Abstract
Glycosylation plays a myriad of roles in the immune system: Certain glycans can interact with specific immune receptors to kickstart a pro-inflammatory response, whereas other glycans can do precisely the opposite and ameliorate the immune response. Specific glycans and glycoforms can themselves become the targets of the adaptive immune system, leading to potent antiglycan responses that can lead to the killing of altered self- or pathogenic species. This hydra-like set of roles glycans play is of particular importance in cancer immunity, where it influences the anticancer immune response, likely playing pivotal roles in tumor survival or clearance. The complexity of carbohydrate biology requires synthetic access to glycoproteins and glycopeptides that harbor homogeneous glycans allowing the probing of these systems with high precision. One particular complicating factor in this is that these synthetic structures are required to be as close to the native structures as possible, as non-native linkages can themselves elicit immune responses. In this Review, we discuss examples and current strategies for the synthesis of natively linked single glycoforms of peptides and proteins that have enabled researchers to gain new insights into glycoimmunology, with a particular focus on the application of these reagents in cancer immunology.
Introduction
Mammalian cell biology cannot be understood without taking post-translational protein glycosylation into account.1,2 This is of particular relevance in the immune system, where glycans play a myriad of roles at all stages of the immune response; from the initial sensing of danger and the preservation of self-cells, the homing of specific effector cell populations to the right locations, to the resolution and dampening of the immune response.3 These can be dependent on either broad classes of glycan structure, or specific glycoforms on specific sites of proteins.4,5
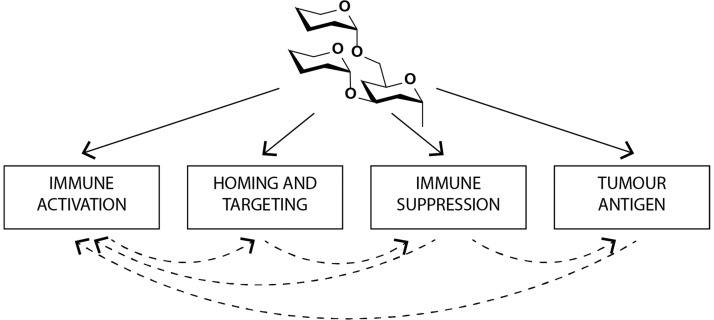
One archetypal example is that of sialyl LewisX-containing glycoproteins. These carbohydrates can interact with the selectin-family of lectins, which are upregulated on the endothelial surface at sites of inflammation.6 In these proteins, the underlying protein scaffold plays a minimal role in binding. It is not until the upregulation of α-1,3-fucosyltransferase (Fuc-TVII) expression (upon receiving an activating stimulus) that the functional ligand sialyl LewisX is produced.7 The introduction of this single monosaccharide converts immune cell surface proteins, such as the P-selectin glycoprotein ligand-1 (PSGL-1), into a glycoform capable of binding the immune cell homing receptors, thereby orchestrating the key step of effector cell mobilization of the immune response (Figure 1).8,9
Figure 1.
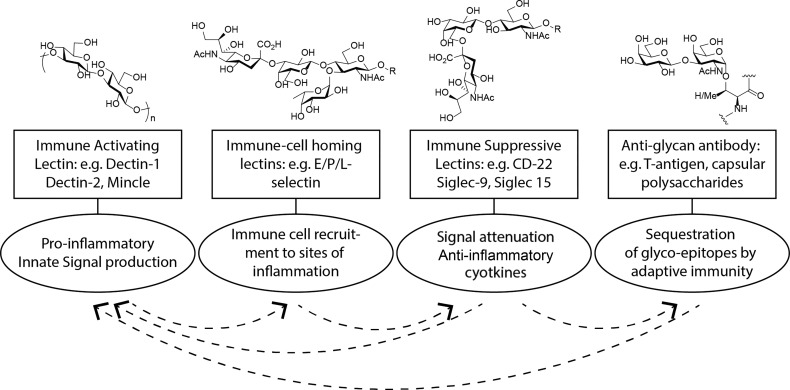
Carbohydrate–protein interactions play a myriad of roles in the immune system. They influence initial pattern recognition, leading to immune activation, as well as the routing of immune cells in the body to immune suppressive effects. They can also be targets of antibody responses, leading to clearance of specific glycans. All these events can affect others in the pathway either synergistically or detrimentally.
Carbohydrates also play other important roles in the initiation of the inflammatory response.10 They are the ligands of a wide array of immune lectins, that upon ligation can initiate the expression of pro-inflammatory cytokines, leading to the initiation of an inflammatory response. For example, the binding of β-1,3- and β-1,6-glucan structures by dectin-1, a transmembrane receptor with a lectin-like carbohydrate binding domain, is a key event in antifungal immunity.11 The ligation of this receptor to these, and other carbohydrate ligands often found on unicellular pathogens, results in the Syk-mediated activation of a variety of innate immune responses. This leads to the secretion of pro-inflammatory cytokines, enhanced phagocytosis, and T-cell skewing to antifungal Th1/Th17 phenotypes.12 Other members of this family of carbohydrate pattern recognition receptors (PRRs) include Mincle,13 Dectin-2,14 the mannose receptor,15 and DC-SIGN,16 and all are of prime importance to the initiation of antipathogenic immune responses.17
The interactions of carbohydrates with proteins can also block or reduce inflammation. It has, for example, emerged that changes in the heavy chain N-glycan (Situated on Asn297) of IgG antibodies adjust the effector functions of the antibody by fine-tuning, among other things, individual FcγR receptor affinities.18,19 A simple fucosylation of the core pentasaccharide of the glycan changes the affinity of the antibody toward Fc receptors dramatically, thereby reducing the ability of an antibody to recruit effector cells. In addition, the enzymatic removal of sialic acids from the antennae of the complex-type glycan can turn the properties of the antibodies from anti-inflammatory to pro-inflammatory.20,21
Some of the aforementioned PRR-lectins can also serve as immune-dampening lectins. For instance, ligation of mannosylated lipoarabinomannan (ManLAM), a mycobacterial cell wall component, to the dendritic cell (DC) C-type lectin receptor DC-SIGN16 masks infection by hindering DC activation and by stimulating secretion of the immunosuppressive cytokine IL-10 in response to the otherwise highly immunogenic bacterial compound LPS.22
Sialic acid binding immunoglobulin-type lectins (Siglecs) are another important class of immune modulatory carbohydrate receptors.23 These lectins, which are found on a wide variety of immune cells, often have immunoreceptor tyrosine-based inhibitory signaling motifs (ITIMs), which can initiate anti-inflammatory signaling cascades.24 On B-cells, Siglec 2 (CD22) can modulate the strength of B-cell receptor signaling,25 with other Siglecs causing this immunosuppressive phenotype.23 This feature is, for example, exploited by Group B Streptococcus aureus, which decorates its surface with ligands for siglec-9 to prevent platelet-mediated killing.26
One interesting aspect of siglec-based immune modulation is that tumors often exploit these receptors for their own immune evasion. For example, in certain tumor types, siglec-9 was shown to modulate the reactivity of a pool of CD8 positive memory T-cells27 (CD8 positive T-cells recognize antigen in MHC-I context to activate and then initiate killing of infected or transformed target cells), and siglec-15 was shown to block these T-cell responses.28 Therapeutic inhibition of the latter showed a similar biological effect to clinical checkpoint inhibitor therapy (e.g., anti-PD-L1).28 Even the broad removal of sialic acids from the tumor surface was shown to enhance antitumor responses.29
The recent discovery of the T cell immunoglobulin and mucin-domain containing protein-3 (TIM-3) offers another example of the immunomodulatory roles of glycans in the tumor microenvironment. TIM-3 is expressed by various immune cell types, and inhibition of this glycoprotein leads to decreased tumor growth in preclinical models, probably through the blockade of an immunomodulatory signal exerted by this protein on cells ranging from DCs to tumor associated macrophages, natural killer (NK)-cells, and T-cells.30 In humans, tumor infiltrating CD8 positive T-cells in colorectal cancer patients undergo apoptosis more readily if they express TIM-3, and this was shown to be dependent on tumor secretion of galectin-9 (which binds to the Tim-3 IgV domain N-linked glycans) in vivo in a mouse model.31
The multiple roles of glycans as well as the heterogeneity of naturally occurring glycans make the study of glycan–protein interactions in the immune system complex. Although analytical methods to determine the glycosylation sequences and glycoform distribution of glycoproteins are now becoming available,32,33 there is an acute need for access to single glycoforms to study the immunological function of glycoproteins in vivo and to address whether specific single glycoforms are required for optimal vaccination strategies.34
The aim of this Review is to illustrate the methodologies and developments for synthesis of single glycoforms of immune-relevant constructs and their use in strategies toward improved treatments against cancer. We focus mainly on native linkages, as recent work has shown that non-native linkages can themselves serve as (part of) the epitope in an adaptive immune response and that the non-native linkages can alter the processing that is required for a peptide to become capable of T-cell activation.35,36
Synthesis of Tn, T, and Sialylated T(n)-Antigens
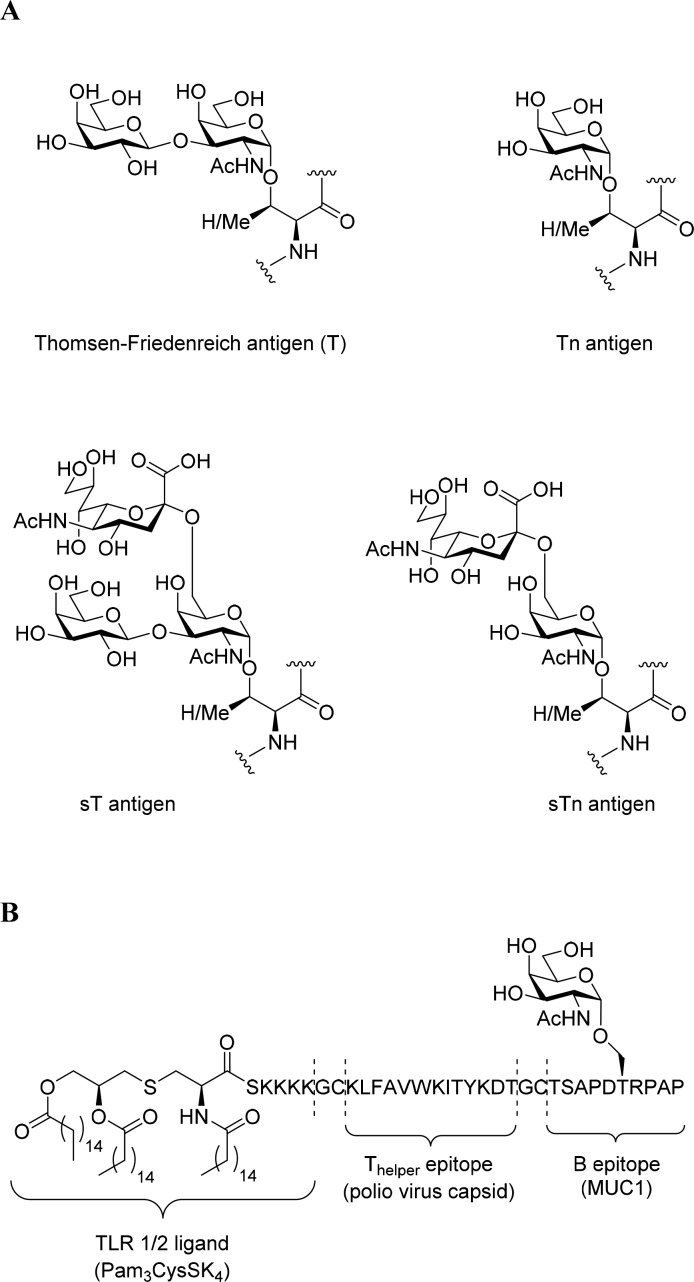
One of the most pursued targets in the study of carbohydrate immunity has arguably been the T/Tn-family of antigens.37 The mucin protein MUC-1, which is normally heavily O-glycosylated, harbors a tandem repeat sequence (HGVTSAPDTRPAPGSTAPPA) and expressed at the site apical side of mucous membranes. In adenocarcinoma, and certain hematopoietic cancers, it is expressed with truncated glycans on all sides of the cell, resulting in humoral (i.e., antibody mediated) response against the protein.38 The simplest of these truncated glycans is a single N-acetylgalactosamine linked to a Ser/Thr-residue, the so-called Tn-antigen, or Gal-β1-3-GalNAc-Ser/Thr (T-antigen) and the 2,6- or 2,3-monosialylated variants of these sugars (Figure 2A). These heavily truncated O-linked glycans promote tumor survival by, e.g., binding the macrophage galactose lectin-1 (MGL-1) and various siglecs to dampen the immune response. MGL-1 is, like other lectins, expressed on tumor-associated macrophages,39 and its presence is associated with poor prognosis and increased cancer growth.40
Figure 2.
A. Chemical structures of the various truncated MUC-1 glycans. B. Chemical structure of the Boons tripartite vaccine.
Aberrantly glycosylated mucins were also found to illicit strong humoral immune responses, with the glycan-containing mucin-repeat regions serving as epitopes for both T- and B-cells. This observation, taken together with the relatively easy synthetic access to the glycans involved, has made MUC-1 a prominent target for cancer immunotherapy, particularly for vaccine strategies.38
Early attempts at exploiting this T- and B-cell recognition potential in a vaccine, such as Tecemotide, which was a liposome-anchored nonglycosylated MUC-1 tandem repeat peptide, did not elicit protective immune responses in clinical trial,41 nor did the first later-phase clinical trials of an α-2,6-sialyl-Tn tandem repeat modified peptide conjugated to the keyhole limpet hemocyanin carrier protein.42,43 This has not deterred the further pursuit of MUC-1 as an immunotherapy target, and virus-based vaccines such as the modified vaccinia Ankara expressing MUC1 and IL-2,44 dendritic cell vaccinations,45 and chimaeric antigen receptor-T-cells (CAR T-cells) targeting this aberrantly glycosylated protein,46,47 are in clinical development.
Extensive synthetic effort has also gone into producing tandem repeat (TR)-peptide based vaccines. For example, the conjugation of the TR-peptides to other T-helper epitope containing proteins, such as ovalbumin,48 or tetanus toxin,49 was reported by Kunz and co-workers to improve antibody titers. This approach has also led to the development of antibodies against aberrantly glycosylated MUC1 as diagnostic tools,50 and preventive immunization in a humanized mouse model expressing human MUC1 led to decreased growth rates of MUC1 expressing tumors.51 Boons and co-workers attempted to improve the vaccine through the direct conjugation of toll-like receptor (TLR)-ligands and peptidic T-helper epitopes to the TR-glycopeptides.52 To avoid carrier and linker-induced immune suppression (leading to vaccine rather than antigen neutralization),53−55 they made a single polypeptide containing the glycosylated TR, as well as a T-helper-epitope from the poliovirus type 1 capsid polypeptide VP1103–115 (KLFAVWKITYKDT)56 (Figure 2B). Antibody titers were further improved when the immunological adjuvant Pam3CysSK4, which efficiently activates antigen presenting cells (APCs) via Toll-like receptor (TLR) 1/2,57,58 was also attached covalently to the epitope. Surprisingly, the effect was significantly higher than for coinjection of epitopes and adjuvant, whereas exchange of this adjuvant for a TLR9 agonist turned out to be detrimental to the ability of the vaccine to elicit the desired antibody response in mice.59
The results after the initial immunization with the vaccine candidate (Figure 2B) were encouraging. The compound was incorporated into phospholipid-based small unilamellar vesicles (SUVs) and used for vaccination. After 4 vaccinations at weekly intervals, anti-MUC-I IgG titers were very high. Only low levels of antibodies directed toward the T-helper-epitope, but robust CD8+ T-cell responses (and antitumor NK-cell responses) were observed.60 The glycan was shown to be very important for vaccination outcome. Both antibody titers, humoral and cellular immunity assays, and tumor burden assessment yielded significantly poorer results when the vaccine candidate was used without the glycan, an observation which has also since been made by Huang and co-workers.61 However, both glycosylated and nonglycosylated peptides were recognized by the produced antibodies. Although antibodies that recognize glycans specifically are well-known,62 most antibodies recognizing the glycosylated TR domain of MUC-1 exhibit increased binding to glycosylated epitopes without actually recognizing the glycan.63,64 This effect has been attributed to a more extended and antibody-accessible conformation imposed on the peptide by the glycan,65 an effect which has also been observed in other MUC-1-derived motifs.66,67
CD8+ T-cell recognition also seems to be indirectly mediated by the glycan, as T-cells from mice immunized with the above tripartite vaccine were better at recognizing both glycosylated and nonglycosylated peptides in vitro. This observation might be explained by the peptide’s mode of binding to MHC-I molecules on APCs, as the glycan belonging to the synthetic MUC-1-epitope SAPDT(O-α-D-GalNAc)RPA has been shown to be buried in the MHC groove rather than being presented toward the T-cell receptor in a solvent exposed position, thereby increasing the binding affinity of the peptide toward the MHC-I molecule instead.65
Taken together with the observations that O-glycans can influence MUC-1 antigen processing,68 but are not necessarily removed in the process,69 it seems likely that the glycan has an indirect role, promoting cross-presentation (presentation of exogenous antigen in MHC-I context) of the glycosylated peptide to CD8+ T-cells.70 Nevertheless, it is clear from the above work that native glycans on a peptide vaccine can prove highly beneficial for its efficacy. Further studies are still needed to clarify some important complications associated with these vaccines, such as tumor escape by immunoediting and evasion.71,72
The most recent clinical developments in the anti-MUC1 vaccine arena have focused on the multivalent delivery of the TR-glycoepitopes,37,73 conjugating to other immune activating lipids.74 It will be highly interesting to follow the progress of such efforts and to see if these strategies will lead to new exciting breakthroughs in the near future.
Other Synthetic O-Glycans for Immune Modulations
While the tumor associated aberrant O-glycosylation motifs of the T and Tn types discussed in the previous section have received the lion’s share of the attention from the synthetic community, other glycosylation motifs have been investigated as well. In one example, β-1,2-mannan containing peptides conjugated to protein carriers were recently explored to induce protection against infections from Candida albicans and other fungi.75 Extensive efforts have also been put into the synthesis of sialyl LewisX glycans as models of the natural human P-selectin ligand P-selecting glycoprotein ligand-1 (PSGL-1).76 These might be able to inhibit the extravasation of lymphocytes into sites of chronic or acute infection, and so might alleviate symptoms of inflammation. Recent clinical trials with the selectin-blocking glycomimetic Rivipansel have provided interesting results for the treatment of vaso-occlusive crises in patients suffering from sickle cell disease. However, future trials will have to ascertain the significance of these results in a statistical sense.77
The synthesis of sialyl LewisX, especially as part of glycopeptide constructs, is made difficult by the nature of the glycoside linkages involved. Specifically, the α2–3 sialylation needed and the easily acid-degradable fucosylation pose large synthetic challenges. However, Wong and co-workers elegantly showed that milligram-scale preparation of a high-binding N-terminal fragment of PSGL-1 was possible using a chemoenzymatic strategy.78,79
The biological evaluations of sialyl LewisX constructs seem to be so far mostly limited to basic in vitro assays. As an example, Kunz and co-workers prepared an N-linked version of sialyl LewisX and successfully tested it as an inhibitor of E-selectin binding to 32Dcl3 neutrophils.80 In another study, they tested non-natively linked, multivalent glycoconjugates containing sialyl LewisX or mixtures of the monosaccharides contained in sialyl LewisX (sialic acid, galactose, and fucose) as binders to endothelial cells and macrophages, and as inhibitors of macrophage migration in vitro.81 However, as selectins are an emerging target for treatment of excessive inflammation in such diseases as metabolic syndrome and even psoriasis,82 we hope that this clinical trial failure does not mark the end of this field.
Synthesis of Chemically Defined Immune-Relevant Glycopeptides and Vaccines via Chemoselective Methods
One drawback of glycopeptide vaccines, despite being powerful tools, is their low immunogenicity in vivo. A range of studies showed that covalently attached adjuvants led to potent T-cell responses,83 and to robust or even higher antibody titers, when compared to separate application of adjuvant and vaccine.52,84 Thus, research has focused on methods to covalently attach adjuvants to glycopeptide building blocks. One convenient way to do this is to employ chemoselective ligation methods which are orthogonal and give high selectivity during coupling reactions.85 The chemoselective ligation methods to obtain glycopeptides have been reviewed extensively,86−89 and will not be discussed exhaustively in this article. Rather than this, we will present a selection of chemoselective ligation methods to prepare adjuvant containing glycopeptide based vaccines with a special focus on O-glycans.
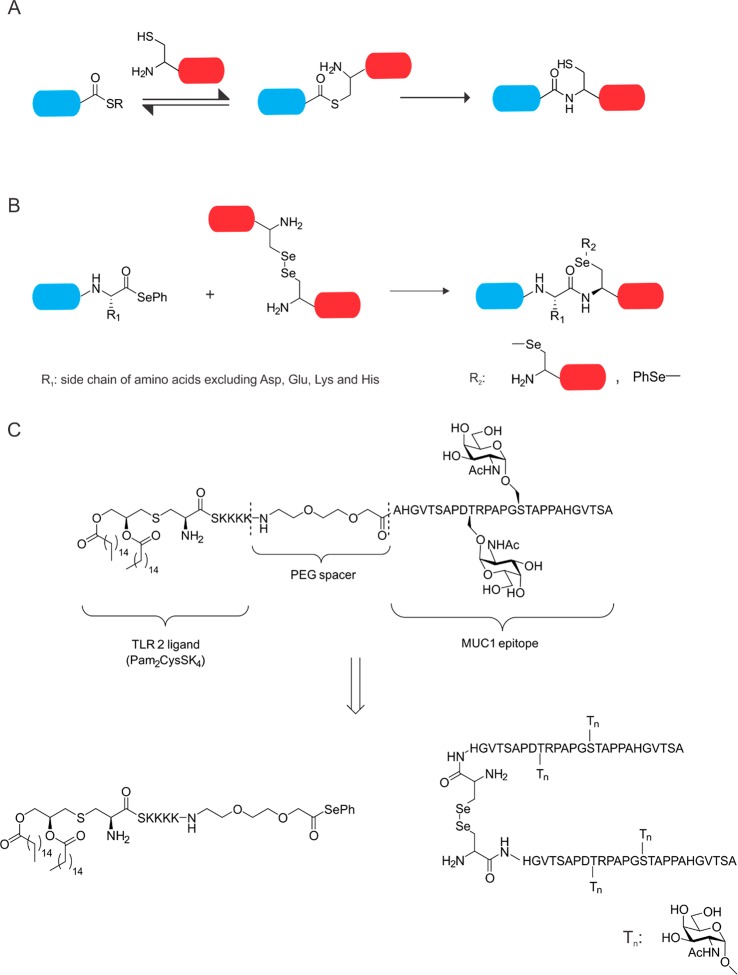
As discussed above, Boons and co-workers reported the first successful synthesis of a self-adjuvating MUC1 cancer vaccine (Figure 2B).52 To this end, they used a chemoselective ligation method to conjugate the adjuvant to the peptide backbone, namely, native chemical ligation (NCL). NCL is a chemoselective reaction between a C-terminal peptide α-thioester and an N-terminal cysteinyl-peptide under aqueous conditions with subsequent purification (Figure 3A).90 C-terminal peptide α-thioesters and their precursors are compatible with standard 9-fluorenylmethoxycarbonyl (Fmoc) solid phase peptide synthesis (SPPS) protocols. Already in 2006, Boons and colleagues developed a method in which liposomes (consisting of dodecylphosphocholine) were used to enhance the solubilization of lipopeptides and assist the ligation of lipophilic lipopeptide thioesters to their cysteinyl reaction partners.91 This was the first demonstration of NCL in glycopeptide vaccine design.
Figure 3.
Native chemical ligation (NCL) and diselenide-selenoester ligation (DSL). A. Schematic representation of NCL, blue cylinder: peptide thioester, red cylinder: N-terminal cysteinyl peptide. B. Schematic representation of DSL, blue cylinder: peptide selenoester, red cylinder: N-terminal diselenide peptide. C. Design and synthesis of a self-adjuvating MUC-1 vaccine.
In the past decade, other NCL inspired chemoselective conjugation methods were reported.92,93 One example worth mentioning is the diselenide-selenoester ligation (DSL), invented by Payne and co-workers.94,95 In this approach, the researchers generate a selenoester at the C-terminus of peptides, whereas the N-terminal selenocysteine dimer of a second peptide enables a not yet fully explored ligation mechanism resulting in the formation of a nascent peptide bond upon deselenization (Figure 3B). This method is highly efficient (quantitative conversion) and rapid (1–10 min).94,96
In the past decade, Payne and co-workers described the synthesis of two and/or three component MUC 1 vaccines (similar to Boons vaccines) using conventional fragment condensation strategies.97−99 Recently, they expanded their chemical toolbox with DSL to synthesize a Tn-antigen bearing mucin tandem repeat peptide covalently linked to the TLR-2 agonist Pam2CysSK4 (adjuvant). In their approach, a Pam2CysSK4 selenoester was synthesized using diphenyl diselenide as a nucleophile (Figure 3C). The glycopeptide with selenocysteine on its N-terminus could be synthesized using standard SPPS protocols and the dimerization occurred spontaneously. Next, the two peptide segments were ligated to each other (2 min, quantitative conversion) with a yield of 62% after purification. With the vaccine in hand, Payne and co-workers performed in vivo experiments by injecting the vaccine candidate subcutaneously into C57BL/6 mice. Adoptive transfer of a mixture of control (PBS treated) or MUC-1 specific splenocytes into vaccinated mice resulted in a significant increase in the MUC-1 specific CTL response. Subsequently, antibody titers in sera from vaccinated mice were assessed, and interestingly, vaccination led to high IgM but not IgG titers. To improve the IgG titer, researchers mixed the vaccine with the pan T-helper epitope PADRE. However, the IgG titers did not increase. Instead, PADRE specific T-cells secreted pro-inflammatory cytokines upon exposure to the self-adjuvating vaccine and external PADRE in mixture, whereas addition of PADRE without the vaccine did not cause the same effect.
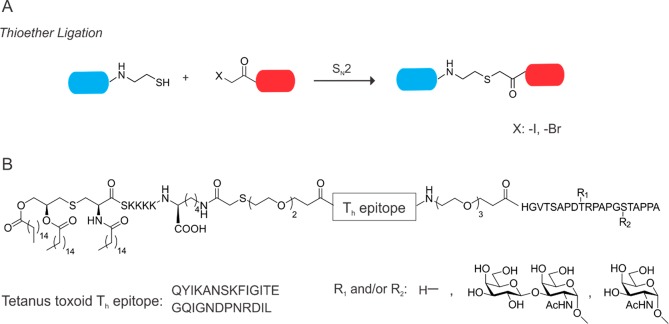
Another interesting approach to obtaining chemically well-defined glycopeptide vaccines has been described by Kunz and co-workers, namely, the thioether ligation (Figure 4A).100 This method relies on the nucleophilicity of sulfhydryl groups. Halogenated acetamides such as iodoacetamide and bromoacetamide have been shown to undergo nucleophilic substitution when reacted with cysteine or other reactive thiols.101 This technique has also been used for peptide ligation strategies prior to the development of NCL.102
Figure 4.
Thioether ligation and its use in glyco-vaccine design. A. Schematic representation of thioether ligation, blue cylinder: C-terminal peptide thiol, red cylinder: halogen-acetopeptide. B. Chemical structure of the Kunz-vaccine.
Kunz and colleagues used their thioether method for the linkage of a lipidated adjuvant, Pam3CysSK4, to a mucine TR peptide, which is attached to a T-helper epitope of the P2 or P4 tetanus toxoid peptide (Figure 4B). In doing so, they obtained a library of two- (without the Pam3Cys adjuvant) and three-component vaccines, which could then be investigated regarding their antibody titers and complement-dependent cytotoxicity (CDC). The three-component vaccine containing P2 Th epitope, Pam3Cys, and the TR peptide initiated an immune response and exhibited high CDC even in the absence of standard adjuvant usage.
These advances demonstrate the power of chemoselective chemical ligation methods as tools for the synthesis of glycopeptide vaccine constructs. With improved methodologies becoming available, so too are detailed studies of glycopeptide vaccines with various adjuvants or helper epitopes becoming increasingly feasible. Collaborative efforts between chemistry and immunology research groups will likely benefit from these new possibilities, spurring hope for the future development of more effective vaccines.
Synthesis of Single-Glycoform N-Glycan Modified Antigens
In comparison to the often short glycans found in aberrant O-linked glycosylation, the synthesis of N-linked glycoproteins and peptides involves larger sugars and a concomitant increase in synthetic complexity.103,104 Their immunological evaluation is therefore less developed than that of the T(n) antigen family. N-Linked glycoproteins all share the same core motif, but are modified with various terminal antennae. The synthesis of single glycoforms for various applications has made great inroads over the past few years, and the application to protein and peptide total synthesis is also beginning to take shape.104 The most commonly used methods have focused on the use of recombinant glycan-remodeling enzymes, total protein synthesis, or the genetic engineering of the glycosylation machinery of whole organisms to yield single glycoforms upon recombinant expression.105 Here, we will give a short overview on the above applications.
Glycoprotein Total Synthesis
The total synthesis of single glycoforms of glycoproteins presents a phenomenal synthetic challenge, which has been solved for very specific cases only. The milestone was the total synthesis of a polyglycosylated single glycoform of erythropoietin by the group of Danishefsky.106,107 Since then, a few other examples of natively linked glycoproteins have been reported, which have been reviewed here.88,89,105,108,109 In the context of this Review—due to their relevance as immune cell signaling moieties—the syntheses of cytokines bears mentioning. Cytokines are crucial for the modulation of inflammation (both acute and chronic) via a complex mechanism.110 They are secreted/expressed by immune cells as a response to pathogens and other dangerous molecules.110 Their small size, combined with often a single glycosylation site, has rendered them attractive targets for glycoprotein total synthesis. Interferon beta (IFN-β),108 the O-glycoprotein interleukin-2 (IL-2),111 granulocyte–macrophage colony-stimulating factor (GM-CSF),112 and IL-6113 have all been synthesized using semisynthetic strategies based on NCL methodology. To take IFN-β as an example, Kajihara and co-workers synthesized it carrying either a sialylated biantennary complex glycan, an asialo-biantennary glycan, or no glycan, and its antitumor activity was assessed highlighting a potential role for sialylation in antitumor activity. The homogeneously sialylated variant showed a slightly more pronounced antitumor effect, although reasons for this effect remain unknown.114 In all, the chemical synthesis of multiple single glycoforms of a single protein on a scale sufficient for immunological evaluation remains a herculean challenge.
Cellular Glycoprotein Expression for Production of Single Glycoform Antigens
Single glycoform cell lines were first developed by the company GlycoFi, who engineered the yeast strain Pichia pastoris in such a manner that it produced single glycoforms of glycoproteins,115 such as the antibody CD20.116 Recently, this methodology has been applied to the production of single glycoforms from Chinese hamster ovary (CHO)-cells, through extensive engineering of the glycosylation machinery of these cells.117,118 This approach, while in principle having the potential to create single glycoforms of antigenic proteins has, to the best of our knowledge, not yet been approached to produce single glycoform cancer antigens. The complexity of the approach must also not be underestimated; with the effort required for each individual glycoform being mammoth-like. Even then, it is still difficult to produce a single glycoform in these optimized sytems.
Chemoenzymatic Glycan Introduction/Remodeling of Peptides
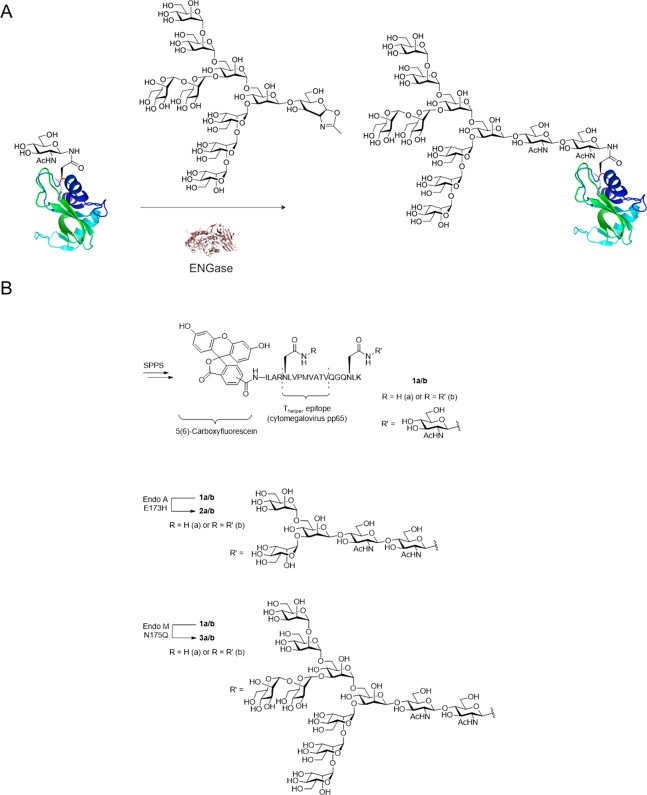
Chemoenzymatic glycoprotein remodeling is the process by which endo-β-N-acetylglucosaminidases (ENGases) are first used to trim down a heterogeneous mixture of glycoforms to a single N-acetylglucosaminyl (GlcNAc) residue (or an αFuc(1 → 6)GlcNac if core fucosylation is present in the parent glycoforms mixture) attached to asparagine.105 This single GlcNAc can then be used as the handle for reintroducing a homogeneous full-length native N-glycan using the same enzyme class to catalyze the reverse reaction (Figure 5A). This affords the desired single glycoform of the protein in question. This type of methodology has been pioneered and reviewed by the groups of Fairbanks119 and Wang,105 and today the ENGases have been mutated to obtain optimal so-called glycosynthases for the attachment of various different glycans with almost no hydrolysis of the product obtained during the enzymatic ligation reaction.119 Of all the above approaches to obtain N-glycosylated peptides, this approach has been the most extensively pursued in an immunological context.
Figure 5.
ENGase catalyzed glycosylation of proteins. A. Scheme of Man9GlcNAcylation of RNase B-GlcNAc via EndoA171A.121 B. Cytomegalovirus pp65 peptide construct prepared and utilized by Fairbanks and co-workers to study antigen uptake and presentation.
Fairbanks and co-workers, for example, synthesized derivatives of a 19-mer peptide derived from cytomegalovirus pp65 protein, containing a known cytotoxic T-cell epitope. Using microwave-assisted SPPS, they synthesized the native peptide as well as variants containing an N-linked GlcNAc on asparagine residues within and adjacent to the T-cell epitope (1a/b, Figure 5B).120 They then used the incorporated GlcNAc residues as targets for ENGase-catalyzed glycosylation using either truncated synthetic glycan oxazolines or full-length high-mannose-type glycan oxazolines derived from soybean flour. This approach yielded chemically defined peptides (Figure 5B, 2a/b and 3a/b) containing single N-glycosylation motifs linked via natural glycosidic bonds rather than by nonhydrolyzable artificial covalent conjugation modes (Figure 5).
The authors went on to show that glycosylated peptides 2a/b and 3a/b targeted antigen presenting cells (APCs), expressing the mannose receptor (MR), significantly better than the nonglycosylated parent peptide. Interestingly, in vitro activation of peptide-specific CTL clones by APCs loaded with the peptide constructs was completely absent when the peptide was glycosylated inside the epitope (2b and 3b), but not when the glycan was exclusively situated outside of the epitope (2a and 3a). This result could indicate that the T-cell clones were specific for peptides that were blocked by the presence of N-glycans in the presented peptide, which were not (fully) removed during antigen processing. This is in contrast to previous experiments that do show their removal during the processing of the peptides for MHC-loading.122,123 An alternative reason could be that the process of glycan introduction and removal—which results in a net Asn to Asp mutation—changed the binding properties of this peptide to such an extent that the T-cell receptors of the clones used no longer recognized this peptide. Indeed, we124,125 and others126 have shown recently that single-atom substitutions can be enough to cause such an effect. Taken together, several factors might play a role regarding N-glycans in antigen processing/presentation, which should be investigated in more detail in the future.
Recently, Wang and co-workers have also used a glycan remodeling approach to produce a peptide-based vaccine. The aim of this vaccine was to elicit an antibody response against the 46 amino acid V3-domain of the HIV-1 ENV glycoprotein, or a 33-mer truncation thereof. They synthesized a cyclic version of this domain carrying a Man9-GlcNAc2-glycan, which they further conjugated to a T-helper epitope and a TLR-ligand.127 Upon vaccination, this construct potently elicited antibodies against the glycan on the epitope,127 which could be further enhanced when the glycan was displayed in a multivalent fashion.35
Concluding Remarks
The above examples serve to demonstrate that, although fully glycosylated proteins are still hardly accessible via synthetic chemistry, synthetic carbohydrate chemistry as of today does provide ready access to some very important targets for immunological and vaccine studies. The bespoke application of these advances in technology may lead to critical findings not only in basic research, but also in translational medicine, i.e., in the development of novel vaccine technology.
The two most obvious caveats associated with the various chemical methodologies discussed in this Review are that, on one hand, the chemical expertise is still very much needed for their successful application; and on the other hand, the heterogeneity of tumors themselves complicate matters, with individual tumors in individual patients likely showing high glycan heterogeneity. However, the recent emergence of patient-specific vaccine strategies may in future also be applied here.61 We therefore strongly encourage the immunology and carbohydrate communities to join forces to an extent that is much larger than what is being done today. Such collaborations will almost certainly lead to many exciting discoveries which will greatly advance our fundamental as well as translational scientific view of the immune system in action. It is tempting to make the perhaps slightly provoking prediction that some of these future discoveries will likely alter the current mechanistic consensuses about glycoimmunology, most of which have been established using non-naturally glycosylated motifs or mixtures of protein glycoforms.
Acknowledgments
M.H.S.M. was funded by a Postdoc Abroad Fellowship from the Lundbeck Foundation. S.I.V.K. was funded by an ERC Starting Grant (639005). C.A. was funded by an ECHO Grant from Dutch Scientific Organization (NWO).
The authors declare no competing financial interest.
References
- Varki A., and Gagneux P. (2015) Biological Functions of Glycans. In Essentials of Glycobiology (Varki A., Cummings R. D., Esko J. D., Stanley P., Hart G. W., Aebi M., Darvill A. G., Kinoshita T., Packer N. H., Prestegard J. H., Schnaar R. L., and Seeberger P. H., Eds.) pp 77–88, Cold Spring Harbor Laboratory Press. [PubMed] [Google Scholar]
- Hart G. W.; Copeland R. J. (2010) Glycomics hits the big time. Cell 143, 672–6. 10.1016/j.cell.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum J. S.; Wearsch P. A.; Cresswell P. (2013) Pathways of antigen processing. Annu. Rev. Immunol. 31, 443–473. 10.1146/annurev-immunol-032712-095910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kooyk Y.; Rabinovich G. A. (2008) Protein-glycan interactions in the control of innate and adaptive immune responses. Nat. Immunol. 9, 593–601. 10.1038/ni.f.203. [DOI] [PubMed] [Google Scholar]
- Dube D. H.; Bertozzi C. R. (2005) Glycans in cancer and inflammation--potential for therapeutics and diagnostics. Nat. Rev. Drug Discovery 4, 477–88. 10.1038/nrd1751. [DOI] [PubMed] [Google Scholar]
- McEver R. P. (2015) Selectins: initiators of leucocyte adhesion and signalling at the vascular wall. Cardiovasc. Res. 107, 331–339. 10.1093/cvr/cvv154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knibbs R. N.; Craig R. A.; Natsuka S.; Chang A.; Cameron M.; Lowe J. B.; Stoolman L. M. (1996) The fucosyltransferase FucT-VII regulates E-selectin ligand synthesis in human T cells. J. Cell Biol. 133, 911–920. 10.1083/jcb.133.4.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Andrian U. H.; Mackay C. R. (2000) T-cell function and migration. Two sides of the same coin. N. Engl. J. Med. 343, 1020–34. 10.1056/NEJM200010053431407. [DOI] [PubMed] [Google Scholar]
- Malý P.; Thall A. D.; Petryniak B.; Rogers C. E.; Smith P. L.; Marks R. M.; Kelly R. J.; Gersten K. M.; Cheng G.; Saunders T. L.; et al. (1996) The α(1,3)Fucosyltransferase Fuc-TVII Controls Leukocyte Trafficking through an Essential Role in L-, E-, and P-selectin Ligand Biosynthesis. Cell 86, 643–653. 10.1016/S0092-8674(00)80137-3. [DOI] [PubMed] [Google Scholar]
- Zhou J. Y.; Oswald D. M.; Oliva K. D.; Kreisman L. S. C.; Cobb B. A. (2018) The Glycoscience of Immunity. Trends Immunol. 39, 523–535. 10.1016/j.it.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown G. D.; Gordon S. (2001) A new receptor for β-glucans. Nature 413, 36–37. 10.1038/35092620. [DOI] [PubMed] [Google Scholar]
- Hardison S. E.; Brown G. D. (2012) C-type lectin receptors orchestrate antifungal immunity. Nat. Immunol. 13, 817. 10.1038/ni.2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams S. J. (2017) Sensing Lipids with Mincle: Structure and Function. Front. Immunol. 8, 1662–1662. 10.3389/fimmu.2017.01662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerscher B.; Willment J. A.; Brown G. D. (2013) The Dectin-2 family of C-type lectin-like receptors: an update. Int. Immunol. 25, 271–7. 10.1093/intimm/dxt006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie E. J.; Su Y. P.; Martinez-Pomares L. (2002) The mannose receptor, a bi-functional lectin with roles in homeostasis and immunity. Trends Glycosci. Glycotechnol. 14, 273–283. 10.4052/tigg.14.273. [DOI] [Google Scholar]
- Garcia-Vallejo J. J.; van Kooyk Y. (2013) The physiological role of DC-SIGN: a tale of mice and men. Trends Immunol. 34, 482–6. 10.1016/j.it.2013.03.001. [DOI] [PubMed] [Google Scholar]
- Dambuza I. M.; Brown G. D. (2015) C-type lectins in immunity: recent developments. Curr. Opin. Immunol. 32, 21–27. 10.1016/j.coi.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L. L.; Suscovich T. J.; Fortune S. M.; Alter G. (2018) Beyond binding: antibody effector functions in infectious diseases. Nat. Rev. Immunol. 18, 46–61. 10.1038/nri.2017.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbison A.; Fadda E. (2019) An atomistic perspective on ADCC quenching by core-fucosylation of IgG1 Fc N-glycans from enhanced sampling molecular dynamics. bioRxiv 701896. [DOI] [PubMed] [Google Scholar]
- Kaneko Y.; Nimmerjahn F.; Ravetch J. V. (2006) Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science 313, 670–3. 10.1126/science.1129594. [DOI] [PubMed] [Google Scholar]
- Anthony R. M.; Nimmerjahn F.; Ashline D. J.; Reinhold V. N.; Paulson J. C.; Ravetch J. V. (2008) Recapitulation of IVIG anti-inflammatory activity with a recombinant IgG Fc. Science 320, 373–6. 10.1126/science.1154315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geijtenbeek T. B. H.; van Vliet S. J.; Koppel E. A.; Sanchez-Hernandez M.; Vandenbroucke-Grauls C. M. J. E.; Appelmelk B.; van Kooyk Y. (2003) Mycobacteria Target DC-SIGN to Suppress Dendritic Cell Function. J. Exp. Med. 197, 7–17. 10.1084/jem.20021229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lübbers J.; Rodríguez E.; van Kooyk Y. (2018) Modulation of Immune Tolerance via Siglec-Sialic Acid Interactions. Front. Immunol. 9, 1. 10.3389/fimmu.2018.02807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macauley M. S.; Crocker P. R.; Paulson J. C. (2014) Siglec-mediated regulation of immune cell function in disease. Nat. Rev. Immunol. 14 (10), 653–666. 10.1038/nri3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara N.; Imamura A.; Yonemizu T.; Akatsu C.; Yang H.; Ueki A.; Watanabe N.; Abdu-Allah H.; Numoto N.; Takematsu H. (2018) CD22-Binding Synthetic Sialosides Regulate B Lymphocyte Proliferation Through CD22 Ligand-Dependent and Independent Pathways, and Enhance Antibody Production in Mice. Front. Immunol. 9, 1. 10.3389/fimmu.2018.00820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiyama S.; Sun J.; Fukahori K.; Ando N.; Wu M.; Schwarz F.; Siddiqui S. S.; Varki A.; Marth J. D.; et al. (2019) Dual actions of group B Streptococcus capsular sialic acid provide resistance to platelet-mediated antimicrobial killing. Proc. Natl. Acad. Sci. U. S. A. 116, 7465–7470. 10.1073/pnas.1815572116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas Q.; Boligan K. F.; Jandus C.; Schneider C.; Simillion C.; Stanczak M. A.; Haubitz M.; Jafari S. M. S.; Zippelius A.; Baerlocher G. M.; et al. (2019) Siglec-9 Regulates an Effector Memory CD8+ T-cell Subset That Congregates in the Melanoma Tumor Microenvironment. Cancer Immunol. Res. 7, 707. 10.1158/2326-6066.CIR-18-0505. [DOI] [PubMed] [Google Scholar]
- Wang J.; Sun J.; Liu L. N.; Flies D. B.; Nie X.; Toki M.; Zhang J.; Song C.; Zarr M.; Zhou X.; et al. (2019) Siglec-15 as an immune suppressor and potential target for normalization cancer immunotherapy. Nat. Med. 25, 656–666. 10.1038/s41591-019-0374-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büll C.; Boltje T. J.; Balneger N.; Weischer S. M.; Wassink M.; van Gemst J. J.; Bloemendal V. R.; Boon L.; van der Vlag J.; Heise T.; et al. (2018) Sialic Acid Blockade Suppresses Tumor Growth by Enhancing T-cell–Mediated Tumor Immunity. Cancer Res. 78, 3574–3588. 10.1158/0008-5472.CAN-17-3376. [DOI] [PubMed] [Google Scholar]
- Das M.; Zhu C.; Kuchroo V. K. (2017) Tim-3 and its role in regulating anti-tumor immunity. Immunol. Rev. 276, 97–111. 10.1111/imr.12520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang C.-W.; Dutta A.; Chang L.-Y.; Mahalingam J.; Lin Y.-C.; Chiang J.-M.; Hsu C.-Y.; Huang C.-T.; Su W.-T.; Chu Y.-Y.; et al. (2015) Apoptosis of tumor infiltrating effector TIM-3+CD8+ T cells in colon cancer. Sci. Rep. 5, 15659–15659. 10.1038/srep15659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y.; Franc V.; Heck A. J. R. (2017) Glycoproteomics: A Balance between High-Throughput and In-Depth Analysis. Trends Biotechnol. 35, 598–609. 10.1016/j.tibtech.2017.04.010. [DOI] [PubMed] [Google Scholar]
- Gray C. J.; Migas L. G.; Barran P. E.; Pagel K.; Seeberger P. H.; Eyers C. E.; Boons G.-J.; Pohl N. L. B.; Compagnon I.; Widmalm G.; et al. (2019) Advancing Solutions to the Carbohydrate Sequencing Challenge. J. Am. Chem. Soc. 141, 14463–14479. 10.1021/jacs.9b06406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L.-X.; Amin M. N. (2014) Chemical and Chemoenzymatic Synthesis of Glycoproteins for Deciphering Functions. Chem. Biol. 21, 51–66. 10.1016/j.chembiol.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai H.; Zhang R.; Orwenyo J.; Giddens J.; Yang Q.; LaBranche C. C.; Montefiori D. C.; Wang L. X. (2018) Multivalent Antigen Presentation Enhances the Immunogenicity of a Synthetic Three-Component HIV-1 V3 Glycopeptide Vaccine. ACS Cent. Sci. 4, 582–589. 10.1021/acscentsci.8b00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buskas T.; Li Y.; Boons G.-J. (2004) The Immunogenicity of the Tumor-Associated Antigen Lewisy May Be Suppressed by a Bifunctional Cross-Linker Required for Coupling to a Carrier Protein. Chem. - Eur. J. 10, 3517–3524. 10.1002/chem.200400074. [DOI] [PubMed] [Google Scholar]
- Gaidzik N.; Westerlind U.; Kunz H. (2013) The development of synthetic antitumour vaccines from mucin glycopeptide antigens. Chem. Soc. Rev. 42, 4421–4442. 10.1039/c3cs35470a. [DOI] [PubMed] [Google Scholar]
- Taylor-Papadimitriou J.; Burchell J. M.; Graham R.; Beatson R. (2018) Latest developments in MUC1 immunotherapy. Biochem. Soc. Trans. 46, 659–668. 10.1042/BST20170400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelissen L. A. M.; Van Vliet S. J. (2016) A Bitter Sweet Symphony: Immune Responses to Altered O-glycan Epitopes in Cancer. Biomolecules 6, 26. 10.3390/biom6020026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahasrabudhe N. M.; van der Horst J. C.; Spaans V.; Kenter G.; de Kroon C.; Bosse T.; van Vliet S. J.; Jordanova E. S. (2019) MGL Ligand Expression Is Correlated to Lower Survival and Distant Metastasis in Cervical Squamous Cell and Adenosquamous Carcinoma. Front. Oncol. 9, 29–29. 10.3389/fonc.2019.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butts C.; Socinski M. A.; Mitchell P. L.; Thatcher N.; Havel L.; Krzakowski M.; Nawrocki S.; Ciuleanu T. E.; Bosquee L.; Trigo J. M.; et al. (2014) Tecemotide (L-BLP25) versus placebo after chemoradiotherapy for stage III non-small-cell lung cancer (START): a randomised, double-blind, phase 3 trial. Lancet Oncol. 15, 59–68. 10.1016/S1470-2045(13)70510-2. [DOI] [PubMed] [Google Scholar]
- Holmberg L. A.; Sandmaier B. M. (2004) Vaccination with Theratope® (STn-KLH) as treatment for breast cancer. Expert Rev. Vaccines 3, 655–663. 10.1586/14760584.3.6.655. [DOI] [PubMed] [Google Scholar]
- Miles D.; Roché H.; Martin M.; Perren T. J.; Cameron D. A.; Glaspy J.; Dodwell D.; Parker J.; Mayordomo J.; Tres A.; et al. (2011) Phase III multicenter clinical trial of the sialyl-TN (STn)-keyhole limpet hemocyanin (KLH) vaccine for metastatic breast cancer. Oncologist 16, 1092–1100. 10.1634/theoncologist.2010-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quoix E.; Lena H.; Losonczy G.; Forget F.; Chouaid C.; Papai Z.; Gervais R.; Ottensmeier C.; Szczesna A.; Kazarnowicz A.; et al. (2016) TG4010 immunotherapy and first-line chemotherapy for advanced non-small-cell lung cancer (TIME): results from the phase 2b part of a randomised, double-blind, placebo-controlled, phase 2b/3 trial. Lancet Oncol. 17, 212–223. 10.1016/S1470-2045(15)00483-0. [DOI] [PubMed] [Google Scholar]
- Kontani K.; Taguchi O.; Ozaki Y.; Hanaoka J.; Sawai S.; Inoue S.; Abe H.; Hanasawa K.; Fujino S. (2003) Dendritic cell vaccine immunotherapy of cancer targeting MUC1 mucin. Int. J. Mol. Med. 12, 493–502. 10.3892/ijmm.12.4.493. [DOI] [PubMed] [Google Scholar]
- Wilkie S.; Picco G.; Foster J.; Davies D. M.; Julien S.; Cooper L.; Arif S.; Mather S. J.; Taylor-Papadimitriou J.; Burchell J. M.; et al. (2008) Retargeting of human T cells to tumor-associated MUC1: the evolution of a chimeric antigen receptor. J. Immunol. 180, 4901–9. 10.4049/jimmunol.180.7.4901. [DOI] [PubMed] [Google Scholar]
- Posey A. D. Jr.; Schwab R. D.; Boesteanu A. C.; Steentoft C.; Mandel U.; Engels B.; Stone J. D.; Madsen T. D.; Schreiber K.; Haines K. M.; et al. (2016) Engineered CAR T Cells Targeting the Cancer-Associated Tn-Glycoform of the Membrane Mucin MUC1 Control Adenocarcinoma. Immunity 44, 1444–54. 10.1016/j.immuni.2016.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerlind U.; Hobel A.; Gaidzik N.; Schmitt E.; Kunz H. (2008) Synthetic vaccines consisting of tumor-associated MUC1 glycopeptide antigens and a T-cell epitope for the induction of a highly specific humoral immune response. Angew. Chem., Int. Ed. 47, 7551–6. 10.1002/anie.200802102. [DOI] [PubMed] [Google Scholar]
- Kaiser A.; Gaidzik N.; Westerlind U.; Kowalczyk D.; Hobel A.; Schmitt E.; Kunz H. (2009) A Synthetic Vaccine Consisting of a Tumor-Associated Sialyl-TN-MUC1 Tandem-Repeat Glycopeptide and Tetanus Toxoid: Induction of a Strong and Highly Selective Immune Response. Angew. Chem., Int. Ed. 48, 7551–7555. 10.1002/anie.200902564. [DOI] [PubMed] [Google Scholar]
- Palitzsch B.; Gaidzik N.; Stergiou N.; Stahn S.; Hartmann S.; Gerlitzki B.; Teusch N.; Flemming P.; Schmitt E.; Kunz H. (2016) A Synthetic Glycopeptide Vaccine for the Induction of a Monoclonal Antibody that Differentiates between Normal and Tumor Mammary Cells and Enables the Diagnosis of Human Pancreatic Cancer. Angew. Chem., Int. Ed. 55, 2894–2898. 10.1002/anie.201509935. [DOI] [PubMed] [Google Scholar]
- Stergiou N.; Gaidzik N.; Heimes A.-S.; Dietzen S.; Besenius P.; Jäkel J.; Brenner W.; Schmidt M.; Kunz H.; Schmitt E. (2019) Reduced Breast Tumor Growth after Immunization with a Tumor-Restricted MUC1 Glycopeptide Conjugated to Tetanus Toxoid. Cancer Immunol. Res. 7, 113–122. 10.1158/2326-6066.CIR-18-0256. [DOI] [PubMed] [Google Scholar]
- Ingale S.; Wolfert M. A.; Gaekwad J.; Buskas T.; Boons G. J. (2007) Robust immune responses elicited by a fully synthetic three-component vaccine. Nat. Chem. Biol. 3, 663–7. 10.1038/nchembio.2007.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pobre K.; Tashani M.; Ridda I.; Rashid H.; Wong M.; Booy R. (2014) Carrier priming or suppression: understanding carrier priming enhancement of anti-polysaccharide antibody response to conjugate vaccines. Vaccine 32, 1423–30. 10.1016/j.vaccine.2014.01.047. [DOI] [PubMed] [Google Scholar]
- Etlinger H. M.; Gillessen D.; Lahm H. W.; Matile H.; Schonfeld H. J.; Trzeciak A. (1990) Use of prior vaccinations for the development of new vaccines. Science 249, 423–5. 10.1126/science.1696030. [DOI] [PubMed] [Google Scholar]
- Sad S.; Rao K.; Arora R.; Talwar G. P.; Raghupathy R. (1992) Bypass of carrier-induced epitope-specific suppression using a T-helper epitope. Immunology 76, 599–603. [PMC free article] [PubMed] [Google Scholar]
- Leclerc C.; Deriaud E.; Mimic V.; van der Werf S. (1991) Identification of a T-cell epitope adjacent to neutralization antigenic site 1 of poliovirus type 1. J. Virol. 65, 711–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabbagh K.; Lewis D. B. (2003) Toll-like receptors and T-helper-1/T-helper-2 responses. Curr. Opin. Infect. Dis. 16, 199–204. 10.1097/00001432-200306000-00003. [DOI] [PubMed] [Google Scholar]
- Oosenbrug T.; van de Graaff M. J.; Ressing M. E.; van Kasteren S. I. (2017) Chemical Tools for Studying TLR Signaling Dynamics. Cell Chem. Biol. 24, 801–812. 10.1016/j.chembiol.2017.05.022. [DOI] [PubMed] [Google Scholar]
- Abdel-Aal A. B.; Lakshminarayanan V.; Thompson P.; Supekar N.; Bradley J. M.; Wolfert M. A.; Cohen P. A.; Gendler S. J.; Boons G. J. (2014) Immune and anticancer responses elicited by fully synthetic aberrantly glycosylated MUC1 tripartite vaccines modified by a TLR2 or TLR9 agonist. ChemBioChem 15 (10), 1508–13. 10.1002/cbic.201402077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshminarayanan V.; Thompson P.; Wolfert M. A.; Buskas T.; Bradley J. M.; Pathangey L. B.; Madsen C. S.; Cohen P. A.; Gendler S. J.; Boons G. J. (2012) Immune recognition of tumor-associated mucin MUC1 is achieved by a fully synthetic aberrantly glycosylated MUC1 tripartite vaccine. Proc. Natl. Acad. Sci. U. S. A. 109 (1), 261–6. 10.1073/pnas.1115166109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X.; Yin Z.; McKay C.; Pett C.; Yu J.; Schorlemer M.; Gohl T.; Sungsuwan S.; Ramadan S.; Baniel C.; et al. (2018) Protective Epitope Discovery and Design of MUC1-based Vaccine for Effective Tumor Protections in Immunotolerant Mice. J. Am. Chem. Soc. 140, 16596–16609. 10.1021/jacs.8b08473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haji-Ghassemi O.; Blackler R. J.; Martin Young N.; Evans S. V. (2015) Antibody recognition of carbohydrate epitopesdagger. Glycobiology 25, 920–52. 10.1093/glycob/cwv037. [DOI] [PubMed] [Google Scholar]
- Karsten U.; Serttas N.; Paulsen H.; Danielczyk A.; Goletz S. (2004) Binding patterns of DTR-specific antibodies reveal a glycosylation-conditioned tumor-specific epitope of the epithelial mucin (MUC1). Glycobiology 14, 681–92. 10.1093/glycob/cwh090. [DOI] [PubMed] [Google Scholar]
- Movahedin M.; Brooks T. M.; Supekar N. T.; Gokanapudi N.; Boons G. J.; Brooks C. L. (2016) Glycosylation of MUC1 influences the binding of a therapeutic antibody by altering the conformational equilibrium of the antigen. Glycobiology 27, 677–687. 10.1093/glycob/cww131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostolopoulos V.; Yuriev E.; Ramsland P. A.; Halton J.; Osinski C.; Li W.; Plebanski M.; Paulsen H.; McKenzie I. F. C. (2003) A glycopeptide in complex with MHC class I uses the GalNAc residue as an anchor. Proc. Natl. Acad. Sci. U. S. A. 100, 15029–15034. 10.1073/pnas.2432220100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coltart D. M.; Royyuru A. K.; Williams L. J.; Glunz P. W.; Sames D.; Kuduk S. D.; Schwarz J. B.; Chen X.-T.; Danishefsky S. J.; Live D. H. (2002) Principles of Mucin Architecture: Structural Studies on Synthetic Glycopeptides Bearing Clustered Mono-, Di-, Tri-, and Hexasaccharide Glycodomains. J. Am. Chem. Soc. 124, 9833–9844. 10.1021/ja020208f. [DOI] [PubMed] [Google Scholar]
- Braun P.; Davies G. M.; Price M. R.; Williams P. M.; Tendler S. J.; Kunz H. (1998) Effects of glycosylation on fragments of tumour associated human epithelial mucin MUC1. Bioorg. Med. Chem. 6, 1531–45. 10.1016/S0968-0896(98)00092-3. [DOI] [PubMed] [Google Scholar]
- Ninkovic T.; Hanisch F. G. (2007) O-glycosylated human MUC1 repeats are processed in vitro by immunoproteasomes. J. Immunol. 179, 2380–8. 10.4049/jimmunol.179.4.2380. [DOI] [PubMed] [Google Scholar]
- Vlad A. M.; Muller S.; Cudic M.; Paulsen H.; Otvos L. Jr.; Hanisch F. G.; Finn O. J. (2002) Complex carbohydrates are not removed during processing of glycoproteins by dendritic cells: processing of tumor antigen MUC1 glycopeptides for presentation to major histocompatibility complex class II-restricted T cells. J. Exp. Med. 196, 1435–46. 10.1084/jem.20020493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norbury C. C. (2016) Defining cross presentation for a wider audience. Curr. Opin. Immunol. 40, 110–116. 10.1016/j.coi.2016.04.003. [DOI] [PubMed] [Google Scholar]
- Lakshminarayanan V.; Supekar N. T.; Wei J.; McCurry D. B.; Dueck A. C.; Kosiorek H. E.; Trivedi P. P.; Bradley J. M.; Madsen C. S.; Pathangey L. B.; et al. (2016) MUC1 Vaccines, Comprised of Glycosylated or Non-Glycosylated Peptides or Tumor-Derived MUC1, Can Circumvent Immunoediting to Control Tumor Growth in MUC1 Transgenic Mice. PLoS One 11, e0145920. 10.1371/journal.pone.0145920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber R. D.; Old L. J.; Smyth M. J. (2011) Cancer Immunoediting: Integrating Immunity’s Roles in Cancer Suppression and Promotion. Science 331, 1565–1570. 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- Glaffig M.; Palitzsch B.; Stergiou N.; Schüll C.; Straßburger D.; Schmitt E.; Frey H.; Kunz H. (2015) Enhanced immunogenicity of multivalent MUC1 glycopeptide antitumour vaccines based on hyperbranched polymers. Org. Biomol. Chem. 13, 10150–10154. 10.1039/C5OB01255D. [DOI] [PubMed] [Google Scholar]
- Li M.; Wang Z.; Yan B.; Yin X.; Zhao Y.; Yu F.; Meng M.; Liu Y.; Zhao W. (2019) Design of a MUC1-based tricomponent vaccine adjuvanted with FSL-1 for cancer immunotherapy. MedChemComm 1. 10.1039/C9MD00254E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao J.; Pan B.; Liao G.; Zhao Q.; Gao Y.; Chai X.; Zhuo X.; Wu Q.; Jiao B.; Pan W.; et al. (2019) Synthesis and immunological studies of β-1,2-mannan-peptide conjugates as antifungal vaccines. Eur. J. Med. Chem. 173, 250–260. 10.1016/j.ejmech.2019.04.001. [DOI] [PubMed] [Google Scholar]
- Pudelko M.; Bull J.; Kunz H. (2010) Chemical and Chemoenzymatic Synthesis of Glycopeptide Selectin Ligands Containing Sialyl Lewis X Structures. ChemBioChem 11, 904–930. 10.1002/cbic.201000029. [DOI] [PubMed] [Google Scholar]
- Telen M. J.; Wun T.; McCavit T. L.; De Castro L. M.; Krishnamurti L.; Lanzkron S.; Hsu L. L.; Smith W. R.; Rhee S.; Magnani J. L.; et al. (2015) H., Randomized phase 2 study of GMI-1070 in SCD: reduction in time to resolution of vaso-occlusive events and decreased opioid use. Blood 125, 2656–2664. 10.1182/blood-2014-06-583351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeller K. M.; Smith M. E. B.; Huang R.-F.; Wong C.-H. (2000) Chemoenzymatic Synthesis of a PSGL-1 N-Terminal Glycopeptide Containing Tyrosine Sulfate and α-O-Linked Sialyl Lewis X. J. Am. Chem. Soc. 122, 4241–4242. 10.1021/ja0004938. [DOI] [Google Scholar]
- Koeller K. M.; Smith M. E. B.; Wong C.-H. (2000) Tyrosine Sulfation on a PSGL-1 Glycopeptide Influences the Reactivity of Glycosyltransferases Responsible for Synthesis of the Attached O-Glycan. J. Am. Chem. Soc. 122, 742–743. 10.1021/ja993820o. [DOI] [Google Scholar]
- Filser C.; Kowalczyk D.; Jones C.; Wild M. K.; Ipe U.; Vestweber D.; Kunz H. (2007) Synthetic Glycopeptides from the E-Selectin Ligand 1 with Varied Sialyl Lewisx Structure as Cell-Adhesion Inhibitors of E-Selectin. Angew. Chem., Int. Ed. 46, 2108–2111. 10.1002/anie.200604442. [DOI] [PubMed] [Google Scholar]
- Moog K. E.; Barz M.; Bartneck M.; Beceren-Braun F.; Mohr N.; Wu Z.; Braun L.; Dernedde J.; Liehn E. A.; Tacke F.; et al. (2017) Polymeric Selectin Ligands Mimicking Complex Carbohydrates: From Selectin Binders to Modifiers of Macrophage Migration. Angew. Chem., Int. Ed. 56, 1416–1421. 10.1002/anie.201610395. [DOI] [PubMed] [Google Scholar]
- Patel M. S.; Miranda-Nieves D.; Chen J.; Haller C. A.; Chaikof E. L. (2017) Targeting P-selectin glycoprotein ligand-1/P-selectin interactions as a novel therapy for metabolic syndrome. Transl. Res. 183, 1–13. 10.1016/j.trsl.2016.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R. J.; Tang C.-w.; Daniels N. J.; Compton B. J.; Hayman C. M.; Johnston K. A.; Knight D. A.; Gasser O.; Poyntz H. C.; Ferguson P. M.; et al. (2014) A self-adjuvanting vaccine induces cytotoxic T lymphocytes that suppress allergy. Nat. Chem. Biol. 10, 943. 10.1038/nchembio.1640. [DOI] [PubMed] [Google Scholar]
- Wilkinson B. L.; Day S.; Chapman R.; Perrier S.; Apostolopoulos V.; Payne R. J. (2012) Synthesis and Immunological Evaluation of Self-Assembling and Self-Adjuvanting Tricomponent Glycopeptide Cancer-Vaccine Candidates. Chem. - Eur. J. 18, 16540–16548. 10.1002/chem.201202629. [DOI] [PubMed] [Google Scholar]
- Hackenberger C. P. R.; Schwarzer D. (2008) Chemoselective Ligation and Modification Strategies for Peptides and Proteins. Angew. Chem., Int. Ed. 47, 10030–10074. 10.1002/anie.200801313. [DOI] [PubMed] [Google Scholar]
- Westerlind U. (2012) Synthetic glycopeptides and glycoproteins with applications in biological research. Beilstein J. Org. Chem. 8, 804–818. 10.3762/bjoc.8.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto R.; Izumi M.; Kajihara Y. (2010) Expanding the Scope of Native Chemical Ligation in Glycopeptide Synthesis. Int. J. Pept. Res. Ther. 16, 191–198. 10.1007/s10989-010-9226-8. [DOI] [Google Scholar]
- Carlo U.; Yasuhiro K. (2018) Recent advances in the chemical synthesis of N-linked glycoproteins. Curr. Opin. Chem. Biol. 46, 130–137. 10.1016/j.cbpa.2018.07.004. [DOI] [PubMed] [Google Scholar]
- Unverzagt C.; Kajihara Y. (2013) Chemical assembly of N-glycoproteins: a refined toolbox to address a ubiquitous posttranslational modification. Chem. Soc. Rev. 42, 4408–4420. 10.1039/c3cs35485g. [DOI] [PubMed] [Google Scholar]
- Dawson P. E.; Muir T. W.; Clark-Lewis I.; Kent S. B. (1994) Synthesis of proteins by native chemical ligation. Science 266, 776–9. 10.1126/science.7973629. [DOI] [PubMed] [Google Scholar]
- Ingale S.; Buskas T.; Boons G.-J. (2006) Synthesis of Glyco(lipo)peptides by Liposome-Mediated Native Chemical Ligation. Org. Lett. 8, 5785–5788. 10.1021/ol062423x. [DOI] [PubMed] [Google Scholar]
- Dunkelmann D. L.; Hirata Y.; Totaro K. A.; Cohen D. T.; Zhang C.; Gates Z. P.; Pentelute B. L. (2018) Amide-forming chemical ligation via O-acyl hydroxamic acids. Proc. Natl. Acad. Sci. U. S. A. 115, 3752–3757. 10.1073/pnas.1718356115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malins L. R.; Payne R. J. (2014) Recent extensions to native chemical ligation for the chemical synthesis of peptides and proteins. Curr. Opin. Chem. Biol. 22, 70–78. 10.1016/j.cbpa.2014.09.021. [DOI] [PubMed] [Google Scholar]
- Mitchell N. J.; Malins L. R.; Liu X.; Thompson R. E.; Chan B.; Radom L.; Payne R. J. (2015) Rapid Additive-Free Selenocystine–Selenoester Peptide Ligation. J. Am. Chem. Soc. 137, 14011–14014. 10.1021/jacs.5b07237. [DOI] [PubMed] [Google Scholar]
- Kulkarni S. S.; Watson E. E.; Premdjee B.; Conde-Frieboes K. W.; Payne R. J. (2019) Diselenide–selenoester ligation for chemical protein synthesis. Nat. Protoc. 14, 2229–2257. 10.1038/s41596-019-0180-4. [DOI] [PubMed] [Google Scholar]
- McDonald D. M.; Hanna C. C.; Ashhurst A. S.; Corcilius L.; Byrne S. N.; Payne R. J. (2018) Synthesis of a Self-Adjuvanting MUC1 Vaccine via Diselenide-Selenoester Ligation-Deselenization. ACS Chem. Biol. 13, 3279–3285. 10.1021/acschembio.8b00675. [DOI] [PubMed] [Google Scholar]
- Wilkinson B. L.; Malins L. R.; Chun C. K. Y.; Payne R. J. (2010) Synthesis of MUC1–lipopeptide chimeras. Chem. Commun. 46, 6249–6251. 10.1039/c0cc01360a. [DOI] [PubMed] [Google Scholar]
- Wilkinson B. L.; Day S.; Malins L. R.; Apostolopoulos V.; Payne R. J. (2011) Self-Adjuvanting Multicomponent Cancer Vaccine Candidates Combining Per-Glycosylated MUC1 Glycopeptides and the Toll-like Receptor 2 Agonist Pam3CysSer. Angew. Chem., Int. Ed. 50, 1635–1639. 10.1002/anie.201006115. [DOI] [PubMed] [Google Scholar]
- McDonald D. M.; Wilkinson B. L.; Corcilius L.; Thaysen-Andersen M.; Byrne S. N.; Payne R. J. (2014) Synthesis and immunological evaluation of self-adjuvanting MUC1-macrophage activating lipopeptide 2 conjugate vaccine candidates. Chem. Commun. 50, 10273–10276. 10.1039/C4CC03510K. [DOI] [PubMed] [Google Scholar]
- Cai H.; Sun Z.-Y.; Huang Z.-H.; Shi L.; Zhao Y.-F.; Kunz H.; Li Y.-M. (2013) Fully Synthetic Self-Adjuvanting Thioether-Conjugated Glycopeptide-Lipopeptide Antitumor Vaccines for the Induction of Complement-Dependent Cytotoxicity against Tumor Cells. Chem. - Eur. J. 19, 1962–1970. 10.1002/chem.201203709. [DOI] [PubMed] [Google Scholar]
- Kenyon G. L.; Bruice T. W. (1977) Novel sulfhydryl reagents. Methods Enzymol. 47, 407–430. 10.1016/0076-6879(77)47042-3. [DOI] [PubMed] [Google Scholar]
- Englebretsen D. R.; Garnham B. C.; Bergman D. A.; Alewood P. F. (1995) A novel thioether linker: Chemical synthesis of a HIV-1 protease analogue by thioether ligation. Tetrahedron Lett. 36, 8871–8874. 10.1016/0040-4039(95)01843-7. [DOI] [Google Scholar]
- Davis B. G. (2002) Synthesis of Glycoproteins. Chem. Rev. 102, 579–602. 10.1021/cr0004310. [DOI] [PubMed] [Google Scholar]
- Gamblin D. P.; Scanlan E. M.; Davis B. G. (2009) Glycoprotein Synthesis: An Update. Chem. Rev. 109, 131–163. 10.1021/cr078291i. [DOI] [PubMed] [Google Scholar]
- Li C.; Wang L.-X. (2018) Chemoenzymatic Methods for the Synthesis of Glycoproteins. Chem. Rev. 118, 8359–8413. 10.1021/acs.chemrev.8b00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Tejada A., Brailsford J., Zhang Q., Shieh J.-H., Moore M. A. S., and Danishefsky S. J. (2015) Total Synthesis of Glycosylated Proteins, In Protein Ligation and Total Synthesis I (Liu L., Ed.) pp 1–26, Springer International Publishing, Cham. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P.; Dong S.; Brailsford J. A.; Iyer K.; Townsend S. D.; Zhang Q.; Hendrickson R. C.; Shieh J.; Moore M. A.; Danishefsky S. J. (2012) At last: erythropoietin as a single glycoform. Angew. Chem., Int. Ed. 51, 11576–11584. 10.1002/anie.201206090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto I.; Tezuka K.; Fukae K.; Ishii K.; Taduru K.; Maeda M.; Ouchi M.; Yoshida K.; Nambu Y.; Igarashi J.; et al. (2012) Chemical Synthesis of Homogeneous Human Glycosyl-interferon-β That Exhibits Potent Antitumor Activity in Vivo. J. Am. Chem. Soc. 134, 5428–5431. 10.1021/ja2109079. [DOI] [PubMed] [Google Scholar]
- Burke H. M.; McSweeney L.; Scanlan E. M. (2017) Exploring chemoselective S-to-N acyl transfer reactions in synthesis and chemical biology. Nat. Commun. 8, 15655–15655. 10.1038/ncomms15655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner M. D.; Nedjai B.; Hurst T.; Pennington D. J. (2014) Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochim. Biophys. Acta, Mol. Cell Res. 1843, 2563–2582. 10.1016/j.bbamcr.2014.05.014. [DOI] [PubMed] [Google Scholar]
- Asahina Y.; Komiya S.; Ohagi A.; Fujimoto R.; Tamagaki H.; Nakagawa K.; Sato T.; Akira S.; Takao T.; Ishii A.; et al. (2015) Chemical Synthesis of O-Glycosylated Human Interleukin-2 by the Reverse Polarity Protection Strategy. Angew. Chem., Int. Ed. 54, 8226–8230. 10.1002/anie.201501847. [DOI] [PubMed] [Google Scholar]
- Zhang Q.; Johnston E. V.; Shieh J.-H.; Moore M. A. S.; Danishefsky S. J. (2014) Synthesis of granulocyte-macrophage colony-stimulating factor as homogeneous glycoforms and early comparisons with yeast cell-derived material. Proc. Natl. Acad. Sci. U. S. A. 111, 2885–2890. 10.1073/pnas.1400140111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reif A.; Siebenhaar S.; Tröster A.; Schmälzlein M.; Lechner C.; Velisetty P.; Gottwald K.; Pöhner C.; Boos I.; Schubert V.; Rose-John S.; et al. (2014) Semisynthesis of Biologically Active Glycoforms of the Human Cytokine Interleukin 6. Angew. Chem., Int. Ed. 53, 12125–12131. 10.1002/anie.201407160. [DOI] [PubMed] [Google Scholar]
- Carlo U.; Yasuhiro K. (2018) Recent advances in the chemical synthesis of N-linked glycoproteins. Curr. Opin. Chem. Biol. 46, 130–137. 10.1016/j.cbpa.2018.07.004. [DOI] [PubMed] [Google Scholar]
- Hamilton S. R.; Bobrowicz P.; Bobrowicz B.; Davidson R. C.; Li H.; Mitchell T.; Nett J. H.; Rausch S.; Stadheim T. A.; Wischnewski H.; et al. (2003) Production of complex human glycoproteins in yeast. Science 301, 1244–6. 10.1126/science.1088166. [DOI] [PubMed] [Google Scholar]
- Li H.; Sethuraman N.; Stadheim T. A.; Zha D.; Prinz B.; Ballew N.; Bobrowicz P.; Choi B.-K.; Cook W. J.; Cukan M.; et al. (2006) Optimization of humanized IgGs in glycoengineered Pichia pastoris. Nat. Biotechnol. 24, 210–215. 10.1038/nbt1178. [DOI] [PubMed] [Google Scholar]
- Yang Z.; Wang S.; Halim A.; Schulz M. A.; Frodin M.; Rahman S. H.; Vester-Christensen M. B.; Behrens C.; Kristensen C.; Vakhrushev S. Y.; et al. (2015) Engineered CHO cells for production of diverse, homogeneous glycoproteins. Nat. Biotechnol. 33, 842. 10.1038/nbt.3280. [DOI] [PubMed] [Google Scholar]
- Tian W.; Ye Z.; Wang S.; Schulz M. A.; Van Coillie J.; Sun L.; Chen Y.-H.; Narimatsu Y.; Hansen L.; Kristensen C.; et al. (2019) The glycosylation design space for recombinant lysosomal replacement enzymes produced in CHO cells. Nat. Commun. 10, 1785. 10.1038/s41467-019-09809-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks A. J. (2017) The ENGases: versatile biocatalysts for the production of homogeneous N-linked glycopeptides and glycoproteins. Chem. Soc. Rev. 46, 5128–5146. 10.1039/C6CS00897F. [DOI] [PubMed] [Google Scholar]
- McIntosh J. D.; Brimble M. A.; Brooks A. E. S.; Dunbar P. R.; Kowalczyk R.; Tomabechi Y.; Fairbanks A. J. (2015) Convergent chemo-enzymatic synthesis of mannosylated glycopeptides; targeting of putative vaccine candidates to antigen presenting cells. Chem. Sci. 6, 4636–4642. 10.1039/C5SC00952A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W.; Li C.; Li B.; Umekawa M.; Yamamoto K.; Zhang X.; Wang L.-X. (2009) Glycosynthases Enable a Highly Efficient Chemoenzymatic Synthesis of N-Glycoproteins Carrying Intact Natural N-Glycans. J. Am. Chem. Soc. 131, 2214–2223. 10.1021/ja8074677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfert M. A.; Boons G. J. (2013) Adaptive immune activation: glycosylation does matter. Nat. Chem. Biol. 9, 776–84. 10.1038/nchembio.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd P. M.; Elliott T.; Cresswell P.; Wilson I. A.; Dwek R. A. (2001) Glycosylation and the immune system. Science 291, 2370–6. 10.1126/science.291.5512.2370. [DOI] [PubMed] [Google Scholar]
- Pawlak J. B.; Hos B. J.; van de Graaff M. J.; Megantari O. A.; Meeuwenoord N.; Overkleeft H. S.; Filippov D. V.; Ossendorp F.; van Kasteren S. I. (2016) The Optimization of Bioorthogonal Epitope Ligation within MHC-I Complexes. ACS Chem. Biol. 11, 3172–3178. 10.1021/acschembio.6b00498. [DOI] [PubMed] [Google Scholar]
- Pawlak J. B.; Gential G. P. P.; Ruckwardt T. J.; Bremmers J. S.; Meeuwenoord N. J.; Ossendorp F. A.; Overkleeft H. S.; Filippov D. V.; van Kasteren S. I. (2015) Bioorthogonal Deprotection on the Dendritic Cell Surface for Chemical Control of Antigen Cross-Presentation. Angew. Chem., Int. Ed. 54, 5628–5631. 10.1002/anie.201500301. [DOI] [PubMed] [Google Scholar]
- Zehn D.; Lee S. Y.; Bevan M. J. (2009) Complete but curtailed T-cell response to very low-affinity antigen. Nature 458, 211–4. 10.1038/nature07657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai H.; Orwenyo J.; Giddens J. P.; Yang Q.; Zhang R.; LaBranche C. C.; Montefiori D. C.; Wang L. X. (2017) Synthetic Three-Component HIV-1 V3 Glycopeptide Immunogens Induce Glycan-Dependent Antibody Responses. Cell Chem. Biol. 24, 1513. 10.1016/j.chembiol.2017.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]