Abstract
Background:
Calcium silicate cements in treatments such as revascularization and apexogenesis are adjacent to blood and pulp tissues. This study evaluated tooth discoloration after treatment with mineral trioxide aggregate (MTA), calcium-enriched mixture (CEM) cement, and Biodentine® in the presence and absence of blood using spectrophotometric analysis.
Materials and Methods:
In this experimental study, A total of 68 extracted permanent anterior teeth were prepared and randomly divided into two groups as follows: the sponge embedded in access cavities was saturated with fresh blood or normal saline using insulin syringe, and then each group was subdivided into the following three cement subgroups: MTA-Angelus®, CEM cement, and Biodentine; these materials with a thickness of 3 mm were placed in the access cavity on the sponge. In the control group, the sponges were saturated in saline and blood in the absence of cements. Discoloration rate was measured by spectrophotometer within the following four intervals: after preparing the cavity and 1 day, 1 month, and 6 months after material placement. ANOVA and Tukey's test were used to assess the effect of blood and materials and time on discoloration. (P < 0.05).
Results:
In general, discoloration rate is significantly higher in blood group than saline group (P < 0.05) and an increase in Δ E is observed over time for the materials in all groups. In this study, discoloration rate in the presence and absence of blood in Biodentine group was lower, and this difference was statistically significant compared to that of MTA group (P < 0.05) but not significant compared to that of the CEM group.
Conclusion:
This study indicated that Biodentine induces the lowest tooth discoloration in the presence and absence of blood, and its discoloration rate is significantly lower than that of MTA. Therefore, it can be suggested that Biodentine can be used more confidently for endodontic treatments with coronal blood contamination such as regeneration and cervical perforation repair in esthetic zone of teeth.
Key Words: Calcium-enriched mixture cement, MTA-Angelus®, tooth discoloration
INTRODUCTION
Coronal discoloration has been reported as an undesirable consequence of endodontic treatments,[1] which has a significant impact on the quality of life of a person.[2] Based on many clinical studies, calcium silicate cements such as mineral trioxide aggregate (MTA) that are applied in endodontic treatments, such as pulp capping, pulpotomy, regeneration, apexogenesis, apexification, perforation repair, and root-end filling, have a high potential for discoloration of dental tissues. This property is a limitation for using these cements, especially in the anterior teeth.[3] The MTA has recently been a common alternative to calcium hydroxide and has comparable clinical results, even better than calcium hydroxide. MTA powder is a mixture of Portland cement and bismuth oxide that consists of fine hydrophilic particles and sets on mixing with water.[4] Recent studies have identified iron, magnesium, and bismuth oxide as elements responsible for the tooth discoloration; in fact, bismuth oxide exposure to dentin collagens is one of the causes of discoloration associated with white MTA (WMTA)-Angelus®. The contamination with blood is another cause of tooth discoloration. MTA without bismuth oxide also causes discoloration in the presence of blood due to porosity of the cement or the hypothesis of heme absorption from hemoglobin.[5] Although MTA can maintain pulp vitality, it has caused patient dissatisfaction with tooth discoloration. To overcome this limitation, new cements that are capable of retaining all the qualities of the MTA while preventing discoloration have been produced.[3] Calcium-enriched mixture (CEM) cement is another calcium silicate cement that was introduced to dentistry in 2006 with applications similar to the MTA but with different chemical compounds. The presence of phosphate in this cement is one of the main chemical differences with the MTA.[4] Optimal clinical and biological outcomes have made this cement suitable for endodontic applications such as pulp capping or perforation repair.[4] Although CEM cement lacks iron and bismuth oxide compared to MTA, it may cause discoloration like MTA. Tooth discoloration following the use of CEM may be related to its constituent chemical components such as calcium oxide, calcium phosphate, calcium carbonate, calcium silicate, calcium sulfate, calcium hydroxide, and calcium chloride. However, the exact mechanism of its discoloration is still unclear.[6]
Biodentine® is a new calcium silicate cement. The powder of Biodentine contains calcium carbonate, tricalcium silicate, and zirconium oxide as radiopacifiers. The liquid contains calcium chloride as an accelerator.[7] The physical properties of Biodentine, compared with the MTA, have been improved by modifying its powder composition and adding accelerator and softener and replacing bismuth oxide with zirconium oxide. Its calcium ion release and Biodentine mineralization ability are better than that of MTA;[4] discoloration induced by Biodentine has not been reported in most studies.[5,7] Therefore, reducing the content of bismuth oxide of MTA could minimize tooth discoloration.[8] The discoloration affects the material both on its surface and internally, but it is reversible with walking bleach technique.[9] This color change exacerbates in the presence of blood and may necessitate a veneer or crown.[9]
Since most of the studies which evaluated the discoloration of tooth did not consider the role of blood in this matter, this ex vivo study evaluated the induced tooth discoloration rate after the application of MTA, CEM, and Biodentine in the presence and absence of blood. These three cements have not yet been compared in any study in the presence of blood.
MATERIALS AND METHODS
In this experimental study, samples consisted of 68 permanent anterior single-rooted teeth extracted due to advanced periodontal disease and had no decay, repair, and crack. The sample size was estimated according to previous studies.[7] The teeth were disinfected in a solution of sodium hypochlorite (5.25%) for 1 h and then immersed in normal saline until testing according to Shokouhinejad et al's. study.[7]
Preparation of specimens
Debris and surface pigments were removed by Ultrasonic Scaler (NSK, Japan), followed by polishing with prophylaxis paste. To standardize the specimens, the apical portion of the root was sectioned by dental diamond bur (#010) (Teeskavan, Iran) perpendicular to the longitudinal axis of the tooth, so that 5 mm of the root remained. After preparing standard access cavities, the shortened root canals were cleaned and shaped by #1–6 Gates Glidden Drills (Dentsply Maillefer, Ballaigues, Switzerland) and rinsed with 5.25% sodium hypochlorite and saline. Next, a cylindrical piece of white sponge was placed from the root end to the cementoenamel junction according to Shokouhinejad et al's. study.[7]
Afterward, in all samples of the groups, the end of the root and dentin surfaces were etched and bonded in cross section and filled with Composite Resin A3 (3M™ ESPE™, USA) as an apical restoration.
Experimental setup
In this study, we used an experimental model used in the previous study.[7] The teeth were randomly divided into two groups of blood and normal saline. Fresh human blood was taken by a trained person from a healthy volunteer. The sponges embedded in 30 samples were saturated with fresh blood and the sponges embedded in another 30 samples were saturated with normal saline using insulin syringe, and then each group was subdivided into three cement subgroups. The tested three cements, namely MTA-Angelus (Angelus, Londrina, PR, Brazil), CEM cement (Yektazist Dandan, Tehran, Iran), and Biodentine (Septodont, Saint-Maur-des-Fosses, France), were prepared according to the manufacturer's instructions, and these cements (with a thickness of 3 mm) were placed in the access cavity on the sponges saturated by blood or normal saline. Then, the contact of material with sponge was ensured through a gentle pressure of the plastic instrument. A wet cotton pellet was placed on the cements for setting. After 24 h when the setting of materials was checked with the light pressure of endodontic explorer, all the cavities were sealed with composite resin A3 (3M ESPE, USA) by 2 mm. In the remaining eight teeth (control group), the sponges were saturated in the normal saline for four teeth and in the blood for four others and were restored with composite in the absence of bioceramics. All the specimens were kept in an incubator at 37°C and 100% humidity.
Color assessment
The discoloration rate of each specimen was measured using a spectrophotometer (Vita Easy shade, Germany) by the same operator. The device was calibrated in accordance with the manufacturer's instructions. Coronal discoloration rate of each specimen was measured by the spectrophotometer within the following four intervals: after preparing the cavity (at the baseline) and at 1 day, 1 month, and 6 months after material placement. In order to reproduce tooth positioning under the device, a 2 mm × 3 mm rectangular window was created by a needle diamond bur (Teeskavan, Iran) in the cervical and middle-thirds of the labial side of the crown and each time, the device tip was placed in the same window as the reference. For repetitive position, the teeth were mounted in a putty mold. The labial side was positioned upward to allow it to stand in front of the device eye. After each analysis, the specimens were removed and placed again in the incubator at 100% humidity.
Color assessment was reported using the L*a*b* system. In each analysis, the evaluation was performed in three replicates for each tooth and then averaged. Next, the tooth discoloration (ΔE) was calculated according to the equation of
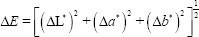
In addition, the brightness value (ΔL) was obtained according to the equation of Lt_ L0; where, L* denotes the lightness ranging from 0 (black) to 100 (white), a* indicates the redness/greenness (negative values: greenness and positive values: redness), and b* shows blueness/yellowness (negative values: blueness and positive values: yellowness).
Statistical analysis
The obtained data was statically analyzed using ANOVA, Tukey's test, and repeated measures analysis of variance to evaluate and compare the effects of three factors (contamination with blood, type of material, and time) on ΔE and ΔL between the groups at different times. P <0.05 was considered to be statistically significant.
RESULTS
ΔE: Based on the findings of Figure 1, there was no significant difference in the discoloration rate after 1-day treatment between different materials adjacent to the saline. After 1 month and 6 months, the color change in the control/saline group and the Biodentine/saline group was lower than that of the other groups in the absence of blood, which was statistically significant with the MTA/saline group (P < 0.05), but no significant difference in discoloration was observed between Biodentine/saline and CEM/saline groups and also between MTA/saline and CEM/saline groups.
Figure 1.
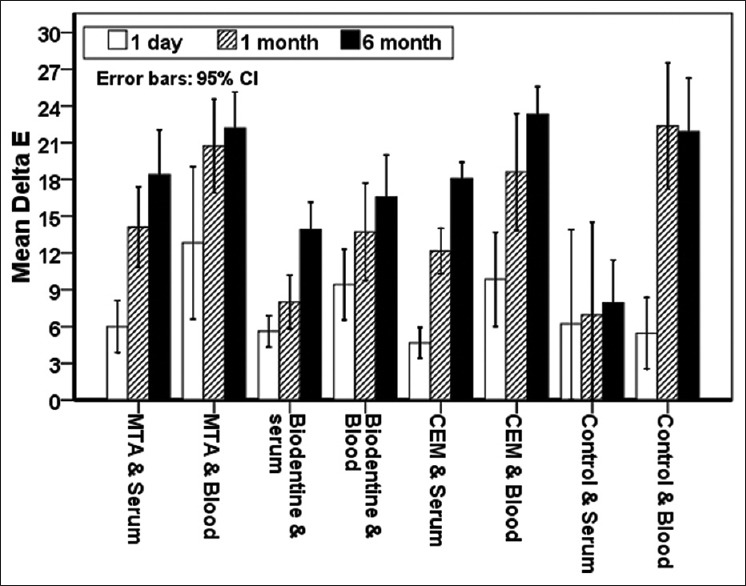
ΔE average in both saline and blood media at desired times.
In the presence of blood, there was no significant color change between the different materials on the 1st day. After a month, the Biodentine/blood group showed significantly less discoloration than MTA/blood group (P < 0.05), and after 6 months, Biodentine/blood group showed significantly less discoloration than MTA/blood and CEM/blood groups (P < 0.05). The highest discoloration rate was observed in control/blood and MTA/blood groups, but not significant compared to the CEM/blood group. In general, discoloration (ΔE) rate is significantly higher in blood group than saline group and over time there is an increase in discoloration (ΔE) for the materials in all groups in the presence of both blood and saline.
ΔL: Based on the findings of Figure 2, ΔL in the blood group was significantly higher than that in the saline group, and this luminosity change was significantly higher in the CEM/blood and MTA/blood groups compared to that of the saline groups (P < 0.05).
Figure 2.
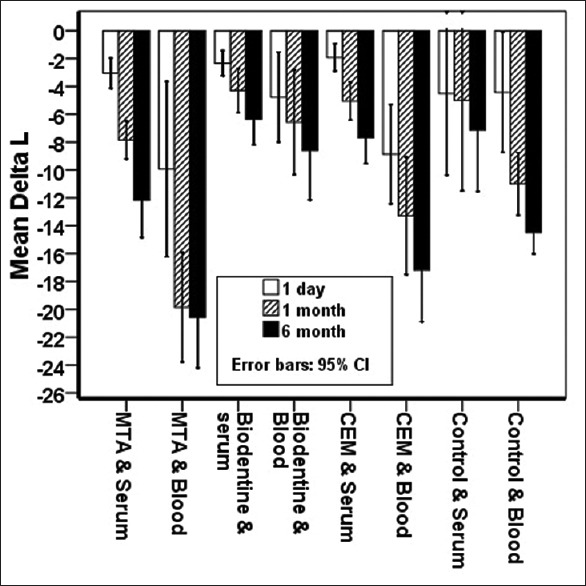
ΔL average in both saline and blood media at desired times.
After 1 month and 6 months, the Biodentine/blood group showed less luminosity change compared to CEM/blood and MTA/blood groups.
Moreover, over time, there is an increase in Δ L for the materials in all groups in the presence of both blood and saline.
DISCUSSION
Based on many clinical studies, it has been observed that some calcium silicate cements have a high potential for discoloration of dental tissues, which is an undesirable consequence of endodontic treatments.[1,3]
This ex vivo study compared the degree of coronal discoloration after the application of three widely used endodontic materials, namely MTA-Angelus, CEM cement, and Biodentine, in the presence and absence of blood in human teeth.
This study examined the extracted human anterior teeth, and according to the study of Shokouhinejad et al.,[7] after the application of cements and their setting, access cavity was restored with composite resin. Some studies did not provide the coronal access cavities and the cements were retrograded through the root canal in the pulp chamber.[8,9] It is obvious that absence of coronal access cavity does not similar to the clinical conditions and makes it difficult to remove residual pulp tissues from the pulp chamber and pulp horns. In this study, we examined the effect of access cavity on tooth discoloration; comparison of Δ E before and after the cavity preparation showed slight color change, but this difference was not statistically significant (P > 0.05). Marciano et al.[10] reported that composite resin did not play a role in the discoloration process of calcium silicate cements.
The present study employed fresh human blood to check the effect of blood on coronal discoloration after using cements to simulate clinical conditions during vital pulp therapy.
Tooth discoloration is usually measured by special instruments such as spectrophotometer. Visual spectrophotometer is considered as a gold standard instrument for color assessment and has been applied successfully in dentistry.[3] According to Dozić et al., spectrophotometer has shown the highest reliability in both in vivo and in vitro conditions;[11] facilitates quantitative measurements of the transmission or reflection of light through the samples; and is a repeatable, precise, and reliable instrument.[3]
The CIE L*a*b* system is a sensitive technique that even analyzes slight color changes. The Δ E, obtained from the values of L*, a*, and b*, shows the difference of final and initial color. ΔE ≥3.3 represents a clinically perceptible discoloration;[3] according to this definition, all materials in the present study showed a visible discoloration at all times, which changed increasingly over time, and also, it was found that all the three cements in the presence of blood caused more severe discoloration than the saline samples, and this difference was significant compared to the saline group.
In the present study, MTA-Angelus exhibited the highest discoloration in the presence of saline. The tooth discoloration associated with WMTA has been reported in various in vivo and in vitro studies.[1] Bismuth oxide, which is added to the MTA to improve its radiopacity, appears to be responsible for respective tooth discoloration.[2] When bismuth oxide reacts with collagen, it will turn into black sediment. In addition, when bismuth oxide is oxidized, its oxygen molecule will become unstable and react with carbon dioxide in the air, resulting in the production of bismuth carbonate that is an agent for discoloration. Another possible mechanism for MTA-induced discoloration is the oxidation of iron contained in the set material, which is related to the phase of calcium aluminoferrite in cement powder.[3] Considering that the interaction between bismuth oxide and sodium hypochlorite is another possible mechanism of discoloration[7] and we also used NaOCL 5.25% when preparing cavities, this agent may be one of the causes of further discoloration of MTA-Angelus.
In the current study, in the absence of blood, MTA-Angelus exhibited the highest discoloration and Biodentine had the lowest discoloration rate. There was a significant difference in the discoloration rate between these two materials. These results are consistent with many studies[1,2,7,12,13,14] and are contradictory with the results of Beatty and Svec[15] as their study showed a higher discoloration for Biodentine compared to ProRoot® MTA.
In Biodentine formulation, zirconium oxide has been used as a radiopacifier instead of bismuth oxide.[13] Most studies[1,2,12,14,16] reported the calcium silicate cements that lack the bismuth oxide in their formulation (such as the Biodentine) and instead contain radiopacifiers such as calcium zirconium complex, zirconium oxide, or titanium oxide have less potential for discoloration.[1]
Marciano et al. reported that zirconium oxide and calcium tungstate exhibit color stability. In their study, zirconium oxide and calcium tungstate in contact with collagen showed no discoloration, whereas bismuth oxide rendered an obvious discoloration in contact with collagen.[10] This finding can explain the color stability of Biodentine reported in other study.[13]
CEM cement also clearly showed less discoloration compared to MTA-Angelus in the presence of saline, but not statistically different; this finding was consistent with a study by Esmaeili et al.[17](but the difference between CEM and MTA was significant in their study) and in contrast to the results of a study by Arman et al.[6] who found no clinical difference in the discoloration rate between the two materials.
Unlike MTA, CEM cement has no iron (Fe) and bismuth oxide in its content.[6] According to studies, both of these factors (iron and bismuth oxide) can cause discoloration.[2,8] Therefore, CEM cement discoloration is expected to be less than MTA. This finding was also found in the present study; the cause of less discoloration of CEM cement compared to MTA-Angelus in our study can be attributed to the absence of iron and bismuth oxide in the structure of CEM cement compared to MTA-Angelus.
However, studies have demonstrated that CEM cement may indicate discoloration through its other constituents (calcium carbonate, calcium oxide, calcium phosphate, calcium silicate, calcium sulfate, calcium hydroxide, and calcium chloride),[6] and this could explain further the discoloration found in CEM cement compared with Biodentine in the present study.
In this study, Biodentine showed less discoloration compared to CEM cement, but not statistically different. No previous study has compared these two materials, and our study for the first time revealed that Biodentine has less discoloration compared to CEM cement.
In the present study, it was found that all the three cements in the presence of blood caused more severe discoloration than the saline samples, and this difference was significant compared to the saline group. These findings are in line with the results of all studies that have applied calcium silicate cements in the presence of blood.[7,9,18]
Felman and Parashos,[9] Shokouhinejad et al.,[7] and Lenherr et al.[18] found that contamination with blood significantly worsens the discoloration associated with calcium silicate cements with or without bismuth oxide or other radiopacifiers, in line with this study.
One of the hypotheses to explain the more severe discoloration in the presence of blood can be hemolysis and absorption of erythrocytes into the structure of the teeth and calcium silicate materials. The lysis of erythrocytes forms products such as iron, hemoglobin, and hematin derivatives that enter the dentinal tubules. On the other hand, blood can induce discoloration due to the penetration into the porosity of the cements.[18,19]
In this study, the discoloration rate of Biodentine was significantly lower in the presence of blood than that in other materials, However, Shokouhinejad et al.[7] reported that all materials caused equal discoloration in the presence of blood. In the presence of blood, the discoloration by MTA was more than CEM, and the discoloration by Biodentine was the least. A hypothesis to explain this finding can be attributed to the difference in the amount of porosity in cements.[19] Blood can cause discoloration with penetration into the porosities of the cements. Less discoloration of CEM in the presence of blood is likely due to its smaller particle size compared to MTA and its more homogeneous structure. Biodentine has also formed less discoloration in the presence of blood due to its denser structure and less porosity compared to MTA.[4]
According to studies, Biodentine has sealing ability similar to MTA and has setting time much shorter than MTA (about 12 min).[1] Considering these advantages compared to MTA and given that the results of this study indicated that Biodentine induces the least tooth discoloration in the presence and absence of blood and its discoloration rate is significantly less compared to that of MTA, it can be suggested that Biodentine can be used more confidently in areas where esthetics is important.
Financial support and sponsorship
Nil.
Conflicts of interest
The authors of this manuscript declare that they have no conflicts of interest, real or perceived, financial or nonfinancial, in this article.
Acknowledgment
The authors would like to be thankful to the Deputy of Research and Technology of Babol University of Medical Sciences for their support.
REFERENCES
- 1.Shokouhinejad N, Khoshkhounejad M, Alikhasi M, Bagheri P, Camilleri J. Prevention of coronal discoloration induced by regenerative endodontic treatment in an ex vivo model. Clin Oral Investig. 2018;22:1725–31. doi: 10.1007/s00784-017-2266-0. [DOI] [PubMed] [Google Scholar]
- 2.Marconyak LJ, Jr, Kirkpatrick TC, Roberts HW, Roberts MD, Aparicio A, Himel VT, et al. A comparison of coronal tooth discoloration elicited by various endodontic reparative materials. J Endod. 2016;42:470–3. doi: 10.1016/j.joen.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 3.Możyńska J, Metlerski M, Lipski M, Nowicka A. Tooth discoloration induced by different calcium silicate-based cements: A systematic review of in vitro studies. J Endod. 2017;43:1593–601. doi: 10.1016/j.joen.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 4.Dawood AE, Parashos P, Wong RH, Reynolds EC, Manton DJ. Calcium silicate-based cements: Composition, properties, and clinical applications. J Investig Clin Dent. 2017;8:e12195. doi: 10.1111/jicd.12195. [DOI] [PubMed] [Google Scholar]
- 5.Torabinejad M, Parirokh M, Dummer PM. Mineral trioxide aggregate and other bioactive endodontic cements: An updated overview – Part II: Other clinical applications and complications. Int Endod J. 2018;51:284–317. doi: 10.1111/iej.12843. [DOI] [PubMed] [Google Scholar]
- 6.Arman M, Khalilak Z, Rajabi M, Esnaashari E, Saati K. In vitro spectrophotometry of tooth discoloration induced by tooth-colored mineral trioxide aggregate and calcium-enriched mixture cement. Iran Endod J. 2015;10:226–30. doi: 10.7508/iej.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shokouhinejad N, Nekoofar MH, Pirmoazen S, Shamshiri AR, Dummer PM. Evaluation and comparison of occurrence of tooth discoloration after the application of various calcium silicate-based cements: An ex vivo study. J Endod. 2016;42:140–4. doi: 10.1016/j.joen.2015.08.034. [DOI] [PubMed] [Google Scholar]
- 8.Kang SH, Shin YS, Lee HS, Kim SO, Shin Y, Jung IY, et al. Color changes of teeth after treatment with various mineral trioxide aggregate-based materials: An ex vivo study. J Endod. 2015;41:737–41. doi: 10.1016/j.joen.2015.01.019. [DOI] [PubMed] [Google Scholar]
- 9.Felman D, Parashos P. Coronal tooth discoloration and white mineral trioxide aggregate. J Endod. 2013;39:484–7. doi: 10.1016/j.joen.2012.11.053. [DOI] [PubMed] [Google Scholar]
- 10.Marciano MA, Costa RM, Camilleri J, Mondelli RF, Guimarães BM, Duarte MA, et al. Assessment of color stability of white mineral trioxide aggregate angelus and bismuth oxide in contact with tooth structure. J Endod. 2014;40:1235–40. doi: 10.1016/j.joen.2014.01.044. [DOI] [PubMed] [Google Scholar]
- 11.Dozić A, Kleverlaan CJ, El-Zohairy A, Feilzer AJ, Khashayar G. Performance of five commercially available tooth color-measuring devices. J Prosthodont. 2007;16:93–100. doi: 10.1111/j.1532-849X.2007.00163.x. [DOI] [PubMed] [Google Scholar]
- 12.Ramos JC, Palma PJ, Nascimento R, Caramelo F, Messias A, Vinagre A, et al. 1-year in vitro evaluation of tooth discoloration induced by 2 calcium silicate-based cements. J Endod. 2016;42:1403–7. doi: 10.1016/j.joen.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 13.Vallés M, Roig M, Duran-Sindreu F, Martínez S, Mercadé M. Color stability of teeth restored with biodentine: A 6-month in vitro study. J Endod. 2015;41:1157–60. doi: 10.1016/j.joen.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 14.Kohli MR, Yamaguchi M, Setzer FC, Karabucak B. Spectrophotometric analysis of coronal tooth discoloration induced by various bioceramic cements and other endodontic materials. J Endod. 2015;41:1862–6. doi: 10.1016/j.joen.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 15.Beatty H, Svec T. Quantifying coronal tooth discoloration caused by Biodentine and endo sequence root repair material. J Endod. 2015;41:2036–9. doi: 10.1016/j.joen.2015.08.032. [DOI] [PubMed] [Google Scholar]
- 16.Camilleri J. Staining potential of neo MTA plus, MTA plus, and biodentine used for pulpotomy procedures. J Endod. 2015;41:1139–45. doi: 10.1016/j.joen.2015.02.032. [DOI] [PubMed] [Google Scholar]
- 17.Esmaeili B, Alaghehmand H, Kordafshari T, Daryakenari G, Ehsani M, Bijani A, et al. Coronal discoloration induced by calcium-enriched mixture, mineral trioxide aggregate and calcium hydroxide: A spectrophotometric analysis. Iran Endod J. 2016;11:23–8. doi: 10.7508/iej.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lenherr P, Allgayer N, Weiger R, Filippi A, Attin T, Krastl G, et al. Tooth discoloration induced by endodontic materials: A laboratory study. Int Endod J. 2012;45:942–9. doi: 10.1111/j.1365-2591.2012.02053.x. [DOI] [PubMed] [Google Scholar]
- 19.Akbulut MB, Terlemez A, Akman M, Buyukerkmen B, Guneser MB, Eldeniz AU. Tooth discoloration effects of calcium silicate based barrier materials used in revascularization and treatment with internal bleaching. J Dent Sci. 2017;12:347–53. doi: 10.1016/j.jds.2017.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]


