Abstract
Due to the great value of amino alcohols, new methods for their synthesis are in high demand. Abundant aliphatic alcohols represent the ideal feedstock for the method development toward this important motif. To date, transition-metal-catalyzed approaches for the directed remote amination of alcohols have been well established. Yet, they have certain disadvantages such as the use of expensive catalysts and limited scope. Very recently, transition-metal-free visible-light-induced radical approaches have emerged as new powerful tools for directed remote amination of alcohols. Relying on 1,5-HAT reactivity, these methods are limited to β- or δ-amination only. Herein, we report a novel transition-metal- and visible-light-free room-temperature radical approach for remote β-, γ-, and δ-C(sp3)–N bond formation in aliphatic alcohols using mild basic conditions and readily available diazonium salt reagents.
A diverse range of natural products, pharmaceuticals, and catalyst designs feature amino alcohols as privileged motifs.1 One of the most powerful strategies toward their synthesis is the directed site-selective C(sp3)–H amination of aliphatic alcohols,2 which are ubiquitous in complex molecules and are inexpensive and sustainable starting materials. The seminal strategy for directed amination of alcohols developed by Du Bois, White, and others relies on insertion of Rh-,3 Ru-,4 Ag-,5 Fe-,6 Mn-,7 Cu-,8 or Co9-based transition-metal-nitrene species A into distal unactivated C(sp3)–H bonds10 (Scheme 1, a). However, the innate metallacyclic intermediates restrict these metal-based methods to γ- and β-site functionalization. Recently, radical approaches for remote functionalization of alcohols11 have gained broad attention due to the discoveries of new mild methods for generation of radicals and their versatile hydrogen atom transfer (HAT) reactivity.12 To date, only two methods on remote site-selective C(sp3)–H amination of alcohols have been developed relying on radical intermediates. In 2017, the Nagib group introduced an elegant metal-free visible-light-induced radical relay for synthesis of β-amino alcohols from their alcohol analogues (Scheme 1, b).13
Scheme 1.
Directed Remote C–H Amination of Alcohols
This method utilizes a novel, easily installable/removable imidate-based chaperone capable of generating N-centered radical species B, which undergoes a 1,5-HAT process producing C. Subsequent 5-endo-trig radical cyclization of 3 and in situ hydrolysis delivers the amination products with exclusive β-selectivity. Later, the Zuo group developed another visible-light-induced protocol to access δ-amino alcohols (Scheme 1, c).14 In this case, Ce-catalyzed generation of alkoxy radicals D directly from alcohols followed by 1,5-HAT and radical addition of E to di-tert-butyl azodiformate leads to δ-selective C–N bond formation. Overall, these radical approaches eliminated the necessity for use of expensive transition metal catalysts and conventional heating for remote C(sp3)–H amination of alcohols.
However, due to the preference of heteroatom-centered radicals for 1,5-HAT,12b the scope of these radical transformations remains limited and γ-amination toward valuable 1,3-amino alcohols has not yet been achieved. Therefore, development of more general metal-free methods featuring benign conditions is highly desired.15 Herein, we report the first transition-metal- and visible-light-free room-temperature protocol for remote β-, γ-, and δ-C(sp3)–N bond formation in aliphatic alcohols using mild basic conditions and readily available diazonium salts (Scheme 1, d). This method employs the ability of the latter to initiate radical reactions in the presence of bases or other reducing agents,16 as well as to trap nucleophilic radical intermediates to form a new C–N bond.17 Thus, previously unknown iodine atom abstraction from the silyl methyl iodide moiety of a tethered alcohol by an aryl radical, formed from the diazonium salt in the presence of base, leads to silyl methyl radical species F. This electrophilic radical,18 due to electronic mismatch, is not predisposed to coupling with the diazonium reagent and instead undergoes a selective 1,6-, 1,5-, or 1,7-HAT process to produce transposed nucleophilic radical species G at the remote C(sp3)–H site of the alcohol.19 The latter would be ultimately trapped by excess diazonium salt, which is known to be a facile process (k = 106 M−1 s−1 for primary alkyl radicals; k ≥ 108 M−1 s−1 for tertiary alkyl radicals),17b and reduced by base to produce β-, γ-, or δ-diazenylated alcohols, accordingly. Thus, the base plays a double duty by reducing both the diazonium cation and its radical adduct.
Continuing our previous work on visible-light-induced Pdcatalyzed remote functionalization of aliphatic alcohols with the employment of Si-based auxiliaries,19a we began our studies on remote amination by subjecting standard substrate 1a to the reactions with different electrophilic reagents. It turned out that when substrate 1a and diazonium salt 2a were mixed in methanol under our blue light/Pd-based conditions, in 1 h the γ-diazenylated Si-protected alcohol 3aa was obtained in 22% GC yield (Table 1, entry 1). To our surprise, the control experiments revealed that this reaction does not require employment of Pd catalyst or visible light to proceed with the same efficiency toward 3aa! (entries 2, 3). The control experiment proved the necessity of base for the success of this transformation (entry 4). Excited by the obtained results, we began optimizing this novel transition-metal- and visible-light-free base-promoted remote amination by screening different solvent systems (see SI for full optimization). We realized that poor solubility of nonpolar silyl ether 1a in methanol was a key problem; therefore a cosolvent was required for better efficiency and reproducibility. The mixture of N,N-dimethylacetamide (DMA) and methanol (1:1) was found to be an optimal solvent system, producing 3aa in 60% yield (entry 5). Testing different bases revealed that inexpensive and decently soluble in methanol HCO2Li·H2O was superior to other inorganic bases (entry 6). Finally, using more diluted solution and decreasing the temperature to 0 °C allowed the reaction to deliver 3aa in 76% GC yield after 2 h (entry 7).
Table 1.
Optimization Table
 | |||||
|---|---|---|---|---|---|
| no. | cat. | base | solvent | cond | yield (3aa),a % |
| 1b | [Pd]c | Cs2CO3 | MeOH | rt, 1 h | 22 |
| 2 | [Pd]c | Cs2CO3 | MeOH | rt, 1 h | 20 |
| 3 | Cs2CO3 | MeOH | rt, 1 h | 23 | |
| 4 | MeOH | rt, 1 h | 0 | ||
| 5 | Cs2CO3 | DMA/MeOH | rt, 1 h | 60 | |
| 6 | HCO2Li·H2O | DMA/MeOH | rt, 30 min | 64 | |
| 7 | HCO2Li·H2O | DMA/MeOHd | 0 °C to rt, 2 h | 76 | |
0.1 mmol scale reaction; GC-MS yield (standard: pentadecane).
Reaction mixture was irradiated with visible light.
5 mol % Pd(OAc)2/10 mol % Xantphos.
0.05 M concentration.
With the optimized conditions in hand, we investigated the scope of diazonium salts (Table 2). Both electron-rich (2b–g) and electron-deficient (2h–l) diazonium salts were reactive toward remote diazenylation of 1a, leading to the desired products 3 in good to excellent yields. We noticed that the yields were generally slightly higher in the case of more electron-rich diazonium salts. Interestingly, ortho-substitution at diazonium salts did not have much effect on the yield of this transformation (entries 3, 5).
Table 2.
Screening of Diazonium Salts
 | ||
|---|---|---|
| no. | Ar | yield of 3 or 4, % |
| 1 | C6H5 (2a) | 84%a (3aa), 68%b (4aa) |
| 2 | p-MeC6H4 (2b) | 86%a (3ba), 81%b (4ba) |
| 3 | o-MeC6H4 (2c) | 69%b (4ca) |
| 4 | m-MeC6H4 (2d) | 83%a (3da) |
| 5 | p,o-diMeC6H3 (2e) | 87%a (3ea) |
| 6 | p,m-diMeC6H3 (2f) | 80%a (3fa) |
| 7 | p-MeOC6H4 (2g) | 81%a (3ga), 64%b (4ga) |
| 8 | p-CF3C6H4 (2h) | 54%b (4ha) |
| 9 | p-CNC6H4 (2i) | 52%b (4ia) |
| 10 | m-ClC6H4 (2j) | 67%a (3ja) |
| 11 | m-FC6H4 (2k) | 73%a (3ka) |
| 12 | p-ClC6H4 (2l) | 76%a (3la) |
0.1 mmol scale reaction; NMR yield (standard: CH2Br2).
0.5 mmol scale reaction, followed by deprotection with aqueous HCl (5 equiv); isolated yield.
The products possessing p-H (4aa), p-Me (4ba), o-Me (4ca), p-MeO (4ga), p-CF3 (4ha), and p-CN (4ia) substituents were isolated as the γ-diazenylated alcohols 4 after in situ deprotection of silicon auxiliary in good yields. Based on these results, p-tolylN2BF4 (2b) was selected as the preferred diazenylating reagent for the remote amination of aliphatic alcohols.
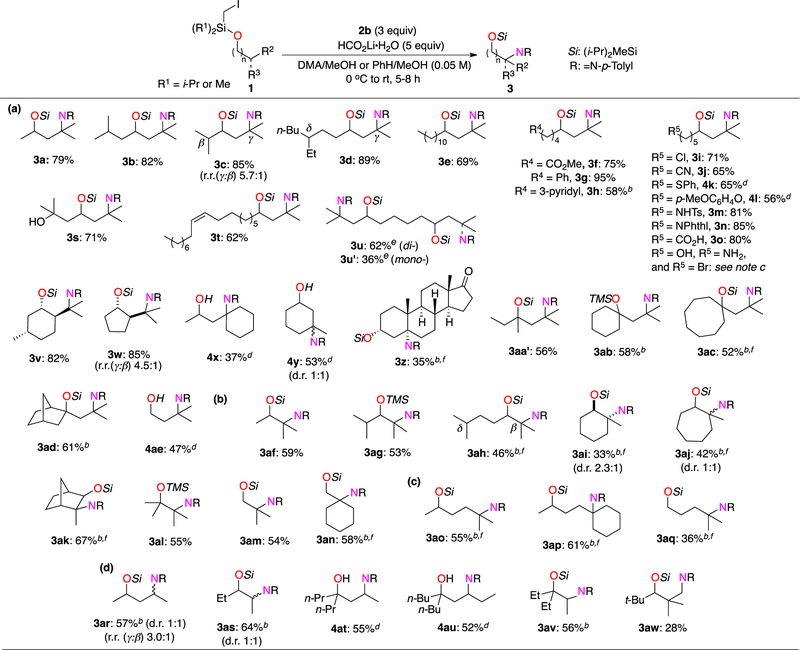
The remote diazenylation of Si-tethered alcohols was found to be quite general (Scheme 2). In contrast to our recently developed Heck relay process,19b the product of premature coupling was never observed in this transformation, due to the electronic mismatch of the silyl methyl radical and diazonium cation.18 First, using the optimized conditions and p-tolylN2BF4 (2b), the scope of γ-diazenylation was examined (Scheme 2, a). Similarly to the standard substrate 1a, its symmetric analogue 1b furnished the corresponding product 3b in excellent yield. Substrates 1c and 1d, which possess competitive Hβ and Hδ sites of abstraction, reacted preferentially at the γ-C–H sites, producing 2c and 2d in high yields. This result is in agreement with the previously observed reactivity preference of the silicon tether toward 1,6-HAT over 1,5-HAT and 1,7-HAT for tertiary sites with similar bond dissociation energies.19a Alcohols possessing an unsubstituted long chain (1e) and −CO2Me and −Ph-substituted chains (1f, 1g, respectively) underwent γ−3° C–H diazenylation reaction in a highly efficient and regioselective manner. Moreover, functional groups such as 3-pyridyl- (3h), chloride-(3i), cyanide- (3j), phenylsulfide- (4k), p-metoxyphenoxide- (4l), N-tosylamide- (3m), N-phthalimide- (3n), carboxylic acid (3o), tertiary alcohol (3s), and alkene (3t) were all tolerated under these conditions (see robustness screening in the SI).20 However, trace or no product was observed with substrates possessing a primary alcohol, amine, or bromide moiety (3p, 3q, and 3s, respectively). Interestingly, this method afforded the double γ-diazenylation of diol 1u: product 3u was isolated together with the monodiazenylated adduct 3u′. (–)-Menthol derivative 1v was selectively diazenylated at the isopropyl group in 82% yield. Reaction of its five-membered analogue 1w was less selective, giving a mixture of γ- and β-diazenylated alcohols 3w in 85% yield (4.5:1 ratio). γ-Diazenylation of C–H bonds of cyclic alcohols 1x–1z, even in the complex setting of androsterone 1z, proceeded uneventfully, delivering products 3k–3m in reasonable yields. Furthermore, cyclic (1ab–ad) and acyclic (1aa′) tertiary alcohols were also compatible, giving rise to products 3aa′–ad. Primary alcohol 1ae furnished the desired product 4ae in 47% yield in the γ-diazenylation reaction followed by one-pot Si-auxiliary deprotection.
Scheme 2. Scope of Transition-Metal-Free Amination of Remote Unactivated C(sp3)–H Sites of Aliphatic Alcohols:a (a) γ-Diazenylation; (b) β-Diazenylation; (c) δ-Diazenylation; (d) Diazenylation at Secondary and Primary C–H Sites.
a0.3 or 0.4 mmol scale reactions. Isolated yield,%. bNMR yield,% (standard: CH2Br2). cWhen R5 = OH (3p): 20% NMR yield; R5 = NH2 (3q): decomp; R5 = Br (3r): decomp. dProducts 3 were deprotected and isolated as free alcohols 4. eCalculated yield, %. fMixture of regio- or stereoisomers: NMR yield of major isomer is represented.
Next, we investigated the scope of the kinetically less favorable β-diazenylation (Scheme 2, b). To our delight, secondary alcohols 1af and 1ag reacted quite efficiently under slightly modified conditions (see SI for details), producing 3af and 3ag in 81% and 53% yields, respectively. Diazenylation of 1ah possessing accessible β−3° C–H, γ−2° C–H, and δ−3° C–H sites preferably occurred at the β-position, thus supporting our earlier conclusion that, for this silicon tether, the 1,5-HAT is more favorable than the 1,7-HAT.19a In addition, γ- and δ-diazenylation products were also observed in this reaction as minor regioisomers. Cyclic (1ai, 1aj) and bicyclic (1ak) alcohols underwent diazenylation reaction at the β-tertiary C–H sites, as well (products 3ai, 3aj, 3ak). Moreover, this protocol was compatible with tertiary (1al) and primary alcohols (1am, 1an), leading to the desired products 3al, 3am, and 3an, respectively. Challenging δ-diazenylation was targeted as well (Scheme 2, c). Gratifyingly, reactions of secondary (1ao, 1ap) and primary (1aq) alcohols led to the desired products as mixtures of δ- and γ-diazenylation products in good yields.
Finally, we applied this methodology for 2° C–H and even 1° C–H site functionalization (Scheme 2, d). Both γ- and β-diazenylation reactions were accomplished for secondary (1ar, 1as) and tertiary (1at, 1au, 1av) alcohols in good yields. Remarkably, this diazenylation protocol was also applicable for unactivated 1° C–H site functionalization, yielding primary diazene 3aw in 28% yield!
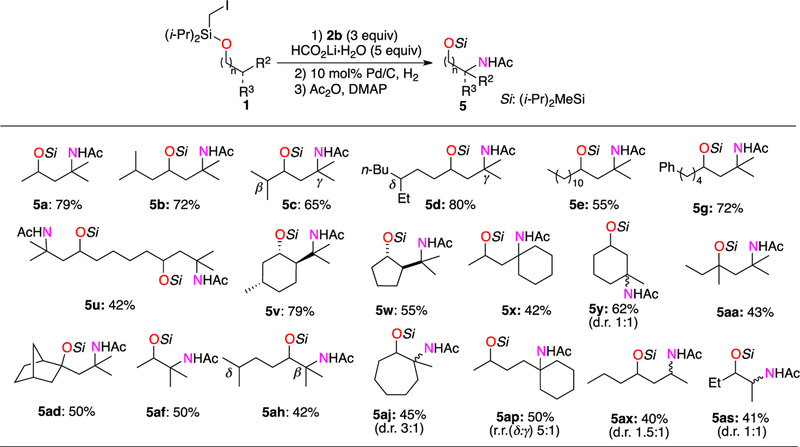
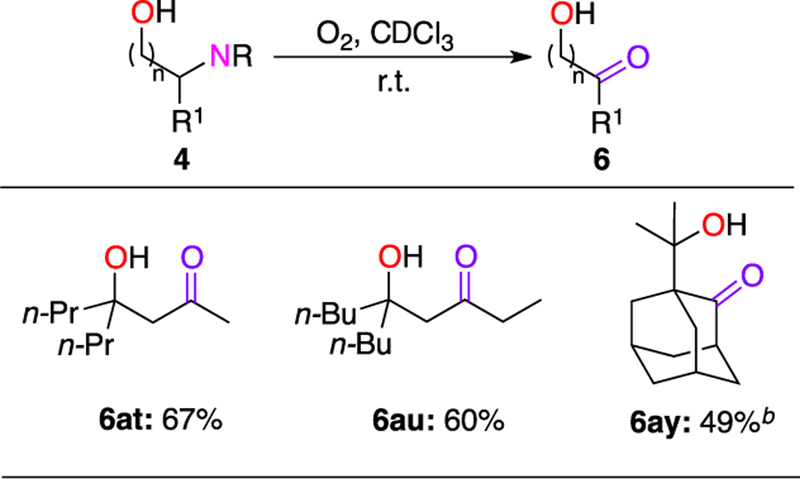
Lastly, we examined the synthetic utility of the obtained β-, γ-, and δ-diazenylated alcohols toward their highly valuable amino alcohol derivatives. Thus, the synthesized substrates were subjected to hydrogenation conditions21 followed by acylation to access the corresponding protected amino alcohols 5 (Scheme 3). To our delight, the diverse range of secondary and tertiary cyclic and acyclic 1,2– 1,3- and 1,4-amino alcohols were obtained in reasonable yields via a three-step procedure starting from Si-tethered alcohols 1. Moreover, it was found that secondary diazenes, which are prone to isomerize into hydrazones, could efficiently be oxidized in the presence of oxygen into the corresponding ketones.22 Thus, this approach can be further used for the remote oxygenation of aliphatic alcohols23 with secondary sites into γ-hydroxy ketones 6 (Scheme 4). This method potentially can be used to generate aldol equivalent products, which are difficult to access by other methods (6ay).
Scheme 3. Transformations of Diazenylated Alcohols: Hydrogenation toward Amino Alcoholsa.
a0.3–0.4 mmol scale reactions. Isolated yield, %.
Scheme 4. Transformations of Diazenylated Alcohols: Oxygenation toward Hydroxy Ketonesa.
a0.2–0.4 mmol scale reactions. Isolated yield, %. bCompound synthesized via a semi-one-pot procedure from the corresponding Si-auxiliary protected alcohol 1ay. Isolated yield over two steps, %.
In summary, we have developed the first classical-radical-initiator-, transition-metal- and visible-light-free, auxiliary-enabled remote C–H functionalization protocol for selective γ-, β-, and δ-C(sp3)–N bond formation in aliphatic alcohols. This method features mild radical conditions and employs readily available diazonium salts to serve a double duty as the I atom abstracting agent and a coupling partner. The reaction is promoted by a weak base, lithium formate, and does not require use of transition metals or radical initiators. The scope of obtained diazenylated alcohols is very broad, and their transformations toward amino alcohols and hydroxy ketones were accomplished with good efficiency. Overall, this is a highly accessible, inexpensive, and practical method for functionalization of remote β-, γ-, and δ-unactivated C–H sites of alcohols, expected to find broad applications in synthesis.
Supplementary Material
ACKNOWLEDGMENTS
We thank the National Institutes of Health (GM120281) and National Science Foundation (CHE-1663779) for the financial support of this work. We also thank visiting researchers Michael Buck and Mirela Sparano and undergraduate researcher Yana McAuliff for their help with preparation of starting materials for this work.
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/jacs.9b04189.
Experimental procedures and compound characterization data (PDF)
The authors declare no competing financial interest.
REFERENCES
- (1).(a) Ager DJ; Prakash I; Schaad DR 1,2-Amino Alcohols and Their Heterocyclic Derivatives as Chiral Auxiliaries in Asymmetric Synthesis. Chem. Rev 1996, 96, 835. [DOI] [PubMed] [Google Scholar]; (b) Bergmeier SC The Synthesis of Vicinal Amino Alcohols. Tetrahedron 2000, 56, 2561. [Google Scholar]; (c) Lait SM; Rankic DA; Keay BA 1,3-Aminoalcohols and Their Derivatives in Asymmetric Organic Synthesis. Chem. Rev 2007, 107, 767. [DOI] [PubMed] [Google Scholar]; (d) Gomes CRB; Moreth M; Cardinot D; Kopke V; Cunico W; Da Silva Lourenio MC; De Souza MVN Synthesis and Antimycobacterial Activity of Novel Amino Alcohols Containing Central Core of the Anti-HIV Drugs Lopinavir and Ritonavir. Chem. Biol. Drug Des 2011, 78, 1031. [DOI] [PubMed] [Google Scholar]; (e) Quiliano M; Mendoza A; Fong KY; Pabón A; Goldfarb NE; Fabing I; Vettorazzi A; López de Cerain A; Dunn BM; Garavito G; Wright DW; Deharo E; Pérez-Silanes S; Aldana I; Galiano S Exploring the scope of new arylamino alcohol derivatives: Synthesis, antimalarial evaluation, toxicological studies, and target exploration. Int. J. Parasitol.: Drugs Drug Resist 2016, 6, 184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).(a) Davies HML; Manning JR Catalytic C-H functionalization by metal carbenoid and nitrenoid insertion. Nature 2008, 451, 417. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Du Bois J Rhodium-Catalyzed C-H Amination. An Enabling Method for Chemical Synthesis. Org. Process Res. Dev 2011, 15, 758. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Roizen JL; Harvey ME; Du Bois J Metal-Catalyzed Nitrogen-Atom Transfer Methods for the Oxidation of Aliphatic C-H Bonds. Acc. Chem. Res 2012, 45, 911. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Jeffrey JL; Sarpong R Intramolecular C(sp3)-H amination. Chem. Sci 2013, 4, 4092. [Google Scholar]; (e) Darses B; Rodrigues R; Neuville L; Mazurais M; Dauban P Transition metal-catalyzed iodine(III)-mediated nitrene transfer reactions: efficient tools for challenging syntheses. Chem. Commun 2017, 53, 493. [DOI] [PubMed] [Google Scholar]; (f) Park Y; Kim Y; Chang S Transition Metal-Catalyzed C-H Amination: Scope, Mechanism, and Applications. Chem. Rev 2017, 117, 9247. [DOI] [PubMed] [Google Scholar]
- (3).(a) Espino CG; Wehn PM; Chow J; Du Bois J Synthesis of 1,3-Difunctionalized Amine Derivatives through Selective C-H Bond Oxidation. J. Am. Chem. Soc 2001, 123, 6935. [Google Scholar]; (b) Espino CG; Fiori KW; Kim M; Du Bois J Expanding the Scope of C-H Amination through Catalyst Design. J. Am. Chem. Soc 2004, 126, 15378. [DOI] [PubMed] [Google Scholar]; (c) Espino CG; Du Bois J A Rh-Catalyzed C-H Insertion Reaction for the Oxidative Conversion of Carbamates to Oxazolidinones. Angew. Chem., Int. Ed 2001, 40, 598. [DOI] [PubMed] [Google Scholar]; (d) Grelier G; Rey-Rodriguez R; Darses B; Retailleau P; Dauban P Catalytic Intramolecular C(sp3)-H Amination of Carbamimidates. Eur. J. Org. Chem 2017, 82, 1880. [DOI] [PubMed] [Google Scholar]; (e) Lebel H; Mamani Laparra L; Khalifa M; Trudel C; Audubert C; Szponarski M; Dicaire Leduc C; Azek E; Ernzerhof M Synthesis of Oxazolidinones: Rhodium-catalyzed C-H Amination of N-Mesyloxycarbamates. Org. Biomol. Chem 2017, 15, 4144. [DOI] [PubMed] [Google Scholar]
- (4).(a) Milczek E; Boudet N; Blakey S Enantioselective C-H Amination Using Cationic Ruthenium(II)-pybox Catalysts. Angew. Chem., Int. Ed 2008, 47, 6825. [DOI] [PubMed] [Google Scholar]; (b) Liang J-L; Yuan S-X; Huang J-S; Yu W-Y; Che C-M Highly Diastereo- and Enantioselective Intramolecular Amidation of Saturated C-H Bonds Catalyzed by Ruthenium Porphyrins. Angew. Chem., Int. Ed 2002, 41, 3465. [DOI] [PubMed] [Google Scholar]; (c) Zhang J-L; Huang J-S; Che C-M Oxidation Chemistry of Poly(ethylene glycol)-Supported Carbonylruthenium(II) and Dioxoruthenium(VI) meso-Tetrakis(pentafluorophenyl)porphyrin. Chem. - Eur. J 2006, 12, 3020. [DOI] [PubMed] [Google Scholar]
- (5).Alderson JM; Phelps AM; Scamp RJ; Dolan NS; Schomaker JM Ligand-Controlled, Tunable Silver-Catalyzed C-H Amination. J. Am. Chem. Soc 2014, 136, 16720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).(a) Liu Y; Guan X; Wong EL-M; Liu P; Huang J-S; Che C-M Nonheme Iron-Mediated Amination of C(sp3)-H Bonds. Quinquepyridine-Supported Iron-Imide/Nitrene Intermediates by Experimental Studies and DFT Calculations. J. Am. Chem. Soc 2013, 135, 7194. [DOI] [PubMed] [Google Scholar]; (b) Paradine SM; White MC Iron-Catalyzed Intramolecular Allylic C-H Amination. J. Am. Chem. Soc 2012, 134, 2036. [DOI] [PubMed] [Google Scholar]
- (7).Paradine SM; Griffin JR; Zhao J; Petronico AL; Miller SM; Christina White M A manganese catalyst for highly reactive yet chemoselective intramolecular C(sp3)-H amination. Nat. Chem 2015, 7, 987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Barman DN; Nicholas KM Copper-Catalyzed Intramolecular C-H Amination. Eur. J. Org. Chem 2011, 2011, 908. [Google Scholar]
- (9).Lu H; Tao J; Jones JE; Wojtas L; Zhang XP Cobalt(II)-Catalyzed Intramolecular C-H Amination with Phosphoryl Azides: Formation of 6- and 7-Membered Cyclophosphoramidates. Org. Lett 2010, 12, 1248. [DOI] [PubMed] [Google Scholar]
- (10).(a) Fraunhoffer KJ; White MC syn-1,2-Amino Alcohols via Diastereoselective Allylic C-H Amination. J. Am. Chem. Soc 2007, 129, 7274. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Rice GT; White MC Allylic C-H Amination for the Preparation of syn-1,3-Amino Alcohol Motifs. J. Am. Chem. Soc 2009, 131, 11707. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Qi X; Rice GT; Lall MS; Plummer MS; White MC Diversification of a β-Lactam Pharmacophore via Allylic C-H Amination: Accelerating Effect of Lewis Acid co-catalyst. Tetrahedron 2010, 66, 4816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).(a) Wu X; Zhang H; Tang N; Wu Z; Wang D; Ji M; Xu Y; Wang M; Zhu C Metal-free alcohol-directed regioselective heteroarylation of remote unactivated C(sp3)-H bonds. Nat. Commun 2018, 9, 3343. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Friese FW; Mück-Lichtenfeld C; Studer A Remote C-H functionalization using radical translocating arylating groups. Nat. Commun 2018, 9, 2808. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Zhu Y; Huang K; Pan J; Qiu X; Luo X; Qin Q; Wei J; Wen X; Zhang L; Jiao N Silver-catalyzed remote Csp3-H functionalization of aliphatic alcohols. Nat. Commun 2018, 9, 2625. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Chen K; Richter JM; Baran PS 1,3-Diol Synthesis via Controlled, Radical-Mediated C-H Functionalization. J. Am. Chem. Soc 2008, 130, 7247. [DOI] [PubMed] [Google Scholar]; (e) Short MA; Blackburn JM; Roizen JL Sulfamate Esters Guide Selective Radical-Mediated Chlorination of Aliphatic C-H Bonds. Angew. Chem 2018, 130, 302. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Sathyamoorthi S; Banerjee S; Du Bois J; Burns NZ; Zare RN Site-selective bromination of sp3 C-H bonds. Chem. Sci 2018, 9, 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).(a) Robertson J; Pillai J; Lush RK Radical translocation reactions in synthesis. Chem. Soc. Rev 2001, 30, 94. [Google Scholar]; (b) Chiba S; Chen H sp3 C-H oxidation by remote H-radical shift with oxygen-and nitrogen-radicals: a recent update. Org. Biomol. Chem 2014, 12, 4051. [DOI] [PubMed] [Google Scholar]; (c) Yan M; Lo JC; Edwards JT; Baran PS Radicals: Reactive Intermediates with Translational Potential. J. Am. Chem. Soc 2016, 138, 12692. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Hu X-Q; Chen J-R; Xiao W-J Controllable Remote C-H Bond Functionalization by Visible-Light Photocatalysis. Angew. Chem., Int. Ed 2017, 56, 1960. [DOI] [PubMed] [Google Scholar]; (e) Green SA; Crossley SWM; Matos JLM; Vásquez-Céspedes S; Shevick SL; Shenvi RA The High Chemofidelity of Metal-Catalyzed Hydrogen Atom Transfer. Acc. Chem. Res 2018, 51, 2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Wappes EA; Nakafuku KM; Nagib DA Directed β CH Amination of Alcohols via Radical Relay Chaperones. J. Am. Chem. Soc 2017, 139, 10204. [DOI] [PMC free article] [PubMed] [Google Scholar]; While this manuscript was under revision, a report on light-free β C–H amination was published:Stateman LM; Wappes EA; Nakafuku KM; Edwards KM; Nagib DA Catalytic β C-H amination via an imidate radical relay. Chem. Sci 2019, 10, 2693.
- (14).Hu A; Guo J-J; Pan H; Tang H; Gao Z; Zuo Z δ-Selective Functionalization of Alkanols Enabled by Visible-Light-Induced Ligand-to-Metal Charge Transfer. J. Am. Chem. Soc 2018, 140, 1612. [DOI] [PubMed] [Google Scholar]
- (15).Samanta R; Matcha K; Antonchick AP Metal-Free Oxidative Carbon-Heteroatom Bond Formation Through C-H Bond Functionalization. Eur. J. Org. Chem 2013, 2013, 5769. [Google Scholar]
- (16).(a) Abrams R; Lefebvre Q; Clayden J Transition Metal Free Cycloamination of Prenyl Carbamates and Ureas Promoted by Aryldiazonium Salts. Angew. Chem., Int. Ed 2018, 57, 13587. [DOI] [PubMed] [Google Scholar]; (b) Hartmann M; Li Y; Studer A Transition-metal-free oxyarylation of alkenes with aryl diazonium salts and TEMPONa. J. Am. Chem. Soc 2012, 134, 16516. [DOI] [PubMed] [Google Scholar]; (c) Naveen N; Sengupta S; Chandrasekaran S Metal-Free S-Arylation of Cysteine Using Arenediazonium Salts. J. Org. Chem 2018, 83, 3562. [DOI] [PubMed] [Google Scholar]; (d) Koziakov D; Jacobi von Wangelin A Metal-free radical aromatic carbonylations mediated by weak bases. Org. Biomol. Chem 2017, 15, 6715. [DOI] [PubMed] [Google Scholar]; (e) Kindt S; Wicht K; Heinrich MR Base-Induced Radical Carboamination of Nonactivated Alkenes with Aryldiazonium Salts. Org. Lett 2015, 17, 6122. [DOI] [PubMed] [Google Scholar]
- (17).(a) Blank O; Heinrich MR Carbodiazenylation of Olefins by Radical Iodine Transfer and Addition to Arenediazonium Salts. Eur. J. Org. Chem 2006, 2006, 4331. [Google Scholar]; (b) Citterio A; Minisci F Free-radical diazocoupling. A new general reaction of diazonium salts. J. Org. Chem 1982, 47, 1759. [Google Scholar]; (c) Cannella R; Clerici A; Pastori N; Regolini E; Porta O One-Pot Four-Component Reaction: Aqueous TiCl3/PhN +2-Mediated Alkyl Radical Addition to Imines Generated in Situ. Org. Lett 2005, 7, 645. [DOI] [PubMed] [Google Scholar]; (d) Cao L; Li C p-MeOC6H4N +2BF4-/TiCl3: a novel initiator for halogen atom-transfer radical reactions in aqueous media. Tetrahedron Lett. 2008, 49, 7380. [Google Scholar]; (e) Matcha K; Antonchick AP Cascade Multicomponent Synthesis of Indoles, Pyrazoles, and Pyridazinones by Functionalization of Alkenes. Angew. Chem., Int. Ed 2014, 53, 11960. [DOI] [PubMed] [Google Scholar]
- (18).Kurandina D; Parasram M; Gevorgyan V Visible Light-Induced Room-Temperature Heck Reaction of Functionalized Alkyl Halides with Vinyl Arenes/Heteroarenes. Angew. Chem., Int. Ed 2017, 56, 14212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).(a) Parasram M; Chuentragool P; Wang Y; Shi Y; Gevorgyan V General, Auxiliary-Enabled Photoinduced Pd-Catalyzed Remote Desaturation of Aliphatic Alcohols. J. Am. Chem. Soc 2017, 139, 14857. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Chuentragool P; Yadagiri D; Morita T; Sarkar S; Parasram M; Wang Y; Gevorgyan V Aliphatic Radical Relay Heck Reaction at Unactivated C(sp3)-H Sites of Alcohols. Angew. Chem., Int. Ed 2019, 58, 1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).(a) Collins KD; Rühling A; Glorius F Application of a robustness screen for the evaluation of synthetic organic methodology. Nat. Protoc 2014, 9, 1348. [DOI] [PubMed] [Google Scholar]; (b) Collins KD; Glorius F A robustness screen for the rapid assessment of chemical reactions. Nat. Chem 2013, 5, 597. [DOI] [PubMed] [Google Scholar]
- (21).Nelson HM; Patel JS; Shunatona HP; Toste FD Enantioselective α-amination enabled by a BINAM-derived phase-transfer catalyst. Chem. Sci 2015, 6, 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).(a) Harej M; Dolenc D Autoxidation of Hydrazones. Some New Insights. J. Org. Chem 2007, 72, 7214. [DOI] [PubMed] [Google Scholar]; (b) Ohkatsu Y; Yoshino K; Tsuruta T Autoxidation of α-Methylbenzylhydrazones. Sekiyu Gakkaishi 1983, 26, 272. [Google Scholar]
- (23).Wappes EA; Vanitcha A; Nagib DA β C-H dihalogenation via iterative hydrogen atom transfer. Chem. Sci 2018, 9, 4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.