Abstract
Background and Aims:
Metabolic abnormalities in T2DM (Type 2 diabetes mellitus) include classic manifestations such as impaired insulin secretion, synthesis and peripheral insulin resistance. The intronic variants rs7903146 and rs12255372 of the TCF7L2 (transcription factor 7-like 2) gene are strongly associated with risk of incidence of T2DM and impaired β-cell functions. Studies addressing the early T2DM onset, and early insulin dependence in T2DM patients of south Tamil Nadu are still lacking, and hence the present study focuses in determining the influence of the TCF7L2 polymorphisms in the incidence and disease course in the T2DM patients of south Tamil Nadu.
Methods:
Anthropometric measurements and biochemical parameters were carried out in early onset (Group A), early onset insulin dependent T2DM patients (Group B) and non-insulin dependent T2DM patients (Group C). PCR, allele specific PCR (ASP), PCR product sequencing strategies were utilized to determine the genotype and the impact of the TCF7L2 SNPs in the T2DM disease course.
Results:
Female T2DM patients with the CT/TT rs7903146 genotype (P = 0.005) and the rs12255372 GT/TT genotype (P = 0.036) exhibited a significantly low mean age for T2DM incidence. Correlation/regression analysis in the T2DM patients revealed that rs12255372 (P = 0.042) is associated with early onset in the Group C patients and the rs7903146 (P = 0.018), rs12255372 (P = 0.026) are associated with insulin dependence in the group B patients.
Conclusion:
Screening for the TCF7L2 polymorphisms will prevent T2DM incidence and enable life style changes, appropriate therapeutic strategies that would help combat the accelerated disease course in the T2DM patients.
Keywords: Age onset, allele specific PCR, direct sequencing, insulin dependence, T2DM, TCF7L2
INTRODUCTION
Impaired insulin secretion, peripheral insulin resistance and increased hepatic glucose output are classic metabolic manifestations in T2DM. While the etiology of T2DM is complex, genetic markers for T2DM are surfacing fast and amidst such markers, the transcription factor 7-like 2 (TCF7L2) gene located on chromosome 10q25.2-q25.3 is reproducibly associated with T2DM.[1,2,3] TCF7L2 physiologically interacts with ß-catenin and functions through the Wnt signaling pathway, and thereby regulates cell morphology, proliferation, motility, oncogenesis, and tumor suppression.[4] The intron 3 variant rs7903146 (IVS3C>T) and the intron 4 variant rs12255372 (IVS4G>T) of the TCF7L2 gene are strongly associated with T2DM incidence, impaired β-cell functions such as insulin secretion, synthesis, processing and modulation of the levels of the insulinotropic hormone GLP-1 (glucagon-like peptide).[5,6]
Earlier studies assessing the impact of rs7903146 on mechanisms pertaining to diabetes reveal that the carriers of the SNP may exhibit a diabetogenic effect by impairing insulin secretion.[7,8] Very recent studies also reiterate that rs7903146 SNP has been associated with an increase in the ratio of proinsulin/insulin and rs12255372 is associated with lower fasting plasma glucose[9,10] levels, thereby indicating that the genetic variants of TCF7L2 may predispose the carriers to the incidence of T2DM, β-cell dysfunctions/insulin in sufficiency. In order to understand the prevalence and influence of TCF7L2 polymorphisms in T2DM in the south Indian population, we had earlier brought to focus that TCF7L2 gene variants are prevalent, incident in the T2DM patients, non-diabetic participants in the south Tamil Nadu population (Ramanathan B et al., Int J Community Med Public Health 2019 Mar; 6 (3):1025-1030). In sequence, the present study aims to further understand the impact of the TCF7L2 gene variants on the age onset of T2DM, and the pancreatic insulin synthesis/secretion efficiency by analyzing if the TCF7L2 rs7903146, rs12255372 carriers of the regional population presented an association with earlier T2DM incidence, early insulin dependency. The results from our study primarily reveal that the T2DM carriers of the TCF7L2 SNPs present an early onset of T2DM, early dependence on insulin therapy and that screening for TCF7L2 rs7903146, rs12255372 will significantly aid disease management, enable well-being in T2DM patients.
METHODS
The present study was carried out in Alpha Health Foundation, Alpha Hospital and Research Center. The proposed study group T2DM patients were enrolled for the study on clearance from the Institutional Ethical Committee (IEC) and upon informed consent from the participants. A total of 90 T2DM patients participated in the present study and they were categorized under three groups. Group A T2DM patients presented with a T2DM onset ≤40 years and had less than 10 years of T2DM onset; Group B T2DM patients presented with T2DM onset ≤ 40 years, had greater than 10 years of T2DM onset and are insulin dependent; group C T2DM patients had greater than 10 years of T2DM onset and were insulin independent. Details pertaining to the patients' age, height, weight, age of T2DM onset, duration of diabetes, medication history, history of comorbidities, and the age at which insulin therapy was initiated and other therapeutic interventions were obtained.
Routine clinical assessments including anthropometric measurements and biochemical tests relevant to glucose, lipid metabolism were carried out according to standard protocols. Genomic DNA was extracted from peripheral blood samples using a QIAamp DNA Blood Mini Kit (QIAGEN India Pvt. Ltd, New Delhi, India) according to the manufacturer's protocol. Quantitative and qualitative (260/280 nm absorbance ratio) assessment of the DNA samples were carried out using a nanodrop, Thermo scientific, India.
Determination of the presence of the TCF7L2 polymorphism rs7903146 was carried out by PCR amplification of a region corresponding to a 318bp product using the forward primer 5'- GGTAATGCAGATGTGATGAGATCT-3', and the reverse primer 5'-AGATGAAATGTAGCAGTGAAGTGC-3'. The PCR conditions pertained to an initial denaturation of 3 min at 94°C followed by 32 cycles of denaturation for 1 min at 94°C, annealing for 1 min at 58°C and extension for 1 min at 72°C and final extension of 5 min at 72°C. A 352bp PCR product encompassing the region pertaining to the rs12255372 SNP was obtained using PCR with the conditions pertaining to the initial denaturation of 5 min at 94°C, followed by 30 cycles of denaturation for 1 min at 94°C, annealing for 30 sec at 52°C and elongation for 1 min at 72°C, with a final elongation of 5 min at 72°C, using the forward primer 5'- TTTTGTTAATGGCTTGCAGGT-3', and the reverse primer 5'- GCGCATGCTAATTTCCTGTC-3'. Allele specific PCR (T allele) was also carried out for the TCF7L2 polymorphism rs12255372 (256bp) using the forward primer 5'-TTTTGTTAATGGCTTGCAGGT-3' and 5'-GGCCTGAGTAATTATCAGAATATGATA-3' reverse primer (conditions corresponded to initial denaturation of 5 min at 94°C, followed by 30 cycles of 1 min denaturation at 94°C, 30 secs annealing at 60°C, 1 min primer extension at 72°C and final extension of 5 min at 72°C).
The PCR products were visualized after electrophoresis using a UV gel documentation system (Medicare, Chennai, India) and further gel extracted, purified and utilized for direct sequencing (Agrigenome, Kochi, Kerala).
Statistical analysis
All statistical analysis were carried out using SPSS version 20.0 for Windows (IBM Chicago, IL, USA) and Graph Pad Prism version 7.04 for Windows (Graph Pad Software, La Jolla California USA).
RESULTS
Recent years have seen a surge in global molecular genetic studies that aim to understand the prevalence, influence of TCF7L2 polymorphisms as they predict the risk of incidence of T2DM in the individuals. Our earlier pilot studies had revealed that a considerable number of T2DM patients and healthy volunteers in the regional population of south Tamil Nadu, presented the TCF7L2 rs7903146, rs12255372 SNPs. Therefore we further aimed to elucidate the impact of these polymorphisms in the T2DM course by means of studying the prevalence, impact of these SNPs in T2DM patients, and determining if the carriers of TCF7L2 SNPs were more likely to require insulin therapy at earlier stages.
The overall mean age, BMI, HbA1c levels, triglyceride levels and the total cholesterol levels in the patients of the study groups are presented in Table 1. As indicated in the table the mean age of the group A patients was 36.3 ± 1.3, BMI 28.7 ± 1.1 kg/m2, HbA1c 9.83 ± 0.4%, TGL 236.28 ± 33.5mg/dl, TC 202.65 ± 11.6 mg/dl; group B presented with a mean age of 47.9 ± 2.0, values of BMI 28.05 ± 1.0 kg/m2, HbA1c levels of 10.4 ± 0.4, TGL levels of 256.5 ± 49.5, and TC levels of 175.4 ± 13.7; group C exhibited a mean age of 57.6 ± 1.7, values of BMI 25.2 ± 0.7 kg/m2, HbA1c 8.82 ± 0.3%, TG levels 201.56 ± 29.8 and TC levels of 162.64 ± 6.1 mg/dl.
Table 1.
Age, BMI, glycemic and lipid profile parameters in the T2DM patients
| Variables | Group A | Group B | Group C |
|---|---|---|---|
| Age (years) | 36.3±1.3 | 47.9±2.0 | 57.6±1.7 |
| BMI (kg/m2) | 28.7±1.1 | 28.05±1.0 | 25.2±0.7 |
| HbA1c (%) | 9.83±0.4 | 10.4±0.4 | 8.82±0.3 |
| TGL (mg/dl) | 236.28±33.5 | 256.5±49.5 | 201.56±29.8 |
| T.CHO (mg/dl) | 202.65±11.6 | 175.4±13.7 | 162.64±6.1 |
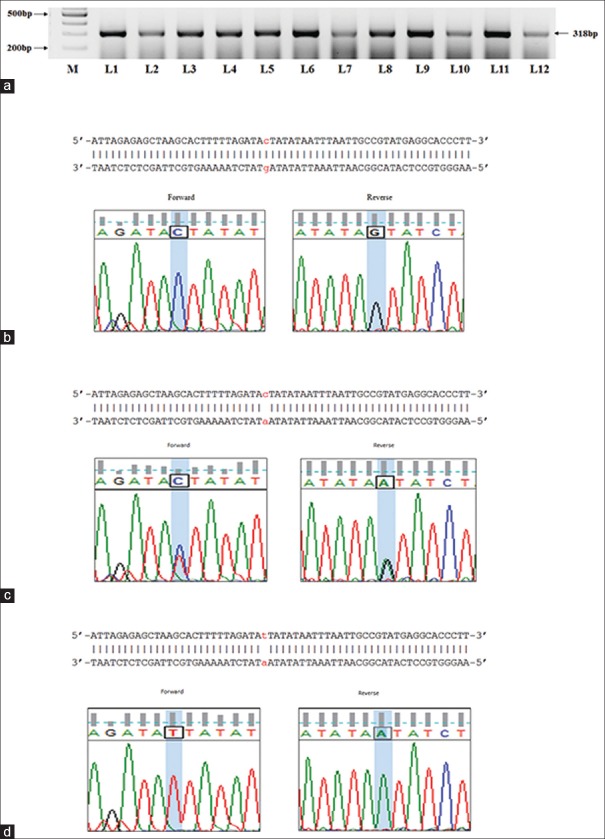
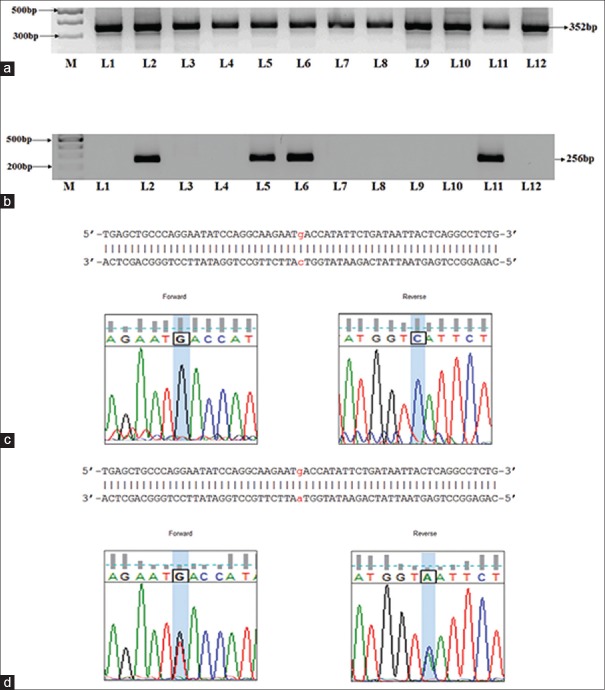
We then assessed the genotypic distribution for rs7903146, rs12255372 in the group A, group B and group C patients. Figure 1 depicts the results that are representative for the rs7903146 PCR amplification, direct sequencing. Figure 1a presents a representative image of the PCR amplified 318bp product which encompasses the genomic region pertaining to the rs7903146 SNP and Figure 1b-d are representative images for the genotypic information. Figure 1b presents the chromatogram image for wild type carriers indicating a CG allelic distribution in the forward and reverse sequences. Similarly Figure 1c and d presents the genotype of a heterozygous carrier (CA), homozygous carrier (TA) in the aligned forward and reverse sequences respectively. Figure 2a presents the 352bp PCR product that encloses the genomic region pertaining to the rs12255372 polymorphism and Figure 2b presents a representative image for the allele specific PCR results that enable identification of the presence of SNP. As indicated in the image L1 and L2 are negative and positive control samples that are represent by the absence and presence of the 256 bp rs12255372 SNP containing region. ASP results for patients presenting the rs12255372 GT/TT alleles are represented in L5, L6 and L11 of the image, thus indicating that ASP can easily and routinely be utilized for the determination of the rs12255372 TCF7L2 SNP. Further sequencing outputs are represented in Figure 2c and d and correspond to sequencing chromatogram images of a wild type carrier GC (Forward, reverse strands) and a heterozygous carrier (GA in the forward and reverse strands respectively). Together, the results obtained demonstrate the reliability and feasibility of the utilization of PCR based strategies, PCR product sequencing in the identification of the rs7903146, rs12255372 in molecular diagnosis.
Figure 1.
Determination of the TCF7L2 rs7903146 SNP incidence in T2DM patients. (a) PCR product that encompasses rs7903146 SNP was electrophoretically separated and visualized in a 1% agarose gel. (b) The forward and reverse sequences were aligned and the wild type alleles (C, G) are presented for a negative patient (c) The forward and reverse sequences of a heterozygous positive patient were aligned and the C, A alleles are shown in the aligned sequence (d) The forward and reverse sequences for a homozygous positive patient were aligned and the T, A alleles are shown in the aligned sequence
Figure 2.
Determination of the TCF7L2 rs12255372 SNP incidence in T2DM patients. (a) PCR product that encompasses rs12255372 SNP was electrophoretically separated and visualized in a 1% agarose gel. (b) Allele Specific PCR was carried to detect the G to T transition using allele specific primers; patients presenting T allele amplified a 256bp product. (c) The forward and reverse sequences were aligned and the wild type G, C alleles are presented for a negative patient (d) The forward and reverse sequences for heterozygous positive patient were aligned and the G, A alleles are shown in the aligned sequence
Based on the genotype prevalence data presented in Table 2, it can be observed that group A presented with a maximum percentage of (74.3%) patients who were positive for the rs7903146, rs12255372 (48.6%) SNPs. It can also be observed that the early onset insulin dependent group B comprised of 63.6% patients positive for rs7903146 and 31.8% positive for rs12255372. The group C non insulin dependent patients presented with the maximum percentage of wild type carriers (51.7%) for rs7903146 and group B presented with a higher percentage of patients negative (68.2%, GG) for the rs12255372 SNP. Further analysis of the anthropometric and clinical parameters in the rs7903146, rs12255372 wild type and hetero/homozygous carriers are presented in Table 3. Association analysis for determining the influence of the SNPs in age, age onset for incidence of T2DM, BMI and parameters associated with glucose, lipid metabolism between the groups and amidst the same groups, in carriers and non-carriers revealed significant differences in total cholesterol levels between group A and group C for the rs7903146 SNP (CC Vs CT/TT genotype; P = 0.027).
Table 2.
Allelic distribution (%) in the study group T2DM patients
| Study Group | rs7903146 | rs12255372 | ||
|---|---|---|---|---|
| CC (%) | CT/TT (%) | GG (%) | GT/TT (%) | |
| Group A | 25.7 | 74.3 | 51.4 | 48.6 |
| Group B | 36.4 | 63.6 | 68.2 | 31.8 |
| Group C | 51.7 | 48.3 | 55.2 | 44.8 |
Table 3.
Age, Age onset, BMI, glycemic and lipid profile parameters based on the genotypic distribution in the T2DM patients
| Variables | Group A | Group B | Group C | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| rs7903146 | rs12255372 | rs7903146 | rs12255372 | rs7903146 | rs12255372 | |||||||
| (CC) | (CT/TT) | (GG) | (GT/TT) | (CC) | (CT/TT) | (GG) | (GT/TT) | (CC) | (CT/TT) | (GG) | (GT/TT) | |
| Age (years) | 38.1±1.7 | 35±1.4 | 38.9±1.2 | 32.3±1.6 | 53.13±1.9 | 45±2.7 | 51.2±1.4 | 39.6±4.2 | 59.4±2.6 | 55.79±2.3 | 58.5±2.5 | 56.62±2.5 |
| Age onset (years) | 34.8±1.1 | 32.3±1.1 | 35.2±0.9 | 29.48±1.4 | 35.38±2.1 | 29.8±2.3 | 34.4±1.6 | 26.7±3 | 42.8±2.59 | 40.18±2.46 | 43.1±2.4 | 42±3 |
| BMI (kg/m2) | 28.6±1.7 | 29.5±1.5 | 27.5±1.1 | 31.1±2.1 | 30.08±1.1 | 26.8±1.4 | 28.2±0.9 | 23.7±2.4 | 25.31±1.2 | 25.25±0.7 | 25.96±1.0 | 24.5±0.9 |
| HbA1c (%) | 9±0.8 | 8.16±1.1 | 9±0.7 | 7.3±1.2 | 10.65±0.6 | 10.2±0.5 | 10.26±0.4 | 9.1±1 | 8.08±0.4 | 7.96±0.5 | 8.51±0.5 | 9.22±0.5 |
| TGL (mg/dl) | 193±47.7 | 275.2±44.6 | 257.3±46.5 | 225.8±48.6 | 281.5±93.1 | 237±54.7 | 293.6±62 | 215.1±46.2 | 161.0±27.2 | 233.3±48 | 202.2±45.5 | 200.6±32.7 |
| T.CHO (mg/dl) | 213.8±21.5 | 200.2±15 | 210.9±15.8 | 193.4±19.0 | 177.4±24.7 | 173.5±15.2 | 168.9±15.8 | 150.9±21 | 254.2±11.3 | 288.8±30.9* | 268.9±26.3 | 268.7±23.7 |
We next proceeded to analyze the impact of the TCF7L2 polymorphisms rs7903146, rs12255372 on the age at which T2DM incidents, and insulin dependency begins based on gender categorization in the study group patients. As indicated in Figure 3a, amidst the assessed population, the TCF7L2 SNP positive female T2DM patients presented with a significantly lower onset age for T2DM [rs7903146 (P < 0.01), rs12255372 (P < 0.05)]. Detailed analysis in the early onset, insulin dependent group B patients indicated that the rs7903146, rs12255372 carriers presented with a lower age for T2DM incidence and insulin dependency in comparison to the wild type carriers [Figure 3b]. Correlation/regression analysis for determining the influence of the SNPs in earlier incidence of T2DM and early insulin dependency reveal that the rs7903146 positive patients exhibited an early dependence on insulin (P = 0.018). The results also indicate that the rs12255372 SNP significantly influences the (P = 0.042) onset age for T2DM in group C patients and the dependence age for insulin therapy in Group B patients (P = 0.026). Taken together, the results from the present study strongly suggest that rs7903146, rs12255372 TCF7L2 carriers present with impaired β-cell efficiency reflecting with an accelerated disease course that requires early dependence on insulin therapy.
Figure 3.
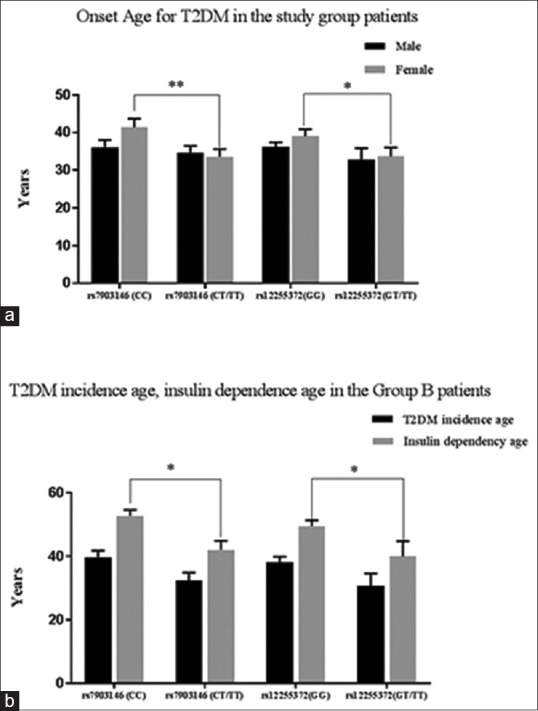
Association of rs7903146, rs12255372 SNPs with early incidence of T2DM and insulin dependency age. (a) Pearson's correlation analysis revealed a significant association with T2DM onset age in rs7903146 (P = 0.005) and rs12255372 (P = 0.036) positive patients. (b) Logistic regression analysis revealed significant association for the rs7903146 (P = 0.018) and rs12255372 (P = 0.026) with insulin dependency age in group B patients
DISCUSSION
Genetic variants from the TCF7L2 gene are strongly associated with T2DM and several studies have demonstrated the influence of rs7903146, rs12255372 with risk of incidence, insulin secretion, β cell efficiency and T2DM obesity.[11,12] In conjunction with several other reports, including ours, the results of the present study indicates that the percentage prevalence of TCF7L2 polymorphisms in the T2DM patients was significantly high.[13]
Earlier research studies addressing the mechanistic impact of TCF7L2 SNPs in detail (insulin synthesis, secretion, glucagon secretion) demonstrated that the TCF7L2 SNP carriers exhibit aberrant insulin dependent outputs. Other subsequent studies have also reported that the TCF7L2 polymorphisms influenced peak insulin response indicating their association with hepatic insulin clearance/processing.[14] Very recent transcriptional network studies have added value to such earlier findings by demonstrating that TCF7L2 regulates pro-insulin production in a genotype dependent manner and that the TCF7L2 SNP carriers present with lower insulin levels, secretion, and thereby mark a relatively aggressive disease course.[15,16] In a parallel track, interesting clinical studies demonstrate that the rs7903146 SNP carriers presented with impaired absolute, relative insulin secretion, increased peripheral sensitivity, elevated rate of endogenous glucose production, and inadequate insulin levels for compensation of insulin resistance. Based on all of these findings, it can be strongly suggested that screening for the TCF7L2 risk allele carriers, and providing appropriate therapeutic interventions for the TCF7L2 carrier T2DM patients would drastically alleviate the burden associated with the T2DM disease.[17,18]
Moving ahead and owing to the lack of knowledge pertaining to the influence of the TCF7L2 polymorphisms in the T2DM disease course in the regional population, we first examined the association between the TCF7L2 SNP carriers and the T2DM incidence age, the disease course, by means of understanding the patient's need for insulin therapy. In conjunction with prior research studies that have reported an association of rs7903146 SNP with early onset T2DM,[17] the results from the present study also indicate that the TCF7L2 rs7903146 and rs12255372 polymorphisms are significantly associated with T2DM incidence age. Such evidences cumulatively highlight that the TCF7L2 SNP carriers may strongly be predisposed to multiple facets in the incidence, progression of T2DM in the Indian/south Tamil Nadu population. It is further observed from the present study that the TCF7L2 rs7903146, rs12233572 polymorphisms are associated with earlier dependence on insulin when compared to the wild type carriers. Such an observation is in line with findings that have associated the presence of TCF7L2 SNPs with lower insulin levels and inefficient β-cell functions[8,11,16] that may resultantly lead to earlier dependence on insulin during the course of T2DM. The current study results thus provide regional evidence for early insulin dependence for the first time, and further draw attention to the fact that T2DM patients with the TCF7L2 polymorphisms may require additional measures to prevent the accelerated disease course. Put together, concurrent detailed and large scale studies are required to introduce life style and therapeutic modulations in the SNP carriers.
CONCLUSION
The present TCF7L2 SNP study in the regional south Tamil Nadu population indicates that active screening measures for the TCF7L2 rs7903146, rs12255372 will not only prevent T2DM incidence, but will also introduce appropriate intervention strategies that would effectively control the accelerated disease course in the SNP carriers.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Cauchi S, Nead KT, Choquet H, Horber F, Potoczna N, Balkau B, et al. The genetic susceptibility to type 2 diabetes may be modulated by obesity status: Implications for association studies. BMC Med Genet. 2008;9:45. doi: 10.1186/1471-2350-9-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–6. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- 3.Tong Y, Lin Y, Zhang Y, Yang J, Zhang Y, Liu H, et al. Association between TCF7L2 gene polymorphisms and susceptibility to type 2 diabetes mellitus: A large Human Genome Epidemiology (HuGE) review and meta-analysis. BMC Med Genet. 2009;10:15. doi: 10.1186/1471-2350-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hansson O, Zhou Y, Renstrom E, Osmark P. Molecular function of TCF7L2: Consequences of TCF7L2 splicing for molecular function and risk for type 2 diabetes. Curr Diab Rep. 2010;10:444–51. doi: 10.1007/s11892-010-0149-8. [DOI] [PubMed] [Google Scholar]
- 5.Grant SF, Thorleifsson G, Reynisdottir I, Benediktsson R, Manolescu A, Sainz J, et al. Variant of transcription factor 7-like 2 (TCF7L2) gene confers risk of type 2 diabetes. Nat Genet. 2006;38:320–3. doi: 10.1038/ng1732. [DOI] [PubMed] [Google Scholar]
- 6.Alami FM, Ahmadi M, Bazrafshan H, Tabarraei A, Khosravi A, Tabatabaiefar MA, et al. Association of the TCF7L2 rs12255372 (G/T) variant with type 2 diabetes mellitus in an Iranian population. Genet Mol Biol. 2012;35:413–7. doi: 10.1590/S1415-47572012005000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dahlgren A, Zethelius B, Jensevik K, Syvanen AC, Berne C. Variants of the TCF7L2 gene are associated with beta cell dysfunction and confer an increased risk of type 2 diabetes mellitus in the ULSAM cohort of Swedish elderly men. Diabetologia. 2007;50:1852. doi: 10.1007/s00125-007-0746-5. [DOI] [PubMed] [Google Scholar]
- 8.Ferreira MC, da Silva MER, Fukui RT, Arruda-Marques MDC, Dos Santos RF. TCF7L2 correlation in both insulin secretion and postprandial insulin sensitivity. Diabetol Metab Syndr. 2018;10:37. doi: 10.1186/s13098-018-0338-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bodhini D, Radha V, Dhar M, Narayani N, Mohan V. The rs12255372 (G/T) and rs7903146 (C/T) polymorphisms of the TCF7L2 gene are associated with type 2 diabetes mellitus in Asian Indians. Metabolism. 2007;56:1174–8. doi: 10.1016/j.metabol.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 10.Klunder-Klunder M, Mejia-Benitez MA, Flores-Huerta S, Burguete-Garcia AI, Garcia-Mena J, Cruz M. rs12255372 variant of TCF7L2 gene is protective for obesity in Mexican children. Arch Med Res. 2011;42:495–501. doi: 10.1016/j.arcmed.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 11.Shokouhi S, Delpisheh A, Haghani K, Mahdizadeh M, Bakhtiyari S. Association of rs7903146, rs12255372, and rs290487 polymorphisms in TCF7L2 gene with type 2 diabetes in an Iranian Kurdish ethnic group. Clin Lab. 2014;60:1269–76. doi: 10.7754/clin.lab.2013.130809. [DOI] [PubMed] [Google Scholar]
- 12.Rosengren AH, Braun M, Mahdi T, Andersson SA, Travers ME, Shigeto M, et al. Reduced insulin exocytosis in human pancreatic beta-cells with gene variants linked to type 2 diabetes. Diabetes. 2012;61:1726–33. doi: 10.2337/db11-1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tan S, Scherag A, Janssen OE, Hahn S, Lahner H, Dietz T, et al. Large effects on body mass index and insulin resistance of fat mass and obesity associated gene (FTO) variants in patients with polycystic ovary syndrome (PCOS) BMC Med Genet. 2010;11:12. doi: 10.1186/1471-2350-11-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chandak GR, Janipalli CS, Bhaskar S, Kulkarni SR, Mohankrishna P, Hattersley AT, et al. Common variants in the TCF7L2 gene are strongly associated with type 2 diabetes mellitus in the Indian population. Diabetologia. 2007;50:63–7. doi: 10.1007/s00125-006-0502-2. [DOI] [PubMed] [Google Scholar]
- 15.Wood AR, Jonsson A, Jackson AU, Wang N, van Leewen N, Palmer ND, et al. Agenome-wide association study of IVGTT-based measures of first-phase insulin secretion refines the underlying physiology of type 2 diabetes variants. Diabetes. 2017;66:2296–309. doi: 10.2337/db16-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou Y, Park SY, Su J, Bailey K, Ottosson-Laakso E, Shcherbina L, et al. TCF7L2 is a master regulator of insulin production and processing. Hum Mol Genet. 2014;23:6419–31. doi: 10.1093/hmg/ddu359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geoghegan G, Simcox J, Seldin MM, Parnell TJ, Stubben C, Just S, et al. Targeted deletion of Tcf7l2 in adipocytes promotes adipocyte hypertrophy and impaired glucose metabolism. Mol Metab. 2019;24:44–63. doi: 10.1016/j.molmet.2019.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kozarova M, Javorsky M, Stancakova A, Dobrikova M, Habalova V, Klimcakova L, et al. Relationship of five type 2 diabetes candidate gene polymorphisms to the age at diagnosis of diabetes in the Slovakian population. Bratisl Lek Listy. 2010;111:150–2. [PubMed] [Google Scholar]