Abstract
Background
Eriocalyxin B (EriB), a diterpenoid isolated from the plant Isodon eriocalyx, has been shown to possess anti-tumor properties. However, few systematic studies of the mechanism underlying the anti-tumor activity of Eriocalyxin B in prostate cancer cells have been published. The aim of this study was to investigate the effect of Eriocalyxin B on prostate cancer cells.
Material/Methods
In the present study, the PC-3 (androgen-independent) and 22RV1 (androgen-dependent) human prostate cancer cell lines were cultured with and without increasing doses of Eriocalyxin B. MTT assay was used to measure cell viability. Western blotting was performed to measure levels of proteins associated with apoptosis and autophagy. Flow cytometry was used to assess changes in cell apoptosis and cycle. Fluorescence microscopy was used to capture images of autophagy-related proteins.
Results
Treatment of human prostate cancer cells with Eriocalyxin B resulted in apoptosis in a dose- and time-dependent manner. Eriocalyxin B also induced autophagy, with elevated LC3B-II protein expression and punctuate patterns. Additionally, autophagy protected prostate cancer cells from apoptosis induced by Eriocalyxin B, which was demonstrated by addition of chloroquine (CQ). Moreover, the results indicated that Eriocalyxin B could inhibit the phosphorylation of Akt and mTOR. Eriocalyxin B induced apoptosis and autophagy by inhibition of the Akt/mTOR pathway.
Conclusions
Eriocalyxin B induces apoptosis and autophagy involving the Akt/mTOR pathway in prostate cancer cells in vitro. These findings provide evidence for Eriocalyxin B as a potent therapeutic for the treatment of prostate cancer.
MeSH Keywords: Apoptosis, Autophagy, Cell Cycle Checkpoints, Prostatic Neoplasms, Proto-Oncogene Proteins c-akt, TOR Serine-Threonine Kinases
Background
Prostate cancer (PCa) is the most commonly diagnosed malignancy in men and is a leading cause of death in male patients. Radical prostatectomy (RP) is considered to be the criterion standard of surgical treatment [1]. However, 15–30% of patients experience a biochemical recurrence after definitive local therapy [2]. Many patients will relapse despite local salvage therapy, with elevated blood levels of prostate-specific antigen (PSA). Therefore, it is necessary to find new treatments or complementary therapeutic methods for prostate cancer.
Autophagy is an important intracellular process that induces degradation of unnecessary or damaged cytoplasmic contents to sustain metabolism and homeostasis [3]. It is characterized by emergence of double-membrane vesicles called autophagosomes [4]. Autophagy is usually considered to be a critical process in protein and organelle quality control. Previous studies have demonstrated that autophagy plays a dual role in neoplastic development [5,6]. Whether autophagy inhibits cancer development or promotes cancer progression seems to depend on the different cell types and conditions of stress [7]. It can function as a suppressor in the early stage of carcinoma and as a promoter in the later stage [8]. Hence, it is important to explore the mechanisms of autophagy in cancer progression, as they may reveal novel means for cancer treatment.
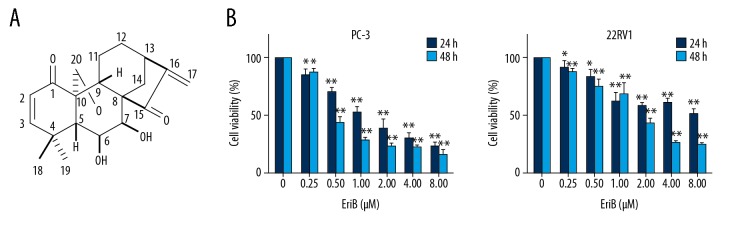
Eriocalyxin B (EriB) (Figure 1A) was isolated from the leaves of the plant Isodon eriocalyx var. Laxiflora, a perennial herb of the Labiatae family, which was used as an anti-bacterial and anti-inflammatory agent [9]. Recent studies show that EriB can suppress the growth of several cancer cell lines. EriB induces apoptosis of SMMC-7721 hepatocellular carcinoma cells through inhibition of NF-κB signaling [10]. In addition, the ability of EriB to downregulate JAK2/STAT3 signaling pathway activation was demonstrated in colon cancer cells [11]. Moreover, EriB-induced apoptosis is associated with NF-κB inactivation and disturbance of the MAPK pathway in t(8;21) leukemia cells, without affecting the proliferation of normal hematopoietic progenitor cells [12].
Figure 1.
Inhibitory effect induced by EriB on cell viability of human prostate cancer cell lines. (A) Eriocalyxin B chemical structure. (B) Various concentrations (0.25–8.0 μM) of EriB-treated PC-3 and 22RV1 cells for 24 and 48 h, after which MTT assay was used to measure cell proliferation rate.
In this study, we first investigated the effect of EriB on prostate cancer cells. The results indicated that EriB had cytostatic potential against prostate cancer cells. We further investigated the underlying mechanisms, and the results suggested that EriB induced apoptosis and autophagy in prostate cancer cells via the Akt/mTOR pathway.
Material and Methods
Materials
Ultrapure water (pH 6.7; Milli-Q, Bedford, MA, USA) was used in all experiments. Eriocalyxin B (purity >98%) (B30248) was purchased from Shanghai YuanYe Biotechnology Co. Thiazolyl blue tetrazolium bromide (MTT, T0793) was purchased from Bio Basic. Antibody against LC3 (NB100-2220, 1: 2000 dilution for WB) was purchased from Novus Biologicals (Colorado, USA). Antibody against GAPDH (E-AB-20059, 1: 2000 dilution for use) was purchased from Elabscience (Wuhan, China). Antibody against Caspase-3 (AC033, 1: 1000 dilution for WB) was purchased from Beyotime (Shanghai, China). Antibodies against Caspase-8 (9746T, 1: 1000 dilution for WB), cleaved Caspase-3 (9664T, 1: 1000 dilution for WB), mTOR (2983, 1: 1000 dilution for WB), p-mTOR (5536T, 1: 1000 dilution for WB), Akt (9272S, 1: 1000 dilution for WB), and p-Akt (9271S, 1: 1000 dilution for WB) were bought from Cell Signaling (Danvers, MA, USA). Antibodies against PARP (AB012801, 1: 2000 dilution for WB) and cleaved PARP (AB012901, 1: 2000 dilution for WB) were purchased from Anhui Duoneng Biotechnology Co. HRP-conjugated anti-rabbit antibody (W4011) was purchased from Promega (Madison, WI, USA). Enhanced chemiluminescence (ECL) kits were purchased from Biological Industries (Kibbutz Beit Haemek, Israel). Chloroquine (C6628) and 3-Methyladenine (M9281) were purchased from Sigma Aldrich (St. Louis, MO). Goat Anti-Rabbit IgG H&L (Alexa Fluor® 488) (ab150077) was purchased from Abcam (Cambridge, UK).
Cell culture
Human prostate cancer cells (PC-3 and 22RV1), obtained from Cell Bank (Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, Shanghai) were grown at 37°C in an atmosphere of 5% CO2 in RPMI-1640 culture medium containing 10% (v/v) fetal bovine serum, 100 U/ml penicillin, and 10 U/ml streptomycin.
MTT assay
To assess the inhibitory effects of EriB, MTT assay was performed to detect the viability of prostate cancer cells. Prostate cancer cells were grown in 96-well plates and incubated for 24 h. Then, they were treated with various concentrations (0.25–8 μM) of EriB. Cells were incubated for 24 h or 48 h at 37°C. Subsequently, 20 μl of MTT solution (5 mg/ml) was added to each well, followed by incubation at 37°C for 4 h. After the medium was removed, 150 μl of DMSO was added to each well to dissolve the formed formazan crystals. Cell viability was measured at 490 nm using a spectrometer (Elx800, BioTek, Winooski, VT, USA).
Western blotting
Cells were harvested and washed twice in ice-cold PBS. RIPA buffer with protease and phosphatase inhibitors was used to extract the total proteins on ice. An equal amount of protein was placed in each lane and separated by electrophoresis on an SDS-polyacrylamide gel, then transferred to a PVDF membrane. After blocking with 5% BSA for 1 h, the PVDF membrane was incubated overnight at 4°C with primary antibodies, then washed with TBST and incubated for 1 h with an HRP-conjugated secondary antibody. Protein bands were visualized by reaction with an enhanced chemiluminescence (ECL) kit.
Apoptosis analysis by flow cytometry
The AnnexinV-FITC/PI apoptosis kit (Vazyme Biotech Co.) was used to assess cell apoptosis. Cells were first collected and then washed twice in ice-cold PBS, then resuspended in 100 ul binding buffer. We added 5 μl Annexin V-FITC and 5 μl PI staining solution to the suspension for 10 min at room temperature in the dark. We added 400 μl binding buffer to each sample, followed by detection by flow cytometry (Becton Dickinson FACSCanto II, USA) within 1 h.
Cell cycle analysis by flow cytometry
The Cell Cycle Analysis kit (shanghai BestBio Biotechnology Co.) was used to assess the cell cycle. Cells were first collected and then washed twice in ice-cold PBS. Ice-cold 70% (v/v) ethanol was used to fix cells at 4°C overnight. Finally, each sample was resuspended in 500 ul PBS with 400 ul PI and 20 ul RNase A, then incubated at 37°C for 30 min in the dark. The samples were analyzed by flow cytometry.
Immunofluorescence analysis
Cells were seeded on glass bottom dishes and treated with EriB. After removing the media, cells were fixed with 4% paraformaldehyde and permeabilized with 0.1%Triton X-100, then incubated overnight with rabbit anti-LC3 antibody at 4°C. Goat Anti-Rabbit IgG H&L was then applied to cells for 1 h in the dark at room temperature. The nucleus was stained with DAPI. Fluorescence images were captured using fluorescence microscopy (Olympus TH4-200, Tokyo, Japan).
Statistical analysis
All statistical analyses were carried out using SPSS 17.0 statistical software (IBM, Armonk, NY, USA) and Prism 7.0 software (GraphPad Software, San Diego, CA, USA). Significant differences between the groups were determined using one-way ANOVA by Bonferroni post hoc analysis. All data are expressed as mean ±SEM. * P<0.05 and ** P<0.01 were considered statistically significant.
Results
EriB suppressed proliferation of prostate cancer cells
The anti-proliferative effect of EriB was first assessed. Prostate cancer cells were treated with various concentrations of EriB for 24 h and 48 h. Cell viability was tested by MTT assay. As shown in Figure 1B, EriB suppressed the growth of PC-3 cells and 22RV1 cells in a dose- and time-dependent manner. After 24–48 h of treatment, the IC50 of EriB in PC-3 cells was 0.46–0.88 μM and the IC50 of EriB in 22RV1 cells was 1.20–3.26 μM. PC-3 cells were more sensitive to EriB than were 22RV1 cells. Hence, EriB concentrations of 0.5 μM (PC-3) and 2 μM (22RV1) were used for the following experiments.
EriB induced apoptosis of prostate cancer cells
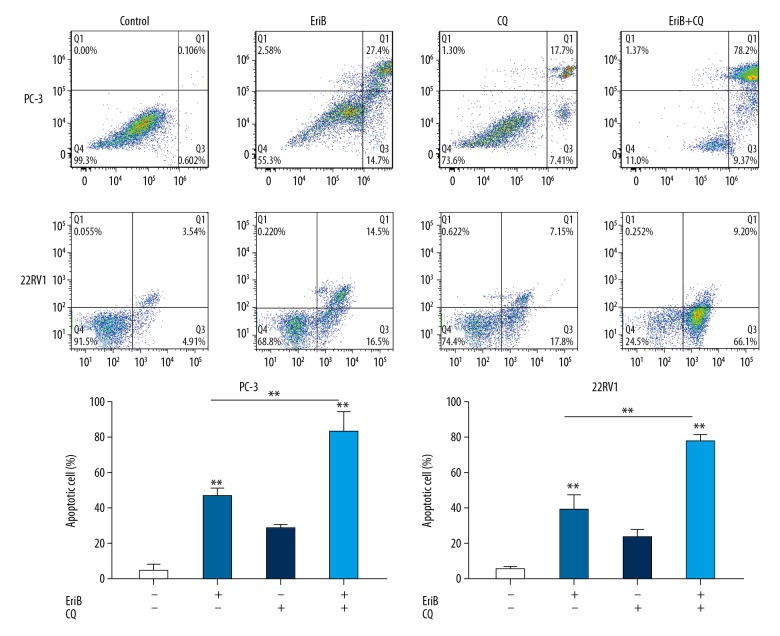
To confirm whether these cells underwent apoptosis in response to EriB, Annexin V/PI staining was performed using flow cytometry analyses. Following treatment with EriB for 48 h, 0.5 μM EriB increased the percentage of early and late apoptotic cells from 0.7% to 42.1% in PC-3 cells, and the apoptosis percentage of 22RV1 cells in the 2-μM EriB group was 31% (Figure 2).
Figure 2.
Apoptosis induced by EriB alone or cotreatment with autophagy inhibitor. EriB-treated PC-3 cells (0.5 μM, 48 h) and 22RV1 cells (2 μM, 48 h). We added 50 μM CQ to culture medium for the last 6 h. Cell apoptosis was assessed by flow cytometry with Annexin V/PI staining.
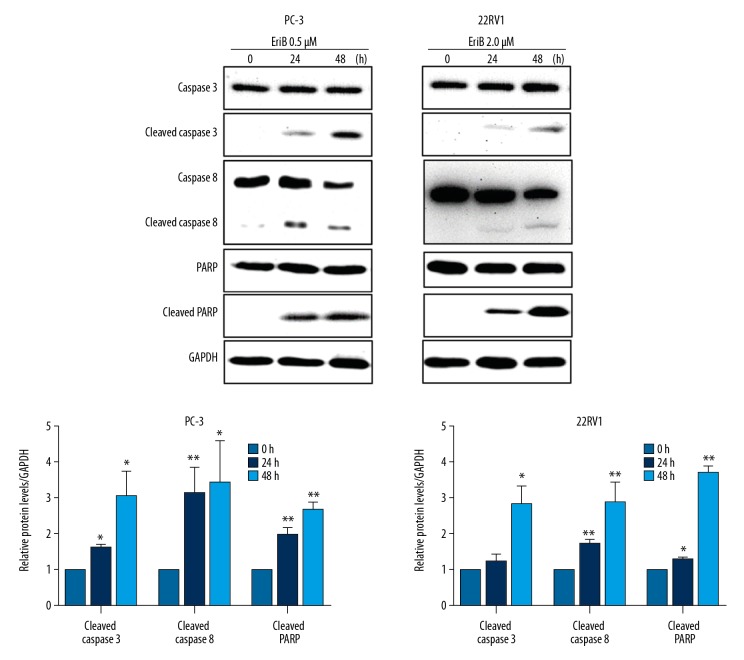
To explore the molecular mechanism of EriB-induced apoptosis, we performed Western blot analysis to assess apoptosis-related proteins. As shown in Figure 3, compared to the vehicle control, higher levels of cleaved caspase-3, caspase-8, and PARP were observed in EriB-treated prostate cancer cells. The results demonstrated that EriB-induced apoptosis is caspase-dependent.
Figure 3.
Apoptosis induced by EriB in human prostate cancer cell lines. PC-3 and 22RV1 cells treated with 0.5 and 2 μM of EriB, respectively, for 24 and 48 h. We assessed caspase-3, caspase-8, and PARP levels by Western blot.
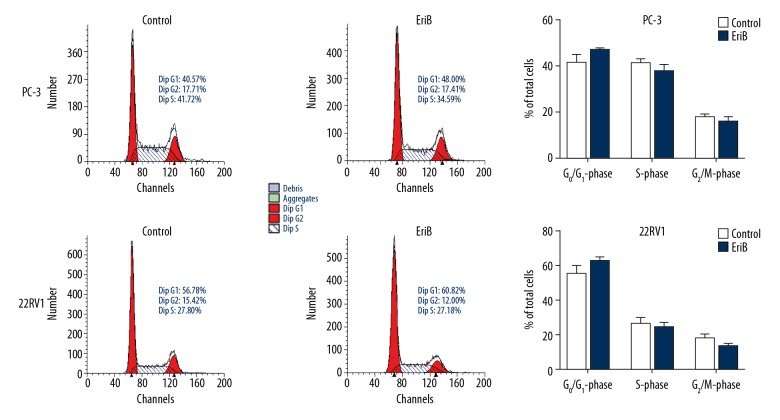
EriB did not induce cell cycle arrest in prostate cancer cells
A previous study indicated that EriB could arrest the cell cycle of SW1116 cells [11]. We analyzed the cell cycle distribution of PC-3 and 22RV1 cells by flow cytometry. The results indicated that EriB had no significant effect on the cell cycle following 48-h treatment (Figure 4).
Figure 4.
Effect of EriB on the cell cycle distribution of human prostate cancer cell lines. PC-3 and 22RV1 cells treated with 0.5 and 2 μM of EriB, respectively, for 48 h. Various phases of the cell cycle were analyzed by flow cytometry.
EriB induced autophagy in prostate cancer cells
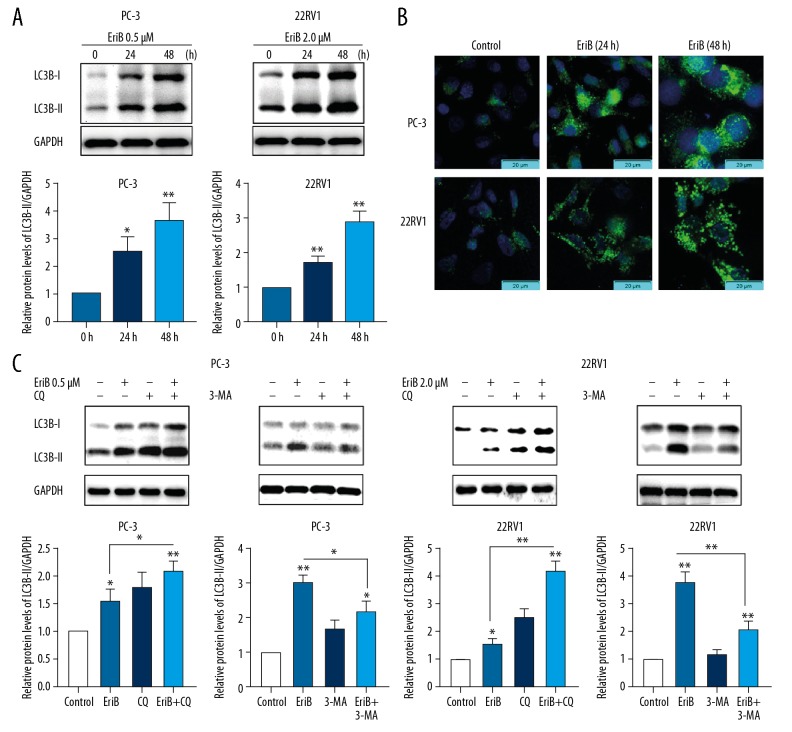
To investigate whether EriB regulated autophagy, the membrane-bound form of LC3 (LC3-II) was examined. LC3-II is an important marker of autophagy [13]. Figure 5 shows that EriB upregulated protein expression levels and punctuate patterns of LC3B-II, indicating that EriB regulated autophagy.
Figure 5.
Regulation by EriB alone or EriB with autophagy inhibitor on the expression of LC3B in human prostate cancer cell lines. (A) PC-3 and 22RV1 cells treated with 0.5 and 2 μM of EriB, respectively, for 24 and 48 h. The proteins of LC3B were detected by Western blot. (B) After staining endogenous LC3 proteins and nucleus with anti-LC3 antibody (FITC, green) and DAPI (blue) respectively. The images of LC3 distribution were captured under a fluorescence microscope. (C) PC-3 and 22RV1 cells were treated with 0.5 and 2 μM, respectively, of EriB for 48 h. We added 50 μM CQ or 5 MM 3-MA to culture medium for the last 6 h. The proteins of LC3B were detected by Western blot.
The increase of autophagosome formation and the suppression of autophagosome turnover promote the expression of LC3-II [3]. To further substantiate this, changes in processed LC3B-II were examined in cells treated with EriB alone or EriB with autophagy inhibitor, 3-methyladenine (3-MA), or chloroquine (CQ) (Figure 5C). These results indicated that combined treatment with EriB and 3-MA inhibited the LC3B-II expression at lower levels than that of EriB treatment alone. In contrast, cells treated concomitantly with CQ and EriB accumulated LC3B-II at higher levels than that of EriB or CQ treatment alone. CQ can inhibit lysosomal protein degradation and the fusion of autophagosomes, which can also elevate LC3B-II levels [14]. These data further support that EriB induces autophagy in prostate cancer cells.
Modulation of EriB-induced apoptosis by autophagy inhibitor
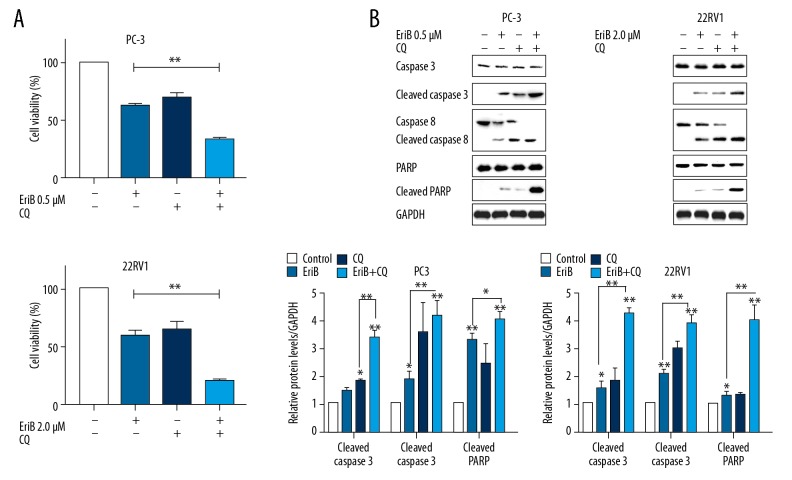
Because it has a cytotoxic or cytoprotective effect in anticancer treatments, autophagy can promote cell survival or induce cell death. The modulation of EriB-induced apoptosis by autophagy inhibitor was investigated. As shown in Figure 6, the proliferation of PC-3 cells and 22RV1 cells was more severely suppressed in concomitant treatment with EriB and CQ. Additionally, caspase-3, caspase-8, and PARP were also investigated by Western blot. Higher expressions of these apoptosis-related proteins were observed in concomitant treatment groups. Addition of CQ enhanced the apoptosis percentage in EriB-treated prostate cancer cells in flow cytometry analysis (Figure 2). These results show that EriB-induced apoptosis were enhanced through concurrent autophagy inhibitor.
Figure 6.
Regulation of EriB-induced apoptosis in human prostate cancer cell lines by autophagy inhibitor. (A) EriB-treated PC-3 cells (0.5 μM, 48 h) and 22RV1 cells (2 μM, 48 h). We added 50 μM CQ to culture medium for the last 6 h, after which MTT assay was used to measure cell viability. (B) The proteins of caspase 3, caspase 8, and PARP were detected by Western blot.
EriB regulated the apoptosis and autophagy of prostate cancer cells via the Akt/mTOR signaling pathway
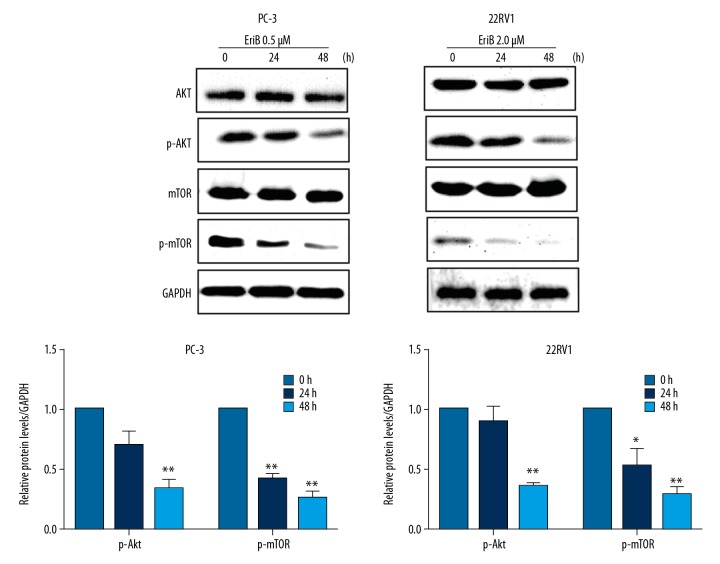
To elucidate the underlying molecular mechanism of EriB-induced apoptosis and autophagy in prostate cancer cells, Western blotting was used to examined the levels of proteins associated with the Akt/mTOR signaling pathway. The results suggested that, compared with the control groups, treatment with EriB downregulated the expression of phosphorylation of Akt and mTOR in PC-3 cells and 22RV1 cells (Figure 7). Previous investigations have demonstrated that apoptosis and autophagy can be initiated by inhibiting Akt and the mTOR signaling pathway [15–17]. These results indicated that the apoptosis and autophagy effect induced by EriB in prostate cancer cells might occur by suppression of Akt and mTOR phosphorylation.
Figure 7.
Inhibition of Akt/mTOR signal pathway by EriB. EriB-treated PC-3 cells (0.5 μM, 24 h) and 22RV1 cells (2 μM, 48 h). mTOR, p-mTOR, Akt, and p-Akt were detected by Western blot.
Discussion
Although several new drugs have recently been approved for prostate cancer, due to delayed diagnosis and increased chemotherapy resistance, the increased survival time with each of these new drugs is only a few months [18]. After chemotherapy and cytoreductive surgery, the 5-year survival rate remains <50% [19], thus leading to a high mortality rate. Consequently, new therapies that increase survival time are urgently needed.
Plants have been used in treatment of various diseases since time immemorial, and a variety of anti-neoplastic agents of natural origin are used in clinical treatments [20]. EriB has been confirmed to be effective against several cancers, but the properties of EriB in promoting apoptosis and autophagy and the molecular mechanism in prostate cancer cells are unclear. Our study shows that EriB induced apoptosis and autophagy in prostate cancer cells via the Akt/mTOR signaling pathway.
Although previous studies reported that EriB induced cell cycle arrest in colon cancer cells and pancreatic adenocarcinoma cells [21,22], a definite negative effect of EriB on the cell cycle in cancer cells has never been reported. The anti-tumor pathway and effect of natural products differ according to the cancer cell types and the drug concentration, which has been confirmed in some other research [23–25]. In this study, we demonstrated that EriB did not induce cell cycle arrest in prostate cancer cells.
Apoptosis and autophagy are closely related to tumorigenesis and tumor development. Promoting apoptosis and autophagy could be an effective strategy for anti-tumor therapy [26,27]. In this study, we first established that EriB inhibited cell proliferation in a time-and dose-dependent manner in PC-3 and 22RV1 cells, as determined by MTT. Then, inducing apoptosis by EriB in 2 prostate cancer cells was observed via annexin V-FITC assay. Western blot analysis demonstrated that EriB led to the cleavage of the apoptosis markers caspase-8, caspase-3, and PARP. Next, we investigated the potential of EriB-induced autophagy. LC3-II is an autophagy-related protein and participates in autophagosome formation. The transformation of LC3-I to LC3-II, observed in the process of autophagy, is an important molecular event [14]. LC3B-II expression was significantly increased, as shown in EriB-treated prostate cancer cells by Western blotting and immunofluorescence analysis. As a PI3-kinase specific inhibitor, 3-MA can block autophagosome formation at an early stage. The effect of CQ in inhibiting the autophagosome and impairing the process of lysosome fusion can block autophagic degradation. In our study, the increasing expression of LC3B-II in prostate cancer cells treated with EriB was inhibited by addition of 3-MA, and was further enhanced by addition of CQ. These results further confirmed that EriB induced autophagy in prostate cancer cells. The cross-talk between apoptosis and autophagy is intricate and may often appear contradictory. Our study showed that combined treatment with an established autophagy inhibitor – CQ – decreased EriB-induced cell viability. The fact that autophagy inhibitor enhanced EriB-induced apoptosis in PC-3 and 22rv1 cells was further demonstrated by Western blotting. Annexin V-FITC assay demonstrated that the addition of CQ increased the percentage of apoptotic cells in prostate cancer cells treated with EriB. These results indicated that autophagy can protect prostate cancer cells against EriB-induced apoptosis.
Although multiple upstream signals control apoptosis and autophagy, Akt/mTOR signaling is a well-known pathway involving simultaneous or sequential regulation of both apoptosis and autophagy. The anti-apoptotic property of Akt signaling was demonstrated by withdrawal of neurotrophic factor in cerebellar granule neurons [28]. Akt signaling acts as anti-apoptotic molecule, as confirmed in many cell death paradigms [29,30]. The anti-autophagic role is another characteristic of Akt signaling, and mTOR is a vital link between Akt and the inhibition of autophagy. As a downstream signaling molecule of Akt, mTOR suppresses autophagy via inhibiting ULK1 complex in response to a sufficient supply of nutrients [31]. In this study, Western blot analysis demonstrated that the phosphorylated protein of Akt/mTOR was obviously decreased in PC-3 and 22RV1 cells treated with EriB. These findings further support that suppression of the Akt/mTOR pathway is involved in apoptosis and autophagy induced by EriB in human prostate cancer cells. mTOR plasmid transfection, which reverses inhibition of the Akt/mTOR pathway by EriB, further confirmed this relationship, and the lack of mTOR plasmid transfection is one of the limitations of this study.
Conclusions
Our study demonstrated that EriB suppresses cell proliferation and induces apoptosis and autophagy in PC-3 and 22RV1 prostate cancer cells by inhibiting the Akt/mTOR pathway. In addition, autophagy inhibitor enhanced EriB-induced apoptosis in prostate cancer cells. The anti-tumor properties of EriB have not been investigated in clinical trials. Further studies of EriB may provide clinical applications for the treatment of prostate cancer.
Abbreviations
- MTT
3-[4, 5-dimethylthiazol-2-yl]-2, 5-diphenyl-tetrazolium bromide
- DMSO
dimethylsulfoxide
- FBS
fetal bovine serum
- PBS
phosphate-buffered saline
- PI
propidium iodide
- PVDF
polyvinyl difluoride
- CQ
chloroquine
- 3-MA
3-methyladeseven
- mTOR
mammalian target of rapamycin
- PARP
poly (ADP-ribose) polymerase
Footnotes
Source of support: This work was supported by the National Natural Science Foundation of China (81630019, 81870519)
Conflict of interest
None.
References
- 1.Mottet N, Bellmunt J, Bolla M, et al. EAU-ESTRO-SIOG guidelines on prostate cancer. part 1: Screening, diagnosis, and local treatment with curative intent. Eur Urol. 2016;71:618–29. doi: 10.1016/j.eururo.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 2.Alva A, Hussain M. Intermittent androgen deprivation therapy in advanced prostate cancer. Curr Treat Options Oncol. 2014;15:127–36. doi: 10.1007/s11864-013-0272-2. [DOI] [PubMed] [Google Scholar]
- 3.Klionsky DJ, Abdelmohsen K, Abe A, et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition) Autophagy. 2016;12:1–222. doi: 10.1080/15548627.2015.1100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hurley JH, Schulman BA. Atomistic autophagy: The structures of cellular self-digestion. Cell. 2014;157:300–11. doi: 10.1016/j.cell.2014.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tanaka S, Hikita H, Tatsumi T, et al. Rubicon inhibits autophagy and accelerates hepatocyte apoptosis and lipid accumulation in nonalcoholic fatty liver disease in mice. Hepatology. 2016;64:1994–2014. doi: 10.1002/hep.28820. [DOI] [PubMed] [Google Scholar]
- 6.Cerezo M, Rocchi S. New anti-cancer molecules targeting HSPA5/BIP to induce endoplasmic reticulum stress, autophagy and apoptosis. Autophagy. 2017;13:216–17. doi: 10.1080/15548627.2016.1246107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moretti L, Yang ES, Kim KW, Lu B. Autophagy signaling in cancer and its potential as novel target to improve anticancer therapy. Drug Resist. 2007;10:135–43. doi: 10.1016/j.drup.2007.05.001. Update. [DOI] [PubMed] [Google Scholar]
- 8.Guo JY, Xia B, White E. Autophagy-mediated tumor promotion. Cell. 2013;155:1216–19. doi: 10.1016/j.cell.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun HD, Lin ZW, Niu FD, et al. Diterpenoids from Isodon eriocalyx var. laxiflora. Phytochemistry. 1995;38:1451–55. doi: 10.1016/0031-9422(94)00815-b. [DOI] [PubMed] [Google Scholar]
- 10.Kong L-M, Deng X, Zuo Z-L, et al. Identification and validation of p50 as the cellular target of eriocalyxin B. Oncotarget. 2014;5:11353–64. doi: 10.18632/oncotarget.2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu Y-M, Chen W, Zhu J-S, et al. Eriocalyxin B blocks human SW1116 colon cancer cell proliferation, migration, invasion, cell cycle progression and angiogenesis via the JAK2/STAT3 signaling pathway. Mol Med Rep. 2016;13:2235–40. doi: 10.3892/mmr.2016.4800. [DOI] [PubMed] [Google Scholar]
- 12.Wang L, Zhao W-L, Yan J-S, et al. Eriocalyxin B induces apoptosis of t(8;21) leukemia cells through NF-κB and MAPK signaling pathways and triggers degradation of AML1-ETO oncoprotein in a caspase-3-dependent manner. Cell Death Differ. 2007;14:306–17. doi: 10.1038/sj.cdd.4401996. [DOI] [PubMed] [Google Scholar]
- 13.Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell. 2010;140:313–26. doi: 10.1016/j.cell.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kabeya Y, Mizushima N, Ueno T, et al. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19:5720–28. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heras-Sandoval D, Pérez-Rojas JM, Hernández-Damián J, Pedraza-Chaverri J. The role of PI3K/Akt/mTOR pathway in the modulation of autophagy and the clearance of protein aggregates in neurodegeneration. Cell Signal. 2014;26:2694–701. doi: 10.1016/j.cellsig.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 16.Wu J, Hu G, Dong Y, et al. Matrine induces Akt/mTOR signalling inhibition-mediated autophagy and apoptosis in acute myeloid leukaemia cells. J Cell Mol Med. 2017;21:1171–81. doi: 10.1111/jcmm.13049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi S, Cao H. Shikonion promotes autophagy in BXPC-3 human pancreatic cancer cells through the PI3K/Akt signaling pathway. Oncol Lett. 2014;8:1087–89. doi: 10.3892/ol.2014.2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rekoske BT, McNeel DG. Immunotherapy for prostate cancer: False promises or true hope. Cancer. 2016;122:3598–607. doi: 10.1002/cncr.30250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang W, Meng Y, Liu N, et al. Insights into chemoresistance of prostate cancer. Int J Biol Sci. 2015;11:1160–70. doi: 10.7150/ijbs.11439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Newman DJ, Cragg GM. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J Nat Prod. 2012;75:311–35. doi: 10.1021/np200906s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu YM, Chen W, Zhu JS, et al. Eriocalyxin B blocks human SW1116 colon cancer cell proliferation, migration, invasion, cell cycle progression and angiogenesis via the JAK2/STAT3 signaling pathway. Mol Med Rep. 2016;13:2235–40. doi: 10.3892/mmr.2016.4800. [DOI] [PubMed] [Google Scholar]
- 22.Li L, Yue GG, Lau CB, et al. Eriocalyxin B induces apoptosis and cell cycle arrest in pancreatic adenocarcinoma cells through caspase- and p53-dependent pathways. Toxicol Appl Pharmacol. 2012;262:80–90. doi: 10.1016/j.taap.2012.04.021. [DOI] [PubMed] [Google Scholar]
- 23.Zhong Z, Tan W, Chen X, Wang Y. Furanodiene, a natural small molecule suppresses metastatic breast cancer cell migration and invasion in vitro. Eur J Pharmacol. 2014;737:1–10. doi: 10.1016/j.ejphar.2014.04.043. [DOI] [PubMed] [Google Scholar]
- 24.Zhong ZF, Yu HB, Wang CM, et al. Furanodiene induces extrinsic and intrinsic apoptosis in doxorubicin-resistant MCF-7 breast cancer cells via NF-κB-independent mechanism. Front Pharmacol. 2017;8:648. doi: 10.3389/fphar.2017.00648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiao Y, Yang FQ, Li SP, et al. Furanodiene induces G2/M cell cycle arrest and apoptosis through MAPK signaling and mitochondria-caspase pathway in human hepatocellular carcinoma cells. Cancer Biol Ther. 2007;6:1044–50. doi: 10.4161/cbt.6.7.4317. [DOI] [PubMed] [Google Scholar]
- 26.Goldar S, Khaniani MS, Derakhshan SM, Baradaran B. Molecular mechanisms of apoptosis and roles in cancer development and treatment. Asian Pac J Cancer Prev. 2015;16:2129–44. doi: 10.7314/apjcp.2015.16.6.2129. [DOI] [PubMed] [Google Scholar]
- 27.Thorburn A, Thamm DH, Gustafson DL. Autophagy and cancer therapy. Mol Pharmacol. 2014;85:830–38. doi: 10.1124/mol.114.091850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dudek H, Datta SR, Franke TF, et al. Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science. 1997;275:661–65. doi: 10.1126/science.275.5300.661. [DOI] [PubMed] [Google Scholar]
- 29.Jin D, Cao M, Mu X, et al. Catalpol inhibited the proliferation of T24 human bladder cancer cells by inducing apoptosis through the blockade of Akt-mediated anti-apoptotic signaling. Cell Biochem Biophys. 2015;71:1349–56. doi: 10.1007/s12013-014-0355-0. [DOI] [PubMed] [Google Scholar]
- 30.Cuconati A, Mills C, Goddard C, et al. Suppression of Akt anti-apoptotic signaling by a novel drug candidate results in growth arrest and apoptosis of hepatocellular carcinoma cells. PLoS One. 2013;8:e54595. doi: 10.1371/journal.pone.0054595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–93. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]