Abstract
Enterovirus A71 (EV-A71) is one of the major causative agents of hand, foot and mouth disease (HFMD) in the world, infecting mostly infants and young children (<5 years of age) in Asia. Approximately 2 million cases of HFMD were reported in China each year, of which approximately 45–50% were due to EV-A71. Most of the HFMD infections caused by EV-A71 usually result in mild symptoms with rashes and ulcers in the mouth. However, virulent strains of EV-A71 can infect the central nervous system and cause severe neurologic diseases, leading to reduced cognitive ability, acute flaccid paralysis and death. The lack of understanding of cellular immunity for long-term protection from the HFMD disease represents a major obstacle for vaccine development. In particular, the role of innate and T cell immunity during HFMD infection remains unclear and there is evidence suggesting the importance of CD4+ and CD8+ T cells for protective immunity. Currently, no US FDA-approved vaccine is available for EV-A71. Although the inactivated vaccines produced in China are highly effective (vaccine efficacy >95%), they lack the cellular immunity required for long-term protection. In this review, we discuss the findings that support the protective roles of innate and T cell immunity against EV-A71 infection, which will provide the knowledge needed for the urgent development of efficacious vaccines that will confer long-term protection.
Keywords: cellular immunity, EV-A71, HFMD, innate immunity, vaccines
Introduction
The immune system is an extremely important defence mechanism against viruses through the recognition of viral antigens. Upon entry of viruses into the human host, the antigen-presenting cell is the first to encounter a pathogen through the recognition of specific viral components by using different types of pattern recognition receptors such as Toll-like receptors (TLRs), nucleotide oligomerization domain-like receptors (NODs) and retinoic acid-inducible gene I-like receptors (RLRs).1 These receptors trigger the activation of antigen-presenting cells by increasing the expression of surface molecules, followed by the production of pro-inflammatory cytokines and chemokines that could further stimulate the downstream intracellular signalling cascades. The cytokines secreted by activated dendritic cells (DCs) are important in influencing the activation of macrophages, and could further stimulate the T helper and cytotoxic T cells as well as stimulating the humoral response to neutralize viruses.2
Enterovirus A71 (EV-A71) is relatively efficient in modulating innate immunity, in particular blocking PRR signalling cascade and type I IFN signalling has become a main EV-A71 viral escape strategy through different proteases (e.g. 2A, 3C, 2C, and 3D).3 EV-A71-derived 2A protein counteracted the antiviral type I IFN response by cleaving MDA5 in infected cells and was confirmed to suppress interferon regulatory factor (IRF)3 signalling through the cleavage of MAVS, resulting in IFN-α/β reduction in HeLa cells.4,5 EV-A71 3C protein disrupted the association of adaptor MAVS and MDA5 in 293T cells transfected with plasmids encoding MDA5-N-Myc, MAVS-Flag, and HA-3C.6 EV-A71 3C protein has been found to block IFN-β production through the TIR-domain-containing adapter-inducing interferon-β (TRIF) in response to endosomal TLR3 activation.7 EV-A71 2A and 3C proteins were shown to interfere with inflammasome assembly through the cleavage of NLRP3 in 293T cells and the 3C protein from EV-A71 suppressed IL-1β secretion by interacting with NLRP3. In contrast, the EV-A71 3D protein bound to NLRP3 by facilitating the assembly of inflammasome complexes, resulting in the secretion of IL-1β in 293T cells.8 EV-A71 2Apro blocked STAT1, STAT2, Jak1 and Tyk2 phosphorylation by reducing IFNAR1 expression in 293T cells transfected with 2Apro.9 In another study, EV-A71 2Apro was shown to attenuate IFN-γ-induced serine phosphorylation of STAT1 by blocking ERK signalling in mouse embryonic fibroblasts (MEFs) transfected with 2Apro along with IFN-γ treatment, while EV-A71 3Dpro attenuation of IFN-γ signalling was accompanied by a STAT1 decrease.10 Cumulative evidence described the correlation of innate immunity with the evasion of EV-A71 and a reduction of type I IFN production, which is an essential cytokine for controlling virus infection.11,12 EV-A71 was also shown to hamper the host innate defence by blocking type I IFN production through the 3C viral protein in mice.13 The 3C protein might directly or indirectly cleave a component involved in IFN signalling and further studies are required to clarify whether the IFN-inhibition effect exerted by the 3C protein was mediated by its proteolytic activity on one of the components of IFN signalling.
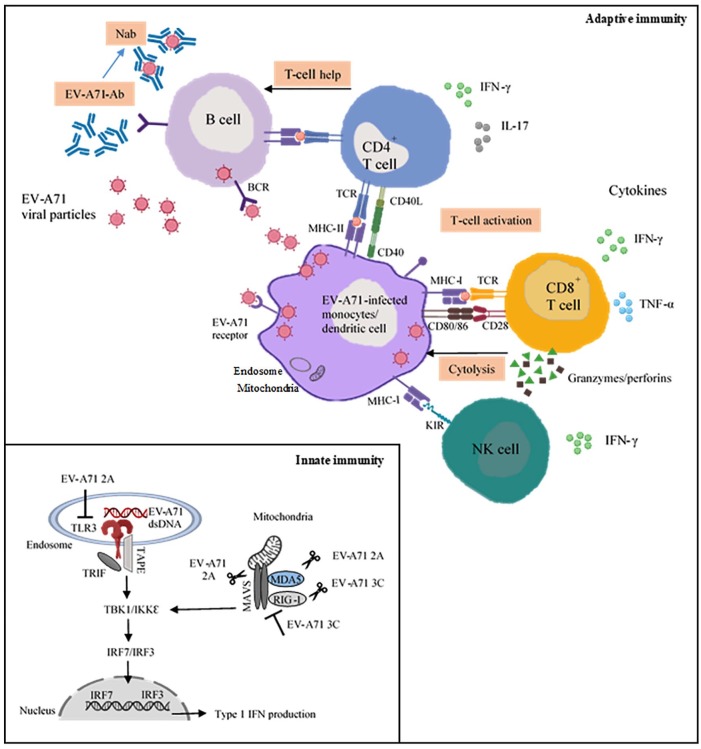
The mechanisms of how the innate immune system detected EV-A71 infection to elicit antiviral immunity have been identified and several receptors have been discovered to be crucial entry factors for EV-A71. For example, the human scavenger receptor B2 (SCARB2) and P-selectin glycoprotein ligand-1 (PSGL-1) have been shown to serve as viral receptors for EV-A71 infection.14,15 Studies using human SCARB2 transgenic mice have further confirmed the importance of human SCARB2 for EV-A71 infection in vivo.16,17 Other cell surface molecules such as sialylated glycans, nucleolin and heparan sulphate glycosaminoglycan have also been shown to play a role in enhancing EV-A71 infection in mammalian cells.18–20 TLR3 has recently been identified to detect EV-A71 infection and this has led to triggering type I IFN β production. Interestingly, the EV-A71 protease 2A was suggested to be involved in subverting TLR3-mediated antiviral defences by impairing the IFN-β secretion upon infection. As there is a marginal effect of EV-A71 2A on the cleavage of TLR3 in vitro, EV-A71 2A might mediate TLR3 downregulation through direct cleavage (Figure 1).21 Another recently discovered cellular entry factor, human tryptophanyl-tRNA synthetase, could be induced by IFN-γ in EV-A71 infection. IFN-γ was shown to induce the expression and membrane translocation of human tryptophanyl-tRNA synthetase which subsequently sensitized cells to EV-A71 infection.22 However, other factors might exist to influence host and EV-A71 interactions in EV-A71 pathogenesis.
Figure 1.
The role of innate and adaptive immune responses to EV-A71. The insert illustrates the involvement of TLR3 in the detection of dsRNA EV-A71 during infection, which further triggers type I IFN signalling pathways. EV-A71 proteases such as 2A and 3C are known to modulate innate immunity by counteracting the type I IFN-mediated antiviral responses.
The induction of B and T cell responses depends on their activation by antigen-presenting cells. DCs play a pivotal role in initiating and linking innate to the adaptive immune response through the recognition of vaccine antigens. The predominant role of B cells in producing antibodies is crucial for binding specifically to a virus. Cytotoxic CD8+ T cells limit the spread of the virus by killing infected cells, while CD4+ T cells are also essential for the induction of high-affinity antibodies and the generation of immune memory cells (Figure 1). Long-term protection requires the persistence of antibodies above protective thresholds and the generation of effective memory cell responses with subsequent virus infections. The roles of CD4+ and CD8+ T lymphocytes have been extensively studied in a variety of model systems and their mechanisms elucidated. However, the contribution of these cell types vary widely among different virus infections. In this review, we will discuss the roles of CD4+ and CD8+ T cells as well as memory T cells in EV-A71 infection.
B and T cell immunity against EV-A71
EV-A71 is one of the major causative agents of hand, foot and mouth disease (HFMD) in infants and children (<5 years old). Most cases of HFMD caused by EV-A71 result in mild symptoms with rashes on the body and ulcers in the mouth. However, virulent strains can infect the central nervous system and induce severe neurologic diseases, leading to acute flaccid paralysis, pulmonary oedema and death. It is speculated that, following the eradication of poliovirus (PV), EV-A71 could fill the niche vacated by PV due to its neurotropic characteristics. It has been suggested that cellular immunity, rather than humoral immunity, was associated with the clinical outcomes of EV-A71 infection as decreased cellular immunity and lower IFN-γ were correlated with the severity of EV-A71 infection, whereas no difference in the neutralizing Ab titres was observed between mild, severe and even fatal cases.23,24 Previous reports showed that the number of circulating immune cells, including T follicular helper cells, type 1 helper, cytotoxic T cells, T helper 17 and 22 cells, were found to increase with the severity of HFMD after EV-A71 infection.25,26 The proportion of human CD45+, CD3+, CD4+, CD8+ cells and activated CD8+ T cells were significantly upregulated in the humanized mice post EV-A71 infection.27 EV-A71-infected neonatal mice, including ICR and C57BL/6 mice, possessed higher numbers of infiltrating CD4+ and CD8+ T cells and CD19+ B cells in their brains after the third day of infection when compared to their uninfected counterparts.28 Activation of CD4+ and CD8+, followed by the secretion of IFN-γ, was shown to mediate immune protection against EV-A71 in mice and humans.28 Another study showed that EV-A71 infection increased the number of CD4+ and CD8+ T lymphocyte cells, which were accompanied by an increase in cytokine-related mRNA expressions of IFN-γ, IL-2 and IL-10 in BALB/c mice,29 suggesting that CD4+ and CD8+ T cells might contribute equally in mediating the protection against EV-A71 infection. However, Tan and colleagues reported that among the four structural antigens (Ags) (VP1, VP2, VP3 and VP4) of EV-A71, only the VP2 Ag carried a broad distribution of immunogenic peptides that dominated T cell responses against EV-A71, and they were mainly IFN-γ-secreting CD4+ T cells.30 The diverse distributions of T cell immunogenic regions in the VP2 protein were distributed from the N- to the C-terminus of the protein in the study subjects. More CD4+ T cell epitopes were present in VP2 than in VP1, VP3 and VP4. Conservancy analysis of the immunogenic peptides revealed that moderately variant peptides were present in the majority in coxsackievirus A16 (CV-A16), suggesting that weak cross-reactivity against CV-A16 might exist, whereas influences from poliovirus vaccination were limited due to the high variability of the peptides between EV-A71 and poliovirus vaccine strains. This study also suggested the presence of a minor population of EV-A71-responsive memory T cells circulating in the peripheral blood that might assist the control of recurrent EV-A71 infections.30
IL-2, IL-4, IL-10 and IFN-γ were the major cytokines that were shown to play crucial roles in immune responses to EV-A71.31,32 The secretion of various pro-inflammatory cytokines, including IFN-γ, IL-8 and IL-17, was enhanced in EV-A71-infected humanized mice, which might contribute to the exacerbation of disease pathogenesis.27 It was reported that Th1 cells/IFN-γ levels were significantly higher in mild and severe HFMD patients, while Th17 cells/IL-17 levels were the highest in severe HFMD patients, suggesting that the imbalance of Th1/Th2 and Th17/Treg were involved in the pathogenesis of EV-A71 infections.33
In addition to T lymphocytes, it was demonstrated that antibody responses were equally important in the protection of mice from EV-A71 infection as treatment with virus-specific antibodies before and after infection significantly reduced the disease severity, mortality and tissue viral loads of mice deficient in B cells.28 Neutralizing antibodies could be sufficient for immune protection, but poorer cellular immunity might lead to severe neurological complications and death. In clinical trials of EV-A71 inactivated vaccines, more than 95% efficacy was observed and the neutralizing antibodies induced a robust protective neutralization response.34,35 High levels of neutralizing antibody (NtAb) titres, together with IFN-γ secretion following vaccination, were able to confer 100% protection against hind limb paralysis from EV-A71 challenge, suggesting that humoral immune response might be able to protect mice from being killed by high viral load.36
Development of monovalent vaccines against EV-A71
Inactivated vaccine
By referring to existing technologies of inactivated polio and hepatitis A vaccines, the development of the inactivated EV-A71 vaccine has progressed rapidly in recent years due to the safety and stability of inactivated vaccines. But inactivated vaccines usually have weak immunogenicity compared to the live attenuated vaccines, because the viruses were killed and would not be able to replicate. Hence, inactivated vaccines might require periodic supplementary doses to increase the antigens to boost protection against viral diseases. To date, three inactivated EV-A71 vaccines have been evaluated by clinical trials and are now licensed in China.37 However, all of these inactivated alum-adjuvant EV-A71 vaccines were based on the C4 subgenotype and all have achieved vaccine efficacy of more than 95% against EV-A71-caused HFMD.38,39 The induction of cross-reactive broadly neutralizing antibodies is critical in protection against other EV-A71 subgenotypes. A neutralization titre of >1:16 is suggestive of cross-protection against other subgenotypes, and a titre of >1:42 was proposed to confer long-term protection.37,40 Chou and colleagues demonstrated that cross-neutralizing antibody responses could be elicited against several EV-A71 subgenotypes B1, B4, B5, C2, C4a and C4b in individuals vaccinated with an inactivated B4 subgenotype EV-A71.41 Moreover, antibodies of individuals receiving two C4 genotype EV-A71 vaccines in clinical trials [ClinicalTrials.gov identifiers: NCT01313715 and NCT01273246] elicited broad cross-neutralizing antibodies against EV-A71 subgenotypes of C2, C4, C5, B4 and B5.42 Clinical phase III studies of children (<5 years) vaccinated with the inactivated EV-A71 C4a [ClinicalTrials.gov identifier: NCT01636245] showed consistent protection against different subgenotypes C2, C4, C5, B4 and B5.43 However, the three inactivated EV-A71 vaccines could not provide cross-protection against other common enteroviruses associated with HFMD. The major challenge remained that the inactivated EV-A71 vaccine did not protect against CV-A16, CV-A6 and CV-A10, which are also major aetiological agents of recent HFMD outbreaks.
Live attenuated vaccine
Live attenuated vaccine (LAV) is created by reducing the virulence of a virus so that it becomes harmless but maintains its antigenicity. Among different platforms, the LAV might serve as a more effective vaccine as it is similar to the natural infection and elicits both humoral and cellular immune responses that can provide lifelong protection. No multiple boosters or adjuvant are required for LAV vaccination. However, LAV can be challenging due to the potential of the vaccine strain to revert to the more virulent strain. LAV cannot be administered to immunocompromised individuals. Proper storage of LAV might be difficult, especially in remote communities, as the activity of LAV depends on stability and viability. An LAV of EV-A71 was developed by Arita and colleagues but it retained neurovirulence in macaques.44 Although their study indicated that the immunization of macaques with the attenuated EV-A71 vaccine had the potential to produce significant titres of neutralizing antibodies against different EV-A71 subgenotypes such as A, B1, B4, C2 and C4, the neurovirulence observed in macaques indicated that safety issues concerning possible reversion of the live attenuated vaccine strain need to be overcome.44 In fact, there are a few successful LAVs that are used today, such as the attenuated yellow fever vaccine, influenza vaccine, chickenpox vaccine and the measles, mumps, and rubella vaccines. Recently, two LAVs against EV-A71 were constructed by Yee and colleagues;36 one is a multiply mutated strain (MMS) and the other, the pIY strain, carried two additional micro-RNAs (Let 7a and MicroRNA 124) in the EV-A71 genome, which carried a deletion in the 5′NTR and a G64R mutation. This study demonstrated that both MMS and pIY strains were genetically stable after 20 serial passes in vitro. Both MMS and pIY vaccine strains showed high immunogenicity with broad protective neutralizing antibodies against several EV-A71 subgenotypes (B3, B4, C1 and C4) and both vaccine strains could confer protection against challenge with a mouse-adapted EV-A71 (MAV) strain,36 thus suggesting that both MMS and pIY are promising LAV candidates against EV-A71 infection. Besides eliciting neutralizing titres ranging from 1:16 to 1:32 against several subgenotypes of EV-A71, significant IFN-γ levels indicating good cellular responses were observed. Both the MMS and pIY vaccine strains induced higher IFN-γ response in mice splenocytes, ranging from 640 SFU/106 T cells to 765 SFU/106 T cells when compared to the inactivated vaccine, which produced only 400 SFU/106 T cells.36
Recombinant vaccine
Considering that the EV-A71 VP1 is highly conserved and carries significant immunogenic B cell epitopes, previous studies have focused on developing recombinant VP1 vaccines. Compared to LAVs and inactivated vaccines, these are safer and more cost-effective. The recombinant VP1 vaccine as a good vaccine candidate was well established in previous studies.45–47 Immunization with recombinant VP1 proteins of EV-A71 expressed from host systems such as Escherichia coli, yeast or baculovirus could induce high levels of VP1-specific IgG antibodies that are able to confer protection against EV-A71 infection.48 Compared with the inactivated virus, recombinant VP1 elicited 32-fold lower titre of EV-A71-specific total IgG and protected against EV-A71 only at a low challenge dose of 230 LD50 per mouse. Survival of challenged mice was at 80% even though similar levels of neutralizing antibodies were elicited as the inactivated virus in serum.49 Immunization with the SP70 synthetic peptide that contains a neutralizing linear epitope from the EV-A71 VP1 capsid protein was able to elicit a neutralizing antibody titre comparable to that obtained with an inactivated vaccine. The passive immunization with the SP70 peptide was also capable of protecting 80% of newborn mice against lethal challenge and elicited cross-protective neutralizing antibodies (1:32) against EV-A71 subgenotypes B2, B5, C2, and C4.50 A novel recombinant tandem multi-linear neutralizing epitope vaccine of EV-A71 was designed and designated as mTLNE. The mTLNE vaccine comprised two well-identified EV-A71 linear neutralizing epitopes from the capsid protein VP1 and one from VP2. The two epitopes VP1-SP55, VP1-SP70 and the VP2-SP28 were sequentially linked by a Gly–Ser linker [(G4S)3] and expressed in E. coli with the thioredoxin (Trx) and His tag at either terminus. Immunization of mice with the recombinant mTLNE protein elicited higher titres of IgG antibodies against the three epitopes, namely VP1-SP55, VP1-SP70 and VP2-SP28, at neutralizing titres of 1:246, 1:1488 and 1:1710, respectively.51 In terms of EV-A71-specific cellular immune response, recombinant mTLNE induced low IFN-γ production but significantly increased the levels of IL-4 and IL-6 in the splenocytes of mTLNE immunized mice. The neutralizing antibodies elicited by the recombinant mTLNE were able to confer 100% protection against the lethal EV-A71 challenge in mice by the passive transfer of the anti-mTLNE sera.51 However, studies with the recombinant vaccines were conducted in mice with the administration of complete and incomplete Freund’s adjuvants.49,51 There are no clinical trials in humans involving the recombinant VP1 protein-based vaccine platform and there is a need to further assess the use of alum as an adjuvant.
Future enterovirus vaccines: bivalent and multivalent vaccines
EV-A71 and CV-A16 bivalent vaccines
As EV-A71 and CV-A16 are the two major pathogens commonly isolated from HFMD patients presenting with clinical manifestations, efforts have been made to develop bivalent vaccines against EV-A71 and CV-A16. A previous study has shown that inactivated EV-A71/CV-A16 bivalent vaccine-induced antibodies capable of neutralizing both viruses and immunization with the bivalent vaccine were able to protect mice against either EV-A71 or CV-A16 lethal infections.52 In contrast, the monovalent vaccine could only protect against one virus. The bivalent inactivated vaccines were shown to elicit high levels of neutralizing antibodies which confer protection upon mice against lethal challenges with both viruses.53
Similar to the inactivated bivalent vaccines, bivalent EV-A71/CV-A16 virus-like particle (VLP) vaccines were able to protect against both viruses as sera from mice immunized with aluminium or CpG-adjuvanted VLP bivalent vaccines were able to neutralize EV-A71 and CV-A16 in vitro.54 The EV-A71/CV-A16-VLP vaccine was able to induce high levels of antibodies and protected mice from lethal challenges from EV-A71 and CV-A16.54,55 Neutralizing antibodies also showed cross-protection against other subgenotypes of EV-A71 and CV-A16.53 Another VLP that has been recently developed against CV-A10 showed that VLPs could efficiently induce antibodies capable of neutralizing CV-A10 infection in vitro.56 However, a monovalent or bivalent vaccine is limited in providing protection only against a single or at most two pathogens capable of causing HFMD. One of the difficulties in developing a broadly protective HFMD vaccine is that the antibodies produced by enteroviruses are efficient when it comes to cross-neutralizing subgenotypes within a single serotype, but generally do not cross-protect across different enterovirus serotypes.
EV-A71/CV-A16/CV-A6 trivalent vaccine
Besides CV-A16, CV-A6 has been increasingly isolated as a major HFMD pathogen in some major outbreaks.57 Recently, a trivalent inactivated EV-A71/CV-A16/CV-A6 vaccine was shown to broadly protect mice against EV-A71, CV-A16 and CV-A6 challenges. Although lower neutralizing antibody levels were detected with the trivalent vaccine when compared to the monovalent vaccine, the levels of neutralizing antibodies were sufficient to provide complete protection against lethal challenge.58
EV-A71/CV-A16/CV-A6/CV-A10 multivalent vaccine
A combination of formalin-inactivated EV-A71, CV-A6, CV-A10 and CV-A16 multivalent vaccine was observed to elicit serotype-specific neutralizing antibody responses in mice and rabbits. Anti-EV-A71 neutralizing antibodies had the highest neutralization titre of 1/708 when compared with those obtained against the other three viruses, which were 1/100, 1/16 and 1/22 for CV-A6, CV-A10 and CV-A16, respectively.59 Recently, Zhang and colleagues60 developed the first VLP-based tetravalent vaccine targeting EV-A71, CV-A16, CV-A10 and CV-A6. Passive transfer of tetravalent vaccine-immunized sera could confer complete protection against lethal infection with any one of the four viruses by inducing broadly neutralizing antibodies against EV-A71, CV-A16, CV-A10 and CV-A6. In parallel with the previous finding, the neutralizing titres reported in this study for monovalent CV-A10 VLP [geometric mean titre (GMT) = 406] and CV-A6 VLP (GMT = 256) were generally lower than the EV-A71 VLP (GMT = 2896) and CV-A16 VLP (GMT = 5793).60 These results indicated a feasible approach for developing a tetravalent HFMD vaccine that could elicit antigen-specific and durable antibody responses. However, the significant difference in neutralization titres against four different EVs indicated that there is antigen interference in both the IV and VLP formulations.59 This required adjustment of the optimal ratios of each specific antigen in the tetravalent vaccine. The SP70 linear epitope from EV-A71 was replaced by the SP70 epitope of CV-A16 in a chimeric VLP to produce a bivalent HFMD vaccine.61 The structural vaccinology approach revealed the possibility of inserting the SP70 epitope of the CV-A16 into the EV-A71-based VLP. For example, Anasir and Poh suggested that in addition to the substitution of the SP70 epitope of EV-A71 with the corresponding CV-A16 SP70 epitope in the EV-A71 VLP, the SP70 epitope from CV-A6 could also be inserted into the BC loop insertion site and another SP70 epitope from CV-A10 could be inserted into the EF loop of VP0 or VP2.62 Therefore, further research is needed to determine the possibility of inserting more epitopes into the same VLP to generate the tetravalent HFMD vaccine. Using a single multivalent VLP might overcome the antigen interference phenomenon observed for the multivalent VLP when each of the VLP components was prepared individually and mixed in the vaccine formulation.
Conclusion
Vaccine is the most effective tool to prevent HFMD, which is prevalent in Asia. Among the 15 licensed vaccines approved by the US Food and Drug Administration against viral infections to date, most of them are live attenuated compared to inactivated vaccines.63 LAVs are good in eliciting lifelong immunity provided by memory T cells, but have the risk of reversion to the wild type. The introduction of multiple mutations in the genome will reduce the likelihood of reversion in the designed vaccine strain. Bivalent inactivated or VLP-based vaccines have been evaluated in the murine model and showed their ability to prevent death in lethal challenges. There is a likelihood that regulatory authorities will accept EV-A71 VLP vaccine provided that it meets all the safety criteria. Tetravalent inactivated or VLP-based vaccines have also been constructed and evaluated in the murine model. However, large-scale production of both multivalent vaccines is laborious and requires complex bioprocesses for purification. Each of the four enteroviruses (EV-A71, CV-A16, CV-A10 and CV-A6) will need to be grown in individual bioreactors and inactivated by formaldehyde before further downstream processing by chromatographic separations. This would incur substantial costs for both vaccine platforms. Moreover, the multivalent vaccine against four different enteroviruses will need to elicit balanced protective immunity as antigenic interference needs to be overcome. The need to adjust the optimum ratio of four VLPs in the tetravalent formulation to overcome antigenic interference could perhaps be achieved by constructing a recombinant VLP carrying the appropriate B cell epitopes. Selection of optimal CD8+ T cell epitopes to be incorporated in the recombinant VLPs could extend the long-term T cell immune responses. The synthetic peptide vaccine has low immunogenicity, which has to be overcome by either strong chemical adjuvants or the applications of suitable self-adjuvating nanoparticles to increase the immunogenicity.
Footnotes
Author note: Part of this work will be presented at Vaccines R&D 2019 (Vaccines Research & Development), 18–20 November 2019 at Boston, MA, USA.
Funding: This study was funded by the Sunway University Research Centre Grant (2019) to the Centre for Virus and Vaccine Research (CVVR).
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Chit Laa Poh  https://orcid.org/0000-0001-8475-6291
https://orcid.org/0000-0001-8475-6291
Contributor Information
Hui Xuan Lim, Centre for Virus and Vaccine Research, School of Science and Technology, Sunway University, Bandar Sunway, Kuala Lumpur, Selangor, Malaysia.
Chit Laa Poh, Centre for Virus and Vaccine Research, School of Science and Technology, Sunway University, Bandar Sunway, Kuala Lumpur, Selangor 47500, Malaysia.
References
- 1. Mogensen TH. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin Microbiol Rev 2009; 22: 240–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Newton AH, Cardani A, Braciale TJ. The host immune response in respiratory virus infection: balancing virus clearance and immunopathology. Semin Immunopathol 2016; 38: 471–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jin Y, Zhang R, Wu W, et al. Innate immunity evasion by enteroviruses linked to epidemic hand–foot–mouth disease. Front Microbiol 2018; 9: 2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Feng Q, Langereis MA, Lork M, et al. Enterovirus 2Apro targets MDA5 and MAVS in infected cells. J Virol 2014; 88: 3369–3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang B, Xi X, Lei X, et al. Enterovirus 71 protease 2Apro targets MAVS to inhibit anti-viral type I interferon responses. PLoS Pathog 2013; 9: e1003231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rui Y, Su J, Wang H, et al. Disruption of MDA5-mediated innate immune responses by the 3C proteins of coxsackievirus A16, coxsackievirus A6, and enterovirus D68. J Virol 2017; 91: pii: e00546-17 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lei X, Sun Z, Liu X, et al. Cleavage of the adaptor protein TRIF by enterovirus 71 3C inhibits antiviral responses mediated by Toll-like receptor 3. J Virol 2011; 85: 8811–8818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang W, Xiao F, Wan P, et al. EV71 3D protein binds with NLRP3 and enhances the assembly of inflammasome complex. PLoS Pathog 2017; 13: e1006123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lu J, Yi L, Zhao J, et al. Enterovirus 71 disrupts interferon signaling by reducing the level of interferon receptor 1. J Virol 2012; 86: 3767–3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang C, Sun M, Yuan X, et al. Enterovirus 71 suppresses interferon responses by blocking Janus kinase (JAK)/signal transducer and activator of transcription (STAT) signaling through inducing karyopherin-alpha1 degradation. J Biol Chem 2017; 292: 10262–10274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu ML, Lee YP, Wang YF, et al. Type I interferons protect mice against enterovirus 71 infection. J Gen Virol 2005; 86(Pt 12): 3263–3269. [DOI] [PubMed] [Google Scholar]
- 12. Sadler AJ, Williams BRG. Interferon-inducible antiviral effectors. Nat Rev Immunol 2008; 8: 559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lee YP, Wang YF, Wang JR, et al. Enterovirus 71 blocks selectively type I interferon production through the 3C viral protein in mice. J Med Virol 2012; 84: 1779–1789. [DOI] [PubMed] [Google Scholar]
- 14. Nishimura Y, Shimojima M, Tano Y, et al. Human P-selectin glycoprotein ligand-1 is a functional receptor for enterovirus 71. Nat Med 2009; 15: 794–797. [DOI] [PubMed] [Google Scholar]
- 15. Yamayoshi S, Yamashita Y, Li J, et al. Scavenger receptor B2 is a cellular receptor for enterovirus 71. Nat Med 2009; 15: 798–801. [DOI] [PubMed] [Google Scholar]
- 16. Fujii K, Nagata N, Sato Y, et al. Transgenic mouse model for the study of enterovirus 71 neuropathogenesis. Proc Natl Acad Sci U S A 2013; 110: 14753–14758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lin YW, Yu SL, Shao HY, et al. Human SCARB2 transgenic mice as an infectious animal model for enterovirus 71. PLoS One 2013; 8: e57591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yang B, Chuang H, Yang KD. Sialylated glycans as receptor and inhibitor of enterovirus 71 infection to DLD-1 intestinal cells. Virol J 2009; 6: 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tan CW, Poh CL, Sam IC, et al. Enterovirus 71 uses cell surface heparan sulfate glycosaminoglycan as an attachment receptor. J Virol 2013; 87: 611–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Su PY, Wang YF, Huang SW, et al. Cell surface nucleolin facilitates enterovirus 71 binding and infection. J Virol 2015; 89: 4527–4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen KR, Yu CK, Kung SH, et al. Toll-like receptor 3 is involved in detection of enterovirus A71 infection and targeted by viral 2A protease. Viruses 2018; 10: pii: E689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yeung ML, Jia L, Yip CCY, et al. Human tryptophanyl-tRNA synthetase is an IFN-gamma-inducible entry factor for Enterovirus. J Clin Invest 2018; 128: 5163–5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chang LY, Hsiung CA, Lu CY, et al. Status of cellular rather than humoral immunity is correlated with clinical outcome of enterovirus 71. Pediatr Res 2006; 60: 466–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yang C, Deng C, Wan J, et al. Neutralizing antibody response in the patients with hand, foot and mouth disease to enterovirus 71 and its clinical implications. Virol J 2011; 8: 306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wu J, Cui D, Yang X, et al. Increased frequency of circulating follicular helper T cells in children with hand, foot, and mouth disease caused by enterovirus 71 infection. J Immunol Res 2014; 2014: 651872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cui D, Zhong F, Lin J, et al. Changes of circulating Th22 cells in children with hand, foot, and mouth disease caused by enterovirus 71 infection. Oncotarget 2017; 8: 29370–29382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ke Y, Liu WN, Her Z, et al. Enterovirus A71 infection activates human immune responses and induces pathological changes in humanized mice. J Virol 2019; 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lin YW, Chang KC, Kao CM, et al. Lymphocyte and antibody responses reduce enterovirus 71 lethality in mice by decreasing tissue viral loads. J Virol 2009; 83: 6477–6483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Issaro N, Wu F, Weng L, et al. Induction of immune responses by a novel recombinant fusion protein of enterovirus A71 in BALB/c mice. Mol Immunol 2019; 105: 1–8. [DOI] [PubMed] [Google Scholar]
- 30. Tan S, Tan X, Sun X, et al. VP2 dominated CD4+ T cell responses against enterovirus 71 and cross-reactivity against coxsackievirus A16 and polioviruses in a healthy population. J Immunol 2013; 191: 1637–1647. [DOI] [PubMed] [Google Scholar]
- 31. Chung YC, Ho MS, Wu JC, et al. Immunization with virus-like particles of enterovirus 71 elicits potent immune responses and protects mice against lethal challenge. Vaccine 2008; 26: 1855–1862. [DOI] [PubMed] [Google Scholar]
- 32. Zhou S-L, Ying X-L, Han X, et al. Characterization of the enterovirus 71 VP1 protein as a vaccine candidate. J Med Virol 2015; 87: 256–262. [DOI] [PubMed] [Google Scholar]
- 33. Li S, Cai C, Feng J, et al. Peripheral T lymphocyte subset imbalances in children with enterovirus 71-induced hand, foot and mouth disease. Virus Res 2014; 180: 84–91. [DOI] [PubMed] [Google Scholar]
- 34. Zhu FC, Meng FY, Li JX, et al. Efficacy, safety, and immunology of an inactivated alum-adjuvant enterovirus 71 vaccine in children in China: a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2013; 381: 2024–2032. [DOI] [PubMed] [Google Scholar]
- 35. Li R, Liu L, Mo Z, et al. An inactivated enterovirus 71 vaccine in healthy children. N Engl J Med 2014; 370: 829–837. [DOI] [PubMed] [Google Scholar]
- 36. Yee PTI, Tan SH, Ong KC, et al. Development of live attenuated Enterovirus 71 vaccine strains that confer protection against lethal challenge in mice. Sci Rep 2019; 9: 4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhu F, Xu W, Xia J, et al. Efficacy, safety, and immunogenicity of an enterovirus 71 vaccine in China. N Engl J Med 2014; 370: 818–828. [DOI] [PubMed] [Google Scholar]
- 38. Wu JT, Jit M, Zheng Y, et al. Routine pediatric enterovirus 71 vaccination in China: a cost-effectiveness analysis. PLoS Med 2016; 13: e1001975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yi EJ, Shin YJ, Kim JH, et al. Enterovirus 71 infection and vaccines. Clin Exp Vaccine Res 2017; 6: 4–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhu W, Jin P, Li J-X, et al. Correlates of protection for inactivated enterovirus 71 vaccine: the analysis of immunological surrogate endpoints. Expert Rev Vaccines 2017; 16: 945–949. [DOI] [PubMed] [Google Scholar]
- 41. Chou AH, Liu CC, Chang JY, et al. Formalin-inactivated EV71 vaccine candidate induced cross-neutralizing antibody against subgenotypes B1, B4, B5 and C4A in adult volunteers. PLoS One 2013; 8: e79783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mao Q, Cheng T, Zhu F, et al. The cross-neutralizing activity of enterovirus 71 subgenotype C4 vaccines in healthy Chinese infants and children. PLoS One 2013; 8: e79599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhang H, An D, Liu W, et al. Analysis of cross-reactive neutralizing antibodies in human HFMD serum with an EV71 pseudovirus-based assay. PLoS One 2014; 9: e100545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Arita M, Nagata N, Iwata N, et al. An attenuated strain of enterovirus 71 belonging to genotype A showed a broad spectrum of antigenicity with attenuated neurovirulence in Cynomolgus monkeys. J Virol 2007; 81: 9386–9395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhang F, Hao C, Zhang S, et al. Oral immunization with recombinant enterovirus 71 VP1 formulated with chitosan protects mice against lethal challenge. Virol J 2014; 11: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Xu J, Zhang C. Human IgG Fc promotes expression, secretion and immunogenicity of enterovirus 71 VP1 protein. J Biomed Res 2016; 30: 209–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kim YI, Song JH, Kwon BE, et al. Pros and cons of VP1-specific maternal IgG for the protection of Enterovirus 71 infection. Vaccine 2015; 33: 6604–6610. [DOI] [PubMed] [Google Scholar]
- 48. Kiener TK, Premanand B, Kwang J. Immune responses to baculovirus-displayed enterovirus 71 VP1 antigen. Expert Rev Vaccines 2013; 12: 357–364. [DOI] [PubMed] [Google Scholar]
- 49. Wu CN, Lin YC, Fann C, et al. Protection against lethal enterovirus 71 infection in newborn mice by passive immunization with subunit VP1 vaccines and inactivated virus. Vaccine 2001; 20(5–6): 895–904. [DOI] [PubMed] [Google Scholar]
- 50. Foo DG, Alonso S, Phoon MC, et al. Identification of neutralizing linear epitopes from the VP1 capsid protein of Enterovirus 71 using synthetic peptides. Virus Res 2007; 125: 61–68. [DOI] [PubMed] [Google Scholar]
- 51. Li Y-X, Zhao H, Cao R-Y, et al. Recombinant tandem multi-linear neutralizing epitopes of human enterovirus 71 elicited protective immunity in mice. Virol J 2014; 11: 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cai Y, Ku Z, Liu Q, et al. A combination vaccine comprising of inactivated enterovirus 71 and coxsackievirus A16 elicits balanced protective immunity against both viruses. Vaccine 2014; 32: 2406–2412. [DOI] [PubMed] [Google Scholar]
- 53. Sun S, Jiang L, Liang Z, et al. Evaluation of monovalent and bivalent vaccines against lethal Enterovirus 71 and Coxsackievirus A16 infection in newborn mice. Hum Vaccin Immunother 2014; 10: 2885–2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gong M, Zhu H, Zhou J, et al. Cryo-electron microscopy study of insect cell-expressed enterovirus 71 and coxsackievirus A16 virus-like particles provides a structural basis for vaccine development. J Virol 2014; 88: 6444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ku Z, Liu Q, Ye X, et al. A virus-like particle based bivalent vaccine confers dual protection against enterovirus 71 and coxsackievirus A16 infections in mice. Vaccine 2014; 32: 4296–4303. [DOI] [PubMed] [Google Scholar]
- 56. Liu Q, Tong X, Huang Z. Towards broadly protective polyvalent vaccines against hand, foot and mouth disease. Microbes Infect 2015; 17: 155–162. [DOI] [PubMed] [Google Scholar]
- 57. Li J, Zhu R, Huo D, et al. An outbreak of coxsackievirus A6-associated hand, foot, and mouth disease in a kindergarten in Beijing in 2015. BMC Pediatr 2018; 18: 277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Caine EA, Fuchs J, Das SC, et al. Efficacy of a trivalent hand, foot, and mouth disease vaccine against enterovirus 71 and coxsackieviruses A16 and A6 in mice. Viruses 2015; 7: 5919–5932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Liu CC, Guo MS, Wu SR, et al. Immunological and biochemical characterizations of coxsackievirus A6 and A10 viral particles. Antiviral Res 2016; 129: 58–66. [DOI] [PubMed] [Google Scholar]
- 60. Zhang W, Dai W, Zhang C, et al. A virus-like particle-based tetravalent vaccine for hand, foot, and mouth disease elicits broad and balanced protective immunity. Emerg Microbes Infect 2018; 7: 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zhao H, Li HY, Han JF, et al. Novel recombinant chimeric virus-like particle is immunogenic and protective against both enterovirus 71 and coxsackievirus A16 in mice. Sci Rep 2015; 5: 7878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Anasir MI, Poh CL. Advances in antigenic peptide-based vaccine and neutralizing antibodies against viruses causing hand, foot, and mouth disease. Int J Mol Sci 2019; 20: pii: E1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Graham BS, Sullivan NJ. Emerging viral diseases from a vaccinology perspective: preparing for the next pandemic. Nat Immunol 2018; 19: 20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]