Abstract
Fatigability is defined as the extent of fatigue in the context of activity and differs from the term used in exercise literature to describe muscle endurance characteristics. Many fatigability measures are available, but no studies have thoroughly evaluated them for adequate incorporation of fatigability concepts. This integrative review provides an overall assessment of existing fatigability measures and then evaluates each in depth. A database search and hand search produced 14 studies for review. Fatigability measurement took three forms: self-reported fatigability, perceived fatigability (self-reported fatigue following a defined performance test), and performance fatigability (performance deterioration). Of 17 measures identified, validity and/or reliability was reported for six (35.3%), and no measure was used in more than one study. Fatigability measures have been correlated with clinical measures, indicating that fatigability should be measured during routine clinical health screening. Refinement of measures and additional fatigability data collection will improve understanding and treatment of fatigue.
Keywords: Fatigability, Fatigue, Physical activity
Introduction
Fatigue refers to global self-reported tiredness, exhaustion, lack of energy, and weariness1 and is a common complaint in older adults, although experienced by people of every age. Fatigue in older adults is associated with poor mobility, functional limitations, and mortality.2,3 Assessing fatigue and its impact on physical activity, however, is challenging given the propensity to modify activities to maintain feelings of fatigue within an acceptable range; referred to as self-pacing.1 For example, different people may rate their fatigue at the same level, however, the impact of similarly-rated fatigue levels on physical activity likely differs from individual to individual. The concept of fatigability addresses this relationship between fatigue and physical activity. Eldadah (2010) defined fatigability as the degree of fatigue experienced during performance of a defined activity, which normalized fatigue to activity level. Understanding fatigability, therefore, can provide insight into the extent to which fatigue actually interferes with physical activity and this is important for evaluating the impact of fatigue on physical activity and vice versa.
Fatigability, a relatively new concept in the geriatric literature, has generated a great deal of research interest. Because this work is still in its early stages, multiple definitions of fatigability exists leading to conceptual confusion and wide variations in measurement. For example, in some studies, fatigability was defined as fatigue in relation to a defined activity of a specific intensity and duration.4,5 Other studies defined fatigability as a change in performance, which included performance deterioration, or self-reported fatigue in response to physical activity, which included changes in perceived exertion.3,6 Two key points about fatigability have emerged from the literature: (1) fatigability is defined as a change in perceived fatigue in the context of activity, and (2) the activity or task must be standardized in terms of duration, intensity, and frequency. Arriving at a clear understanding of fatigability is important, as fatigue, physical inactivity, and the resulting fatigability likely play a role in the development of frailty, a common geriatric syndrome.7
Many measures are available for measuring fatigability, and these measures have been applied in various ways depending on the conceptual definition of fatigability used by researchers. Moreover, fatigability measurement is challenging because fatigue as a subjective symptom must be self-reported. Conversely, although physical activity may be self-reported, objective measurement of physical activity is preferred because it is more precise. In addition, there is no consensus about how to best measure fatigability, and there has been no systematic evaluation of how well each measure incorporates fatigability concepts.
Therefore, this review of fatigability instruments and measurement techniques was performed to help identify reliable and valid measures for use in future research. This integrative review was conducted in two phases. The purpose of phase one was to perform an overall assessment of fatigability measurement characteristics and research findings. Phase two was intended to provide an in-depth evaluation of individual fatigability measures in order to determine (1) how fatigue and activity were quantified; (2) how a fatigability score was calculated, including the type of scaling used; and (3) whether reliability and validity testing was reported.
Methods
Publications included in the integrative review were identified through literature searches of PubMed, CINAHL, and Embase using the combined terms “fatigability AND fatigue.” The option “Limit to terms indexed in article as major focus” was chosen for the Embase search in an effort to limit the articles to only those that primarily focused on fatigue and fatigability. The references of the studies obtained through computer indexing were examined to locate any additional articles not indexed in the literature databases. Only quantitative studies published between January 2010 and January 2016 were included in this review. The year 2010 was selected because fatigability was newly defined at that time in the fifth Bedside-to-Bench conference of the American Geriatrics Society.8 This conference defined fatigability as “a phenotype describing the change in fatigue level as a function of the change in intensity, duration, or frequency of activity” (p. 969). This definition contrasts with previous definitions of fatigability that relied mainly on the physiological phenomenon of skeletal muscle fatigability. Articles were included in the review if they met the following criteria: (1) quantitative research published in English, (2) research participants included adult patients or healthy controls, and (3) fatigability was conceptualized as perceived fatigue in the context of a defined activity level. Abstracts, unpublished studies, and review papers were not included in the review.
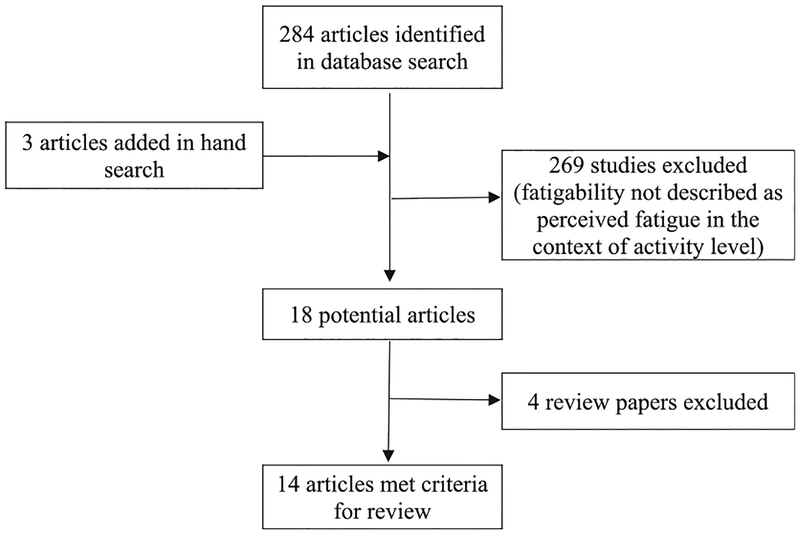
The search of the databases yielded the following results: 267 articles in PubMed, 74 articles in Embase, and 83 articles in CINAHL. The abstracts for all the articles were reviewed, and substantial overlapping of articles among the databases was found. After duplicates were removed, 284 articles were identified as potentially relevant. Three additional articles were identified by hand-searching the reference lists of the 284 identified articles. Of the 284 articles, 14 met the inclusion criteria and were included in this study (Fig. 1).
Fig. 1.
Flowchart of search and selection strategy.
Results
General study characteristics
During an initial review of the 14 research articles, their methodological characteristics were assessed (Table 1). Most of the studies (85.7%) examined fatigability in an elderly population, but only two (14.3%) focused on fatigability in patients with chronic illness. The sample sizes varied considerably among the studies, with some enrolling as few as 17 subjects and some as many as 1,181; however, most studies (71%) enrolled fewer than 100 subjects. In terms of design, 10 of the studies (71.5%) used a cross-sectional design, three studies (21.4%) employed a retrospective research design, and only one measured fatigability at two time periods using a prospective design. Perceived fatigability, which is defined as self-reported fatigue following a defined performance test, was the measure most frequently used in the studies (71.4%): five studies (35.7%) measured fatigability as both perceived fatigability and performance fatigability (performance deterioration), and the other five (35.7%) measured only perceived fatigability in their research.
Table 1.
General characteristics of fatigability studies.
| Study characteristic | N | % |
|---|---|---|
| Sample Population | ||
| Elderly | 12 | 85.7 |
| Chronic illness patients | 2 | 14.3 |
| Sample size | ||
| ≥100 subjects | 4 | 28.5 |
| <100 subjects | 10 | 71.5 |
| Research design | ||
| Cross-sectional | 10 | 71.5 |
| Prospective | 1 | 7.1 |
| Retrospective | 3 | 21.4 |
| Fatigability measurement | ||
| Self-reported fatigability (a) | 2 | 14.3 |
| Perceived fatigability--self-reported fatigue following a defined performance Test (b) | 5 | 35.7 |
| Performance fatigability--Performance deterioration (c) | 1 | 7.1 |
| (a) & (b) | 1 | 7.1 |
| (b) & (c) | 5 | 35.7 |
Phase one
Overall, 14 research articles met the criteria for inclusion in the review. They are described in detail in Table 2. The purpose of phase one was to characterize fatigability measurement in recent research, and such measurement was found to take one of three forms: (1) self-reported fatigability, (2) perceived fatigability (self-reported fatigue following a defined performance test), and (3) performance fatigability (performance deterioration). For both perceived and performance fatigability, measurements employed performance-based assessments.
Table 2.
Overview of fatigability studies.
| Study and subjects | Design | Study aim | Measures used to assess fatigability | Major findings |
|---|---|---|---|---|
| Barbosa et al., 2015 N = 48 Elderly women |
Cross-sectional | To determine the relationship between perceived fatigability and oxygen consumption (VO2), carbon dioxide production, respiratory exchange ratio, and the energy cost of walking in older women. | • Fatigability • Severity of perceived fatigability • Severity of performance fatigability |
Severity of perceived fatigability was correlated with greater VO2, low physical activity, low walking distance, and severity of performance fatigability. |
| Buchowski et al., 2013 N = 17 Elderly individuals |
Cross-sectional | To measure changes in performance and fatigue across a series of standardized physical tasks that required different levels of energy expenditure and to explore the relationships among these measures. | • Changes in perceived fatigue • Changes in performance • Performance fatigability severity • Perceived fatigability severity |
Performance and perceived fatigability severity scores were correlated, and both perceived and performance fatigability severity scores were associated with physical activity-related energy expenditure. |
| Glynn et al., 2015 N = 1,013; 483 (for validation) Elderly individuals (aged ≥60) |
Cross-sectional | To describe the development of the Pittsburgh Fatigability Scale and to establish its reliability and concurrent and convergent validity against performance measures. | • The Pittsburgh Fatigability Scale instrument | Physical fatigability scores of the instrument, adjusted for age, gender, and race, were greater for those with high performance fatigability, slow gait speed, worse physical function, and lower fitness. |
| Gonzales et al., 2015 N = 45 Elderly individuals (aged 60–78) |
Cross- sectional | To test the hypothesis that central (carotid artery) and peripheral (superficial femoral artery, SFA) arterial stiffness would be associated with perceived fatigability during walking in older adults | • Perceived fatigability | The SFA β-index was identified as an independent predictor of perceived fatigability. |
| Keyser et al., 2015 N = 13 Physically inactive patients with interstitial lung disease |
Prospective (one-group pretest-posttest design) | To examine the hypothesis that variability in cardiorespiratory function could exert a modulating influence on fatigability that is independent of variability in the maximum capacity of the system. | • Performance fatigability | Performance fatigability was reduced 11% in patients with interstitial lung disease after aerobic exercise training. |
| Lin, Roiland, Heffner et al., 2014 N = 49 Elderly individuals (aged 75+) with vascular risk |
Cross-sectional | To test a new way of measuring objective mental fatigability by examining its association with perceived mental fatigability and to identify associated psychological, physiological, and situational predictors. | • Objective mental fatigability • Perceived mental fatigability |
Objective mental fatigability and perceived mental fatigability were correlated, and they were not associated with the same factor. |
| Lin, Roiland, Polesskaya et al., 2014 N = 55 Elderly individuals |
Retrospective | To investigate the effect of fatigability on cognitive processes and inflammatory response (IL-6) following an acute cognitive stress task in older adults | • Perceived fatigability | The high fatigability cluster had higher levels of IL-6 response than the low fatigability cluster. After controlling for multiple covariates, fatigability was found to moderate the relationship between processing speed and IL-6 reactivity. |
| Manty et al., 2012 N = 1,181 Elderly individuals (nonagen-arians) |
Cross-sectional | To evaluate the prevalence and associated health factors of indoor mobility- related fatigability among nonagenarians | • Two items of the Avlund Mobility-Tiredness Scale | Fatigability was associated with cardiovascular diseases, musculoskeletal pain, medications, walking speed, and depressive symptoms. |
| Murphy & Smith, 2010 N = 60 (40 osteoarthritis patients and 20 controls) |
Cross-sectional | To present a new measurement method for fatigability and begin to test its validity. | • The increase in fatigue severity after a period of high activity (1 standard deviation above mean activity) measured using accelerometers | Subjects with osteoarthritis (OA) were about four times more likely to have an increase in fatigue after a high-activity interval than controls. Among subjects with OA, average fatigue and fatigability were not highly related. Fatigability was most strongly associated with body mass index, OA severity, and knee strength, but fatigue was most strongly associated with reported physical function, pain, and vitality. |
| Richardson et al., 2014 N = 36 Elderly individuals |
Cross-sectional | To examine the hypotheses that (a) slower preferred gait speed is associated with higher energetic requirements during walking and higher levels of fatigability and (b) preferred gait speed is not associated with fatigue. | • Situational Fatigue Scale • Rating of perceived exertion (RPE) at the end of a 400-meter walk • RPE after walking at standard speed (0.72 m/s) on a treadmill • RPE after walking at subject-preferred speed on a treadmill |
Slow walkers reported higher RPE after walking and greater overall fatigability on the scale but no differences in fatigue. |
| Santanasto et al., 2014 N = 30 Elderly individuals |
Cross-sectional | To examine the effects of higher and lower fatigability on lower skeletal muscle oxidative capacity. | • RPE using the Borg scale after a 5-min treadmill walk | Subjects with high fatigability required a higher proportion of VO2 peak to walk at 0.72 m/s compared to those with low fatigability. |
| Schnelle et al., 2012 N = 43 Elderly individuals |
Descriptive, cross-sectional | To document the stability, concurrent validity, and clinical correlates of two fatigability severity measures recommended by the American Geriatrics Society. | • Perceived fatigability severity • Performance fatigability severity |
Perceived fatigability severity was related to performance fatigability severity, “tired today,” gait speed, activity counts, fatigue severity, frailty, and gender. Performance fatigability severity was related to perceived fatigability severity, “tired today,” gait speed, activity counts, fatigue severity, and frailty. |
| Simonsick et al., 2014 N = 605 Elderly individuals |
Retrospective (cross-sectional analysis of data from the Baltimore Longitudinal Study of Aging) | To evaluate the criterion validity of two measures of fatigability defined as (a) perceived effort to perform a standardized task and (b) performance deterioration. | • Perceived exertion • Performance deterioration |
High fatigability in perceived exertion was associated with tiredness, weakness, and reported and observed mobility deficits. High fatigability in performance deterioration was strongly associated with self-reported fatigue and walking ability but weakly associated with performance-based mobility measures. |
| Simonsick et al., 2016 N = 602 Elderly individuals |
Retrospective (cross-sectional analysis of data from the Baltimore Longitudinal Study of Aging) | To extend previous work by examining (a) free thyroxine (FT-4) levels in men and women spanning a 30-year age range from 68 to 97 and (b) their association with functional mobility, fitness, and fatigue. | • RPE using the Borg scale after a slow-paced 5-min walk (0.67 m/s) | Adjusting for gender, age, race, height, weight, exercise, and smoking, researchers found that reported walking ability, usual and rapid gait speed, 400-meter time, fatigability, and reported energy level were less favorable with increasing FT4 levels. |
Self-reported fatigability
Three studies used a self-reported measure of fatigability.4,9,10 One study developed a self-reported instrument specifically for fatigability.4 The other two studies used instruments originally developed to measure fatigue in specific activities of daily life.11,12 The numbers of items in the three instruments varied from two to 13.
Perceived fatigability (self-reported fatigue following a defined performance test)
In 10 of 14 studies (71.4%), perceived fatigability was measured using a self-reported fatigue score following a defined performance test. Self-reported fatigue was measured using several types of scales such as rating of perceived exertion (RPE) on the Borg scale (ranging from 6 to 20), and physical activity was used for defined performance tests except in two studies.13,14
Performance fatigability (performance deterioration)
In six studies, performance fatigability was measured as performance deterioration by quantifying decreasing speed of activity3,15–17 or prolonged reaction time.6,14
Research findings (fatigability-related factors)
Among the 14 studies, the fatigability measures in three studies were shown to be valid and reliable3,4,17 and to have significant relationships with symptoms, physical functioning, and biological measures. Five studies reported significant associations between their perceived fatigability measure (self-reported fatigue following a defined performance test) and performance fatigability measure (performance deterioration).3,6,14,15,17 Examination of the studies revealed that perceived symptoms, tiredness,3,17 perceived fatigue severity,17 musculoskeletal pain,9,18 and depressive symptoms9 were significantly related to fatigability. With respect to biomarkers, increases in cytokine interleukin 6 (IL-6)14 and free thyroxine (FT-4)19 were significantly related to high fatigability. Several studies examined the relationship between fatigability and physical functioning; fatigability was found to be significantly related to walking speed,4,9,10,17 knee strength,18 physical activity counts,15,17 and frailty.17 In addition, energy expenditure6 and oxygen consumption (VO2)5,15 during physical activities were significantly related to fatigability.
Phase two
During phase two, these 17 measures that conceptualized fatigability as a change in perceived fatigue in the context of activity were subjected to in-depth evaluation. Table 3 summarizes the characteristics of these measures, and Table 4 provides detailed information on each measure as well as reference information.
Table 3.
Characteristics of measures that conceptualized perceived fatigue in the context of activity.
| Measure characteristic | N | % |
| Assessment method | ||
| Performance-based | 14 | 82.4 |
| Self-report | 3 | 17.6 |
| Number of fatigue items | ||
| Single item | 12 | 70.6 |
| Multiple items | 5 | 29.4 |
| Activity type | ||
| Walking test | 9 | 52.9 |
| Physical task | 4 | 23.5 |
| Cognitive task | 2 | 11.8 |
| Physical and cognitive tasks | 2 | 11.8 |
| Fatigability output | ||
| Scoring | ||
| Ratio of fatigue score to activity level | 4 | 23.5 |
| Fatigue score | 13 | 76.5 |
| Level of measurement | ||
| Ratio | 6 | 35.3 |
| Interval | 4 | 23.5 |
| Ordinal | 2 | 11.8 |
| Nominal | 5 | 29.4 |
| Reliability/validity reported | ||
| Yes | 6 | 35.3 |
| No | 11 | 64.7 |
Table 4.
Fatigability measures representing perceived fatigue in the context of activity.
| Measure | Components | Fatigability output | Level of measurement | Validity/Reliability | |
|---|---|---|---|---|---|
| Perceived fatigue | Activity | ||||
| Severity of perceived fatigability (Barbosa et al., 2015) | • 7-point scale • 1 “extremely energetic” to 7 “extremely tired” • Immediately after activity |
• 6-min walking test (6MWT) | Ratio of fatigue to distance walked in the 6MWT | Ratio | Not reported |
| Changes in perceived fatigue (Buchowski et al., 2013) | • 7-point scale • 1 “extremely more energetic” to 7 “extremely more tired” (4 “neither more tired or energetic”) • After defined physical activity task |
• Physical activity tasks (e.g., moving objects with hands while sitting, moving a 0.5-kg weight while standing, walking across a 3-m room with turns | Fatigue score | Ordinal | Not reported |
| Perceived fatigability severity (Buchowski et al., 2013) | • 7-point scale • 1 “extremely more energetic” to 7 “extremely more tired” (4 “neither more tired or energetic”) • After defined physical activity task |
• Physical activity tasks (e.g., moving objects with hands while sitting, moving a 0.5-kg weight while standing, walking across a 3-m room with turns) | Ratio of measured fatigue score to energy expenditure for each task | Ratio | Not reported |
| The Pittsburgh Fatigability Scale (Glynn et al., 2015) (instrument) | • 6-point scale • 0 “No fatigue” to 5 “Extremely fatigue” • Separate assessment of mental and physical fatigue |
• 10 items for defined activity • Sedentary to moderate and high-intensity activity |
• Summed mental fatigue scores and summed physical fatigue scores • Each score could range from 0 to 50 |
Ratio |
Reliability • Cronbach’s alpha=0.88 • Test-retest reliability=0.86Validity • Good overall scale score discrimination in correctly classifying persons with high performance deterioration (AUCs, 0.68, p<.001) and high perceived exertion (AUCs, 0.73, p<.001). |
| Perceived fatigability (Gonzales et al., 2015) | • 7-point scale • 1 “extremely more energetic” to 7 “extremely more tired” (4 “neither more tired or energetic”) • After defined physical activity task |
• 400-m walk | Ratio of fatigue score to energy expenditure during 400-m walk | Ratio | Not reported |
| Perceived fatigability (Lin, Roiland, Polesskaya et al., 2014) | • 18 items of visual analogue scale to evaluate fatigue severity (VAS-F) • Respond on a 10-cm analogue rating line ranging from 0 cm (not at all) to 10 cm (extremely) • Assessment before and after cognitive tests |
• Cognitive tests (e.g., trail making test, Stroop color naming, digit span backward) | • Results of cluster analysis of difference in fatigue before and after cognitive tests • Results reported as high or low fatigability |
Nominal | VAS-F internal consistency reliability • Before tests: 0.88 • After tests: 0.94 |
| Perceived fatigability (Lin, Roiland, Heffner et al., 2014) | • 18 items of VAS-F • Respond on a 10-cm analogue rating line ranging from 0 cm (not at all) to 10 cm (extremely) • Assessment before and after 1- back tests |
• 1-backtests • Including 10 visual sessions and 10 auditory sessions |
• Yes/no responses • Use of repeated measure Anova • Significant main effect reported as fatigability (Yes) |
Nominal | VAS-F internal consistency reliability • Before tests: 0.88 • After tests: 0.94 |
| Mobility-Tiredness Scale (Manty et al., 2012) (instrument) | • Feeling fatigue or not feeling fatigue | • 2 items for defined activity (e.g., transferring from a chair or bed and walking indoors without another person’s help) | Yes/no responses | Nominal | Not reported |
| Changes in fatigue during high activity (Murphy & Smith, 2010) | • 5-point fatigue scale • Range of 0–4 (scale values not described) • Assessment at six specific time points each day (waking; +2, +6, +10, and +14 h; and 30 min before bed). |
• Free-living activity • 4 h of high activity (1 SD above mean activity) |
Difference in fatigue before and after high activity | Ratio | Not reported |
| Situational Fatigue Scale (Richardson et al., 2014) (instrument) | • 6-point scale • 0 “not fatigued at all” to 5 “extremely fatigued” |
• 9 items for mental fatigue activity (e.g., reading magazines or newspapers for 1 h) • 4 items for physical fatigue activity (e.g., jogging for 20 min) |
Summed ratings for each item | Ratio |
Internal consistency reliability • Overall (Cronbach’s alpha = 0.90) • Mental fatigue items (Cronbach’s alpha = 0.89) • Physical fatigue items (Cronbach’s alpha = 0.88)Criterion validity • Moderate correlation with Fatigue Assessment Instrument (FAI): r = 0.47, p < .001 |
| Rating of perceived exertion (RPE) at the end of a 400-m walk (Richardson et al., 2014) | • RPE on Borg scale • 6 “No exertion at all” to 20 “Maximal exertion” • Immediately after walking test |
• 400-m walk | RPE score | Interval | Not reported |
| RPE after walking at standard speed (0.72 m/s) on a treadmill (Richardson et al., 2014) | • RPE on Borg scale • 6 “No exertion at all” to 20 “Maximal exertion” • Immediately after walking test |
• 5 min of treadmill walking at 0.72 m/s | RPE score | Interval | Not reported |
| RPE after walking at subject-preferred speed on a treadmill (Richardson et al., 2014) | • RPE on Borg scale • 6 ‘No exertion at all” to 20 “Maximal exertion” • Immediately after walking test |
• Walking at preferred gait speed for 5 min | RPE score | Interval | Not reported |
| RPE after walking test (Santasasto et al., 2014) | • RPE on Borg scale • 6 “No exertion at all” to 20 “Maximal exertion” • Immediately after walking test |
• 5 min of treadmill walking at 0.72 m/s and 0% grade | • High fatigability: RPE ≥10 • Low fatigability: RPE <10 |
Nominal | Not reported |
| Perceived fatigability severity (Schnelle et al., 2012) | • 7-point scale • 1 “extremely energetic” to 7 “extremely tired” • Immediately after walking test |
• 10-min walking test (10MWT) | Ratio of fatigue score to walking distance during 10MWT | Interval |
Concurrent validity • Perceived fatigability severity measure was highly correlated with performance fatigability severity |
| Perceived exertion (Simonsick et al., 2014) | • RPE on Borg scale • 6 “No exertion at all” to 20 “Maximal exertion” • Immediately after walking test |
• 5 min of treadmill walking at 0.67 m/s | High fatigability: RPE ≥ 10 Low fatigability: RPE <10 |
Nominal |
Concurrent validity • Consistently strong associations with fatigue symptomsPredictive validity Robust association between perceived exertion and reported and observed function whether an RPE of 10 was used as a threshold or RPE was used as a continuous measure |
| RPE after walking test (Simonsick et al., 2016) | • RPE on Borg scale • 6 “No exertion at all” to 20 “Maximal exertion” • Immediately after walking test |
• 5 min of treadmill walking at 0.67 m/s | Fatigue score | Interval | Not reported |
Assessment of fatigue and activity
Among the 17 fatigability measures, 12 (70.6%) used a single item to quantify fatigue; the remaining five measures used multiple items for this purpose.4,9,10,13,14 Most frequently used to quantify fatigue was an item providing an RPE on the Borg scale (ranging from 6 to 20, with 6 meaning “no exertion at all” and 20 meaning “maximal exertion”) after a performance activity.3,5,10,19 Regarding the activity components used, activity type, intensity, and duration differed among measures. More than half the measures (52.9%) used a walking test to quantify activity, but the walking protocol differed among these studies; specifically, the tests varied based on the number of meters walked (i.e., a 400-meter walking test),10,20 walking duration (6, 5, or 10 min),10,15,17 and walking duration with a fixed speed (0.72 m/s).3,10,19 Other measures used a defined activity such as a physical task,6,9 a cognitive task,13,14 or both4,10 or employed free-living activity.18
Calculation methods for fatigability scores and types of scaling
Scoring of measures was examined to identify how fatigability scores were calculated. Most of the studies (76.5%) used a raw fatigue score immediately after activity performance; only four measures considered activity levels such as energy expenditure or walking distance during a test in calculating the fatigability score (e.g., the ratio of fatigue to activity level).6,17,20 Among the measures, levels of fatigability measurement varied from nominal to ratio. Six measures (35.3%) used a ratio level with a fatigue scale ranging from 0 to 418 or the ratio of the fatigue score to the activity level.6,15,17,20 In addition, five measures (29.4%) used nominal levels to measure fatigability in terms of RPE categories of high (RPE ≥ 10) and low (RPE < 10).3,5
Validity and reliability test information
Validity and/or reliability information was reported for only six of the 17 measures (35.3%). For example, for the Pittsburgh Fatigability Scale (PFS),4 one study reported its reliability (Cronbach’s alpha = 0.88) and its concurrent and convergent validity. PFS validity was established using several criterion variables (high perceived exertion, high performance deterioration, slow gait speed, worse physical function, and lower fitness) with least-squared means (standard error) and area under the ROC curve (AUC) values (0.68–0.74, p < .001).
Discussion
Identifying the most important aspects of fatigability measurement has important research implications for moving geriatric science forward, particularly as it relates to fatigue and physical activity. The concept of fatigability emerged in conferences on aging in 2010 to address fatigue problems in older adults.21 Since that time, a groundswell of research opinion has emerged that it is now necessary to comprehensively examine fatigability measurement.
This integrative review had several key findings. Most fatigability research to date has employed a sample of healthy older adults and has employed two or three measures to assess fatigability. The measures applied fell into three categories: self-reported fatigability, perceived fatigability, and performance fatigability (performance deterioration). In addition, 17 of 25 fatigability measures identified in the review conceptualized perceived fatigue in the context of activity, and no measure was used in more than one study.
Overall assessment of fatigability research
From a population perspective, most studies included in this review employed a sample population of healthy elderly people (85.7%). Thus, the next steps in the development of fatigability should include a wider range of subjects experiencing both problematic fatigue and low physical activity.22–24 For example, cancer patients have reported severe fatigue and low physical activity levels in their daily life, making this a promising population for fatigability research.
In terms of research design, most studies included in this review used a cross-sectional rather than longitudinal design. Fatigability studies employing a longitudinal design will further our understanding of the underlying mechanisms of fatigability as well as its characteristics in a given population across an extended time period. For example, a longitudinal study will offer the advantage of being able to examine effects of exercise or treatment on fatigability by observing fatigability patterns in a given individual and across a specific population. The review findings revealed that most of the studies had a sample size consisting of fewer than 100 subjects; research with larger sample sizes will allow for greater power to detect differences and increased ability to generalize the findings.
In the 14 studies reviewed, eight of which (57.1%) measured fatigability using more than one measure, with a perceived fatigability measure (self-reported fatigue following a defined performance test) used most frequently. These studies have appropriately incorporated the two essential components of fatigability, since it is defined as perceived fatigue in the context of defined activity. In addition to perceived fatigability, researchers have measured performance fatigability by assessing changes in walking speed or prolonged reaction time.3,6,13,15,17 Performance fatigability objectively represents an individual’s physical fatigue in terms of decreases in speed, and this approach is similar to that previously used to measure fatigability in exercise and neurology studies. However, this approach does not include an individual’s perceived fatigue as part of the fatigability measure. Thus, this type of measure is useful for quantifying physical fatigue in the context of activity but does not adequately capture fatigability.
Major research findings in recent fatigability studies have focused on relationships between fatigability measures and clinical measures such as perceived symptoms, disease-related biomarkers, and physical functioning. Given the significant correlations observed between fatigability and clinical measures, each of which is indicative or predictive of health problems, the findings support the potential usefulness of fatigability measures in clinical research. For example, accurate assessment of fatigability provides an opportunity for early identification of appropriate interventions to prevent decreased physical functioning. People with high fatigability have been found to exhibit low physical activity and low gait speed, which were related to reduced muscle mass9 or muscle strength.18 In the clinical context, exploring how quickly an individual reaches a given level of fatigue during activity would provide a potentially valuable indicator for developing a tailored exercise interventions of nutrition, exercise training, dietary supplements or strategies for approaching daily physical task.
Measurement of fatigability
Among the 25 measures identified in the studies reviewed, 17 (68%) conceptualized perceived fatigue in the context of activity. However, these measures quantified the perceived fatigue and activity components in different ways. Only three of the measures were self-report instruments, while the remaining 14 were performance-based assessment measures.
Of the three self-report fatigability measures—the PFS, the Avlund Movility-Tiredness (MOB-T) scale, and Situational fatigue scale (SFS)—only the PFS was developed to measure fatigability in older adults.4 The two remaining fatigability instruments were derived from previously used fatigue measures that asked respondents to score their fatigue after a defined activity. It is difficult to determine which of these three measures is most useful as a self-report fatigability instrument because none of them was used in more than one study. However, the PFS instrument is preferable over the others in that this measure has reported validity for an elderly population, incorporates an activity component with a broad range of intensities and durations, and normalizes activities to an intensity level. However, on the PFS, item scores are summed to obtain a fatigability score, which is not appropriate because the items address different intensities of activity. More accurate measurement of fatigability levels could be achieved if the item scores were weighted according to the intensity of activity addressed.
The activity components of measures varied from walking tests to physical and/or cognitive tasks. For example, a walking test was frequently used as the activity component; however, measures differed in terms of whether they employed walking speed, duration, distance, or a combination of these. Some researchers applied a walking test by limiting the duration (6 or 10 min) or distance (400 m) while using a standardized walking speed.3,10,19 However, fatigability researchers should consider whether such walking activities could trigger changes in perceived fatigue in their research sample. The raw fatigue score obtained after such activities is a key component of the fatigability score, and thus selecting an appropriate activity component is essential. For example, the speed of 0.67 m/s was commonly used as the walking intensity in fatigability measures for older adults under the rationale that this speed is suitable for distinguishing frail from non-frail individuals in this population.7 Thus, the activity component in fatigability research should be selected with careful consideration of the target population.
One study used free-living physical activity as the activity component of its fatigability measure and repeatedly assessed real-time fatigue using the ecological momentary assessment method.18 This method offers many benefits to researchers assessing patterns of fatigue, physical activity, and fatigability within the day and across the week. For example, the data obtained can be generalized to real-life situations because the assessment occurs in a person’s natural environment within the daily routine. Moreover, the ecological momentary assessment method avoids recall bias, which is a weakness of most instruments. However, in one study that used the ecological momentary assessment, the researchers did not explain why they chose four hours of high intensity physical activity in free-living individuals for their fatigability measure.18 Also, these researchers assumed that high activity (1 SD above the mean activity level) increased fatigue in individuals, but they did not consider the effects of lower activity levels on fatigue.
Finally, with regard to calculation of fatigability scores, one study categorized each participant’s RPE as indicating either high or low fatigability.3 A cutoff RPE of 10 was used for categorization purposes; this value was not explicitly defined, but a value of 9 was defined as “very light” exertion. The cutoff RPE of 10 was based on previous training intensity research that employed an RPE of 11 to limit exertion during training.25 In addition, the walking intensity of the measure used by Simonsick et al. (2014) in their study was 0.67 m/s, which was chosen to indicate whether older adult participants were frail or not. However, the fatigability concept includes fatigue and physical activity but not the condition referred to as frailty. Frailty in older adults is a state of vulnerability to declines in health or function26,27 and is a clinical syndrome in which three or more of these criteria are present: slowed walking speed, un intentional weight loss, fatigue or poor endurance, muscle weakness, and low physical activity.7 Furthermore, although the RPE cutoff of 10 and the walking speed of 0.67 m/s used by Simonsick et al. (2014) may have been high enough to measure fatigability accurately in older adults, these values do not appear to be suitable for categorizing fatigability in other populations. Further research is needed to identify criteria specific to other populations.
Limitations
This review has two limitations that should be acknowledged. One is that the literature searches were confined to studies published in English between January 2010 and January 2016. It is possible that relevant studies were published in other languages. The second limitation is that abstracts, unpublished studies, and review papers were not included in the review. Given the recent interest in fatigability as a potential means of improving understanding of fatigue and physical activity, it is possible that unpublished studies have examined this concept.
Conclusion
The concept of fatigability is still in the early stages of research and measurement. For this reason, no gold standard exists for fatigability measurement in research or clinical practice. Among the 14 reviewed studies published from 2010 to 2016, 17 fatigability measures conceptualized perceived fatigue in the context of activity, but none of these measures was applied more than once. Moreover, most of these measures were developed for elderly populations, and validity and reliability information was reported for only a small number of measures. Therefore, selecting a proper measurement for examining fatigability in other populations is problematic and requires careful consideration.
Based on the study results, a fatigability measure should incorporate perceived fatigue as well as a defined physical activity that minimizes potential problems with self-pacing during the activity. Using this type of measure, researchers can effectively assess the change in perceived fatigue during physical activity and can also identify the effects of interventions for reducing fatigue, thus contributing to development of individually tailored interventions for specific populations. In addition, most current measures should be refined to more fully incorporate the fatigability concept, and fatigability data should be collected in broader populations.
References
- 1.Eldadah BA. Fatigue and fatigability in older adults. PM R. 2010;2(5):406–413. 10.1016/j.pmrj.2010.03.022. [DOI] [PubMed] [Google Scholar]
- 2.Moreh E, Jacobs JM, Stessman J. Fatigue, function, and mortality in older adults. J Gerontol A Biol Sci Med Sci. 2010;65(8):887–895. 10.1093/gerona/glq064. [DOI] [PubMed] [Google Scholar]
- 3.Simonsick EM, Schrack JA, Glynn NW, Ferrucci L. Assessing fatigability in mobility-intact older adults. J Am Geriatr Soc. 2014;62(2):347–351. 10.1111/jgs.12638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glynn NW, Santanasto AJ, Simonsick EM, et al. The pittsburgh fatigability scale for older adults: development and validation. J Am Geriatr Soc. 2015;63(1):130–135. 10.1111/jgs.13191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Santanasto AJ, Glynn NW, Jubrias SA, et al. Skeletal muscle mitochondrial function and fatigability in older adults. J Gerontol A Biol Sci Med Sci. 2014, doi:glu134 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buchowski MS, Simmons SF, Whitaker LE, et al. Fatigability as a function of physical activity energy expenditure in older adults. Age (Dordr). 2013;35(1):179–187. 10.1007/s11357-011-9338-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–M156. [DOI] [PubMed] [Google Scholar]
- 8.Alexander NB, Taffet GE, Horne FM, et al. Bedside-to-Bench conference: research agenda for idiopathic fatigue and aging. J Am Geriatr Soc. 2010;58(5):967–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manty M, de Leon CF, Rantanen T, et al. Mobility-related fatigue, walking speed, and muscle strength in older people. J Gerontol A Biol Sci Med Sci. 2012;67(5):523–529. 10.1093/gerona/glr183. [DOI] [PubMed] [Google Scholar]
- 10.Richardson CA, Glynn NW, Ferrucci LG, Mackey DC. Walking energetics, fatigability, and fatigue in older adults: the study of energy and aging pilot. J Gerontol A Biol Sci Med Sci. 2015;70(4):487–494. 10.1093/gerona/glu146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang C, Wu C. The situational fatigue scale: a different approach to measuring fatigue. Qual Life Res. 2005;14(5):1357–1362. [DOI] [PubMed] [Google Scholar]
- 12.Avlund K, Era P, Davidsen M, Gause-Nilsson I. Item bias in self-reported functional ability among 75-year-old men and women in three nordic localities. Scand J Soc Med. 1996;24(3):206–217. [DOI] [PubMed] [Google Scholar]
- 13.Lin F, Roiland R, Heffner K, Johnson M, Chen DG, Mapstone M. Evaluation of objective and perceived mental fatigability in older adults with vascular risk. J Psychosom Res. 2014;76(6):458–464. 10.1016/j.jpsychores.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin F, Roiland R, Polesskaya O, et al. Fatigability disrupts cognitive processes’ regulation of inflammatory reactivity in old age. Am J Geriatr Psychiatry. 2014;22(12):1544–1554. 10.1016/j.jagp.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barbosa JF, Bruno SS, Cruz NS, de Oliveira JS, Ruaro JA, Guerra RO. Perceived fatigability and metabolic and energetic responses to 6-minute walk test in older women. Physiotherapy. 2015, doi: S0031-9406(15)03820-1 [pii]. [DOI] [PubMed] [Google Scholar]
- 16.Keyser RE, Christensen EJ, Chin LM, et al. Changes in fatigability following intense aerobic exercise training in patients with interstitial lung disease. Respir Med. 2015;109(4):517–525. 10.1016/j.rmed.2015.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schnelle JF, Buchowski MS, Ikizler TA, Durkin DW, Beuscher L, Simmons SF. Evaluation of two fatigability severity measures in elderly adults. J Am Geriatr Soc. 2012;60(8):1527–1533. 10.1111/j.1532-5415.2012.04062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murphy SL, Smith DM. Ecological measurement of fatigue and fatigability in older adults with osteoarthritis. J Gerontol A Biol Sci Med Sci. 2010;65(2):184–189. 10.1093/gerona/glp137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simonsick EM, Chia CW, Mammen JS, Egan JM, Ferrucci L. Free thyroxine and functional mobility, fitness, and fatigue in euthyroid older men and women in the baltimore longitudinal study of aging. J Gerontol A Biol Sci Med Sci. 2016;71(7):961–967. 10.1093/gerona/glv226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gonzales JU, Wiberg M, Defferari E, Proctor DN. Arterial stiffness is higher in older adults with increased perceived fatigue and fatigability during walking. Exp Gerontol. 2015;61:92–97. 10.1016/j.exger.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 21.Alexander NB, Taffet GE, Horne FM, et al. Bedside-to-bench conference: research agenda for idiopathic fatigue and aging. J Am Geriatr Soc. 2010;58(5):967–975. 10.1111/j.1532-5415.2010.02811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blanchard CM, Courneya KS, Stein K, American Cancer Society’s SCS-II. Cancer survivors’ adherence to lifestyle behavior recommendations and associations with health-related quality of life: results from the american cancer society’s SCS-II. J Clin Oncol. 2008;26(13):2198–2204. 10.1200/JCO.2007.14.6217. [DOI] [PubMed] [Google Scholar]
- 23.Stevinson C, Steed H, Faught W, et al. Physical activity in ovarian cancer survivors: associations with fatigue, sleep, and psychosocial functioning. Int J Gynecol Cancer. 2009;19(1):73–78. 10.1111/IGC.0b013e31819902ec. [DOI] [PubMed] [Google Scholar]
- 24.Wang XS, Zhao F, Fisch MJ, et al. Prevalence and characteristics of moderate to severe fatigue: a multicenter study in cancer patients and survivors. Cancer. 2014;120(3):425–432. 10.1002/cncr.28434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Birk TJ, Birk CA. Use of ratings of perceived exertion for exercise prescription. Sports Med. 1987;4(1):1–8. [DOI] [PubMed] [Google Scholar]
- 26.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381(9868):752–762. 10.1016/S0140-6736(12)62167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Torpy JM, Lynm C, Glass RM. JAMA patient page. frailty in older adults. JAMA. 2006;296(18):2280, doi: 296/18/2280 [pii]. [DOI] [PubMed] [Google Scholar]