Abstract
Objective and Aims:
Overweight/obese children are at risk of developing type 2 diabetes mellitus. Random glucose elevations provide early warning signs of glycemic dysregulation. To assess random blood glucose (RBG) concentrations and risk factors associated with prediabetes in children aged 3-18 years from six Indian regions.
Method:
Multicenter, cross sectional, observational school-based study; multi-stage stratified random sampling was carried out. Height and weight measured; body mass index (BMI) was computed. RBG measured using a glucometer. National sample survey was used for dietary patterns. Data were analyzed using SPSS 25.0 for Windows.
Setting:
Study centers were from Maharashtra, Gujarat, Chhattisgarh, Assam, Tamil Nadu and Punjab from 40 selected schools.
Participant:
Children aged 3-18 years were measured.
Results:
Data on 14339 subjects (7413 boys) were analyzed. Prevalence of obesity was 5.8% and overweight-10.6%. Overall, 1% had low (<3 mmol/L), 93.7% in reference range (3.9-7.2 mmol/L) and 5.3% had elevated RBG (>7.2 mmol/L). With increasing mean BMI, there was increase in RBG concentrations. Children from Tamil Nadu were more likely to have RBG outside reference range compared to other regions (P < 0.05). Assam and Punjab had highest prevalence of RBG and BMI within reference range. Energy intake partly explained regional variations. Multivariate analysis showed male gender, urban residency, age >10 yrs (girls) and 13 yrs (boys), and overweight or obesity were predictive of prediabetes.
Conclusion:
Increased prevalence of overweight, obesity and prediabetes in Indian children are a matter of concern. Regional differences suggest that strategies to prevent obesity and combat perturbations in blood sugar may have to be customized.
Keywords: Children, Indian, overweight, prediabetes, random blood glucose
INTRODUCTION
Prevalence of diabetes in India is increasing, possibly due to a rise in obesity and changing lifestyles with nutrition transition; our earlier studies have indicated an increase in the risk of metabolic syndrome in children and adolescents.[1] The overall prevalence of overweight and obesity has been reported to be 18.2-23.9% in Indian children and adolescents.[2] Asian-Indians have been reported to have an increased susceptibility to insulin resistance from infancy, which is a forerunner of type 2 diabetes.[3,4]
Reports from at risk countries such as the Middle East suggest that half the adolescents with blood glucose concentrations in the prediabetes range (>7.8 mmol/L) are overweight/obese.[5] Thus, pediatric obesity also has immediate health effects such as prediabetes.[6] Recognizing prediabetes in children and adolescent populations can potentially reduce the risk of developing diabetes.
The American Diabetic Association (ADA) proposes that fasting blood glucose is appropriate for screening for prediabetes/type 2 DM;[7] and the World Health Organisation (WHO) accepts measurements of glucose in capillary blood.[8] Fasting capillary blood glucose has thus been used for screening for diabetes.[9] However, glucose homeostasis is usually tightly regulated and when individuals' transition from normal glucose metabolism to prediabetes and diabetes, glycemic variability increases.[10] Studies thus suggest that random glucose elevations provide an early warning sign of glycemic dysregulation.[11,12]
In India, the cuisine varies widely across the country according to the region, culture and tradition.[13] The share of energy intake contributed by cereals has been reported to vary from 57% for rural India to 48% for urban India; the contribution of cereals varies across the major states from 42% (Punjab) to 70% (Odisha) in the rural sector, and from 39% (Haryana) to 60% (Odisha and Bihar) in the urban sector.[14] These patterns of food across states and urban and rural areas are likely to have a varied impact on blood sugar concentrations.
There are a few studies that have looked at impaired glucose concentrations and its relation with obesity from some states of India. In a population-based study from southern India, the overall prevalence of dysglycaemia was 3.7%, which increased to 12.7% in girls with abdominal obesity.[15] In a study from North India, the prevalence of impaired fasting glucose and impaired glucose tolerance among obese adolescents was 6.5% and 5.5%, respectively.[16] However, larger multi centric studies across states have not been conducted. Therefore the aim of our study was to assess random blood glucose (RBG) concentrations and risk factors associated with prediabetes in school children aged 3-18 years from 6 major Indian regions. Our specific objectives were:
To assess RBG concentrations in 3-18 years old children from 6 different regions of India including urban and rural areas and to explore regional differences
To evaluate relationship of BMI and RBG concentrations across regions
To investigate selected risk factors associated with elevated RBG in 3-18 year olds.
METHODS
Study design and subjects
This was a part of a multicenter, cross sectional, observational school-based study. Sampling was carried out by adopting a multi-stage stratified random sampling procedure. Of the Indian states, 6 states were randomly selected (census data 201, http://www.censusindia.gov.in/pca/Searchdata.aspx) on percentage of population in the age group 3-18 years. At the second stage, six cities (one/state), were selected. From the neighboring villages around the selected cities, six villages, one village near each city was randomly selected. The study centers thus selected were Maharashtra (Pune, Ranjangaon), Gujarat (Bilimora, Gandevi), Chhattisgarh (Raipur, Kurud), Assam (Diphu, Manja), Tamil Nadu (Chennai, Urapakkam) and Punjab (Mohali, Lalru). At the third stage, list of schools in cities was made. Village schools were Government Public schools catering to underprivileged children. Ethics committee approval was obtained from the Institutional ethics committee. Health authorities, schools and parents gave written consent and assent was obtained from children (older than 7 years).
All children were examined by a pediatrician and children's medical records were reviewed. Children with 1) diabetes, 2) any serious illness, 3) suffering from chronic disorders, and 4) children with height or weight below 3rd or above 97th percentiles were excluded. A total of 40 schools were studied. Average yearly fees from the paying schools were Rs. 20,748/- (around $320, € 260 and ≤227) (per capita income for 2016 = Rs. 93,231/yr (around $1310, € 1148 and ≤994) [Figure 1].[17]
Figure 1.
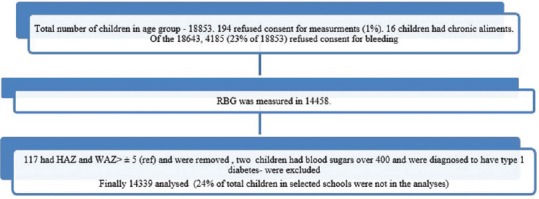
Consort Diagram of children studied
Data collection
Data collection was performed from July 2016 to October 2017. All sites used the same set of measuring equipment which were calibrated daily; glucometers and strips from the same manufacturer were used to assess RBG. All staff received training at the beginning of the study and retraining was performed at each center.
Anthropometry
Height and weight were measured and Body Mass Index (BMI) was computed (weight/height m2) and height, weight and BMI Z-scores were calculated.[18] BMI Z-scores were classified as underweight, normal weight, overweight and obese.[18]
Testing for capillary Random blood glucose concentrations
RBG estimation was performed using a glucometer (Glucometer Elite XL; Bayer Corp, Mishawaka, Ind). Manufacturer's protocol was used to calibrate Glucometers (True results, product code – NIP0051, http://www.niproindia.com/Product1.aspx??enc = 7rlEjTfaCziVRTEMUwMvtg).
Testing was performed as per manufacturers' instructions (http://www.accu-check.in/download/file/fid/16726). Ring finger of non-dominant hand was used to collect blood sample. The finger was massaged for assuring appropriate blood circulation. The finger was then cleaned with a spirit swab (70% ethanol) and pricked with sterile single use safety lancet (https://diabetic24.com/medisafe-solo-disposable-lancets-1-5mm-29G). The first drop of blood was discarded and the second capillary whole blood drop was used to measure RBG using glucometer strip. The blood drop was placed on a reagent strip and inserted into the blood glucose monitoring system and the reading was recorded. Students with an RBG less than <3.9 mmol/L were advised to have two teaspoons of sugar, followed by a snack. Parents of children with RBG below <3.9 mmol/L and above >7.2 mmol/L were called and children with RBG of ≥11.1 mmol/L were referred to a pediatrician.
Dietary patterns
Nutrition data from national sample survey for each state for rural and urban areas was used to represent dietary patterns.[19] Food group wise consumption of cereals (wheat/rice/millet/based) and non-cereals (refined/processed) products were considered to define dietary patterns. These data were expressed as percentage of calories from cereals and miscellaneous foods and beverages; data were considered as a surrogate measure of children's dietary habits [Table 1].
Table 1.
Percentage contribution of cereals and miscellaneous foods and beverages to calorie consumption: major States, 2011-12
| State | Percentage of calories from cereals | Percentage of calories from miscellaneous foods and beverages | ||
|---|---|---|---|---|
| Rural | Urban | Rural | Urban | |
| Assam | 67.4 | 59.4 | 7.5 | 8.9 |
| Chhattisgarh | 65.7 | 58.3 | 9.6 | 9.1 |
| Gujarat | 48.1 | 41.4 | 7.8 | 8.2 |
| Maharashtra | 49.7 | 42.7 | 10.1 | 12.1 |
| Punjab | 42.5 | 41.8 | 8.6 | 7.6 |
| Tamil Nadu | 53.3 | 46.2 | 12.1 | 15.1 |
Source: National sample survey data of 68th nutrition round (2011-12)[21]
Statistical methods
Data were analyzed using SPSS 25.0 for Windows (IBM SPSS, Bangalore, India). Height, weight, BMI and RBG measurements were tested for normality. Parametric tests for normal variables and nonparametric tests for non-normal variables were used. χ2 test was used to test the differences in proportions of BMI categories and RBG groups across different regions. To study association of weight status with RBG, correlation analysis was performed. Percentage energy intakes from cereals and miscellaneous foods [Table 1] were used as categorical variables to assess the role of diet in increasing risk for prediabetes. A generalized linear model was fitted to identify risk factors for prediabetes at significance level of P < 0.05. MATLAB 2017a was used to generate Figures 1-3 (MATLAB 2017a, The MathWorks, Inc., Natick, Massachusetts, United States).
Figure 3.
Center-wise Comparison of Proportion of subjects in different BMI categories
RESULTS
18853 children and adolescents (3-18 years) from 6 regions (12 sites – 6 rural and 6 urban regions were included in the study) were in the selected schools [Figure 1]. Of these, 14474 consented. 16 children were excluded because of chronic ailments, 2 children were found to have blood sugars over 22.2 mmol/L and were further investigated (fasting and post prandial sugars, c-peptide and glycated hemoglobin concentrations), and 117 were excluded as HAZ and or WAZ were ≥5 SD. Thus, data on 14339 subjects (7413 boys, 6926 girls) were analyzed.
Table 2 illustrates anthropometric characteristics and mean RBG concentrations in 14339 children and adolescents from urban and rural areas in the 6 centers. There were significant differences between mean HAZ, WAZ, BMIZ and RBG concentrations between most urban/rural areas (P < 0.05). Prevalence of obesity was 5.8%, overweight 10.6%, 74.2% subjects had BMI within reference range and 9.4% were underweight. Overall, 1% children had low RBG (<3.9 mmol/L), 93.7% had RBG in the range of 3.9-7.2 mmol/L and 5.3% had impaired/elevated RBG (>7.2 mmol/L)[20] (Maharashtra 5%, Gujarat 5.3%, Tamil Nadu 11.4%, Punjab 2.6%, Chhattisgarh- 3.4% and Assam- 2.3%).
Table 2.
Anthropometric characteristics and mean random blood glucose concentrations of study population
| Centre | Parameters | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Age (yr) | Height (cm) | Weight (kg) | BMI (kg/ht-m2) | HAZ†* | WAZ† | BMIZ† | RBG (mmol/L)† | |||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| Maharashtra | |||||||||||||||||
| Urban* Pune |
968 | 10.6 | 2.8 | 139.9 | 16.6 | 34.4 | 14.3 | 16.8 | 3.9 | 0.03 | 1.2 | -0.2 | 1.1 | -0.4 | 1.2 | 5.49 | 0.98 |
| Rural Ranjangaon |
1569 | 9.3 | 2.4 | 127.5 | 13.6 | 23.5 | 8.2 | 14.1 | 2.2 | -0.8 | 12 | -1.2 | 1.1 | -1.2 | 1.1 | 5.89 | 0.81 |
| Gujarat | |||||||||||||||||
| Urban* Billimora |
1045 | 10.2 | 3.1 | 134.4 | 17.8 | 30.8 | 13.6 | 16.3 | 3.7 | -0.4 | 0.9 | -0.5 | 1.1 | -0.4 | 1.1 | 5.93 | 0.72 |
| Rural Gandevi |
1332 | 13.1 | 2.6 | 146.2 | 13.7 | 35.9 | 11.4 | 16.4 | 3.3 | -0.7 | 1 | -1 | 1 | -0.9 | 1 | 6.04 | 0.69 |
| Tamil Nadu | |||||||||||||||||
| Urban* Chennai |
1409 | 13.4 | 2.5 | 152.5 | 14 | 45 | 15.3 | 18.9 | 4.3 | -0.1 | 1 | -0.1 | 1.1 | -0.1 | 1.2 | 6.53 | 1.0 |
| Rural Urapakkam |
1477 | 10.9 | 3.4 | 140.4 | 18.7 | 36.3 | 16.1 | 17.5 | 4.3 | 0.01 | 1 | -0.08 | 1.1 | -0.2 | 1.2 | 6.15 | 0.65 |
| Punjab | |||||||||||||||||
| Urban* Mohali |
901 | 13.5 | 3.1 | 150.4 | 16.6 | 41.4 | 14.7 | 17.7 | 3.5 | -0.4 | 1.1 | -0.5 | 1 | -0.4 | 1 | 5.44 | 0.73 |
| Rural Lalru |
770 | 13.6 | 3.3 | 148 | 17.7 | 38.4 | 13.7 | 16.9 | 3.3 | -0.6 | 1 | -0.9 | 1 | -0.8 | 0.9 | 5.54 | 0.83 |
| Chhattisgarh | |||||||||||||||||
| Urban* Raipur |
1433 | 12 | 2.3 | 147.1 | 13 | 41.2 | 14.2 | 18.6 | 4.4 | 0.01 | 1 | 0.04 | 1.1 | 0.03 | 1.2 | 5.65 | 0.64 |
| Rural Kurud |
1220 | 13.8 | 3.2 | 145.6 | 15.3 | 36.7 | 11.2 | 16.9 | 2.9 | -1 | 1 | -1.1 | 0.9 | -0.8 | 0.8 | 5.91 | 0.72 |
| Assam | |||||||||||||||||
| Urban* Diphu |
1003 | 10.8 | 3.1 | 138.2 | 16.3 | 33.5 | 12.9 | 16.9 | 3.6 | -0.3 | 0.9 | -0.3 | 1 | -0.3 | 1 | 5.86# | 0.79 |
| Rural Manja |
1212 | 12.7 | 3.0 | 144.5 | 15.4 | 38.1 | 12 | 17.7 | 3.1 | -0.7 | 1 | -0.6 | 0.9 | -0.4 | 0.8 | 5.83 | 0.66 |
| Sub-total | |||||||||||||||||
| Urban* | 6759 | 11.9 | 3.0 | 144.7 | 16.8 | 38.6 | 15.1 | 17.7 | 4.1 | -0.2 | 1 | -0.2 | 1.1 | -0.2 | 1.1 | 5.86 | 0.91 |
| Rural | 7580 | 12 | 3.4 | 141 | 17.3 | 34.1 | 13.4 | 16.4 | 3.5 | -0.6 | 1.1 | -0.8 | 1.1 | -0.7 | 1.1 | 5.92 | 0.74 |
| Total | 14339 | 11.7 | 3.5 | 141.3 | 18.6 | 35.4 | 14.7 | 16.9 | 3.8 | -0.4 | 1.1 | -0.5 | 1.1 | -0.5 | 1.1 | 5.89 | 0.83 |
†HAZ, WAZ, BAZ and RBG were significantly different in all urban and rural centers except in Assam (marked as #), *P<0.01
To explore the relationship of RBG with increasing BMIZ, tertiles for each of the BMIZ groups (underweight, within reference range, overweight and obese) were computed and mean BMI-Z for each tertile was plotted against mean RBG for each of these groups [Figure 2]. The Pearson's Correlation coefficient between BMI-Z and RBG was found to be r = 0.62 (P = 0.03); with increasing mean BMI, there was an increase in RBG.
Figure 2.
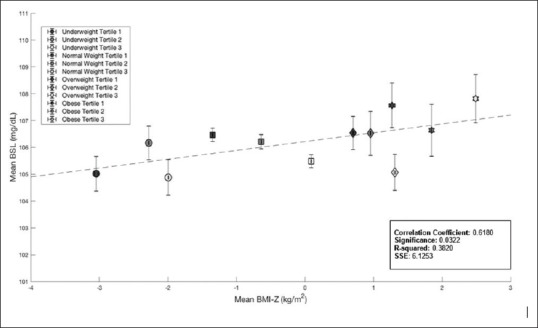
BMI-Z Means vs. RBG Means
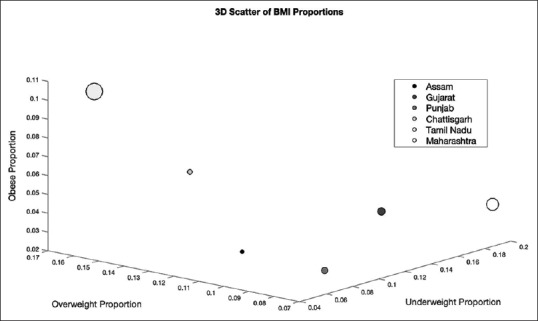
To assess region-wise risk of BMI outside the reference range (i.e. <18 kg/m2 adult equivalent and >23 and 28 kg/m2 equivalent) and low or borderline high RBG (i.e <3.9 mmol/L and above >7.2 mmol/L), a 3-D scatter plot of the proportion of individuals who were obese, overweight, and underweight in each region was computed [Figure 3]. Regions falling in the upper left quadrant of the figure have higher risk for obesity and being overweight and low risk for being underweight while regions falling in the lower right quadrant of the figure had low risk for obesity and being overweight and high risk for being underweight. To observe the effects of RBG on BMI, the size of the markers was made to represent the proportion of individuals with low and borderline RBG. The proportion of individuals with low, borderline, and high RBG for each regions were determined by using cut-offs of <3.9 mmol/L, 3.9-7.2 mmol/L and >7.2 mmol/L.[20]
Tamil Nadu and Chhattisgarh both fell in the upper left quadrant, indicating that both regions had a higher risk for overweight/obesity. Tamil Nadu was found to have the highest proportion of overweight/obese individuals (16.2% and 10.3% respectively), followed by Chhattisgarh (12.3% and 7.1%, respectively). A two-sample Chi-squared test for proportions was conducted to quantify differences between BMI groups among regions. Children from Tamil Nadu were significantly more likely to be overweight/obese than children from all other regions (P < 0.01). Children in Chhattisgarh were significantly more likely to be overweight/obese than children in all other regions except Tamil Nadu (P < 0.01). Importantly, the marker size of Tamil Nadu was quite large, indicating that RBG outside the reference range, along with overweight/obesity were prevalent. A similar two-sample Chi-squared test for proportions was conducted to quantify differences between RBG outside the reference range among different regions. Children in Tamil Nadu (both urban and rural) were significantly more likely to have RBG outside the reference range (11.6%) than children from all other regions (P < 0.01).
Both Maharashtra and Gujarat fell in the lower right quadrant, indicating that both had a higher risk of underweight. Maharashtra was observed to have the highest proportion of underweight children (19.1%), followed by Gujarat (13.6%). Children in Maharashtra were significantly more likely to be underweight than children from other regions (P < 0.01). Children in Gujarat were significantly more underweight than children in all other regions with the exception of Maharashtra (P < 0.01). Importantly, the marker size of Maharashtra was also quite large, indicating that RBG outside the reference range along with underweight are prevalent in Maharashtra. Children in Maharashtra were significantly more likely to have RBG outside reference range (8.5%) than children from all other regions with the exception of Tamil Nadu (P < 0.001).
To examine distribution of subjects with BMI and RBG in the reference range, the proportion of subjects with BMI and RBG (in the reference range) was individually calculated and plotted [Figure 4]. Tamil Nadu and subsequently Maharashtra were found to have the lowest proportion of RBG (88.3% and 91.4%, respectively) and BMI within the reference range. Tamil Nadu was found to have a significantly lower proportion of RBG within the reference range than all other regions (P < 0.001). Similarly, Maharashtra was found to have a significantly lower proportion of RBG within the reference range than all other regions except Tamil Nadu (P < 0.001). Assam and subsequently Punjab had the highest prevalence of RBG (97.4% and 96.5%, respectively) and BMI within the reference range. A two-sample Chi-square test for proportions revealed that the proportion of individuals with RBG within the reference range in Assam was significantly higher than all other regions with the exception of Chhattisgarh (P < 0.001).
Figure 4.
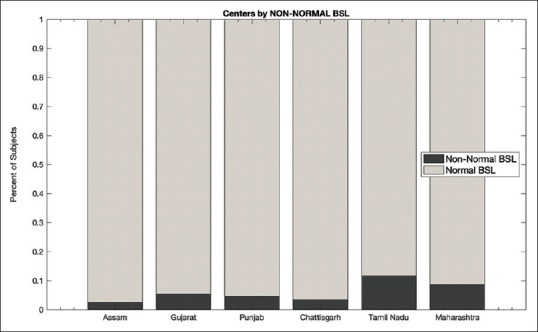
Comparison of Children and Adolescents from various centers with RBG and BMI within reference range
We performed multivariate analysis of factors related to prediabetes, viz.; residence, gender, age and BMI category. Male gender, urban residency, age >10 yrs (girls) and >13 yrs (boys), and overweight/obesity were predictive of prediabetes. Dietary patterns indicated that Tamil Nadu and Assam had higher whereas Maharashtra had lower odds ratios for prediabetes (P < 0.05). Similarly, percent calories from miscellaneous foods and beverages showed higher odds ratios for Tamil Nadu, Gujarat and Chhattisgarh for elevated RBG concentrations [Table 3].
Table 3.
Generalized linear regression model for factors associated with elevated blood glucosea among Indian children and adolescents
| Parameter | Model I OR (95%CI) | P | Model II OR (95%CI) | P | Model III OR (95%CI) | P |
|---|---|---|---|---|---|---|
| Urban residency | 1.34 (1.25, 1.43) | 0.0001 | 1.32 (1.23, 1.42) | 0.0001 | 1.32 (1.23, 1.417) | 0.0001 |
| Boys | 1.21 (1.12, 1.29) | 0.0001 | 1.19 (1.1, 1.27) | 0.0001 | 1.19 (1.11,1.285) | 0.0001 |
| Age >10 yr girls or age >13 y boys | 1.31 (1.13, 1.52) | 0.0001 | 1.296 (1.1, 1.52) | 0.002 | 1.57 (1.34, 1.84) | 0.0001 |
| Overweight | 1.167 (1.0,1.36) | 0.046 | - | - | ||
| Obese | 1.38 (1.15, 1.65) | 0.001 | - | - | ||
| Percent calories from cereals (Reference low- Gujarat and Punjab) | - | |||||
| Maharashtra | 0.856* (0.753, 0.974) | 0.019 | ||||
| Assam | 1.285** (1.13, 1.46) | 0.0001 | ||||
| Tamil Nadu | 5.02** (4.39, 5.74) | 0.0001 | ||||
| Percent calories from Miscellaneous foods and Beverages (Reference low Assam and Punjab) | - | |||||
| Tamil Nadu | 6·23** (5.48, 7.08) | 0.0001 | ||||
| Gujarat | 2.357** (2.08, 2.67) | 0.0001 | ||||
| Chhattisgarh | 1.138* (1.01, 1.278) | 0.029 |
*Significant if P <0.05, **Significant if P <0.001. a: Random blood sugar >7.2 mmol/L. Odds ratios (OR) and 95% confidence intervals (CI) obtained from generalized linear model. Model I: Independent variables: Residence, gender, age group, BMI group. Model II: Independent variables: Residence, gender, age group, percent calories from cereal intake. Model III: Independent variables: Residence, gender, age group, percent calories from miscellaneous foods and beverages intake
DISCUSSION
In our study population, 1% children had low, 93.7% had RBG in the range of 3.9-7.2 and 5.3% had RBG >7.2 mmol/L. There were significant differences among different regions of the country and also urban children showed a higher risk for elevated RBG. We observed prevalence of overweight to be 10.6% and obesity 5.8%; as the BMI increased, the RBG concentrations increased. Children from Tamil Nadu were significantly more likely to be overweight/obese and were more likely to have RBG outside the reference range than children from other regions. Maharashtra and Gujarat had higher risk of having underweight children. RBG outside the reference range along with underweight were prevalent in Maharashtra. Assam and Punjab had the highest prevalence of RBG and BMI within reference range. Energy intake partly explained regional variations in RBG. Male gender, urban residence, age >10 yrs in girls and >13 yrs in boys, and overweight/obesity were predictive for raised RBG.
In a study on South Indian 5-10-year-old children, authors found that prevalence of prediabetes was 3.7% and diabetes was 0.6%.[21] Prevalence of prediabetes of 4.2% has been reported in adolescent and teenage students from the Middle East, based on RBG measurements;[5] both these results are close to what we found (1.93%). Overweight/obese children are known to have increased risk of impaired glucose tolerance.[22] A Brazilian study concluded that overweight and resistance to insulin in adolescent girls was likely to be a risk factor in development of type 2 diabetes in adult life.[23] In their study, authors found that insulin resistance was the best predictor of plasma glucose concentrations at two hours in an oral glucose tolerance test.
Factors including age, gender as well as socio-economic status have been linked to obesity. The transition from living in a rural vs urban area has been associated with increased levels of obesity due to changes in lifestyles.[24] Rural-urban differences in prevalence of obesity in India have been described by others and similar to our study, higher prevalence of obesity has been found in urban areas.[25,26] Higher prevalence of diabetes in urban population as compared to rural population also suggests higher blood glucose concentrations in urban populations.[27] We have found higher blood glucose in urban area (6.1% versus 4.7%) than in rural children.
There are some reports of regional differences of overweight/obesity from various states of India. A study by Gregori et al. (to investigate factors responsible for obesity), authors report varying prevalence of overweight/obesity regionally. They found that the prevalence in the South Indian states, Bangalore, Chennai and Hyderabad was 8%, 13% and 28% respectively, while Mumbai and Surat (western India) had 42% and 19% resp., Kolkata (East) and New Delhi (North) had 37% and 11%, respectively.[28] While studies and reports differ in the prevalence of overweight and obesity, possibly because of the cut-offs used for screening for overweight/obesity and the populations selected for studies, what is clear is that increasing prevalence is a definite worry and that there are regional differences thus suggesting that strategies to combat overweight/obesity may have to be different in different regions.
Data on perturbations in regional prevalence of prediabetes in Indian children are scarce. A study on the prevalence of diabetes and prediabetes in Indian adults which was conducted in 15 states of India reports overall prevalence of diabetes of 7.3% and prediabetes of 10.3%. Authors also report variation in prevalence of prediabetes: 6.0% in Mizoram to 14.7% in Tripura, both regions being in the East. Punjab (North) had a prevalence of 8.2%, Gujarat (West) of 10.2% and Karnataka (South) 11.7%.[29] Numbers in current study showed that highest prevalence of prediabetes was in Tamil Nadu (4.5%) and lowest in Assam (0.52%).
The strength of our study is we provide data on RBG on a large sample of children from 6 Indian states, including both rural and urban areas. We believe that we have described regional variations in prevalence of raised RBG in Indian children for the first time. Further, we have explored in detail the relationships between overweight/obesity and RBG in our study population. However, our study has several limitations. Though the study covers a large area of the country, selection of healthy children may have introduced a downward bias in the estimate of prediabetes prevalence. Another limitation of the study is that we used RBG to define elevated blood glucose in place of IFG. However, often, younger children have difficulty in fasting before blood testing. We also did not perform sexual maturity staging, and data on physical activity were collected on a subset, however, we have not considered it in the current analysis. Similarly, dietary data for cereal and miscellaneous food and beverage intake were used from a National nutritional survey rather than from dietary records of individual children.
To conclude, the present study provides data on RBG in a large nationwide random sample of 3 to 18 years old Indian children and adolescents and adds that the prevalence of elevated RBG was 5.3%. The increased prevalence of overweight obesity and the prevalence of prediabetes in Indian children are a matter of concern. Urgent attention is required towards prevalence of obesity and prediabetes in Indian children particularly in adolescents from urban areas. Regional differences suggest that strategies to prevent obesity and combat perturbations in blood sugar may have to be different in different regions.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) has/have given his/her/their consent for his/her/their clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Partial Funding (Fellowship) from University Grant Commission, India to - Smruti Vispute, Rubina Mandlik and Sonal Palande.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
All authors are grateful to participant families and school authorities.
REFERENCES
- 1.Khadilkar AV, Chiplonkar SA, Pandit DS, Kinare AS, Khadilkar VV. Metabolic risk factors and arterial stiffness in Indian children of parents with metabolic syndrome. J Am Coll Nutr. 2012;31:54–62. doi: 10.1080/07315724.2012.10720009. [DOI] [PubMed] [Google Scholar]
- 2.Khadilkar VV, Khadilkar AV, Cole TJ, Chiplonkar SA, Pandit D. Overweight and obesity prevalence and body mass index trends in Indian children. Int J Pediatr Obes. 2011;6:e216–24. doi: 10.3109/17477166.2010.541463. [DOI] [PubMed] [Google Scholar]
- 3.Yajnik CS, Lubree HG, Rege SS, Naik SS, Deshpande JA, Deshpande SS, et al. Adiposity and hyperinsulinemia in Indians are present at birth. J Clin Endocrinol Metab. 2002;87:5575–80. doi: 10.1210/jc.2002-020434. [DOI] [PubMed] [Google Scholar]
- 4.Narayan KM, Fagot-Campagna A, Imperatore G. Type 2 diabetes in children: A problem lurking for India? Indian Pediatr. 2001;387:701–4. [PubMed] [Google Scholar]
- 5.Mamtani R, Lowenfels AB, Sheikh J, Cheema S, Al-Hamaq A, Matthis SA, et al. Adolescent prediabetes in a high-risk Middle East country: A cross-sectional study. JRSM Open. 2014;5:2054270414536550. doi: 10.1177/2054270414536550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rao PV. Type 2 diabetes in children: Clinical aspects and risk factors. Indian J Endocrinol Metab. 2015;19:S47–50. doi: 10.4103/2230-8210.155401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.American Diabetes Association. Standards of medical care in diabetes—2010. Diabetes Care. 2010;33(Suppl 1):S11–61. doi: 10.2337/dc10-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.WHO Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia: Report of a WHO/IDF consultation. Geneva: WHO; 2006. [Last accessed on 2019 Jan 22]. Available from: www.who.int/diabetes/publications/Definition%20and%20diagnosis%20of%20 diabetes_new.pdf . [Google Scholar]
- 9.Report of the World Health Organization and International Diabetes Federation meeting, 2003, 1-48, 2003. [Last accessed on 2019 Jan 22]. Available from: https://www.who.int/diabetes/publications/en/screening_mnc03.pdf .
- 10.Kahn SE, Cooper ME, Del Prato S. Pathophysiology and treatment of type 2 diabetes: Perspectives on the past, present, and future. Lancet. 2014;383:1068–1083. doi: 10.1016/S0140-6736(13)62154-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bowen ME, Xuan L, Lingvay I, Halm EA. Random blood glucose: A robust risk factor for type 2 diabetes. J Clin Endocrinol Metab. 2015;100:1503–10. doi: 10.1210/jc.2014-4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rolka DB, Narayan KM, Thompson TJ, Goldman D, Lindenmayer J, Alich K, et al. Performance of recommended screening tests for undiagnosed diabetes and dysglycemia. Diabetes Care. 2001;24:1899–903. doi: 10.2337/diacare.24.11.1899. [DOI] [PubMed] [Google Scholar]
- 13.Green R, Milner J, Joy EJ, Agrawal S, Dangour AD. Dietary patterns in India: A systematic review. Br J Nutr. 2016;116:142–8. doi: 10.1017/S0007114516001598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.NSS Report No. 560 (68/1.0/3): Nutritional Intake in India, 2011-12, Government of India, Ministry of Statistics and Programme Implementation, National Statistical Organization, National Sample Survey Office, October2014. [Last accessed on 2019 Feb 09]. Available from: http://www.indiaenvironmentportal.org.in/files/file/nutritional%20intake%20in%20India%202011-12.pdf .
- 15.Ranjani H, Sonya J, Anjana RM, Mohan V. Prevalence of glucose intolerance among children and adolescents in urban South India (ORANGE-2) Diabetes Technol Ther. 2013;15:13–9. doi: 10.1089/dia.2012.0236. [DOI] [PubMed] [Google Scholar]
- 16.Tandon N, Garg MK, Singh Y, Marwaha RK. Prevalence of metabolic syndrome among urban Indian adolescents and its relation with insulin resistance (HOMA-IR) J Pediatr Endocrinol Metab. 2013;26:1123–30. doi: 10.1515/jpem-2013-0020. [DOI] [PubMed] [Google Scholar]
- 17. [Last accessed on 2019 Jan 23]. Available from: https://data.worldbank.org/country/india .
- 18.Khadilkar VV, Yadav S, Agrawal KK, Tamboli S, Banerjee M, Cherian A, et al. Revised IAP growth charts for height, weight and body mass index for 5- to 18-year-old Indian children. Indian Pediatr. 2015;52:47–55. doi: 10.1007/s13312-015-0566-5. [DOI] [PubMed] [Google Scholar]
- 19. [Last accessed on 2019 Jan 30]. Available from: http://www.indiaenvironmentportal.org.in/files/file/nutritional%20intake%20in%20India%202011-12.pdf .
- 20.Saudek CD, Herman WH, Sacks DB, Bergenstal RM, Edelman D, Davidson MB. A new look at screening and diagnosing diabetes mellitus. J Clin Endocrinol Metab. 2008;93:2447–53. doi: 10.1210/jc.2007-2174. [DOI] [PubMed] [Google Scholar]
- 21.Narayanappa D, Rajani HS, Mahendrappa KB, Prabhakar AK. Prevalence of prediabetes in school-going children. Indian Pediatr. 2011;48:295–9. doi: 10.1007/s13312-011-0061-6. [DOI] [PubMed] [Google Scholar]
- 22.Lambert M, Delvin EE, Levy E, O'Loughlin J, Paradis G, Barnett T, et al. Prevalence of cardiometabolic risk factors by weight status in a population-based sample of Quebec children and adolescents. Can J Cardiol. 2008;24:575–83. doi: 10.1016/s0828-282x(08)70639-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alvarez MM, Vieira AC, Moura AS, da Veiga GV. Insulin resistance in Brazilian adolescent girls: Association with overweight and metabolic disorders. Diabetes Res Clin Pract. 2006;74:183–8. doi: 10.1016/j.diabres.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 24.Ziraba AK, Fotso JC, Ochako R. Overweight and obesity in urban Africa: A problem of the rich or the poor? BMC Public Health. 2009;9:465–73. doi: 10.1186/1471-2458-9-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mohan B, Kumar N, Aslam N, Rangbulla A, Kumbkarni S, Sood NK, et al. Prevalence of sustained hypertension and obesity in urban and rural school going children in Ludhiana; Urban rural and regional differences. Indian Heart J. 2004;56:310–4. [PubMed] [Google Scholar]
- 26.Pathak S, Modi P, Labana U, Khimyani P, Joshi A, Jadeja R, et al. Prevalence of obesity among urban and rural school going adolescents of Vadodara, India: A comparative study. Int J Contemp Pediatr. 2018;5:1355–9. [Google Scholar]
- 27.Aung WP, Htet AS, Bjertness E, Stigum H, Chongsuvivatwong V, Kjøllesdal MK, et al. Urban–rural differences in the prevalence of diabetes mellitus among 25–74-year-old adults of the Yangon Region, Myanmar: Two cross-sectional studies. BMJ Open. 2018;8:e020406. doi: 10.1136/bmjopen-2017-020406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gregori D, Gulati A, Paramesh EC, Arockiacath P, Comoretto R, Paramesh H, et al. Cross regional analysis of multiple factors associated with childhood obesity in India: A national or local challenge? Indian J Pediatr. 2014;81:5–16. doi: 10.1007/s12098-014-1550-0. [DOI] [PubMed] [Google Scholar]
- 29.Anjana RM, Deepa M, Pradeepa R, Mahanta J, Narain K, Das HK, et al. Prevalence of diabetes and prediabetes in 15 states of India: Results from the ICMR-INDIAB population-based cross-sectional study. Lancet Diabetes Endocrinol. 2017;5:585–96. doi: 10.1016/S2213-8587(17)30174-2. [DOI] [PubMed] [Google Scholar]


