Abstract
Objective:
Malignant insulinoma is an extremely uncommon tumor that is usually accompanied by severe hypoglycemia that is difficult to manage. At this time, the long-term effect of 177Lu-DOTATATE (lutetium [Lu-177]-DOTA-Tyr3-octreotate) on this tumor is not well known.
Methods:
We report a case of severe, life-threatening, and refractory hypoglycemia associated with malignant insulinoma treated with 177Lu-DOTATATE.
Results:
A 51-year-old woman was referred because of severe, life-threatening, and refractory hypoglycemia due to malignant insulinoma. The patient had been treated unsuccessfully with chemotherapy, targeted therapies, and symptomatic therapy with diazoxide, steroids, and somatostatin analogues without success. 177Lu-DOTATATE adequately controlled her hypoglycemia after the other conventional treatments failed.
Conclusion:
177Lu-DOTATATE was effective in providing rapid and long-term symptomatic control of the hypoglycemia and significantly improved the quality of life of the patient.
INTRODUCTION
Malignant insulinoma is an uncommon tumor (5 to 10% of all insulinomas) that is usually accompanied by severe hypoglycemia and a short life expectancy (10-year survival is <10%) (1). Its clinical management is complex and constitutes a real therapeutic challenge (2). The evidence on radionuclide treatment in these tumors is scarce with <20 reported clinical cases so far (3–7).
We report a clinical case with metastatic malignant insulinoma accompanied by severe, life-threatening, and refractory hypoglycemia that was early and adequately controlled with radionuclide therapy after failure of conventional treatment, such as debulking surgery, systemic chemotherapy, novel targeted drugs (multiple tyrosine kinase inhibitors, sunitinib and mammalian target of rapamycin complex 1 inhibitors, everolimus), steroids, diazoxide, and somatostatin analogs. Radionuclide treatment was well tolerated and associated with a long-term stabilization of the disease.
CASE REPORT
A 51-year-old woman was referred to us for severe and refractory hypoglycemia. She had been diagnosed with a pancreatic neuroendocrine tumor (pNET) in 2013 at another center. Presurgical abdominal computed tomography showed a hypodense area in the tail of the pancreas with multiple liver metastases and large (up to 14 cm) ovarian masses. She underwent exploratory laparotomy with bilateral adnexectomy. Pathology determined a large cell neuroendocrine carcinoma with a Ki-67 index of 60% and a post-surgical OctreoScan (using 111In-DTPA-octreotide scintigraphy) revealed hypercaptant lesions in the pancreatic tail.
Therapy was started with lanreotide autogel at 120 mg/28 days and sunitinib at 37.5 mg/day, achieving stabilization of the pancreatic and hepatic lesions. After tumor progression, she received 6 cycles of chemotherapy (using cisplatin and etoposide) with partial response (50% size reduction) of the pancreatic lesion and liver stabilization.
After the third cycle of chemotherapy, she began to have severe and repeated hypoglycemic episodes with neuroglycopenic symptoms, loss of consciousness, and seizures. Blood glucose levels were low (capillary blood glucose usually <30 mg/dL) throughout the day despite continued oral intakes of food. It was even necessary to institute anticonvulsant treatment to avoid seizures. A directed biopsy of the pancreatic and hepatic lesions was performed, resulting in a G2 neuroendocrine tumor (NET) with a Ki-67 index between 2 and 20%. She started everolimus (10 mg/day) for 3 months, and later received methylprednisolone (32 mg/day), diazoxide (150 mg/day), and octreotide long-acting release (30 mg/2 weeks).
On her first visit 4 years later, she continued having frequent hypoglycemic episodes despite frequent oral food intake and therapy with diazoxide, steroids, and somatostatin analogs. In the last 2 years, her weight had increased by 30 kg. On physical examination, her weight was 93 kg and her body mass index was 36.8 kg/m2. She showed a cushingoid phenotype with hirsutism and significant edema in the lower limbs. Her capillary blood glucose was 20 mg/dL. Analytical study showed severe hyperinsulinemic hypoglycemia with a blood glucose of 20 mg/dL (normal range, 70 to 110 mg/dL), serum insulin of 132 μU/mL (normal range, 3 to 27 μU/mL), and serum C peptide of 18.7 ng/mL (normal range, 0.8 to 5.2 ng/mL).
Computed tomography imaging showed a pancreatic tail lesion (62 × 60 × 45 mm) with multiple pulmonary, hepatic and peritoneal metastases. Scintigraphy using Tektrotyd (99mTc-EDDA/HYNIC-Tyr3-octreotide) revealed a pathological deposit in the pancreatic tail and multiple pathological deposits in the liver compatible with metastases. In 2017, the first dose (200 mCi) of lutetium (Lu-177)-DOTA-Tyr3-octreotate (177Lu-DOTATATE) was administered. After 10 days, the patient reported a clear subjective symptomatic improvement with reduced hypoglycemia, adequate control of capillary blood glucose throughout the day, and no need to take diazoxide. At this time, the only therapy used was glucocorticoid replacement dose and long-acting release octreotide at 30 mg/3 weeks.
In total she was treated with 4 doses of 177Lu-DOTATATE. Eighteen months after the first dose of 177Lu-DOTATATE the patient continued without hypoglycemia and with normal serum glucose concentrations (Fig. 1). A 177Lu-DOTATATE scan after the fourth dose showed radiological stability of the lesions (Fig. 2). This therapy was well-tolerated without acute and chronic side effects. An abdominal computed tomography scan at her last visit showed stable disease. The patient gave her signed consent for the publication of the clinical case.
Fig. 1.
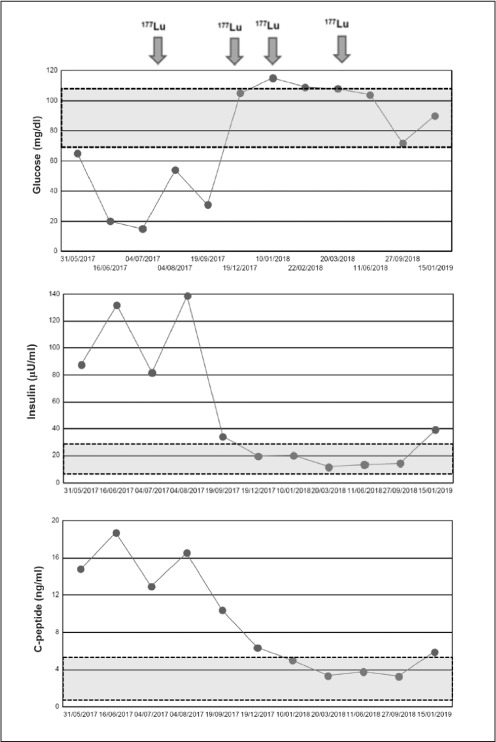
Serum glucose, insulin, and C peptide concentrations before and throughout the treatment with 4 doses of 177Lu-DOTATATE therapy. Grey area indicates normal range for each analyte.
Fig. 2.
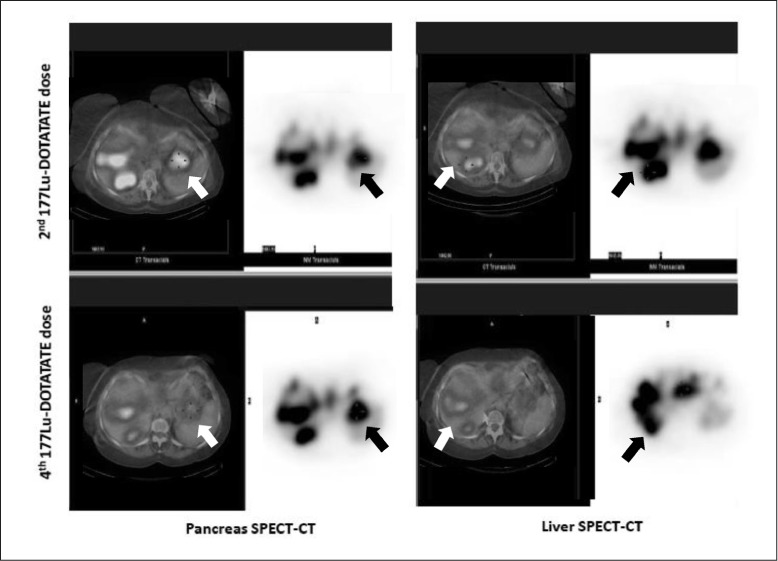
Comparative study of body scans after second (top) and fourth (bottom) 177Lu-DOTATATE dose. Single-photon emission computerized tomography with computed tomography scans of the pancreas (left) and liver (right).
DISCUSSION
Peptide receptor radionuclide therapy (PRRT) is an attractive therapeutic option in progressive somatostatin receptor (SSTR)-positive metastatic NETs with homogenous SSTR expression. According to the last European Neuroendocrine Tumor Society Consensus Guidelines update for the management of distant metastatic disease of intestinal, pancreatic, and bronchial neuroendocrine neoplasms (8), PRRT can be considered as a second-line therapy in nonfunctional, low-grade intestinal (midgut) NETs. This includes cases with tumor type G1 with low tumor burden and no symptoms that are SSTR positive with advanced loco-regional disease or distant metastases after failure of somatostatin analog therapy.
These recommendations are based on the results of the first multicenter, randomized controlled phase III trial known as the NETTER-1 study, a registrational trial of 177Lu-DOTATATE in progressive midgut NETs which showed a significant prolongation of progression-free survival compared to high-dose (60 mg/month), long-acting release octreotide (9). PRRT is also included in the guidelines for the management of SSTR-positive pNETs with advanced loco-regional disease or distant metastases (8). PRRT can be used as a third-line therapy after failure of somatostatin analogs and later everolimus or sunitinib in non-functional (G1, low G2, low tumor burden) pNETs or after cytotoxic chemotherapy and later everolimus or sunitinib in non-functional (G2, high tumor burden) pNETs. In relation to pNETs with functional activity such as insulinoma, PRRT may be recommended as a second-line therapy after failure of diazoxide to control hypoglycemia (8).
Given the low prevalence of malignant insulinoma, the experience with PRRT is very limited. Only isolated case reports (6,7) or small series of patients have been reported (3–5) so far. In 2008, Kwekkeboom et al (3) reported the clinical response of 5 patients with malignant insulinomas treated with 177Lu-DOTATATE. They found partial response in 3 patients and stable disease in another. In 2010, Ong et al (4) used 177Lu-DOTATATE in 2 men with malignant insulinomas, 1 of which was also treated with everolimus, and achieved successful normoglycemia, facilitating safe discharge from the hospital. Both men also had regression in the size and number of hepatic metastases. A series of 5 patients reported by van Schaik et al (5) found PRRT achieved stable disease for a mean period of 27 months without hypoglycemic episodes. These beneficial effects have also been observed in isolated clinical cases from other hospital centers (6,7).
In our patient, PRRT allowed her to withdraw from diazoxide treatment and pharmacological doses of steroids while maintaining an adequate control of glycemia and facilitating weight loss and correction of edema. In addition, 177Lu-DOTATATE treatment stabilized her lesions 18 months after the first dose. Our clinical case, unlike those previously described, specifically evaluated not only the clinical response related to hypoglycemia, but also the biochemical and hormonal responses (glucose, insulin and C peptide) to the treatment with 177Lu-DOTATATE in the long term. PRRT with 177Lu-DOTATATE has been well-tolerated without acute side effects and no serious hematological, liver, or renal toxicities (4,5). Similarly, our patient tolerated 177Lu-DOTATATE treatment without acute or chronic severe adverse effects and she maintained adequate renal and hematopoietic function.
CONCLUSION
Treatment with 177Lu-DOTATATE seems to be effective in the management of severe, life-threatening, and refractory hypoglycemia associated with malignant insulinoma. It can help achieve and maintain long-term euglycemia after failure of other supportive therapies and has good tolerance. Therefore, we suggest its use in early stages of the symptomatic disease in order to improve the quality of life of the patient and promote tumor growth stabilization.
Abbreviations
- 177Lu-DOTATATE
lutetium (Lu-177)-DOTA-Tyr3-octreotate
- NET
neuroendocrine tumor
- pNET
pancreatic neuroendocrine tumor
- PRRT
peptide receptor radionuclide therapy
- SSTR
somatostatin receptor
Footnotes
DISCLOSURE
The authors have no multiplicity of interest to disclose.
REFERENCES
- 1.Service FJ. Recurrent hyperinsulinemic hypoglycemia caused by an insulin-secreting insulinoma. Nat Clin Pract Endocrinol Metab. 2006;2:467–470. doi: 10.1038/ncpendmet0263. [DOI] [PubMed] [Google Scholar]
- 2.Iglesias P, Díez JJ. Management of endocrine disease: a clinical update on tumor-induced hypoglycemia. Eur J Endocrinol. 2014;170:147–157. doi: 10.1530/EJE-13-1012. [DOI] [PubMed] [Google Scholar]
- 3.Kwekkeboom DJ, de Herder WW, Kam BL et al. Treatment with the radiolabeled somatostatin analog [177 Lu-DOTA 0,Tyr3] octreotate: toxicity, efficacy, and survival. J Clin Oncol. 2008;26:2124–2130. doi: 10.1200/JCO.2007.15.2553. [DOI] [PubMed] [Google Scholar]
- 4.Ong GS, Henley DE, Hurley D, Turner JH, Claringbold PG, Fegan PG. Therapies for the medical management of persistent hypoglycaemia in two cases of inoperable malignant insulinoma. Eur J Endocrinol. 2010;162:1001–1008. doi: 10.1530/EJE-09-1010. [DOI] [PubMed] [Google Scholar]
- 5.van Schaik E, van Vliet EI, Feelders RA et al. Improved control of severe hypoglycemia in patients with malignant insulinomas by peptide receptor radionuclide therapy. J Clin Endocrinol Metab. 2011;96:3381–3389. doi: 10.1210/jc.2011-1563. [DOI] [PubMed] [Google Scholar]
- 6.Costa R, Costa R, Bacchi CE, Almeida Filho P. Metastatic insulinoma managed with radiolabeled somatostatin analog. Case Rep Endocrinol. 2013;2013 doi: 10.1155/2013/252159. 252159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Makis W, McCann K, McEwan AJ. Metastatic insulinoma pancreatic neuroendocrine tumor treated with 177Lu-DOTATATE induction and maintenance peptide receptor radionuclide therapy: a suggested protocol. Clin Nucl Med. 2016;41:53–54. doi: 10.1097/RLU.0000000000001023. [DOI] [PubMed] [Google Scholar]
- 8.Pavel M, O'Toole D, Costa F et al. ENETS Consensus Guidelines Update for the Management of Distant Metastatic Disease of Intestinal, Pancreatic, Bronchial Neuroendocrine Neoplasms (NEN) and NEN of Unknown Primary Site. Neuroendocrinology. 2016;103:172–185. doi: 10.1159/000443167. [DOI] [PubMed] [Google Scholar]
- 9.Strosberg J, El-Haddad G, Wolin E et al. Phase 3 trial of (177) Lu-Dotatate for midgut neuroendocrine tumors. N Engl J Med. 2017;376:125–135. doi: 10.1056/NEJMoa1607427. [DOI] [PMC free article] [PubMed] [Google Scholar]


