Abstract
Background
Due to shared risk factors between coronary artery disease (CAD) and cerebrovascular disease, patients with a history of transient ischemic attack (TIA) or stroke are at greater risk of developing CAD which may require percutaneous coronary intervention (PCI). However, there remains a paucity of research examining outcomes after PCI in these patients.
Methods and Results
We analyzed consecutive patients who underwent PCI between 1/1/13–3/31/16 at 47 Michigan hospitals and identified those with a history of TIA/stroke. We used propensity score matching to adjust for differences in baseline characteristics and compared in-hospital outcomes between patients with and without a history of TIA/stroke. We compared rates of 90-day readmission and long-term mortality in a subset of patients. Among 98,730 patients who underwent PCI, 10,915 had a history of TIA/stroke. After matching (n=10,618 per group), a history of TIA/stroke was associated with an increased risk of in-hospital stroke (aOR 2.04; 95% CI: 1.41–2.96; p<0.001). There were no differences in the risks of other in-hospital outcomes. In a subset of patients with post-discharge data, a history of TIA/stroke was associated with increased risks of 90-day readmission (aOR 1.22, 95% CI: 1.09–1.38; p<0.001) and long-term mortality (HR 1.23, 95% CI: 1.07–1.43; p=0.005).
Conclusions
A history of TIA/stroke was common in patients who underwent PCI and was associated with increased risks of in-hospital stroke, 90-day readmission, and long-term mortality. Given the devastating consequences of post-PCI stroke, patients with a history of TIA/stroke should be counseled on this increased risk prior to undergoing PCI.
Keywords: transient ischemic attack, stroke, percutaneous coronary intervention, outcomes research, cerebrovascular accident
Due to shared risk factors between coronary artery disease (CAD) and cerebrovascular disease, patients with a history of transient ischemic attack (TIA) or stroke frequently undergo PCI. Indeed, approximately one in ten patients undergoing PCI have a history of TIA or stroke.1,2 This common subgroup of patients with CAD may be a higher-risk cohort compared to those without a history of TIA or stroke based on the findings from two recent clinical trials, TRITON-TIMI 38 and TRA 2P-TIMI 50. In these trials, patients with a history of stroke were more likely to experience harm from treatment with prasugrel and vorapaxar, respectively.3,4 Thus, both agents are contraindicated in these patients.5,6 This has led to renewed interest in understanding the risks of short- and long-term outcomes after PCI in patients with a history of TIA or stroke.
Among the few studies evaluating the relationship between a history of TIA or stroke and PCI outcomes, the majority have been limited by single-center study designs,7–9 or an evaluation of in-hospital outcomes only.7,9 Given the recent evidence from RCTs suggesting that patients with a history of stroke are at an increased risk of adverse events,3,4 along with the relatively high prevalence of patients with a history of TIA or stroke undergoing PCI, it is important to understand whether such patients are at an increased risk of in-hospital and post-discharge outcomes after PCI in real-world practice. Therefore, we sought to examine this relationship using a large, contemporary, clinical registry of all PCIs performed at non-federal hospitals in the state of Michigan.
Methods
We performed a retrospective analysis using data collected by the Blue Cross Blue Shield of Michigan Cardiovascular Consortium (BMC2). This is a prospective, multicenter, statewide registry of patients who underwent PCI at all non-federal hospitals in Michigan. A more detailed description of the registry has been described previously.10,11 We evaluated consecutive patients who underwent PCI between January 1, 2013, and March 31, 2016, at 47 PCI-capable hospitals in Michigan. Among this group, we stratified patients by whether they had a history of TIA or stroke. TIA was defined as a loss of neurological function that was abrupt in onset but with complete return of function within 24 hours. Stroke was defined as a loss of neurological function with residual symptoms at least 72 hours after onset. A history of TIA or stroke was determined by review of the medical record by each institution’s trained registry abstractors. Further details regarding the abstraction and auditing process of registry records has been previously described.11
The University of Michigan Institutional Review Board approved the study and determined that it met the definition of research not requiring informed consent. The analytic and statistical methods will be made available through Milan Seth (mcseth@med.umich.edu) to other researchers for purposes of reproducing the results or replicating the procedure. However, the study data set cannot be shared due to our data use agreements.
Primary In-Hospital Outcomes
We compared post-PCI in-hospital outcomes among patients with and without a history of TIA or stroke. All in-hospital outcomes were assessed during the index hospitalization where PCI was performed. The primary outcomes included death due to any cause, stroke, transfusion, bleeding, and major bleeding. Post-PCI stroke was defined as a loss of neurological function caused by an ischemic or hemorrhagic event with residual symptoms lasting at least 24 hours after onset or leading to death. Transfusion was defined as transfusion of whole or packed red blood cells from the start of the procedure to the time of discharge. Bleeding was defined as any suspected or confirmed bleeding event within 72 hours of PCI that was associated with any of the following: 1) drop of ≥3 g/dL; 2) transfusion of whole or packed red blood cells; or 3) procedural intervention/surgery at the bleeding site to stop/reverse, or correct the bleeding. Major bleeding was defined as a drop in baseline hemoglobin of >5g/dL.12 In an exploratory analysis, we also examined whether vascular access site used for PCI modified the association between a patient’s history of TIA or stroke and post-PCI in-hospital stroke.
Post-discharge 90-day Readmission and Long-Term Mortality
Among a subset of patients who could be linked to administrative Medicare claims, we performed a supplemental analysis to evaluate post-discharge outcomes among patients with and without a history of TIA or stroke. The Medicare data was made available through collaboration with the Michigan Value Collaborative.13 The Michigan Value Collaborative constructs 90-day episodes of care using Medicare administrative claims. We linked our clinical registry data to PCI episodes through indirect matching of the index PCI procedure using multiple variables including hospital and operator National Provider Identifier numbers; admission, discharge, and procedure dates for the index hospitalization; and patient gender and date of birth.14,15 After linking the data, we were able to determine post-discharge outcomes such as 90-day readmission and long-term mortality (due to any cause). Readmission was defined as admission to an acute care hospital for any reason within 90 days after discharge from the incident hospitalization or stay when PCI was performed. In addition, only the first readmission was counted per each PCI event. Post-discharge mortality, even beyond the 90-day episode, was obtained from the Medicare beneficiary file which includes the date of death for deceased beneficiaries.
Statistical Analysis
Baseline characteristics were compared between patients with and without a history of TIA or stroke using Pearson chi-square or Fisher’s exact test for categorical variables and Student t tests for continuous variables. For the analysis of in-hospital outcomes, propensity score matching and multivariable analysis were used to adjust for differences in baseline characteristics between those with and without a history of TIA or stroke. A list of the 31 clinical variables can be found in Supplementary Table S1. We required exact matching on coronary artery disease presentation such as non-ST elevation MI and ST-elevation MI. Patients with a history of TIA or stroke were matched in a 1:1 manner to patients without a history of TIA or stroke using a greedy matching algorithm.16
Post-discharge outcomes were analyzed using the unmatched linked data set consisting of clinical and claims-based variables. We used a backward and forward stepwise selection routine with Akaike information criterion to select predictors included in final logistic regression and Cox proportional hazards models used to evaluate the outcomes of 90-day readmission and long-term mortality, respectively.17 The variables included in the logistic regression and Cox proportional hazards models are listed in Supplementary Tables S2 and S3, respectively. Kaplan-Meier curves were used to display differences in cumulative mortality between the two groups of patients. A p-value <0.05 was considered to be statistically significant. All analyses were performed using R version 3.2.1.18
Results
Patient Characteristics
Between January 2013 and March 2016, a total of 98,730 consecutive patients underwent PCI at 47 hospitals in Michigan. Of these, 10,915 (11.1%) had a history of TIA or stroke prior to PCI. Prior to matching, patients with a history of TIA or stroke were more likely to be older, female, and have more cardiovascular comorbidities (Table).
Table.
Baseline characteristics of the unmatched and matched cohort
| Before Matching | After Matching | ||||||
|---|---|---|---|---|---|---|---|
| Characteristics | No History of TIA or stroke (n=87,815) | History of TIA or stroke (n=10,915) | p-value | No History of TIA or stroke (n=10,618) | History of TIA or stroke (n=10,618) | p-value | |
| Age | 64.84 ± 11.94 | 69.19 ± 11.31 | 0.001 | 69.56 ± 11.23 | 69.23 ± 11.29 | 0.031 | |
| Female sex | 27,986/87,815 (31.9%) | 4,404/10,915 (40.3%) | 0.001 | 4,294/10,618 (40.4%) | 4,274/10,618 (40.3%) | 0.780 | |
| White race | 75,929/87,815 (86.5%) | 8,830/10,915 (80.9%) | 0.001 | 8,654/10,618 (81.5%) | 8,590/10,618 (80.9%) | 0.261 | |
| Black race | 9,220/87,815 (10.5%) | 1,842/10,915 (16.9%) | 0.001 | 1,758/10,618 (16.6%) | 1,790/10,618 (16.9%) | 0.556 | |
| Comorbidities | |||||||
| Current/Recent Smoker (w/in 1 year) | 25,670/87,784 (29.2%) | 2,587/10,910 (23.7%) | 0.001 | 2,503/10,618 (23.6%) | 2,511/10,618 (23.6%) | 0.897 | |
| Hypertension | 73,972/87,800 (84.3%) | 10,378/10,913 (95.1%) | 0.001 | 10,215/10,618 (96.2%) | 10,112/10,618 (95.2%) | 0.001 | |
| *Dyslipidemia | 69,993/87,760 (79.8%) | 9,794/10,908 (89.8%) | 0.001 | 9,640/10,618 (90.8%) | 9,540/10,618 (89.8%) | 0.020 | |
| Family History of Premature CAD | 13,788/87,788 (15.7%) | 1,456/10,912 (13.3%) | 0.001 | 1,401/10,618 (13.2%) | 1,421/10,618 (13.4%) | 0.686 | |
| Prior MI | 29,337/87,798 (33.4%) | 5,186/10,915 (47.5%) | 0.001 | 5,054/10,618 (47.6%) | 5,056/10,618 (47.6%) | 0.978 | |
| Prior Heart Failure | 13,831/87,786 (15.8%) | 3,481/10,912 (31.9%) | 0.001 | 3,310/10,618 (31.2%) | 3,401/10,618 (32.0%) | 0.179 | |
| Prior Valve Surgery/Procedure | 1,520/87,778 (1.7%) | 361/10,907 (3.3%) | 0.001 | 368/10,618 (3.5%) | 353/10,618 (3.3%) | 0.570 | |
| Prior PCI | 39,325/87,794 (44.8%) | 5,955/10,914 (54.6%) | 0.001 | 5,914/10,618 (55.7%) | 5,816/10,618 (54.8%) | 0.176 | |
| Prior CABG | 15,013/87,789 (17.1%) | 2,898/10,912 (26.6%) | 0.001 | 2,842/10,618 (26.8%) | 2,840/10,618 (26.7%) | 0.975 | |
| Currently on Dialysis | 1,863/87,749 (2.1%) | 654/10,906 (6.0%) | 0.001 | 554/10,618 (5.2%) | 639/10,618 (6.0%) | 0.011 | |
| Cerebrovascular Disease | 5,127/87,792 (5.8%) | 10,389/10,912 (95.2%) | 0.001 | 1,017/10,618 (9.6%) | 10,113/10,618 (95.2%) | 0.001 | |
| Peripheral Arterial Disease | 12,259/87,798 (14.0%) | 3,122/10,912 (28.6%) | 0.001 | 2,908/10,618 (27.4%) | 3,048/10,618 (28.7%) | 0.033 | |
| Chronic Lung Disease | 16,070/87,797 (18.3%) | 3,239/10,911 (29.7%) | 0.001 | 3,101/10,618 (29.2%) | 3,158/10,618 (29.7%) | 0.391 | |
| Diabetes Mellitus | 33,057/87,789 (37.7%) | 5,739/10,913 (52.6%) | 0.001 | 5,632/10,618 (53.0%) | 5,602/10,618 (52.8%) | 0.680 | |
| Heart Failure w/in 2 Weeks | 10,257/87,785 (11.7%) | 2,078/10,909 (19.0%) | 0.001 | 1,995/10,618 (18.8%) | 2,047/10,618 (19.3%) | 0.363 | |
| Cardiomyopathy or Left Ventricular Systolic Dysfunction | 8,761/87,793 (10.0%) | 1,594/10,914 (14.6%) | 0.001 | 1,562/10,618 (14.7%) | 1,569/10,618 (14.8%) | 0.892 | |
| Coronary artery disease presentation | |||||||
| No symptom, no angina | 3,157/87,794 (3.6%) | 445/10,912 (4.1%) | 0.011 | 433/10,618 (4.1%) | 433/10,618 (4.1%) | 1.000 | |
| Symptom unlikely to be ischemic | 2,037/87,794 (2.3%) | 293/10,912 (2.7%) | 0.018 | 290/10,618 (2.7%) | 290/10,618 (2.7%) | 1.000 | |
| Stable angina | 7,793/87,794 (8.9%) | 917/10,912 (8.4%) | 0.101 | 895/10,618 (8.4%) | 895/10,618 (8.4%) | 1.000 | |
| Unstable angina | 39,667/87,794 (45.2%) | 5,178/10,912 (47.5%) | 0.001 | 5,086/10,618 (47.9%) | 5,086/10,618 (47.9%) | 1.000 | |
| Non-STEMI | 20,274/87,794 (23.1%) | 2,911/10,912 (26.7%) | 0.001 | 2,863/10,618 (27.0%) | 2,863/10,618 (27.0%) | 1.000 | |
| ST-Elevation MI (STEMI) or equivalent | 14,866/87,794 (16.9%) | 1,168/10,912 (10.7%) | 0.001 | 1,051/10,618 (9.9%) | 1,051/10,618 (9.9%) | 1.000 | |
| Pre-procedural and procedural variables | |||||||
| Cardiogenic Shock within 24 Hours | 1,668/87,795 (1.9%) | 241/10,912 (2.2%) | 0.027 | 209/10,618 (2.0%) | 229/10,618 (2.2%) | 0.334 | |
| Cardiac Arrest w/in 24 Hours | 1,828/87,790 (2.1%) | 182/10,913 (1.7%) | 0.004 | 152/10,618 (1.4%) | 173/10,618 (1.6%) | 0.240 | |
| IABP | 2,166/87,797 (2.5%) | 248/10,915 (2.3%) | 0.214 | 261/10,618 (2.5%) | 232/10,618 (2.2%) | 0.186 | |
| Other Mechanical Ventricular Support | 927/87,786 (1.1%) | 159/10,912 (1.5%) | 0.001 | 143/10,615 (1.3%) | 152/10,615 (1.4%) | 0.598 | |
| Arterial Access Site: Femoral | 59,614/87,804 (67.9%) | 7,995/10,914 (73.3%) | 0.001 | 7,557/10,616 (71.2%) | 7,774/10,617 (73.2%) | 0.001 | |
| Arterial Access Site: Radial | 27,940/87,804 (31.8%) | 2,864/10,914 (26.2%) | 0.001 | 3,021/10,616 (28.5%) | 2,792/10,617 (26.3%) | 0.001 | |
| Pre-PCI Left Ventricular Ejection Fraction | 51.93 ± 12.84 | 50.23 ± 13.88 | 0.001 | 50.42 ± 13.87 | 50.24 ± 13.90 | 0.414 | |
| Cardiogenic Shock at Start of PCI | 1,886/87,769 (2.1%) | 237/10,913 (2.2%) | 0.876 | 205/10,618 (1.9%) | 224/10,618 (2.1%) | 0.354 | |
| Pre-Procedure Creatinine | 1.14 ± 0.94 | 1.42 ± 1.46 | 0.001 | 1.38 ± 1.38 | 1.42 ± 1.46 | 0.056 | |
| Pre-Procedure Hemoglobin | 13.51 ± 1.93 | 12.63 ± 2.04 | 0.001 | 12.65 ± 2.04 | 12.64 ± 2.04 | 0.580 | |
| In-hospital outcomes | |||||||
| Stroke | 244/87,770 (0.3%) | 91/10,913 (0.8%) | 0.001 | 43/10,612 (0.4%) | 86/10,616 (0.8%) | 0.001 | |
| RBC/Whole Blood Transfusion | 2,042/87,769 (2.3%) | 471/10,913 (4.3%) | 0.001 | 462/10,611 (4.4%) | 449/10,616 (4.2%) | 0.654 | |
| NCDR bleeding | 2,869/87,768 (3.3%) | 470/10,913 (4.3%) | 0.001 | 419/10,612 (3.9%) | 451/10,616 (4.2%) | 0.270 | |
| Major bleeding | 852/71,933 (1.2%) | 96/9,342 (1.0%) | 0.184 | 96/9,142 (1.1%) | 93/9,222 (1.0%) | 0.780 | |
| Discharge Status: Deceased | 1,353/87,815 (1.5%) | 259/10,915 (2.4%) | 0.001 | 227/10,618 (2.1%) | 251/10,618 (2.4%) | 0.267 | |
All percentages represent frequencies. Where nominal values are used, they are presented as mean +− standard deviation.
Abbreviations: NCDR = National Cardiovascular Data Registry; Non-STEMI=Non ST-elevation myocardial infarction; STEMI=ST-elevation myocardial infarction; PCI=percutaneous coronary intervention.
In-Hospital Outcomes
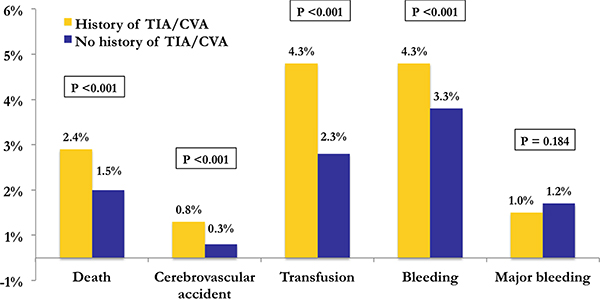
Patients with a history of TIA or stroke had significantly higher rates of post-PCI adverse outcomes including death (2.4% vs 1.5%; p<0.001), stroke (0.8% vs 0.3%; p<0.001), blood transfusion (4.3% vs 2.3%; p<0.001), and bleeding (4.3% vs 3.3%; p<0.001; Figure 1). However, there was no significant difference in the rate of major bleeding (1.0% vs 1.2%; p=0.184).
Figure 1: In-hospital outcome rates in the unmatched cohort.
Bar graph of primary in-hospital outcomes of patients with (yellow) and without (blue) a history of TIA or stroke in the unmatched cohort. Outcome rates and p-values are noted above each bar.
Abbreviations: None
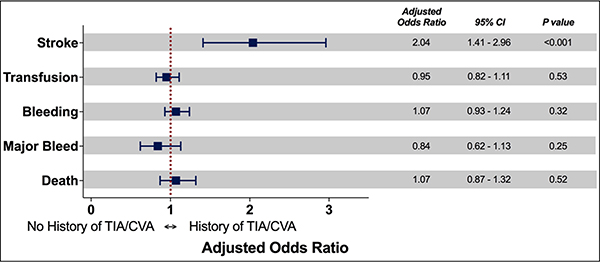
After propensity score matching, 10,618 patients were included in each group. The absolute standardized differences were <10% on all matched variables, indicating acceptable covariate balance between the two groups (Figure 2). Among the matched cohort, patients with a history of TIA or stroke had a significantly higher risk of post-PCI stroke (adjusted odds ratio [aOR]: 2.04; 95% CI: 1.41–2.96; p<0.001). In contrast to the findings from the unmatched analysis, there were no significant differences in the risk of transfusion (aOR: 0.95; 95% CI: 0.82–1.11; p=0.53), bleeding (aOR: 1.07; 95% CI: 0.93–1.24; p=0.32), major bleeding (aOR: 0.84; 95% CI: 0.62–1.13; p=0.25), or death (aOR: 1.07; 95% CI: 0.87–1.32; p=0.52) (Figure 3).
Figure 2: Plot of absolute standardized differences before and after matching.
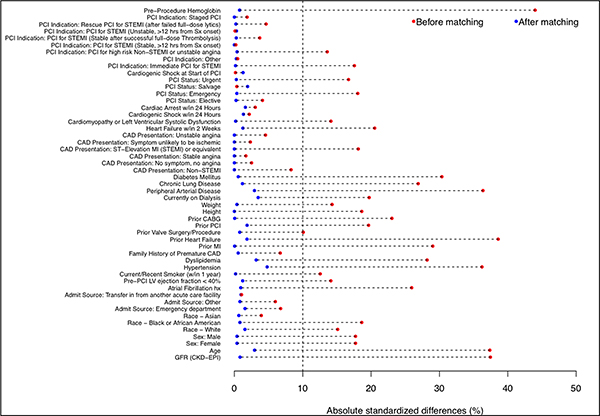
Absolute standardized differences before and after matching patients with and without a history of TIA or stroke.
Abbreviations: CABG = coronary artery bypass grafting; CAD = coronary artery disease; CVD = cerebrovascular disease; HF = heart failure; LVEF = left ventricular ejection fraction; MI = myocardial infarction; PAD = peripheral artery disease; PCI = percutaneous coronary intervention; STEMI = ST-segment elevation myocardial infarction; Sx = symptoms.
Figure 3: Adjusted odds ratios of in-hospital outcomes in the matched cohort.
Forest plot demonstrating adjusted odds ratios and confidence intervals of in-hospital outcomes in the matched cohort.
Abbreviations: CI = confidence interval
The association between a patient’s history of TIA or stroke and post-PCI in-hospital stroke was not significantly modified by the vascular access site chosen for PCI. Among patients with a history of TIA or stroke, the rate of post-PCI in-hospital stroke was 0.93% and 0.77% for patients who underwent radial versus femoral PCI, respectively.
Post-Discharge 90-Day Readmission and Long-Term Mortality
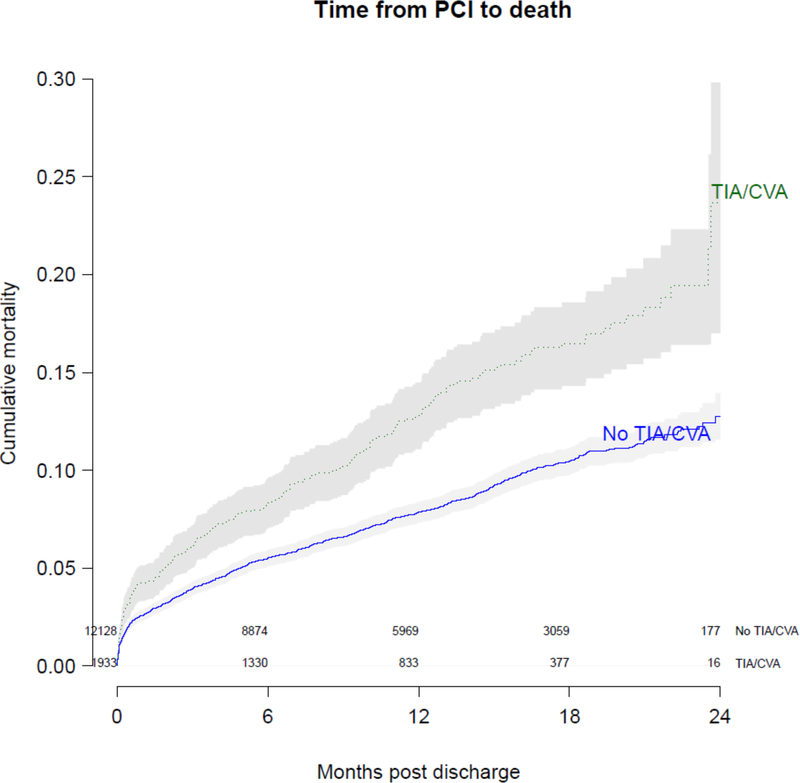
A total of 14,061 patients who underwent PCI in the BMC2 PCI registry were linked to administrative Medicare claims. The baseline characteristics of these patients can be found in Supplementary Table S4. Among this subgroup of patients, 1,933 (13.6%) had a history of TIA or stroke. Patients with a history of TIA or stroke had an increased risk of 90-day all-cause readmission (aOR 1.22; 95% CI: 1.09–1.38; p<0.001) and an increased hazard of mortality (hazard ratio 1.23, 95% CI: 1.07–1.43; p=0.005) compared with patients without a history of TIA or stroke. The unadjusted survival curves for patients with and without a history of TIA or stroke are shown in Figure 4.
Figure 4: Kaplan-Meier curve of long-term mortality among Medicare patients who underwent PCI.
Curve demonstrates cumulative long-term mortality in a subset of patients with (green dashed line) and without a history of transient ischemic attack (TIA) or cerebrovascular accident (CVA) (blue solid line). The solid gray bars represent the 95% confidence interval around the estimates of cumulative mortality.
Abbreviations: None
Discussion
There are two primary findings from our study. First, a history of TIA or stroke was common among patients who underwent PCI and was associated with a two-fold increased risk of post-PCI in-hospital stroke without significant differences in the risks of in-hospital bleeding, transfusion, or death. Second, among the subset of patients who could be linked to Medicare claims data, patients with a history of TIA or stroke experienced an increased risk of 90-day readmission and long-term mortality after PCI compared with patients without a history of TIA or stroke.
The absolute incidence of post-PCI stroke in our study was low (0.36%), similar to findings from prior research with incidences ranging between 0.15%−0.42%.2,9,19,20 However, patients with a history of TIA or stroke had a two-fold increased risk of post-PCI stroke. Prior studies have evaluated the relationship between a history of TIA or stroke and post-PCI stroke and arrived at conflicting conclusions. For instance, similar to the findings from our study, Upadhya et al and Li et al demonstrated that patients with a history of TIA or stroke have an increased risk of post-PCI stroke.9,21 Whereas, Zhang et al and Sasao et al found no significant differences in rates of post-PCI stroke in patients with and without a history of TIA or stroke.7,8 Our findings underscore the importance of appropriately counseling patients with a history of TIA or stroke on the risks of post-PCI in-hospital stroke. An open conversation of patient-specific risks and benefits of PCI is integral to the process of shared decision-making. At the very least, patients with a history of TIA or stroke undergoing diagnostic coronary angiography with possible ad hoc PCI should routinely be informed of their increased risk of post-procedural in-hospital stroke.
We also found that patients with a history of TIA or stroke had a higher risk of 90-day readmission and long-term mortality. This may be related to the fact that atherosclerotic disease is present in at least two critical arterial beds in these patients, thus increasing the vulnerability of these patients to devastating or fatal events.22,23 Similar to our findings, Li et al found that patients with a history of TIA or stroke had an increased risk of long-term mortality after PCI.21 However, their study did not evaluate the relationship with readmission, a costly event associated with adverse patient outcomes.24 Moreover, in the current era of healthcare payment reform including the implementation of bundled payment models, effectively identifying patients with an increased risk of readmission, and preventing these costly events will be increasingly important.25–27 More broadly, given the signals of harm seen in this population in two contemporary randomized controlled trials of antithrombotic therapies,3,4 the increased risk of post-discharge adverse outcomes may be related to the challenges of long-term antithrombotic therapy in this population. The mechanisms responsible for the increased risk of post-discharge complications in these patients remains unclear, highlighting the need for further research in this prevalent, high-risk population.
Study Limitations
Our study has several important limitations. First, although we attempted to account for differences in baseline characteristics among patients with and without a history of TIA or stroke through propensity score matching, we were unable to account for all potential confounders given the observational nature of the study. Second, all hospitals participating in the BMC2 PCI clinical registry are engaged in statewide collaborative quality improvement initiatives. Therefore, our findings may not be generalizable to hospitals in other states that do not participate in such initiatives.28 Finally, among patients with a history of TIA or stroke, it may have been difficult to differentiate whether post-PCI stroke symptoms were due to new cerebrovascular events or if they were due to post-stroke recrudescence of symptoms. Recrudescence of stroke symptoms may be caused by the use of sedating medications frequently used during PCI.29 This may have led to an overestimation of the incidence of post-PCI stroke in the subgroup of patients with a history of TIA or stroke. However, our definition of post-PCI stroke required symptoms to be present for at least 24 hours, whereas among patients with symptoms due to post-stroke recrudescence, the majority have resolution of symptoms within 24 hours.29
Conclusion
In this retrospective observational analysis of patients who underwent PCI, a history of TIA or stroke prior to PCI was common and associated with a two-fold increased risk of post-PCI in-hospital stroke without significant differences in the risks of in-hospital bleeding, transfusion, or death. Our study provides important real-world evidence that a history of TIA or stroke is associated with adverse outcomes after PCI. Although the absolute increase in the risk of post-PCI stroke is small and may not dissuade practitioners from recommending PCI, given the possible devastating consequences of post-PCI stroke, we believe that patients with a history of TIA or stroke should be counseled on this increased risk in the current era of shared decision-making. In addition, further research is needed to understand the mechanisms responsible for the increased risk of post-PCI stroke and post-discharge adverse outcomes in this high-risk subgroup of patients so that effective strategies can be developed to reduce this risk.
Supplementary Material
What is Known.
Patients with a history of TIA or stroke are at greater risk of developing CAD that may require PCI.
What the Study Adds.
Using data from a large, multicenter clinical registry, we found that a history of TIA or stroke is common (11.1%) among patients undergoing PCI.
In addition, a history of TIA or stroke was associated with a higher risk of post-PCI stroke, 90-day readmission, and long-term mortality compared with patients without a history of TIA or stroke.
Such patients should be appropriately counseled on these risks prior to PCI.
Acknowledgements
The authors are indebted to all the study coordinators, investigators, and patients who participated in the BMC2 registry.
Sources of Funding
Dr. Sukul is supported by the National Institutes of Health T32 postdoctoral research training grant (T32-HL007853). This work was supported by the Blue Cross Blue Shield of Michigan and Blue Care Network as part of the Blue Cross Blue Shield of Michigan Value Partnerships program. The funding source supported data collection at each site and funded the data-coordinating center but had no role in study concept, interpretation of findings, or in the preparation, final approval or decision to submit the manuscript.
Disclosures
Hitinder S. Gurm receives research funding from Blue Cross Blue Shield of Michigan, the National Institutes of Health and is a consultant for Osprey Medical.
None of the authors have any relationships with industry directly relevant to this study.
Abbreviations
- PCI
percutaneous coronary intervention
- TIA
transient ischemic attack
- BMC2
Blue Cross Blue Shield of Michigan Cardiovascular Consortium
- Aor
adjusted odds ratio
- HR
hazard ratio
Footnotes
Disclaimer: Although Blue Cross Blue Shield of Michigan (BCBSM) and BMC2 work collaboratively, the opinions, beliefs and viewpoints expressed by the authors do not necessarily reflect the opinions, beliefs and viewpoints of BCBSM or any of its employees.
References
- 1.Aggarwal A, Dai D, Rumsfeld JS, Klein LW, Roe MT. Incidence and Predictors of Stroke Associated With Percutaneous Coronary Intervention. Am J Cardiol. 2009;104:349–353. [DOI] [PubMed] [Google Scholar]
- 2.Didier R, Gaglia MA, Koifman E, Kiramijyan S, Negi SI, Omar AF, Gai J, Torguson R, Pichard AD, Waksman R. Cerebrovascular accidents after percutaneous coronary interventions from 2002 to 2014: Incidence, outcomes, and associated variables. Am Heart J. 2016;172:80–87. [DOI] [PubMed] [Google Scholar]
- 3.Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, Neumann F-J, Ardissino D, De Servi S, Murphy SA, Riesmeyer J, Weerakkody G, Gibson CM, Antman EM. Prasugrel versus Clopidogrel in Patients with Acute Coronary Syndromes. N Engl J Med. 2007;357:2001–2015. [DOI] [PubMed] [Google Scholar]
- 4.Morrow DA, Braunwald E, Bonaca MP, Ameriso SF, Dalby AJ, Fish MP, Fox KAA, Lipka LJ, Liu X, Nicolau JC, Ophuis AJO, Paolasso E, Scirica BM, Spinar J, Theroux P, Wiviott SD, Strony J, Murphy SA. Vorapaxar in the Secondary Prevention of Atherothrombotic Events. N Engl J Med. 2012;366:1404–1413. [DOI] [PubMed] [Google Scholar]
- 5.Vorapaxar [package insert]. Merck & Co; [Internet]. [cited 2017 Nov 27];Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/204886s000lbl.pdf [Google Scholar]
- 6.Prasugrel [package insert]. Eli Lilly and Company; [Internet]. [cited 2017 Nov 27];Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/022307s002lbl.pdf [Google Scholar]
- 7.Zhang M, Guddeti RR, Wang S, Wang J, Xin M, Chen S-J, Kang J-P, Lv Q, Ma C-S, Liu J-H. Prior ischemic stroke is not associated with worse clinical outcomes in patients undergoing percutaneous coronary intervention. Clin Invest Med. 2014;37:196–202. [DOI] [PubMed] [Google Scholar]
- 8.Sasao H, Fujiwara H, Horiuchi N, Shirasaki S, Sakai I, Tsuchida K, Murai H. Comparison of Long-Term Clinical Outcomes after Drug-Eluting Stent Implantation in Patients with Coronary Artery Disease with and without Prior Cerebral Infarction. Ann Vasc Dis. 2015;8:79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Upadhya B, Sane DC, Applegate RJ, Kutcher MA, Gandhi SK, Deliargyris EN. Differences in baseline characteristics and in-hospital outcomes in patients with or without prior stroke undergoing percutaneous coronary intervention. J Invasive Cardiol. 2005;17:243–247. [PubMed] [Google Scholar]
- 10.Kline-Rogers E, Share D, Bondie D, Rogers B, Karavite D, Kanten S, Wren P, Bodurka C, Fisk C, Mcginnity J, Wright S, Fox S, Eagle KA, Moscucci M. Development of a Multicenter Interventional Cardiology Database: The Blue Cross Blue Shield of Michigan Cardiovascular Consortium (BMC2) Experience. J Intervent Cardiol. 2002;15:387–392. [DOI] [PubMed] [Google Scholar]
- 11.Moscucci M, Rogers EK, Montoye C, Smith DE, Share D, O’Donnell M, Maxwell-Eward A, Meengs WL, Franco ACD, Patel K, McNamara R, McGinnity JG, Jani SM, Khanal S, Eagle KA. Association of a Continuous Quality Improvement Initiative With Practice and Outcome Variations of Contemporary Percutaneous Coronary Interventions. Circulation. 2006;113:814–822. [DOI] [PubMed] [Google Scholar]
- 12.NCDR® CathPCI Registry® v4.4 Coder’s Data Dictionary. [cited 2017 Oct 25];Available from: https://www.ncdr.com/WebNCDR/docs/public-data-collection-documents/cathpci_v4_codersdictionary_4-4.pdf
- 13.Herrel LA, Syrjamaki JD, Linsell SM, Miller DC, Dupree JM. Identifying Drivers of Episode Cost Variation With Radical Prostatectomy. Urology. 2016;97:105–110. [DOI] [PubMed] [Google Scholar]
- 14.Parsh J, Seth M, Briguori C, Grossman P, Solomon R, Gurm HS. The optimal definition of contrast-induced acute kidney injury for prediction of inpatient mortality in patients undergoing percutaneous coronary interventions. Am Heart J. 2016;175:160–167. [DOI] [PubMed] [Google Scholar]
- 15.Song C, Sukul D, Seth M, Dupree JM, Khandelwal A, Dixon SR, Wohns D, LaLonde T, Gurm HS. Ninety-Day Readmission and Long-Term Mortality in Medicare Patients (≥65 Years) Treated With Ticagrelor Versus Prasugrel After Percutaneous Coronary Intervention (from the Blue Cross Blue Shield of Michigan Cardiovascular Consortium). Am J Cardiol. 2017;120:1926–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gu XS, Rosenbaum PR. Comparison of Multivariate Matching Methods: Structures, Distances, and Algorithms. J Comput Graph Stat. 1993;2:405–420. [Google Scholar]
- 17.Bozdogan H Model selection and Akaike’s Information Criterion (AIC): The general theory and its analytical extensions. Psychometrika. 1987;52:345–370. [Google Scholar]
- 18.Team RC. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; Available online at: www.R-Project.org 2014. [Google Scholar]
- 19.Korn-Lubetzki I, Farkash R, Pachino RM, Almagor Y, Tzivoni D, Meerkin D. Incidence and Risk Factors of Cerebrovascular Events Following Cardiac Catheterization. J Am Heart Assoc. 2013;2:e000413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dukkipati S, O’Neill WW, Harjai KJ, Sanders WP, Deo D, Boura JA, Bartholomew BA, Yerkey MW, Sadeghi HM, Kahn JK. Characteristics of cerebrovascular accidents after percutaneous coronary interventions. J Am Coll Cardiol. 2004;43:1161–1167. [DOI] [PubMed] [Google Scholar]
- 21.Li Y-J, Rha S-W, Chen K-Y, Jin Z, Minami Y, Wang L, Dang Q, Poddar KL, Ramasamy S, Park J-Y, Oh DJ, Jeong MH, the Korea Acute Myocardial Infarction Registry Investigators. Clinical characteristics and mid-term outcomes of acute myocardial infarction patients with prior cerebrovascular disease in an Asian population: Lessons from the Korea Acute Myocardial Infarction Registry. Clin Exp Pharmacol Physiol. 2010;37:581–586. [DOI] [PubMed] [Google Scholar]
- 22.Cox AJ, Hsu F-C, Agarwal S, Freedman BI, Herrington DM, Carr JJ, Bowden DW. Prediction of mortality using a multi-bed vascular calcification score in the Diabetes Heart Study. Cardiovasc Diabetol. 2014;13:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elias-Smale SE, Odink AE, Wieberdink RG, Hofman A, Hunink MGM, Krestin GP, Koudstaal PJ, Breteler MMB, van der Lugt A, Witteman JCM. Carotid, aortic arch and coronary calcification are related to history of stroke: The Rotterdam Study. Atherosclerosis. 2010;212:656–660. [DOI] [PubMed] [Google Scholar]
- 24.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among Patients in the Medicare Fee-for-Service Program. N Engl J Med. 2009;360:1418–1428. [DOI] [PubMed] [Google Scholar]
- 25.Guduguntla V, Syrjamaki JD, Ellimoottil C, Miller DC, Prager RL, Norton EC, Theurer P, Likosky DS, Dupree JM. Drivers of Payment Variation in 90-Day Coronary Artery Bypass Grafting Episodes. JAMA Surg. 2018;153:14–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ellimoottil C, Li J, Ye Z, Dupree JM, Min HS, Kaye D, Herrel LA, Miller DC. Episode-Based Payment Variation for Urological Cancer Surgery. Urology. 2017;111:78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Birkmeyer JD, Gust C, Baser O, Dimick JB, Sutherland JM, Skinner JS. Medicare payments for common inpatient procedures: implications for episode-based payment bundling. Health Serv Res. 2010;45:1783–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Share DA, Campbell DA, Birkmeyer N, Prager RL, Gurm HS, Moscucci M, Udow-Phillips M, Birkmeyer JD. How A Regional Collaborative Of Hospitals And Physicians In Michigan Cut Costs And Improved The Quality Of Care. Health Aff (Millwood). 2011;30:636–645. [DOI] [PubMed] [Google Scholar]
- 29.Topcuoglu MA, Saka E, Silverman SB, Schwamm LH, Singhal AB. Recrudescence of Deficits After Stroke: Clinical and Imaging Phenotype, Triggers, and Risk Factors. JAMA Neurol. 2017;74:1048–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.