Abstract
Objective:
To describe a case of sequential bilateral adrenal infarction and hemorrhage resulting in an unusual pattern of adrenal function over time.
Methods:
A 50-year-old male with autoimmune antiphospholipid syndrome (APS) presented to the emergency room with severe abdominal pain. Diagnostic studies performed included contrast-enhanced computerized tomographic (IV-CT) imaging of abdomen and pelvis, and laboratory assessment of the hypothalamic-pituitary-adrenal axis.
Results:
IV-CT of abdomen and pelvis on day 1 showed acute left adrenal gland infarction; cortisol level was 19.9 μg/dL and serum sodium was 133 mEq/L. The patient subsequently developed hyponatremia and hypotension. Repeat IV-CT of abdomen and pelvis on day 3 showed hemorrhagic conversion of the left infarcted adrenal gland and a new right adrenal gland infarction. Cosyntropin stimulation test (CST) confirmed primary glucocorticoid insufficiency. Plasma renin activity and the serum aldosterone level were within normal limits with normokalemia. At 7-month follow-up, CST demonstrated cortisol and aldosterone deficiency.
Conclusion:
Adrenal infarction is a rare complication of APS but is the most common endocrine complication. Evidence of bilateral adrenal infarction on imaging does not predict the type of adrenal dysfunction that might ensue, as demonstrated in this case. Thorough evaluation of glucocorticoid, mineralocorticoid, and androgen deficiency should be conducted both at the time of the event and in follow-up.
INTRODUCTION
Adrenal insufficiency (AI) is generally classified into 2 categories: central AI caused by an insult to the pituitary/hypothalamus in which only glucocorticoid synthesis is affected, or primary AI (PAI), due to a direct insult to both adrenal glands, in which glucocorticoid, mineralocorticoid, and androgen synthesis are affected. In developed countries, the etiology of PAI is most often autoimmune disease (70 to 90%) (1). There are multiple, less frequent causes of PAI; however, the focus of this report is PAI caused by bilateral adrenal infarction.
The clinical presentation of AI depends on whether the cause is central or primary and if the onset is acute or chronic. In cases of bilateral adrenal injury from infarction and/or hemorrhage causing PAI, the most common presenting symptoms include hypotension or shock (>90%), abdominal, flank, back, or lower chest pain (86%), fever (66%), nausea and vomiting (42%), and confusion (42%) (2). Characteristic laboratory findings of bilateral adrenal infarction/hemorrhage (BAI/H) are a decrease in hemoglobin/hematocrit and electrolyte/metabolic abnormalities that can include hyponatremia, hyperkalemia, mild acidemia, and evidence of volume contraction.
To diagnose AI, a fasting, morning plasma cortisol level is a useful screening tool with levels <3 μg/dL highly indicative of AI and levels >19 μg/dL ruling out AI (3). The 250 μg cosyntropin stimulation test (CST) is the most widely used dynamic test to evaluate for adrenal insufficiency (4). Although physicians should be aware of changing cortisol cut-off levels with the advent of new assays, in this 60-minute test, a peak cortisol level of greater than or equal to 18 to 20 μg/dL is considered evidence of preserved adrenal function.
CASE REPORT
A 50-year-old Hispanic man with past medical history of antiphospholipid syndrome (APS) diagnosed 5 years earlier, multiple deep vein thromboses, and type 2 diabetes, presented to the emergency room (day 1) with severe abdominal pain associated with nausea and multiple episodes of vomiting that started acutely the previous night. The patient was taking warfarin for APS since his initial diagnosis; however, upon hospital admission he admitted to nonadherence to warfarin for the previous month. This patient had no prior history of hypothalamic-pituitary-adrenal insult.
Physical examination was significant for abdominal tenderness in the epigastric and left upper quadrant areas. Computed tomography imaging enhanced with intravenous contrast (IV-CT) of abdomen and pelvis showed hypo-enhancement of the left adrenal gland compared to a normally enhancing right adrenal gland, suggesting acute left adrenal ischemic infarction (Fig. 1 A and B). Day 1 laboratory results were significant for mild hyponatremia (sodium [Na+] 133 mEq/L) without hyperkalemia (potassium [K+] 3.7 mEq/L), acidemia, or any signs of volume contraction (Fig. 2). Serum cortisol level on admission was 19.9 μg/dL. The patient was started on an intravenous (IV) infusion of heparin and normal saline. Throughout day 2, gradual worsening of hyponatremia was noted. By day 3, despite improved abdominal pain and resolution of nausea and vomiting, the serum Na+ level further decreased to 121 mEq/L, and blood pressure dropped from 166/82 to 113/75 mm Hg. The patient became febrile (peak temperature 103.0°F), but further infectious work-up was negative. Day 3 repeat cortisol and adrenocorticotropic hormone (ACTH) levels were 1.9 μg/dL and 310 pg/mL, respectively; dihydroepiandosterone (DHEA) was undetectable, plasma aldosterone level was normal, and plasma renin activity (PRA) was not elevated (Table 1).
Fig. 1.
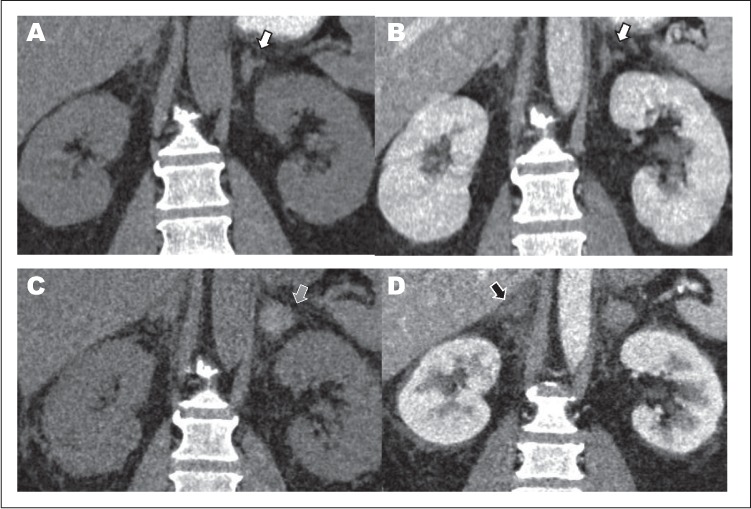
Computed tomography (CT) scan of coronal view of adrenal glands. Day 1: A, Pre-contrast. B, Post-contrast showing no enhancement within the left adrenal gland (white arrow). Day 3: C, Pre-contrast showing high density hemorrhage on the left (grey arrow). D, Post-contrast showing no enhancement within right adrenal glands (black arrow).
Fig. 2.
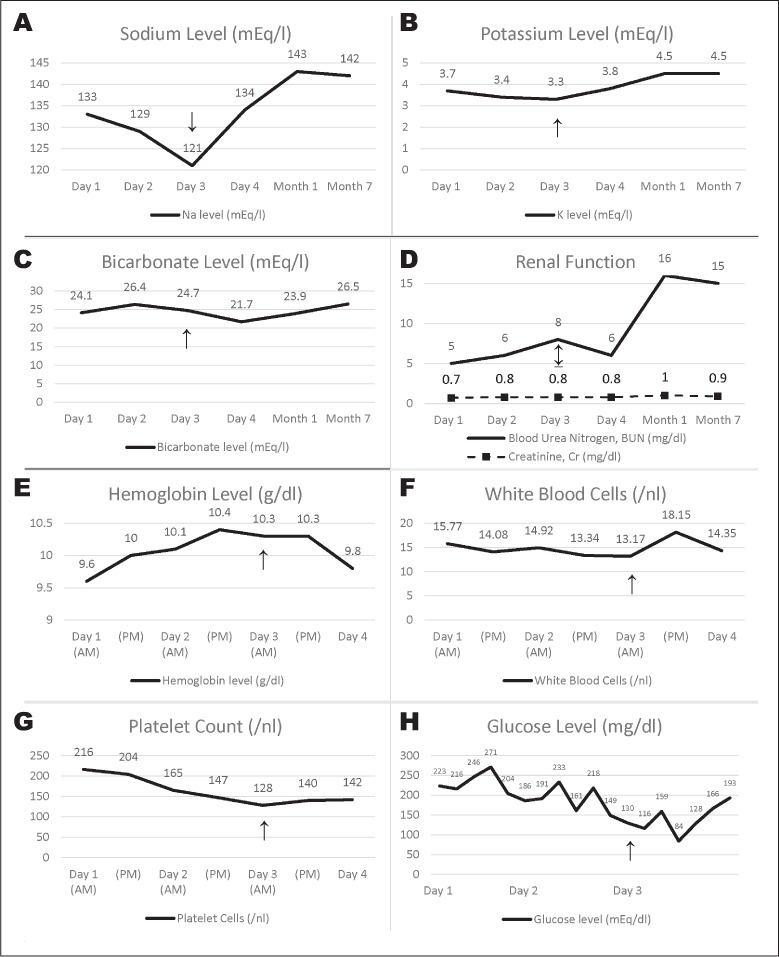
Serum chemistry and blood cell count results at different time points. A, Sodium level. B, Potassium level. C, Bicarbonate level. D, Creatinine and blood urea nitrogen level. E, Hemoglobin level. F, White blood cells. G, Platelet count. H, Glucose level (arrows indicated detection of hemorrhagic conversion of previous left adrenal infarction and concurrent right adrenal infarction on day 3).
Table 1.
Hormone Levels at Different Time Points Postinfarction/Hemorrhage
| Laboratory parametera | Time point | Value |
|---|---|---|
| Plasma renin activity (PRA) (0.16–5.3 ng/mL/hour) | Day 3 | 1.3 |
| Week 4 | 4.3 | |
| 6-months | 2.6 | |
| 7-months | 6.1 | |
| Serum aldosterone (1–21 ng/dL) | Day 3 | 9.6 |
| Week 4 | 3.6 | |
| 6-months | <3.0 | |
| 7-monthsb (0, 60 min) | <3.0, 4.3 | |
| Plasma adrenocorticotropic hormone (ACTH) (5–46 pg/mL) | Day 3, before hydrocortisone administration | 310 |
| Day 3, after hydrocortisone administration | 46 | |
| 6-months | 21 | |
| 7-months | 40 | |
| Serum cortisol (6.2–19.4 μg/dL) | Day 1 | 19.9 |
| Day 3, before hydrocortisone administration | 0.15 | |
| Week 4 | <0.04 | |
| 6-months | 5.7 | |
| 7-monthsb (0, 60 min) | 0.79, 0.39 | |
| Dehydroepiandrosterone (DHEA)(31–701 ng/dL) | Day 3 | <20 |
| 6-months | <20 | |
| Metanephrines (44–261 pg/mL) | Day 3 | <25 |
| 6-months | <25 | |
| Normetanephrine (128–484 pg/mL) | Day 3 | 140 |
| 6-months | 69 |
aRange of normal values shown in brackets.
b7-month values for aldosterone and cortisol represent results of a cosyntropin stimulation test.
On day 3, the patient received a one-time IV infusion of hydrocortisone 100 mg and underwent repeat IV-CT of abdomen and pelvis, which revealed a high-density hemorrhage in the left infarcted adrenal gland and evidence of a new right adrenal infarction (Fig. 1 C and D). By day 4, the Na+ level normalized and results of a CST showed a peak serum cortisol level of 0.18 μg/dL at 60 minutes, confirming AI. The patient did not experience hyperkalemia at any point during hospitalization.
On day 14, the patient was discharged with prescriptions for daily, oral hydrocortisone 15 mg in the morning/10 mg in the afternoon, fludrocortisone 0.1 mg, and warfarin 5 mg.
Four weeks post-discharge, outpatient laboratory results showed undetectable am cortisol and normal aldosterone and PRA levels (Table 1). Fludrocortisone was discontinued. Six months post-discharge repeat labs showed detectable am cortisol, low aldosterone, normal PRA, and normal electrolytes (Table 1). The patient denied symptoms of mineralocorticoid deficiency. Fludrocortisone was not re-initiated given its long half-life and the need for repeat CST with evaluation for cortisol and aldosterone. Instead, the patient's hydrocortisone dose was temporarily increased to 15 mg twice daily. A CST conducted at 7-month follow-up demonstrated cortisol and aldosterone deficiency; serum Na+ and K+ were within normal limits (Table 1).
DISCUSSION
Although adrenal infarction and hemorrhage are rare, there are several case reports of these events in patients with APS (5–8). In a review of endocrine disorders and APS, PAI due to adrenal infarction/hemorrhage was cited as the most common endocrine manifestation of APS (9).
The adrenal glands are vulnerable to infarction and hemorrhage. The adrenal blood supply, rich in vasculature, is described as a “vascular dam” as several arterial branches that supply a subcapsular plexus drain into a single vein (Fig. 3) (10). The IV-CT results showed that our patient experienced sequential infarction of the left and right adrenal glands occurring 2 days apart with ensuing hemorrhage in the left gland (right gland might have hemorrhaged; repeat computed tomography not performed).
Fig. 3.
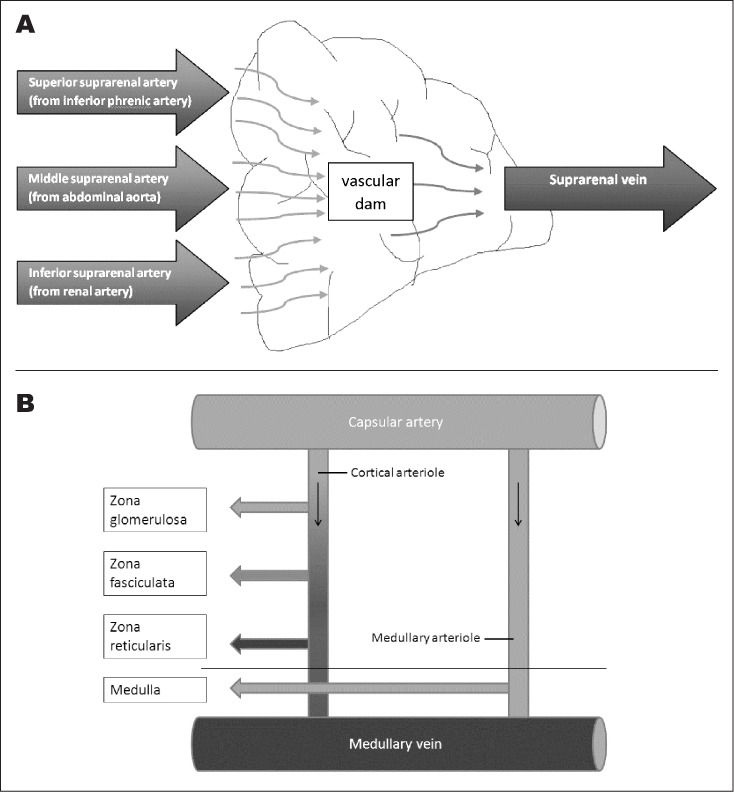
A, Anatomy of arterial and venous blood supply of adrenal glands describing “vascular dam.” Adrenal glands have a relatively small amount of venules draining to suprarenal vein compared to arterioles from 3 different arteries. B, Cortical arteriole and medullary arteriole supplying the cortex and medulla, respectively.
The available literature on adrenal infarction primarily consists of case reports, a few small retrospective case series, and older articles that assess the pathology of infarcted adrenals from various causes. These reports all propose that adrenal vein thrombosis is the initial insult to the adrenal, especially in the setting of a hypercoagulable state such as APS, followed by hemorrhagic infarction, although hemorrhage might not always occur (8,11,12). Several reports, including a 16-patient, retrospective case series of BAI/H in APS, document simultaneous BAI/H (8). We identified 2 case reports that document sequential adrenal infarction occurring 3 and 4 days apart (12,13). A third case report documents simultaneous BAI/H (7); however, the patient in this case did not present to a health-care provider until 3 days after the onset of symptoms by which time sequential BAI/H could have presented as simultaneous BAI/H on imaging. Thus, sequential BAI/H might be more common than is reported if time to presentation is considered (7,8,13).
Despite evidence of a left adrenal infarct on presentation, our patient's normal appearing right adrenal gland, absence of any clinical signs of AI, and day 1 cortisol level of 19.9 μg/dL did not suggest acute AI (14). Progressive worsening of hyponatremia and decreasing blood pressure over the hospital stay, however, raised suspicion of acute AI (Fig. 2 A) and was likely representative of his new, bilateral adrenal damage.
Of interest, is that this patient initially experienced hyponatremia without hyperkalemia (Fig. 2) suggesting preserved zona glomerulosa (mineralocorticoid/aldosterone) function despite apparent zona fasciculata (glucocorticoid/cortisol) and zona reticularis (androgen/DHEA) insufficiency. Characteristic laboratory features of aldosterone deficiency are hyponatremia, hyperkalemia, and an increase in PRA, however, this patient only demonstrated hyponatremia. Hyponatremia occurs in patients with isolated cortisol deficiency, for instance, due to ACTH deficiency, at least in part resulting from hypersecretion of antidiuretic hormone (ADH) (15). Cortisol directly suppresses ADH secretion and has negative feedback on corticotropin-releasing hormone, an ADH secretagogue. Without cortisol, hypersecretion of ADH results in free water retention and hyponatremia.
To our knowledge, intact mineralocorticoid function in the setting of deficient glucocorticoid function has not been reported previously in the setting of PAI due to APS. An older postmortem case series of adrenal infarction due to thrombosis of the venous supply from various causes not including APS, reports that “occasionally there is a rim of surviving zona glomerulosa or of outer zona fasciculata” (11). This might account for the temporary preservation of mineralocorticoid function in our patient.
However, review of the aforementioned reports shows that mineralocorticoid function is often not assessed in cases of BAI/H. Despite treatment with fludrocortisone in 13/16 patients in the 16-patient case series, aldosterone levels were only assessed in 6 patients. Similarly, in 4 case reports that describe 5 cases of BAI/H, aldosterone levels were measured in 1 case (7,12,13,16). Fludrocortisone therapy was prescribed in this case and in one other; all 5 patients were treated with a glucocorticoid. Our case highlights the importance of mineralocorticoid function assessment.
Our patient's laboratory data demonstrate that postinfarction, not all adrenal function may be lost acutely, and dysfunction may evolve over time as related to the pattern of insult to the adrenal blood supply (Fig. 3). In addition, there are reports of patients that have recovered both glucocorticoid and mineralocorticoid function over time (2 years) (8,16).
Therefore, adrenal function should be monitored over many months, even up to 2 years or longer, until glucocorticoid, mineralocorticoid and androgen function, are shown to be deficient or sufficient on multiple occasions over time.
CONCLUSION
We report a case of a patient with APS who developed BAI/H that resulted in acute impairment of glucocorticoid and androgen production, while mineralocorticoid production was preserved for several months. Given selective preservation of and the possibility of further deterioration or recovery of adrenal function post BAI/H, a full adrenal hormonal evaluation should be performed not only at the time of presentation, but, repeatedly over many months.
ACKNOWLEDGMENT
We thank our colleague, Dr. Ulrich K. Schubart, for his invaluable feedback in the preparation of this manuscript. We thank the research participant for his consent to release his medical information for publications.
Abbreviations
- ADH
antidiuretic hormone
- AI
adrenal insufficiency
- APS
antiphospholipid syndrome
- BAI/H
bilateral adrenal infarction/hemorrhage
- CST
cosyntropin stimulation test
- IV-CT
computed tomography imaging enhanced with intravenous contrast
- PAI
primary adrenal insufficiency
- PRA
plasma renin activity
Footnotes
DISCLOSURE
The authors have no multiplicity of interest to disclose.
REFERENCES
- 1.Zelissen PM, Bast EJ, Croughs RJ. Associated autoimmunity in Addison's disease. J Autoimmun. 1995;8:121–30. doi: 10.1006/jaut.1995.0009. [DOI] [PubMed] [Google Scholar]
- 2.Rao RH, Vagnucci AH, Amico JA. Bilateral massive adrenal hemorrhage: early recognition and treatment. Ann Intern Med. 1989;110:227–235. doi: 10.7326/0003-4819-110-3-227. [DOI] [PubMed] [Google Scholar]
- 3.Grinspoon SK, Biller BM. Clinical review 62: laboratory assessment of adrenal insufficiency. J Clin Endocrinol Metab. 1994;79:923–931. doi: 10.1210/jcem.79.4.7962298. [DOI] [PubMed] [Google Scholar]
- 4.Nieman LK. Dynamic evaluation of adrenal hypofunction. J Endocrinol Invest. 2003;26:74–82. [PubMed] [Google Scholar]
- 5.Presotto F, Fornasini F, Betterle C, Federspil G, Rossato M. Acute adrenal failure as the heralding symptom of primary antiphospholipid syndrome: report of a case and review of the literature. Eur J Endocrinol. 2005;153:507–14. doi: 10.1530/eje.1.02002. [DOI] [PubMed] [Google Scholar]
- 6.Batt NM, Malik D, Harvie M, Sheth H. Non-haemorrhagic, bilateral adrenal infarction in a patient with antiphospholipid syndrome along with lupus myocarditis. BMJ Case Rep. 2016:2016. doi: 10.1136/bcr-2016-216364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Godfrey RL, Clark J, Field B. Bilateral adrenal haemorrhagic infarction in a patient with antiphospholipid syndrome. BMJ Case Rep. 2014:2014. doi: 10.1136/bcr-2014-207050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramon I, Mathian A, Bachelot A et al. Primary adrenal insufficiency due to bilateral adrenal hemorrhage-adrenal infarction in the antiphospholipid syndrome: long-term outcome of 16 patients. J Clin Endocrinol Metab. 2013;98:3179–3189. doi: 10.1210/jc.2012-4300. [DOI] [PubMed] [Google Scholar]
- 9.Mehdi AA, Salti I, Uthman I. Antiphospholipid syndrome: endocrinologic manifestations and organ involvement. Semin Thromb Hemost. 2011;37:49–57. doi: 10.1055/s-0030-1270071. [DOI] [PubMed] [Google Scholar]
- 10. TS . The adrenal cortex. In: Bloodworth JB Jr, editor. Endocrine Pathology: General and Surgical. 2nd ed. Baltimore, MD: Williams & Wilkins; 1982. pp. 419–471. [Google Scholar]
- 11.Fox B. Venous infarction of the adrenal glands. J Pathol. 1976;119:65–89. doi: 10.1002/path.1711190202. [DOI] [PubMed] [Google Scholar]
- 12.Riddell AM, Khalili K. Sequential adrenal infarction without MRI-detectable hemorrhage in primary antiphospholipid-antibody syndrome. AJR Am J Roentgenol. 2004;183:220–222. doi: 10.2214/ajr.183.1.1830220. [DOI] [PubMed] [Google Scholar]
- 13.Caron P, Chabannier MH, Cambus JP, Fortenfant F, Otal P, Suc JM. Definitive adrenal insufficiency due to bilateral adrenal hemorrhage and primary antiphospholipid syndrome. J Clin Endocrinol Metab. 1998;83:1437–1439. doi: 10.1210/jcem.83.5.4833. [DOI] [PubMed] [Google Scholar]
- 14.Rotman-Pikielny P, Rouach V, Chen O, Gur HG, Limor R, Stern N. Serum cortisol levels in patients admitted to the department of medicine: prognostic correlations and effects of age, infection, and comorbidity. Am J Med Sci. 2006;332:61–67. doi: 10.1097/00000441-200608000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Vantyghem MC, Balavoine AS, Wemeau JL, Douillard C. Hyponatremia and antidiuresis syndrome. Annales d'endocrinologie. 2011;72:500–512. doi: 10.1016/j.ando.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 16.Kolinioti A, Tsimaras M, Stravodimos G, Komporozos V. Acute adrenal insufficiency due to adrenal hemorrhage complicating colorectal surgery: report of two cases and correlation with the antiphospholipid antibody syndrome. Int J Surg Case Rep. 2018;51:90–94. doi: 10.1016/j.ijscr.2018.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]