Abstract
The preponderance of temporomandibular joint (TMJ) degenerative disorders in women and their early onset during reproductive years have implicated female sex hormones, particularly 17-β estradiol (E2), in the pathogenesis of these disorders. Nevertheless, the mechanisms by which E2 contributes to TMJ degenerative disorders and the reasons for its targeted effects on the TMJ but not other joints remain poorly understood. Here, we developed an ovariectomized mouse model in which systemic E2 concentrations mimicked those in cycling women, and we determined the effect of E2 on the targeted turnover of TMJ fibrocartilage matrix via E2-induced matrix metalloproteinases MMP9 and MMP13. Infusion of E2 and progesterone (P4; hormone control) over 7 d resulted in 5- and 8-fold greater serum E2 and P4 levels relative to controls, respectively, achieving systemic hormone levels similar to high baseline levels in cycling women. Administration of E2 but not P4 caused a significant loss of TMJ collagen and glycosaminoglycans, which was accompanied by amplification of ERα and specific increases in MMP9 and MMP13 expression. This dose of E2 had no effect on knee meniscus fibrocartilage, demonstrating the specificity of the degradative effect of E2. Dose-response experiments showed a greater sensitivity and a higher peak induction of MMP9 and MMP13 in TMJ fibrocartilaginous cells than knee meniscus cells to E2, providing an explanation for the differential responses of these tissues to E2. Using MMP9- and MMP13-null mice, we observed no discernible effects of each proteinase individually to E2-mediated TMJ matrix loss but noted a significant compensatory reciprocal induction of each MMP by E2 in the absence of the other. The redundancy in E2’s induction of MMP9 and MMP13 suggests that the proteinases may together contribute to E2-mediated TMJ fibrocartilage loss. These results advance our understanding of E2-mediated upregulation of MMP9 and MMP13 on fibrocartilage matrix turnover targeted to the TMJ.
Keywords: temporomandibular joint disorder, extracellular matrix, matrix metalloproteinases, estrogen receptors, collagen, glycosaminoglycans
Introduction
Degenerative disease (DD) or osteoarthritis (OA) of the temporomandibular joint (TMJ) is a common clinicopathologic finding within a highly prevalent spectrum of ailments known as temporomandibular disorders. These disorders are accompanied by pain and limitation in jaw movements that adversely affect activities such as eating and speech (Scrivani et al. 2008; Schiffman et al. 2014). Since the etiology and pathogenesis of TMJ DD/OA are unknown, no effective etiology-specific therapies are available. Nevertheless, their preponderance in women and early onset during reproductive years (Scrivani et al. 2008; Wang et al. 2008; Maixner et al. 2011; Murphy et al. 2013), as opposed to similar conditions in appendicular joints that primarily afflict postmenopausal women, have implicated the role of female sex hormones, particularly 17-β estradiol (E2), in targeted TMJ DD/OA in this cohort of subjects (Landi et al. 2005; Maixner et al. 2011). Several findings provide support for this postulate. First, elevated serum E2 levels have been found in young female subjects with TMJ symptoms as compared with age-matched asymptomatic controls (Landi et al. 2004, 2005). Similarly, men with TMJ DD/OA have significantly higher systemic E2 levels than asymptomatic controls (Landi et al. 2005). Second, estrogen receptors (ERs) have been localized to the human TMJ (Abubaker et al. 1993) and are more highly expressed in female than male TMJ fibrocartilages (Milam et al. 1987; Wang et al. 2009). Third, ERα polymorphisms that enhance ERα levels are associated with the prevalence and severity of TMJ DD/OA (Lee et al. 2006; Kang et al. 2007; Ribeiro-Dasilva et al. 2009; Stemig et al. 2015), indicating that signaling initiated by E2 may contribute to the pathogenesis of TMJ DD/OA. Finally, systemic administration of E2 aggravates monosodium iodoacetate-induced rat TMJ DD/OA (Wang et al. 2013). Despite these findings, a conclusive association between E2 and TMJ degeneration has not been established, nor have the molecular mechanisms by which E2 mediates the onset and progression of TMJ DD/OA been elucidated. Also, the reasons for the specificity of these effects of E2 on the TMJ as opposed to that on appendicular joints is not known.
Cartilage or fibrocartilage degradation is the most common and early hallmark of DD/OA, including those of the TMJ. The degradation of the fibrocartilage extracellular matrix (ECM), which is composed primarily of type I collagen and glycosaminoglycans (GAGs), is caused by an array of proteolytic enzymes, particularly matrix metalloproteinases (MMPs). Among the 25 members of the MMP family, MMP9 and MMP13 are crucial proteinases implicated in cartilaginous ECM degradation and are upregulated in TMJ degenerative disorders (Leonardi et al. 2008; Wadhwa and Kapila 2008; Troeberg and Nagase 2012; Loreto et al. 2013). While the contribution of specific MMPs to inflammation-mediated cartilage degeneration is well known (Troeberg and Nagase 2012; Yang et al. 2013) and we have demonstrated that E2 induces MMP9 and MMP13 in TMJ fibrocartilage cells in vitro (Ahmad et al. 2018), the effects of E2-induced MMPs on TMJ matrix loss in vivo have not been investigated. The aims of our study were 1) to develop a mouse model for sustained delivery of E2 resulting in desired systemic E2 concentrations and 2) to use this model to decipher the effect of E2 on the specific turnover of TMJ fibrocartilage ECM as well as the role of E2-induced MMP9 and MMP13 in the targeted degradation of the TMJ. Our findings demonstrate that E2 but not progesterone (P4) at systemic concentrations similar to those found in cycling women contributes to a significant loss of TMJ but not knee meniscus (KM) fibrocartilage collagen and GAG concomitant to the upregulation of MMP9 and MMP13 in TMJ fibrocartilage. These findings suggest the potential vulnerability of compromised TMJ fibrocartilaginous tissue to further degenerative changes during specific phases of the menstrual cycle.
Materials and Methods
Animal Procedures and Tissue GAG and Collagen Quantification
All procedures on 12-wk-old female wild-type (WT), MMP9-/-, and MMP13-/- C57BL/6J mice (Jackson Laboratory) were conducted following approval from the Institutional Animal Care and Use Committee. Three days after bilateral ovariectomy, blood was collected, and the animals were allowed to equilibrate for another 4 d when Alzet osmotic pumps (model 1007D; 0.5 µL/h) containing 100 µL of solution to deliver 3 µg/kg/d of E2 (Sigma-Aldrich) or 0.15 mg/kg/d of P4 or phosphate buffered saline (PBS) vehicle were subcutaneously implanted in the upper backs. After 7 d, blood was collected; the mice were euthanized; and bilateral TMJ discs and KM fibrocartilages were retrieved. Serum E2 and P4 concentration was measured with enzyme-linked immunosorbent assays (ELISAs) (Cayman Chemical). After the tissue was lyophilized in a SpeedVac (Labconco Corporation), tissue dry weight was determined (Hashem et al. 2006); tissues were digested with 3 mg/mL of pepsin (Sigma-Aldrich) in 0.5M acetic acid at 45 °C; and total GAG and collagen were assayed by dimethyl methylene blue and hydroxyproline assays, respectively.
RNA Extraction, Gene Array, and Real-time qRT-PCR Analysis
TMJ discs were homogenized, and total RNA was extracted with a Qiagen RNAeasy Mini Kit and either subjected to ECM RT2 Profiler PCR Array (Extracellular Matrix and Adhesion Molecules, 330231) as described previously (Wrighton et al. 2014) or reverse transcribed with an Ominiscript RT Kit (Qiagen) with random hexamer primers for real-time qRT-PCR (quantitative reverse transcription polymerase chain reaction; PRISM 7500, Applied Biosystems). The array results were analyzed with SABiosciences data analysis software. The relative content of mRNA in each sample was quantified as described previously (Ahmad et al. 2012) with GAPDH (glyceraldehyde-3-phosphate dehydrogenase) used as an internal control.
Fibrochondrocyte Isolation and Culture
KM and TMJ discs were isolated from 10-wk-old female C57BL/6J mice, and fibrochondrocytes were isolated and cultured in α-MEM supplemented with 10% fetal bovine serum (Ahmad et al. 2012). Approximately 70% to 80% of confluent passage 3 to 4 fibrochondrocytes were washed and maintained in phenol- and serum-free medium (α-MEM with 0.2% lactalbumin hydrolysate) for 4 h, washed, and incubated in fresh medium with 0 to 20 ng/mL of E2. Cell-conditioned media were collected after 48 h and concentrated with Amicon Ultra-4 centrifugal filters (Millipore) for Western blot analyses.
Protein Assays and Western Blots
Protein concentrations in the media or TMJ disc digests was determined by bicinchoninic acid (BCA) assay. Western blot was performed with anti-MMP13 antibody (ab51072) and anti-MMP9 antibody (228402) as described previously (Ahmad et al. 2018). The blots were reprobed with anti-actin for the tissue digests and anti-MMP2 (ab37150) antibody for the media, which were used as internal controls.
Statistical Analysis
Descriptive data were extracted as mean ± SD collagen or GAG content (µg/mg dry weight) or relative fold change for MMPs and ERs and analyzed by 1-way analysis of variance, followed by Sidak’s multiple-comparisons test with GraphPad Prism 7.04 software. The sample size for in vivo studies was 5 to 6 animals in each group. In vitro experiments were repeated 3 times in triplicate wells from cells pooled from 3 mice and data were analyzed as described earlier. P < 0.05 was considered statistically significant.
Results
Establishment of an OVX Mouse Model for Systemic E2 and P4 Administration
To determine the desired systemic concentrations of E2 and P4 (hormone control) consistent with peak systemic midfollicular and midluteal levels in cycling women, a series of pilot studies were performed on ovariectomized (OVX) mice involving administration of a range of doses of hormones via subcutaneously implanted osmotic pumps. We found that administration of 3 µg/kg/d of E2 resulted in systemic E2 concentrations of 94.3 ± 9.2 versus 19.9 ± 3.0 pg/mL in PBS OVX control mice (P < 0.01). Similarly, P4 administration at 0.15 mg/kg/d resulted in systemic P4 concentrations of 950.9 ± 105.1 versus 15.2 ± 5.3 pg/mL in PBS-administered OVX mice (P < 0.01).
Administration of E2 but Not P4 Contributes to Targeted Loss of TMJ Fibrocartilage ECM
Administration of E2 resulted in a 40% to 50% reduction (P < 0.05) in TMJ fibrocartilage collagen and GAG (Fig. 1A, B) content but had no discernible effects on KM fibrocartilaginous matrices (Fig. 1C, D). P4 administration did not contribute to any significant changes in TMJ and KM fibrocartilage collagen and GAG.
Figure 1.
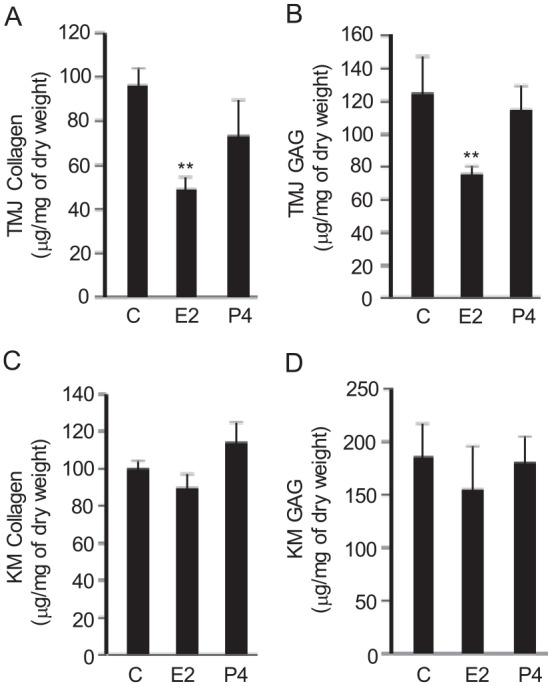
Administration of E2 causes targeted loss of collagen and glycosaminoglycan (GAG) in mouse temporomandibular joint (TMJ) but not knee meniscus fibrocartilage. Seven days following ovariectomy, osmotic pumps containing phosphate buffered saline (control; C), 17-β estradiol (E2; 3 µg/kg/d), or progesterone (P4; 0.15 mg/kg/d as hormone control) were implanted subcutaneously in 12-wk-old female mice. One week after the pumps were implanted, the mice were euthanized and the tissues retrieved and assayed for collagen and GAG. Collagen (A) and GAG (B) content in TMJ disc was significantly reduced by E2 but not by P4 infusion. E2 did not modulate any changes in collagen (C) and GAG (D) content in knee meniscus (KM) fibrocartilage. Results are shown as mean ± SD from 5 mice. **P < 0.01 vs. control or P4-treated mice.
E2 Enhances ERα, MMP9, and MMP13 Expression in TMJ Fibrocartilage
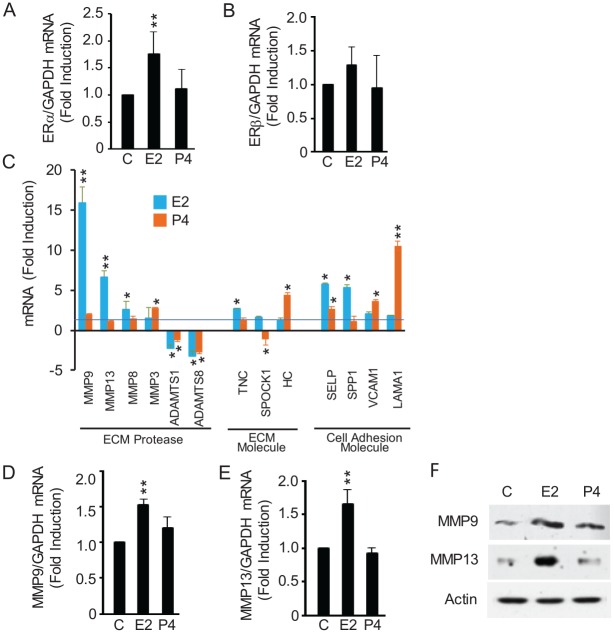
Because E2 exerts its functions primarily through ERα and ERβ (Levin and Hammes 2016), we explored the effects of systemic E2 on these ERs in TMJ fibrocartilage. Elevated levels of systemic E2 but not P4 significantly increased ERα but not ERβ transcription in TMJ fibrocartilage (Fig. 2A, B).
Figure 2.
E2 enhances the expression of ERα and specific MMPs in TMJ fibrocartilage in vivo. Mice were treated as in Figure 1. Total RNA and protein were extracted from TMJ fibrocartilage and assayed by real-time qRT-PCR, gene array, and Western blots. (A, B) E2 but not P4 enhanced ERα mRNA expression, while neither hormone modulated ERβ mRNA expression. (C) RT2 Profiler gene array data of the 13 assayed mRNAs (out of 84) that are significantly modulated by E2 and/or P4 demonstrate the specificity and magnitude of E2’s induction of MMP9 and MMP13. (D–F) Real-time qRT-PCR and representative Western blot confirming E2’s induction of MMP9 and MMP13 in vivo. Results are presented as mean ± SD from 5 mice. *P < 0.05 and **P < 0.01 vs. control. C, control (phosphate buffered saline); E2, 17-β estradiol; ER, estrogen receptor; MMP, matrix metalloproteinase; P4, progesterone; qRT-PCR, quantitative reverse transcription polymerase chain reaction; TMJ, temporomandibular joint. Abbreviations of genes in panel C are as follows: ADAMTS1 and ADAMTS8: a disintegrin and metalloproteinase with thrombospondin motifs 1 and 8; HC: hemolytic complement; Lama1: laminin alpha 1; Selp: selectin platelet; SPOCK1: SPARC (osteonectin), CWCV and Kazal like domains proteoglycan 1; Spp1: secreted phosphoprotein 1; TNC: tenascin C; Vcam1: vascular cell adhesion molecule 1.
To determine the potential molecular mechanism underlying E2-mediated TMJ fibrocartilage ECM loss, we next assessed E2’s modulation of MMPs, ECM, and adhesion molecules in TMJ disc fibrocartilage. Of the 84 ECM/MMP/cell adhesion genes assayed via PCR array, 13 genes showed significant induction or repression in TMJ disc fibrocartilage after 7 d of exposure to E2 or P4 (Fig. 2C). These included significant induction of MMP9, MMP13, MMP8, TNC, and SPP1 by E2 but not P4; enhanced expression of MMP3, HC, VCAM, and LAMA1 by P4 but not E2; and enhanced expression of SELP by E2 and P4. Additionally, ADAMTS1 and ADAMTS8 were repressed by both hormones, and SPOCK1 was inhibited by P4. Among the assayed genes, E2’s most dramatic induction was of the 2 proteinases MMP9 and MMP13 of 16- and 7-fold, respectively. In contrast, MMP9 and MMP13 were not modulated by P4. Real-time qRT-PCR (Fig. 2D, E) and Western blot analysis (Fig. 2F) showed significant induction of MMP9 and MMP13 in the TMJ fibrocartilage of mice administered E2 but not P4, providing confirmation of the gene array findings. Thus, among 84 assayed genes, E2 but not P4 specifically induces MMP9 and MMP13 in vivo, thereby pointing to their potential contribution to E2-mediated TMJ fibrocartilage matrix turnover.
Abrogation of MMP9 or MMP13 Does Not Mitigate E2-Mediated Collagen Loss, Which May Be Due to Compensatory Reciprocal Increase of One MMP in the Absence of the Other
We next undertook studies with MMP9-/- and MMP13-/- mice to identify any causal link between E2-induced MMP9 and MMP13 and TMJ fibrocartilage collagen loss. Unexpectedly, E2 treatment of either of these MMP-null mice resulted in a loss of TMJ disc collagen to a similar extent as in control WT mice (Fig. 3A, B). To understand the basis for this observation, we assayed MMP9 and MMP13 mRNA expression in TMJ tissue from the mice. These assays revealed E2’s significantly greater induction of MMP13 in MMP9-/- mice and that of MMP9 in MMP13-/- mice than in WT mice (Fig. 3C, D), suggesting that each of these proteinases compensates for the absence of the other in response to E2. This compensatory redundancy of the 2 MMPs may explain the similarities in E2-mediated collagen loss in WT and each of the MMP-null mice, which requires validation through the use of double MMP9 and MMP13 knockout models.
Figure 3.
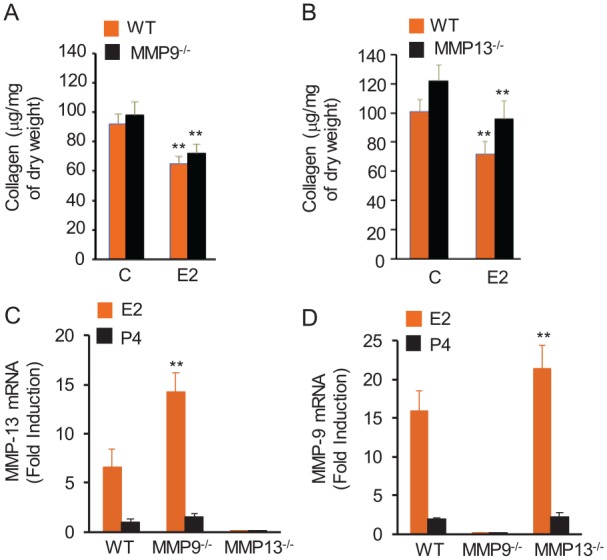
Disruption of MMP9 or MMP13 fails to rescue E2-mediated loss of collagen in mouse TMJ fibrocartilage, which may be attributed to a compensatory increase in one MMP in the absence of the other MMP. Ovariectomized WT, MMP9-, and MMP13-null mice were treated as described in Figure 1. TMJ fibrocartilage was retrieved, and protein extracted for collagen assays and total RNA was prepared for the RT2 Profiler PCR Array. E2-mediated reduction in TMJ fibrocartilage collagen was not significantly different between WT and MMP9-null mice (A) or between MMP13-null and WT mice (B). MMP9-null mice showed amplified expression of MMP13 in TMJ fibrocartilage in response to E2 (C), while mice lacking MMP13 showed a markedly enhanced induction of MMP9 mRNA by E2 relative to vehicle-treated control mice (D). P4 does not modulate the expression of either MMP in WT or null mice. Results are presented as mean ± SD from 5 mice. **P < 0.01 vs. control or the levels in WT mice. C, control (phosphate buffered saline); E2, 17-β estradiol; MMP, matrix metalloproteinase; P4, progesterone; TMJ, temporomandibular joint; WT, wild type.
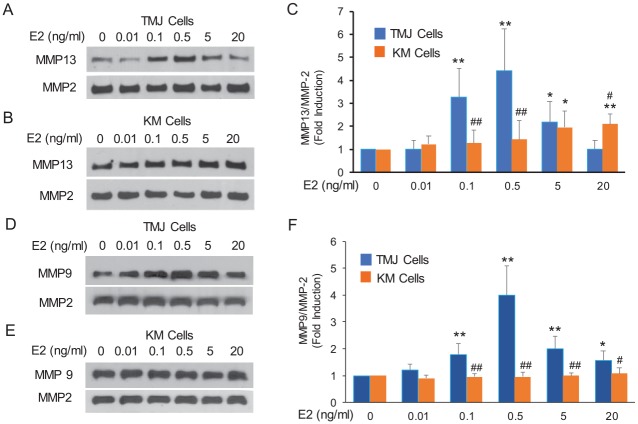
TMJ Fibrocartilage Cells Are More Sensitive Than KM Cells in MMP9 and MMP13 Induction by E2
To understand the reason for the differences between TMJ and KM fibrocartilage matrix loss in response to E2, we explored the sensitivity of the primary cells in the modulation of MMP13 and MMP9 by E2. TMJ fibrocartilaginous cells treated to increasing concentrations of E2 showed a statistically significant peak 3.2- to 4.4-fold induction of MMP13 at 0.1 and 0.5 ng/mL of E2 (Fig. 4A, C). In contrast, MMP13 expression was significantly increased only about 2-fold in KM cells at 5 and 20 ng/mL of E2 (Fig. 4B, C). Similarly E2 at concentration ranges of 0.1 to 20 ng/mL significantly increased MMP9 expression with a peak induction of ~4-fold at 0.5 ng/mL of E2 (Fig. 4D, F). However, none of the E2 doses had any effect on MMP9 expression in KM cells (Fig. 4E, F). These results indicate the greater sensitivity of TMJ than KM fibrocartilage cells in the induction of MMP13 and MMP9 by E2.
Figure 4.
TMJ fibrocartilaginous cells are more sensitive to E2 than KM cells in the induction of MMP13 and MMP9. Early-passage fibrocartilaginous cells were cultured in serum-free, phenol-free media without or with increasing concentrations of E2 for 48 h. Cell-conditioned media was retrieved and assayed by Western blots for MMP13 and MMP9. MMP2 was used as an internal control, since our gene array results and E2 dose-response experiments showed little change in its expression by E2. Representative Western blots for MMP13 and MMP9 for TMJ fibrocartilaginous (A and D) and KM (B and E) fibrocartilaginous cells. Histograms of mean ± SD of pooled data from Western blots show significantly greater sensitivity and higher peak induction of MMP13 (C) and MMP9 (F) in the TMJ versus the KM fibrocartilaginous cells in response to E2. *P < 0.05 and **P < 0.01 vs. vehicle-treated control. #P < 0.05 and ##P < 0.01 vs. the same doses of E2-treated TMJ cell groups. E2, 17-β estradiol; KM, knee meniscus; MMP, matrix metalloproteinase; TMJ, temporomandibular joint.
Discussion
Because of 1) the preponderance of TMJ disorders in women during reproductive years, 2) the association of TMJ DDs in men and women with elevated levels of E2 (Landi et al. 2004; Landi et al. 2005), and 3) our previous findings showing the induction of MMPs by E2 in TMJ fibrocartilaginous cells in vitro, we initially undertook a series of trials to establish a mouse model to attain systemic E2 levels mimicking those found during the luteal phase in cycling women. Using OVX mice to minimize the confounding effects of the variations in hormones during the menstrual cycle, we found that the continuous infusion of 3 µg/kg/d of E2 or 0.15 mg/kg/d of P4 resulted in mean systemic concentrations of 94 pg/mL and 951 pg/mL, respectively, of these hormones. These serum hormone concentrations are consistent with peak systemic midluteal E2 and P4 concentrations in cycling women and for E2 in gonadal intact female mice (Marsh et al. 2011; Nilsson et al. 2015). Our studies demonstrated that exposure to these concentrations of E2 but not P4 resulted in a significant loss of TMJ fibrocartilage collagen and GAG, an early phenotype of DD/OA. Neither E2 nor P4 had any discernible effects on KM fibrocartilaginous matrices. Since the loss of key ECM molecules is a precursor to OA, these findings suggest that E2 at levels found in vivo may predispose the TMJ to targeted degenerative TMJ disorders.
The loss of cartilaginous matrices, including those of the TMJ in DD/OA, is believed to be mediated by matrix-degrading enzymes of the MMP family. Of these MMPs, MMP9 and MMP13 are the 2 primary enzymes responsible for cartilage ECM breakdown, as shown in clinical and animal studies (Leonardi et al. 2008; Wadhwa and Kapila 2008; Troeberg and Nagase 2012; Loreto et al. 2013; Yang et al. 2013). Additionally both these MMPs are upregulated in degenerative TMJ disorders (Leonardi et al. 2008; Loreto et al. 2013). While the upregulation of MMP9 and MMP13 in OA has primarily been attributed to their induction by proinflammatory cytokines (Burrage et al. 2006), our study demonstrates for the first time the in vivo induction of MMP9 and MMP13 by E2. Because E2’s induction of MMP9 and MMP13 occurred in parallel with loss of fibrocartilage ECM, we used global MMP9- and MMP13-null mice to explore the role of each MMP in directly contributing to the E2-mediated TMJ fibrocartilage degradation. Unexpectedly, the global disruption of MMP9 or MMP13 had no statistically significant effect in the rescue of E2-induced loss of collagen from the TMJ fibrocartilage. While various reasons, including the inherent limitations of global knockout mice, may explain these observations, the most compelling explanation for this lack of effect of absence of either MMP to E2-mediated fibrocartilage ECM loss is likely related to the compensatory reciprocal upregulation of one MMP in the absence of the other. Similar observations on the redundancy in the effects of these MMPs have been observed in developing cartilage, wherein the early developmental defects are rescued as the animal matures due to this redundancy (Page-McCaw et al. 2007). The concept that >1 MMP contributes to fibrocartilage matrix degradation is also supported by our previous observations in which a pan-MMP inhibitor blocked the loss of TMJ fibrocartilage matrices in explants exposed to relaxin (Naqvi et al. 2005). If the assumption that both MMPs contribute to fibrocartilage matrix loss is validated through additional in vivo studies, these proteinases together may serve as effective targets for ameliorating E2-mediated cartilage matrix loss with agents such as CAS204140-01-02 that specifically block MMP9 and MMP13 (Cheng et al. 2000) without the side effects related to the use of pan-MMP inhibitors.
While the presence of both ERs in TMJ and KM fibrocartilages (Wang et al. 2013) implies that they are target sites for the potential effects of E2, the ECM turnover responses to E2 are significantly different between these tissues. Thus, while systemic E2 administered at concentrations found in cycling women diminishes the collagen and GAG content of the TMJ fibrocartilage, it has no effect on the collagenous and proteoglycan content of the KM. This finding may be attributed to the higher expression levels of ERs in TMJ than in KM fibrocartilage (Wang et al. 2009), possibly contributing to greater sensitivity of the TMJ than knee fibrocartilages to E2. In support of this postulate, our findings show the dose-dependent bell-shaped upregulation of MMP9 and MMP13 in TMJ fibrocartilage that peaks at ~4-fold of baseline levels at 0.5 ng/mL of E2. In contrast, in KM fibrocartilaginous cells, the peak induction of MMP13 at about 2-fold greater levels than in controls is observed only at 5 and 20 ng/mL of E2, while none of the concentrations of E2 had any effect on MMP9 expression levels. Interestingly, the peak induction of both these MMPs in TMJ fibrocartilaginous cells in vitro occurs within the physiologic concentration range of E2, which was also replicated in our model. Our findings of the minimal MMP-inductive effects of E2 on KM fibrocartilaginous cells explain our in vivo results in KM fibrocartilage and are supported by observations of others showing that a high but not low dose of E2 causes cartilage loss in rabbit knee OA (Tsai and Liu 1993) and that this effect is blocked by an estrogen antagonist, tamoxifen (Tsai and Liu 1992).
Previously, we demonstrated that E2 induces MMP9 and MMP13 via ERα in TMJ fibrocartilaginous cells in vitro (Ahmad et al. 2018). Now we show that E2-mediated loss of cartilage matrices is accompanied by amplification of ERα but not ERβ, suggesting that E2-mediated overexpression of ERα may contribute to further enhancing the sensitivity of this tissue to E2, thereby potentially amplifying the effects of E2 on matrix turnover in the TMJ. Similarly, the higher expression levels of ERα in female than male murine and primate TMJ fibrocartilages (Milam et al. 1987; Wang et al. 2009) with the consequently greater sensitivity of female TMJ tissue to the adverse effects of E2 may explain the greater predilection of TMJ DD/OA in women than men.
In summary, our study demonstrates that E2 but not P4 at systemic concentrations similar to those found in cycling women contributes to a significant loss of collagen and GAG in the TMJ fibrocartilage but not KM fibrocartilage. Such enhanced loss of ECM by E2 is a likely precursor to DD/OA targeted to the TMJ. E2-mediated loss of collagen and GAGs was paralleled by enhanced expression of 2 specific MMPs, MMP9 and MMP13, known to degrade these matrix molecules in cartilage. Furthermore, while we did not find that absence of either MMP9 or MMP13 mitigated the E2-mediated loss of ECM, we observed a compensatory reciprocal upregulation of each MMP in the absence of the other, implying that both proteinases may be critical to E2-mediated TMJ matrix loss. While the findings provide a possible reason for the association between elevated levels of E2 and TMJ DD/OA in women and men and although the systemic levels of E2 achieved in our experiments are similar to midluteal and midfollicular concentrations in cycling women and estrous in mice, the E2 levels achieved represent those of hyperestrogenism particularly for the mice. Future studies based on models with cycling E2 levels and limited durations of elevated E2 levels would provide additional insights on the possible role of E2 in TMJ DDs. These findings lay the foundation for further studies to identify the precise contributions of both MMPs with double knockout mice and ERs in the targeted loss of TMJ fibrocartilage by E2, which could help in designing and implementing effective etiology-specific treatments for E2-mediated TMJ DD/OA.
Author Contributions
Y. Park, contributed to conception, data acquisition, analysis, and interpretation, drafted the manuscript; S. Chen, contributed to conception, design, data acquisition, analysis, and interpretation, drafted the manuscript; N. Ahmad, T. Hayami, contributed to design and data acquisition, critically revised the manuscript; S. Kapila, contributed to conception, design, data acquisition, analysis, and interpretation, critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Footnotes
This study was supported by grant R01 DE018455 from the National Institutes of Health / National Institute of Dental and Craniofacial Research to S. Kapila.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Abubaker AO, Raslan WF, Sotereanos GC. 1993. Estrogen and progesterone receptors in temporomandibular joint discs of symptomatic and asymptomatic persons: a preliminary study. J Oral Maxillofac Surg. 51(10):1096–1100. [DOI] [PubMed] [Google Scholar]
- Ahmad N, Chen S, Wang W, Kapila S. 2018. 17beta-estradiol induces MMP-9 and MMP-13 in TMJ fibrochondrocytes via estrogen receptor alpha. J Dent Res. 97(9):1023–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad N, Wang W, Nair R, Kapila S. 2012. Relaxin induces matrix-metalloproteinases-9 and -13 via RXFP1: induction of MMP-9 involves the PI3k, ERK, Akt and PKC-zeta pathways. Mol Cell Endocrinol. 363(1–2):46–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrage PS, Mix KS, Brinckerhoff CE. 2006. Matrix metalloproteinases: role in arthritis. Front Biosci. 11:529–543. [DOI] [PubMed] [Google Scholar]
- Cheng M, De B, Pikul S, Almstead NG, Natchus MG, Anastasio MV, McPhail SJ, Snider CE, Taiwo YO, Chen L, et al. 2000. Design and synthesis of piperazine-based matrix metalloproteinase inhibitors. J Med Chem. 43(3):369–380. [DOI] [PubMed] [Google Scholar]
- Hashem G, Zhang Q, Hayami T, Chen J, Wang W, Kapila S. 2006. Relaxin and beta-estradiol modulate targeted matrix degradation in specific synovial joint fibrocartilages: progesterone prevents matrix loss. Arthritis Res Ther. 8(4):R98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SC, Lee DG, Choi JH, Kim ST, Kim YK, Ahn HJ. 2007. Association between estrogen receptor polymorphism and pain susceptibility in female temporomandibular joint osteoarthritis patients. Int J Oral Maxillofac Surg. 36(5):391–394. [DOI] [PubMed] [Google Scholar]
- Landi N, Lombardi I, Manfredini D, Casarosa E, Biondi K, Gabbanini M, Bosco M. 2005. Sexual hormone serum levels and temporomandibular disorders: a preliminary study. Gynecol Endocrinol. 20(2):99–103. [DOI] [PubMed] [Google Scholar]
- Landi N, Manfredini D, Lombardi I, Casarosa E, Bosco M. 2004. 17-Beta-estradiol and progesterone serum levels in temporomandibular disorder patients. Minerva Stomatol. 53(11–12):651–660. [PubMed] [Google Scholar]
- Lee DG, Kim TW, Kang SC, Kim ST. 2006. Estrogen receptor gene polymorphism and craniofacial morphology in female TMJ osteoarthritis patients. Int J Oral Maxillofac Surg. 35(2):165–169. [DOI] [PubMed] [Google Scholar]
- Leonardi R, Loreto C, Barbato E, Caltabiano R, Lombardo C, Musumeci G, Lo Muzio L. 2008. MMP-13 (collagenase 3) localization in human temporomandibular joint discs with internal derangement. Acta Histochem. 110(4):314–318. [DOI] [PubMed] [Google Scholar]
- Levin ER, Hammes SR. 2016. Nuclear receptors outside the nucleus: extranuclear signalling by steroid receptors. Nat Rev Mol Cell Biol. 17(12):783–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreto C, Leonardi R, Musumeci G, Pannone G, Castorina S. 2013. An ex vivo study on immunohistochemical localization of MMP-7 and MMP-9 in temporomandibular joint discs with internal derangement. Eur J Histochem. 57(2):e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maixner W, Diatchenko L, Dubner R, Fillingim RB, Greenspan JD, Knott C, Ohrbach R, Weir B, Slade GD. 2011. Orofacial pain prospective evaluation and risk assessment study—the OPPERA study. J Pain. 12(11 Suppl):T4–T11.e1–e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh EE, Shaw ND, Klingman KM, Tiamfook-Morgan TO, Yialamas MA, Sluss PM, Hall JE. 2011. Estrogen levels are higher across the menstrual cycle in African-American women compared with caucasian women. J Clin Endocrinol Metab. 96(10):3199–3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milam SB, Aufdemorte TB, Sheridan PJ, Triplett RG, Van Sickels JE, Holt GR. 1987. Sexual dimorphism in the distribution of estrogen receptors in the temporomandibular joint complex of the baboon. Oral Surg Oral Med Oral Pathol. 64(5):527–532. [DOI] [PubMed] [Google Scholar]
- Murphy MK, MacBarb RF, Wong ME, Athanasiou KA. 2013. Temporomandibular disorders: a review of etiology, clinical management, and tissue engineering strategies. Int J Oral Maxillofac Implants. 28(6):e393–e414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi T, Duong TT, Hashem G, Shiga M, Zhang Q, Kapila S. 2005. Relaxin’s induction of metalloproteinases is associated with the loss of collagen and glycosaminoglycans in synovial joint fibrocartilaginous explants. Arthritis Res Ther. 7(1):R1–R11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson ME, Vandenput L, Tivesten A, Norlen AK, Lagerquist MK, Windahl SH, Borjesson AE, Farman HH, Poutanen M, Benrick A, et al. 2015. Measurement of a comprehensive sex steroid profile in rodent serum by high-sensitive gas chromatography-tandem mass spectrometry. Endocrinology. 156(7):2492–2502. [DOI] [PubMed] [Google Scholar]
- Page-McCaw A, Ewald AJ, Werb Z. 2007. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 8(3):221–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro-Dasilva MC, Peres Line SR, Leme Godoy dos Santos MC, Arthuri MT, Hou W, Fillingim RB, Rizzatti Barbosa CM. 2009. Estrogen receptor-alpha polymorphisms and predisposition to TMJ disorder. J Pain. 10(5):527–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffman E, Ohrbach R, Truelove E, Look J, Anderson G, Goulet JP, List T, Svensson P, Gonzalez Y, Lobbezoo F, et al. ; International RDC/TMD Consortium Network, International Association for Dental Research; Orofacial Pain Special Interest Group, International Association for the Study of Pain. 2014. Diagnostic criteria for temporomandibular disorders (DC/TMD) for clinical and research applications: recommendations of the International RDC/TMD Consortium Network* and Orofacial Pain Special Interest Group. J Oral Facial Pain Headache. 28(1):6–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scrivani SJ, Keith DA, Kaban LB. 2008. Temporomandibular disorders. N Engl J Med. 359(25):2693–2705. [DOI] [PubMed] [Google Scholar]
- Stemig M, Myers SL, Kaimal S, Islam MS. 2015. Estrogen receptor-alpha polymorphism in patients with and without degenerative disease of the temporomandibular joint. Cranio. 33(2):129–133. [DOI] [PubMed] [Google Scholar]
- Troeberg L, Nagase H. 2012. Proteases involved in cartilage matrix degradation in osteoarthritis. Biochim Biophys Acta. 1824(1):133–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai CL, Liu TK. 1992. Inhibition of estradiol-induced early osteoarthritic changes by tamoxifen. Life Sci. 50(25):1943–1951. [DOI] [PubMed] [Google Scholar]
- Tsai CL, Liu TK. 1993. Estradiol-induced knee osteoarthrosis in ovariectomized rabbits. Clin Orthop Relat Res. (291):295–302. [PubMed] [Google Scholar]
- Wadhwa S, Kapila S. 2008. TMJ disorders: future innovations in diagnostics and therapeutics. J Dent Educ. 72(8):930–947. [PMC free article] [PubMed] [Google Scholar]
- Wang J, Chao Y, Wan Q, Zhu Z. 2008. The possible role of estrogen in the incidence of temporomandibular disorders. Med Hypotheses. 71(4):564–567. [DOI] [PubMed] [Google Scholar]
- Wang W, Hayami T, Kapila S. 2009. Female hormone receptors are differentially expressed in mouse fibrocartilages. Osteoarthritis Cartilage. 17(5):646–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XD, Kou XX, Meng Z, Bi RY, Liu Y, Zhang JN, Zhou YH, Gan YH. 2013. Estrogen aggravates iodoacetate-induced temporomandibular joint osteoarthritis. J Dent Res. 92(10):918–924. [DOI] [PubMed] [Google Scholar]
- Wrighton PJ, Klim JR, Hernandez BA, Koonce CH, Kamp TJ, Kiessling LL. 2014. Signals from the surface modulate differentiation of human pluripotent stem cells through glycosaminoglycans and integrins. Proc Natl Acad Sci U S A. 111(51):18126–18131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CC, Lin CY, Wang HS, Lyu SR. 2013. Matrix metalloproteases and tissue inhibitors of metalloproteinases in medial plica and pannus-like tissue contribute to knee osteoarthritis progression. PloS One. 8(11):e79662. [DOI] [PMC free article] [PubMed] [Google Scholar]