancers, cervical adenocarcinoma, melanoma, neuroblastoma and colorectal cancer [3–6, 8–13, 20–25] (Fig. 6). Table 2 summarizes the results of DSF and/ or its combinations used in a variety of biological systems including purified enzyme, human cancer cell models, animal model and patients.
Table 2.
Effects of Disulfiram in different biological systems.
| Model | Compounds | Concentration | Effects | |
|---|---|---|---|---|
| Cell Lines | Hep3B cells | DSF and DSF-PL-80 nanoparticles | 1–6 μM | Upregulation of BAX, downregulation of Bcl-2 |
| C8161, C8146A, M1205, W3211, melanocytes | DSF and copper | 0.17 μM - 1μM Cu | Decrease cell viability | |
| SUM149 | DSF and copper | 17 μM DSF - 10 μM Cu | Cell death depending XIAP and eIF4G1 downregulation, reduced the proportion of ALDH+ cells | |
| MCF10A | DSF – Cu | > 10 μM | Cell death | |
| MDA-IBC-3 | DSF – Cu | > 10 μM | Cell death | |
| Raji | DSF – Cu | 1 μM Cu – 3.3 μM DSF | ROS-JNK activation, Nrf2 inhibition, NF-κB inhibition | |
| Molt4 | DSF – Cu | 1 μM Cu – 0.435 μM DSF | ROS-JNK activation, Nrf2 inhibition, NF-κB inhibition | |
| C8161 | DSF – SOD | 0.15 μM DSF – 250 Unit/mL | Extracellular H2O2 generation, PARP cleavage | |
| V600E-BRaf mutant A375 | DSF – SOD | 0.15 μM DSF – 250 Unit/mL | Extracellular H2O2 generation | |
| SKBR3 | DSF - SOD | 0.1 μM DSF – 600 unit/mL SOD | PARP cleavage | |
| Crl1619 | DSF - Zn and DSF - CU | 0.5 μM DSF – 0.2-10 μM Cu and Zn | Inhibition of activation of transcription factor /cyclic AMP responsive element binding protein | |
| Chinese Hamster fibroblast V79 | DSF | 200 μM | Oxidative stress and apoptosis, increased GSH and GSSG, elevated protein carbonyl, increased Catalase, GR | |
| H2373, H2452, H2595, H2714 and H2461 | DSF – Cu | 0.1 μM to 10 μM complex | Apoptosis, increasing levels of ubiquitinated proteins, | |
| HT116GEM, H630GEM, MCF-7 | DSF- Cu | 0.3 μM to 25 μM complex | Inhibition of NF-κB, potentiation of toxic effect of gemcitabine | |
| NSCLC | DSF- Cu | 0.2 μM Cu – 100 μM DSF | Inhibition of growth | |
| Primary astrocytes | DSF- Cu | 1 μM Cu – 10 μM DSF | Oxidative stress, activation of JNK | |
| SK-N-BE(2c) | DSF | < 4 μM Cu plus 10 μM DSF | Induction of apoptosis | |
| SK-N-BE(2c) | DSF - Cu - Radiation | 0.34 μM complex | Synergistically effects | |
| PANC-1, AsPC1,BxPC-3,MIAPaCa-2, CCD-27sk | 2 mM ATO – 100 mM Ascorbic acid - 0,25 mM DSF | Aponecrosis, increased ROS, decrease ATP | ||
| c81–46A, c81–61, c83–2C | DSF | 25 nM | Apoptosis redox related | |
| Huh1, Huh7, PLC/PRF/5, Huh6 | DSF | 0.1 – 10 μM | Inhibition of tumorigenicity, ROSp38-MAPK pathway | |
| M-14, SK-Mel-19, SK-Mel-94, SK-Mel-173, Yusac, WM-278, WM-35, WM-39, WM-1552c, 451Lu | DSF – Cu | 50 – 250 nM DSF plus 250 nM Cu | Induction of ROS, inhibition of proliferation | |
| KMS-18, KMS-27, ARH-77 | DSF – Cu | 0.5 μM DSF plus 0.5 μM Cu | Induction of ROS, inhibition of cell proliferation | |
| U251MG,U87MG, U373MG | DSF – Cu | 464.9 μM DSF plus 1 μM Cu | Increased cytotoxic effect, induction of ROS, modulation of Bcl2 family, inhibition of ALDH, inhibition of NF-κB | |
| ALDH-positive cancer-stemlike cells | DSF – Cu | 0.5 μM DSF plus 1 μM Cu | Inhibition of growth | |
| U251MG, U87MG, U373MG | DSF – Cu / gemcitabine | 196.4 μM DSF plus 1 μM Cu plus 17.1 μM gemcitabine | Increased cytotoxic effect, induction of ROS, modulation of Bcl2 family, inhibition of ALDH, inhibition of NF-κB | |
| ALDH-positive cancer-stemlike cells | DSF – Cu / gemcitabine | 0.5 μM DSF plus 1 μM Cu plus 1 μM gemcitabine | Inhibition of growth | |
| TRAMP-C1 mouse, C57BL/6 mouse | DSF – Cu | 150 nM DSF plus 20 μM Cu | Elevated oxidative stress, diminished GSH | |
| Human tumor cell lines: leukemia, breast, colonrectal, lung, ovarian, renal cancer | DSF | 0.5 – 2-5 μM DSF | Induction of apoptosis | |
| Human tumor cells from patients: leukemia, breast, colonrectal, lung, ovarian, renal cancer | DSF | 0.5 – 2-5 μM DSF | Induction of apoptosis | |
| C8161, A375, C8146a | DSF | 0.17 μM – 84 nM | Facilitates intracellular Cu uptake, induces apoptosis | |
| MCF7 | DSF – PLGA-PEG nanoparticles | 250 nM DSF encapsulated | Induce reactive oxygen species formation, induce apoptosis and inhibit cell proliferation | |
| MDA-MB-157, MDA-MB-231,BT20,MCF7,T47D, BT474,MCF10A | DSF-Cu | 10 μM | Inhibition of PI3K, retard tumor growth, accumulation of ubiquitinated proteins | |
| OVCAR-3, SKOV-3, OVMZ-30, OVMZ-31, OVMZ-37, OVMZ-38 | DSF-Cu | 0-5 μM DSF – 1 μM Cu - | Cell death, increased ROS, induction of Heat Shock Protein, | |
| OVCAR-3, SKOV-3, OVMZ-30, OVMZ-31, OVMZ-37, OVMZ-38 | DSF-Cu / auranofin | 250 nM DSF – 250 nM Cu - 1 μM AUR | Cell death, synergistic effect | |
| HL-60, HL60/DOX | DSF- Cu | 1.11 – 0.44 μM | Activation of JNK/c-jun pathway, induce cytotoxicity in Doxorubicin resistant cells | |
| CD133+ nestin+ phenotype Human Pituitary Adenoma cells | DSF | 10 μM | Sensitized human pituitary adenoma cells and stem-like cells to TMZ, via the ubiquitin-proteasomal MGMT protein elimination route | |
| Animal Model | Mice | DSF – Cu | 2.88 ng DSF / 20 mg and 0.012 mg Cu / 20 mg per 10 days | Decrease growth tumor |
| Melanomas transplanted in severe combined immunodeficient mice | DSF – Zn | 200 mg/Kg/day DFS plus 1000 ppm Zn | Inhibition of growth and angiogenesis | |
| Mice | DSF – PLGA-PEG nanoparticles | 6 mg DSF/kg iv | Decrease in the breast cancer tumor growth rate | |
| Human breast tumor xenograft: female athymic nude mice | DSF | 30 mg/Kg | Inhibition of tumorigenicity | |
| Mitochondria isolation from the liver of Wistar rats | DSF | 78 ng/ mg mitochondrial protein | Mitochondrial injury Ca-dependent | |
| Murine MPM cell-derived tumors | DSF – Cu | 50 mg/kg DSF-Cu by daily | Inhibited growth of murine MPM cellderived tumors in vivo. | |
| Xenograft transplantation-Nonobese diabetic/severe combined immunodeficiency (NOD/ SCID) mic | DSF | 50 mg DSF /Kg | Inhibition of tumor volume | |
| Tumor xenografts | DSF | 200 mg/Kg DSF | Inhibition of growth | |
| Patient | IBC patient samples | DSF and copper | Altered redox response | |
| Patient with stage IV metastatic ocular melanoma | DSF - Zn | 250 mg /day DSF and 50 mg/day Zn | Reduction in size of tumor | |
| Phase I study | DSF/Temozolomide | 500–1000mg +150–200mg | Limited proteasome inhibition on peripheral blood cells | |
3.1. Role of Reactive Oxygen Species (ROS)
Many studies using DTCs suggest that these compounds (especially Disulfiram) act as a promising anti-cancer drug. Although the involved mechanism of action is still not fully defined, most researchers converge that ROS is an important mechanism to induce cancer cell death after treatment with dithiocarbamates with or without metals. The involved signaling pathways are known to be redox sensitive, and the MAPK (mitogen-activated protein kinases) and NF-κB (nuclear factor kappa B) are the most studied. Many of the redox effects are mediated either directly or indirectly by these signaling pathways, which contain many sensitive targets to oxidative stress. A variety of dithiocarbamic derivatives have been studied in cell culture models, showing their effectiveness in the redox regulation of apoptosis as either a prooxidant or an antioxidant, depending on the environment in which the compound is administered (Fig. 6, Table 2).
There are studies using a dithiocarbamate alone in different cancer cell lines. In this case DTC acts as an antioxidant or a mimetic Cu/ZnSOD compound, inducing apoptosis and necrosis, depending on its dosage, via activating NF-κB/JNK pathway and altering mitochondrial pathways that induce oxidative stress. HL-60 premyelocytic leukemia cells [50] are one of such examples. Similar studies have been done onhepatocarcinoma, ovarian, breast, colon, lung and melanoma cancer cell lines. DTCs show greater potency than cisplatin; however, DTCs have no or little effect on normal cells [51–55]. DTCs induce down-regulation of inducible nitric oxide synthase (iNOS) [28] and induction of ROS, p21, Sp1 and MnSOD, but do not cause any changes in Cu-ZnSOD and Bak/Bax levels [56, 57].
PDTC was also evaluated as a prooxidant compound in U937 hematopoietic human cancer cell line. PDTC decreases levels of SOD1 transcripts, protein and promoter activity, associated with an increase in oxidative stress through JNK/AP-1 pathways [58]. Also, DSF-Cu induces cytotoxicity in lymphoid malignant cells by inhibition of NF-κB and JNK pathway [59] (Fig. 6). Authors of another study also report the effectiveness of PDTC-Cu in NCI-H196 than NCI-H889 lung cancer cells, but not in normal human embryonal lung fibroblast cells. Response in NCI-H196 cells was ROS- and copper-dependent, which was determined by addition of NAC and Bathocuproine disulfonate, respectively. They also found that PDTC down-regulated the expression of ATP7A (a copper efflux transporter) but did not have any effect on CTR1 (copper uptake transporter). Furthermore, PDTC and cisplatin were observed to act synergistically in inducing cytotoxicity in NCI-H196 cells [60]. Similar effect was observed in HL-60 cells. PDTC complexes with Cu, but not with Fe or Pb, decreased mitochondrial membrane potential, and increased cytochrome c release and ROS. A pathway involving JNK, NF-κB and AP-1 was also found to be activated [61]. In human metastatic melanoma treated with DSF, apoptosis was shown to be ROS-dependent, associated with decreased mitochondrial membrane polarization [62].
It has also been reported that PDTC inhibits growth in p53-positive pancreatic cancer cells, with a greater potency than gemcitabine, in an ROS- and Zinc-dependent manner; involvement of nuclear translocation of the mitochondrial factor AIF but not caspase was observed. In vivo studies on PaCa44 cells xenografts in nude mice show that PDTC also reduced tumor volume without any apparent form of toxicity [63, 64]. Another group did a similar study using pancreatic cancer cells with mutated RAS; PDTC induced aponecrosis through the generation of ROS and decreased intracellular ATP. Reduction and even elimination of tumors in vivo by PDTC were observed in nude mice with PANC-1 xenografts [65].
It has also been shown that PDTC downregulates the specific DNA-binding of p53 in a copper-dependent fashion [66]. PDTC induces JNK activation in various cancer cells (Jurkat, HEK293, LNCaP, and TsuPr1). This activation was copper-dependent [53]. Treatment of neuroblastoma and glioma cells with DSF and radiotherapy showed a synergistic effect, which was more apparent in the presence of copper [67].
DSF has also been evaluated in melanoma, malignant pleural mesothelioma, non-small cell lung, breast, colon, prostate, ovarian, and squamous lung carcinoma, over normal cells (melanocytes and astrocytes). All experiments showed that DSF plus copper works as a potent anticancer drug without any effect on normal cells [67–77].
Like other dithiocarbamates, DSF is cytotoxic in Glioblastoma multiforme cells (GBM) in a copper-dependent manner. DSF is able to induce ROS, activates JNK and p38 pathways, and inhibits NF-κB [78]. Treatment of multiple myeloma cells with DSF plus copper induced mitochondrial membrane potential loss, ROS induction and executioner caspase activation [79]. Also, when tested in melanoma cells, DSF induced copper-dependent ROS and apoptosis, without any effect on melanocytes, via activation of extrinsic apoptotic pathways evaluated by caspase-8 cleavage [44]. Subtoxic doses of DSF along with H2O2 (without copper) were able to induce cell death in melanoma cells, indicating that ROS is important in disulfiram response [74]. DSF impairs the permeability of mitochondria membrane (IMM) in a Ca++-independent manner, releasing signaling molecules from mitochondria by which then induces DNA strand breakage, suggesting that an intrinsic, ROS-dependent pathway is important for apoptosis by dithiocarbamates [72].
There is evidence that a minor population of tumor cells, similar to normal stem cells and called cancer stem cells or tumor-initiating cells, organizes a cellular hierarchy and shows pronounced tumorigenic activity in xenograft transplantations. Sphere formation assays on hepatocarcinona cells (Huh1, Huh6 and Huh7) and xenograft transplantation were treated with DSF. Results showed that DSF suppressed the anchorage-independent sphere formation in hepatocarcinona cells through the activation of reactive oxygen species (ROS)-p38 MAPK pathway but not through regular ROS-JNK pathway [80].
3.2. As an Inhibitor of Aldehyde Dehydrogenase (ALDH)
Aldehyde dehydrogenases (ALDHs) are a family of 19 isoenzymes of NAD(P)+-dependent enzymes involved in detoxification of endogenous and exogenous aldehyde substrates to their respective carboxylic acids. ALDH activity is constitutively expressed in mammalian tissues, mainly in the liver, kidney, uterus, and brain. One or more ALDH isoenzymes are responsible for their activity and expression of these isoenzymes in some cells can be induced under stress conditions. ALDH activity has been used as a CSC marker in many tumors [81–86].
DSF is the best-known ALDH irreversible inhibitor, which has been used in the alcoholic patients. Inhibition by DSF induces accumulation of acetaldehyde after ethanol ingestion leading to a number of unpleasant effects collectively known as the disulfiram reaction (Fig. 6). In the models of cancer cells, the drug resistance can be associated with the transcriptional activation of ALDH1 expression [82].
Of all human ALDH isozymes, only ALDH1A1, ALDH1B1, ALDH2, ALDH3A1, ALDH3B1 and ALDH7A1 have been characterized biochemically. These enzymes have relative substrate specificity (overlapping spectrum of substrates) and it is difficult to precisely delineate isozyme-specific effects. Pharmacological inhibition is known only for ALDH2 enzyme involved in the metabolism of alcohol and ALDH1A1 and ALDH3A1 anticancer drugs [82].
In the mechanisms that can explain the inhibition of ALDH by DSF, the formation of a intramolecular disulfide bond by two mechanisms in involved: (i) between DSF and an active site thiol in the enzyme; or (ii) between the active site thiol and thiol of another cysteine residue via an unstable mixed disulfide adduct [82, 83].
Disulfiram has more affinity and potency for ALDH1 than ALDH2 (Table 3). To elaborate, in the ALDH1 enzyme, there exists a hydrophobic tunnel through which natural substrate enters along with DSF to exert the inhibitory effects [84]. DSF can be the inhibitor of ALDH via reduction of a disulfide bond and liberation of DTC (a metabolic product of DSF that can also be the inhibitor of ALDH in vivo but not in vitro. DSF is converted to other potent inhibitors of mitochondrial ALDH2 in vivo, S-methyl-N,N-diethyldithiocarbamate (DETC) and S-methyl-N,N-diethyldithiocarbamate (Me-DDTC), which are formed by hepatic thiol methyltransferases. The products of catalysis of DSF via cytochrome P-450 such as Me-DDTC, DETC-sulfoxide (DETC-SO), S-methyl-N,N-diethylthiocarbamate-sulfoxide (MeDDTC-SO) and -sulfone (Me-DDTC-SO2) also inhibit mitochondrial ALDH. The inhibition induced by all of these compounds is irreversible because of carbamylation of the catalytic Cys302 residue [20, 73, 81, 82].
Table 3.
Effect of Disulfiram in purified systems.
| Enzyme | Concentration of Enzyme in Assay | DSF or DFS Complex Value | Effect |
|---|---|---|---|
| Cytoplasmic aldehyde dehydrogenase from sheep liver | 0.1–0.2 μM | IC50: 0.14–0,2 μM DSF | Inactivation GSH dependent |
| Aldehyde dehydrogenase from Saccharomyces cerevisiae | 1 mg/mL | IC50: 20 μM DSF | Total inhibition |
| Human Mitochondrial Aldehvde Dehydrogenase | 160 μM | IC50: 0.1 – 4 μM DSF | Inhibition |
| Rat liver mitochondrial Aldehyde Dehydrogenase | Data not reported | 7.4 μM DSF | Inhibition |
| Rat Liver Mitochondrial Aldehyde Dehydrogenase | 10 μM | IC50: 36.4 μM DSF | Inhibition |
| Inhibition of 20S proteasome from rabbit | Data not reported | IC50: 7.6 μM complex DSF- Cu | Inhibition |
| Porcine kidney enzyme (pkBADH) | 0.65 μM | Range: 10–30 μM DSF Ki: 0.4 −2.4 min−1 |
Inactivation NADPH dependent |
| Human Glutathione S-Transferase | 20 nM | IC50: 5 μM DSF | Reversible inhibition and time-dependent inactivation |
| Rat Glutathione S-Transferase | 25 nM | IC50: 43 μM DSF | Reversible inhibition and time-dependent inactivation |
| hALDH1A1 purified | 10 μM | 0.15 μM | Irreversible inhibition |
| hALDH2 npurified | 10 μM | 1.45 μM | Irreversible inhibition |
In the last few years, high expression levels in ALDH enzymes were observed in different cancers, suggesting that this enzyme is important in cancer cell survival and progression [20]. In the case of cancer, two isoenzymes are the most important ALDH1A1 and ALDH1A3, and are associated with the development, progression and prognosis of cancers and tumor perpetuation and invasion [82]. ALDH1A1 positivity is found in several cancers such as lung, cervical carcinoma, ovarian, advanced squamous cell head-neck, nasopharyngeal carcinoma, bladder cancer and uterine endometrioid adenocarcinoma, and ALDH1A3 activity is observed in breast cancer, cholangiocarcinoma, prostate cancer and gliomas [13, 84].
It has been found that when tested in cell-free systems, DSF can inhibit activities of different enzymes, including ALDH, 20S proteasome and GST. The dosage and potency of DSF are summarized in Table 3 and Fig. (6).
3.3. Inhibitor of Proteasomes
Ubiquitin-proteasome system (UPS) is the most important mechanism for degradation of endogenous proteins. This system is essential for a coordinated expression of cell cycle-regulatory and apoptosis-controlling proteins. Proteasome inhibition in cancer cells is proved to be a novel anticancer strategy, as proteasome inhibitors selectively induce massive disturbances in protein homeostasis and accumulation of polyubiquitinated proteins and cytotoxic protein aggregates in cancer over normal cells. In cancer, safely administered with acceptable side-effects proteasome inhibitors have been developed as antitumor activity.
Several proteasome inhibitors have been approved by the FDA as anticancer drugs and used for the treatment of some cancers (such as multiple myeloma). Importantly, disulfiram and its combination with copper were also shown to have a strong inhibitory effect on cancer proteasomes [87–91] (Fig. 6).
3.3.1. As an Inhibitor of 20S Proteasome
The studies which tested whether dithiocarbamates act as inhibitor of proteasome started with PDTC, but not with DSF. PDTC/Cu and PDTC/Zn complexes were able to inhibit the chymotrypsin-like activity of a purified 20S proteasome with IC50 values of 13.8 μM for Zn-PDTC and 5.3 μM for Cu-PDTC. These complexes were also able to inhibit proteasome activity in prostate and breast cancer cells.
Even zinc and copper complexes kill tumor cells via proteases pathways, they use different mechanisms. Zinc complexes activate calpain independent of caspase-3 pathways and copper complexes activate both proteases, calpain and caspase-3 [6, 92].
A study using complex DSF with cadmium in human breast cancer model MCF-10DCIS and MCF-10A, suggests that in this case, Cd can act as a proteasome-inhibitor and cancer cell apoptosis inducer inhibiting chymotrypsin-like activity of 20S proteasome more potently than a carcinogen being much more sensitive in human breast cancer MCF10DCIS cells than immortalized but non-tumorigenic human breast MCF-10A cells [26, 93].
Other studies have also shown that DSF complex with copper shows similar effects as cadmium complexes inducing apoptotic cell death by inhibition of proteasomal activity in cancer cells but not in normal cells [26, 93–95]. Furthermore, cancer cells with copper concentration than patients (MDA-MB-231) show proteasome inhibition in the presence of DSF alone, suggesting that copper is a endogenous element in the cells capable of forming a complex with DSF and inducing cell death [94]. Similar effect was observed in mice bearing MDA-MB-231 tumor xenografts treated with DSF inhibiting around 74% of tumor growth, proteasome and inducing apoptosis in vivo [94]. These studies are important in order to demonstrate that copper and cadmium have similar activity as inductor of apoptosis via proteasomal activity and a synergistic effect was observed when nontoxic doses of DSF were administrated [26, 93–95].
In addition to DSF, other dithiocarbamates as pyrrolidine dithiocarbamate (PDTC) and diethyldithiocarbamate (DEDTC) also form toxic complexes with metals, showing inhibitory activity of proteasome and inducing apoptosis as anticancer compounds. In this order, a study on structure-activity relationships using eight PDTC with different substitutions in the pyrrolidine ring was performed. Results suggest low effect of proteasome inhibitory activity or antiproliferative activity in human breast cancer cells with the substitution of piperidine-PDTC, showing a decrease in activity with morpholine-PDTC substitution and no effects on piperazine-PDTC substitution. Similar results were obtained with copper-PDTC-substitution[87, 92, 96].
3.3.2. As an Inhibitor of 19S-associated DUBs
The ubiquitin-proteasome system (UPS) is a tightly regulated process responsible for maintenance of protein homeostasis in the cells. The development of cancer treatment with 19S-associated DUBs target is becoming an important anticancer strategy, e.g., therapy with bortezomib, a drug used for the treatment of multiple myeloma, and acts as an inhibitor of 20S proteasome peptidases and 19S deubiquitinases (DUBs) [97–99].
Another important example is copper-pyrithione (CuPT) complex; a copper-based proteasome inhibitor specifically inhibits the ubiquitin-proteasome system (UPS) via targeting both 19S proteasome-specific DUBs and 20S proteolytic peptidases different than bortezomib. This complex acts by inhibiting UCHL5 and USP14 activities and also inhibits tumor growth in vivo and induces cytotoxicity in vitro and ex vivo [6, 100].
Studies using Aurofin (Aur), a gold-based antirheumatic drug, as a new approach in Phase II clinical trial to treat cancer, suggest the mechanism of this drug to be proteasome-inhibitory effect similar to that of bortezomib/Velcade (Vel) but via DUBs UCHL5 and USP14 rather than the 20S proteasome. It also selectively induces cytotoxicity and inhibition of tumor growth in vivo and also in cancer cells from acute myeloid leukemia patients. This study reports the inhibition of proteasome-associated DUBs as promising anti-tumor effects [101]. Also DSF and Aur were shown to synergistically induce apoptosis and tumor growth in hepatoma cancer cells by caspase activation in an ROS-independent manner [97, 98].
3.4. Other Pathways Contributing to DSF’s Anti-cancer Activity
In glioma cells, DSF-Cu exerts an ROS-dependent antiangiogenic activity through EGFR/Src/VEGF pathway [99]. Another group suggests that DSF can exert this effect by inhibition of MMP-2 or MMP9 [102].
Complex DSF-copper in the presence of glutathione can decrease transcription factor binding to the cyclic AMP-responsive element suggesting the important role of dithiocarbamates as an inductor of S-glutathionylation of some transcription factors.
Disulfiram also plays a role in angiogenesis. In a study on melanomas transplanted in severe combined immunodeficient mice, DSF induced inhibition of growth in a Zn-dependent manner [70, 103, 104].
4. CLINICAL TRIALS
Disulfiram has previously been documented to potentiate the effect of several chemotherapy agents in vitro, including: cisplatin, gemcitabine, temozolomide, paclitaxel, docetaxel, cyclophosphamide, 5-fluorouracil, doxorubicin, sunitinib, and BCNU. Currently, disulfiram is being tested in multiple clinical trials in different cancers. There are 42 studies registered, 12 of which are related to cancer (Table 4).
Table 4.
Clinical trials using disulfiram.
| Identifier | Study type | Interaction with another drugs | Type of cancer | First Year |
|---|---|---|---|---|
| Efficacy Study Phase I/II | DSF | Stage IV melanoma | 2005 | |
| Multi-institutional Translational Clinical Trial | DSF | Men With Recurrent Prostate Cancer | 2010 | |
| Randomized Phase 2 Trial | DSF - cisplatin | Mouse model of lung cancer (non-small cell lung cancer) | 2006 | |
| Safety/Efficacy Study | DSF plus Arsenic Trioxide | Metastatic Melanoma | 2007 | |
| Interventional- Safety/Efficacy Study | DSF and chelated zinc | Refractory disseminated malignant melanoma | 2010 | |
| Phase I Study | DSF and Copper Gluconate | Refractory Solid Tumors Involving the Liver | 2008 | |
| Phase II clinical trial | DSF/Copper | Glioblastoma multiform | 2013 | |
| Randomized Controlled Trial | DSF and copper | Recurrent Glioblastoma | 2016 | |
| Multi-institutional, phase I/II study | DSF/Copper with Concurrent Radiation Therapy and Temozolomide | Patients with presumed glioblastoma multiforme | 2016 | |
| A Pharmacodynamic Study of Proteasome Inhibition | DSF/temozolomide | Glioblastoma | 2013 | |
| Safety/Efficacy Study | DSF - Temozolomide | Recurrent Glioblastoma | 2016 | |
| Phase 1 Trial and Randomized, Double-Blinded, Placebo-Controlled Expansion Cohort Study | DSF and Gemcitabine Hydrochloride | Patients With Unresectable Solid Tumors or Metastatic Pancreatic Cancer | 2016 |
In the most of these studies, DSF is administrated as coadyuvant in order to obtain non-toxic doses and the anticancer effect.
A Phase II, multicenter, randomized, double-blinded study assessed the safety and efficacy of the addition of disulfiram to cisplatin and vinorelbine. Disulfiram was administered at a dose of 40 mg three times daily. An increase in survival was noted for the disulfiram group, but however, there were only two long-term survivors [87].
A phase I study aimed to evaluate DSF alone or in combination with temozolomide in glioblastoma (GBM) patients after standard chemoradiotherapy. Disulfiram can be safely combined with temozolomide but can cause reversible neurological toxicities [105]. Herein the authors provided strong rationale for the clinical use of DSF (and Cu) in combination with current standard-of care in newly diagnosed and in patients with recurrent tumors that have acquired resistance to temozolomide.
An open-label, dose escalation trial of disulfiram in men with non-metastatic recurrent prostate cancer (PCa) after local therapy was conducted. Two cohorts received DSF daily. A minority of patients had transient global peripheral blood mononuclear cell demethylation changes. Given the toxicities and no clinical benefits, further development of disulfiram should not be pursued in this population [89].
A prospective randomized study was conducted wherein patients with Cisplatin (CP)-sensitive malignancies were assigned to therapy with CP alone or CP and DSF. Group with CP and DSF experienced higher grades of toxicity, and there was no difference in nephrotoxicity between the two groups. The authors concluded that, contrary to the previously published reports, DSF does not afford significant nephroprotection against CP and, in fact, enhances gastrointestinal and ototoxicity [106].
4.1. Disulfiram as a Potent Drug for Drug-resistant Cancers
Cancer stem cells (CSCs) can explain the phenomena of resistance of some drugs in cancer cells. These cells can have enriched levels of aldehyde dehydrogenase (ALDH), a marker expressed in certain stem/progenitor cells, and are highly drug-resistant. In studies using CFPAC-1, MIA, PaCa-2, PANC-1, and AsPc-1 (pancreatic cancer cell lines) as well as hTERT-HPNE (normal pancreatic ductal epithelial cell line) with high level of ALDH, the authors evaluated DSF as an anticancer drug in resistant cells. Most ALDH-high cancer cells were sensitive to disulfiram, such as an ALDH inhibitor, when tested in vitro. Furthermore, in vivo xenograft studies showed that the effect of disulfiram was additive to that of low-dose gemcitabine when applied in combination [107].
Glioblastomas (GBM) is a good model to study resistant cancer, however, DSF inhibited the growth of BT74 and GBM4 primary cell lines without any effect on normal human astrocytes [108].
Another important use of disulfiram is their synergistic effect when administrated with cisplatin. Using human lung adenoma cell line A549 and cisplatin-resistant A549DDP cells as a model, combination of cisplatin with DSF had a synergistic effect and decreased cell survival even in resistant cells. In this research to obtain better effects, a poly(L-glutamic acid) graft polyethylene glycol-cisplatin complex (PGA-CisPt) was administrated alone or with DSF, showing similar synergistic effects [108–110]. To translate the effects in the cells to the organisms, Balb/C nude mice with A549DDP xenografts were used, where PGA-CisPt inhibited tumor growth with low toxicity via ROS-dependent pathways as DSF, evidenced by the reduction of intracellular glutathione levels, inhibition of NF-κB activity, and changes in apoptosis-related proteins Bcl-2 and Bax [108–110].
In addition, DSF/Cu complex was reported to be able to reverse the chemoresistance in Dox-resistant leukemia HL60 cells. An important combination of dithiocarbamate in chemoresistant cells as in Dox-resistant leukemia HL60 cells, reported DSF-copper reversed resistance to Dox in these cells. The mechanism of action involved inhibition of NFκB activity, BCL-2 and activation of JNK-MAPK pathway. [82, 111].
4.2. Disulfiram in Combinations with Other Drugs
Many drugs have been studied in combination with DSF; some of them were previously explained as Cisplatin, doxycycline or some inhibitors of ALDH specially in resistant-cells. However, DFS can also act in different model cells lines as a coadjuvant or as a synergistic drug with another anticancer drug.
A study using some drugs in combination as adjuvants (artesunate, auranofin, captopril, copper gluconate, disulfiram, toconazole, nelfinavir, sertraline and temozolomide) showed that the best combination was disulfiram with the strongest potential benefit in glioblastoma [112].
Combination of DSF and auranofin can be a good choice for ovarian cancer treatment. These drugs induce cell death in a ROS- and copper-dependent manner. The mechanism of action involves induction of the inducible heat shock proteins HSP70, HSP40, and HSP32 and further enhancement of the cytotoxic effect of disulfiramn through inhibition of thioredoxin system by auranofin [113].
An important pathway in DSF toxicity is PI3K/PTEN/AKT that contributes to breast cancer. Studies demonstrated that DSF and Cu inhibited breast growth via PIK#CA, with diminished expression of PTEN and activation of AKT in a dose- and time-dependent manner in different cell lines with or without PIK3CA mutations. In this study, inhibitor of these pathways specifically PI3K, LY294002 in combination with complex DSF-Cu was synergistically observed to be an inhibitor of growth in breast cancer cell line MDA-MB-231. Also this inhibition was observed in breast tumor xenograft in nude mice induced by MDA-MB-231 cells, expressing mutant PIK3CA-H1047R and PIK3CA-E545K without effects when DSF or LY294002 was administrated alone [6, 114].
DSF and Aurofin were investigated in combination obtaining a synergistic effect in hepatoma cells in vitro and in vivo, associated to caspase activation, endoplasmic reticulum (ER) stress, and reactive oxygen species (ROS) production. A Combination DSF-Cu with temozolomide was used in Glioblastoma inducing inhibition of proteasome activity and augmenting the therapeutic effects of DNA damaging agents (TMZ and radiation). DSF-Cu should be considered as an adjuvant therapy for the treatment of patients with glioblastoma in both newly diagnosed and recurrent settings [97, 98, 101, 115].
Oral DSF as a cancer therapeutic has been tested in several completed and on-going trials without positive results due to its extreme instability under physiological conditions. DSF is quickly degraded in the gastrointestinal system, through the hepatic first-pass effect and in the blood stream. As a result, orally administered DSF does not reach tumor tissues at therapeutic concentrations. In order to obtain more stability of DSF, researchers have studied a stable loading of DSF using PLGA nanoparticles. DSF can be administrated in these nanoparticles showing unstained drug release patterns. This is a good strategy to combat the resistance of cisplatin in the tumor microenvironment using nanoparticles-loaded disulfiram (NPs-DSF) as a modulator [116].
Similar research was done in breast cancer cell. A folate-receptor-targeted poly (lactide-co-Glycolide) (PLGA)-Polyethylene glycol (PEG) nanoparticle was developed for encapsulation and delivery of disulfiram into MCF7 breast cancer cells and Hep3B cell lines [110, 117].
Other drugs administrated with disulfiram are cyclophosphamide, cisplatin and vinorelbine which were well tolerated and appeared to prolong survival in patients with newly diagnosed non-small cell lung cancer [118].
CONCLUSION
Disulfiram is an old anti-alcoholism drug which has been proved to be a promising anticancer agent. DSF is non-toxic, and its activity can be enhanced when used with metals like copper or zinc. Disulfiram can regulate different pathways in cancer cells, including: induction of ROS, inhibition of proteasome, alteration of MAPK or MMP pathways, making it possible to inhibit various types or stages of cancer. DSF is a very safe drug in both human cancer trials and alcoholism treatment and is well-tolerated with few side effects, even with long-term use.
Most studies utilizing DSF or DSF in combination with metal are focusing on inhibition of tumor growth and cancers metastasis. However, some of the clinical trials did not obtain satisfactory results. This could be due to the inappropriate therapeutic design and/or other factors such as the instability of the DSF or DSF derived complex in vivo as discussed above. Improved efficacy is expected to be achieved once these technical problems are solved.
Additionally, another therapeutic scenario should be considered for clinical applications of DSF or DSF/Cu complex in cancer therapy. ALDH1 and ALDH3 are important and well-studied stem cell markers. ALDH+ subpopulation in tumor has been confirmed to harbor strong self-renewal and tumor initiating capability. Given the fact that ALDHs are the major targets of DSF and DSF/Cu, it is possible that DSF or DSF/Cu complex will be an ideal therapeutic reagent to target cancer stem cells, which are responsible for tumor recurrence and chemoresistance. The only limitation of this approach is that ALDH+ subpopulation may not be the only stem cell subpopulation in tumor mass. Thus, to achieve the enhanced therapeutic effect, a combination therapy is needed to be identified to simultaneously target multiple stem cell populations instead of a single treatment of DSF. This approach, when in combination with chemotherapy, is expected to achieve better overall survival or disease free survival in cancer patients.
Disulfiram is an inexpensive and safe drug; if its addition to chemotherapy could be shown to prolong survival of patients, an effective regimen could be established and used widely, even in resource-poor countries. Today, the average drug costs around $2.6 billion to bring to the market and takes between 10 and 20 years from synthesis to application for FDA approval. As DSF has already been synthesized and developed, the time and cost for DSF repositioning to include cancer therapy would likely be far less than the development of a novel compound. This makes DSF an extremely cost-effective treatment option, especially when compared to current cancer drugs. DSF is showing great promise in research studies as an effective anticancer drug. In addition to the mounting evidence of its ability to inhibit the proteasome, survival pathways, and induce apoptosis in cancer cells, DSF has prominent advantages if used as a repurposed drug, Overall, continued research on DSF is highly recommended in the pursuit of its use as an effective, low-toxic, and highly cost-effective anticancer therapy.
ACKNOWLEDGEMENTS
This work was partially supported by National Cancer Institute grant R21CA184788 (to Q. Ping Dou), National Institutes of Health grant P30 CA022453 (to the Karmanos Cancer Institute at Wayne State University), the National Natural Science Foundation of China (Grant No. 81772492/H1615), and University of Cartagena Grant to internship Res No. 3487-2015 (to M. Viola-Rhenals).
Footnotes
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- [1].American Cancer Society. Cancer Facts & Figures, 2016., 2016.
- [2].Hanahan D; Weinberg RA Hallmarks of cancer: The next generation. Cell, 2011, 144(5), 646–674. [DOI] [PubMed] [Google Scholar]
- [3].Boyd P; Major I; Wang W; McConville C Development of disulfiram-loaded vaginal rings for the localised treatment of cervical cancer. Eur. J. Pharm. Biopharm, 2014, 88(3), 945–953. [DOI] [PubMed] [Google Scholar]
- [4].McConville C; Tawari P; Wang W Hot melt extruded and injection moulded disulfiram-loaded PLGA millirods for the treatment of glioblastoma multiforme via stereotactic injection. Int. J. Pharm, 2015, 494(1), 73–82. [DOI] [PubMed] [Google Scholar]
- [5].Sedlacek J; Martins L; Danek P; Pombeiro A; Cvek B Diethyldithiocarbamate complexes with metals used as food supplements different effects in cancer cells. J. Appl. Bio-med, 2014, 12, 301–308. [Google Scholar]
- [6].Fasehee H; Dinarvand R; Ghavamzadeh A; Esfandyari-Manesh M; Moradian H; Faghihi S; Ghaffari SH Delivery of disulfiram into breast cancer cells using folate-receptor-targeted PLGA-PEG nanoparticles: in vitro and in vivo investigations. J. Nanobiotechnology, 2016, 14, 32–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Diehl A; Ulmer L; Mutschler J; Herre H; Krumm B; Croissant B; Mann K; Kiefer F Why is disulfiram superior to acamprosate in the routine clinical setting? A retrospective long-term study in 353 alcohol-dependent patients. Alcohol Alcohol, 2010, 45(3), 271–277. [DOI] [PubMed] [Google Scholar]
- [8].Yang Y; Deng Q; Feng X; Sun J Use of the disulfiram/copper complex for breast cancer chemoprevention in MMTV-erbB2 transgenic mice. Mol. Med. Rep, 2015, 12(1), 746–752. [DOI] [PubMed] [Google Scholar]
- [9].Lun X; Wells JC; Grinshtein N; King JC; Hao X; Dang NH; Wang X; Aman A; Uehling D; Datti A; Wrana JL; Easaw JC; Luchman A; Weiss S; Cairn-cross JG; Kaplan DR; Robbins SM; Senger DL Disulfiram when combined with copper enhances the therapeutic effects of temozolomide for the treatment of glioblastoma. Clin. Cancer Res, 2016, 22(15), 3860–3875. [DOI] [PubMed] [Google Scholar]
- [10].Yang Y; Zhang K; Wang Y; Li M; Sun X; Liang Z; Wang L; Chen L; Yang H; Zhu L Disulfiram chelated with copper promotes apoptosis in human breast cancer cells by impairing the mitochondria functions. Scanning, 2016, 38(6), 825–836. [DOI] [PubMed] [Google Scholar]
- [11].Wickström M; Danielsson K; Rickardson L; Gullbo J; Nygren P; Isaksson A; Larsson R; Lövborg H Pharmacological profiling of disulfiram using human tumor cell lines and human tumor cells from patients. Biochem. Pharmacol, 2007, 73(1), 25–33. [DOI] [PubMed] [Google Scholar]
- [12].Yip NC; Fombon IS; Liu P; Brown S; Kannappan V; Armesilla AL; Xu B; Cassidy J; Darling JL; Wang W Disulfiram modulated ROS-MAPK and NFκB pathways and targeted breast cancer cells with cancer stem cell-like properties. Br. J. Cancer, 2011, 104(10), 1564–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Li Y; Fu SY; Wang LH; Wang FY; Wang NN; Cao Q; Wang YT; Yang JY; Wu CF Copper improves the anti-angiogenic activity of disulfiram through the EGFR/Src/VEGF pathway in gliomas. Cancer Lett, 2015, 369(1), 86–96. [DOI] [PubMed] [Google Scholar]
- [14].Orrenius S; Nobel CS; van den Dobbelsteen DJ; Burkitt MJ; Slater AF Dithiocarbamates and the redox regulation of cell death. Biochem. Soc. Trans, 1996, 24(4), 1032–1038. [DOI] [PubMed] [Google Scholar]
- [15].Warshawsky A; Rogachev I; Patil Y; Baszkin A; Weiner L; Gressel J Copper-specific chelators as synergists to herbicides: 1. Amphiphilic dithiocarbamates, synthesis, transport through lipid bilayers, and inhibition of Cu/Zn superoxide dismutase activity. Langmuir, 2001, 17, 5621–5635. [Google Scholar]
- [16].Nami SA; Ullah I; Alam M; Lee DU; Sarikavakli N Synthesis, characterization, molecular docking and biological studies of self-assembled transition metal dithiocarbamates of substituted pyrrole-2-carboxaldehyde. J. Photo-chem. Photobiol. B, 2016, 160, 392–399. [DOI] [PubMed] [Google Scholar]
- [17].Victoriano L The reactivity of metal species towards thiuram sulfides: An alternative route to the syntheses of metal dithiocarbamates. Coord. Chem. Rev, 2000, 196, 383–398. [Google Scholar]
- [18].Kanchi S; Singh P; Bisetty K Dithiocarbamates as hazardous remediation agent: A critical review on progress in environmental chemistry for inorganic species studies of 20th century. Arab. J. Chem, 2014, 7, 11–25. [Google Scholar]
- [19].Lewis DJ; Deshmukh P; Tedstone AA; Tuna F; O’Brien P On the interaction of copper(II) with disulfiram. Chem. Commun. (Camb), 2014, 50(87), 13334–13337. [DOI] [PubMed] [Google Scholar]
- [20].Burkitt MJ; Bishop HS; Milne L; Tsang SY; Provan GJ; Nobel CS; Orrenius S; Slater AF Dithiocarbamate toxicity toward thymocytes involves their copper-catalyzed conversion to thiuram disulfides, which oxidize glutathione in a redox cycle without the release of reactive oxygen species. Arch. Biochem. Biophys, 1998, 353(1), 73–84. [DOI] [PubMed] [Google Scholar]
- [21].Nobel CS; Burgess DH; Zhivotovsky B; Burkitt MJ; Orrenius S; Slater AF Mechanism of dithiocarbamate inhibition of apoptosis: Thiol oxidation by dithiocarbamate disulfides directly inhibits processing of the caspase-3 proenzyme. Chem. Res. Toxicol, 1997, 10(6), 636–643. [DOI] [PubMed] [Google Scholar]
- [22].Vigee G; Selbin J Iron (III) diethyldithiocarbamate- a new volatile metal chelate. J. Inorg. Nucl. Chem, 1970, 32, 2431–2433. [Google Scholar]
- [23].Kane S; Lazo P; Ylli F; Stafilov T; Qarri F; Marku E Separation of heavy metal from water samples--The study of the synthesis of complex compounds of heavy metal with dithiocarbamates. J. Environ. Sci. Health A Tox. Hazard Subst. Environ. Eng, 2016, 51(4), 335–340. [DOI] [PubMed] [Google Scholar]
- [24].Nardon C; Fregona D Gold(III) complexes in the oncological preclinical arena: From aminoderivatives to peptidomimetics. Curr. Top. Med. Chem, 2016, 16(3), 360–380. [DOI] [PubMed] [Google Scholar]
- [25].Ferreira I; de Lima G; Paniago E; Pinheiro C; Wardell J; Wardell S Study of metal dithiocarbamate complexes, Part V. Metal complexes of [S2CN(CH2CH(OMe)2]: A standard dimeric zinc dithiocarbamate structural motive, a rare cadmium dithiocarbamate coordination polymer, and a hydrated sodium dithiocarbarmate complex, with a[Na2O2] core and chain. Inorg. Chim. Acta, 2016, 441, 137–145. [Google Scholar]
- [26].Cvek B; Milacic V; Taraba J; Dou QP Ni(II), Cu(II), and Zn(II) diethyldithiocarbamate complexes show various activities against the proteasome in breast cancer cells. J. Med. Chem, 2008, 51(20), 6256–6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Rogachev I Kampel V; Gusis V; Cohen N; Gressel J; Warshawsky A Synthesis, properties, and use of copper-chelating amphiphilic dithiocarbamates as synergists of oxidant-generating herbicides. Pestic. Biochem. Physiol, 1998, 60, 133–145. [Google Scholar]
- [28].Januchowski R; Wojtowicz K; Zabel M The role of aldehyde dehydrogenase (ALDH) in cancer drug resistance. Biomed. Pharmacother, 2013, 67(7), 669–680. [DOI] [PubMed] [Google Scholar]
- [29].Rhenals MV; Strasberg-Rieber M; Rieber M Nitric oxide donors or nitrite counteract copper-[dithiocarbamate](2)-mediated tumor cell death and inducible nitric oxide synthase down-regulation: Possible role of a nitrosyl-copper [dithiocarbamate](2) complex. J. Med. Chem, 2010, 53(4), 1627–1635. [DOI] [PubMed] [Google Scholar]
- [30].Byung-Hoon L; Yun-Seon S; Jongsei P; Jae-Chun R Metabolism and pharmacokinetics of S-(N,N-diethyldithiocarbamoyl)_N-acetyl-L-cysteine in rats. Arch. Pharm. Res, 1994, 17, 458–433. [DOI] [PubMed] [Google Scholar]
- [31].Jamuna Rani P; Thirumaran S; Ciattini S Synthesis and characterization of Ni(II) and Zn(II) complexes of (furan-2-yl)methyl(2-(thiophen-2-yl)ethyl)dithiocarbamate (ftpedtc): X-ray structures of [Zn(ftpedtc)2(py)] and [Zn(ftpedtc)Cl(1,10-phen)]. Spectrochim. Acta A Mol. Bio-mol. Spectrosc, 2015, 137, 1164–1173. [DOI] [PubMed] [Google Scholar]
- [32].Cen D; Brayton D; Shahandeh B; Meyskens FL Jr; Farmer PJ Disulfiram facilitates intracellular Cu uptake and induces apoptosis in human melanoma cells. J. Med. Chem, 2004, 47(27), 6914–6920. [DOI] [PubMed] [Google Scholar]
- [33].Mothes M; Petzold H; Jakob A; Ruffer T,. Lang H Dithiocarbamate copper (I) and silver (I) complexes: Synthesis, structure and thermal behavior. Inorg. Chim. Acta, 2015, 429, 227–236. [Google Scholar]
- [34].Díaz A; Ortiz M; Sánchez I; Cao R; Mederos A; Sanchiz J; Brito F Interactions of nitric oxide with copper(II) dithiocarbamates in aqueous solution. J. Inorg. Biochem, 2003, 95(4), 283–290. [DOI] [PubMed] [Google Scholar]
- [35].Mederos A; Cachapa A; Hernandez-Molina R; Armas MT; Gili P; Sokolov M; Gonzalez-Platas J; Brito F Theoretical and spectrophotometrical study of the interaction of nitric oxide with copper (II) dithiocarbamates. Inorg. Chem. Commun, 2003, 6, 498–502. [Google Scholar]
- [36].Martindale JL; Holbrook NJ Cellular response to oxidative stress: Signaling for suicide and survival. J. Cell. Physiol, 2002, 192(1), 1–15. [DOI] [PubMed] [Google Scholar]
- [37].Shackelford RE; Kaufmann WK; Paules RS Oxidative stress and cell cycle checkpoint function. Free Radic. Biol. Med, 2000, 28(9), 1387–1404. [DOI] [PubMed] [Google Scholar]
- [38].Jomova K; Valko M Advances in metal-induced oxidative stress and human disease. Toxicology, 2011, 283(2–3), 65–87. [DOI] [PubMed] [Google Scholar]
- [39].Birben E; Sahiner UM; Sackesen C; Erzurum S; Kalayci O Oxidative stress and antioxidant defense. World Allergy Organ. J, 2012, 5(1), 9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Fenton H Oxidation of tartaric acid in presence of iron. J. Chem. Soc, 1894, 65, 899–910. [Google Scholar]
- [41].Kehrer JP The Haber-Weiss reaction and mechanisms of toxicity. Toxicology, 2000, 149(1), 43–50. [DOI] [PubMed] [Google Scholar]
- [42].Haber F The catalytic decomposition of hydrogen peroxide by iron salts. Proc. Roy. Soc, 1934, 147, 332–351. [Google Scholar]
- [43].Brüning A; Kast RE Oxidizing to death: disulfiram for cancer cell killing. Cell Cycle, 2014, 13(10), 1513–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Morrison BW; Doudican NA; Patel KR; Orlow SJ Disulfiram induces copper-dependent stimulation of reactive oxygen species and activation of the extrinsic apoptotic pathway in melanoma. Melanoma Res, 2010, 20(1), 11–20. [DOI] [PubMed] [Google Scholar]
- [45].Mankhetkorn S; Abedinzadeh Z; Houee-Levin C Anti-oxidant action of sodium diethyldithiocarbamate: Reaction with hydrogen peroxide and superoxide radical. Free Radic. Biol. Med, 1994, 17(6), 517–527. [DOI] [PubMed] [Google Scholar]
- [46].Zanocco AL; Pavez R; Videla LA; Lissi EA Anti-oxidant capacity of diethyldithiocarbamate in a metal independent lipid peroxidative process. Free Radic. Biol. Med, 1989, 7(2), 151–156. [DOI] [PubMed] [Google Scholar]
- [47].Klotz LO; Kröncke KD; Buchczyk DP; Sies H Role of copper, zinc, selenium and tellurium in the cellular defense against oxidative and nitrosative stress. J. Nutr, 2003, 133(5)(Suppl. 1), 1448S–1451S. [DOI] [PubMed] [Google Scholar]
- [48].Hosni M; Meskini N; Prigent AF; Anker G; Joulain C; el Habib R; Lagarde M Diethyldithiocarbamate (ditiocarb sodium) effect on arachidonic acid metabolism in human mononuclear cells. Glutathione peroxidase-like activity. Biochem. Pharmacol, 1992, 43(6), 1319–1329. [DOI] [PubMed] [Google Scholar]
- [49].Townsend DM; Tew KD; Tapiero H The importance of glutathione in human disease. Biomed. Pharmacother, 2003, 57(3–4), 145–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Kimoto-Kinoshita S; Nishida S; Tomura TT Diethyldithiocarbamate can induce two different type of death: Apoptosis and necrosis mediating the differential MAP kinase activation and redox regulation in HL60 cells. Mol. Cell. Biochem, 2004, 265(1–2), 123–132. [DOI] [PubMed] [Google Scholar]
- [51].Tan YS; Ooi KK; Ang KP; Akim AM; Cheah YK; Halim SN; Seng HL; Tiekink ER Molecular mechanisms of apoptosis and cell selectivity of zinc dithiocarbamates functionalized with hydroxyethyl substituents. J. Inorg. Biochem, 2015, 150, 48–62. [DOI] [PubMed] [Google Scholar]
- [52].Nobel CI; Kimland M; Lind B; Orrenius S; Slater AF Dithiocarbamates induce apoptosis in thymocytes by raising the intracellular level of redox-active copper. J. Biol. Chem, 1995, 270(44), 26202–26208. [DOI] [PubMed] [Google Scholar]
- [53].Chen YR; Shrivastava A; Tan TH Down-regulation of the c-Jun N-terminal kinase (JNK) phosphatase M3/6 and activation of JNK by hydrogen peroxide and pyrrolidine dithiocarbamate. Oncogene, 2001, 20(3), 367–374. [DOI] [PubMed] [Google Scholar]
- [54].Allensworth JL; Evans MK; Bertucci F; Aldrich AJ; Festa RA; Finetti P; Ueno NT; Safi R; McDonnell DP; Thiele DJ; Van Laere S; Devi GR Disulfiram (DSF) acts as a copper ionophore to induce copper-dependent oxidative stress and mediate anti-tumor efficacy in inflammatory breast cancer. Mol. Oncol, 2015, 9(6), 1155–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Han YH; Park WH The effects of N-acetyl cysteine, buthionine sulfoximine, diethyldithiocarbamate or 3-amino-1,2,4-triazole on antimycin A-treated Calu-6 lung cells in relation to cell growth, reactive oxygen species and glutathione. Oncol. Rep, 2009, 22(2), 385–391. [PubMed] [Google Scholar]
- [56].Viola-Rhenals M; Rieber MS; Rieber M Suppression of survival in human SKBR3 breast carcinoma in response to metal-chelator complexes is preferential for copper-dithiocarbamate. Biochem. Pharmacol, 2006, 71(6), 722–734. [DOI] [PubMed] [Google Scholar]
- [57].Viola-Rhenals M; Rieber MS; Rieber M Role of peroxidases, thiols and Bak/Bax in tumor cell susceptibility to Cu[DEDTC]2. Biochem. Pharmacol, 2007, 74(6), 841–850. [DOI] [PubMed] [Google Scholar]
- [58].Riera H; Afonso V; Collin P; Lomri A A Central Role for JNK/AP-1 Pathway in the pro-oxidant effect of pyrrolidine dithiocarbamate through superoxide dismutase 1 gene repression and reactive oxygen species generation in hematopoietic human cancer cell line U937. PLoS One, 2015, 10(5), e0127571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Zha J; Chen F; Dong H; Shi P; Yao Y; Zhang Y; Li R; Wang S; Li P; Wang W; Xu B Disulfiram targeting lymphoid malignant cell lines via ROS-JNK activation as well as Nrf2 and NF-kB pathway inhibition. J. Transl. Med, 2014, 12, 163–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Tahata S; Yuan B; Kikuchi H; Takagi N; Hirano T; Toyoda H Cytotoxic effects of pyrrolidine dithiocarbamate in small-cell lung cancer cells, alone and in combination with cisplatin. Int. J. Oncol, 2014, 45(4), 1749–1759. [DOI] [PubMed] [Google Scholar]
- [61].Chen SH; Lin JK; Liang YC; Pan MH; Liu SH; Lin-Shiau SY Involvement of activating transcription factors JNK, NF-kappaB, and AP-1 in apoptosis induced by pyrrolidine dithiocarbamate/Cu complex. Eur. J. Pharmacol, 2008, 594(1–3), 9–17. [DOI] [PubMed] [Google Scholar]
- [62].Cen D; Gonzalez RI; Buckmeier JA; Kahlon RS; Tohidian NB; Meyskens FL Jr. Disulfiram induces apoptosis in human melanoma cells: A redox-related process. Mol. Cancer Ther, 2002, 1(3), 197–204. [PubMed] [Google Scholar]
- [63].Donadelli M; Dalla Pozza E; Scupoli MT.; Costanzo C; Scarpa A; Palmieri M Intracellular zinc increase inhibits p53−/− pancreatic adeno-carcinoma cell growth by ROS/AIF-mediated apoptosis. Biochim. Biophys. Acta, 2009, 1793, 273–280. [DOI] [PubMed] [Google Scholar]
- [64].Donadelli M; Dalla Pozza E; Costanzo C; Scupoli MT.; Piacentini P; Scarpa A; Palmieri M Increased stability of P21WAF1/CIP1 mRNA is required for ROS/ERK-dependent pancreatic adenocarcinoma cell growth inhibition by pyrrolidine dithiocarbamate. Biochim. Biophys. Acta, 2006, 1763, 917–926. [DOI] [PubMed] [Google Scholar]
- [65].Dinnen RD; Mao Y; Qiu W; Cassai N; Slavkovich VN; Nichols G; Su GH; Brandt-Rauf P; Fine RL Redirecting apoptosis to aponecrosis induces selective cytotoxicity to pancreatic cancer cells through increased ROS, decline in ATP levels, and VDAC. Mol. Cancer Ther, 2013, 12(12), 2792–2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Verhaegh GW; Richard MJ; Hainaut P Regulation of p53 by metal ions and by antioxidants: Dithiocarbamate down-regulates p53 DNA-binding activity by increasing the intracellular level of copper. Mol. Cell. Biol, 1997, 17(10), 5699–5706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Rae C; Tesson M; Babich JW; Boyd M; Sorensen A; Mairs RJ The role of copper in disulfiram-induced toxicity and radiosensitization of cancer cells. J. Nucl. Med, 2013, 54(6), 953–960. [DOI] [PubMed] [Google Scholar]
- [68].Chen SH; Liu SH; Liang YC; Lin JK; Lin-Shiau SY Oxidative stress and c-Jun-amino-terminal kinase activation involved in apoptosis of primary astrocytes induced by disulfiram-Cu(2+) complex. Eur. J. Pharmacol, 2001, 414(2–3), 177–188. [DOI] [PubMed] [Google Scholar]
- [69].Duan L; Shen H; Zhao G; Yang R; Cai X; Zhang L; Jin C; Huang Y Inhibitory effect of Disulfiram/copper complex on non-small cell lung cancer cells. Biochem. Biophys. Res. Commun, 2014, 446(4), 1010–1016. [DOI] [PubMed] [Google Scholar]
- [70].Guo X; Xu B; Pandey S; Goessl E; Brown J; Armesilla AL; Darling JL; Wang W Disulfiram/copper complex inhibiting NFkappaB activity and potentiating cytotoxic effect of gemcitabine on colon and breast cancer cell lines. Cancer Lett, 2010, 290(1), 104–113. [DOI] [PubMed] [Google Scholar]
- [71].Cheriyan VT; Wang Y; Muthu M; Jamal S; Chen D; Yang H; Polin LA; Tarca AL; Pass HI; Dou QP; Sharma S; Wali A; Rishi AK Disulfiram suppresses growth of the malignant pleural mesothelioma cells in part by inducing apoptosis. PLoS One, 2014, 9(4), e93711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Balakirev MY; Zimmer G Mitochondrial injury by disulfiram: two different mechanisms of the mitochondrial permeability transition. Chem. Biol. Interact, 2001, 138(3), 299–311. [DOI] [PubMed] [Google Scholar]
- [73].Grosicka-Maciag E; Kurpios-Piec D; Grzela T; Czeczot H; Skrzycki M; Szumilo M Rahden-Staron I Protective effect of N-acetyl-L-cysteine against disulfiram-induced oxidative stress and apoptosis in V79 cells. Toxicol. Appl. Pharmacol, 2010, 248, 10–16. [DOI] [PubMed] [Google Scholar]
- [74].Brar SS; Grigg C; Wilson KS; Holder WD Jr; Dreau D; Austin C; Foster M; Ghio AJ; Whorton AR; Stowell GW; Whittall LB; Whittle RR; White DP; Kennedy TP Disulfiram inhibits activating transcription factor/cyclic AMP-responsive element binding protein and human melanoma growth in a metal-dependent manner in vitro, in mice and in a patient with metastatic disease. Mol. Cancer Ther, 2004, 3(9), 1049–1060. [PubMed] [Google Scholar]
- [75].Calderon-Aparicio A; Strasberg-Rieber M; Rieber M Disulfiram anti-cancer efficacy without copper overload is enhanced by extracellular H2O2 generation: Antagonism by tetrathiomolybdate. Oncotarget, 2015, 6(30), 29771–29781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Cen D; Brayton D; Shahandeh B; Meyskens FL Jr; Farmer PJ Disulfiram facilitates intracellular Cu uptake and induces apoptosis in human melanoma cells. J. Med. Chem, 2004, 47(27), 6914–6920. [DOI] [PubMed] [Google Scholar]
- [77].Denoyer D; Pearson HB; Clatworthy SA; Smith ZM; Francis PS; Llanos RM; Volitakis I; Phillips WA; Meggyesy PM; Masaldan S; Cater MA Copper as a target for prostate cancer therapeutics: Copper-ionophore pharmacology and altering systemic copper distribution. Oncotarget, 2016, 7(24), 37064–37080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Liu P; Brown S; Goktug T; Channathodiyil P; Kannappan V; Hugnot JP; Guichet PO; Bian X; Armesilla AL; Darling JL; Wang W Cytotoxic effect of disulfiram/copper on human glioblastoma cell lines and ALDH-positive cancer-stem-like cells. Br. J. Cancer, 2012, 107(9), 1488–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Conticello C; Martinetti D; Adamo L; Buccheri S; Giuffrida R; Parrinello N; Lombardo L; Anastasi G; Amato G; Cavalli M; Chiarenza A; De Maria R; Giustolisi R; Gulisano M; Di Raimondo F Disulfiram, an old drug with new potential therapeutic uses for human hematological malignancies. Int. J. Cancer, 2012, 131(9), 2197–2203. [DOI] [PubMed] [Google Scholar]
- [80].Chiba T; Suzuki E; Yuki K; Zen Y; Oshima M; Miyagi S; Saraya A; Koide S; Motoyama T; Ogasawara S; Ooka Y; Tawada A; Nakatsura T; Hayashi T; Yamashita T; Kaneko S; Miyazaki M; Iwama A; Yokosuka O Disulfiram eradicates tumor-initiating hepatocellular carcinoma cells in ROS-p38 MAPK pathway-dependent and -independent manners. PLoS One, 2014, 9(1), e84807–e84817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Alison MR; Lin WR; Lim SM; Nicholson LJ Cancer stem cells: In the line of fire. Cancer Treat. Rev, 2012, 38(6), 589–598. [DOI] [PubMed] [Google Scholar]
- [82].Koppaka V; Thompson DC; Chen Y; Ellermann M; Nicolaou KC; Juvonen RO; Petersen D; Deitrich RA; Hurley TD; Vasiliou V Aldehyde dehydrogenase inhibitors: A comprehensive review of the pharmacology, mechanism of action, substrate specificity, and clinical application. Pharmacol. Rev, 2012, 64(3), 520–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Shen ML; Lipsky JJ; Naylor S Role of disulfiram in the in vitro inhibition of rat liver mitochondrial aldehyde dehydrogenase. Biochem. Pharmacol, 2000, 60(7), 947–953. [DOI] [PubMed] [Google Scholar]
- [84].Tomita H; Tanaka K; Tanaka T; Hara A Aldehyde dehydrogenase 1A1 in stem cells and cancer. Oncotarget, 2016, 7(10), 11018–11032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Kitson TM The inactivation of aldehyde dehydrogenase by disulfiram in the presence of glutathione. Biochem. J, 1981, 199(1), 255–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Brien J Loomis c. Aldehyde dehydrogenase inhibitors as alcohol-sensitizing drugs: A pharmacological perspective. Trends Pharmacol. Sci, 1985, 6, 477–480. [Google Scholar]
- [87].Nechushtan H; Hamamreh Y; Nidal S; Gotfried M; Baron A; Shalev YI; Nisman B; Peretz T; Peylan-Ramu N A phase IIb trial assessing the addition of disulfiram to chemotherapy for the treatment of metastatic non-small cell lung cancer. Oncologist, 2015, 20(4), 366–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Huang J; Campian JL; Gujar AD; Tran DD; Lockhart AC; DeWees TA; Tsien CI; Kim AH A phase I study to repurpose disulfiram in combination with temozolomide to treat newly diagnosed glioblastoma after chemoradiotherapy. J. Neurooncol, 2016, 128(2), 259–266. [DOI] [PubMed] [Google Scholar]
- [89].Schweizer MT; Lin J; Blackford A; Bardia A; King S; Armstrong AJ; Rudek MA; Yegnasubramanian S; Carducci MA Pharmacodynamic study of disulfiram in men with non-metastatic recurrent prostate cancer. Prostate Cancer Prostatic Dis, 2013, 16(4), 357–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Verma S; Stewart DJ; Maroun JA; Nair RC A randomized phase II study of cisplatin alone versus cisplatin plus disulfiram. Am. J. Clin. Oncol, 1990, 13(2), 119–124. [DOI] [PubMed] [Google Scholar]
- [91].Kim SK; Kim H; Lee DH; Kim TS; Kim T; Chung C; Koh GY; Kim H; Lim DS Reversing the intractable nature of pancreatic cancer by selectively targeting ALDH-high, therapy-resistant cancer cells. PLoS One, 2013, 8(10), e78130–e78141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Milacic V; Chen D; Giovagnini L; Diez A; Fregona D; Dou QP Pyrrolidine dithiocarbamate-zinc(II) and - copper(II) complexes induce apoptosis in tumor cells by inhibiting the proteasomal activity. Toxicol. Appl. Pharmacol, 2008, 231(1), 24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Li L; Yang H; Chen D; Cui C; Dou QP Disulfiram promotes the conversion of carcinogenic cadmium to a proteasome inhibitor with pro-apoptotic activity in human cancer cells. Toxicol. Appl. Pharmacol, 2008, 229(2), 206–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Chen D; Cui QC; Yang H; Dou QP Disulfiram, a clinically used anti-alcoholism drug and copper-binding agent, induces apoptotic cell death in breast cancer cultures and xenografts via inhibition of the proteasome activity. Cancer Res, 2006, 66(21), 10425–10433. [DOI] [PubMed] [Google Scholar]
- [95].Yu Z; Wang F; Milacic V; Li X; Cui QC; Zhang B; Yan B; Dou QP Evaluation of copper-dependent proteasome-inhibitory and apoptosis-inducing activities of novel pyrrolidine dithiocarbamate analogues. Int. J. Mol. Med, 2007, 20(6), 919–925. [PubMed] [Google Scholar]
- [96].Wang F; Zhai S; Liu X; Li L; Wu S; Dou QP; Yan B A novel dithiocarbamate analogue with potentially decreased ALDH inhibition has copper-dependent proteasome-inhibitory and apoptosis-inducing activity in human breast cancer cells. Cancer Lett, 2011, 300(1), 87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Huang H; Liao Y; Liu N; Hua X; Cai J; Yang C; Long H; Zhao C; Chen X; Lan X; Zang D; Wu J; Li X; Shi X; Wang X; Liu J Two clinical drugs deubiquitinase inhibitor auranofin and aldehyde dehydrogenase inhibitor disulfiram trigger synergistic anti-tumor effects in vitro and in vivo. Oncotarget, 2016, 7(3), 2796–2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Huang H; Liao Y; Liu N; Cai J; Wang X; Liu J Novel use of clinical drugs: Deubiquitinase inhibitor auranofin and disulfiram show synergistic anti-tumor effects in vitro and in vivo. Cancer Cell Microenviron, 2016, 3, e1199–e1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].D’Arcy P; Linder S Proteasome deubiquitinases as novel targets for cancer therapy. Int. J. Biochem. Cell Biol, 2012, 44(11), 1729–1738. [DOI] [PubMed] [Google Scholar]
- [100].Lui N; Liu C; Li X; Liao S; Song W; Yang C; Zhao C; Huang H; Guan L; Zhang P; Lui S; Hua X; Chen X; Zhou P; Lan X; Yi S; Wang S; Wang X; Dou PQ; Lui J Anovel proteasome inhibitor suppresses tumor growth via targeting both 19S proteasome deubiquitinases and 20S proteolytic peptidases. Sci. Rep, 2014, 10, 5240–5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Liu N; Li X; Huang H; Zhao C; Liao S; Yang C; Liu S; Song W; Lu X; Lan X; Chen X; Yi S; Xu L; Jiang L; Zhao C; Dong X; Zhou P; Li S; Wang S; Shi X; Dou PQ; Wang X; Liu J Clinically used antirheumatic agent auranofin is a proteasomal deubiquitinase inhibitor and inhibits tumor growth. Oncotarget, 2014, 5(14), 5453–5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Shian SG; Kao YR; Wu FY; Wu CW Inhibition of invasion and angiogenesis by zinc-chelating agent disulfiram. Mol. Pharmacol, 2003, 64(5), 1076–1084. [DOI] [PubMed] [Google Scholar]
- [103].Lin J; Haffner MC; Zhang Y; Lee BH; Brennen WN; Britton J; Kachhap SK; Shim JS; Liu JO; Nelson WG; Yegnasubramanian S; Carducci MA Disulfiram is a DNA demethylating agent and inhibits prostate cancer cell growth. Prostate, 2011, 71(4), 333–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Murphy MJ; Wilson A; Trumpp A More than just proliferation: Myc function in stem cells. Trends Cell Biol, 2005, 15(3), 128–137. [DOI] [PubMed] [Google Scholar]
- [105].Huang J; Campian JL; Gujar AD; Tran DD; Lockhart AC; DeWees TA; Tsien CI; Kim AH A phase I study to repurpose disulfiram in combination with temozolomide to treat newly diagnosed glioblastoma after chemoradiotherapy. J. Neurooncol, 2016, 128(2), 259–266. [DOI] [PubMed] [Google Scholar]
- [106].Verma S; Stewart DJ; Maroun JA; Nair RC A randomized phase II study of cisplatin alone versus cisplatin plus disulfiram. Am. J. Clin. Oncol, 1990, 13(2), 119–124. [DOI] [PubMed] [Google Scholar]
- [107].Kim SK; Kim H; Lee DH; Kim TS; Kim T; Chung C; Koh GY; Kim H; Lim DS Reversing the intractable nature of pancreatic cancer by selectively targeting ALDH-high, therapy-resistant cancer cells. PLoS One, 2013, 8(10), e78130–e78141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Triscott J; Lee C; Hu K; Fotovati A; Berns R; Pambid M; Luk M; Kast RE; Kong E; Toyota E; Yip S; Toyota B; Dunn SE Disulfiram, a drug widely used to control alcoholism, suppresses the self-renewal of glioblastoma and over-rides resistance to temozolomide. Oncotarget, 2012, 3(10), 1112–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Kona FR; Buac D; M Burger A Disulfiram, and disulfiram derivatives as novel potential anticancer drugs targeting the ubiquitin-proteasome system in both preclinical and clinical studies. Curr. Cancer Drug Targets, 2011, 11(3), 338–346. [DOI] [PubMed] [Google Scholar]
- [110].Song W; Tang Z; Lei T; Wen X; Wang G; Zhang D; Deng M; Tang X; Chen X Stable loading and delivery of disulfiram with mPEG-PLGA/PCL mixed nanoparticles for tumor therapy. Nanomedicine (Lond.), 2016, 12(2), 377–386. [DOI] [PubMed] [Google Scholar]
- [111].Xu B; Shi P; Fombon IS; Zhang Y; Huang F; Wang W; Zhou S Disulfiram/copper complex activated JNK/c-jun pathway and sensitized cytotoxicity of doxorubicin in doxorubicin resistant leukemia HL60 cells. Blood Cells Mol. Dis, 2011, 47(4), 264–269. [DOI] [PubMed] [Google Scholar]
- [112].Kast RE; Boockvar JA; Brüning A; Cappello F; Chang WW; Cvek B; Dou QP; Duenas-Gonzalez A; Efferth T; Focosi D; Ghaffari SH; Karpel-Massler G; Ketola K; Khoshnevisan A; Keizman D; Magné N; Marosi C; McDonald K; Muñoz M; Paranjpe A; Pourgholami MH; Sardi I; Sella A; Srivenugopal KS; Tuccori M; Wang W; Wirtz CR; Halatsch ME A conceptually new treatment approach for relapsed glioblastoma: coordinated undermining of survival paths with nine repurposed drugs (CUSP9) by the international initiative for accelerated improvement of glioblastoma care. Oncotarget, 2013, 4(4), 502–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Papaioannou M; Mylonas I; Kast RE; Brüning A Disulfiram/copper causes redox-related proteotoxicity and concomitant heat shock response in ovarian cancer cells that is augmented by auranofin-mediated thioredoxin inhibition. Oncoscience, 2013, 1(1), 21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Zhang H; Chen D; Ringler J; Chen W; Cui QC; Ethier SP; Dou QP; Wu G Disulfiram treatment facilitates phosphoinositide 3-kinase inhibition in human breast cancer cells in vitro and in vivo. Cancer Res, 2010, 70(10), 3996–4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Ayala-Castro HG; Valenzuela-Soto EM; Figueroa-Soto CG; Muñoz-Clares RA Complex, unusual conformational changes in kidney betaine aldehyde dehydrogenase suggested by chemical modification with disulfiram. Arch. Biochem. Biophys, 2007, 468(2), 167–173. [DOI] [PubMed] [Google Scholar]
- [116].Song W; Tang Z; Shen N; Yu H; Jia Y; Zhang D; Jiang J; He C; Tian H; Chen X Combining disulfiram and poly(l-glutamic acid)-cisplatin conjugates for combating cisplatin resistance. J. Control. Release, 2016, 231, 94–102. [DOI] [PubMed] [Google Scholar]
- [117].Hoda M; Pajaniradje S; Shakya G; Mohankumar K; Rajagopalan R Anti-proliferative and apoptosis-triggering potential of disulfiram and disulfiram-loaded polysorbate 80-stabilized PLGA nanoparticles on hepatocellular carcinoma Hep3B cell line. Nanomedicine (Lond)., 2016, 12(6), 1641–1650. [DOI] [PubMed] [Google Scholar]
- [118].Gamelli RL; Ershler WB; Hacker MP; Foster RS The effect of disulfiram on cyclophosphamide-mediated myeloid toxicity. Cancer Chemother. Pharmacol, 1986, 16(2), 153–155. [DOI] [PubMed] [Google Scholar]