Abstract
Background
Gegen qinlian decoction (GGQLD) is a form of traditional Chinese medicine used for hundreds of years for its efficacy in treating diabetes. However, the mechanisms underlying the therapeutic effects of GGQLD on diabetes are still not clear. We aimed to evaluate the effect of GGQLD on hepatic insulin resistance (IR) through silent information regulator1 (SIRT1)/forkhead box O1 (FOXO1) in an IR mouse model.
Material/Methods
A high-fat diet (HFD) mouse model was established and GGQLD was administrated by oral gavage. Metabolic parameters were detected, including body weights, triglyceride, fasting glucose, fasting insulin and HOMA-IR index, glucose intolerance, and insulin resistance. HE-stained sections were used to observe the histopathology of liver tissue. For in vitro study, GGQLD-medicated serum was used to treat palmitic acid-stimulated HepG2 cells. The glycogen synthesis and downstream SIRT1/FOXO1 signaling pathways were examined. Specific siRNAs were used to knock down SIRT1 in HepG2 cells.
Results
GGQLD administration significantly decreased body weights, triglyceride level, fasting glucose level, fasting insulin level, and HOMA-IR index, and improved IR in HFD mice. GGQLD enhanced SIRT1 expression and suppressed the expression of Ac-FOXO1 in liver tissues. Further, GGQLD-medicated serum promoted SIRT1 upregulation and suppressed Ac-FOXO1 levels in palmitate-stimulated HepG2 cells. GGQLD-medicated serum also increased the protein expression of PPARγ and reduced the expression of FABP4 in palmitate-stimulated HepG2 cells.
Conclusions
We found that GGQLD alleviates insulin resistance through SIRT1-dependent deacetylation of FOXO1.
MeSH Keywords: Diabetes Mellitus, Forkhead Transcription Factors, Hep G2 Cells, Insulin Resistance, Sirtuin 1
Background
Diabetes and its chronic complications have become a global public health problem that seriously affects human health. A recent study has shown that there are 425 million diabetic patients worldwide [1]. Clinical statistics show that over 90% of diabetes cases are type 2 diabetes mellitus (T2DM). Insulin resistance is responsible for the pathogenesis of T2DM and can be found in muscle, adipose tissue, and liver, among which hepatic IR is a key mediator of the progression of T2DM [2,3].
Traditional Chinese medicine has been commonly used in China for millennia and is a subject of increasing global interest. Herbal extracts contain various chemical components that can treat diabetes by targeting various pathological pathways, controlling complex disease systems. Gegen Qinlian Decoction (GGQLD) is a famous prescription in traditional Chinese medicine, which was originally described in “Shanghan Lun” compiled by Zhong-Jing Zhang. GGQLD is formulated from 4 herbs: Radix puerariae, Radix scutellariae, Rhizoma coptidis, and Radix glycyrrhizae. In previous studies, it has been found that various parts of GGQLD and their isolated components were effective in treating diabetes [4–6].
SIRT1, an NAD+-dependent deacetylase, is involved in the improvement of several metabolic diseases. SIRT1 can modulate hepatic glucose metabolism by interacting with PGC1a [7] and promotes fat mobilization [8]. SIRT1 can directly increase insulin sensitivity in the liver [9] and has been closely linked to insulin resistance in many different pathological conditions. SIRT1 can regulate the insulin pathway by enhancing the phosphorylation of insulin receptor [10]. FOXO1 is an important downstream factor of insulin resistance in the progression of T2DM. Under the condition of insulin resistance, the phosphorylation level of FOXO1 decreases, and the inhibition of FOXO1 expression can improve insulin sensitivity of the liver [11]. Multiple studies have suggested a link between FOXO1 and SIRT1 [12]. SIRT1 was shown to regulate the activity of FOXO1 by deacetylating of FOXO1, which promotes the nuclear retention of FOXO1 and maintains the FOXO1-induced signaling pathway [12–15].
GGQLD can treat lipid metabolism and inflammation by stimulating the SIRT1 pathway [16]. The evidence presented above suggests that GGQLD acts through the SIRT1/FOXO1 pathway to regulate liver insulin sensitivity in T2DM. To achieve this, GGQLD was used to treat T2DM in C57BL/6J mice. We used pioglitazone, which can reduce insulin resistance by stimulating PPARγ, as a positive control treatment. Based on multiple parameters such as body weight, triglyceride, fasting glucose, fasting insulin, and HOMA-IR index we found that IR in high-fat diet (HFD) mice was significantly improved by oral GGQLD administration. We also studied the molecular mechanisms using HepG2 cells and found that GGQLD acts on the SIRT1/FOXO1 pathway to improve hepatic insulin resistance.
Material and Methods
Preparation of GGQLD and pioglitazone
All herbs used in the study were purchased from the Affiliated Hospital of Integrated Traditional Chinese and Western Medicine, Nanjing University of Chinese Medicine. GGQLD was composed of 4 herbs – Radix puerariae (batch number: 20170301), Radix scutellariae (batch number: 1610009), Rhizoma coptidis (batch number: 1703015), and Radix glycyrrhizae (batch number: 170109) – at a ratio of 8: 3: 3: 2 (w/w/w/w). The preparation of GGQLD was made according to the method described in the literature [17]. Pioglitazone was purchased from MedChem Express, China (Cat. No: HY-14601) and configured to a suspension with a final concentration of 2 mg/ml. Each medicine was stored at 4°C until use.
Animals
Forty-eight 12-week-old SPF male C57BL/6J mice weighing 18–20 g were obtained from Shanghai Slack Laboratory Animal Co. (Shanghai, China). The guidelines for the use of laboratory animals were followed for all experiments. Animals were maintained in a climate-controlled facility (temperature 22±2°C) with a 12-h light/dark cycle. C57BL/6J mice were split into a normal fat diet (NFD) group (n=12) and an HFD group (n=36). Mice in the NFD group were fed a standard chow diet, while mice in the HFD group were fed a diet containing 60% fat, 14.1% protein, and 25.9% carbohydrate for 6 weeks. HFD group mice were equally divided into 3 groups: an HFD group (n=12) that received saline (10 ml/kg/day), a GGQLD group (n=12) that received GGQLD (10 g/kg/day), and a PIO group (n=12) that received pioglitazone (20 mg/kg/day). The NFD group (n=12) were also administrated with saline (10 ml/kg/day). All these doses were given via oral gavage daily over 8 weeks. At the end of the experiment, 10 mice in each group survived because of gastric administration. Body weight was measured every 2 weeks. Food was removed 2 h before measuring the weight. The Animal Ethics Committee of Jiangsu Province Hospital on Integration of Chinese and Western Medicine approved this study (license No. SCXK (Shanghai) 2012-0002).
Preparation of drug-containing serum
We obtained 8-week-old male Sprague-Dawley rats (200±20 g) from the Comparative Medicine Centre of Yangzhou University (license number: SCXK (Su) 2017-0007) and housed them in an environment with temperature 22±2°C, humidity 55±5%, and a 12-h light/dark cycle. Animals had free access to water and food.
Healthy SD rats were randomly divided into 2 groups: a GGQLD-treated group and a normal saline group (n=25 each). The rats were intragastrically administered GGQLD or saline at 8 a.m. and 8 p.m., respectively, for 4 consecutive days. The administered dosage of GGQLD was 6 ml/100 g/d body weight, at the decoction concentration of 1 g/ml. The administered dosage of normal saline was 10 ml/kg/d.
Abdominal aortic blood samples were taken after the last dose. The serum samples were inactivated using a water bath and sterilized, and then stored at −80°C until use. The serum containing drug was named GGQLD-medicated serum.
Determination of metabolic parameters
IPGTT and IPITT were tested at the 8th week of treatment. We performed IPGTT and IPITT according to methods described in the literature [18]. After mice were sacrificed, blood samples were taken and centrifuged. The serum was stored at −20°C. The levels of serum triglycerides (TG) and plasma insulin were measured by ELISA Assay Kit (ALPCO, USA). Glucose concentrations were determined from the tail vein by glucose monitor.
Histological analysis
Fresh liver tissues were collected upon experiment termination, fixed in 10% formaldehyde, and subjected to paraffin embedding. Paraffin sections then underwent HE staining (Zeping, Beijing, China) to investigate the architecture. An Olympus microscope and digital camera (BX20, Beijing, China) were used to visualize samples with the NIS Element SF 4.00.06 software (Beijing, China). Per group, liver samples from 3–5 mice were prepared, stained, and analyzed.
HPLC-MS analysis
The authentic standards were purchased from Chengdu Biopurity Phytochemicals (Chengdu, China). The contents of puerarin, tables berberine, coptisine hydrochloride, drug root base, daidzein, bar martin, berberine, scutellarin, baicalein, and gutellarin in GGQLD- and GGQLD-medicated serum samples were assessed via LTQ Orbitrap-MS (Thermo Fisher Scientific, CA, USA) coupled with an HPLC model U3000 device (Dionex, CA, USA). Negative ion mode in the 100–1000 m/z mass range was used for full-scan mass spectra acquisition.
Cell culture and treatment
The human hepatocellular carcinoma (HepG-2) cells were from the ATCC and were grown using DMEM containing 10% FBS (Gibco, Carlsbad, CA, USA), penicillin (100 units/mL), and streptomycin (100ug/mL) at 37°C under 5% CO2, in a 95% humidified atmosphere. HepG2 cells were seeded in 6-well plates (3×105 cells/well). After HepG2 cells grew to 60% confluence, they were subjected to 6-h starvation, then treated with or without 0.25 mM palmitic acid (Sigma, USA) or palmitic acid together with GGQLD-medicated serum or palmitate together with pioglitazone for 24 h.
HepG2 cells were transfected with specific siRNA using HiPerFect Transfection Reagent (QIAGEN, Germany). Cells transfected with scrambled siRNA were used as negative control. The sequence of siRNA targeting SIRT1 was 5′-CAGGATTATTGTATTTACGTT-3′. At 48 h after transfection, the cells were treated as detailed above.
RT-PCR assay
After treatment, RNA from mice liver or HepG2 cells were extracted. Absorbance at 260 and 280 nm was used to assess RNA purity and concentration. A ReverTra Ace qPCR RT Kit (Toyobo, Japan) was then used for reverse transcription, followed by RT-PCR reactions based on provided directions. Triplicate samples were analyzed, with GAPDH mRNA used for normalization and relative expression calculated via the 2−ΔΔCt method. Primers used in these analyses are shown in Table 1.
Table 1.
Primers used in qRT-PCR.
| Gene | Primers (5′→3′) |
|---|---|
| SIRT1 | Forward: GAAGTATGACAAAGATGA |
| Reverse: AGAGCTTCTTGGAGACTG | |
| FOXO1 | Forward: ACAATCTGTCCCTACACAG |
| Reverse: AAATTTGCTAAGAGCCGAGAPDH | |
| GAPDH | Forward: CAAGATTGTCAGCAATGCAT |
| Reverse: TCACTGCCACTCAGAAGA C |
Western blot
Proteins were prepared according to the method described in the literature [19]. The supernatants were collected and then quantitated for protein determination using the PIERCE BCA protein assay kit (Thermo Fisher Scientific). Denatured protein samples were separated on 12% SDS-polyacrylamide gels (SDS-PAGE), and transferred to nitrocellulose membranes (0.45 um; Millipore, MA, USA). The membranes were blocked with 5% skimmed milk and incubated with the following primary antibodies overnight at 4°C: anti-SIRT1, anti-FOXO1, anti-Acetylated-Lysine, anti-FABP4, anti-PPARγ, and anti-β-actin. All antibodies were from CST (USA) and were used at a 1: 1000 dilution. Blots were subsequently washed and incubated with the corresponding peroxidase-conjugated secondary antibodies (1: 3000; CST, USA) at room temperature. The target protein bands were scanned and quantified via Gel Doc2000 (Bio-Rad, USA).
Statistical analysis
All data are presented as means±standard deviation (mean±SD). Samples were analyzed via t tests and one-way analyses of variance (ANOVA) for 2 and more than 2 groups, respectively. P<0.05 was the significance threshold.
Results
HPLC Analysis of GGQLD
The major components of GGQLD extract and GGQLD-medicated serum were identified and quantified by HPLC (Figure 1) with standard reference compounds (Figure 1A). The contents of puerarin (RT=3.37 min), tables berberine (RT=6.59 min), coptisine hydrochloride (RT=6.64 min), drug root base (RT=6.78 min), daidzein (RT=7.56 min), bar martin (RT=7.97 min), berberine (RT=8.08 min), scutellarin (RT=8.81 min), baicalein (RT=10.82 min), and gutellarin (RT=14.23 min) in GGQLD extract were identified (Figure 1B). The contents of puerarin (RT=3.37 min), coptisine hydrochloride (RT=6.64 min), daidzein (RT=7.56 min), berberine (RT=8.08 min), scutellarin (RT=8.81 min), baicalein (RT=10.82 min), and gutellarin (RT=14.23 min) were found in GGQLD-medicated serum (Figure 1C), which indicates these contents are the bioactive compounds responsible for the therapeutic effects of GGQLD.
Figure 1.

HPLC-MS chromatograms of GGQLD extracts. (A) The HPLC-MS chromatogram of the standards. (B) The HPLC-MS chromatogram of GGQLD extract. (C) The HPLC-MS chromatogram of GGQLD-medicated serum. Each number indicates a specific component: 1 puerarin; 2 tables berberine; 3 coptisine hydrochloride; 4 drug root base; 5 daidzein; 6 bar martin; 7 berberine; 8 scutellarin; 9 baicalein; 10 gutellarin.
Effect of GGQLD on glycolipid metabolism indexes
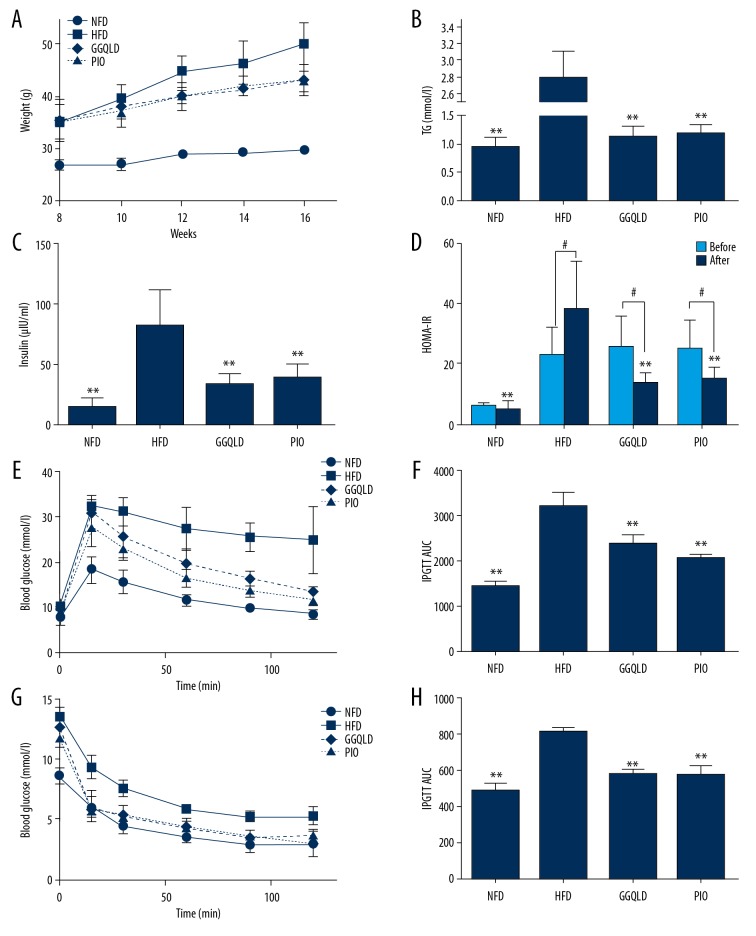
Body weight was monitored once a week during the experimental period. Six weeks later, body weight and HOMA-IR in HFD group were significantly higher than those in the NFD group (Figure 2A, 2B). After further high-fat diet feeding and medication for 8 weeks, GGQLD treatment significantly slowed the weight gain compared with the HFD group (Figure 3A), which was similar to the effect of pioglitazone. The serum levels of triglyceride (TG) and insulin were much higher than those in the NFD group but were decreased in the GGQLD treatment and pioglitazone treatment groups (Figure 3B, 3C). In addition, HOMA-IR, which was markedly increased in the HFD group, was significantly reduced by GGQLD or pioglitazone treatment (Figure 3D). The area under the curve (AUC) of IPGTT and IPITT in the HFD group were significantly increased compared with the NFD group, and GGQLD significantly decreased the AUC compared with that of the HFD group (Figure 3E–3H).
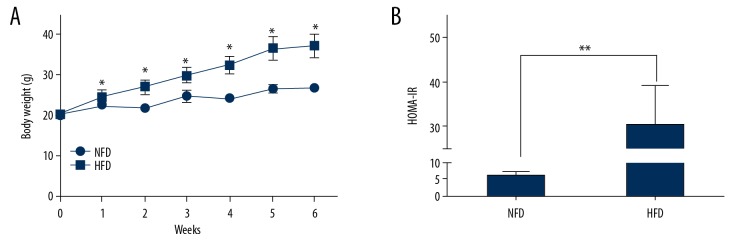
Figure 2.
HFD increased body weight and HOMA-IR. C57BL/6J mice were assigned to either the NFD group (n=12) or the HFD group (n=36) fed with standard chow or HFD, as detailed in Methods. The 2 groups had similar average body weights at the beginning of the experiment. (A) Body weight was measured weekly during the experiment. (B) HOMA-IR was measured in the mice 6 weeks after HFD treatment. * P<0.05 vs. NFD; ** P<0.01 vs. NFD.
Figure 3.
GGQLD improved body weight, glucose and lipid metabolism, and IR in HFD mice. The mice fed a HFD for 6 weeks were subsequently assigned to 3 subgroups (for each subgroup, n=12) with different treatments for an additional 8 weeks (saline-treated, GGQLD-treated, pioglitazone-treated, with pioglitazone used as a positive control). (A) Body weight was measured every 2 weeks during the treatment period. Serum triglyceride (TG) (B), serum insulin (C), and HOMA-IR (D) levels were measured at the end of the experiment. IPGTT (E) and IPITT (G) were determined at the 8th week of the treatment. Area under the curve (AUC) levels of IPGTT (F) and IPITT (H) were calculated. ** P<0.01 vs. HFD, # P<0.05 vs. before.
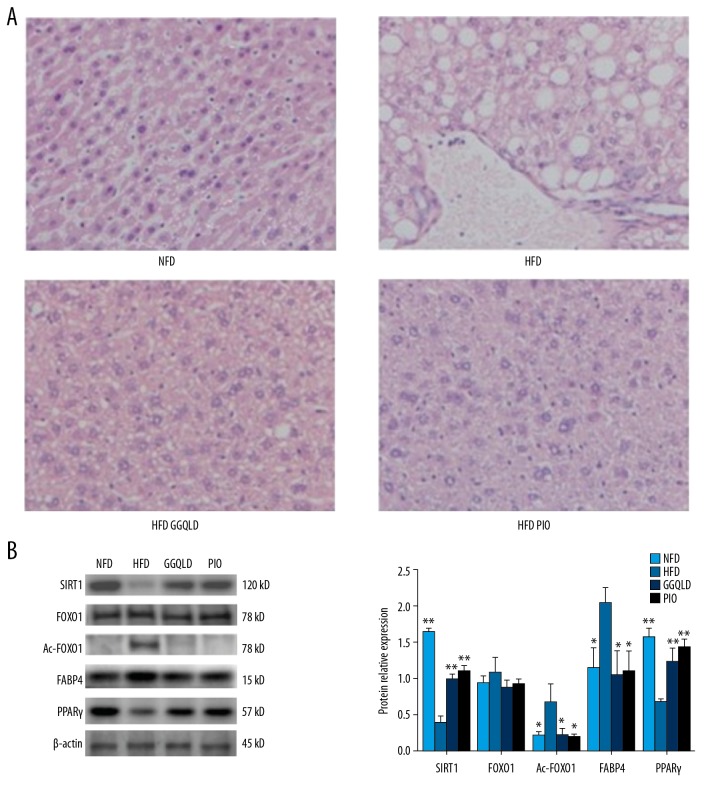
GGQLD attenuated the pathological changes in HFD mouse liver tissues and affected the SIRT1 and Ac-FOXO1 protein expression
A large number of hepatocytes with steatosis and ballooning degeneration were found in the HFD group, while GGQLD or pioglitazone treatment reduced cytoplasmic vacuolation (Figure 4A). The expression of SIRT1 was significantly lower in the HFD group (P<0.01) and the expression of Ac-FOXO1 was greatly increased (P<0.05) compared with the NFD group (Figure 4B). The expression of SIRT1 in the HFD-GGQLD group was higher (P<0.01), and the expression of Ac-FOXO1 was lower (Figure 4B) compared with the HFD group. The expression of PPARγ in the HFD-GGQLD group was much higher (P<0.01), and the expression of FABP4 was lower.
Figure 4.
GGQLD improved histopathology and affected the expression levels of key molecules in the SIRT1/FOXO1 signaling pathway in liver. (A) Liver tissues were subjected to paraffin sectioning and HE staining. For each group, liver samples from 3–5 mice were used. Representative images are shown (×200). (B) Total proteins of liver tissues were extracted and subjected to Western blot analysis for the detection of SIRT1, FOXO1, Ac-FOXO1, FABP4, and PPARγ. Representative images are shown (left). For quantification, optical densities of the bands were determined. β-actin was used as a loading control. Normalized protein expression levels were calculated. Data from 3 independent experiments were used for statistics, and results are expressed as the mean±SEM (right). * P<0.05 vs. HFD, ** P<0.01 vs. HFD.
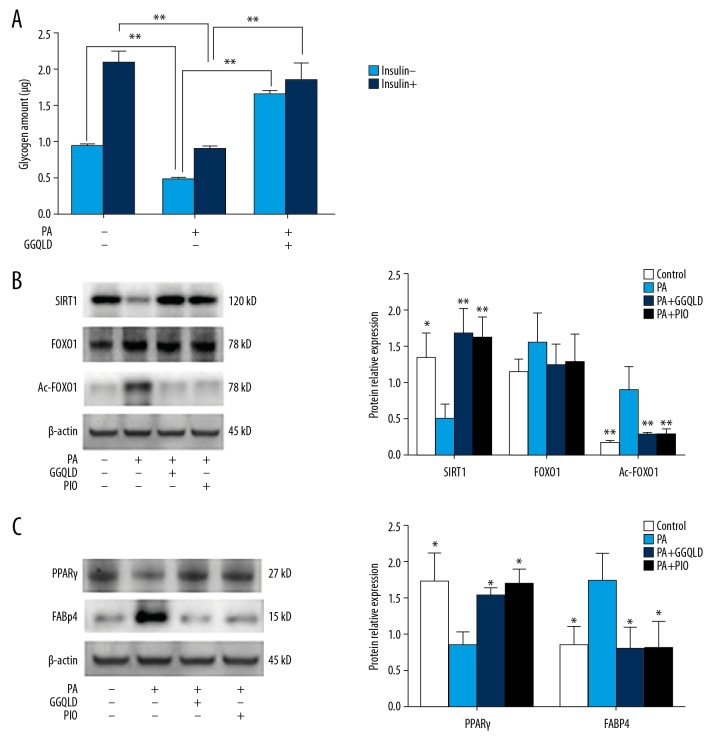
GGQLD-medicated serum promotes glycogen synthesis and inhibits Ac-FOXO1 protein expression
After being treated with palmitic acid, the glycogen synthesis of HepG2 cells decreased significantly, regardless of the addition of insulin. However, the addition of the GGQLD-medicated serum restored the decreased glycogen synthesis of HepG2 cells (Figure 5A). The expression of SIRT1 decreased and the level of Ac-FOXO1 increased significantly after stimulation with palmitic acid (Figure 5B). However, when cotreated with the GGQLD-medicated serum, the expression of SIRT1 was increased and the expression of Ac-FOXO1 was decreased significantly (Figure 5B).
Figure 5.
GGQLD-mediated serum promoted glycogen synthesis and affected the key molecules in the SIRT1/FOXO1 signaling pathway. (A) HepG2 cells were treated with medium, PA alone, or PA + GGQLD-medicated serum with or without the presence of insulin. Then, glycogen synthesis was measured as indicated in Methods. (B) HepG2 cells were treated with medium, PA alone, PA + GGQLD-medicated serum, or PA+PIO. Western blots for SIRT1, FOXO1, and Ac-FOXO1, and (C) for the detection of PPARγ and FABP4. Representative images are shown (B left, C left). For quantification, optical densities of the bands were determined. β-actin was used as loading controls. Normalized protein expression levels were calculated. Data from 3 independent experiments were used for statistics and results were expressed as the mean±SEM (B righ, C right). PA – palmitic acid. PIO – pioglitazone. * P<0.05 vs. HFD, ** P<0.01 vs. HFD or their respective control indicated by line.
Palmitic acid reduced the expression of PPARγ in HepG2 cells, while GGQLD, as well as pioglitazone, increased the expression of PPARγ. The expression of FABP4 was increased after palmitic acid stimulation, and GGQLD or pioglitazone treatment reduced FABP4 expression levels in palmitic acid-treated HepG2 cells (Figure 5C).
GGQLD-medicated serum reduces Ac-FOXO1 by promoting xxpression of SIRT1 in HepG2 cells
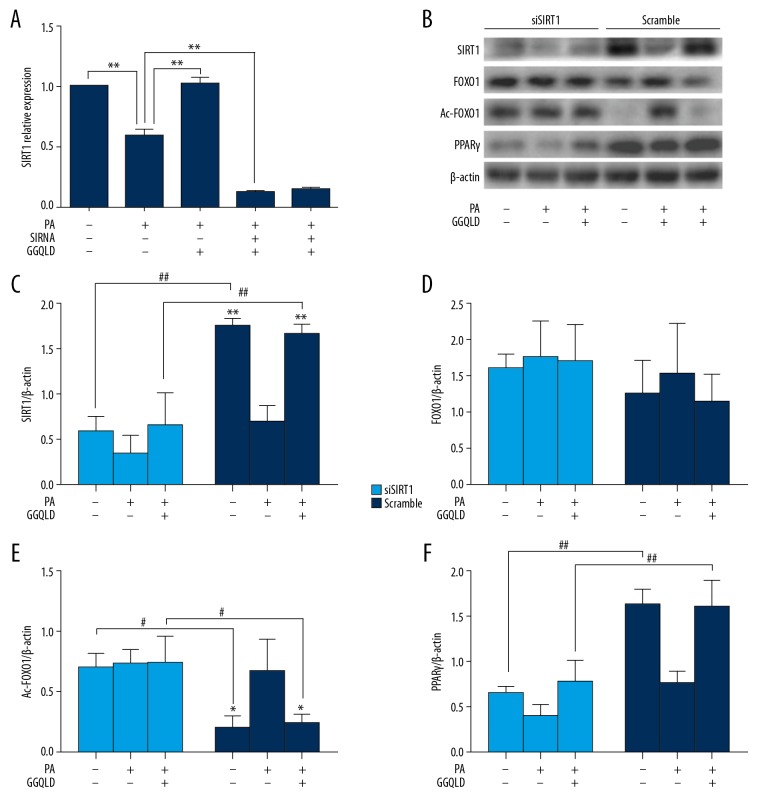
To prove that the increase of SIRT1 mediated the decrease of Ac-FOXO1 in GGQLD-medicated serum-treated HepG2 cells, we silenced SIRT1 by specific siRNAs in HepG2 cells and studied the expression of Ac-FOXO1 and PPARγ in these cells. After the transfection of HepG2 with SIRT1 siRNA, the relative expressions of SIRT1 mRNA and protein in HepG2 cells were significantly reduced (Figure 6A–6C). The Ac-FOXO1 protein level was increased (Figure 6B, 6E) compared with the scrambled siRNA group. Palmitate alone could no longer affect the expression of SIRT1 (Figure 6B, 6D), Ac-FOXO1 (Figure 6B, 6E) and PPARγ (Figure 6B, 6F). What’s more, GGQLD-medicated serum also showed no effects on FOXO1, Ac-FOXO1 and PPARγ (Figure 6B, 6D, 6E, 6F) in SIRT1-silenced HepG2 cells.
Figure 6.
GGQLD-medicated serum functioned through regulating the expression of SIRT1 in palmitate acid-treated HepG2 cells. HepG2 cells were transfected with SIRT1 siRNA or scrambled control siRNA. Then, the cells were treated with medium, PA alone, or PA+GGQLD medicated serum, as indicated. (A) RNAs were extracted and SIRT1 mRNA expression was measured by qRT-PCR. (B) Western blots for SIRT1, FOXO1, Ac-FOXO1, and PPARγ. Representative images are shown. (C–F) For quantification, optical densities of the bands were determined. β-actin was used as loading control. Normalized protein expression levels were calculated. Data from 3 independent experiments were used for statistics, and results are expressed as the mean±SEM. PA – palmitic acid. # P<0.05 vs, Scramble. ** P<0.01 vs. PA alone. ## P<0.01 vs. Scramble.
Discussion
Herein, we investigated the effect of GGQLD on IR in a T2DM mouse model. Animal experiments showed that GGQLD could reduce body weight, decrease triglyceride, and improve glucose metabolism and IR in C57BL/6J mice on HFD. The above results demonstrate that GGQLD indeed has therapeutic effects in treating T2DM.
We also found that the protein expression levels of SIRT1 and PPARγ in the liver tissues of HFD mice were significantly decreased, while GGQLD could restore them. Consistent with this, the protein expression levels of Ac-FOXO1 in the liver tissues of HFD mice were increased, while GGQLD inhibited the expression of Ac-FOXO1. In addition, GGQLD-medicated serum restored the expression of SIRT1 and PPARγ, which was decreased in palmitic acid-treated HepG2 cells.
Accordingly, GGQLD-medicated serum inhibited the level of Ac-FOXO1 in palmitic acid-treated HepG2 cells. We also found that GGQLD counteracted the induction of FABP4, an indicator of insulin resistance [20], by palmitic acid. Therefore, our results demonstrated that, in T2DM, GGQLD enhanced the expression of SIRT1 and subsequently reduced the level of Ac-FOXO1, as did pioglitazone, in vitro and in vivo. PPARγ has the function of regulating insulin sensitivity, and FABP4 plays a key role in the regulation of PPARγ transcription [21]. Our study also demonstrated that GGQLD up-regulates PPARγ by inhibiting FABP4, which further indicated GGQLD relieves insulin resistance and oxidative stress.
A previous study showed that SIRT1 agonist can improve insulin sensitivity in the liver of Zucker fa/fa rats, and the sensitivity of insulin secretion in response to blood glucose was decreased after SIRT1 knockout [22]. To further prove that GGQLD improved hepatic insulin resistance through the SIRT1-FOXO1 signaling pathway, we used siRNA to knock down SIRT1 in HepG2 cells. The results showed that, with SIRT1 being silenced, the expression level of Ac-FOXO1 in HepG2 cells showed no obvious changes when treated with palmitate together with GGQLD-medicated serum compared to palmitate-treated HepG2 cells, thus suggesting that GGQLD functions through regulating the SIRT1-FOXO1 signaling pathway.
Numerous herbs in GGQLD have been believed to benefit insulin resistance, such as berberine, puerarin, and glycyrrhizin [23,24], which may contribute to the therapeutic effects of GGQLD on insulin resistance. Although in the past, GGQLD was widely used to treat diarrhea [25], and previous modern pharmacology studies of the monomer of GGQLD indicated it can improve glucose and lipid metabolism disorder. For example, puerarin can improve mitochondria performance in muscle and promote the oxidation of fatty acids, which thus prevents the accumulation of intramyocellular lipids in diabetic rats [26]. Baicalin can treat obesity and IR via the Akt/AS160/GLUT4 and P38MAPK/PGC1α/GLUT4 pathways [27]. Berberine can also antagonize the increase of blood glucose caused by exogenous glucose, significantly reduce the blood sugar of 4 alloxan diabetic mice, improve the blood glucose and IR of spontaneous diabetic mice, and stimulate the regeneration of islet beta cells [28,29]. The above-mentioned studies indicate that GGQLD may be of benefit in T2D therapy.
Conclusions
In conclusion, our results demonstrate that hepatic IR plays a crucial role in the progression of T2DM, and GGQLD can be used to improve insulin resistance in liver tissues by upregulating SIRT1 and downregulating Ac-FOXO1. GGQLD is a potentially useful medicine for treatment of T2DM.
Footnotes
Source of support: This study was supported by the National Natural Science Foundation of China (No. 81603585)
Conflicts of interest
None.
References
- 1.Cho NH, Shaw JE, Karuranga S, et al. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;38:271–81. doi: 10.1016/j.diabres.2018.02.023. [DOI] [PubMed] [Google Scholar]
- 2.Melmed S, Polonsky K, Larsen PR, et al. Williams textbook of endocrinology. 13th edition. 2017. [Google Scholar]
- 3.Liu Z, Patil IY, Jiang T, et al. High-fat diet induces hepatic insulin resistance and impairment of synaptic plasticity. PLoS One. 2015;10:e0128274. doi: 10.1371/journal.pone.0128274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang C, Chi Y, Li J, et al. FAM3A activates PI3K p110alpha/Akt signaling to ameliorate hepatic gluconeogenesis and lipogenesis. Hepatology. 2014;59:1779–90. doi: 10.1002/hep.26945. [DOI] [PubMed] [Google Scholar]
- 5.Zhang CH, Xu GL, Liu YH, et al. Anti-diabetic activities of Gegen Qinlian Decoction in high-fat diet combined with streptozotocin-induced diabetic rats and in 3T3-L1 adipocytes. Phytomedicine. 2013;20:221–29. doi: 10.1016/j.phymed.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Q, Xu G, Li J, et al. Metabonomic study on the plasma of streptozotocin-induced diabetic rats treated with Ge Gen Qin Lian Decoction by ultra high performance liquid chromatography-mass spectrometry. J Pharm Biomed Anal. 2016;120:175–80. doi: 10.1016/j.jpba.2015.12.031. [DOI] [PubMed] [Google Scholar]
- 7.Li H, Zhao L, Zhang B, et al. A network pharmacology approach to determine active compounds and action mechanisms of ge-gen-qin-lian decoction for treatment of type 2 diabetes. Evid Based Complement Alternat Med. 2014;2014 doi: 10.1155/2014/495840. 495840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodgers JT, Lerin C, Haas W, et al. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434:113–18. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 9.Picard F, Kurtev M, Chung N, et al. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature. 2004;429:771–76. doi: 10.1038/nature02583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao Y, Jiang X, Ma H, et al. SIRT1 and insulin resistance. J Diabetes Complications. 2016;30:178–83. doi: 10.1016/j.jdiacomp.2015.08.022. [DOI] [PubMed] [Google Scholar]
- 11.Sun C, Zhang F, Ge X, et al. SIRT1 improves insulin sensitivity under insulin-resistant conditions by repressing PTP1B. Cell Metab. 2007;6:307–19. doi: 10.1016/j.cmet.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 12.Sin TK, Yung BY, Siu PM. Modulation of SIRT1-Foxo1 signaling axis by resveratrol: Implications in skeletal muscle aging and insulin resistance. Cell Physiol Biochem. 2015;35:541–52. doi: 10.1159/000369718. [DOI] [PubMed] [Google Scholar]
- 13.Li Y, Ma Z, Jiang S, et al. A global perspective on FOXO1 in lipid metabolism and lipid-related diseases. Prog Lipid Res. 2017;66:42–49. doi: 10.1016/j.plipres.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 14.Yang Y, Duan W, Li Y, et al. New role of silent information regulator 1 in cerebral ischemia. Neurobiol Aging. 2013;34:2879–88. doi: 10.1016/j.neurobiolaging.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 15.Sasaki T, Kitamura T. Roles of FoxO1 and Sirt1 in the central regulation of food intake. Endocr J. 2010;57:939–46. doi: 10.1507/endocrj.k10e-320. [DOI] [PubMed] [Google Scholar]
- 16.Daitoku H, Hatta M, Matsuzaki H, et al. Silent information regulator 2 potentiates Foxo1-mediated transcription through its deacetylase activity. Proc Natl Acad Sci USA. 2004;101:10042–47. doi: 10.1073/pnas.0400593101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi Z, Li Z, Zhang S, et al. Subzero-temperature liquid-liquid extraction coupled with UPLC-MS-MS for the simultaneous determination of 12 bioactive components in traditional Chinese medicine Gegen-Qinlian Decoction. J Chromatogr Sci. 2015;53:1407–13. doi: 10.1093/chromsci/bmu226. [DOI] [PubMed] [Google Scholar]
- 18.Guo Y, Li JX, Mao TY, et al. Targeting Sirt1 in a rat model of high-fat diet-induced non-alcoholic fatty liver disease: Comparison of Gegen Qinlian decoction and resveratrol. Exp Ther Med. 2017;14:4279–87. doi: 10.3892/etm.2017.5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maeda N, Shimomura I, Kishida K, et al. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med. 2002;8:731–37. doi: 10.1038/nm724. [DOI] [PubMed] [Google Scholar]
- 20.Jiang X, Zhou H, Shi M, et al. Bu-shen-zhu-yun decoction promotes synthesis and secretion of FSHbeta and LHbeta in anterior pituitary cells in vitro. Biomed Pharmacother. 2018;102:494–501. doi: 10.1016/j.biopha.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 21.Bosquet A, Guaita-Esteruelas S, Saavedra P, et al. Exogenous FABP4 induces endoplasmic reticulum stress in HepG2 liver cells. Atherosclerosis. 2016;249:191–99. doi: 10.1016/j.atherosclerosis.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 22.Ayers SD, Nedrow KL, Gillilan RE, et al. Continuous nucleocytoplasmic shuttling underlies transcriptional activation of PPARgamma by FABP4. Biochemistry. 2007;46:6744–52. doi: 10.1021/bi700047a. [DOI] [PubMed] [Google Scholar]
- 23.Luu L, Dai FF, Prentice KJ, et al. The loss of Sirt1 in mouse pancreatic beta cells impairs insulin secretion by disrupting glucose sensing. Diabetologia. 2013;56:2010–20. doi: 10.1007/s00125-013-2946-5. [DOI] [PubMed] [Google Scholar]
- 24.Ghosh N, Ghosh R, Mandal V, et al. Recent advances in herbal medicine for treatment of liver diseases. Pharm Biol. 2011;49:970–88. doi: 10.3109/13880209.2011.558515. [DOI] [PubMed] [Google Scholar]
- 25.Zheng P, Ji G, Ma Z, et al. Therapeutic effect of puerarin on non-alcoholic rat fatty liver by improving leptin signal transduction through JAK2/STAT3 pathways. Am J Chin Med. 2009;37:69–83. doi: 10.1142/S0192415X09006692. [DOI] [PubMed] [Google Scholar]
- 26.Ling X, Xiang Y, Tang Q, et al. Comparative pharmacokinetics of eight major bioactive components in normal and bacterial diarrhea mini-pigs after oral administration of Gegen Qinlian Decoction. J Chromatogr B Analyt Technol Biomed Life Sci. 2017;1044–45:132–41. doi: 10.1016/j.jchromb.2017.01.015. [DOI] [PubMed] [Google Scholar]
- 27.Chen XF, Wang L, Wu YZ, et al. Effect of puerarin in promoting fatty acid oxidation by increasing mitochondrial oxidative capacity and biogenesis in skeletal muscle in diabetic rats. Nutr Diabetes. 2018;8:1. doi: 10.1038/s41387-017-0009-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fang P, Yu M, Zhang L, et al. Baicalin against obesity and insulin resistance through activation of AKT/AS160/GLUT4 pathway. Mol Cell Endocrinol. 2017;448:77–86. doi: 10.1016/j.mce.2017.03.027. [DOI] [PubMed] [Google Scholar]
- 29.Chen C, Zhang Y, Huang C. Berberine inhibits PTP1B activity and mimics insulin action. Biochem Biophys Res Commun. 2010;397:543–47. doi: 10.1016/j.bbrc.2010.05.153. [DOI] [PubMed] [Google Scholar]