Abstract
Background
Dermatophytosis is a fungal infectious disease caused by dermatophytes, which produce protease and keratinase to digest keratin, leading to the colonization, invasion, and infection of the stratum corneum of the skin, hair shafts, and nails. Trichophyton interdigitale belongs to Trichophyton mentagrophytes complex, which is the common pathogen causing dermatophytosis. Fungal keratitis, also called keratomycosis, is an infectious disease of cornea.
Case presentation
Here, we report a case of simultaneous dermatophytosis and keratomycosis caused by Trichophyton interdigitale. A 67-year-old man presented with extensive erythema all over the body since 4 years ago, fungal infection of left eye for 2 years, and loss of vision in the eye. These symptoms had become aggravated in the last month. Dermatological examinations showed extensive erythematous plaques with clear borders and scales, scattered red papules with ulceration, and scabs throughout the body. Onychomycosis was observed on the nails of left hand, conjunctival infection with secretion and loss of vision were noted in left eye. Hyaline septate hyphae were observed under direct microscopic examination, fungal culture and internal transcribed spacer sequencing revealed T. interdigitale. Histopathological examination suggested infectious granuloma. A diagnosis of dermatophytosis and keratomycosis caused by T. interdigitale with loss of vision in left eye was made. The patient was treated with luliconazole cream (two applications per day) and itraconazole (100 mg, BID, PO). Complete clinical remission was achieved after 1 month. Subsequently, the patient underwent left eye enucleation in the ophthalmology department.
Conclusions
In the present study, we reported a case of simultaneous dermatophytosis and keratomycosis caused by T. interdigitale, and reviewed the literature on corneal infection caused by Trichophyton. A total of 10 articles with 45 patients were published between 1973 and 2018. The pathogen of 27 patient were identified to species level. There were T. schoenleinii (17), T. mentagrophytes (4), T. verrucosum (3), T. rubrum (1), T. erinacei (1), and T. interdigitale (1). Five patients had corneal trauma, one had contact lens use history. Direct microscopic examination, fungal culture, and analysis of physiological characteristics were the main methods of identification. Early diagnosis and prompt treatment may help improve the management and outcomes.
Keywords: Dermatophytosis, Keratomycosis, Trichophyton interdigitale, Case report
Background
Dermatophytosis is a fungal infectious disease caused by dermatophytes, which produce protease and keratinase to digest keratin, leading to the colonization, invasion, and infection of the stratum corneum of the skin, hair shafts, and nails [1]. Tinea corporis is a common dermatophytosis that involves smooth skin, except for the scalp, hair, palms, nails, and genital area. The risk factors for extensive dermatophytosis include genetic defects [2, 3], chronic diseases [4, 5], immunosuppressive therapy [5, 6], and misdiagnosis or delayed diagnosis [7, 8]. Trichophyton interdigitale is a strictly anthropophilic species that belongs to the Trichophyton mentagrophytes complex, which is the common pathogen causing dermatophytosis [9]..
Fungal keratitis, also called keratomycosis, is an infectious disease of the cornea. Low awareness and delayed diagnosis of this condition lead to complications that can result in permanent loss of vision, and even necessitate enucleation [10, 11].
In the present study, we report a case of simultaneous dermatophytosis and keratomycosis caused by T. interdigitale, which ultimately led to the permanent loss of vision in one eye.
Case presentation
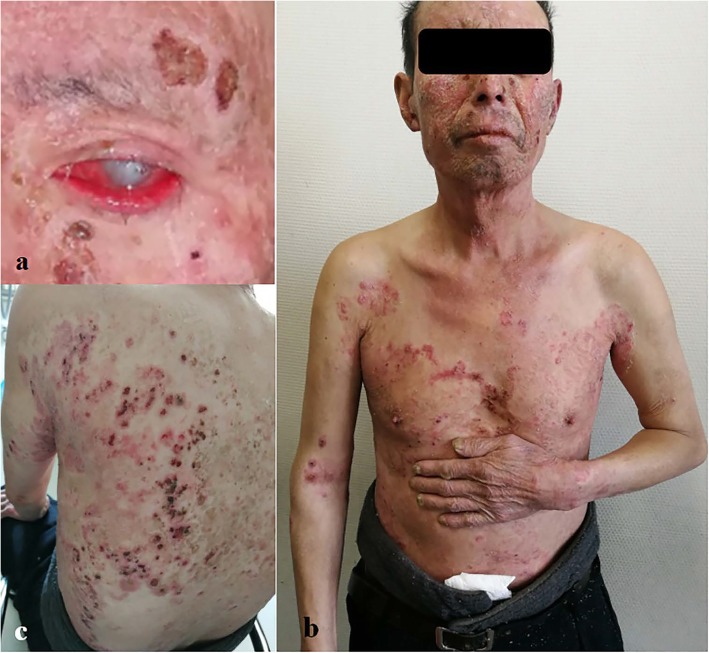
A 67-year-old man was admitted to the dermatology department of our hospital with multiple ringworm lesions on his face, trunk, and limbs. The lesions first appeared within inches of his left eyebrow 4 years ago, and then gradually extended across face, trunk and limbs. Two years ago, he was diagnosed with fungal keratitis at a local hospital. About 1 year ago, he lost the vision in his left eye. Cutaneous symptoms had become aggravated in the last month. Dermatological examinations showed extensive erythematous plaques with clear borders and scales, scattered red papules with ulceration, and scabs throughout the body. Onychomycosis was observed on the nails of his left hand. An ophthalmological examination showed conjunctival infection with secretion, corneal ulcer, and loss of vision in the left eye (Fig. 1). The patient complained of mild itchiness over the lesions and pain in left eye. He had no history of diabetes, eye trauma, or any other significant medical disorders. A history of high-risk behaviors (e.g., multiple sex partners and intravenous drug abuse) for acquired immunodeficiency was not present. He had been diagnosed with fungal keratitis complicated by iridocyclitis in other hospitals, and had received irregular antifungal treatments, such as itraconazole and terbinafine. He had a history of multidrug treatment, including corticosteroid, because of the misdiagnosed with psoriasis and eczema.
Fig. 1.
a Conjunctival infection with secretion, corneal ulcer, and loss of vision in the left eye. b and c Extensive erythematous plaques with clear borders and scales, scattered red papules with ulceration, and scabbing throughout the body; onychomycosis can be observed on the nails of the patient’s left hand
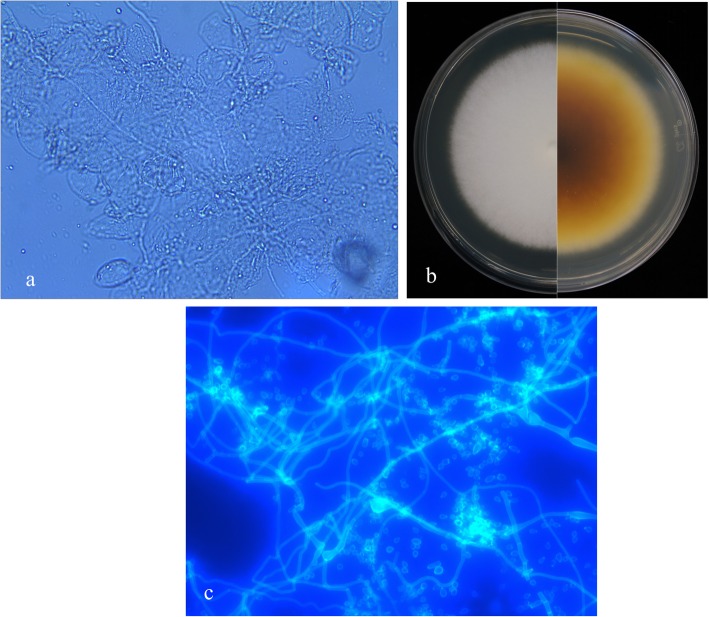
Direct microscopy with 10% potassium hydroxide revealed hyaline septate hyphae (Fig. 2a). Biopsy specimens from the skin lesions, nail and corneal scrapings were inoculated on Sabouraud dextrose agar containing chloromycetin at 28 °C, and white downy colonies grew. These isolates were then subcultured on potato dextrose agar plates, which showed a medium growth rate and produced colonies with white powdery surfaces (Fig. 2b). Slide culture revealed branched septate hyphae and masses of spherical-to-pyriform microconidia (Fig. 2c). For accurate identification, we extracted genomic DNA of the strains and performed polymerase chain reaction (PCR) assays targeting the internal transcribed spacer region (ITS) using primers and amplification conditions described previously [2]. PCR products were sequenced and compared in CBS (www.cbs.knaw.nl) and GenBank database (https://blast.ncbi.nlm.nih.gov/). The ITS sequence of isolates shared 99% identity with the reference sequence for T. interdigitale (CBS 428.63T). The minimum inhibitory concentrations (MICs) of eight antifungal agents (SigmaAldrich, St Louis, MO, USA) were determined using Clinical and Laboratory Standards Institute methodology [12]. T. mentagrophytes (ATCC MYA 4439), Candida parapsilosis (ATCC 22019), and Candida krusei (ATCC 6258) were used as quality controls. The MICs of the antifungal drugs terbinafine, micafungin, caspofungin, posaconazole, voriconazole, itraconazole, fluconazole, and amphotericin B were ≤ 0.03 μg/mL, ≤0.03 μg/mL, 0.25 μg/mL, ≤0.03 μg/mL, 0.06 μg/mL, 0.06 μg/mL, 4 μg/mL, and 1 μg/mL, respectively. QC results were under normal ranges. Histopathological examination of biopsy specimen revealed parakeratosis, mild acanthosis, dense dermal blood vessels, and lymphocyte and plasma cell infiltration (Fig. 3). Laboratory tests revealed decreased levels of IgG (4.07 g/L; normal range: 7.51–15.60 g/L), IgA (0.59 g/L; normal range: 0.82–4.53 g/L), CD3 + CD4+ T-cells (15.6%; normal range: 27.0–57.0%), CD4+/CD8+ T-cells (0.20%; normal range: 1.06–2.66%), increased levels of CD3+ T-cells (96.3%; normal range: 61.0–77.0%) and CD3 + CD8+ T-cells (78.5%; normal range: 14.0–34.0%). The other test results were all within normal ranges or negative. Because of the severe clinical manifestations, the abnormal T-cell subsets and immunoglobulin levels, we suspected a genetic defect in the immune response to fungal infections. After fully explaining his options to the patient and his family, written informed consent from the patient for molecular genetic studies was obtained, according to the rules of the Clinical Research Ethics Committee of the Second Hospital of Jilin University. Genomic DNA was extracted from the peripheral blood of the patient. We analyzed all the exons encoding caspase recruitment domain-containing protein 9 (CARD9) [13] and signal transducer and activator of transcription 3 (STAT3) [14], which have previously been linked to invasive fungal infections. And no disease-causing mutation was found in these two genes.
Fig. 2.
a Direct microscopy showing hyaline septate hyphae. b Potato dextrose agar plates incubated at 28 °C showing medium growth rate and colonies with white powdery surfaces. The bottom of the colonies turned yellowish-brown, while the surface became granular over time. c Slide cultures with calcofluor white stain (× 40) reveal branched septate hyphae and masses of spherical-to-pyriform microconidia
Fig. 3.
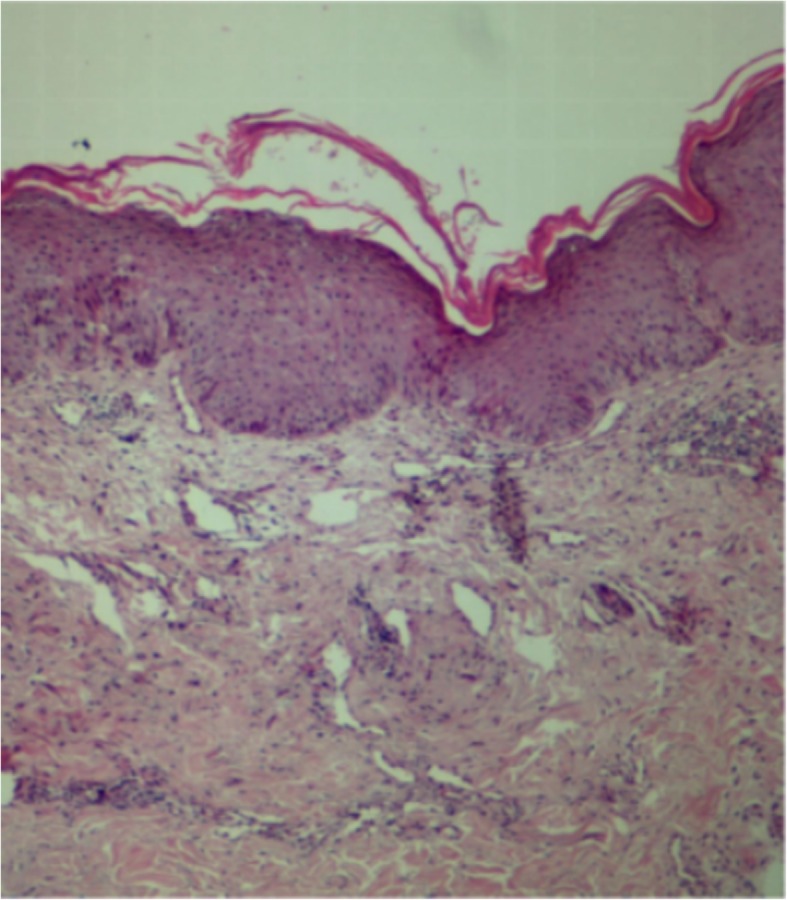
Histopathological examination of a biopsy specimen reveals parakeratosis, mild acanthosis, dense dermal blood vessels, and lymphocyte and plasma cell infiltration (hematoxylin and eosin; × 100)
Diagnosis and treatment
Considering the clinical manifestations and examinations, a diagnosis of dermatophytosis and keratomycosis caused by T. interdigitale with loss of vision in the left eye were made. The patient was treated with luliconazole cream (two applications per day) and itraconazole (100 mg BID, PO) for 1 month. A significant improvement was observed after 14 days (Fig. 4). Subsequently, the patient presented to the ophthalmology department for left eye enucleation. There has been no recurrence during 3 months of follow-up.
Fig. 4.
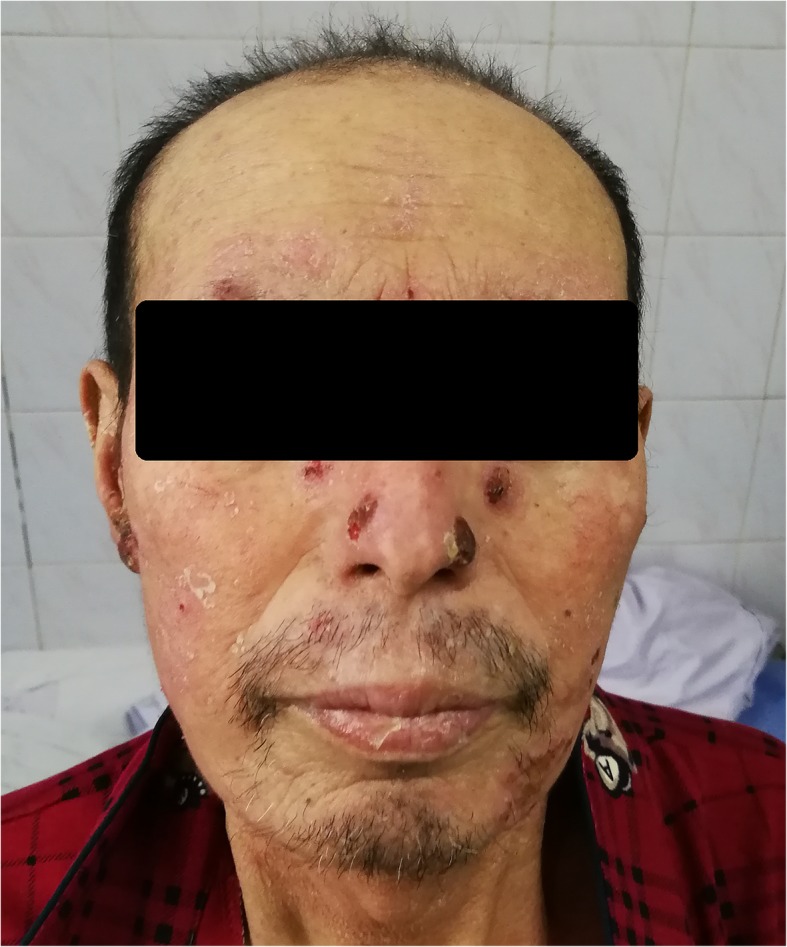
Significant improvement was observed after 14 days
Discussion and conclusions
Tinea corporis is a common type of dermatophytosis that infects smooth skin, except for the scalp, hair, palms, nails, and genital area. When the pathogens invade the stratum corneum of the skin, they cause a mild inflammatory reaction, consisting of erythema, papules, and blisters, followed by ringworm lesions with obvious scales. The source of the infection is typically contact with contaminated items, infected animals [15–18] or spread from an adjacent skin lesion. The risk factors for extensive dermatophytosis involving large parts of the body include genetic defects [2, 3], chronic diseases, such as diabetes, chronic hepatitis, kidney disease, and malignant tumors [4, 5], immunosuppressive therapy, long-term use of corticosteroid [5, 6], and misdiagnosis [7, 8]. The diagnosis of dermatophytosis is mainly based on clinical manifestations and direct microscopic examination. The pathogens can be identified based on culture morphology, physiological characteristics, and molecular sequencing.
T. interdigitale belongs to the division Ascomycota, order Onygenales, family Arthrodermataceae, and genus Trichophyton. It is a strictly anthropophilic species of the T. mentagrophytes complex [9]. Along with T. rubrum, T. interdigitale is a main causative agent of dermatophytosis, and can even cause dermatophytous granuloma [19] and eye infections [20].
Keratomycosis is a vision-threatening corneal fungal infectious disease that occurs all over the world, and is associated with progressive keratolysis, perforation, scleral extension, and endophthalmitis [21]. Poor visual outcomes are correlated to delays in clinical diagnosis, the virulence of fungal organisms, and limitations in effective antifungal agents [10, 11]. Corneal trauma, the injudicious/unreasonable use of corticosteroids or immunosuppressants, immunodeficiency diseases such as diabetes and AIDS, and the overuse of contact lenses are the major risk factors for the development of fungal keratitis [22]. At present, up to 56 genera and 105 pathogenic fungi that can cause fungal keratitis have been identified [23]. Aspergillus, Fusarium, and Candida species remain the most common organisms isolated worldwide [24]. In China, Fusarium is the main pathogen of fungal keratitis, followed by Aspergillus, Penicillium, and Curvularia. Keratomycosis caused by dermatophytes is very rare [20, 25, 26]. Nevertheless, Trichophyton spp. are an important entity implicated in fungal keratitis. Case reports from around the world have designated it as a dangerous pathogen [26]. Correct identification, definite diagnosis, prompt and appropriate clinical management play important roles in improving the prognosis of patients with fungal keratitis [27].
In this paper, we reviewed the literatures on fungal keratitis caused by Trichophyton spp. to help clinicians and researchers recognize that this genus is capable of infecting the eyes, and is a potent etiological agent of fungal keratitis. All published case reports and retrospective analyses on Trichophyton-related fungal keratitis were identified through an extensive search of the PubMed, MEDLINE, CNKI, and Wanfang databases by using different sets of key words, viz. Trichophyton, fungal keratitis, and keratomycosis, in both the English and Chinese. After removing duplicate reports, we had a total of 10 articles with 45 patients, published between 1973 and 2018 (Table 1). In 18 patients, pathogen identification was performed down to the genus level, while in the remaining 27 patients, it was performed down to the species level. Among these 27 patients, 17 patients had infections caused by T. schoenleinii, 4 patients caused by T. mentagrophytes, 3 patients caused by T. verrucosum, and 1 patient each had infections caused by T. rubrum, T. erinacei, and T. interdigitale. Five patients had a clear history of corneal trauma, and one patient had a long history of contact lens use. Direct microscopic examination, fungal culture, and analysis of physiological characteristics were the main methods of identification. In 2001, Tang et al. reported a case of keratitis with left eyelids infection caused by T. mentagrophytes [20], And in 2014, Jin KW et al. reported a case of keratomycosis with onychomycosis [32]. In addition to these two cases, other literature reports were no co-existence of skin or nails infections, or the history is unclear. To the best of our knowledge, this is the first complete case report on simultaneous dermatophytosis and keratomycosis.
Table 1.
Literature review of studies on fungal keratitis caused by Trichophyton spp.
| Year | Place | Species (Numbers of patients) | Cause | Identification method | Co-existence infections | Reference |
|---|---|---|---|---|---|---|
| 1973 | India, Jaipur | Trichophyton spp. (1) | Trauma | – | No | [28] |
| 2001 | Anhui, China | T. mentagrophytes (1) | – | Culture | Eyelids infection | [20] |
| 2003 | Oman | T. mentagrophytes (1) | Trauma | Culture + urease test | No | [22] |
| 2005 | Zhejiang, China | T. mentagrophytes (2); T. verrucosum (1) | – | Culture | Unclear | [27] |
| 2006 | Riyadh, Saudi Arabia | T. schoenleinii (5) | – | Smear + culture + histopathology | No | [29] |
| 2010 | Croatia | Trichophyton spp. (1) | Contact lens use | Culture + histopathology | No | [30] |
| 2011 | Saudi Arabia | Trichophyton spp. (1) | Trauma | Smear + culture + histopathology | No | [31] |
| 2012 | Saudi Arabia | T. schoenleinii (12); T. verrucosum (2); Trichophyton spp. (14) | – | Direct smear + culture | Unclear | [21] |
| 2014 | Delhi, India | T. rubrum (1); T. erinacei (1) | Trauma | Culture + biochemical identification | No | [26] |
| 2014 | Korea | Trichophyton spp. (1) | – | Smear + culture | Onychomycosis | [32] |
| 2018 | Changchun, China | T. interdigitale (1) | – | Smear + culture + ITS sequencing | Dermatophytosis | Present study |
In recent years, with the development of molecular biological technologies, it has been found that species such as T. rubrum and T. violaceum, which are clinically different and very easy to distinguish in culture, are nevertheless molecularly very similar. T. soudanense and T. yaoundei are difficult to distinguish from T. violaceum with standard barcoding genes. Moreover, species that have long been regarded as a single species, have now been identified as a complex consisting of several molecularly similar species, such as the T. rubrum complex and T. mentagrophytes complex [9]. Depending on the type of host, the T. mentagrophytes complex includes the anthropophilic species T. interdigitale and T. tonsurans and the zoophilic species T. mentagrophytes and T. equinum [9]. In line with the latest taxonomic changes, previous clinical isolates of T. mentagrophytes should be renamed T. interdigitale, since the zoophilic T. mentagrophytes rarely infects humans and mainly causes infections in rats and camels [33]. Therefore, we speculate that corneal infections that were reported in the past as having been caused by T. mentagrophytes were in reality caused by the same species reported in the present study.
With the gradual deepening of the research on the relationship between immunodeficiency and fungal infections, an increasing number of studies have confirmed that genetic mutations in the innate immune system may lead to invasive fungal infections. Recent studies have shown that inherited CARD9 [2, 3, 13] and STAT3 [14] mutations predispose to deep dermatophytosis. In our patient, no disease-related mutation was found in the exons of CARD9 or STAT3. Furthermore, none of the patient’s family members showed any symptoms of fungal infection. However, the patient’s T-cell function was abnormal, the existence of acquired immunodeficiency remains to be verified in this patient. The patient has a long history dating back to 4 years before the current visit. Although it was diagnosed as fungal keratitis in a local hospital 2 years ago, the condition was worsened because the patient did not follow the doctor’s advice. In addition, the patient has been misdiagnosed as psoriasis, eczema, and glucocorticoids have been used locally and systematically. Therefore, we speculate that poor economic and sanitary conditions, insufficient attention to the disease, and long-term misdiagnosis and corticosteroid used history were mainly responsible for the prolonged course, extensive lesions and poor prognosis. We repeatedly attempted to elicit a history of corneal trauma from the patient, but he firmly denied it. Since the self-reported primary skin lesion was on the left eyebrow, it is likely that the corneal infection was caused by the spread of the local skin infection.
Trichophyton species have been reported to secrete a variety of proteases and collagenases that hydrolyze keratin, collagen, and gelatin. The cornea and conjunctiva are histopathologically homologous to the epidermis. Although the former two have no horny layer, they can express keratin, and all three structures can be infected by Trichophyton species. Through the secretion of an extracellular collagenase with keratinolytic potential, Trichophyton species can cause severe stromalysis, leading to loss of vision [32]. Any breach of the corneal epithelial cell layer facilitates the penetration of fungi into the stroma. This further damages eye integrity and results in loss of function. Invasion of the anterior chamber heralds the onset of complications, and surgery is often required to eliminate the infection. Few studies have investigated the mechanisms underlying the dermatophyte infection of the human cornea. Hence, further clinical observations and experimental research are needed.
In the present study, we have reported a case of simultaneous dermatophytosis and keratomycosis caused by T. interdigitale, and reviewed the literature on corneal infection caused by Trichophyton species. Early diagnosis and prompt treatment may help improve the management and outcomes. Potassium hydroxide examination is a rapid, simple, and essential investigation for this condition. Mycological culture not only further confirms the diagnosis but also provides credible evidence to correct assertions when the result of potassium hydroxide microscopy is negative. Early diagnosis and aggressive medical treatment are of the utmost importance to improve therapeutic outcomes.
Acknowledgements
Not applicable.
Abbreviations
- CARD9
Caspase recruitment domain-containing protein 9
- ITS
Internal transcribed spacer region
- PCR
Polymerase chain reaction
- STAT3
Signal transducer and activator of transcription 3
Authors’ contributions
LJ and FL, as the clinical physician, analyzed the patient data regarding the Clinical manifestations and laboratory tests, then given the diagnosis and treatment. YX and SL are Responsible for peripheral blood DNA extraction and exon analysis. BW is Mainly responsible for Informed consent and follow-up. MZ performed culture, identification, antifungal-drug sensitivity test and was a major contributor in writing the manuscript. All authors read and approved the final manuscript.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
We obtained written informed consent from the patient for molecular genetic studies, according to the rules of the Clinical Research Ethics Committee of the Second Hospital of Jilin University, protocol number 2018–018.
Consent for publication
Written informed consent for publication of this Case report was obtained from the patient. A copy of the consent form is available for review by the Editor of this journal.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mingrui Zhang, Email: zhangmr16@mails.jlu.edu.cn.
Lanxiang Jiang, Email: jianglanxiang@soho.com.
Fuqiu Li, Phone: 13039123758, Email: lifuqiu1234@126.com.
Yangchun Xu, Email: yangchunxu0701@sina.com.
Sha Lv, Email: tuzicaoye@163.com.
Bing Wang, Email: wangbing513562@163.com.
References
- 1.Ismail MT, Al-Kafri A. Epidemiological survey of dermatophytosis in Damascus, Syria, from 2008 to 2016. Curr Med Mycol. 2016;2:32–36. doi: 10.18869/acadpub.cmm.2.3.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lanternier F, Pathan S, Vincent QB, Liu L, Cypowyj S, Prando C, et al. Deep dermatophytosis and inherited CARD9 deficiency. N Engl J Med. 2013;369:1704–1714. doi: 10.1056/NEJMoa1208487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoshikawa FS, Yabe R, Iwakura Y, de Almeida SR, Saijo S. Dectin-1 and Dectin-2 promote control of the fungal pathogen Trichophyton rubrum independently of IL-17 and adaptive immunity in experimental deep dermatophytosis. Innate Immun. 2016;22:316–324. doi: 10.1177/1753425916645392. [DOI] [PubMed] [Google Scholar]
- 4.Wang E, Zhang X, Zhang Q, Fu Y. A case of systemic lupus erythematosus complicating multiplex tinea corporis. J Pract Dermatol. 2010;3:174–175. [Google Scholar]
- 5.Xiong X, Hou X, Qi W. Clinical analysis of 13 cases of extensive tinea corporis. Chin J Leprosy Skin Dis. 2008;24:435. [Google Scholar]
- 6.Li G, Chen H, Jiang Y, Zeng X. A case of psoriasis vulgaris complicated with extensive tinea corporis. J Clin Dermatol. 2009;38:601. [Google Scholar]
- 7.Gao Y, Gao Z, Ju Q, Li M. Adult tinea capitis and tinea corporis due to Trichophyton violaceum: a case report. Chin J Mycol. 2015;10:297–298. [Google Scholar]
- 8.Liu W, Li X, Tang X, Ye J, Luo Q. A case of extensive tinea corporis. Chin J Dermatovenereol Integr Tradit Western Med. 2013;12:57–58. [Google Scholar]
- 9.Graser Y, Monod M, Bouchara JP, Dukik K, Nenoff P, Kargl A, et al. New insights in dermatophyte research. Med Mycol. 2018;56:2–9. doi: 10.1093/mmy/myx141. [DOI] [PubMed] [Google Scholar]
- 10.Hu LT, Du ZD, Zhao GQ, Jiang N, Lin J, Wang Q, et al. Role of TREM-1 in response to Aspergillus fumigatus infection in corneal epithelial cells. Int Immunopharmacol. 2014;23:288–293. doi: 10.1016/j.intimp.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 11.Li C, Zhao GQ, Che CY, Li N, Lin J, Xu Q, et al. Expression of dectin-1 during fungus infection in human corneal epithelial cells. Int J Ophthalmol. 2014;7:34–37. doi: 10.17816/OV2014134-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Institute CalS. Reference Method for broth dilution antifungal susceptibility testing of filamentous fungi. Approved standard-2nd Edn. In: CLSI Document, vol. M38-A2. Wayne; 2008.
- 13.Glocker EO, Hennigs A, Nabavi M, Schaffer AA, Woellner C, Salzer U, et al. A homozygous CARD9 mutation in a family with susceptibility to fungal infections. N Engl J Med. 2009;361:1727–1735. doi: 10.1056/NEJMoa0810719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simpson JK, Frobel P, Seneviratne SL, Brown M, Lowe DM, Grimbacher B, et al. Invasive dermatophyte infection with Trichophyton interdigitale is associated with prurigo-induced pseudoperforation and a signal transducer and activator of transcription 3 mutation. Br J Dermatol. 2018;179:750–754. doi: 10.1111/bjd.15781. [DOI] [PubMed] [Google Scholar]
- 15.Li J, Yu C, Wang S, Yu Y, Zhang F. A case of extensive tinea corporis complicated with kerion caused by Trichophyton mentagrophytes. Chin J Leprosy Skin Dis. 2018;3:173–174. [Google Scholar]
- 16.Zhu X, Lu H, Liang P. A case of atypical extensive tinea corporis. J Diagn Ther Dermato-venereol. 2015;22:245–246. [Google Scholar]
- 17.Jing D. A case of misdiagnosis of psoriasis with atypical extensive tinea corporis. Chin J Mycol. 2007;2:35. [Google Scholar]
- 18.Li DM, Feng X. A case of extensive tinea corporis caused by Microsporum gallinae. Chin J Mycol. 2007;2:156–157. [Google Scholar]
- 19.Yue X, Wang A, Wan Z, Li R. A case of dermatophyte granuloma. Chin J Mycol. 2007;1:286–287. [Google Scholar]
- 20.Tang H, Hu B, Zhu Y, Gao Y, Wei G, Zhao Z. Deep localized cutaneous infection with Trichophyton mentagrophytes in skin eye: a case report. J Clin Dermatol. 2001;30:123–124. [Google Scholar]
- 21.Alkatan H, Athmanathan S, Canites CC. Incidence and microbiological profile of mycotic keratitis in a tertiary care eye hospital: a retrospective analysis. Saudi J Ophthalmol. 2012;26:217–221. doi: 10.1016/j.sjopt.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shenoy R, Shenoy UA, al Mahrooqui ZH. Keratomycosis due to Trichophyton mentagrophytes. Mycoses. 2003;46:157–158. doi: 10.1046/j.1439-0507.2003.00863.x. [DOI] [PubMed] [Google Scholar]
- 23.Kalkanci A, Ozdek S. Ocular fungal infections. Curr Eye Res. 2011;36:179–189. doi: 10.3109/02713683.2010.533810. [DOI] [PubMed] [Google Scholar]
- 24.Srinivasan M, Gonzales CA, George C, Cevallos V, Mascarenhas JM, Asokan B, et al. Epidemiology and aetiological diagnosis of corneal ulceration in Madurai, South India. Br J Ophthalmol. 1997;81:965–971. doi: 10.1136/bjo.81.11.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang F, Li L, Dou X, Ma L, Fang L, Zhang Q, et al. Adult tinea capitis, tinea corporis and acute corneo-conjunctivits caused by Microsporum canis: a case report. J Clin Dermatol. 2009;38:175–177. [Google Scholar]
- 26.Sharma Y, Jayachandran JS. Keratomycosis: Etiology, Risk Factors and Differential Diagnosis- A Mini Review on Trichophyton spp. J Clin Diagn Res. 2014;8:Dd01–Dd02. doi: 10.7860/JCDR/2014/9029.5044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qiu WY, Yao YF, Zhu YF, Zhang YM, Zhou P, Jin YQ, et al. Fungal spectrum identified by a new slide culture and in vitro drug susceptibility using Etest in fungal keratitis. Curr Eye Res. 2005;30:1113–1120. doi: 10.1080/02713680500423671. [DOI] [PubMed] [Google Scholar]
- 28.Kulshrestha OP, Bhargava S, Dube MK. Keratomycosis: a report of 23 cases. Indian J Ophthalmol. 1973;21:51–55. [PubMed] [Google Scholar]
- 29.Mohammad A, Al-Rajhi A, Wagoner MD. Trichophyton fungal keratitis. Cornea. 2006;25:118–122. doi: 10.1097/01.ico.0000164834.77291.51. [DOI] [PubMed] [Google Scholar]
- 30.Mravicic I, Dekaris I, Gabric N, Romac I, Glavota V, Sviben M. Trichophyton Spp. fungal keratitis in 22 years old female contact lenses wearer. Coll Antropol. 2010;34(Suppl 2):271–274. [PubMed] [Google Scholar]
- 31.Jastaneiah SS, Al-Rajhi AA, Abbott D. Ocular mycosis at a referral center in Saudi Arabia: a 20-year study. Saudi J Ophthalmol. 2011;25:231–238. doi: 10.1016/j.sjopt.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jin KW, Jeon HS, Hyon JY, Wee WR, Suh W, Shin YJ. A case of fungal keratitis and onychomycosis simultaneously infected by Trichophyton species. BMC Ophthalmol. 2014;14:90. doi: 10.1186/1471-2415-14-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Graser Y, Kuijpers AF, Presber W, De Hoog GS. Molecular taxonomy of Trichophyton mentagrophytes and T. tonsurans. Med Mycol. 1999;37:315–330. doi: 10.1046/j.1365-280X.1999.00234.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.