Abstract
Neural EGFL-like 1 (Nell-1) is a well-studied osteogenic factor that has comparable osteogenic potency with the Food and Drug Administration–approved bone morphogenic protein 2 (BMP-2). In this review, which aims to summarize the advanced Nell-1 research in the past 10 y, we start with the correlation of structural and functional relevance of the Nell-1 protein with the identification of a specific receptor of Nell-1, contactin-associated protein-like 4 (Cntnap4), for osteogenesis. The indispensable role of Nell-1 in normal craniofacial and appendicular skeletal development and growth was also defined by using the newly developed tissue-specific Nell-1 knockout mouse lines in addition to the existing transgenic mouse models. With the achievements on Nell-1’s osteogenic therapeutic evaluations from multiple preclinical animal models for local and systemic bone regeneration, the synergistic effect of Nell-1 with BMP-2 on osteogenesis, as well as the advantages of Nell-1 as an osteogenic protein with antiadipogenic, anti-inflammatory, and provascularized characteristics over BMP-2 in bone tissue engineering, is highlighted, which lays the groundwork for the clinical trial approval of Nell-1. At the molecular level, besides the mitogen-activated protein kinase (MAPK) signaling pathway, we emphasize the significant involvement of the Wnt/β-catenin pathway as well as the key regulatory molecules Runt-related transcription factor 2 (Runx2) in Nell-1-induced osteogenesis. In addition, the involvement of Nell-1 in chondrogenesis and its relevant pathologies have been revealed with the participation of the nuclear factor of activated T cells 1 (Nfatc1), Runx3, and Indian hedgehog (Ihh) signaling pathways, although the mechanistic insights of Nell-1’s osteochondrogenic property will be continuously evolving. With this perspective, we elucidate some emerging and novel functional properties of Nell-1 in oral-dental and neural tissues that will be the frontiers of future Nell-1 studies beyond the context of bone and cartilage. As such, the therapeutic potential of Nell-1 continues to evolve and grow with continuous pursuit.
Keywords: osteogenesis, chondrocytes, cartilage, bone remodeling, bone regeneration, osteoporosis
Introduction
It has been 2 decades since the first report of neural EGFL-like 1 (Nell-1) function, in which Nell-1 overexpression was associated with human craniosynostosis (Ting et al. 1999). The research efforts of the first decade were focused on the osteogenic property of Nell-1, the potential usage of Nell-1 in bone regeneration, and the Nell-1-mediated mitogen-activated protein kinase (MAPK) signaling pathway (Bokui et al. 2008; Zhang et al. 2010). In the past 10 y, the functional impacts and mechanistic understandings of Nell-1 in modulating skeletal development and growth, as well as in promoting osteochondral tissue regeneration, have been significantly expanded and advanced. In this article, the major milestones of the continuous research on Nell-1’s roles and its therapeutic potential in skeletal tissues and beyond are reviewed with emphases on 1) the novel finding of Nell-1 binding partners for osteogenesis, 2) the indispensable role of Nell-1 in normal craniofacial and appendicular skeletogenesis, 3) the extended and improved therapeutic applications of Nell-1 for osteoporotic conditions and osteoarthritis, and 4) the emerging roles of Nell-1 in tissues beyond bone and cartilage.
Further Understanding of Nell-1 Protein Structure and Functional Relevance
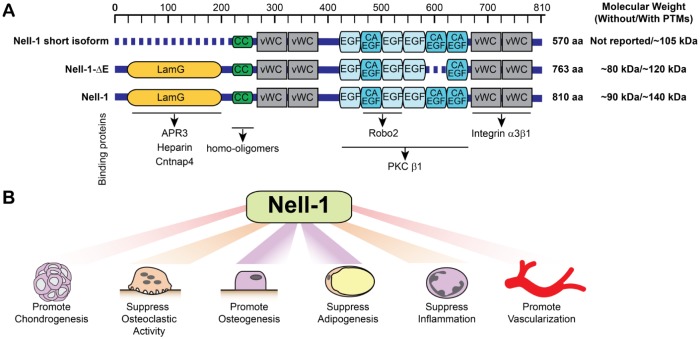
As reviewed previously (Zhang et al. 2010), Nell-1 contains 810 amino acids with a molecular weight of ~90 kDa immediately after translation and ~140 kDa with posttranslational modifications. To date, there are 2 shorter isoforms of Nell-1 being identified with functions, and several binding partners of the Nell-1 protein have been distinguished in the past decade (Fig. 1A).
Figure 1.
The domains of Nell-1 and Nell-1’s isoforms as well as the known functions of Nell-1. (A) Nell-1 has a length of 810 amino acids and is composed by a secretion signal peptide, an NH2-terminal von Willebrand factor C (vWC) domain, 4 vWC domains, and 6 epidermal growth factor–like (EGF) domains. Nell-1 short isoform, also known as Nell-1 570, is an N-terminal-truncated Nell-1 isoform. Nell-1-ΔE is lacking 1 calcium-binding EGF-like domain. The molecular weight of the full-length Nell-1 and its isoforms before and after posttranslational modifications (PTMs) based on previous publications are stated in the figure. The LamG domain of Nell-1 can bind to apoptosis-related protein 3 (APR3), heparin, and contactin-associated protein-like 4 (Cntnap4). The CC region is in charge of the formation of homo-oligomers. The EGF domains can bind to protein kinase C βI (PKCβI), while the second and third EGF domains can also bind to roundabout 2 (Robo2). The last 2 vWC domains can bind to integrin α3β1. (B) The current known functions of Nell-1.
Binding Partners of Nell-1
Since Nell-1 mainly functions as a secreted protein, great efforts have been placed on finding the specific functional receptor(s) of Nell-1 (Fig. 1A). Nell-1 was initially known as a binding protein to protein kinase C βI that may result in Nell-1’s phosphorylation (Kuroda and Tanizawa 1999). Apoptosis-related protein 3 (APR3) has been identified with the predominant co-localization of Nell-1/APR3 on the nuclear envelope of human osteosarcoma cell lines and may be partially responsible for promoting osteoblastic differentiation and suppressing cell proliferation (Zou et al. 2011). It was later reported that Nell-1 can promote osteoblastic cell adhesion and differentiation through binding to integrin α3β1 with use of the last 2 von Willebrand factor C domains on its C-terminal (Nakamura et al. 2014). In addition, 2 positively charged patches in the TSPN domain of Nell-1 have moderate heparin-binding activity, and the interaction of Nell-1 with heparan sulfate proteoglycans on the cell surface may assist in Nell-1-integrin binding (Takahashi et al. 2015). Nell-1 was recently found to bind to roundabout 2 (Robo2) through its second and third EGF domains (Yamamoto et al. 2019). Up to this point, none of the aforementioned Nell-1 binding proteins have been recognized as the specific functional receptors of Nell-1. This is because either the cell membrane protein–like integrin β1 is known to bind with a broad range of molecules or the bindings to Robo2 and APR3 are not naturally present on the cell surface.
Identifying Contactin-Associated Protein-like 4 as a Nell-1-Specific Receptor
More recently, a breakthrough was made in pursuit of identifying cell surface-specific receptors of Nell-1. The physical high-affinity ligand-receptor-like binding between contactin-associated protein-like 4 (Cntnap4) and Nell-1 was identified without artificially forcing the overexpression of Nell-1 or Cntnap4 in the osteogenic cells. Functionally, depleting Cntnap4 expression diminished Nell-1-induced osteogenesis without affecting the osteogenic effects of bone morphogenic protein 2 (BMP-2). More interestingly, the specific Cntnap4 inactivation in mouse Wnt1-expressing cells has cleidocranial dysplasia–like calvarial phenotype at the neonatal stage that is quite similar, if not identical, to the calvarial defects in N-ethyl-N-nitrosourea (ENU)–induced Nell-1-deficient (Nell-16R/6R) mice (Li, Zheng, Ha, et al. 2018). Thus, Cntnap4 can be defined as the only cell surface–specific receptor of Nell-1 identified thus far to be responsible for osteogenesis.
Interestingly, during the surface plasmon resonance assay, when immobilizing the CM5 sensor chips with the extracellular portion of Cntnap4 (Cntnap4extra), the solution phase of Nell-1 showed strong binding affinity to the chips (Li, Zheng, Ha, et al. 2018); however, no binding signals were detected when flowing the Cntnap4extra solution through the Nell-1 immobilized chips (unpublished data). Considering the controversies about the quaternary structure of Nell-1, in which the homodimer, homotrimer, homotetramer, and homopentamer have all been suggested (Nakamura et al. 2014), these phenomena might implicate a dynamic change of the quaternary structure of Nell-1. Specifically, Nell-1 might present as homodimers and homotrimers as the stable and nonfunctioning forms, and when a cell surface–specific receptor becomes available, these homodimers and homotrimers are able to form homopentamers as the functioning form. Based on the fact that Nell-1 binds to Cntnap4 via the LamG domain (Li, Zheng, Ha, et al. 2018) and the coiled-coil region of Nell-1 is in charge of homo-oligomers (Nakamura et al. 2014), a 3-dimensional binding structure of functioning Nell-1 and Cntnap4 was predicted as a reference for future studies (Fig. 2).
Figure 2.
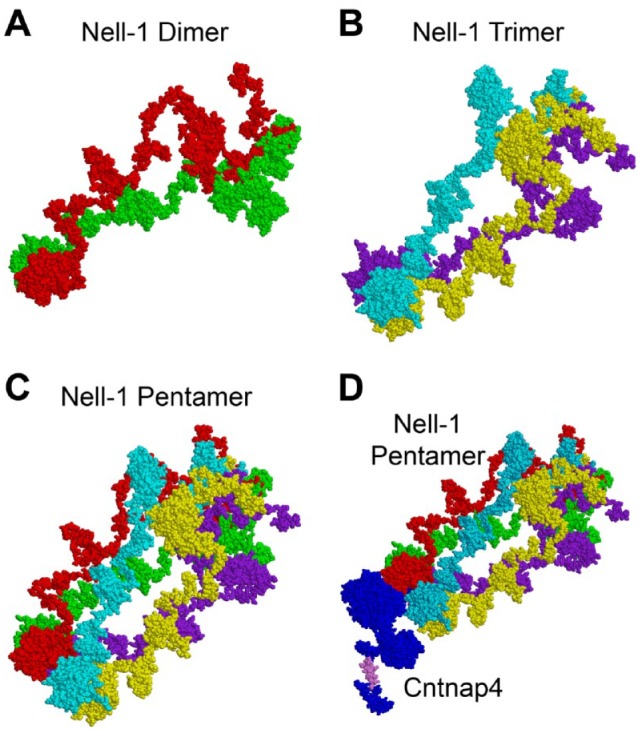
Homopentamer might be the functional format of Nell-1. The predicted 3-dimensional structure of Nell-1 as a homodimer (A), homotrimer (B), and homopentamer (C) and that of the Nell-1 pentamer and Cntnap4 complex (D) were predicted by using the PATCHDOCK server (http://bioinfo3d.cs.tau.ac.il/PatchDock/php.php) and I-TASSER server (https://zhanglab.ccmb.med.umich.edu/I-TASSER/).
Nell-1 Isoforms with Distinct Functions
Two isoforms of Nell-1 have been reported. The first one is an N-terminal-truncated Nell-1 short isoform, also known as Nell-1 570 (Fig. 1A). Nell-1 570 can significantly stimulate mesenchymal stromal cell proliferation and osteogenic differentiation, and the effect of Nell-1 570 on proliferation is age-dependent via upregulating the Notch pathway (Meyers et al. 2019). Another isoform of Nell-1 is known as Nell-1-ΔE, which is lacking 1 calcium-binding EGF-like domain and exhibits similar subcellular localization and expression patterns as Nell-1. However, Nell-1-ΔE, but not Nell-1, was found being capable of interacting with enolase 1 in the extracellular spaces of osteogenic linear cell lines (Zhao et al. 2018). It is worth noting that there are likely more Nell-1 isoforms to be identified and functionally characterized in future studies, as multiple Nell-1 transcripts are listed in the NCBI database.
Indispensable Role of Nell-1 for Normal Skeletal Development and Growth
The global Nell-1 overexpression transgenic mice exhibited craniosynostosis-like phenotypes, while the global knockout of Nell-1 led to perinatal lethality in homozygous newborns and a set of developmental skeletal deformities, as reviewed previously (Zhang et al. 2010). For Nell-1+/6R mice, even though they appear normal at a young age, the osteoporotic phenotype is apparent when the mice are older than 6 mo (James et al. 2015; Table 1).
Table 1.
Phenotypes of Genetically Modified Nell-1 Mice.
| Strain | CMV-Nell-1 Overexpression | ENU-Induced Nell-1 Deficiency | Nell-1CMVKO | Nell-1Wnt1KO | Nell-1Col1KO | Nell-1Col2a1KO |
|---|---|---|---|---|---|---|
| Life span | Die shortly after birth with severe craniofacial anomalies cases | Nell-16R/6R mice die perinatally. Nell-1+/6R mice have similar life span to that of WT controls. | Die at birth with extremely severe KO cases but no significant changes for the majority of KO mice | Shorter in KOs with hydrocephalus | No change | No change |
| Whole body | No observable extracranial skeletal anomalies | The Nell-16R/6R mice presented CDD-like phenotype, short body length, compressed cervical intervertebral spaces, anomalous curvature in the cervical spine, and severe deformity of the ribcage. | Not analyzed | Smaller in KOs with hydrocephalus | No significant difference at birth, osteoporotic phenotypes later | Dwarfism, shorter femur and tibia, premature osteoporosis with earlier onset at 1 mo old |
| Skull | ||||||
| General | A large protuberance in the paramedial parietal area with completely closed sagittal and posterior-frontal sutures and partially closed coronal sutures; neural tube defects | Enlarged cranial vaults, less mineralized calvarial bones, widened sutures, and severe underdevelopment of middle ear bones and auricular bony capsule | Cranial skeletal hypoplasia at neonatal stage for the most KO mice | Shorter; some (5.4%) mice develop hydrocephalus at around 40 d old; frontonasal hypoplasia | 19.4% in heterozygous KO and 7.7% in homozygous KO develop hemifacial microsomia-like phenotype | Not analyzed |
| Skull vault | Thickened, disorganized ridges of calvarial ridges with closing / overlapping osteogenic fronts | Enlarged sagittal suture width, reduction of parietal bone thickness, and lower BMD and BV/TV | Wider frontal, sagittal, and coronal sutures and lower BMD and BV/TV | Wider frontal, sagittal, and coronal sutures; lower BMD and BV/TV of calvarial bones; significantly reduced thickness in frontonasal bones | Curved nose, maxilla, and frontal bones; delayed fusion of frontal and sagittal sutures; lower BMD | Not analyzed |
| Skull base | Not analyzed | Delayed maturation of auricular capsule, midear bones, chondrocranium | Not analyzed | No detectable changes at neonatal stage but premature ossification and fusion of intrasphenoidal synchondrosis and/or sphenoid-occipital synchondrosis in hydrocephalic cases | Lower BMD | Not analyzed |
| Maxilla | Not analyzed | Not analyzed | Not analyzed | Shortened | Shortened, deviated | Not analyzed |
| Mandible | Not analyzed | Change of growth direction of the temporomandibular joint condyle | Smaller mandible and lower BMD and BV/TV; lacking of whole mandible with extremely severe KO cases | Micrognathia with lower BMD and BV/TV | Micrognathia, deviated | Not analyzed |
| Femur/tibia | ||||||
| Trabecular bone | No observable anomalies | The 18-mo-old Nell-1+/6R mice exhibited osteoporotic phenotype. | Not analyzed | Not analyzed | Lower BV/TV, BMD | Lower BMD, BV/TV, Tb.Th, Tb.N; larger Tb.Sp. |
| Cortical bone | No observable anomalies | Cortical bone malformations in neonatal Nell-16R/6R mice | Not analyzed | Not analyzed | Not analyzed | Lower BMD, Ct.Th. |
| Histology | No abnormal histology | Nell-1+/6R mice showed a marked reduction in Sca-1+ MPCs, which temporally preceded the development of the low-BMD phenotype. | Marked reduction in Sca-1+ MPCs within the bone marrow compartment | Not analyzed | Not analyzed | Delayed / reduced mineralization at secondary ossification centers, thinner epiphyseal plates |
| Lumbar | ||||||
| Trabecular bone | No observable anomalies | Less mineralized trabeculae in the central zone of vertebral bodies | Not analyzed | Not analyzed | Not analyzed | Not analyzed |
| Cortical bone | No observable anomalies | Cortical bone malformations | Not analyzed | Not analyzed | Not analyzed | Not analyzed |
| Histology | No abnormal histology | Nell-16R/6R mice have reduced extracellular matrix in the intervertebral spaces, delayed ossification at the center of vertebral bodies, and fewer and thinner trabeculae. The 18-mo-old Nell-1+/6R mice have a significantly reduced osteoblast number per bone perimeter and a significantly increased osteoclast number per bone perimeter in the vertebrae. | Not analyzed | Not analyzed | Not analyzed | Not analyzed |
| Mechanism | Nell-1 accelerates osteoblast differentiation. Overexpression of Nell-1 promotes apoptosis in calvarial osteoblasts and neural cells. | Nell-1 is an important growth factor for regulation of osteochondral differentiation by regulating Runx2 and Sox9 expression within the calvarium. | Not analyzed | Nell-1 is a pivotal modulator of cranial neural crest cells, partially via activating the Wnt/β-catenin pathway. Nell-1 is also critical in regulating cerebrospinal fluid homeostasis through structural abnormalities and misplaced expression of vimentin and prealbumin / transthyretin in the degenerative choroid plexus epithelial cells and ependymal cells. | Not analyzed | Nell-1 inactivation in chondrocytes inhibits Ihh signaling, predominantly altering the Ihh-PTHrP feedback loop. |
| Conclusion | Nell-1 transgenic animals exhibited CS-like phenotypes that ranged from simple to compound synostoses at birth and NTDs at a relatively late gestation stage. | Nell-1 is required for normal craniofacial membranous and endochondral skeletal development. | Nell-1 is a key factor for normal craniofacial skeletal development. | Nell-1 is a pivotal modulator of cranial neural crest cells that is essential for the normal development and growth of the cranial vault. Nell-1 is also critical in regulating cerebrospinal fluid homeostasis. | Not analyzed | Nell-1 is a pivotal modulator of epiphyseal homeostasis and endochondral ossification. |
| References | Zhang et al. 2002; Zhang et al. 2006 | Desai et al. 2006; Zhang et al. 2012; James et al. 2015; Qin et al. 2016; James et al. 2017 | James et al. 2017; Chen et al. 2019 | Chen et al. 2019 | Unpublished data | Qi et al. 2019 |
BMD, bone mineral density; BV/TV, bone volume/total volume; CDD, cleidocranial dysplasia; CMV, cytomegalovirus; CS, craniosynostosis; Ct.Th., average cortical thickness; ENU, N-ethyl-N-nitrosourea; KO, knockout; MPC, mesenchymal progenitor cell; NTD, neural tube defect; Tb.N, trabecular number; Tb.Th, trabecular thickness; Tb.Sp, trabecular separation; WT, wild type.
Evidently, the results obtained from these murine models were far from sufficient due to the nonphysiologically high expression level of Nell-1 in CMV-Nell-1 mice and the neonatal lethality for precluding postnatal evaluation in Nell-16R/6R mice. The Cre/loxP recombination system was recently used to successfully generate floxed Nell-1 mice (James et al. 2017). In contrast to Nell-16R/6R, the majority of Nell-1CMVKO mice were born alive and demonstrated various degrees of hypoplastic craniofacial skeletal defects (Table 1).
To further define Nell-1’s specific effects in craniofacial and appendicular bones, several driver mouse lines were preferentially selected to make relevant conditional Nell-1 knockout mice models (Table 1). With Nell-1 being inactivated in cranial neural crest cells, Nell-1Wnt1KO mice exhibited wider cranial sutures and lower bone mineral density of calvarial bones. Some Nell-1Wnt1KO mice developed hydrocephalus at around 40 d old, accompanied with a shorter life span, short body length, and premature ossification and fusion of cranial base synchondrosis. This modulatory role of Nell-1 on cranial neural crest cells is partial via activating the Wnt/β-catenin pathway (Chen et al. 2019). With the deleted Nell-1 gene in the preosteoblastic cell lineages, the Nell-1Col1KO mice again represented a delayed fusion of cranial sutures. The Nell-1Col1KO mice also presented with systemically low bone mineral density, curved nasal and frontal bones, as well as retrognathic and deviated maxilla and mandible (unpublished data). The Nell-1Col2a1KO mice manifested dwarfism and premature osteoporosis with delayed development and reduced mineralization at secondary ossification centers (Qi et al. 2019), indicating the direct effects of Nell-1 to chondrocytes and endochondral bone formation. The onset of osteoporotic phenotypes was much earlier in Nell-1Col2a1KO mice than in Nell-1+/6R mice, which may implicate the importance of Nell-1 dosage during skeletal growth. Mechanistically, these phenotypic changes are likely the results of inhibition of Ihh signaling and alternation of the Ihh-PTHrP feedback loop in chondrocytes of the growth plate (Qi et al. 2019; Table 1). In addition to the cell-autonomous effects of Nell-1 inactivation in cranial neural crest cells and chondrocytes in Nell-1Wnt1KO and Nell-1Col2a1KO mice, respectively, the non–cell autonomous influences on osteoclasts have been observed in both mutant mouse lines, with significantly higher levels of TRAP staining detected in calvarial and mandibular bones in neonatal Nell-1Wnt1KO mice and in metaphyseal trabecular bones in 1- and 3-mo-old Nell-1Col2α1KO mice (Chen et al. 2019; Qi et al. 2019). However, the underlying mechanisms of the non–cell autonomous effects of Nell-1 inactivation remain left to be investigated in future studies.
Notably, a clinical case report of a 3-year-old Japanese girl who presented with short stature, relative macrocephaly, and delayed closure of cranial fontanelles and sutures was revealed with the 11p14.1-p15.3 deletion involving NELL-1 (Li, Zheng, Ha, et al. 2018). Given the highly conserved Nell-1 gene in rodent and human species (Zhang et al. 2010), the significant overlapping of pathologic phenotypes between this patient and the aforementioned Nell-1 conditional knockout mice highly suggested the indispensable roles of Nell-1 in the normal development and growth of human intramembranous and endochondral bones. The significant findings from these genetically modified Nell-1 mouse models will facilitate the development of Nell-1 as a potential therapeutic modality to patients with craniofacial anomalies and osteoporotic conditions.
Extensive Preclinical Experimentations in Nell-1’s Therapeutic Potential in Bone Tissue Engineering
Since the last review, one of the major research efforts on Nell-1 has been characterized by its therapeutic potentials in the area of bone tissue regeneration with different animal models, including the nonhuman primate, by the combination of different cells and/or scaffolds as well as through Nell-1 protein modifications for more effective systemic delivery (Appendix Table 1).
Updates of Nell-1 Application to Craniofacial Bone Regenerations
Nell-1 has comparable osteogenic capacity with BMPs for craniofacial bone regeneration in mouse models and may also act synergistically with BMPs to promote local bone formation with better quality not only in calvarial bones and distracted palatal suture as previously reviewed (Zhang et al. 2010) but also in the models of maxillary sinus floor elevation (Xia et al. 2011). Very recently, a preclinical study used pDNA-NELL-1 in combination with bone marrow mesenchymal stem cells and 3-dimensionally–printed bioactive glass block/chitosan nanoparticles composites for alveolar bone regeneration in a rhesus monkey. Not only did this significantly promote alveolar bone regeneration, but the regenerative new bone tissues were extremely close to the normal bone in quality (Zhang et al. 2018). Thus, the efficacious clinical application of Nell-1 for craniofacial skeletal regeneration would be well anticipated in the foreseeable future.
Improved Efficacy of Nell-1 in Promoting Spinal Fusion under the Osteoporotic Condition and in Nonhuman Primate
The effects of Nell-1 delivered by the demineralized bone matrix on spinal fusion has been extensively validated in rat and sheep models (Zhang et al. 2010). However, the effective dose of Nell-1 for nonosteoporotic rat spinal fusion models can achieve only low spinal fusion rates in the osteoporotic rats. Administration of the Nell-1 protein in combination with human perivascular stem cells (hPSCs) could significantly improve the spinal fusion rate among the osteoporotic rats (Lee et al. 2015). This finding unveiled the unrivaled potential of Nell-1 in a combination of hPSCs in treating patients with osteoporosis. Significantly, a nonhuman primate model has been successfully completed, resulting in a 100% fusion rate at 3 mo postoperation at a Nell-1 dose of 1.7 mg/mL without any detectable adverse effects (James et al. 2017). With the promising preclinical experimentations, Nell-1 has just received Human Research Ethics Committee approval for the first center of a multicenter pilot clinical trial in Australia to evaluate the safety and efficacy of NELL-1 and demineralized bone matrix in adults with degenerative disc disease (https://www.businesswire.com/news/home/20190325005038/en/Bone-Biologics-Receives-Human%C2%A0Research-EthicsCommittee-HREC).
Modifications of the Nell-1 Protein for Lasting Osteogenic Effects by Systemic Delivery
Local delivery of Nell-1 was effective in most experimental animal models, including promoting the healing of segmental defects in long bones (Li et al. 2011; Xue et al. 2011; Zhu, Song, et al. 2011; Tanjaya et al. 2018), preventing the collapse of the femoral head (Fan et al. 2013), as well as recovering the local low bone mineral density in osteoporotic animals (Kwak et al. 2013; James et al. 2015). However, as osteoporosis involves the whole-body skeletal system, local administration was not an ideal solution. The full exploration of Nell-1 systemic delivery was initiated with intravenous (IV) injection of Nell-1 through the mouse tail vein. Impressively, IV injection of Nell-1 every 48 h for 4 wk was found to induce significant bone formation in osteoporotic mice either ovarectomized (James et al. 2015) or Nell-1 deficiency (James et al. 2017). However, the high frequency of IV injection makes this approach impractical for potential clinical usage. Thus, PEGylation has been introduced to prolong the Nell-1 half-life from 5.5 to 15.5 h in vivo and to reduce the injection frequency from every 48 h to every 7 d (Kwak et al. 2015). More important, PEGylation could increase the distribution of Nell-1 to bone tissues in vivo when compared with naked Nell-1 (Kwak et al. 2015). Later, an effective single-dose intraperitoneal administration of PEGylated Nell-1 was developed (Tanjaya et al. 2016). All these efforts made it possible to further investigate the therapeutic effect of Nell-1 on the bone loss caused by microgravity during space travel (https://www.nasa.gov/mission_pages/station/research/experiments/explorer/Investigation.html?#id=2017).
Advantageous Characteristics of Nell-1 for Bone Tissue Engineering over BMP-2
BMP-2 has been increasingly used in current orthopedic practice on certain indications. However, the adverse effects of the clinical application of BMP-2 have also attracted more attention in the field (James et al. 2016).
High-dose BMP-2 induced large bony masses with a mature bone margin and a central cavity filled with primarily fatty marrow tissue (James et al. 2016). On the contrary, Nell-1 has been demonstrated as having an antiadipogenic effect (Pakvasa et al. 2017) (Fig. 1B, Table 2). When Nell-1 is combined with BMPs, Nell-1 actually can reverse the proadipogenic effects of BMPs to improve the quality of regenerated bone (Pakvasa et al. 2017). More recently, several genome-wide association analyses suggested that Nell-1 may be associated with regular or pathologic lipid/lipoprotein metabolism (Kraja et al. 2013; Rudkowska et al. 2014) and is a hallmark of obesity (de Luis et al. 2018). Thus, the function of Nell-1 in adipogenesis, as well as lipid metabolism, is worth further investigation.
Table 2.
Comparison of Nell-1 and BMP-2 in Bone Regeneration.
| Nell-1 | BMP-2 | |
|---|---|---|
| Osteogenesis | Promote | Promote |
| Chondrogenesis | Promote | Promote |
| Adipogenesis | Inhibit | Promote |
| Inflammation | Inhibit | Promote |
| Mesenchymal stem cell migration | Promote | — |
| Endothelial cell migration | Promote | Promote |
| Angiogenesis | Promote | Promote |
| Tumorigenesis | Unclear | Promote |
| Ectopic bone formation | No | Yes |
| Osteoclast activation | Inhibit | Promote |
| Chemotaxis | — | Promote |
Notably, BMP-2-induced inflammation could be suppressed by Nell-1 in a femoral bone onlay model (Pakvasa et al. 2017). Not surprising, Nell-1 gene single-nucleotide polymorphisms has been linked to inflammatory bowel disease (Franke et al. 2007) and idiopathic pulmonary fibrosis (Bauer et al. 2015). Furthermore, Nell-1 upregulation exhibited protective functions during inflammatory damage in human mitral valve interstitial cells (Chen et al. 2018).
Nell-1 also stimulates angiogenic effects at early stages of bone regeneration and enhances mesenchymal stem cell migration (Fahmy-Garcia et al. 2018). For instance, Nell-1 could improve the formation of the tubular-like structure of the human umbilical vein endothelial cells (Fahmy-Garcia et al. 2018). Nell-1 promoted the secretion of vascular endothelial growth factor (VEGF) in human pericytes (Zhang et al. 2011) and perivascular stem cells (Askarinam et al. 2013) in vitro and enhanced blood vessel formation with a high expression level of VEGF when the cells were implanted intramuscularly. In addition, Nell-1 treatment could increase the vascularity in a nonhuman primate lumbar vertebral spinal fusion model (James et al. 2017).
Potential Concerns of Nell-1 for Bone Tissue Engineering Application
Tumorigenesis is one of the safety concerns of BMP-2 (James et al. 2016). This is also important for Nell-1 because bone regeneration after tumor resection is important for bone tissue engineering (Tian et al. 2017). Hypermethylation of Nell-1 has been identified in colorectal cancer (Tham et al. 2014), gastric cancer (Gao et al. 2015), and Barrett’s esophagus (Wang et al. 2019), as well as in poor prognosis in early-stage esophageal adenocarcinoma (Jin et al. 2007). In addition, loss of Nell-1 expression has been found in Hodgkin lymphoma (Slovak et al. 2011) and renal cell carcinoma (Nakamura et al. 2015). On the contrary, overexpression of Nell-1 has been found in pediatric gastrointestinal stromal tumors (Agaram et al. 2008) and rhabdomyosarcoma (Tombolan et al. 2017). Notably, Nell-1 expression has also been detected in all types of bone tumors and cartilage-forming tumors. Among benign bone tumors, strong and diffuse staining of Nell-1 was observed. In contrast, a relative reduction in Nell-1 staining was observed in osteosarcoma with large variation among tumors (Shen, LaChaud, Khadarian, et al. 2015). In addition, Nell-1 expression did not significantly vary by the type and grade of the cartilage-forming tumors (Shen, LaChaud, Shrestha, et al. 2015). These associations of Nell-1 expression with tumor tissues make the effects of Nell-1 in tumorigenesis inconclusive. However, Nell-1 increased the chemotherapeutic sensitivity of 95-D lung cancer stem‑like cells (Zhai et al. 2019), had a negative effect on myoblast invasion (Rapa et al. 2012), and inhibited renal cell carcinoma cell migration and adhesion (Nakamura et al. 2015). These limited functional studies indicated that Nell-1 may act as a tumor suppressor candidate. Taken together, efforts are certainly needed to further define Nell-1’s functional property related to malignancies, particularly its pros and cons in promoting bone regeneration after removal of osteosarcoma in human patients.
Improved Understanding of Major Signaling Pathways of Nell-1 in Osteogenesis
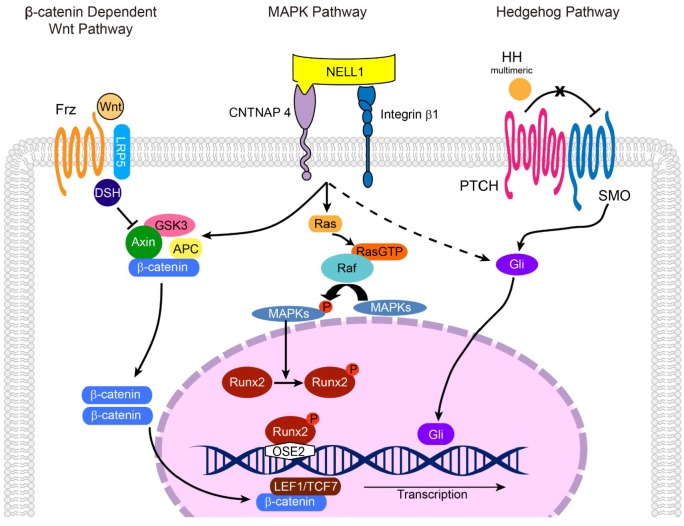
As previously reviewed (Zhang et al. 2010), during osteogenesis, Nell-1 and Runx2 regulate each other, and Nell-1 functions through the phosphorylation of JNK and ERK1/2 by Ras but not through Smad signaling or p38. In addition, in Saos-2 cells, osterix downregulates Nell-1 by affecting the binding of RNA polymerase II to the Sp1 sites of the Nell-1 promoter. Additionally, Nell-1 increases the expression and nuclear translocation of β-catenin in osteoblasts and osteoclasts, which increases osteoblast differentiation and inhibits osteoclast-directed bone resorption (James et al. 2015). Nell-1 also relies on Wnt/β-catenin signaling to induce Sca-1+ (stem cell antigen 1) mesenchymal progenitor cell expression in bone maintenance and repair (James et al. 2017; Figs. 3, 4). In addition, Nell-1 increases preosteoblast mineralization and Pi influx with activation of Pit-1 and Pit-2 channels, with significantly increased Pit-2 production (Cowan et al. 2012).
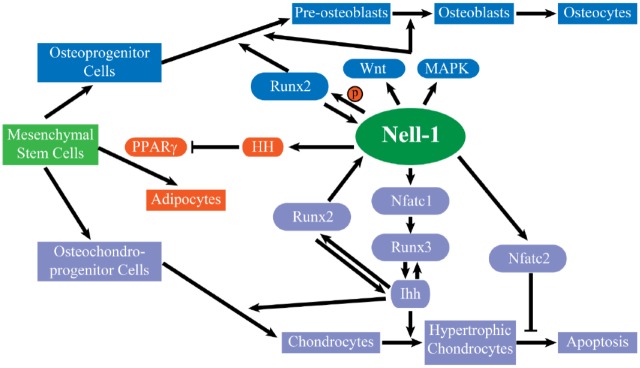
Figure 3.
The function of Nell-1 in osteogenesis, chondrogenesis, and adipogenesis. During the osteogenic differentiation of the mesenchymal stem cells, Nell-1 promotes the differentiation of osteoprogenitor cells to preosteoblasts and osteoblasts. Nell-1 and Runx2 regulate each other, and Nell-1 mainly actives canonical Wnt and MAPK signaling. During the chondrogenic differentiation of the mesenchymal stem cells, Nell-1 promotes the chondrocytes’ formation and maturation through the Runx2-Nell-1-Nfatc1-Runx3-Ihh signaling cascades. Nell-1 inhibits the adipogenesis by inhibition of the major adipogenic transcription factor peroxisome proliferator-activated receptor γ (PPARγ), which is a Hedgehog-dependent effect in our study.
Figure 4.
The mechanism of Nell-1 in osteogenesis. Nell-1 could bind to Cntnap4 and integrin α3β1 on the surface of osteogenic linear cell, while Cntnap4 serves as the specific functional receptor-like protein of Nell-1. Nell-1 could activate the canonical Wnt, ERK1/2, and JNK MAPK signaling, as well as HH signaling pathways during the osteogenesis. Cntnap4 is known to be participate in Nell-1’s activation via the Wnt and MAPK signaling pathways. Nell-1 could promote the phosphorylation of Runx2, while Runx2 regulates the expression of Nell-1 directly by binding to the OSE2 sites of Nell-1 promoter.
Besides the pro-osteogenic and antiosteoclastic effects, Nell-1 inhibits the adipogenic differentiation of 3T3-L1 preadipocytes as well as mesenchymal stromal cells via inhibition of the major adipogenic transcription factors peroxisome proliferator-activated receptor γ and CCAAT/enhancer binding protein, which is a hedgehog-dependent mechanism (James et al. 2011). Applying cyclopamine or smoothened agonist with Nell-1 could enhance the pro-osteogenic and antiadipogenic effects (Lee et al. 2017; Fig. 3).
Nell-1’s Potential Therapeutic Effects in Cartilage-Related Defects
Nell-1 Is a Direct Effector in Chondrogenesis
Nell-1 functional knockout significantly reduced expression of cartilage-related genes (Desai et al. 2006; Qi et al. 2019). In vitro, Nell-1 promoted cartilaginous nodule formation with rabbit auricular chondrocytes (Lee et al. 2010) and enhanced the chondrogenic differentiation of human bone mesenchymal stem cells (Wang et al. 2017) and hPSCs (Li et al. 2016). In vivo, Nell-1 promoted cartilage regeneration in a rabbit femoral condylar cartilage defect (Siu et al. 2012), while Nell-1-modified bone marrow mesenchymal stem cells accelerated native articular cartilage and subchondral bone repair in goat mandibular condyle (Zhu, Zhang, et al. 2011).
With Runx2 and Nell-1 regulating each other during osteogenesis and Runx2 acting as a key regulator in chondrogenesis, the next logical progression was to investigate the regulatory and functional relationships between the two during chondrogenesis. During chondrogenesis, Nell-1 had a similar spatiotemporal expression pattern as Runx2, and Nell-1 acted as a downstream target of Runx2 in chondrocytes (Li et al. 2017). Further investigation showed that Nell-1 regulated chondrocyte maturation through Runx3-mediated Ihh signaling and that nuclear factor of activated T cells 1 (Nfatc1) functioned as the direct response of Nell-1, which could bind to the promoter of Runx3 (Li, Zheng, Zhang, et al. 2018; Fig. 3). These findings elucidate the mode of action of Nell-1 in chondrogenesis, although the specific chondrocyte cell surface receptor of Nell-1 remains unknown.
Therapeutic Significance of Nell-1 to Osteoarthritis
Recently, a significant association was found between Nell-1 and ankylosing spondylitis with peripheral arthritis (Polo et al. 2019). In fact, aging Nell-1+/6R mice represented a severe arthritic change in the knee joints, and intra-articular injection of interleukin 1β induced more severe cartilage damage and higher inflammatory marker expression in young Nell-1+/6R mice than in their wild-type littermates. Promisingly, intra-articular injection of Nell-1 largely restored the interleukin 1β–induced arthritic changes at molecular and functional levels. In vitro experiments proved that Nell-1 not only promoted chondrogenesis but also inhibited inflammation through Runx1 (Li 2019). The dual function of Nell-1 with articular chondrocytes opens a new avenue for the investigation of disease-modifying antiarthritic drugs.
Novel Functional Properties of Nell-1 in Tissues beyond Bone and Cartilage
Nell-1 in the Oral-Dental System
Strong expression of Nell-1 was detected in human dental follicles (Lee et al. 2013), while various expression levels and patterns of Nell-1 were revealed during murine molar development (Tang et al. 2013). In well-developed teeth, Nell-1 was mainly expressed in the body and process of odontoblasts and endothelial cells of the blood vessels (Tang et al. 2013). In vitro, Nell-1 promoted the osteogenic differentiation of human periodontal ligament stem cells (Chen et al. 2012) and the odontoblastic differentiation of human dental pulp cells (Han et al. 2019). In vivo, besides the aforementioned alveolar bone defect repair in a rhesus monkey model (Zhang et al. 2018), local application of Nell-1 into the buccal mucosa region of the orthodontic tooth accelerated corticotomy-assisted tooth movement in a rat model (Wang et al. 2018). The exposed rat pulps capped with Nell-1 plus BMP-2 had superior ability in inducing reparative dentin formation with dentin tubules and in reducing the inflammatory cell response as compared with BMP-2 alone (Wu et al. 2019). Additionally, Nell-1 promoted neural marker expression in rat dental pulp tissues when the tissues were exposed to an inflammatory environment (Han et al. 2019). Thus, aside from effects on the craniofacial skeleton, Nell-1 has crucial functions to the tooth and periodontal tissues.
Nell-1 in Neural Development and Disorders
With the Nell-1 gene being originally identified from a human fetal brain cDNA library, Nell-1 was initially considered a potentially important neural regulator. Indeed, Nell-1 is expressed in neuroblastoma cell lines, glioblastoma cell lines, and adult brain and even at a much higher level in the embryonic inferior olive and spinal cord (Li, Zheng, Ha, et al. 2018). However, the function of Nell-1 in the neural system has not yet been well investigated, although extreme Nell-1 overexpression may cause massive apoptosis of neural cells in the developing brain (Zhang et al. 2006). The recently distinguished association between the single-nucleotide polymorphisms of Nell-1 and autism (Li, Zheng, Ha, et al. 2018), bipolar disorder (Mathieu et al. 2015), and depression (Lin et al. 2018) further emphasizes the potential importance of Nell-1 in the nervous system. Lately, the identification of the functional ligand-receptor-like binding between Cntnap4 and Nell-1 not only opens the new avenue of Cntnap4’s potentials in the skeletal system but also strongly propels the investigation of Nell-1’s roles in neural development and disorders (Li, Zheng, Ha, et al. 2018). Thus, it is expected that future major progress of Nell-1 research will be achieved in the field of neuroscience as well as in neuroskeletal biology.
Conclusion
The remarkable research progress as it pertains to Nell-1 has been made on numerous fronts in the past decade. Particularly, identifying Cntnap4 as a cell surface receptor of Nell-1 opens new frontiers for Nell-1’s emerging roles in the nervous system. With the establishment of the floxed Nell-1 mouse line, the indispensable roles of Nell-1 in osteochondrogenesis have been further validated. The potent and safe delivery of the modified Nell-1 protein for treating osteoporotic conditions has resulted in the first clinical trial in humans. The significance of Nell-1 in the pathogenesis of osteoarthritis has been recognized in terms of its intrinsic role and anti-inflammatory potential. Moreover, the novel functional properties of Nell-1 in oral-dental tissues are beginning to emerge, although further investigations are needed. Undoubtedly, the Nell-1 conditional knockout system shows great promise in unlocking further findings within and outside the bone field. The clinical applications of Nell-1 therapeutics may be in reach for more indications than just skeletal diseases with the continuous worldwide pursuit from institutional/biotech research communities and pharmaceutical entities.
Author Contributions
C. Li, X. Zhang, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; Z. Zheng, contributed to data acquisition, analysis, and interpretation, drafted the manuscript; A. Nguyen, contributed to data interpretation, drafted and critically revised the manuscript; K. Ting, C. Soo, contributed to conception, design, and data interpretation, critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplemental Material
Supplemental material, DS_10.1177_0022034519882000 for Nell-1 Is a Key Functional Modulator in Osteochondrogenesis and Beyond by C. Li, X. Zhang, Z. Zheng, A. Nguyen, K. Ting and C. Soo in Journal of Dental Research
Footnotes
A supplemental appendix to this article is available online.
This study was financially supported by the National Institutes of Health–National Institute of Arthritis and Musculoskeletal and Skin Diseases (grants R01AR066782 and R01AR068835) and the National Institutes of Health–National Center for Advancing Translational Sciences, University of California–Los Angeles, Clinical and Translational Science Institute (grant UL1TR001881). The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
C.L., X.Z., Z.Z., K.T., and C.S. are inventors of NELL-1-related patents. X.Z., K.T., and C.S. are also founders and/or past board members of Bone Biologics Inc. / Bone Biologics Corp., who sublicense NELL-1 patents from the UC Regents and hold equity in the company. Bone Biologics Inc. / Bone Biologics Corp. did not provide financial support for the current study. All of the other authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
ORCID iD: C. Li  https://orcid.org/0000-0001-6331-6983
https://orcid.org/0000-0001-6331-6983
References
- Agaram NP, Laquaglia MP, Ustun B, Guo T, Wong GC, Socci ND, Maki RG, DeMatteo RP, Besmer P, Antonescu CR. 2008. Molecular characterization of pediatric gastrointestinal stromal tumors. Clin Cancer Res. 14(10):3204–3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askarinam A, James AW, Zara JN, Goyal R, Corselli M, Pan A, Liang P, Chang L, Rackohn T, Stoker D, et al. 2013. Human perivascular stem cells show enhanced osteogenesis and vasculogenesis with Nel-like molecule I protein. Tissue Eng Part A. 19(11-12): 1386–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer Y, Tedrow J, de Bernard S, Birker-Robaczewska M, Gibson KF, Guardela BJ, Hess P, Klenk A, Lindell KO, Poirey S, et al. 2015. A novel genomic signature with translational significance for human idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol. 52(2):217–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokui N, Otani T, Igarashi K, Kaku J, Oda M, Nagaoka T, Seno M, Tatematsu K, Okajima T, Matsuzaki T, et al. 2008. Involvement of MAPK signaling molecules and Runx2 in the NELL1-induced osteoblastic differentiation. FEBS Lett. 582(2):365–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Liu YJ, Shi SG, Chen FM, Cai C, Li B, Wang J, Shi L, Li Y, Liu ZY, et al. 2012. Osteogenic differentiation of human periodontal ligament stem cells expressing lentiviral nel-like protein 1. Int J Mol Med. 30(4):863–869. [DOI] [PubMed] [Google Scholar]
- Chen H, Zhang Z, Zhang L, Wang J, Zhang M, Zhu B. 2018. miR-27a protects human mitral valve interstitial cell from TNF-α-induced inflammatory injury via up-regulation of NELL-1. Braz J Med Biol Res. 51(6):e6997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Yu M, Wang H, Kim JK, Qi H, Ha P, Jiang W, Chen E, Luo X, Needle R, et al. 2019. Cumulative inactivation of Nell-1 in wnt1 expressing cell lineages results in craniofacial skeletal hypoplasia and postnatal hydrocephalus. Cell Death Diff [epub ahead of print 3 Oct 2019] in press. doi: 10.1038/s41418-019-0427-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan CM, Zhang X, James AW, Kim TM, Sun N, Wu B, Ting K, Soo C. 2012. Nell-1 increases pre-osteoblast mineralization using both phosphate transporter Pit1 and Pit2. Biochem Biophys Res Commun. 422(3):351–357. [DOI] [PubMed] [Google Scholar]
- de Luis DA, Almansa R, Aller R, Izaola O, Romero E. 2018. Gene expression analysis identify a metabolic and cell function alterations as a hallmark of obesity without metabolic syndrome in peripheral blood, a pilot study. Clin Nutr. 37(4):1348–1353. [DOI] [PubMed] [Google Scholar]
- Desai J, Shannon ME, Johnson MD, Ruff DW, Hughes LA, Kerley MK, Carpenter DA, Johnson DK, Rinchik EM, Culiat CT. 2006. Nell1-deficient mice have reduced expression of extracellular matrix proteins causing cranial and vertebral defects. Hum Mol Genet. 15(8):1329–1341. [DOI] [PubMed] [Google Scholar]
- Fahmy-Garcia S, van Driel M, Witte-Buoma J, Walles H, van Leeuwen J, van Osch G, Farrell E. 2018. NELL-1, HMGB1, and CCN2 enhance migration and vasculogenesis, but not osteogenic differentiation compared to BMP2. Tissue Eng Part A. 24(3–4):207–218. [DOI] [PubMed] [Google Scholar]
- Fan M, Jiang WX, Wang AY, Peng J, Zhang L, Xu WJ, Lu SB. 2013. Combined effects of NEL-like type 1 gene and zoledronate in preventing collapse of the femoral head [in Chinese]. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 35(5):553–560. [DOI] [PubMed] [Google Scholar]
- Franke A, Hampe J, Rosenstiel P, Becker C, Wagner F, Hasler R, Little RD, Huse K, Ruether A, Balschun T, et al. 2007. Systematic association mapping identifies NELL1 as a novel IBD disease gene. PLoS One. 2(8):e691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao C, Zhang Q, Kong D, Wu D, Su C, Tong J, Chen F, Zhang Q. 2015. MALDI-TOF mass array analysis of Nell-1 promoter methylation patterns in human gastric cancer. Biomed Res Int. 2015:136941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Q, Wang Q, Wu J, Li M, Fang Y, Zhu H, Wang X. 2019. Nell-1 promotes the neural-like differentiation of dental pulp cells. Biochem Biophys Res Commun. 513(2):515–521. [DOI] [PubMed] [Google Scholar]
- James AW, LaChaud G, Shen J, Asatrian G, Nguyen V, Zhang X, Ting K, Soo C. 2016. A review of the clinical side effects of bone morphogenetic protein-2. Tissue Eng Part B Rev. 22(4):284–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James AW, Pan A, Chiang M, Zara JN, Zhang X, Ting K, Soo C. 2011. A new function of Nell-1 protein in repressing adipogenic differentiation. Biochem Biophys Res Commun. 411(1):126–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James AW, Shen J, Tsuei R, Nguyen A, Khadarian K, Meyers CA, Pan HC, Li W, Kwak JH, Asatrian G, et al. 2017. Nell-1 induces Sca-1+ mesenchymal progenitor cell expansion in models of bone maintenance and repair. JCI Insight. 2(12):92573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James AW, Shen J, Zhang X, Asatrian G, Goyal R, Kwak JH, Jiang L, Bengs B, Culiat CT, Turner AS, et al. 2015. Nell-1 in the treatment of osteoporotic bone loss. Nat Commun. 6:7362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Z, Mori Y, Yang J, Sato F, Ito T, Cheng Y, Paun B, Hamilton JP, Kan T, Olaru A, et al. 2007. Hypermethylation of the Nel-like 1 gene is a common and early event and is associated with poor prognosis in early-stage esophageal adenocarcinoma. Oncogene. 26(43):6332–6340. [DOI] [PubMed] [Google Scholar]
- Kraja AT, Borecki IB, Tsai MY, Ordovas JM, Hopkins PN, Lai CQ, Frazier-Wood AC, Straka RJ, Hixson JE, Province MA, et al. 2013. Genetic analysis of 16 NMR-lipoprotein fractions in humans, the GOLDN study. Lipids. 48(2):155–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda S, Tanizawa K. 1999. Involvement of epidermal growth factor-like domain of NELL proteins in the novel protein-protein interaction with protein kinase C. Biochem Biophys Res Commun. 265(3):752–757. [DOI] [PubMed] [Google Scholar]
- Kwak J, Zara JN, Chiang M, Ngo R, Shen J, James AW, Le KM, Moon C, Zhang X, Gou Z, et al. 2013. NELL-1 injection maintains long-bone quantity and quality in an ovariectomy-induced osteoporotic senile rat model. Tiss Eng Part A. 19(3–4):426–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak JH, Zhang Y, Park J, Chen E, Shen J, Chawan C, Tanjaya J, Lee S, Zhang X, Wu BM, et al. 2015. Pharmacokinetics and osteogenic potential of pegylated NELL-1 in vivo after systemic administration. Biomaterials. 57:73-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HS, Lee J, Kim SO, Song JS, Lee JH, Lee SI, Jung HS, Choi BJ. 2013. Comparative gene-expression analysis of the dental follicle and periodontal ligament in humans. PLoS One. 8(12):e84201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M, Siu RK, Ting K, Wu BM. 2010. Effect of Nell-1 delivery on chondrocyte proliferation and cartilaginous extracellular matrix deposition. Tissue Eng Part A. 16(5):1791–1800. [DOI] [PubMed] [Google Scholar]
- Lee S, Wang C, Pan HC, Shrestha S, Meyers C, Ding C, Shen J, Chen E, Lee M, Soo C, et al. 2017. Combining smoothened agonist and Nel-like protein-1 enhances bone healing. Plast Reconstr Surg. 139(6):1385–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Zhang X, Shen J, James AW, Chung CG, Hardy R, Li C, Girgius C, Zhang Y, Stoker D, et al. 2015. Brief report: human perivascular stem cells and Nel-like protein-1 synergistically enhance spinal fusion in osteoporotic rats. Stem Cells. 33(10):3158–3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C. 2019. Thomas M. Graber award of special merit: neural EGFL like 1 as an anti-inflammatory disease-modifying anti-arthritic drug. Paper presented at: 2019 American Association of Orthodontics Annual Session; Los Angeles, CA. [Google Scholar]
- Li C, Jiang J, Zheng Z, Lee KS, Zhou Y, Chen E, Culiat CT, Qiao Y, Chen X, Ting K, et al. 2017. Neural EGFL-like 1 is a downstream regulator of Runt-related transcription factor 2 in chondrogenic differentiation and maturation. Am J Pathol. 187(5):963–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Zheng Z, Ha P, Chen X, Jiang W, Sun S, Chen F, Asatrian G, Berthiaume EA, Kim JK, et al. 2018. Neurexin superfamily cell membrane receptor contactin-associated protein like-4 (Cntnap4) is involved in neural EGFL-like 1 (Nell-1)–responsive osteogenesis. J Bone Miner Res. 33(10):1813–1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Zheng Z, Zhang X, Asatrian G, Chen E, Song R, Culiat C, Ting K, Soo C. 2018. Nfatc1 is a functional transcriptional factor mediating Nell-1-induced Runx3 upregulation in chondrocytes. Int J Mol Sci. 19(1):E168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CS, Zhang X, Peault B, Jiang J, Ting K, Soo C, Zhou YH. 2016. Accelerated chondrogenic differentiation of human perivascular stem cells with Nell-1. Tissue Eng Part A. 22(3–4):272–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Zara JN, Siu RK, Lee M, Aghaloo T, Zhang X, Wu BM, Gertzman AA, Ting K, Soo C. 2011. Nell-1 enhances bone regeneration in a rat critical-sized femoral segmental defect model. Plast Reconstr Surg. 127(2):580–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin E, Kuo PH, Liu YL, Yu YW, Yang AC, Tsai SJ. 2018. A deep learning approach for predicting antidepressant response in major depression using clinical and genetic biomarkers. Front Psychiatry. 9:290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieu F, Etain B, Dizier MH, Lajnef M, Lathrop M, Cabon C, Leboyer M, Henry C, Bellivier F. 2015. Genetics of emotional reactivity in bipolar disorders. J Affect Disord. 188:101–106. [DOI] [PubMed] [Google Scholar]
- Meyers CA, Sun Z, Chang L, Ding C, Lu A, Ting K, Pang S, James AW. 2019. Age dependent effects of NELL-1 isoforms on bone marrow stromal cells. J Orthopaedics. 16(2):175–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura R, Oyama T, Tajiri R, Mizokami A, Namiki M, Nakamoto M, Ooi A. 2015. Expression and regulatory effects on cancer cell behavior of NELL1 and NELL2 in human renal cell carcinoma. Cancer Sci. 106(5):656–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Hasebe A, Takahashi K, Iijima M, Yoshimoto N, Maturana AD, Ting K, Kuroda S, Niimi T. 2014. Oligomerization-induced conformational change in the C-terminal region of Nel-like molecule 1 (NELL1) protein is necessary for the efficient mediation of murine MC3T3-E1 cell adhesion and spreading. J Biol Chem. 289(14):9781–9794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakvasa M, Alverdy A, Mostafa S, Wang E, Fu L, Li A, Oliveira L, Athiviraham A, Lee MJ, Wolf JM, et al. 2017. Neural EGF-like protein 1 (NELL-1): signaling crosstalk in mesenchymal stem cells and applications in regenerative medicine. Genes Dis. 4(3):127–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polo YLBJ, Szczypiorska M, Bartolome N, Campos J, Flores-Robles BJ, Sanz J, Fernandez-Espartero C, Clavaguera T, Andrus RF, Artieda M, et al. 2019. Clinical and genetic characteristics of ankylosing spondylitis patients with peripheral arthritis at disease onset. Clin Exp Rheumatol. 37(2):215–221. [PubMed] [Google Scholar]
- Qi H, Kim JK, Ha P, Chen X, Chen E, Chen Y, Li J, Pan HC, Yu M, Mohazeb Y, et al. 2019. Inactivation of Nell-1 in chondrocytes significantly impedes appendicular skeletogenesis. J Bone Miner Res. 34(3):533–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin XY, Zhao HX, Zhang Q, Chen F, Lin JX. 2016. Nell-1: a novel highly efficient and specific growth factor [in Chinese]. Beijing Da Xue Xue Bao Yi Xue Ban (Journal of Peking University Health Sciences). 48(2):380–383. [PubMed] [Google Scholar]
- Rapa E, Hill SK, Morten KJ, Potter M, Mitchell C. 2012. The over-expression of cell migratory genes in alveolar rhabdomyosarcoma could contribute to metastatic spread. Clin Exp Metastasis. 29(5):419–429. [DOI] [PubMed] [Google Scholar]
- Rudkowska I, Guenard F, Julien P, Couture P, Lemieux S, Barbier O, Calder PC, Minihane AM, Vohl MC. 2014. Genome-wide association study of the plasma triglyceride response to an n-3 polyunsaturated fatty acid supplementation. J Lipid Res. 55(7):1245–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, LaChaud G, Khadarian K, Shrestha S, Zhang X, Soo C, Ting K, Dry SM, James AW. 2015. NELL-1 expression in benign and malignant bone tumors. Biochem Biophys Res Commun. 460(2):368–374. [DOI] [PubMed] [Google Scholar]
- Shen J, LaChaud G, Shrestha S, Asatrian G, Zhang X, Dry SM, Soo C, Ting K, James AW. 2015. NELL-1 expression in tumors of cartilage. J Orthop. 12 Suppl 2:S223–S229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu RK, Zara JN, Hou Y, James AW, Kwak J, Zhang X, Ting K, Wu BM, Soo C, Lee M. 2012. Nell-1 promotes cartilage regeneration in an in vivo rabbit model. Tissue Eng Part A. 18(3–4):252–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slovak ML, Bedell V, Hsu YH, Estrine DB, Nowak NJ, Delioukina ML, Weiss LM, Smith DD, Forman SJ. 2011. Molecular karyotypes of Hodgkin and Reed-Sternberg cells at disease onset reveal distinct copy number alterations in chemosensitive versus refractory Hodgkin lymphoma. Clin Cancer Res. 17(10):3443–3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Imai A, Iijima M, Yoshimoto N, Maturana AD, Kuroda S, Niimi T. 2015. Mapping the heparin-binding site of the osteoinductive protein NELL1 by site-directed mutagenesis. FEBS Lett. 589(24 Pt B):4026–4032. [DOI] [PubMed] [Google Scholar]
- Tang R, Wang Q, Du J, Yang P, Wang X. 2013. Expression and localization of Nell-1 during murine molar development. J Mol Histol. 44(2):175–181. [DOI] [PubMed] [Google Scholar]
- Tanjaya J, Lord EL, Wang C, Zhang Y, Kim JK, Nguyen A, Baik L, Pan HC, Chen E, Kwak JH, et al. 2018. The effects of systemic therapy of PEGylated NEL-like protein 1 (NELL-1) on fracture healing in mice. Am J Pathol. 188(3):715–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanjaya J, Zhang Y, Lee S, Shi J, Chen E, Ang P, Zhang X, Tetradis S, Ting K, Wu B, et al. 2016. Efficacy of intraperitoneal administration of PEGylated NELL-1 for bone formation. BioRes Open Access. 5(1):159–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tham C, Chew M, Soong R, Lim J, Ang M, Tang C, Zhao Y, Ong SY, Liu Y. 2014. Postoperative serum methylation levels of TAC1 and SEPT9 are independent predictors of recurrence and survival of patients with colorectal cancer. Cancer. 120(20):3131–3141. [DOI] [PubMed] [Google Scholar]
- Tian H, Zhao J, Brochmann EJ, Wang JC, Murray SS. 2017. Bone morphogenetic protein-2 and tumor growth: diverse effects and possibilities for therapy. Cytokine Growth Factor Rev. 34:73–91. [DOI] [PubMed] [Google Scholar]
- Ting K, Vastardis H, Mulliken JB, Soo C, Tieu A, Do H, Kwong E, Bertolami CN, Kawamoto H, Kuroda S, et al. 1999. Human NELL-1 expressed in unilateral coronal synostosis. J Bone Miner Res. 14(1):80–89. [DOI] [PubMed] [Google Scholar]
- Tombolan L, Poli E, Martini P, Zin A, Romualdi C, Bisogno G, Lanfranchi G. 2017. NELL1, whose high expression correlates with negative outcomes, has different methylation patterns in alveolar and embryonal rhabdomyosarcoma. Oncotarget. 8(20):33086–33099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Wu Y, Yu H, Jiang L, Fang B, Guo Q. 2018. The effects of NELL on corticotomy-assisted tooth movement and osteogenesis in a rat model. Biomed Mater Eng. 29(6):757–771. [DOI] [PubMed] [Google Scholar]
- Wang C, Hou W, Guo X, Li J, Hu T, Qiu M, Liu S, Mo X, Liu X. 2017. Two-phase electrospinning to incorporate growth factors loaded chitosan nanoparticles into electrospun fibrous scaffolds for bioactivity retention and cartilage regeneration. Mater Sci Eng C Mater Biol Appl. 79:507–515. [DOI] [PubMed] [Google Scholar]
- Wang Z, Kambhampati S, Cheng Y, Ma K, Simsek C, Tieu AH, Abraham JM, Liu X, Prasath V, Duncan M, et al. 2019. Methylation biomarker panel performance in esophacap cytology samples for diagnosing Barrett’s esophagus: a prospective validation study. Clin Cancer Res. 25(7):2127–2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Wang Q, Han Q, Zhu H, Li M, Fang Y, Wang X. 2019. Effects of Nel-like molecule-1 and bone morphogenetic protein 2 combination on rat pulp repair. J Mol Histol. 50(3):253–261. [DOI] [PubMed] [Google Scholar]
- Xia L, Xu Y, Chang Q, Sun X, Zeng D, Zhang W, Zhang X, Zhang Z, Jiang X. 2011. Maxillary sinus floor elevation using BMP-2 and NELL-1 gene-modified bone marrow stromal cells and TCP in rabbits. Calcif Tissue Int. 89(1):53–64. [DOI] [PubMed] [Google Scholar]
- Xue J, Peng J, Yuan M, Wang A, Zhang L, Liu S, Fan M, Wang Y, Xu W, Ting K, et al. 2011. Nell1 promotes high-quality bone regeneration in rat femoral distraction osteogenesis model. Bone. 48(3):485–495. [DOI] [PubMed] [Google Scholar]
- Yamamoto N, Kashiwagi M, Ishihara M, Kojima T, Maturana AD, Kuroda S, Niimi T. 2019. Robo2 contains a cryptic binding site for neural EGFL-like (NELL) protein 1/2. J Biol Chem. 294(12):4693–4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai Y, Wei R, Sha S, Lin C, Wang H, Jiang X, Liu G. 2019. Effect of NELL1 on lung cancer stem-like cell differentiation. Oncol Rep. 41(3):1817–1826. [DOI] [PubMed] [Google Scholar]
- Zhang J, Chen Y, Xu J, Wang J, Li C, Wang L. 2018. Tissue engineering using 3D printed nano-bioactive glass loaded with NELL1 gene for repairing alveolar bone defects. Regen Biomater. 5(4):213–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Cowan CM, Jiang X, Soo C, Miao S, Carpenter D, Wu B, Kuroda S, Ting K. 2006. Nell-1 induces acrania-like cranioskeletal deformities during mouse embryonic development. Lab Invest. 86(7):633–644. [DOI] [PubMed] [Google Scholar]
- Zhang X, Kuroda S, Carpenter D, Nishimura I, Soo C, Moats R, Iida K, Wisner E, Hu FY, Miao S, et al. 2002. Craniosynostosis in transgenic mice overexpressing Nell-1. J Clin Invest. 110(6):861–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Peault B, Chen W, Li W, Corselli M, James AW, Lee M, Siu RK, Shen P, Zheng Z, et al. 2011. The Nell-1 growth factor stimulates bone formation by purified human perivascular cells. Tissue Eng Part A. 17(19-20):2497–2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Ting K, Pathmanathan D, Ko T, Chen W, Chen F, Lee H, James AW, Siu RK, Shen J, et al. 2012. Calvarial cleidocraniodysplasia-like defects with ENU-induced Nell-1 deficiency. J Craniofac Surg. 23(1):61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zara J, Siu RK, Ting K, Soo C. 2010. The role of NELL-1, a growth factor associated with craniosynostosis, in promoting bone regeneration. J Dent Res. 89(9):865–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Qin X, Zhang Q, Zhang X, Lin J, Ting K, Chen F. 2018. Nell-1-ΔE, a novel transcript of Nell-1, inhibits cell migration by interacting with enolase-1. J Cell Biochem. 119(7):5725–5733. [DOI] [PubMed] [Google Scholar]
- Zhu S, Song D, Jiang X, Zhou H, Hu J. 2011. Combined effects of recombinant human BMP-2 and NELL-1 on bone regeneration in rapid distraction osteogenesis of rabbit tibia. Injury. 42(12):1467–1473. [DOI] [PubMed] [Google Scholar]
- Zhu S, Zhang B, Man C, Ma Y, Hu J. 2011. NEL-like molecule-1-modified bone marrow mesenchymal stem cells/poly lactic-co-glycolic acid composite improves repair of large osteochondral defects in mandibular condyle. Osteoarthritis Cartilage. 19(6):743–750. [DOI] [PubMed] [Google Scholar]
- Zou X, Shen J, Chen F, Ting K, Zheng Z, Pang S, Zara JN, Adams JS, Soo C, Zhang X. 2011. NELL-1 binds to APRr3 affecting human osteoblast proliferation and differentiation. FEBS Lett. 585(15):2410–2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, DS_10.1177_0022034519882000 for Nell-1 Is a Key Functional Modulator in Osteochondrogenesis and Beyond by C. Li, X. Zhang, Z. Zheng, A. Nguyen, K. Ting and C. Soo in Journal of Dental Research