Abstract
The maintenance of immune homeostasis requires the delicate balance between response to foreign antigens and tolerance to self. As such, when this balance is disrupted, immunodeficiency or autoimmunity may manifest. The adaptor molecule known as Act1 is a critical mediator of IL-17 receptor family signaling. This chapter will detail the current understanding of Act1’s role in signal transduction as well as address the fundamental role of Act1 in autoimmunity. At the molecular level Act1 interacts with IL-17R through the conserved SEFIR domain, binds TRAF proteins and exerts E3 ubiquitin ligase activity. In in vivo models, Act1 deficiency provides protection against experimental autoimmune diseases, such as colitis and EAE. Yet mice lacking in Act1 develop spontaneous autoimmune diseases. Indeed, the utility of Act1 seems to rely on the specific cell type expression that may determine the pathway that Act1 mediates.
Keywords: Act1, IL-17, IL-17 receptor, Experimental Autoimmune Encephalomyelitis (EAE), IL-25 (IL-17E), Asthma
1. Introduction
Autoimmune diseases result from the imbalance between self-tolerance and immune-reactivity in which auto-reactive lymphocytes that escape from selection cause damage to the body itself (Fry et al. 1989; Rocha and von Boehmer 1991; Carlow et al. 1992). The etiology of autoimmune diseases is complex, has both a genetic and an environmental component, and can be systemic like SLE (systemic lupus erythematous) or organ specific like multiple sclerosis, Sjogren’s disease, and diabetes (Steinman 2001; Cooper et al. 2002; Compston and Coles 2008).
CD4+ T helper cells play essential roles in immune responses and autoimmunity. Classically, CD4+ T helper cells were divided into two lineages, T helper 1 (Th1) and T helper 2 (Th2). Th1 cells secrete IFNγ and promote cellular immunity while Th2 cells secrete IL-4, IL-5, and IL-13 for humoral immunity. Recently, a new lineage of T helper cells that secrete IL-17, termed Th17 cells (Kolls and Linden 2004; Harrington et al. 2005; Park et al. 2005; Iwakura and Ishigame 2006), have been found to play a major role in protection against bacterial and fungal infections (Iwakura et al. 2008; Conti et al. 2009; Curtis and Way 2009). IL-17 is a pro-inflammatory cytokine that up-regulates inflammatory genes expression in various cells such as fibroblasts, endothelial cells, and epithelial cells. More importantly, IL-17 levels were elevated in various autoimmune diseases such as multiple sclerosis, asthma, inflammatory bowel disease, psoriasis, and rheumatoid arthritis (Hemdan et al. 2010; Matusevicius et al. 1999). In this chapter, we will focus on autoimmune diseases mediated by IL-17-producing cells and the current proposed mechanisms of IL-17 signaling.
2. IL-17 Family
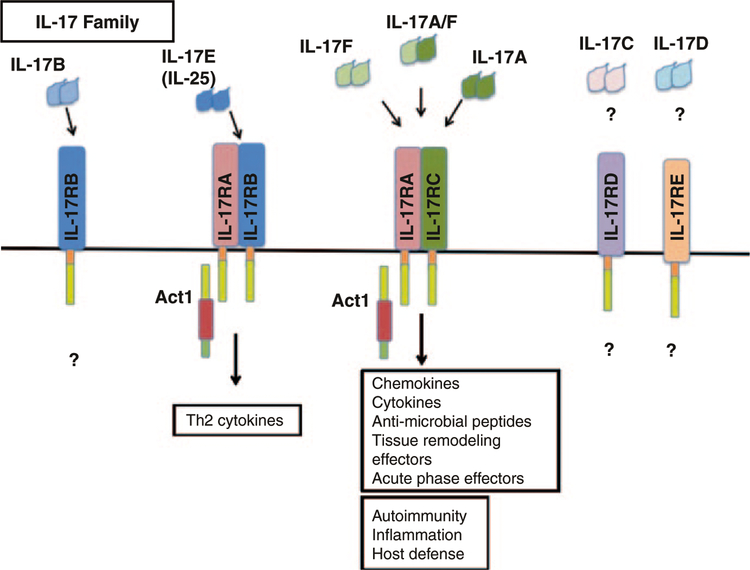
IL-17, also known more specifically as IL-17A, is part of the IL-17 family of six cytokines that includes IL-17B, IL-17C, IL-17D, IL-17E, and IL-17F, all of which share structural similarities. These cytokines are predicted to form homodimeric interactions, or—in the case of IL-17A and IL-17F—heterodimeric interactions prior to signaling on its respective receptors (Fig. 1) (Moseley et al. 2003; Gaffen 2009). Although much less is known of IL-17B-D, they appear to have similar signaling components to IL-17A, IL-17E, and IL-17F. IL-17A and IL-17F share the most homology and are secreted by Th17 cells. In fact, IL-17A and IL-17F can signal through the same receptor. IL-17E, also known as IL-25, is involved in airway inflammation and promotes an allergic response that includes Th2 cytokine production and eosinophilic recruitment (Claudio et al. 2009; Swaidani et al. 2009).
Fig. 1. IL-17 cytokine and receptor family.
The IL-17 cytokine family includes IL-17 A–F, which are predicted to form homo- and hetero-dimeric interactions that are necessary for signaling. There are also five IL-17 receptor subunits, of which IL-17RA, -RC, and -RB are the best described. The receptor subunit, IL-17RA, is common for IL-17A, IL-17F, and IL-17E (IL-25) driven gene expression. IL-17A and IL-17F bind the receptor complex IL-17RA/IL-17RC to drive inflammatory gene expression. IL-25 binds to the IL-17RA/IL-17RB complex to mediate its effects on Th2 homeostasis
There are five known receptors of the IL-17 family (IL-17RA through IL-17RE) (Moseley et al. 2003; Gaffen 2009). Although the pairing of ligand to receptor is not completely clear, we know that IL-17A signals through the IL-17RA/IL-17RC complex while IL-25 signals IL-17RA/IL-17RB complex. Currently, IL-17RA and IL-17RC are found on the surface of many types of cells including epithelial cells, fibroblasts, endothelial cells, astrocytes, and macrophages. Though we know that IL-17RA is expressed on T cells due to the T cells’ ability to respond to IL-17, it is unclear whether T cells also express IL-17RC (O’Connor et al. 2009). IL-25R (formed by IL-17RA/RB) more widely known for its expression on epithelial cells, is also found on the surface of effector T cells (Angkasekwinai et al. 2007) and NKT cells (Terashima et al. 2008; Stock et al. 2009), and can in fact induce the expression of Th2 cytokines (Fort et al. 2001).
3. Discovery of Act1
Under the control of various inflammatory and pathogen derived stimuli, the transcription factor NFκB is a central mediator of gene expression. Upon its activation, NFκB modulates the expression of many target genes including those for cytokines, chemokines, and cell surface receptors among many others. Under normal conditions NFκB is sequestered in the cytoplasm by IkB inhibitory proteins. Upon stimulation with various extracellular stimuli (including Toll-like receptor ligands, IL-1 and TNFα), the IkB protein is phosphorylated by IkB kinase (IKK) thereby releasing NFκB and allowing its translocation to the cell nucleus. The signaling pathways that activate NFκB converge at the IKK complex that is composed of three subunits, the catalytic subunits IKKα and -β and the regulatory subunit IKKγ (Li and Verma 2002).
During a search for NFκB signaling proteins, Act1/CIKS was discovered to activate NFκB. Using an NFκB-dependent selectable marker, Act1 (NFκB activator 1) was discovered due to its ability to activate NFκB (Li et al. 2000). Over-expression of Act1 leads to constitutive activation of NFκB as well as JNK. Furthermore it was shown that Act1 activates IKK through a helix-loop-helix (HLH) domain in its N-terminal portion. Simultaneously, Act1 was also cloned by Leonardi et al. (2000), through yeast two-hybrid screening based on its interaction with IKKg. Likewise, in their study Act1 (referred to as CIKS, connection to IKK and SAPK/JNK) was also found to activate NFκB and JNK.
Initial examination of the amino acid sequence of Act1 revealed that there is an HLH domain, which was functionally important for the interaction with IKK, and two TRAF-binding domains. The TNF-receptor associated factor (TRAF) family consists of six members, TRAF 1–6. Further studies explored the interaction of the TRAF family members with Act1. CD40, a member of the TNF-receptor superfamily, is expressed on CD4 cells, B cells, and epithelial cells. Upon CD40L stimulation the recruitment of TRAF proteins is necessary for NFκB induction. It was found that upon CD40 stimulation, Act1 is recruited to CD40 and that it also interacts with TRAF3 (Qian et al. 2002). One group reported on immunoprecipitation studies with over-expression constructs of Act1 and they reported on its association with TRAF6 through its TRAF-binding domain, however functional data was not explored (Kanamori et al. 2002). The interaction between TRAF6 and Act1 was not fully elucidated until recently.
4. STIR Domain Superfamily
In 2003, Novatchkova et al. reported on the homology of a protein initially described from zebrafish known as similar expression of fibroblast-growth-factor genes, or SEF (Novatchkova et al. 2003). In the zebrafish SEF acts as an inhibitor of FGF signaling. Interestingly, they report that in mammals the closest non-orthologous homologue of SEF is found in the IL-17 family of receptors. The sequence of homology in the cytoplasmic region of IL-17R is referred to as the SEFIR (SEFs and IL-17Rs) domain. What’s more is this search also revealed the SEFIR domain was present in Act1.
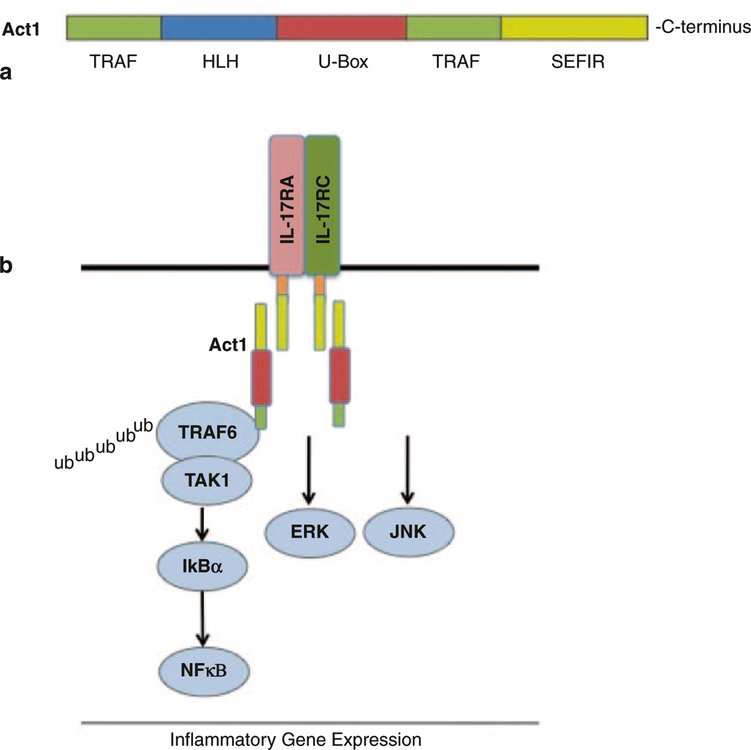
The SEFIR domain is closely related to the TIR (Toll/interleukin-1 receptors) domain expressed in Toll-like and IL-1 receptors. Due to this similarity, the STIR (SEFIR and TIR) domain family as it is referred now, consists of the IL-17 receptor subunits including IL-17RA, IL-17RC, IL-17RB, and Act1. The STIR domain family provided a foundation implicating the possible involvement of Act1 in IL-17R signaling. The Act1 and IL-17 interaction was predicted to be analogous to the involvement of the MyD88 adaptor protein in TIR and IL-1 signaling (Fig. 2).
Fig. 2. Act1 structure and IL-17 signaling cascade.
a The structure of Act1 consists of two TRAF binding domains that mediate TRAF6 interactions following IL-17 stimulation. Moreover, the U-Box E3 ligase domain is functionally important for mediating the ubiquitination of TRAF6. The helix–loop–helix (HLH) domain and SEFIR domain mediate Act1 protein–protein interactions. Furthermore, the SEFIR domain is necessary for Act1 interaction with IL-17R subunits and for IL-17-dependent NFκB activation. b The IL-17A signaling cascade depends on SEFIR–SEFIR domain (shown in yellow) interaction between the IL-17R subunits and Act1. Following this, Act1 exerts its E3 ligase activity by mediated K63-linked ubiquitination of TRAF6, allowing its interaction with TAK1 and subsequent NFκB activation. IL-17 activation of the ERK and JNK pathways are also Act1 dependent, however the exact mechanism leading to their activation is yet to be elucidated
5. Act1 is a U-box Type E3 Ubiquitin Ligase
Ligand-receptor binding is the initiating factor that sets a series of downstream signaling events into action. Signaling events are dependent on protein interactions and specific modifications. Following the identification of the STIR domain superfamily, Act1 was found to be a critical mediator in the IL-17 signaling pathway. Studies conducted by Li and Dong revealed the requirement for Act1 in the IL-17 pathway (Chang et al. 2006; Qian et al. 2007). The structure of Act1 provides insight to the mechanism of signal mediation. Taken together the different domains of Act1—HLH domain at the N-terminus, two TRAF-binding domains, and a coiled-coil domain at its C-terminus—suggest protein–protein interactions. It was indeed shown that Act1 is recruited to IL-17R upon IL-17 stimulation through SEFIR–SEFIR domain interaction, followed by recruitment of the TGFβ Activated Kinase 1 (TAK1) and TRAF6, leading to NFκB activation.
Interestingly, further investigation of Act1 revealed an essential U-box domain that is common in protein E3-ubiquitin ligases. Much like protein phosphorylation, protein ubiquitination is an important modification required for many signaling events. Protein ubiquitination is a sequential process involving the activities of three types of enzymes (E1–E3): the ATP-dependent activity of the ubiquitin-activating enzyme (E1); the acceptance of the activated ubiquitin by the ubiquitin-conjugating enzyme (E2); and an ubiquitin protein ligase (E3), which binds to the E2 and facilitates the conjugation of ubiquitin on the target protein. There are three families of E3 ligases that have been described: RING (really interesting new gene), HECT (homology to E4AP C terminus), and U-box. The conjugation of ubiquitin to different lysine residues can mark proteins for either proteasomal degradation or promote protein–protein interactions if linked on Lys48 or Lys63, respectively.
The study by Liu et al. (2009) examined a region within Act1 from residues 273– 338 that is homologous to the U-box domain in E3-ligases. In vitro assays revealed that Act1 exhibited E3-ubiquitin ligase activity. Further analysis was aimed at determining the substrate for Act1 ligase activity. It was previously reported that IL-17 signaling and activation of NFκB was dependent on TRAF6 (Schwandner et al. 2000). Upon stimulation with IL-17 ubiquitinated forms of TRAF6 are detectable in wild-type cells but not in cells lacking Act1. In vitro ubiquitination assays using TRAF6 as a substrate showed that Act1 catalyzed the Lys63-linked ubiquitination of TRAF6 and that this was dependent on the U-box domain of Act1. Furthermore the ubiquitination of TRAF6 by Act1 was required for the IL-17-dependent activation of NFκB.
The recruitment of Act1 to the IL-17R is through its SEFIR domain and the U-box E3 ligase activity is the second functional domain of Act1. The U-box domain of Act1 is required for IL-17-dependent activation of NFκB that occurs through the ubiquitination of TRAF6. TRAF6 may not be the only molecule that Act1 can exert its E3 ligase activity and this was suggested in the seminal work by Liu et al. (2009). Interestingly in this study it was found that in TRAF6 deficient cells, IL-17-dependent activation of NFκB and JNK was abolished; however, ERK phosphorylation was intact, but this activation was still dependent on the U-box of Act1. These data allude to another downstream component of IL-17-dependent ERK activation that relies on the E3 ligase activity of Act1.
6. Epithelial Requirement of Act1 in IL-17 and IL-25 Mediated Lung Inflammation
Allergic asthma results from a chronic inflammatory response in the lungs with prevailing CD4+ T cells occupying the airways as well as eosinophils and neutrophils, excessive production of mucus and IgE/IgG production. Once activated, the CD4+ T cells differentiate to distinct effector subsets. The CD4+ Th2 cells produce IL-4, IL-5, and IL-13 and act to mediate the humoral and allergic immune responses. In human and in several mouse models of antigen-induced asthma, it is well established that Th2 cells are critical mediators of the immune condition of the lung. However, prior to T cell activation, resident APCs must present antigens detected at the mucosal surface. Therefore, the mucosal surface is not just a passive barrier but is actually required for the orchestration of an appropriate immune response.
Recently, lung epithelium-derived cytokines, IL-25 (IL-17E), IL-33, and TSLP have been found to promote Th2 responses. IL-25 has been demonstrated to promote the differentiation of naïve T cells to effector Th2 cells in an IL-4 and STAT6 dependent manner (Angkasekwinai et al. 2007). Additionally IL-25 can also act directly on the epithelial compartment. It was reported that allergens induce the expression of IL-25 in the epithelium and that increased IL-25 expression can promote Th2 immunity (Angkasekwinai et al. 2007). Importantly, intranasal administration of IL-25 leads to an increase in Th2-driving cytokines—IL-4, IL-5, IL-13, TSLP—and eotaxin and eosinophilia. Although IL-25 is the most divergent IL-17 family member, we and another group found that Act1 interacts with IL-17RB and is required for IL-25-induced responses (Claudio et al. 2009; Swaidani et al. 2009). In the study by Swaidani et al. (2009), Act1 was specifically deleted in the epithelial compartment, which abolished IL-25-induced cytokine production and eosinophilia. Moreover, it is important to note that in human asthmatic tissue, the expression levels of IL-25 as well as IL-25R (IL-17RB) were found to be elevated (Wang et al. 2007). These studies provide insight into the role of IL-25 signaling in epithelium on the initiation and maintenance of allergic responses.
It has also been shown that IL-17 is also involved in allergic airway inflammation. In contrast to IL-25, when IL-17 is injected to the mouse airway there is a dramatic increase in chemokine expression of KC (CXCL1) and IL-6, which is followed by an accumulation of neutrophils. The administration of IL-17 primarily acts on the epithelial compartment as shown by Act1 deletion from the epithelial compartment leading to an abrogated cytokine and neutrophil response.
Besides mediating the direct induction of distinct airway cellularity and cytokine/chemokine production by IL-17 and IL-25, Act1 is also important in antigen-induced asthma. In the asthma challenge model, mice are immunized with OVA and are subsequently challenged two weeks later with OVA aerosol. Mice that are deficient in Act1 in the epithelial compartment have reduced airway eosinophilia/neutrophilia and reduced cytokine/chemokine production. It is important to note that there is no difference in OVA-specific IgE or IgG production or airway hyper-responsiveness in the epithelial-deleted Act1 mouse. This observation may be due to the essential role of Act1 in other immune cell types. Overall, these findings demonstrate the utility of a common signaling component, Act1, and its role in the epithelial compartment. How Act1 mediates the diverse immune response by IL-17 and IL-25 is yet to be determined, but most likely it may be explained by the specific signaling mediated by different receptor subunits.
7. Act1 in CNS Resident Cells
Multiple sclerosis (MS) is generally accepted as an autoimmune disease where pathogenic T cells specific for myelin antigens elicit damage to the central nervous system (CNS), causing demyelination of axons and subsequent axonal damage. The brain and CNS have always been deemed an immune privilege site, an area to which the immune system lacks access. The pathogenesis of MS is thus baffling and the question remains as to how T cells gain access to the CNS. Th17 cells have been proposed to act either on glial cells or directly on endothelial cells to promote blood brain barrier (BBB) breakdown and subsequent Th17 cell access to the CNS (Kebir et al. 2007). Although the exact mechanism of this BBB breakdown is still unknown, recent evidence indicate that different cellular compartments may contribute to disease progression (Benveniste 1997; Chavarria and Alcocer-Varela 2004).
Classically, MS was believed to be mediated by pathogenic Th1 cells, exerting its damage via the secretion of IFNγ. In earlier experiments, scientists hypothesized that if IFNγ is involved in the pathogenesis of this disease, then the disease should be ameliorated when this cytokine is blocked. However, the opposite was seen. In fact, blocking IFNγ actually exacerbated MS disease severity (Billiau 1995). The discovery of the involvement of Th17 cells helped to explain this phenomenon. After years of research, scientists now know that both Th1 and Th17 cells play major roles in the pathogenesis of MS (El-behi et al. 2010; Steinman 2001). It is now believed that Th1 cells act as initiators of MS, whereas Th17 cells serve to promote disease progression. In addition, the two cytokines exhibit regulatory roles on each other: IFNγ inhibits STAT3 expression, which is needed for Th17 differentiation, while IL-17 inhibits the expression of the Th1 signature transcription factor, T-bet (Komiyama et al. 2006; O’Connor et al. 2009). Thus, by blocking IFNγ, the inhibition of STAT3 is lost, allowing Th17 differentiation to occur and disease severity to progress.
In the animal model of MS, experimental autoimmune encephalomyelitis (EAE), mice deficient in IL-17 exhibit less disease severity upon disease induction, again indicating the importance of this cytokine in the pathogenesis of EAE. Likewise, in mice deficient in the IL-17 receptor, mice also exhibit decreased disease severity, indicating the importance of IL-17-mediated signaling in disease pathogenesis (Hu et al. 2010). As indicated earlier, endothelial cells, epithelial cells, astrocytes, macrophages, and neurons all express receptors for IL-17. It seems that in IL-17-mediated EAE, both cells that are producing IL-17 and cells that are responding to IL-17 play major roles in disease progression and pathogenesis. This highlights the importance of the interaction between cells of the immune system with non-immune cells, a delicate balance that helps to maintain homeostasis.
In the absence of Act1, in vitro studies show that IL-17-dependent gene expression of pro-inflammatory cytokines such as KC, IL-6, and MIP-2 is markedly diminished (Qian et al. 2007). In mice deficient in Act1, mice exhibit decreased disease severity upon EAE induction, consistent with the idea that Act1 is essential in IL-17-mediated signaling. Thus, to determine which cellular compartment contributes most to MS pathogenesis, Kang et. al. deleted Act1 in specific cellular compartments. The absence of Act1 in endothelial cells, macrophages, or microglials did not alter the disease process, indicating that IL-17 signaling in these cells do not play a major role in Th17-induced EAE pathogenesis. However, mice with Act1 deleted in neuroectoderm-derived cells exhibit reduced disease severity and delayed onset of disease (Kang et al. 2010). In fact, Act1 deficiency in these cells resulted in decreased immune infiltration in the brain during the disease process, even though the number of Th17 cells remains unchanged compared to wild type mice. This suggests that IL-17 signaling through one (or more) of these cells—astrocytes, oligodendrocytes, or neurons—play a major role in immune cell recruitment and subsequent demyelination and axonal injury. Perhaps it is this feed-forward mechanism through IL-17 signaling in the neuroectoderm-derived cells, resulting in the amplification of inflammatory response that eventually contributes to disease progression.
8. Act1, The Double-Edged Sword
We have provided several lines of evidence that Act1 is necessary for IL-17-mediated inflammatory responses. But what we’ve lacked to mention up to this point is that Act1 deficiency actually leads mice to develop spontaneous autoimmune disease. This observation of spontaneous autoimmune disease in Act1 deficient mice is quite baffling, given that they display resistance to EAE disease induction, an autoimmune disease itself. This intriguing observation adds another layer of complexity to the role of Act1.
In fibroblasts, endothelial cells, epithelial cells, astrocytes, and macrophages, Act1 serves as a component of the IL-17 receptor-signaling cascade. However, in B cells, Act1 serves as a negative regulator of CD40–CD40L and BAFF-BAFFR signaling to control B cell maturation and survival, respectively (Giltiay et al. 2010; Qian et al. 2002, 2004). The loss of Act1 thus results in an increase in B cell population, culminating in splenomegaly, lymphadenopathy, hypergammaglobulinemia, and autoantibody production. In fact, BALB/c mice develop Sjogren-like disease as early as 3 weeks of age, while C57BL/6 mice exhibit autoimmune phenotype by 9 months of age (Qian et al. 2008). This observation of autoimmune phenotype has also been seen in mice with a spontaneous mutation in the Act1 gene (Matsushima et al. 2010).
In addition to the increase in B cell population, loss of Act1 results in an increased number of Th17 cells. The mechanism of this hyper Th17 response is currently unclear and is still under investigation. It is exciting to note that three recent independent cohort studies of psoriasis patients found a genetic mutation in Act1 that predisposes them to develop this autoimmune disease (Ellinghaus et al. 2010; Hüffmeier et al. 2010; Strange et al. 2010). Psoriasis is a skin disease characterized by epidermal hyper-proliferation and chronic inflammation of the skin. Th17 cells have been found to be mediators in psoriasis. Future studies are required to investigate the molecular mechanisms for the precise role of Act1 in modulating Th17 cells and autoimmunity.
These observations in both mouse and human subjects suggest a dual role of Act1 in modulating the immune response. First, as a component in IL-17 and IL-25-mediated signaling cascades, Act1 serves as a positive role in carrying out inflammatory and allergic reactions. Second, as a negative regulator in B cells, Act1 serves as a keeper that prevents hyper B cell functions to eventually lead to autoimmune phenotypes. Third, due to the complexity of the immune system and the intricate interactions between different cells, receptors, and molecules, Act1’s role as a positive effector in one pathway may lead to its indirect role as a negative effector in another pathway. On one hand, loss of Act1 alleviates autoimmune disease, on the other it exacerbates it. The delicate balance of Act1’s role in the immune system reflects the intricate interactions that are necessary to maintain immune homeostasis and regulate a balance between protection against pathogens and tolerance to self.
References
- Angkasekwinai P, Park H, Wang YH, Wang YH, Chang SH, Corry DB, Liu YJ, Zhu Z, Dong C. (2007). Interleukin 25 promotes the initiation of proallergic type 2 responses. J Exp Med 204, 1509–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste EN (1997). Role of macrophages/microglia in multiple sclerosis and experimental allergic encephalomyelitis. J Mol Med 75, 165–173. [DOI] [PubMed] [Google Scholar]
- Billiau A. (1995). Interferons in multiple sclerosis: warnings from experiences. Neurology 45, S50–S53. [DOI] [PubMed] [Google Scholar]
- Carlow DA, Teh SJ, van Oers NS, Miller RG, Teh HS. (1992). Peripheral tolerance through clonal deletion of mature CD4-CD8+ T cells. Int Immunol 4, 599–610. [DOI] [PubMed] [Google Scholar]
- Chang SH, Park H, Dong C. (2006). Act1 adaptor protein is an immediate and essential signaling component of interleukin-17 receptor. J Biol Chem 281, 35603–35607. [DOI] [PubMed] [Google Scholar]
- Chavarria A and Alcocer-Varela J (2004). Is damage in central nervous system due to inflammation? Autoimmun Rev 3, 251–260. [DOI] [PubMed] [Google Scholar]
- Claudio E, Sønder SU, Saret S, Carvalho G, Ramalingam TR, Wynn TA, Chariot A, Garcia-Perganeda A, Leonardi A, Paun A, Chen A, Ren NY, Wang H, Siebenlist U. (2009). The adaptor protein CIKS/Act1 is essential for IL-25-mediated allergic airway inflammation. J Immunol 182, 1617–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compston A and Coles A (2008). Multiple sclerosis. Lancet 372, 1502–1517. [DOI] [PubMed] [Google Scholar]
- Conti HR, Shen F, Nayyar N, Stocum E, Sun JN, Lindemann MJ, Ho AW, Hai JH, Yu JJ, Jung JW, Filler SG, Masso-Welch P, Edgerton M, Gaffen SL. (2009). Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med 206, 299–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper GS, Dooley MA, Treadwell EL, St Clair EW, Gilkeson GS. (2002). Risk factors for development of systemic lupus erythematosus: allergies, infections, and family history. J Clin Epidemiol 55, 982–989. [DOI] [PubMed] [Google Scholar]
- Curtis MM and Way SS (2009). Interleukin-17 in host defence against bacterial, mycobacterial and fungal pathogens. Immunology 126, 177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-behi M, Rostami A, Ciric B. (2010). Current views on the roles of Th1 and Th17 cells in experimental autoimmune encephalomyelitis. J Neuroimmune Pharmacol 5, 189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellinghaus E, Ellinghaus D, Stuart PE, Nair RP, Debrus S, Raelson JV, Belouchi M, Fournier H, Reinhard C, Ding J, Li Y, Tejasvi T, Gudjonsson J, Stoll SW, Voorhees JJ, Lambert S, Weidinger S, Eberlein B, Kunz M, Rahman P, Gladman DD, Gieger C, Wichmann HE, Karlsen TH, Mayr G, Albrecht M, Kabelitz D, Mrowietz U, Abecasis GR, Elder JT, Schreiber S, Weichenthal M, Franke A. (2010). Genome-wide association study identifies a psoriasis susceptibility locus at TRAF3IP2. Nat Genet 42, 991–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fort MM, Cheung J, Yen D, Li J, Zurawski SM, Lo S, Menon S, Clifford T, Hunte B, Lesley R, Muchamuel T, Hurst SD, Zurawski G, Leach MW, Gorman DM, Rennick DM. (2001). IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity 15, 985–995. [DOI] [PubMed] [Google Scholar]
- Fry AM, Jones LA, Kruisbeek AM, Matis LA. (1989). Thymic requirement for clonal deletion during T cell development. Science 246, 1044–1046. [DOI] [PubMed] [Google Scholar]
- Gaffen SL (2009). Structure and signalling in the IL-17 receptor family. Nat Rev Immunol 9, 556–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giltiay NV, Lu Y, Allman D, Jørgensen TN, Li X. (2010). The adaptor molecule Act1 regulates BAFF responsiveness and self-reactive B cell selection during transitional B cell maturation. J Immunol 185, 99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. (2005). Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol 6, 1123–1132. [DOI] [PubMed] [Google Scholar]
- Hemdan NY, Birkenmeier G, Wichmann G, Abu El-Saad AM, Krieger T, Conrad K, Sack U. (2010). Interleukin-17-producing T helper cells in autoimmunity. Autoimmun Rev 9, 785–792. [DOI] [PubMed] [Google Scholar]
- Hu Y, Ota N, Peng I, Refino CJ, Danilenko DM, Caplazi P, Ouyang W. (2010). IL-17RC is required for IL-17A-and IL-17F-dependent signaling and the pathogenesis of experimental autoimmune encephalomyelitis. J Immunol 184, 4307–4316. [DOI] [PubMed] [Google Scholar]
- Hüffmeier U, Uebe S, Ekici AB, Bowes J, Giardina E, Korendowych E, Juneblad K, Apel M, McManus R, Ho P, Bruce IN, Ryan AW, Behrens F, Lascorz J, Böhm B, Traupe H, Lohmann J, Gieger C, Wichmann HE, Herold C, Steffens M, Klareskog L, Wienker TF, Fitzgerald O, Alenius GM, McHugh NJ, Novelli G, Burkhardt H, Barton A, Reis A. (2010). Common variants at TRAF3IP2 are associated with susceptibility to psoriatic arthritis and psoriasis. Nat Genet 42, 996–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwakura Y and Ishigame H (2006). The IL-23/IL-17 axis in inflammation. J Clin Invest 116, 1218–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwakura Y, Nakae S, Saijo S, Ishigame H. (2008). The roles of IL-17A in inflammatory immune responses and host defense against pathogens. Immunol Rev 226, 57–79. [DOI] [PubMed] [Google Scholar]
- Kanamori M, Kai C, Hayashizaki Y, Suzuki H. (2002). NF-kappaB activator Act1 associates with IL-1/Toll pathway adaptor molecule TRAF6. FEBS Lett 532, 241–246. [DOI] [PubMed] [Google Scholar]
- Kang Z, Altuntas CZ, Gulen MF, Liu C, Giltiay N, Qin H, Liu L, Qian W, Ransohoff RM, Bergmann C, Stohlman S, Tuohy VK, Li X. (2010). Astrocyte-restricted ablation of interleukin-17-induced Act1-mediated signaling ameliorates autoimmune encephalomyelitis. Immunity 32, 414–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kebir H, Kreymborg K, Ifergan I, Dodelet-Devillers A, Cayrol R, Bernard M, Giuliani F, Arbour N, Becher B, Prat A. (2007). Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat Med 13, 1173–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolls JK and Linden A (2004). Interleukin-17 family members and inflammation. Immunity 21, 467–476. [DOI] [PubMed] [Google Scholar]
- Komiyama Y, Nakae S, Matsuki T, Nambu A, Ishigame H, Kakuta S, Sudo K, Iwakura Y. (2006). IL-17 plays an important role in the development of experimental autoimmune encephalomy-elitis. J Immunol 177, 566–573. [DOI] [PubMed] [Google Scholar]
- Leonardi A, Chariot A, Claudio E, Cunningham K, Siebenlist U. (2000). CIKS, a connection to Ikappa B kinase and stress-activated protein kinase. Proc Natl Acad Sci U S A 97, 10494–10499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q and Verma IM (2002). NF-kappaB regulation in the immune system. Nat Rev Immunol 2, 725–734. [DOI] [PubMed] [Google Scholar]
- Li X, Commane M, Nie H, Hua X, Chatterjee-Kishore M, Wald D, Haag M, Stark GR. (2000). Act1, an NF-kappa B-activating protein. Proc Natl Acad Sci U S A 97, 10489–10493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Qian W, Qian Y, Giltiay NV, Lu Y, Swaidani S, Misra S, Deng L, Chen ZJ, Li X. (2009). Act1, a U-box E3 ubiquitin ligase for IL-17 signaling. Sci Signal 2, ra63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushima Y, Kikkawa Y, Takada T, Matsuoka K, Seki Y, Yoshida H, Minegishi Y, Karasuyama H, Yonekawa H. (2010). An atopic dermatitis-like skin disease with hyper-IgE-emia develops in mice carrying a spontaneous recessive point mutation in the Traf3ip2 (Act1/CIKS) gene. J Immunol 185, 2340–2349. [DOI] [PubMed] [Google Scholar]
- Matusevicius D, Kivisäkk P, He B, Kostulas N, Ozenci V, Fredrikson S, Link H. (1999). Interleukin-17 mRNA expression in blood and CSF mononuclear cells is augmented in multiple sclerosis. Mult Scler 5, 101–104. [DOI] [PubMed] [Google Scholar]
- Moseley TA, Haudenschild DR, Rose L, Reddi AH. (2003). Interleukin-17 family and IL-17 receptors. Cytokine Growth Factor Rev 14, 155–174. [DOI] [PubMed] [Google Scholar]
- Novatchkova M, Leibbrandt A, Werzowa J, Neubüser A, Eisenhaber F. (2003). The STIR-domain superfamily in signal transduction, development and immunity. Trends Biochem Sci 28, 226–229. [DOI] [PubMed] [Google Scholar]
- O’Connor W Jr, Kamanaka M, Booth CJ, Town T, Nakae S, Iwakura Y, Kolls JK, Flavell RA. (2009). A protective function for interleukin 17A in T cell-mediated intestinal inflammation. Nat Immunol 10, 603–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. (2005). A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol 6, 1133–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Y, Zhao Z, Jiang Z, Li X. (2002). Role of NF kappa B activator Act1 in CD40-mediated signaling in epithelial cells. Proc Natl Acad Sci U S A 99, 9386–9391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Y, Qin J, Cui G, Naramura M, Snow EC, Ware CF, Fairchild RL, Omori SA, Rickert RC, Scott M, Kotzin BL, Li X. (2004). Act1, a negative regulator in CD40-and BAFF-mediated B cell survival. Immunity 21, 575–587. [DOI] [PubMed] [Google Scholar]
- Qian Y, Liu C, Hartupee J, Altuntas CZ, Gulen MF, Jane-Wit D, Xiao J, Lu Y, Giltiay N, Liu J, Kordula T, Zhang QW, Vallance B, Swaidani S, Aronica M, Tuohy VK, Hamilton T, Li X. (2007). The adaptor Act1 is required for interleukin 17-dependent signaling associated with autoimmune and inflammatory disease. Nat Immunol 8, 247–256. [DOI] [PubMed] [Google Scholar]
- Qian Y, Giltiay N, Xiao J, Wang Y, Tian J, Han S, Scott M, Carter R, Jorgensen TN, Li X. (2008). Deficiency of Act1, a critical modulator of B cell function, leads to development of Sjogren’s syndrome. Eur J Immunol 38, 2219–2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha B and von Boehmer H (1991). Peripheral selection of the T cell repertoire. Science 251, 1225–1228. [DOI] [PubMed] [Google Scholar]
- Schwandner R, Yamaguchi K, Cao Z. (2000). Requirement of tumor necrosis factor receptor-associated factor (TRAF)6 in interleukin 17 signal transduction. J Exp Med 191, 1233–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman L. (2001). Multiple sclerosis: a two-stage disease. Nat Immunol 2, 762–764. [DOI] [PubMed] [Google Scholar]
- Stock P, Lombardi V, Kohlrautz V, Akbari O. (2009). Induction of airway hyperreactivity by IL-25 is dependent on a subset of invariant NKT cells expressing IL-17RB. J Immunol 182, 5116–5122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange A, Capon F, Spencer CC, Knight J, Weale ME, Allen MH, Barton A, Band G, Bellenguez C, Bergboer JG, Blackwell JM, Bramon E, Bumpstead SJ, Casas JP, Cork MJ, Corvin A, Deloukas P, Dilthey A, Duncanson A, Edkins S, Estivill X, Fitzgerald O, Freeman C, Giardina E, Gray E, Hofer A, Hüffmeier U, Hunt SE, Irvine AD, Jankowski J, Kirby B, Langford C, Lascorz J, Leman J, Leslie S, Mallbris L, Markus HS, Mathew CG, McLean WH, McManus R, Mössner R, Moutsianas L, Naluai AT, Nestle FO, Novelli G, Onoufriadis A, Palmer CN, Perricone C, Pirinen M, Plomin R, Potter SC, Pujol RM, Rautanen A, Riveira-Munoz E, Ryan AW, Salmhofer W, Samuelsson L, Sawcer SJ, Schalkwijk J, Smith CH, Ståhle M, Su Z, Tazi-Ahnini R, Traupe H, Viswanathan AC, Warren RB, Weger W, Wolk K, Wood N, Worthington J, Young HS, Zeeuwen PL, Hayday A, Burden AD, Griffiths CE, Kere J, Reis A, McVean G, Evans DM, Brown MA, Barker JN, Peltonen L, Donnelly P, Trembath RC. (2010). A genome-wide association study identifies new psoriasis susceptibility loci and an interaction between HLA-C and ERAP1. Nat Genet 42, 985–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaidani S, Bulek K, Kang Z, Liu C, Lu Y, Yin W, Aronica M, Li X. (2009). The critical role of epithelial-derived Act1 in IL-17-and IL-25-mediated pulmonary inflammation. J Immunol 182, 1631–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terashima A, Watarai H, Inoue S, Sekine E, Nakagawa R, Hase K, Iwamura C, Nakajima H, Nakayama T, Taniguchi M. (2008). A novel subset of mouse NKT cells bearing the IL-17 receptor B responds to IL-25 and contributes to airway hyperreactivity. J Exp Med 205, 2727–2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YH, Angkasekwinai P, Lu N, Voo KS, Arima K, Hanabuchi S, Hippe A, Corrigan CJ, Dong C, Homey B, Yao Z, Ying S, Huston DP, Liu YJ. (2007). IL-25 augments type 2 immune responses by enhancing the expansion and functions of TSLP-DC-activated Th2 memory cells. J Exp Med 204, 1837–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]